Abstract
The basidiomycetous fungal pathogen Cryptococcus neoformans is adapted to survive challenges in the soil and environment and within the unique setting of the mammalian host. A C. neoformans mutant was isolated with enhanced virulence in a soil amoeba model that nevertheless exhibits dramatically reduced growth at mammalian body temperature (37°C). This mutant phenotype results from an insertion in the ECA1 gene, which encodes a sarcoplasmic/endoplasmic reticulum (ER) Ca2+-ATPase (SERCA)-type calcium pump. Infection in murine macrophages, amoebae (Acanthamoeba castellanii), nematodes (Caenorhabditis elegans), and wax moth (Galleria mellonella) larvae revealed that the eca1 mutants are virulent or hypervirulent at permissive growth temperatures but attenuated at 37°C. Deletion mutants lacking the entire ECA1 gene were also hypersensitive to the calcineurin inhibitors cyclosporin and FK506 and to ER and osmotic stresses. An eca1Δ cna1Δ mutant lacking both Eca1 and the calcineurin catalytic subunit was more sensitive to high temperature and ER stresses than the single mutants and exhibited reduced survival in C. elegans and attenuated virulence towards wax moth larvae at temperatures that permit normal growth in vitro. Eca1 is likely involved in maintaining ER function, thus contributing to stress tolerance and virulence acting in parallel with Ca2+-calcineurin signaling.
Nonobligate human pathogens confront and must survive diverse environmental conditions. The ability to survive and proliferate in various challenging environmental niches, including in environmental microbial predators, is hypothesized to contribute to the ability of Cryptococcus neoformans, a basidiomycete fungus of global distribution, to cause disease in humans (15, 37, 49). The success of this pathogen is also attributed to its ability to survive at mammalian body temperature, which is an uncommon trait among fungal species. Tolerance to thermal stress (caused by body temperature) and oxidative stresses (from mammalian host defenses) is critical for successful infection. C. neoformans mutants with increased sensitivity to oxidative or nitrosative challenge, including those lacking superoxide dismutase Sod1 or Sod2 (18, 35), alternative oxidase Aox1 (2), flavohemoglobin denitrosylase Fhb1 (23), thiol peroxidase Tsa1 (48), or thioredoxin Trx1 (47), exhibit attenuated virulence. Temperature-sensitive mutants have been isolated in C. neoformans, including cna1 (calcineurin catalytic subunit), cnb1 (calcineurin regulatory subunit), cpa1 (cyclophilin A), and ras1 strains, and all of these have reduced virulence in mammalian host models (3, 29, 55, 73).
Calmodulin and calcineurin are central mediators of calcium signaling and respond to a multiplicity of stresses. Calmodulin is a Ca2+ sensor that undergoes conformational changes upon Ca2+ binding and transduces this Ca2+ signal to other proteins, such as calcineurin and Ca2+/calmodulin-dependent protein kinases (CaMK), via protein-protein interactions. Calcineurin is a highly conserved Ca2+/calmodulin-activated serine/threonine protein phosphatase that exists as a heterodimer of a catalytic A subunit and a regulatory B subunit. It is the target of the immunosuppressive drugs cyclosporin (CsA), which binds to cyclophilin A, and FK506, which binds to FKBP12. Calcineurin transduces Ca2+ signaling by activating the transcription factors NFAT in mammalian T cells and Crz1 in the yeasts Saccharomyces cerevisiae and Candida albicans (21, 36, 39). In C. neoformans, calcineurin is required for mating, morphogenesis, growth at 37°C, and virulence in murine models (20, 29, 55). However, the downstream signaling events regulated by calcineurin and the factors affecting calcineurin function are still largely unknown in C. neoformans and other pathogenic fungi.
Cellular Ca2+ homeostasis is maintained through the coordinated actions of Ca2+ pumps on the plasma, vacuolar, Golgi apparatus, and endoplasmic reticulum (ER) membranes. The sarcoplasmic/endoplasmic Ca2+-ATPases (SERCAs) are a family of ER Ca2+ pumps that are highly conserved in eukaryotic organisms. The ER is the cellular compartment for protein posttranslational processing, such as folding, glycosylation, assembly of protein complexes, and transport to the Golgi apparatus. ER function requires the maintenance of high Ca2+ concentrations, an oxidative milieu in the lumen, and the activity of molecular chaperones (reviewed in reference 12). The ER is also a major site of Ca2+ storage, with release of Ca2+ into the cytosol in response to certain stimuli. SERCAs are important in replenishing Ca2+ levels in the ER by transporting Ca2+ from the cytosol to the ER and thereby play critical roles in maintaining Ca2+ homeostasis in both the ER and the cytosol. In mammals, SERCAs are important for muscle relaxation and deficiencies in SERCAs are linked to multiple genetic diseases (46, 74). In the plant Arabidopsis thaliana, the ECA1 gene encodes a SERCA that is required for growth under low Ca2+ or high Mn2+ concentrations (75). In the parasite Leishmania mexicana amazonesis, a SERCA-type Ca2+ pump (Lmaa1) is involved in virulence (59). In the basidiomycete plant pathogen Ustilago maydis, ECA1 encodes a SERCA required for growth at high temperature and hyphal development (1).
Forward genetic screens are a powerful approach to identify new gene functions. Recently we identified a component in the Ca2+ signaling pathway of C. neoformans (i.e., calmodulin) through an insertional mutagenic screen of temperature-sensitive mutants (42). Here we report the identification of the C. neoformans gene ECA1, encoding a SERCA-type Ca2+ pump, by screening a collection of insertion mutants in an amoeba model of virulence. Genetic analysis indicated that ECA1 is involved in tolerance to multiple stresses and is required for growth at 37°C. Inhibition or mutation of the Ca2+-activated protein phosphatase calcineurin aggravates eca1Δ mutant phenotypes. Infection in four host models suggests that Eca1 contributes to virulence in a temperature-dependent manner: at 37°C, the eca1 mutants are hypovirulent, yet at temperatures permissive for in vitro growth the eca1 mutants have wild-type or enhanced virulence. Our results further extend our understanding of the connections between Ca2+ signaling with stress tolerance and virulence and suggest that the maintenance of virulence of C. neoformans in the environment is a trait of greater complexity than simple positive selection in microbial predators.
MATERIALS AND METHODS
Strains and growth conditions.
The C. neoformans strains used in this study are listed in Table 1. The serotype A MATα strain H99 and congenic MATα KN99α and MATa KN99a strains were used as the wild type (53, 57). The calcineurin subunit A cna1Δ deletion strains were as reported previously (40). The collection of ∼590 insertional mutants was generated previously by biolistic transformation of strain H99 with plasmid pCH233 conferring resistance to nourseothricin (38). C. neoformans was grown in YPD (1% yeast extract, 1% Bacto peptone, 2% dextrose) liquid or solid (supplemented with 2% agar) medium. For treatments, compounds were added after the medium was autoclaved. The macrophage cell line J774A.1 (ATCC TIB-67) is derived from a murine (BALB/c, haplotype H-2d) reticulum sarcoma (58). Macrophage cells were maintained at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 1% nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% NCTC-109 medium (Invitrogen). The cell line was used between passages 4 and 15. The amoeba Acanthamoeba castellanii (ATCC 30234) was maintained at 22°C in PYG medium (ATCC medium 712) following ATCC instructions. Galleria mellonella (wax moth) larvae were obtained from Vanderhorst, Inc. (St. Marys, OH) and maintained in wood shavings at room temperature (22°C). The standard Caenorhabditis elegans strain N2 Bristol was propagated on Escherichia coli strain OP50 lawns on nematode growth medium (NGM) at 25°C.
