Abstract
The causative agent of Lyme disease, Borrelia burgdorferi, is naturally resistant to its host's alternative pathway of complement-mediated killing. Several different borrelial outer surface proteins have been identified as being able to bind host factor H, a regulator of the alternative pathway, leading to a hypothesis that such binding is important for borrelial resistance to complement. To test this hypothesis, the development of B. burgdorferi infection was compared between factor H-deficient and wild-type mice. Factor B- and C3-deficient mice were also studied to determine the relative roles of the alternative and classical/lectin pathways in B. burgdorferi survival during mammalian infection. While it was predicted that B. burgdorferi should be impaired in its ability to infect factor H-deficient animals, quantitative analyses of bacterial loads indicated that those mice were infected at levels similar to those of wild-type and factor B- and C3-deficient mice. Ticks fed on infected factor H-deficient or wild-type mice all acquired similar numbers of bacteria. Indirect immunofluorescence analysis of B. burgdorferi acquired by feeding ticks from the blood of infected mice indicated that none of the bacteria had detectable levels of factor H on their outer surfaces, even though such bacteria express high levels of surface proteins capable of binding factor H. These findings demonstrate that the acquisition of host factor H is not essential for mammalian infection by B. burgdorferi and indicate that additional mechanisms are employed by the Lyme disease spirochete to evade complement-mediated killing.
Borrelia burgdorferi is maintained in nature through an infectious cycle between a variety of vertebrate reservoir hosts and infected Ixodes sp. ticks. B. burgdorferi readily disseminates throughout the vertebrate body from the tick bite site to cause persistent systemic infection. The infectious dose of B. burgdorferi is very low, with as few as 20 spirochetes being capable of causing infection and disease in immunocompetent animals (12). The ability of such low numbers of spirochetes to establish infection reflects an innate ability of these bacteria to efficiently overcome vertebrate immune systems.
The complement system is a critical component of vertebrate immune systems that functions to eliminate invading microorganisms. The alternative pathway of complement is activated independent of antibodies and serves as an early defense mechanism before the production of antibodies by the humoral immune response. The activation of the complement system triggers a proteolytic cascade that leads to C3 being cleaved and the deposition of C3b on surfaces of invading microorganisms, resulting in both opsonization and the formation of membrane attack complexes (MAC). The alternative pathway requires a serine protease, factor B, to be cleaved to an active form (Bb) upon binding C3b to form the C3/C5 convertase and ultimately the MAC. Humans and other vertebrates protect their own cells from damage by the complement system by covering their surfaces with complement regulatory proteins that inactivate C3b. Factor H is the major host serum protein involved in the negative regulation of the alternative pathway. Unfortunately for humans, many microbial pathogens have evolved mechanisms to exploit the host's regulatory proteins in order to evade complement-mediated killing, including the binding of factor H (47).
Virulent B. burgdorferi strains are highly resistant to complement-mediated killing by the alternative pathway, as evidenced by the low number of spirochetes required for mammalian infection and the abilities of many cultured Lyme disease spirochetes to withstand incubation in >50% normal serum (18, 19, 39, 43, 46, 64). Cultured B. burgdorferi is capable of binding host factor H to its surface, which has been proposed to be the mechanism by which it evades the killing effects of complement. Five borrelial proteins that have abilities to interact with factor H, which are collectively termed CRASPs (complement regulator-acquiring surface proteins), have been characterized to date. CRASP-1 is encoded on lp54 by cspA and is distinct from the other CRASPs (41). B. burgdorferi bacteria deficient in CRASP-1 were found to be sensitive to killing by serum in culture (21). High levels of CRASP-1 are expressed by essentially every bacterium during transmission from tick to mammal as well as from mammal to tick (84), although production is repressed within the first month of mammalian infection (54). CRASP-2, encoded on lp28-3 by cspZ, can bind factor H (34, 44, 45), and preliminary studies indicate that it is also expressed during mammal-to-tick transmission (T. Bykowski and B. Stevenson, unpublished results). CRASP-3, -4, and -5 are members of the Erp protein family and are encoded on the spirochete's resident cp32 prophages (4, 5, 36, 44, 77). Erp proteins and CRASP-3, -4, and -5 are also expressed during both transmission stages and are produced throughout chronic mammalian infection (58-61). Despite this wealth of information, the functionality of these proteins during mammalian and tick infections has not previously been examined.
To directly test the importance of factor H binding in mammalian infection, we assessed the ability of B. burgdorferi to infect and persist within factor H-deficient (Cfh−/−) mice. The relative abilities of Lyme disease spirochetes to transmit from Cfh−/− and wild-type mice to feeding ticks were also assessed. The role of the alternative pathway in controlling B. burgdorferi infection was further explored through infection studies of both factor B-deficient (Bf−/−) and C3-deficient (C3−/−) mice. The binding of factor H by bacteria during various stages of infecting mice and ticks was examined using immunofluorescence analysis (IFA).
MATERIALS AND METHODS
Bacteria and growth conditions.
Preliminary mouse infection studies used B. burgdorferi strain N40, a clonal, infectious, and wild-type isolate (13). All subsequent mouse and tick infection studies utilized B. burgdorferi strain B31-MI-16, an infectious clone of the sequenced, nonclonal strain B31-MI (24, 31, 60). Clone B31-MI-16 contains all of the plasmids carried by its parent culture (60). For all purposes, bacteria were grown in Barbour-Stoenner-Kelly II (BSK-II) (9) at 34°C to mid-exponential phase (approximately 107 bacteria/ml).
Mouse strains.
Cfh−/− mice (factor H deficient, lack regulation of alternative pathway) (66) and Bf−/− mice (factor B deficient, no alternative pathway activity) (53) have been backcrossed onto a C57BL/6 genetic background for 10 generations. The genotypes of these mice were initially confirmed by M. Botto, who developed the Cfh−/− strain, and then reconfirmed by M. E. Woodman following completion of these studies. Both the Cfh−/− and Bf−/− strains have been used extensively by others for a variety of infection and other studies (2, 23, 32, 56, 63, 66, 67, 82, 90, 91). The Botto laboratory provided these mouse strains to the Stevenson and Wooten laboratories, where the experiments described herein were performed. C57BL/6 mice deficient in C3 (C3−/−) (no complement activity) were obtained from Jackson Laboratory (Bar Harbor, ME) (86). Wild-type C57BL/6 mice were obtained from Harlan Sprague Dawley (Indianapolis, IN).
Detection of C3 in mouse sera.
Serum samples were collected from uninfected wild-type, Cfh−/−, Bf−/−, and C3−/− mice and examined by immunoblotting for the presence of C3. A 2-μl aliquot of each serum sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electrotransferred to a nitrocellulose membrane, and blocked with 5% nonfat dry milk in Tris-buffered saline-Tween 20 (20 mM Tris [pH 7.5], 150 mM NaCl, 0.05% [vol/vol] Tween 20) overnight at 4°C. C3 was detected by incubation with goat anti-human C3 polyclonal antiserum (Quidel, San Diego, CA), diluted 1:2,000 in Tris-buffered saline-Tween 20, for 1 h at room temperature. The membrane was then washed and incubated with bovine anti-goat horseradish peroxidase conjugate (Santa Cruz Biotechnology, Santa Cruz, CA), and bound antibodies were visualized by enhanced chemiluminescence (Pierce, Rockford, IL).