TABLE 1.
C. neoformans strains used in this study
| Strain | Genotype | Parent strain/background | Source or reference |
|---|---|---|---|
| H99 | Wild-type MATα | 57 | |
| KN99α | Wild-type MATα | 53 | |
| KN99a | Wild-type MATa | 53 | |
| AI-5C9 | eca1::NAT MATα | H99 | 38 |
| WF04-11/eca1-11 | eca1::NAT MATα | H99 | This study |
| WF04-26/eca1-26 | eca1::NAT MATα | H99 | This study |
| AI137 | eca1::NAT MATa | WF04-11 × KN99a | This study |
| AI150 | eca1::NAT ECA1-NEO MATα | WF04-11 | This study |
| KK8 | cna1::NEO MATa | KN99a | 40 |
| WF06-1 | cna1::NEO eca1::NAT MATα | WF04-11 × KK8 | This study |
Identification and disruption of ECA1.
The hypervirulent mutant AI-5C9 was identified in a collection of insertion mutants by screening for altered virulence in amoebae, using the virulence assay as described previously (65) and below. The genome insertion junction of AI-5C9 was cloned by inverse PCR and sequenced. The ECA1 gene was identified in serotype A and D strains by performing BLASTn searches of the Duke University C. neoformans serotype A genome database (http://cneo.genetics.duke.edu) and The Institute for Genomic Research (TIGR) serotype D database (45) with the sequence of the insertion junction. Targeted replacement of the ECA1 open reading frame in strain H99 was achieved by biolistic transformation with an overlap PCR product in which a NAT cassette was flanked by a 1-kb fragment from upstream of the ECA1 start codon and a 1-kb fragment from downstream of the ECA1 stop codon. Transformants were selected on YPD agar medium plates containing 100 mg/liter nourseothricin at 30°C and then screened by PCR for an eca1Δ strain, and the deletion was confirmed by Southern and Northern blot analyses. The eca1Δ mutation was complemented by reintroducing a wild-type version of the gene. ECA1 was amplified from strain JEC21 and cloned into plasmid pPZP-NEO1 (72). Agrobacterium tumefaciens strain EHA105 was transformed with this plasmid by electroporation and used to transform C. neoformans to neomycin resistance using methods reported previously (38).
Generation of an eca1Δ cna1Δ double mutant.
The eca1Δ mutant (NATR) in the MATα background (parent strain H99) was crossed with a cna1Δ (NeoR) mutant in the MATa background (parent strain KN99a) on V8 agar medium. Basidiospores were dissected 9 days after mating and were allowed to germinate on YPD medium at 30°C. The isolates were then tested for the presence of both nourseothicin and neomycin resistance markers, and the presence of both eca1 and cna1 mutations in these strains was confirmed by PCR analysis.
RNA extraction and Northern blot analysis.
Total RNA was extracted from C. neoformans with the TRIzol reagent (Invitrogen), separated by electrophoresis in a 1.2% denaturing agarose gel, and transferred to nylon membrane (Hybond H+; Amersham Biosciences, United Kingdom). The blot was hybridized with 32P-labeled probes in the ULTRAhyb buffer (Ambion, Austin, TX) according to the manufacturer's instructions. Autoradiograph images were analyzed with NIH Image software.
Virulence assays.
Virulence assays were conducted in four model systems as described previously: amoeba A. castellanii (65), murine macrophage cell line J774A.1 (26, 27), the nematode C. elegans (50, 71), and G. mellonella (wax moth) larvae (52). For most assays, C. neoformans cells were cultured in YPD medium at 30°C to mid-to-late log phase, and the cells were washed three times with phosphate-buffered saline (PBS) and resuspended in PBS to the desired concentrations as the inocula. Cell numbers were determined by counting with a hemocytometer. For C. elegans assays, C. neoformans strains were cultured overnight and spread onto brain heart infusion (BHI) agar to create lawns.
For the amoeba virulence assay, A. castellanii was cultured in PYG medium in 96-well microtiter plates to a density of 1 × 105 cells/well, and C. neoformans cells were added to the amoebae at a multiplicity of infection (MOI) of 2:1. The amoeba-yeast coculture was incubated at either 30 or 37°C for 24 or 48 h before being lysed with ice-cold H2O containing 0.05% sodium dodecyl sulfate. Serial dilutions of the lysate were plated on YPD, and the CFU served as an indicator of C. neoformans virulence.
For the macrophage assay, J774A.1 cells were grown in 96-well microtiter plates to a density of 1 × 105 cells/well, and C. neoformans cells were added to the macrophages at an MOI of 2:1, in the presence of 1 μg/ml monoclonal antibody 18B7 (provided by Arturo Casadevall [14]), 50 U/ml of recombinant mouse gamma interferon (Roche Molecular Biochemicals, Mannheim, Germany), and 0.3 μg/ml lipopolysaccharide (Sigma-Aldrich, St. Louis, MO). After 1 h of incubation at 37°C in the presence of 10% CO2, unattached C. neoformans cells were washed off by aspiration with fresh medium and the macrophage-yeast coculture was incubated for a further 24 h, at which time the macrophages were lysed with ice-cold H2O containing 0.05% sodium dodecyl sulfate and serial dilutions of the lysate were plated on YPD. CFU served as the measure of C. neoformans virulence.
Three C. elegans system endpoints were used to examine the virulence properties of the C. neoformans strains: nematode survival, nematode progeny production, and fungal burden within nematodes. C. elegans worms were monitored for survival on C. neoformans lawns as described previously (50, 51). Fungal strains were grown in 2 ml of YPD medium with kanamycin (45 μg/ml), ampicillin (100 μg/ml), and streptomycin (100 μg/ml) with shaking at 30°C for 24 to 48 h. Lawns were prepared by spreading 10 μl of each culture on 35-mm tissue culture plates containing BHI agar with the same antibiotics. The plates were incubated at 30°C for 24 h and then at 25°C for 24 h. Approximately 60 nematodes at the L4 stage were transferred from NGM seeded with E. coli strain OP50 to each of four BHI plates per C. neoformans strain. The plates were incubated at 25°C, and the worms were examined for survival at 24-h intervals with a Nikon SMZ645 dissecting microscope. At each interval, inviable worms were counted and removed.
For C. elegans progeny quantification, C. neoformans lawns were prepared as described above. One nematode at the L4 stage was moved from NGM plates seeded with E. coli OP50 to each of 24 lawns per C. neoformans strain. At 24-h intervals, living worms were transferred to new lawns for each strain, and at 72 h the living worms were removed. Each BHI agar pad was inverted onto a 100-mm tissue culture plate containing NGM agar with streptomycin (100 μg/ml) and seeded with E. coli. The plates were incubated at 25°C for 48 h, and the progeny were counted. Only worms that survived all 3 days were included in calculating the total progeny laid over the course of 72 h.
The C. neoformans burdens within infected C. elegans were quantified as described previously (34), but with 10 animals at the L4 stage in each group and only one 48-h time point of exposure to Cryptococcus lawns prepared on BHI plates as described above. After exposure, animals were washed twice in 8-μl drops of M9 medium on a BHI agar plate containing antibiotics as described above, in order to remove surface cryptococcal cells. Each group of 10 worms was then placed in 40 μl of M9 medium with 1% Triton X-100 and ground with a pestle. The volume was adjusted to 600 μl with M9 medium containing 1% Triton X-100, and the final suspension was serially diluted and plated on YPD agar containing the same antibiotics. The plates were incubated for 48 h at 30°C, and CFU were counted.
For virulence in the wax moth assay, each G. mellonella larva was injected in the terminal pseudopod with C. neoformans cells (1 × 105 in 5 μl PBS). Larvae were incubated at 30 or 37°C, and virulence was measured by scoring the survival of the larvae every 24 h.
RESULTS
Isolation of a hypervirulent, temperature-sensitive insertion mutant.
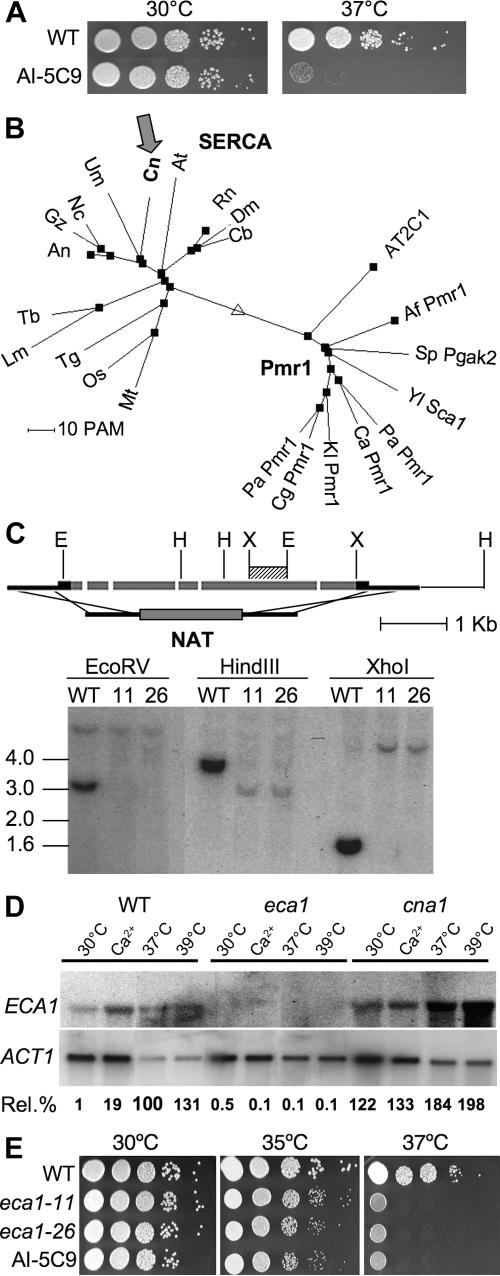
Nonmammalian models have been developed to test virulence properties of C. neoformans and other human pathogens, leading to their use in forward genetic screening to identify attenuated mutants and thereby genes required for virulence. During a screen for C. neoformans strains with altered virulence in amoeba, one hypervirulent strain (AI-5C9) was identified out of 590 insertional mutants examined (Fig. 1), and its in vitro phenotypes were analyzed for known virulence attributes. Capsule and melanin were produced at wild-type levels. Strain AI-5C9 displayed normal growth at 30°C on YPD medium or the PYG medium used in amoeba interactions. However, when cultured at 37°C, growth of mutant AI-5C9 was significantly reduced compared with that of the wild-type strain, H99 (Fig. 1 and 2A). Although strain AI-5C9 exhibits reduced growth at 37°C, the cells display normal cellular morphology even after being cultured at 37°C for 2 days (data not shown). Because of its paradoxical increase in virulence in amoeba yet reduced growth rate at mammalian body temperature, the mutant strain was further characterized.
FIG. 1.
eca1 mutation enhances virulence in the amoeba A. castellanii. Cryptococcal yeast cells of the wild type (WT), eca1 insertion mutant AI-5C9, and two independent eca1Δ deletion mutants (eca1-11 and eca1-26) were inoculated into amoeba cell culture at an MOI of 2:1 (left panels) or into amoeba-free medium (PYG; right panels), and were cultured at either 30°C (top panels) or 37°C. A total of 1 × 106 cryptococcal cells were inoculated into amoebae or medium (gray bars), and fungal cells were isolated 24 h (white bars) or 48 h (hatched bars) after incubation, with growth measured by plating on YPD medium and counting CFU after culture at 30°C for 2 days. *, P < 0.05.
FIG. 2.
Characterization of C. neoformans Eca1. (A). Temperature-sensitive growth of eca1 insertion mutant AI-5C9. Ten-fold dilutions of AI-5C9 and the wild-type (WT) H99 cells were plated on YPD agar and cultured at 25°C or 37°C for 2 days. (B) Phylogenetic analysis of Eca1 and its homologs. Amino acid sequences of C. neoformans Eca1 (Cn) and P-type Ca2+ pumps from other organisms were aligned using the MultAlin program (17). A phylogenetic tree was deduced to depict the evolutionary relationships of the homologs. All of the SERCA-type pumps cluster at the left node (labeled SERCA), and the fungal Pmr1 or mammalian AT2C1 pumps cluster at the right node (labeled Pmr1). (C). Schematic diagram of the targeted deletion of the ECA1 gene. The genomic DNA structure of ECA1 is illustrated as gray boxes for the exons, and the entire coding region was replaced with a nourseothricin resistance cassette (NAT). Shown are the results of Southern analysis of genomic DNA from two independent isolates, the eca1-11 (lanes 11) and eca1-26 (lanes 26) mutants, and parental strain H99 (WT). Genomic DNA was digested with EcoRV, HindIII, or XhoI. The probe used is indicated by the hatched bar. (D) Northern blot analysis of ECA1 expression. Total RNAs were isolated from wild-type, eca1, and cna1 cells cultured at 30°C in YPD medium (30°C) or YPD medium supplemented with 20 mM of CaCl2 (Ca20) and cells cultured in YPD medium at 37°C (37°C), and the Northern blot was hybridized with 32P-labeled probes for actin (ACT1) and ECA1. The autoradiography images were analyzed with NIH Image software to quantify the expression levels. Transcription of ECA1 in each sample was normalized by the ACT1 gene and compared with the 37°C-treated wild-type sample, the value of which was set to 100%. The relative levels of ECA1 expression are listed below the images (Rel. %). (E). Deletion of ECA1 resulted in temperature-sensitive phenotypes identical to that of AI-5C9. Ten-fold serial dilutions of eca1-11, eca1-26, and wild-type strains were plated on YPD agar and cultured at 30°C, 35°C, or 37°C for 2 days.