Mouse infection studies.
The doses and route of injection used in these studies result in a progression of infection comparable to that following infection by natural tick bite (28, 74). Needle injection of cultured bacteria was utilized to ensure reproducible infection by known quantities of B. burgdorferi, which is impossible to achieve by using a tick bite route of infection.
In a preliminary experiment, 16 each of wild-type and Cfh−/− 4- to 6-week-old female mice were injected intradermally with 2 × 103 B. burgdorferi strain N40 bacteria. Groups of 8 Cfh−/− mice were killed after 2 or 4 weeks of infection, as were also 10 wild-type mice after 2 weeks and 6 wild-type mice after 4 weeks of infection. Tibiotarsal joints, ear pinnae, and hearts were removed immediately, and total DNA was extracted as previously described (22).
Based upon the results of the above-described pilot study, a more comprehensive study was undertaken, using a well-characterized derivative of B. burgdorferi strain B31. Groups of female wild-type, Cfh−/−, Bf−/−, and C3−/− mice, 4 to 6 weeks old, were infected by subcutaneous injection with 5 × 104 B. burgdorferi B31-MI-16 bacteria. Cohorts of eight mice of each strain were killed at 1, 2, or 4 weeks postinoculation, numbers of mice that have been reported to consistently provide statistically significant values for animal infection studies (22, 49, 70, 88). Ear pinnae, tibiotarsal joints, and hearts were immediately harvested, placed in liquid nitrogen, and stored at −80°C.
All experimental protocols used in the handling and infections of mice were approved by the Institutional Animal Care and Use Committees and the Institutional Biosafety Committees of the University of Kentucky and the University of Toledo Health Sciences Campus. Prior to their use in these studies, mice were tested by institutional veterinarians and determined to be free of pathogenic bacteria, viruses, nematodes, and other infectious agents.
Quantification of B. burgdorferi during mammalian infection.
Total DNA was extracted from individual tissues using Puregene genomic DNA purification kits (Gentra Systems, Inc., Minneapolis, MN). After purification, the DNA content of each sample was determined by measuring the absorbance at 260 nm and a working concentration of 50 ng/μl water was created for each.
Quantitative PCR was performed using a LightCycler thermal cycler (Roche Applied Science, Indianapolis, IN), as described previously (48, 62). The B. burgdorferi recA gene and the mouse nidogen gene were targeted for amplification to determine the number of spirochetes in each tissue sample (62). Briefly, each amplification was performed with 25 ng of sample DNA in a 10-μl final volume containing Platinum Taq polymerase (Invitrogen, Carlsbad, CA) (final dilution of 1:10 into enzyme diluent; Idaho Technology, Salt Lake City, UT), 0.8 mM deoxynucleoside triphosphates (Idaho Technology), 1× PCR buffer (final concentration, 3 mM MgCl2) (Idaho Technology), 0.5 μM of each oligonucleotide primer, and 1× SYBR green (Molecular Probes, Eugene, OR) (final dilution of 1:10,000 in Tris-EDTA buffer [10 mM Tris-HCl containing 1 mM EDTA-Na2, pH 8.0]; Promega, Madison, WI). A negative control that lacked template was included with each assay to ensure that reagents were not contaminated with extraneous DNA. Dilutions of B31-MI-16 and uninfected wild-type mouse DNA were also included to generate standard curves to quantify the amount of each respective DNA in the samples. All DNA samples were analyzed in triplicate. Melting curves were analyzed for each reaction to ensure specificity of the PCR product. The amplification program for B. burgdorferi recA consisted of an initial denaturation step at 95°C for 2 min, followed by 45 amplification cycles comprised of 95°C for 1 s, 60°C for 4 s, 72°C for 10 s, and 82°C for 3 s. The amplification program for mouse nidogen consisted of an initial denaturation step at 95°C for 2 min, followed by 40 amplification cycles comprised of 95°C for 1 s, 60°C for 4 s, 72°C for 10 s, and 84°C for 3 s (48, 62). The oligonucleotide primers used to detect B. burgdorferi recA were nTM17.F (5′-GTG GAT CTA TTG TAT TAG ATG AGG CTC TCG-3′) and nTM17.R (5′-GCC AAA GTT CTG CAA CAT TAA CAC CTA AAG-3′). The oligonucleotide primers used to detect mouse nidogen were nido.F (5′-CCA GCC ACA GAA TAC CAT CC-3′) and nido.R (5′-GGA CAT ACT CTG CTG CCA TC-3′) (62, 89). Data were reported as numbers of spirochete genomes per 1,000 mouse cell genomes.
Measurement of antibody concentrations during mammalian infection.
Serum was prepared from each B. burgdorferi-infected mouse at the indicated times postinfection. B. burgdorferi-specific immunoglobulin (Ig) was detected in each serum sample by enzyme-linked immunosorbent assay as previously described (48). Briefly, microtiter plates were coated with 5 μg/ml sonicated B. burgdorferi B31-MI-16 or goat antibody to mouse IgG, IgM, and IgA (Southern Biotechnology Associates, Birmingham, AL). Serum dilutions were added to the microtiter plates for 90 min at 37°C and then washed to remove unbound material. Bound Ig was detected using horseradish peroxidase-conjugated antibodies to murine IgG or IgM (Southern Biotechnology). Quantification of Ig was determined by comparing standard curves for purified IgG and IgM (Southern Biotechnology) (48).
Tick rearing and infection.
Ixodes scapularis egg masses were obtained from the Department of Entomology at Oklahoma State University (Stillwater, OK) and held at room temperature in a chamber at 95% relative humidity until hatching to larvae. Larval acquisition of B. burgdorferi from infected mice was examined by feeding approximately 200 naïve larvae each on wild-type and Cfh−/− mice that were previously infected with B. burgdorferi (see above). Larval ticks were allowed to feed to repletion and naturally detach from the mice. Some ticks were dissected immediately after detachment, while the remaining ticks were placed in a humidified chamber to molt into nymphal ticks. Studies of B. burgdorferi transmission from infected ticks to mice were then performed by allowing 20 infected nymphs per mouse to feed on naïve wild-type and Cfh−/− mice. Engorging nymphal ticks were forcibly removed with fine forceps after feeding on the mice for 72 h (60, 84).
Analysis of factor H binding in ticks and tick bite sites.