The ECA1 gene encodes a SERCA-type calcium pump.
Segregation analysis of the progeny produced by mating the AI-5C9 mutant with the congenic MATa strain KN99a confirmed that the NAT resistance marker is linked with the temperature-sensitive phenotype. Strain AI-5C9 mated normally with KN99a, producing wild-type amounts of viable basidiospores. Of those progeny analyzed, 6/13 were nourseothricin resistant and temperature sensitive and 7/13 were nourseothricin sensitive and temperature resistant, indicating 100% genetic linkage. Likewise, in crosses between a eca1 and α eca1 mutants, mating occurred to produce filaments and basidiospores (data not shown). The site of insertion of the plasmid into the C. neoformans genome was identified by inverse PCR and DNA sequencing. The AI-5C9 mutant carries an insertion in a gene encoding a predicted protein of 1,006 amino acids that belongs to the P-type calcium ATPase family. The corresponding gene in the serotype D strain JEC21 genome, which has been sequenced and annotated (45), is CNH02370. Although its closest homolog in Saccharomyces cerevisiae is Pmr1, which is a Ca2+ pump localized to the Golgi apparatus, the C. neoformans-encoded protein is more closely related to SERCA pumps in basidiomycetous fungi and other organisms, including plants, worms, fruit flies, mice, and humans. This is illustrated by the phylogenetic analysis of the deduced amino acid sequences of the C. neoformans gene and related P-type calcium pumps (Fig. 2B). The closest homolog of CNH02370 in the GenBank database is the ECA1 gene from U. maydis (E = 0.0) (1), and the C. neoformans gene was therefore named ECA1. Alignment of the Eca1 amino acid sequence with that of rabbit SERCA1, whose structure has been solved by X-ray crystallography and well studied, showed that not only do they share a high degree of similarity in protein primary structure (54% identity, 68% similarity), but the overall domain structure and critical amino acid residues, such as the nucleotide binding sites and a phosphorylation site (DKTGT), are all conserved.
The ECA1 gene was deleted by homologous recombination to generate two independent eca1Δ deletion mutants (Fig. 2C). Southern blot analysis confirmed that the ECA1 coding region was deleted, resulting in a null mutant (Fig. 2C). ECA1 expression was undetectable in the eca1Δ mutants, as shown by Northern blotting (Fig. 2D), as expected for deletion mutants. The ECA1 transcript was induced after treatment of wild-type cells at 37°C for 1 h, and its expression was similar to that of the wild type in another temperature-sensitive C. neoformans strain background (calcineurin cna1 mutant). When two independent eca1Δ strains (eca1-11 and eca1-26) were cultured at the permissive growth temperature (30°C) or elevated temperatures (35°C and 37°C), both eca1Δ isolates displayed the same enhanced virulence in amoebae and temperature sensitivity as the original AI-5C9 insertion mutant (Fig. 1 and 2E). The eca1Δ mutants grew at a rate similar to that of the wild type at 30°C. At 35°C, the mutants grew more slowly than the wild type, while at 37°C their growth was dramatically inhibited (Fig. 2E). An eca1Δ deletion mutant was crossed to the congenic strain KN99a, and 24 progeny were analyzed. The 8 nourseothricin resistance mutants were all temperature sensitive, while the 16 nourseothricin-sensitive strains grew like the wild type at 37°C, indicative of genetic linkage between the eca1 mutation and the temperature-sensitive growth phenotype. A wild-type copy of ECA1 was reintroduced into the eca1Δ strain, and it complemented the temperature-sensitive phenotype (data not shown). Together, these results confirm that Eca1 regulates virulence in amoebae and is important for C. neoformans growth at high temperatures.
The eca1Δ mutant is hypersensitive to calcineurin inhibitors.
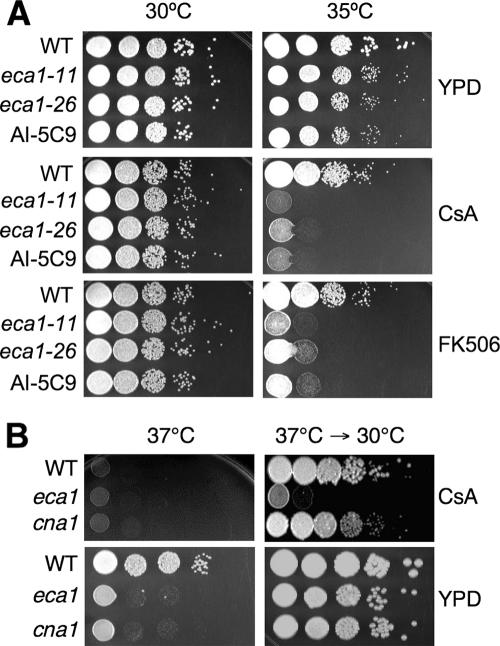
Previous research on C. neoformans has identified a set of temperature-sensitive mutants affected in the Ca2+-calmodulin-calcineuin A and B pathway; thus, it seemed probable that Eca1 might govern calcineurin signaling. The temperature-sensitive phenotype of eca1 mutants is shared with cna1 or cnb1 calcineurin mutants. The eca1 phenotype may result from either increased cytosolic Ca2+ causing overactivation of calcineurin or even reduced Ca2+ availability in response to stresses if Ca2+ derived from the ER is used in signaling. However, eca1 mutants are also hypersensitive to calcineurin inhibitors, indicating that any interaction with Eca1 and calcineurin is not within a simple linear pathway (Fig. 3A). At the semipermissive temperature of 35°C, both CsA and FK506 inhibited the growth of eca1Δ mutants similarly. At 37°C, the growth of wild-type and eca1Δ cells was completely inhibited by CsA (Fig. 3B) or FK506 (not shown), displaying the temperature-sensitive phenotype associated with impaired calcineurin signaling caused by these drugs. However, when the cells were returned to 30°C and allowed to grow for 2 days, eca1Δ mutant cells were not able to recover, while both H99 and cna1Δ cells recovered and resumed growth. Interestingly, when eca1Δ mutant cells were treated at 37°C for 2 days in the absence of CsA, they were able to recover after being shifted back to 30°C (Fig. 3B). Therefore, at 37°C, while CsA is fungistatic to H99, it becomes fungicidal to eca1Δ mutant cells. This indicates that eca1 mutant cells are viable but fail to proliferate at 37°C, calcineurin is required for eca1 mutant cell viability, and Eca1 and calcineurin function at least in parallel pathways in C. neoformans.
FIG. 3.
Growth and viability of the eca1 mutants are reduced by calcineurin inhibitors. (A). Ten-fold serial dilutions of eca1-11, eca1-26, the insertion mutant AI-5C9, and wild-type (WT) cells were plated on YPD agar or YPD medium supplemented with CsA or FK506 and cultured at 30°C or 35°C for 2 days. (B). Wild-type, eca1, or cna1 cells were plated on YPD medium or YPD medium supplemented with CsA and cultured at 37°C for 2 days before being transferred to 30°C and cultured for 2 more days.
eca1Δ is hypersensitive to ER and osmotic stresses.