After drop-off or forcible removal, larval and nymphal tick midguts were immediately dissected. Often during forcible removal of a feeding tick, a piece of skin remained attached to the hypostome. Those bite-site biopsy samples were carefully dissected away from the tick and also examined. At least six ticks of each life stage were examined. At least two distinct mice were used for tick feeding/transmission of each tick life stage. Multiple tick bite sites from four different wild-type C57BL/6 and Cfh−/− mice were examined. All tick midguts and skin samples were dissected into 10 μl phosphate-buffered saline (PBS) on glass slides and allowed to air dry overnight. Control slides with cultured B. burgdorferi were prepared by growing the bacteria to mid-exponential phase and then incubated with either 40% or 100% nonimmune wild-type mouse or human serum for 30 min at 34°C or examined without incubation in any additional serum. Ten-microliter aliquots of such cultured bacteria were spread on glass slides and allowed to air dry overnight.
Slides were then fixed and permeabilized in acetone for 15 min and allowed to air dry. Slides were blocked overnight in PBS containing 0.2% bovine serum albumin (BSA) at 4°C. Slides were then incubated in goat anti-human factor H polyclonal antiserum (Quidel) diluted 1:1,000 in PBS-0.2% BSA for 1 h at room temperature. Slides were washed in PBS-0.2% BSA and incubated for 1 h at room temperature with rabbit polyclonal antiserum raised against B. burgdorferi total membrane proteins, diluted 1:40,000 in PBS-0.2% BSA (60). Slides were washed and incubated simultaneously with 1:1,000 dilutions of Alexa Fluor 488-labeled donkey anti-goat IgG and Alexa Fluor 594-labeled donkey anti-rabbit IgG (Molecular Probes) for 45 min at room temperature. Slides were then washed, dried, and mounted in ProLong antifade mounting medium (Molecular Probes) (60, 84). Slides were analyzed at ×400 magnification using a BX51 epifluorescence microscope (Olympus, Melville, NY) and a Retiga 200R Fast 1394 imaging system (QImaging, Burnaby, BC, Canada). To quantify the number of spirochetes acquired by ticks from infected wild-type and Cfh−/− mice, labeled bacteria were counted in 25 random fields per slide, with a minimum of three slides being analyzed per mouse strain (60, 84).
Statistical analysis.
All analyses were performed by K. Tucker, a professional statistician (79, 87). An analysis of variance was performed in SAS version 9.1 (SAS Institute, Cary, NC), using the GLM procedure to compare mean spirochete and antibody levels across strains of mice. Spirochete and antibody levels were analyzed separately at each time point, and spirochete values were normalized using a log10 transformation. For those models with significant P values (P < 0.05), Tukey's test was performed to determine which strains were different from one another.
RESULTS
Quantification of B. burgdorferi infection of mouse tissues.
To determine the contribution of factor H binding on the ability of B. burgdorferi to survive in the mammalian host, spirochete numbers were compared following infection of wild-type and homozygous factor H-deficient (Cfh−/−) mice. Although Cfh−/− mice are largely unable to control the activation of C3 (66), they continually produce additional C3 and so contain reduced, but detectable, levels of plasma C3 (66; data not shown).
In a pilot study, 16 each of Cfh−/− and wild-type C57BL/6 mice were infected with B. burgdorferi strain N40. Half of the factor H-deficient mice were killed after 2 weeks of infection and the remaining half after 4 weeks of infection. Ten of the wild-type mice were killed after 2 weeks and the remaining six after 4 weeks. Quantitative PCR analyses of the animals' tibiotarsal joints indicated that B. burgdorferi successfully infected the factor H-deficient mice, with tissue burdens that were equal to or greater than those found for wild-type animals (data not shown).
Following that unanticipated result, a more detailed study was undertaken to better characterize B. burgdorferi infection of Cfh−/− mice. This study utilized the well-characterized B. burgdorferi strain B31-MI, a strain for which all of the surface proteins capable of interacting with host factor H have been biochemically and temporally characterized (4, 21, 29, 33, 34, 37, 40-42, 44, 60, 71, 77, 84; Bykowski and Stevenson, unpublished). To further examine the role of the complement system in controlling B. burgdorferi infection, mice deficient in either factor B (Bf−/−) or C3 (C3−/−) were also examined. Cohorts of eight animals of each strain were examined after 1, 2, or 4 weeks of infection.
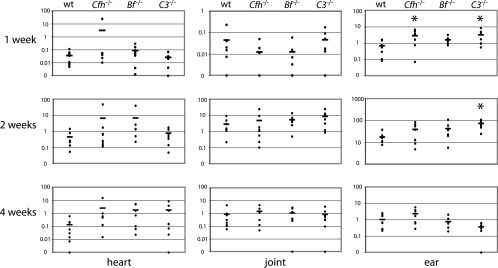
At 1 week postinfection, all animals from all tested strains were infected, but the B. burgdorferi levels were relatively low in all tissues (Fig. 1). At that time point, only minimal levels of spirochete-specific IgM were detectable (Fig. 2). No significant differences in bacterial loads were found in heart and joint tissues of Cfh−/− mice compared to those in wild-type animals, although higher numbers of bacteria were detected within ear tissues (P < 0.05). Likewise, C3−/− mice also possessed higher B. burgdorferi numbers in ear tissues than did wild-type mice (P < 0.05), but bacterial levels were similar in both heart and joint tissues of wild-type and C3−/− mice. Bf−/− mice did not demonstrate statistically significant differences in the bacterial loads of any tested tissue compared to wild-type mice.
FIG. 1.
B. burgdorferi numbers in heart, joint, and ear tissues of wild-type (wt), Cfh−/−, Bf−/−, and C3−/− mice following 1, 2, or 4 weeks of infection, as determined by quantitative PCR. y axis values indicate numbers of B. burgdorferi bacteria per 1,000 mouse cells. Each dot represents an individual mouse, and the bars indicate the averages. Asterisks indicate tissues of complement-deficient mice in which spirochete numbers were significantly different from those of wild-type mice (P < 0.05).
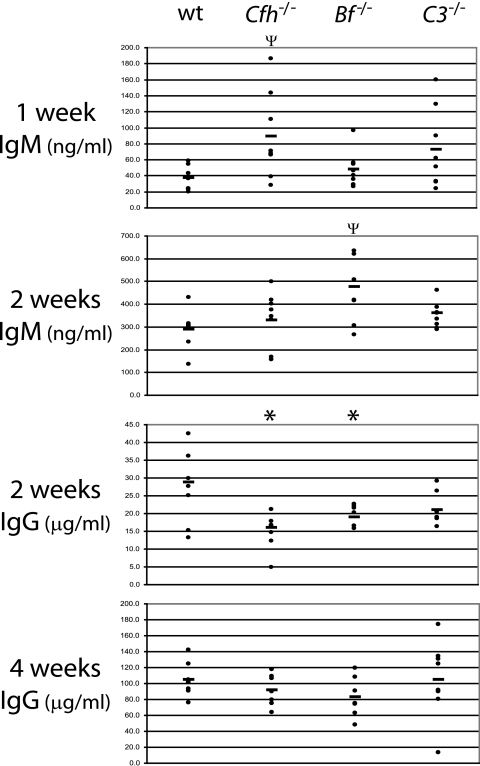
FIG. 2.