Because SERCA pumps such as Eca1 are responsible for transporting Ca2+ into the ER and Ca2+ ions are required for correct folding of newly synthesized proteins and for protein secretion, we tested whether the eca1 mutant is sensitive to ER stress. First we examined whether the eca1Δ mutation might affect tolerance to calcium stress. Indeed, elevated levels of calcium (100 mM or 250 mM) in YPD medium inhibited the growth of the eca1Δ mutant strains at either 30°C or 35°C (Fig. 4A). On the other hand, depletion of Ca2+ with 10 mM of the calcium chelator EGTA also inhibited growth of the eca1Δ strain. Although either high concentrations of Ca2+ or Ca2+ depletion inhibited the growth of the wild-type strain H99, the inhibition of eca1Δ mutant cells was much more severe. This indicates that Eca1 is involved in Ca2+ homeostasis and protection against Ca2+ stresses.
FIG. 4.
Deletion of ECA1 results in hypersensitivity to multiple stresses. (A) Ten-fold dilutions of wild-type (WT), eca1, or cna1 cells were plated on YPD medium or YPD medium supplemented with 100 mM or 250 mM CaCl2, 10 mM EGTA, 0.1 μg/ml tunicamycin (Tm), or 1.5 M NaCl and cultured at 30°C or 35°C for 2 days. (B) Wild-type, eca1, or cna1 cells were cultured in RPMI medium (pH 7.0) containing 10-fold serial dilutions of fludioxonil and cultured at 30°C for 2 days. Growth was determined by measuring the optical density at 595 nm. The graph shows the percentage of growth relative to cells cultured in medium containing no fludioxonil.
We also tested ER stress by adding tunicamycin to the growth medium to disrupt protein N glycosylation. Treatment with 100 nM tunicamycin moderately inhibited the eca1Δ mutant at 30°C and severely inhibited its growth at 35°C (Fig. 4A). This hypersensitivity indicates that Eca1 is involved in tolerance to ER stress.
To test whether the temperature sensitivity of the eca1 mutants was due to overactive CaMKs, the eca1 strain was treated with the CaMK-specific inhibitor KN-93 and the noninhibitory analog KN-92 (68). YPD solid medium was inoculated with a lawn of cells, a 6-mm filter paper disk soaked in either analog (10 μl at 5 mM) or the dimethyl sulfoxide solvent was placed on the lawn, and the strains were grown at 30°C and 37°C. Treatment with the drug was unable to rescue the temperature sensitivity of the eca1Δ mutant strain (data not shown), suggesting that the temperature sensitivity is more likely due to ER stress rather than enhanced Ca2+-calmodulin-dependent kinase activity. However, one caveat in this experiment is the assumption that these molecules are efficiently taken up by C. neoformans as in other fungi.
Ca2+ is an important second messenger regulating numerous cellular functions, including osmotic stress tolerance. We examined whether the eca1Δ mutant has any defect in osmostress tolerance by treatment with a high concentration of NaCl. We found that at 30°C, the eca1Δ mutant is as sensitive to 1.5 M NaCl as the wild-type strain, H99 (Fig. 4A). However, at 35°C, while the growth of H99 was also further inhibited by NaCl, the eca1Δ mutant showed an even higher level of sensitivity. We also treated the eca1Δ mutant with fludioxonil, an antifungal drug that causes osmotic stress by activating the Hog1 mitogen-activated protein kinase and inducing the intracellular accumulation of glycerol (32, 40, 54, 76). While more than 10 mg/ml fludioxonil is required to inhibit growth by 80% in the wild type, less than 1 mg/ml was required to achieve 80% growth inhibition of the eca1Δ mutant at 30°C (Fig. 4B).
Mutation of calcineurin (cna1Δ) aggravates the phenotypes conferred by eca1Δ mutation.
To examine further the relationship between Eca1 and calcineurin, we constructed an eca1Δ cna1Δ double mutant strain by crossing the nourseothricin-resistant eca1::NAT mutant in the H99 (MATα) background with the neomycin-resistant cna1::NEO strain in the KN99a background (MATa). The eca1Δ cna1Δ strain was tested for temperature sensitivity, as well as sensitivity to ER stresses and hyperosmolarity. When cultured on YPD medium or YPD medium containing 100 mM CaCl2, 5 mM EGTA, 0.1 mM tunicamycin, or 1.5 M NaCl, the double mutant displayed a more severe sensitivity than the eca1Δ mutant alone. The eca1Δ cna1Δ mutant was also more sensitive to high temperature than the eca1Δ mutant (Fig. 5B), which is consistent with the increased sensitivity of a single eca1Δ strain when treated with calcineurin inhibitors (see Fig. 3). These findings provide further evidence that Eca1 functions in parallel with calcineurin, and Eca1 may also function upstream or with other Ca2+-dependent proteins or processes to control calcium activation by calmodulin.
FIG. 5.
Calcineurin mutation exacerbates eca1 mutant phenotypes. (A) Ten-fold serial dilutions of the wild-type (WT), eca1, cna1, and eca1 cna1 mutant cells were plated on YPD medium or YPD medium supplemented with EGTA (5 mM), tunicamycin (Tm; 0.1 μg/ml), CaCl2 (250 mM), or NaCl (1.5 M) and cultured at 30°C for 2 days. (B). Ten-fold serial dilutions of the wild-type or eca1, cna1, and eca1Δ cna1Δ mutant cells were plated on YPD medium and cultured at 37°C for 2 or 4 days.
Temperature-dependent roles of Eca1 in C. neoformans virulence.
Using the amoeba A. castellanii as described previously (65), the ECA1 gene was identified in C. neoformans in a screen for mutants with altered virulence (Fig. 1), and the data presented thus far show that the Eca1 Ca2+ pump is involved in stress tolerance. We further investigated a role for Eca1 in virulence and its interaction with calcineurin. First we examined the virulence phenotype of eca1 mutants in the murine macrophage infection model. Cells of two independent eca1Δ mutant strains, the insertion mutant AI-5C9, and the wild-type strain H99 were inoculated into macrophage J774A.1 cultured cells and incubated at 37°C in the presence of 5% CO2. Virulence was measured by the growth of C. neoformans cells as defined by CFU at the 24-h time point. As shown in Fig. 6A, growth of the eca1 mutants was significantly lower than that of the wild type. This demonstrates that the eca1 mutants exhibit reduced virulence in the murine macrophage model. However, the reduced virulence of eca1 mutants may simply reflect their reduced proliferation or viability at elevated temperature, as indicated by the growth of the eca1 mutants in DMEM at 37°C (Fig. 6A) and as observed in previous experiments.
FIG. 6.
The Eca1 SERCA pump is required for virulence in a murine macrophage model. Cryptococcal yeast cells of the wild type (WT), the eca1 insertion mutant (AI-5C9), and two eca1Δ deletion mutants (eca1-11 and eca1-26) were inoculated into macrophage J774A.1 cultured cells at an MOI of 2:1 (left panel) or into macrophage-free medium (DMEM; right panel). The cryptococcal strains were inoculated at 1 × 106 cells (gray bars), and after 24 h (white bars) of incubation, growth was measured by plating on YPD and counting CFU after culture at 30°C for 2 days. *, P < 0.05.
Because experiments with murine models of cryptococcosis are performed at 37°C, to analyze the contribution of Eca1 to C. neoformans virulence, we took advantage of wax moth (G. mellonella) larvae (52), an alternative host model that can grow at temperatures ranging from 22 to 37°C, and the nematode C. elegans (growth at 25°C). For the wax moth larva infection model, 1 × 105 C. neoformans cells were injected into the wax moth larvae and the larvae were incubated at 30°C or 37°C. The virulence of each strain was measured by the death/survival of the larvae injected with it. At 30°C, 80 to 90% of the larvae infected with wild-type or eca1 mutant cells died by day 13, whereas 70 to 90% of the larvae infected with the cna1 or eca1 cna1 mutant cells survived (Fig. 7; P < 0.05, Mann-Whitney test), showing that while calcineurin does contribute to virulence in this model at 30°C, Eca1 does not. However, when incubated at 37°C, the wild-type strain killed all moth larvae within 6 days, whereas the eca1 strain showed reduced virulence, and the cna1 or eca1 cna1 mutants exhibited an even greater attenuation in virulence compared to the wild type (P < 0.05, Mann-Whitney test), in accord with the temperature-sensitive phenotype of the eca1, cna1, and eca1 cna1 mutant strains.
FIG. 7.
Temperature influences the role of C. neoformans Eca1 SERCA pump and calcineurin in virulence in the wax moth G. mellonella. Ten wax moth larvae were injected with 1 × 105 cells of the wild-type or eca1Δ, cna1Δ, or eca1Δ cna1Δ strains and incubated at 30 or 37°C, and survival was monitored daily.
Three virulence assay endpoints were used to assess the role of Eca1 and calcineurin in virulence in the model nematode C. elegans, to assess the role of temperature sensitivity in virulence, and to study whether the hypervirulent phenotype of eca1 mutants was amoeba specific. The C. elegans-C. neoformans interaction experiments were performed at 25°C. For the strains tested, no major differences were observed in the assay that relied on the fungus’ ability to kill the nematode (data not shown). However, in a second assay measuring the generation of nematode progeny after being infected with C. neoformans, differences were observed (Fig. 8). This assay relies on more virulent strains having a detrimental effect of nematode fertility, such that more progeny are produced from nematodes infected with attenuated strains compared to those infected with wild-type strains. Inoculation with the eca1Δ cna1Δ double mutant strain resulted in higher numbers of progeny compared to the wild type or the single cna1Δ mutant (P < 0.005, Mann-Whitney test) and also higher numbers of progeny from worms infected with the eca1 mutant strain compared to the wild type (P < 0.05). Microscopic examination of the nematodes revealed that the C. neoformans yeast cells were present in the intestines of nematodes when exposed to the wild type or eca1Δ or cna1Δ mutants, but rarely for the eca1Δ cna1Δ double mutant (Fig. 8), although there was distension of the proximal intestine in this case, suggesting that the eca1Δ cna1Δ strain survived passage through the nematode grinder and was subsequently lysed in the intestine. CFU from the nematodes infected with these strains confirmed the microscopic observations, with fewer CFU being produced from the nematodes fed the eca1Δ cna1Δ mutant compared to other strains (P < 0.05). The intestines of the nematodes infected with the eca1Δ mutant also appeared to contain fewer C. neoformans cells, and infection with the eca1Δ mutant allowed more progeny to be produced than with the wild type. However, there was no statistically significant difference between CFU emanating from the wild type and eca1 mutants. Thus, analysis of virulence in the nematode C. elegans reveals that only the eca1Δ cna1Δ double mutant shows a dramatic decrease in fungal viability within nematodes and the fertility of the infected nematodes, while the eca1 or cna1 single mutants have little or no effect compared to the wild type.
FIG. 8.
Eca1 SERCA pump and calcineurin additively contribute to virulence in the nematode C. elegans. Micrographs of nematode intestines containing C. neoformans yeast cells. The grinder is to the right in each image. Bar = 20 μM. Shown is the generation of C. elegans progeny after exposure to C. neoformans strains (white bars). CFU are the average yeast colonies (± standard error) from individual C. elegans worms fed C. neoformans, with worms harvested from three plates (gray bars). The eca1 single and eca1 cna1 double mutants show statistically significant differences from the wild type. *, P < 0.05.
Taken together, the virulence phenotypes from multiple models at different temperatures suggest that both Eca1 and Cna1 contribute to virulence by supporting growth at mammalian body temperature; however, mutation of both genes has an additive effect and reduces virulence at room temperature.
DISCUSSION
Pathogenic fungi that normally exist outside the human host face challenges in transitioning from one environmental niche to another, particularly from the environment into the human host. Many rely on the ability to sense these changes via conserved signaling cascades to adapt successfully to new conditions. One such signaling pathway is that mediated by Ca2+-calmodulin-calcineurin. Calcium signaling is important for C. neoformans and other human-pathogenic fungi to cope with stress and for their virulence (41).
In this study, we identified a new gene, ECA1, involved in Ca2+ homeostasis in C. neoformans. The gene is part of a conserved family of SERCAs that regulate Ca2+ levels in the ER lumen in many eukaryotes. In C. neoformans, this gene regulates resistance to various stresses and growth at 37°C and functions in parallel with and upstream of the calcineurin pathway. The closest homolog is the ECA1 gene of the related basidiomycete plant-pathogenic fungus U. maydis, in which the ECA1 gene was identified by analysis of a temperature-sensitive mutant (1). The growth phenotypes of the U. maydis and C. neoformans eca1 mutants are very similar. One exception is that in U. maydis the altered cell morphology is clearly attributable to overactive CaMKs (1). However, in C. neoformans eca1 mutants there is no defect in cellular morphology and a CaMK inhibitor did not rescue the temperature sensitivity of eca1. Rather, we hypothesize that many of the C. neoformans eca1 phenotypes are due to impaired ER function resulting in misregulation of protein processing, suggesting a divergence in function between these two basidiomycetes. We performed a pilot microarray study to elucidate the effects of eca1 deletion on gene transcription in C. neoformans (W. Fan, K. Kojima, and J. Heitman, unpublished data). While we were able to confirm the temperature regulation of transcription by subsequent Northern blot experiments for genes encoding proteins such as opsin, the cruciform DNA binding protein Hmp1, an oxidoreductase (GenBank accession no. CNC03730), ribosomal protein L35, and an Ero1-like protein (data not shown), no Eca1-dependent transcriptional response was identified, suggesting that other microarray experiments or approaches will be required to elucidate Eca1 functions.