B. burgdorferi-specific antibody levels in wild-type (wt), Cfh−/−, Bf−/−, and C3−/− mice following 1, 2, or 4 weeks of infection, as determined by enzyme-linked immunosorbent assay. Each dot represents an individual mouse, and the bars indicate the averages. Asterisks indicate tissues of complement-deficient mice in which antibody levels were significantly different from those of wild-type mice (P = 0.002). Ψ indicates tissues of complement-deficient mice in which antibody levels were marginally different from those of wild-type mice (P = 0.053 or P = 0.052).
By 2 weeks postinfection, the spirochete numbers had increased in all tissues of all mouse strains (Fig. 1) and substantial antibody levels were detected (Fig. 2). Although not statistically significant, the Cfh−/− mice trended toward higher levels of B. burgdorferi, with 6.55, 4.94, and 39.2 spirochetes, than did wild-type mice, with 0.45, 2.88, and 17.6 spirochetes per 1,000 murine cells in the heart, joint, and ear tissues, respectively. One Cfh−/− mouse had exceptionally high bacterial numbers in the heart, which caused the average to increase dramatically; exempting this sample from calculations reduced the average to 0.54 spirochetes per 1,000 murine heart cells. The tissues from Bf−/− mice also possessed higher numbers of B. burgdorferi bacteria than did those from wild-type mice, although none were significantly different. As in the Cfh−/− studies, one Bf−/− heart tissue sample contained extraordinarily high numbers of spirochetes. Notably, the joints and ears of the exceptional Cfh−/− and Bf−/− mice contained spirochete levels similar to those of all other mice of that strain, suggesting that these individual mice were not simply injected with higher doses than the other mice. The C3−/− mice had 0.79, 8.75, and 73.7 spirochetes per 1,000 murine cells in the heart, joint, and ear tissues, respectively, but only the numbers in ear tissues were significantly different from those in wild-type mice (P < 0.05).
Between 2 and 4 weeks postinfection, the spirochete numbers were shown to decrease in all tissues (Fig. 1) and robust antibody responses were evident (Fig. 2). With the production of antibodies, the classical pathway could play a role in controlling the spirochetes. However, no significant differences were seen in numbers of spirochetes in any tissues of wild-type or complement-deficient mice.
B. burgdorferi-specific antibody responses in mice.
To address any potential influence that the classical pathway of complement activation might have on the observed spirochete levels, the B. burgdorferi-specific antibody levels were determined in the infected wild-type, Cfh−/−, Bf−/−, and C3−/− mice after 1, 2, or 4 weeks of infection. At 1 week postinfection, the levels of B. burgdorferi-specific IgM were measured. Cfh−/− mice had serum IgM levels that were marginally higher than those of wild-type mice (P = 0.053) (Fig. 2). No significant amounts of B. burgdorferi-specific IgG were detected at this time in any mouse strain (data not shown). By 2 weeks postinfection, substantial increases were detected in both IgM and IgG levels for all mouse strains. Marginally significant differences in IgM levels (P = 0.052) were detected between Bf−/− and wild-type mice. Cfh−/− and Bf−/− mice had significantly lower IgG levels than wild-type mice (P = 0.002), but no significant differences were detected in C3−/− mice. By 4 weeks postinfection, no differences in B. burgdorferi-specific IgG levels were detected between any mouse strains. Thus, the only distinguishable effect of the complement deficiencies was a slight delay in switching from IgM to IgG, much as was previously observed (30, 81). Importantly, there was no relationship between high antibody levels and low B. burgdorferi numbers, or vice versa, in any individual mouse. Uninfected control samples from all mouse strains possessed similar, low levels of IgM (∼15.0 ng/ml) that recognized B. burgdorferi, most likely indicative of natural antibody (14), but there were no detectable levels of B. burgdorferi-specific IgG.
Examination of interactions between B. burgdorferi and host factor H in ticks and skin bite wounds.
As described above, B. burgdorferi is capable of binding factor H from serum through a number of spirochete-encoded proteins. However, all previous analyses of factor H binding were performed in vitro, using either cultured bacteria or recombinant proteins and purified factor H or serum. To determine whether B. burgdorferi actually binds factor H during the natural infectious cycle, we examined individual bacteria by IFA during different stages of mammal-to-tick transmission. Previous studies have shown that B. burgdorferi CRASPs are able to bind murine factor H (3, 77; J. Hellwage, unpublished results), and consistent with those in vitro studies, IFA of B. burgdorferi incubated in either 40% (data not shown) or 100% (Fig. 3A) fresh, normal mouse serum indicated factor H binding. B. burgdorferi incubated in human serum also bound factor H, with IFA intensities similar to those for mouse factor H (data not shown). Factor H binding was also detected, albeit to a weaker extent, when we examined B. burgdorferi cultured in BSK-II (contains 6% rabbit serum but no additional source), indicating that factor H bound to bacteria can be identified by IFA even at low serum concentrations (Fig. 3B).
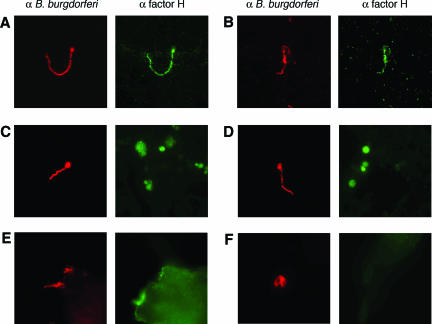
FIG. 3.
Representative IFA images of B. burgdorferi interactions with factor H in culture and during tick and mammal infections. At least six ticks of each life stage were examined, with an average of 20 bacteria detected in each dissected tick. For each tick life stage, at least two distinct mice were used for tick feeding/transmission. Tick bite sites from at least four different wild-type and Cfh−/− mice were examined. Goat anti-human factor H polyclonal antiserum (α factor H) labeled those spirochetes with bound factor H, while rabbit polyclonal antiserum raised against B. burgdorferi total membrane proteins (α B. burgdorferi) labeled all spirochetes in the same field. (A and B) Cultured B. burgdorferi incubated in 100% mouse serum (A) or in BSK-II culture medium (which contains 6% rabbit serum as an essential ingredient) (B). (C and D) B. burgdorferi in midguts of naïve larval I. scapularis ticks immediately following completion of feeding on infected mice. Autofluorescent components of the digesting blood meal are visible in the anti-factor H (green light) channel and were also apparent in midguts of larvae fed on factor H-deficient mice (data not shown). (E and F) Skin samples at tick bite sites from wild-type (E) and factor H-deficient (F) mice.
During mammalian infection, B. burgdorferi bacteria are widely dispersed at low concentrations throughout their host, which prevents the direct assessment of factor H acquisition within the mammalian host (69). However, when larval ticks acquire B. burgdorferi from an infected vertebrate host, large numbers of bacteria become concentrated in the tick midgut. Moreover, essentially 100% of the bacteria express high levels of CRASP-1 and Erp proteins (60, 61, 84). Feeding tick larvae, full of fresh blood and newly acquired B. burgdorferi, were dissected and examined by IFA for the presence of factor H on the bacteria. Surprisingly, none of the bacteria acquired by tick larvae during natural feeding exhibited factor H binding on their surfaces (Fig. 3C and D and data not shown). B. burgdorferi bacteria within midguts of larval ticks that fed on wild-type mice were as devoid of IFA signals as bacteria in ticks fed on Cfh−/− mice (data not shown). Furthermore, the numbers of B. burgdorferi bacteria detected in larval ticks that fed on infected wild-type and Cfh−/− mice were similar, indicating that factor H is not critical during mammalian infection and during acquisition of borreliae from the host and that the bacteria can evade complement-mediated killing by other means.
In the next stage of the B. burgdorferi life cycle, infected nymphal ticks transmit bacteria to a new host during their blood meal. Spirochetes within feeding nymph midguts express low levels of all CRASPs on their surfaces (60, 84). Consistent with these data, factor H could not be detected on any B. burgdorferi bacteria in the midguts of the feeding, blood-filled nymphs (data not shown).
During transmission from feeding nymphs to the skin of the host, B. burgdorferi bacteria dramatically increase the expression of surface proteins capable of binding factor H, such that essentially 100% of transmitted bacteria produce CRASP-1 and Erp proteins (35, 60, 84). However, none of the B. burgdorferi bacteria detected in mouse skin during transmission appeared to bind factor H above the background staining in the skin (Fig. 3E). An unavoidable caveat to this final analysis is that mouse tissues are covered in factor H, and bacteria are too infrequently found in the skin to allow analysis separate from the host tissue. Thus, it is possible that newly transmitted B. burgdorferi bacteria might bind host factor H to some extent. B. burgdorferi bacteria were also detected in the skin of factor H-deficient mice following feeding by infected ticks, demonstrating that the entire B. burgdorferi life cycle, from murine infection to acquisition by larval ticks to transmission of tick nymphs back into mice, can occur in the absence of host factor H (Fig. 3F). As would be expected, the B. burgdorferi bacteria within the skin of factor H-deficient mice did not yield anti-factor H IFA signals.
DISCUSSION
As are many other pathogenic organisms, virulent B. burgdorferi strains are generally resistant to their host's alternative pathway of complement. The spirochete can express several surface proteins capable of binding factor H, which has been hypothesized to afford the bacteria with protection against host complement (4, 5, 21, 34-36, 41, 44, 45, 47, 55, 58-61, 77, 84). We therefore anticipated that B. burgdorferi would be impaired in its ability to infect and/or persist within animals deficient in factor H. To the contrary, Cfh−/− mice were infected at levels similar to those of wild-type animals. Infection levels were also similar to those of mice lacking factor B or C3, which cannot utilize the alternative pathway or any pathway of complement activation, respectively. For most mouse tissues and durations of infection, spirochete loads in any complement-deficient animal did not significantly differ from those observed in wild-type mice. After 1 and 2 weeks of infection, elevated numbers of spirochetes were detected in ear tissues of C3−/− mice, but not Bf−/− mice, suggesting the involvement of a complement activation pathway other than the alternative pathway during early stages of mammalian infection. For comparison, a study with complement-deficient mice demonstrated that innate immunity to Streptococcus pneumoniae infection is mediated primarily by the classical complement pathway (23), whereas the alternative pathway is essential for murine resistance to Pseudomonas aeruginosa (63) and both are important for resistance to Streptococcus pyogenes (90). B. burgdorferi loads in Cfh−/− mice did not differ significantly from those in wild-type mice, with the exception of those in ear tissues after 1 week of infection, indicating that factor H is not essential for protecting B. burgdorferi from clearance by mammalian hosts. One possible explanation for these observations is that the function of factor H is redundant to the functions of other host proteins capable of regulating the alternative pathway. B. burgdorferi may produce additional substances that afford it with protection against complement, such as a putative slime layer (43) and an apparent CD59-like protein that obstructs MAC formation (65). Several pathogens are known to bind other host components or produce their own, novel substances to aid in the protection against complement (10, 11, 15, 47, 51, 57, 68). A large number of studies have clearly indicated that B. burgdorferi expresses a repertoire of proteins during mammalian infection different from the one it expresses during laboratory cultivation (6, 20, 27, 50, 75, 78), so it is highly probable that other, as-yet-unidentified bacterial factors protect the spirochete from complement in vivo. Supporting those alternative hypotheses are the Lyme disease borreliae that are virulent for humans and other mammals yet lack the ability to bind factor H (5, 45, 55, 85).
Several studies have demonstrated that the relative infectivity of Lyme disease spirochetes for humans and other animals does not always correlate with their ability to withstand the direct bactericidal effects of normal serum in vitro (5, 18, 19, 43, 46, 83). This suggests that serum ex vivo does not necessarily replicate conditions experienced by B. burgdorferi during actual infection. For example, murine serum is known to contain inhibitors of both the alternative and classical pathways which become activated upon the isolation of serum from mice (7, 73), which renders mouse serum largely unusable for in vitro assays. For those reasons, we were unable to directly examine B. burgdorferi interactions in vitro using isolated sera from the different complement-deficient mouse strains.
To further explore the role of factor H in the natural course of B. burgdorferi infection, individual bacteria were examined during various stages of host vector infection. Bacteria acquired by ticks feeding on infected animals express all known surface proteins capable of binding factor H (60, 84; Bykowski and Stevenson, unpublished). However, none of the examined bacteria acquired by feeding tick larvae had detectable levels of factor H on their surfaces. Our control studies, along with parallel studies utilizing cultured organisms, indicate that B. burgdorferi is capable of binding mouse factor H (3, 77; Hellwage, unpublished). Blood meal digestion by ticks occurs intracellularly, and tick midgut cells are not known to secrete any protease which might degrade factor H (1, 8, 76). These data suggest that B. burgdorferi CRASPs may bind host components other than factor H, which preclude CRASP-factor H binding. Supporting that hypothesis, the borrelial factor H-binding proteins CRASP-1 (CspA, open reading frame [ORF] BBA68), ErpA (CRASP-5, ORF BBL39/BBP38), ErpC (CRASP-4), and ErpP (CRASP-3, ORF BBN38) all bind additional serum proteins (34a, 37, 54; our unpublished results).
As noted above, no significant differences in bacterial levels were detected between Bf−/− and wild-type mouse tissues at any times postinfection, suggesting that complement activation via the alternative pathway does not play a significant role in controlling B. burgdorferi infection. In contrast, C3-deficient mice developed significantly increased bacterial loads in ear tissues during the first 2 weeks of infection, which may reflect reduced MAC formation and associated lysis and/or a lack of opsonic C3 breakdown products in those tissues (19, 80). However, no differences in bacterial loads were detected in either heart or joint tissue. These data suggest that the classical and/or lectin-binding pathways may affect spirochete numbers in some tissues even before the elicitation of a substantial humoral response. Our study and others have shown that IgM antibodies that bind B. burgdorferi are naturally present in uninfected mammals (14), which could account for the observed bacterial increases in C3−/− mice. Some antibodies can effectively kill B. burgdorferi even in the absence of complement (25, 26, 72). Other innate and adaptive immune responses may also contribute to the control of B. burgdorferi infection and account for the abilities of even C3-deficient mice to control bacterial numbers (17, 22, 38, 52). The borrelial numbers we observed in the complement-deficient mice support previous studies that indicate that the complement system plays a relatively minor role in controlling B. burgdorferi in the host (16, 48).