We initially hypothesized that the temperature-sensitive phenotype of eca1Δ cells could be related to a role upstream in calmodulin-calcineurin signaling. Mutation of ECA1 should impair Ca2+ import into the ER, increasing cytosolic Ca2+ levels and overactivating calcineurin. However, the eca1 cna1 double mutant is more severely impaired than either eca1 or cna1 single mutants for a number of phenotypes, including temperature sensitivity and virulence, suggesting that Eca1 functions in parallel with calcineurin. We consider it likely that Eca1 also plays a role upstream of calcineurin by regulating cytosolic Ca2+ levels. Calcineurin is required for virulence in all human-pathogenic fungi in which it has been studied, but its roles in virulence differ between species. In C. albicans, calcineurin mutants grow like the wild type at 37°C but are killed by calcium in serum (4, 8, 9, 61). In A. fumigatus, calcineurin mutants exhibit severe morphological defects (22, 66). In C. neoformans, calcineurin mutants cannot grow at 37°C (29, 55). Here we show that C. neoformans calcineurin mutants exhibit wild-type virulence at room temperature in C. elegans but reduced virulence in the wax moth at 30°C (a temperature at which there are no growth defects in vitro). Thus, based on the results in the wax moth, the virulence defect in mammalian models may not be attributed solely to temperature sensitivity in C. neoformans. This study highlights the use of nonmammalian models in understanding the genetic mechanisms governing virulence, especially when one phenotype of mutating a gene is temperature sensitivity. We note that because the cna1 mutant was fully virulent in C. elegans, but not in the wax moth, this indicates that virulence determinants can differ between model heterologous hosts, providing unique vantage points from which to dissect branched signaling pathways controlling virulence.
Many of the phenotypes of eca1 mutants are similar to those caused by calcineurin mutation, suggesting that conditions leading to both loss and gain of calcineurin activity might similarly perturb cellular physiology. Another component of calcium homeostasis that has been recently identified is the Cch1 voltage-gated Ca2+ channel that is required for calcium uptake in C. neoformans and predicted to be in the plasma membrane (44). Strains with cch1 mutations show sensitivity to calcium chelation, like eca1 mutants, but no temperature sensitivity. Thus, in the absence of Cch1 other mechanisms must exist to provide sufficient Ca2+ to activate calcineurin. Similarly, other components of calcium-calcineurin signaling show variation in phenotype when mutated, such as mutants of the calcineurin binding protein (CBP1), which exhibit wild-type growth at high temperature but a mating defect like calcineurin mutants (30), or a calmodulin mutant that has similar temperature sensitivity and mating defects to calcineurin mutation but is also hypersensitive to the calcineurin inhibitor FK506 (42). Taken together, these and other results suggest that variation exists between the phenotypes caused by perturbations to calcium homeostasis in C. neoformans and other fungi.
While SERCAs are crucial for maintaining Ca2+ homeostasis in many eukaryotes, including animals, plants, protists, and fungi like C. neoformans and U. maydis, organisms can function without this class of Ca2+ pump. For example, the model eukaryote S. cerevisiae and related hemiascomycete yeasts do not contain a SERCA protein, so intracellular calcium concentrations in these fungi are regulated by other Ca2+ transporters. One is Pmc1, which is localized to the vacuolar membrane, and the key role in S. cerevisiae of the vacuole, where most Ca2+ is stored, in maintaining Ca2+ levels has recently been demonstrated by screening the yeast deletion collection for strains that accumulate (or do not accumulate) ions; the majority of strains affected in Ca2+ levels were mutants that impair vacuole function (24). A second transporter, Pmr1, is localized to the Golgi apparatus, thereby controlling Ca2+ within these post-ER vesicles and also affecting Ca2+ levels within the ER itself (10). S. cerevisiae does contain a P-type ATPase (Cod1/Spf1) localized to the ER to regulate calcium levels (19, 69), but, to the best of our knowledge, there is no evidence that mutants of this gene exhibit interactions with the calcineurin pathway. Mutation of any of these transporters affects calcium homeostasis and reduces viability of S. cerevisiae under stress conditions. Despite different calcium transporter compositions, in both C. neoformans and S. cerevisiae (and the related hemiascomycete pathogens C. albicans and Candida glabrata) impairment of both calcineurin and ER functions leads to additive deleterious effects in these fungi (10), showing a commonality in overall cellular functions.
One hypothesis as to how C. neoformans maintains its ability to infect the human host is that it is under constant selection in the environment by predators, such as amoebae, nematodes, and insects (63). Passage of the fungus through the slime mold Dictyostelium discoideum enhanced virulence in a murine model of disease (64). Several genes required for virulence in the mammalian host are also required for virulence in heterologous hosts (31, 50). Furthermore, two recent screens for strains with attenuated virulence in C. elegans identified the KIN1 and ROM2 genes, which were subsequently shown to be required for virulence in murine models of cryptococcosis (51, 71). The original AI-5C9 eca1 mutant strain was identified as hypervirulent in a screen for strains with altered virulence in an amoeba model of the disease. Subsequent studies show that the magnitude of virulence depends on the temperature of the experiment, as the eca1 mutant has a growth and virulence defect at elevated temperatures but retains wild-type or enhanced virulence at room temperature. While mutation of ECA1 would produce a C. neoformans strain that is better adapted to survive predation by certain environmental hosts like Acanthamoeba castellanii and still be virulent in nematodes and wax moth, it would not survive in the mammalian host or under other stressful conditions. Thus, selection for virulence in this amoeba species by an eca1 mutation would decrease mammalian virulence. A reverse situation exists for the carbonic anhydrase Can2, whose deletion yields a strain that proliferates better than the wild type in a rabbit intracranial model of infection yet is unable to grow outside the animal host in the low CO2 concentrations present in the atmosphere (6). Thus, mutations towards enhanced virulence must be balanced with other selective pressures acting on C. neoformans.
The proposal of Steenbergen et al. (65) for a role for environmental predators as enhancing C. neoformans virulence has stimulated considerable debate (see e.g., reference 43). While the differences in virulence for eca1 and cna1 mutants in diverse host systems could be interpreted as a consequence of these being nonnatural hosts, we argue in favor of alternative systems as virulence models for this fungus. As noted previously, the C. neoformans interaction with amoeba has been observed in nature (13, 16) and the fungus lives in soil, guano, and other debris coinhabited by other microbial predators, such as nematodes and insects. The Cryptococcus genus clusters with other basidiomycetes that are insect pathogens or insect associated (62). C. neoformans has been isolated from the intestines of beetles (7, 67). The wax moth is a common problem for beekeepers, and C. neoformans has been isolated from beehives in Turkey (25). Thus, C. neoformans could associate with these species in nature. Further support for studies with alternative hosts derives from analysis of Legionnaires’ disease, in which it is well established that infectious Legionella pneumophila bacteria are maintained within amoebae in water sources (28, 60). Importantly, laboratory-isolated mutants of L. pneumophila exhibit reduced virulence in both amoebae and macrophages, in macrophages but not amoebae, or in amoebae but not macrophages (e.g., references 11 and 33 and reviewed in reference 70), representing a similar situation to that observed with C. neoformans eca1 mutants. The differences in virulence of the eca1 and cna1 mutants in each model system may reflect underlying differences in host conditions (e.g., Ca2+ concentrations) in each model. This is analogous to recent discoveries showing differing virulence properties of C. albicans calcineurin mutants depending on host tissue, in that calcineurin is required for systemic but not vaginal infection (5, 56). These studies demonstrate the potential complexity in the host-pathogen-environment interactions that represent challenges for future research to understand and control infectious disease.