In summary, the results demonstrate that host factor H is not critical for B. burgdorferi to efficiently infect the mammalian host. B. burgdorferi bacteria can infect and persist at similar levels within multiple tissues of Cfh-deficient and wild-type animals. Moreover, B. burgdorferi bacteria acquired by feeding ticks from infected hosts did not bind detectable amounts of factor H, despite their production of surface proteins capable of binding that host complement regulator. Thus, B. burgdorferi CRASPs may perform additional functions other than factor H binding in vivo, and this spirochetal pathogen must evade complement-mediated killing by some alternative mechanism(s). It is intriguing that mice effectively controlled spirochete infections even in the absence of the key complement component C3, demonstrating that complement-independent mechanisms restrict B. burgdorferi proliferation during mammalian infection. Elucidating those mechanisms will provide important insight into the complex pathogenesis of Lyme disease.
Acknowledgments
This study was funded by U.S. National Institutes of Health grant R01-AI44254 to Brian Stevenson and American Heart Association Scientist Development grant 0335148N to R. Mark Wooten. Michael E. Woodman and Jennifer C. Miller were supported in part by NIH Training Grant in Microbial Pathogenesis T32-AI49795.
We thank Catherine Brissette, Logan Burns, Sean Riley, and Kate von Lackum for their technical assistance and helpful comments on this work and the manuscript.
The laboratories of B. Stevenson and R. M. Wooten contributed equally to this work.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 9 April 2007.
REFERENCES
- 1.Akov, S. 1982. Blood digestion in ticks, p. 197-211. In F. D. Obenchain and R. Galun (ed.), Physiology of ticks. Pergamon Press, Elmsford, NY.
- 2.Alexander, J. J., M. C. Pickering, M. Haas, I. Osawe, and R. J. Quigg. 2005. Complement factor H limits immune complex deposition and prevents inflammation and scarring in glomeruli of mice with chronic serum sickness. J. Am. Soc. Nephrol. 16:52-57. [DOI] [PubMed] [Google Scholar]
- 3.Alitalo, A., T. Meri, T. Chen, H. Lankinen, Z.-Z. Cheng, T. S. Jokiranta, I. J. T. Seppälä, P. Lahdenne, P. S. Hefty, D. R. Akins, and S. Meri. 2004. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J. Immunol. 172:6195-6201. [DOI] [PubMed] [Google Scholar]
- 4.Alitalo, A., T. Meri, H. Lankinen, I. Seppälä, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 5.Alitalo, A., T. Meri, L. Rämö, T. S. Jokiranta, T. Heikkilä, I. J. T. Seppälä, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anguita, J., M. N. Hedrick, and E. Fikrig. 2003. Adaptation of Borrelia burgdorferi in the tick and the mammalian host. FEMS Microbiol. Rev. 27:493-504. [DOI] [PubMed] [Google Scholar]
- 7.Appelmelk, B. J., A. M. J. J. Verweij-Van Vught, J. J. Maaskant, L. G. Thijs, and D. M. MacLaren. 1992. Murine ascitic fluids contain varying amounts of an inhibitor that interferes with complement-mediated effector functions of monoclonal antibodies. Immunol. Lett. 33:135-138. [DOI] [PubMed] [Google Scholar]
- 8.Balashov, Y. S. 1972. Bloodsucking ticks (Ixodoidea)—vectors of diseases of man and animals. Misc. Publ. Entomol. Soc. Am. 8:161-376. [Google Scholar]
- 9.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes, M. G., and A. A. Weiss. 2001. BrkA protein of Bordetella pertussis inhibits the classical pathway of complement after C1 deposition. Infect. Immun. 69:3067-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barondess, J. J., and J. Beckwith. 1995. bor gene of phage λ, involved in serum resistance, encodes a widely conserved outer membrane lipoprotein. J. Bacteriol. 177:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barthold, S. W. 1991. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J. Infect. Dis. 163:419-420. [DOI] [PubMed] [Google Scholar]
- 13.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 14.Belperron, A. A., and L. K. Bockenstedt. 2001. Natural antibody affects survival of the spirochete Borrelia burgdorferi within feeding ticks. Infect. Immun. 69:6456-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berggård, K., E. Johnsson, E. Morfeldt, J. Persson, M. Stalhammar-Carlemalm, and G. Lindahl. 2001. Binding of human C4bp to the hypervariable region of M-protein: a molecular mechanism of phagocytosis resistance in Streptococcus pyogenes. Mol. Microbiol. 42:539-551. [DOI] [PubMed] [Google Scholar]
- 16.Bockenstedt, L. K., S. Barthold, K. Deponte, N. Marcantonio, and F. S. Kantor. 1993. Borrelia burgdorferi infection and immunity in mice deficient in the fifth component of complement. Infect. Immun. 61:2104-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173:2003-2010. [DOI] [PubMed] [Google Scholar]
- 18.Brade, V., I. Kleber, and G. Acker. 1992. Differences of two Borrelia burgdorferi strains in complement activation and serum resistance. Immunobiology 185:453-465. [DOI] [PubMed] [Google Scholar]
- 19.Breitner-Ruddock, S., R. Würzner, J. Schulze, and V. Brade. 1997. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med. Microbiol. Immunol. 185:253-260. [DOI] [PubMed] [Google Scholar]
- 20.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks, C. S., S. R. Vuppala, A. M. Jett, A. Alitalo, S. Meri, and D. R. Akins. 2005. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol. 175:3299-3308. [DOI] [PubMed] [Google Scholar]
- 22.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 99:16969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 25.Coleman, J. L., R. C. Rogers, and J. L. Benach. 1992. Selection of an escape variant of Borrelia burgdorferi by use of bacteriocidal monoclonal antibodies to OspB. Infect. Immun. 60:3098-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman, J. L., R. C. Rogers, P. A. Rosa, and J. L. Benach. 1994. Variations in the ospB gene of Borrelia burgdorferi result in differences in monoclonal antibody reactivity and in production of escape variants. Infect. Immun. 62:303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Silva, A. M., E. Fikrig, E. Hodzic, F. S. Kantor, S. R. Telford, and S. W. Barthold. 1998. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J. Infect. Dis. 177:395-400. [DOI] [PubMed] [Google Scholar]
- 28.de Souza, M. S., A. L. Smith, D. S. Beck, L. J. Kim, G. M. Hansen, and S. W. Barthold. 1993. Variant responses of mice to Borrelia burgdorferi depending on the site of intradermal inoculation. Infect. Immun. 61:4493-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Hage, N., K. Babb, J. A. Carroll, N. Lindstrom, E. R. Fischer, J. C. Miller, R. D. Gilmore, Jr., M. L. Mbow, and B. Stevenson. 2001. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology 147:821-830. [DOI] [PubMed] [Google Scholar]
- 30.Fischer, M. B., M. Ma, S. Goerg, X. Zhou, J. Xia, O. Finco, S. Han, G. Kelsoe, R. G. Howard, T. L. Rothstein, E. Kremmer, F. S. Rosen, and M. C. Carroll. 1996. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J. Immunol. 157:549-556. [PubMed] [Google Scholar]
- 31.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidmann, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 32.Giacomin, P. R., H. Wang, D. L. Gordon, M. Botto, and L. A. Dent. 2005. Loss of complement activation and leukocyte adherence as Nippostrongylus brasiliensis develops within the murine host. Infect. Immun. 73:7442-7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilmore, R. D., Jr., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann, K., C. Corvey, C. Skerka, M. Kirschfink, M. Karas, V. Brade, J. C. Miller, B. Stevenson, R. Wallich, P. F. Zipfel, and P. Kraiczy. 2006. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol. Microbiol. 61:1220-1236. [DOI] [PubMed] [Google Scholar]
- 34a.Haupt, K., R. Wallich, P. Kraiczy, V. Brade, C. Skerka, and P. F. Zipfel. Binding of human FHR-1 to serum resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J. Infect. Dis., in press. [DOI] [PubMed]
- 35.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, J. D. Radolf, and D. R. Akins. 2001. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect. Immun. 69:3618-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hellwage, J., T. Meri, T. Heikkilä, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppälä, and S. Meri. 2001. The complement regulatory factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 37.Hovis, K. M., E. Tran, C. M. Sundy, E. Buckles, J. V. McDowell, and R. T. Marconi. 2006. Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis. Infect. Immun. 74:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu, L. T., and M. S. Klempner. 1997. Host-pathogen interactions in the immunopathogenesis of Lyme disease. J. Clin. Immunol. 17:354-365. [DOI] [PubMed] [Google Scholar]
- 39.Kochi, S. K., R. C. Johnson, and A. P. Dalmasso. 1993. Facilitation of complement-dependent killing of the Lyme disease spirochete, Borrelia burgdorferi, by specific immunoglobulin G Fab antibody fragments. Infect. Immun. 61:2532-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraiczy, P., K. Hartmann, J. Hellwage, C. Skerka, V. Brade, P. F. Zipfel, R. Wallich, and B. Stevenson. 2004. Immunological characterization of the complement regulator factor H-binding CRASP and Erp proteins of Borrelia burgdorferi. Int. J. Med. Microbiol. 293(Suppl. 37):152-157. [DOI] [PubMed] [Google Scholar]
- 41.Kraiczy, P., J. Hellwage, C. Skerka, H. Becker, M. Kirschfink, M. M. Simon, V. Brade, P. F. Zipfel, and R. Wallich. 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 279:2421-2429. [DOI] [PubMed] [Google Scholar]
- 42.Kraiczy, P., J. Hellwage, C. Skerka, M. Kirschfink, V. Brade, P. F. Zipfel, and R. Wallich. 2003. Immune evasion of Borrelia burgdorferi: mapping of a complement inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur. J. Immunol. 33:697-707. [DOI] [PubMed] [Google Scholar]
- 43.Kraiczy, P., K.-P. Hunfeld, S. Breiner-Ruddock, R. Würzner, G. Acker, and V. Brade. 2000. Comparison of two laboratory methods for the determination of serum resistance in Borrelia burgdorferi isolates. Immunobiology 201:406-419. [DOI] [PubMed] [Google Scholar]
- 44.Kraiczy, P., C. Skerka, V. Brade, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 46.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2001. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int. Immunopharmacol. 1:393-401. [DOI] [PubMed] [Google Scholar]
- 47.Kraiczy, P., and R. Würzner. 2006. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol. Immunol. 43:31-44. [DOI] [PubMed] [Google Scholar]
- 48.Lawrenz, M. B., R. M. Wooten, J. F. Zachary, S. M. Drouin, J. J. Weis, R. A. Wetsel, and S. J. Norris. 2003. Effect of complement component C3 deficiency on experimental Lyme borreliosis in mice. Infect. Immun. 71:4432-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazarus, J. J., M. J. Meadows, R. E. Lintner, and R. M. Wooten. 2006. IL-10 deficiency promotes increased Borrelia burgdorferi clearance predominantly through enhanced innate immune responses. J. Immunol. 177:7076-7085. [DOI] [PubMed] [Google Scholar]
- 50.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindahl, G., U. Sjöbring, and E. Johnsson. 2000. Human complement regulators: a major target for pathogenic microorganisms. Curr. Opin. Immunol. 12:44-51. [DOI] [PubMed] [Google Scholar]
- 52.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 72:3195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsumoto, M., W. Fukuda, A. Circolo, J. Goellner, J. Strauss-Schoenberger, X. Wang, S. Fujita, T. Hidvegi, D. D. Chaplin, and H. R. Colten. 1997. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc. Natl. Acad. Sci. USA 94:8720-8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDowell, J. V., K. M. Hovis, H. Zhang, E. Tran, J. Lankford, and R. T. Marconi. 2006. Evidence that BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect. Immun. 74:3030-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDowell, J. V., J. Wolfgang, E. Tran, M. S. Metts, D. Hamilton, and R. T. Marconi. 2003. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect. Immun. 71:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehlhop, E., and M. S. Diamond. 2006. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J. Exp. Med. 203:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meri, T., S. J. Cutler, A. M. Blom, S. Meri, and T. S. Jokiranta. 2006. Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect. Immun. 74:4157-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller, J. C., K. Narayan, B. Stevenson, and A. R. Pachner. 2005. Expression of Borrelia burgdorferi erp genes during infection of non-human primates. Microb. Pathog. 39:27-33. [DOI] [PubMed] [Google Scholar]
- 59.Miller, J. C., and B. Stevenson. 2006. Borrelia burgdorferi erp genes are expressed at different levels within tissues of chronically infected mammalian hosts. Int. J. Med. Microbiol. 296(Suppl. 40):185-194. [DOI] [PubMed] [Google Scholar]
- 60.Miller, J. C., K. von Lackum, K. Babb, J. D. McAlister, and B. Stevenson. 2003. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect. Immun. 71:6943-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller, J. C., K. von Lackum, M. E. Woodman, and B. Stevenson. 2006. Detection of Borrelia burgdorferi gene expression during mammalian infection using transcriptional fusions that produce green fluorescent protein. Microb. Pathog. 41:43-47. [DOI] [PubMed] [Google Scholar]
- 62.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mueller-Ortiz, S. L., S. M. Drouin, and R. A. Wetsel. 2004. The alternative activation pathway and complement component C3 are critical for a protective immune response against Pseudomonas aeruginosa in a murine model of pneumonia. Infect. Immun. 72:2899-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patarakul, K., M. F. Cole, and C. A. N. Hughes. 1999. Complement resistance in Borrelia burgdorferi strain 297: outer membrane proteins prevent MAC formation at lysis susceptible sites. Microb. Pathog. 27:25-41. [DOI] [PubMed] [Google Scholar]
- 65.Pausa, M., V. Pellis, M. Cinco, P. Giulianini, G. Presani, S. Perticarari, R. Murgia, and F. Tedesco. 2003. Serum-resistant strains of Borrelia burgdorferi evade complement-mediated killing by expressing a CD59-like complement inhibitory molecule. J. Immunol. 170:3214-3222. [DOI] [PubMed] [Google Scholar]
- 66.Pickering, M. C., H. T. Cook, J. Warren, A. E. Bygrave, J. Moss, M. J. Walport, and M. Botto. 2002. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in factor H. Nat. Genet. 31:424-428. [DOI] [PubMed] [Google Scholar]
- 67.Pickering, M. C., J. Warren, K. L. Rose, F. Carlucci, Y. Wang, M. J. Walport, H. T. Cook, and M. Botto. 2006. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc. Natl. Acad. Sci. USA 103:9649-9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pramoonjago, P., M. Kaneko, T. Kinoshita, E. Ohtsuba, J. Takeda, K. Hong, R. Inagi, and K. Inoue. 1992. Role of TraT protein, an anticomplementary protein produced in Escherichia coli by R100 factor, in serum resistance. J. Immunol. 148:827-836. [PubMed] [Google Scholar]
- 69.Quin, L. R., S. Carmicle, S. Dave, M. K. Pangburn, J. P. Evenhuis, and L. S. McDaniel. 2005. In vivo binding of complement regulator factor H by Streptococcus pneumoniae. J. Infect. Dis. 192:1996-2003. [DOI] [PubMed] [Google Scholar]
- 70.Richardson, B. A., and J. Overbaugh. 2005. Basic statistical considerations in virological experiments. J. Virol. 79:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rossmann, E., V. Kitiratschky, H. Hofmann, P. Kraiczy, M. M. Simon, and R. Wallich. 2006. Borrelia burgdorferi complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes is expressed in humans and induces antibody responses restricted to nondenatured structural determinants. Infect. Immun. 74:7024-7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadziene, A., M. Jonsson, S. Bergström, R. K. Bright, R. C. Kennedy, and A. G. Barbour. 1994. A bactericidal antibody to Borrelia burgdorferi is directed against a variable region of the OspB protein. Infect. Immun. 62:2037-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sassi, F., F. Hugo, M. Muhly, A. Khaled, and S. Bhakdi. 1987. A reason for the cytolytic inefficiency of murine serum. Immunology 62:145-147. [PMC free article] [PubMed] [Google Scholar]
- 74.Schaible, U. E., L. Gern, R. Wallich, M. D. Kramer, M. Prester, and M. M. Simon. 1993. Distinct patterns of protective antibodies are generated against Borrelia burgdorferi in mice experimentally inoculated with high and low doses of antigen. Immunol. Lett. 36:219-226. [DOI] [PubMed] [Google Scholar]
- 75.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sonenshine, D. E. 1991. Biology of ticks, vol. 1. Oxford University Press, New York, NY.
- 77.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stevenson, B., K. von Lackum, S. P. Riley, A. E. Cooley, M. E. Woodman, and T. Bykowski. 2006. Evolving models of Lyme disease spirochete gene regulation. Wien. Klin. Wochenschr. 118:643-652. [DOI] [PubMed] [Google Scholar]
- 79.Stoute, J. A., J. Gombe, M. R. Withers, J. Siangla, D. McKinney, M. Onyango, J. F. Cummings, J. Milman, K. Tucker, L. Soisson, A. Stewart, J. A. Lyon, E. Angov, A. Leach, J. Cohen, K. E. Kester, C. F. Ockenhouse, C. A. Holland, C. L. Diggs, J. Wittes, D. G. Heppner, Jr., and the MSP-1 Malaria Vaccine Working Group. 2007. Phase 1 randomized double-blind safety and immunogenicity trial of Plasmodium falciparum malaria merozoite surface protein FMP1 vaccine, adjuvanted with AS02A, in adults in western Kenya. Vaccine 25:176-184. [DOI] [PubMed] [Google Scholar]
- 80.Suhonen, J., K. Hartiala, H. Tuominen-Gustafsson, and M. K. Viljanen. 2002. Sublethal concentrations of complement can effectively opsonize Borrelia burgdorferi. Scand. J. Immunol. 56:554-560. [DOI] [PubMed] [Google Scholar]
- 81.Test, S. T., J. Mitsuyoshi, C. C. Connolly, and A. H. Lucas. 2001. Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide. Infect. Immun. 69:3031-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Dam, A. P., A. Oei, R. Jaspars, C. Fijen, B. Wilske, L. Spanjaard, and J. Dankert. 1997. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun. 65:1228-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.von Lackum, K., J. C. Miller, T. Bykowski, S. P. Riley, M. E. Woodman, V. Brade, P. Kraiczy, B. Stevenson, and R. Wallich. 2005. Borrelia burgdorferi regulates expression of complement regulator-acquiring surface protein 1 during the mammal-tick infection cycle. Infect. Immun. 73:7398-7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wallich, R., J. Pattathu, V. Kitiratschky, C. Brenner, P. F. Zipfel, V. Brade, M. M. Simon, and P. Kraiczy. 2005. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect. Immun. 73:2351-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wessels, M. R., P. Butko, M. Ma, H. B. Warren, A. L. Lage, and M. C. Carroll. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 92:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Withers, M. R., D. McKinney, B. R. Ogutu, J. N. Waitumbi, J. B. Milman, O. J. Apollo, O. G. Allen, K. Tucker, L. A. Soisson, C. Diggs, A. Leach, J. Wittes, F. Dubovsky, V. A. Stewart, S. A. Remich, J. Cohen, W. R. Ballou, C. A. Holland, J. A. Lyon, E. Angov, J. A. Stoute, S. K. Martin, and D. G. Heppner. 2006. Safety and reactogenicity of an MSP-1 malaria vaccine candidate: a randomized phase Ib dose-escalation trial in Kenyan children. PLoS Clin. Trials 1:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 89.Yang, L., J. H. Weis, E. Eichwald, C. P. Kolbert, D. H. Persing, and J. J. Weis. 1994. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuste, J., S. Ali, S. Sriskandan, C. Hyams, M. Botto, and J. S. Brown. 2006. Roles of the alternative complement pathway and C1q during innate immunity to Streptococcus pyogenes. J. Immunol. 176:6112-6120. [DOI] [PubMed] [Google Scholar]
- 91.Yuste, J., M. Botto, J. C. Paton, D. W. Holden, and J. S. Brown. 2005. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J. Immunol. 175:1813-1819. [DOI] [PubMed] [Google Scholar]