Acknowledgments
We thank Charles Hall for help with phylogenetic analysis, Arturo Casadevall for the 18B7 antibody, and Alejandro Aballay for suggesting improvements to the manuscript.
The research was supported by NIAID T32 grant AI52080 to A.I., NIAID K08 grant AI63084 and an Ellison Medical Foundation New Scholar Award to E.M., and NIAID R01 grants AI042159 and AI063443 to J.H.
Editor: A. Casadevall
Footnotes
Published ahead of print on 14 May 2007.
REFERENCES
- 1.Adamíková, L., A. Straube, I. Schulz, and G. Steinberg. 2004. Calcium signaling is involved in dynein-dependent microtubule organization. Mol. Biol. Cell 15:1969-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhter, S., H. C. McDade, J. M. Gorlach, G. Heinrich, G. M. Cox, and J. R. Perfect. 2003. Role of alternative oxidase gene in pathogenesis of Cryptococcus neoformans. Infect. Immun. 71:5794-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352-365. [DOI] [PubMed] [Google Scholar]
- 4.Bader, T., B. Bodendorfer, K. Schröppel, and J. Morschhäuser. 2003. Calcineurin is essential for virulence in Candida albicans. Infect. Immun. 71:5344-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bader, T., K. Schröppel, S. Bentink, N. Agabian, G. Köhler, and J. Morschhäuser. 2006. Role of calcineurin in stress resistance, morphogenesis, and virulence of a Candida albicans wild-type strain. Infect. Immun. 74:4366-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahn, Y.-S., G. M. Cox, J. R. Perfect, and J. Heitman. 2005. Carbonic anhydrase and CO2 sensing during Cryptococcus neoformans growth, differentiation, and virulence. Curr. Biol. 15:2013-2020. [DOI] [PubMed] [Google Scholar]
- 7.Baroni, F. D. A., C. R. Paula, É. G. da Silva, F. C. Viani, I. N. G. Rivera, M. T. B. de Oliveira, and W. Gambale. 2006. Cryptococcus neoformans strains isolated from church towers in Rio de Janeiro City, RJ, Brazil. Rev. Inst. Med. Trop. Sao Paulo 48:71-75. [DOI] [PubMed] [Google Scholar]
- 8.Blankenship, J. R., and J. Heitman. 2005. Calcineurin is required for Candida albicans to survive calcium stress in serum. Infect. Immun. 73:5767-5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blankenship, J. R., F. L. Wormley, M. K. Boyce, W. A. Schell, S. G. Filler, J. R. Perfect, and J. Heitman. 2003. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2:422-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonilla, M., K. K. Nastase, and K. W. Cunningham. 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 21:2343-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brieland, J. K., J. C. Fantone, D. G. Remick, M. LeGendre, M. McClain, and N. C. Engleberg. 1997. The role of Legionella pneumophila-infected Hartmannella vermiformis as an infectious particle in a murine model of Legionnaire's disease. Infect. Immun. 65:5330-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brostrom, M. A., and C. O. Brostrom. 2003. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium 34:345-363. [DOI] [PubMed] [Google Scholar]
- 13.Bunting, L. A., J. B. Neilson, and G. S. Bulmer. 1979. Cryptococcus neoformans: gastronomic delight of a soil ameba. Sabouraudia 17:225-232. [DOI] [PubMed] [Google Scholar]
- 14.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L.-A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casadevall, A., and J. Perfect. 1998. Cryptococcus neoformans. American Society for Microbiology Press, Washington, DC.
- 16.Castellani, A. 1930. An amoeba growing in cultures of a yeast: preliminary note. J. Trop. Med. Hyg. 33:160. [Google Scholar]
- 17.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox, G. M., T. S. Harrison, H. C. McDade, C. P. Taborda, G. Heinrich, A. Casadevall, and J. R. Perfect. 2003. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect. Immun. 71:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronin, S. R., R. Rao, and R. Y. Hampton. 2002. Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J. Cell Biol. 157:1017-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz, M. C., D. S. Fox, and J. Heitman. 2001. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 20:1020-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cyert, M. S. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 311:1143-1150. [DOI] [PubMed] [Google Scholar]
- 22.da Silva Ferreira, M. E., T. Heinekamp, A. Härtl, A. A. Brakhage, C. P. Semighini, S. D. Harris, M. Savoldi, P. F. de Gouvêa, M. H. de Souza Goldman, and G. H. Goldman. 2007. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 44:219-230. [DOI] [PubMed] [Google Scholar]
- 23.de Jesús-Berríos, M., L. Liu, J. C. Nussbaum, G. M. Cox, J. S. Stamler, and J. Heitman. 2003. Enzymes that counteract nitrosative stress promote fungal virulence. Curr. Biol. 13:1963-1968. [DOI] [PubMed] [Google Scholar]
- 24.Eide, D. J., S. Clark, T. M. Nair, M. Gehl, M. Gribskov, M. L. Guerinot, and J. F. Harper. 2005. Characterization of the yeast ionome: a genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome Biol. 6:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ergin, Ç., M. Ilkit, and O. Kaftanoǧlu. 2004. Detection of Cryptococcus neoformans var. grubii in honeybee (Apis mellifera) colonies. Mycoses 47:431-434. [DOI] [PubMed] [Google Scholar]
- 26.Fan, W., P. R. Kraus, M.-J. Boily, and J. Heitman. 2005. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot. Cell 4:1420-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fields, B. S. 1996. The molecular ecology of legionellae. Trends Microbiol. 4:286-290. [DOI] [PubMed] [Google Scholar]
- 29.Fox, D. S., M. C. Cruz, R. A. Sia, H. Ke, G. M. Cox, M. E. Cardenas, and J. Heitman. 2001. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 39:835-849. [DOI] [PubMed] [Google Scholar]
- 30.Fox, D. S., and J. Heitman. 2005. Calcineurin-binding protein Cbp1 directs the specificity of calcineurin-dependent hyphal elongation during mating in Cryptococcus neoformans. Eukaryot. Cell 4:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchs, B. B., and E. Mylonakis. 2006. Using non-mammalian hosts to study fungal virulence and host defense. Curr. Opin. Microbiol. 9:346-351. [DOI] [PubMed] [Google Scholar]
- 32.Fujimura, M., N. Ochiai, M. Oshima, T. Motoyama, A. Ichiishi, R. Usami, K. Horikoshi, and I. Yamaguchi. 2003. Putative homologs of SSK22 MAPKK kinase and PBS2 MAPK kinase of Saccharomyces cerevisiae encoded by os-4 and os-5 genes for osmotic sensitivity and fungicide resistance in Neurospora crassa. Biosci. Biotechnol. Biochem. 67:186-191. [DOI] [PubMed] [Google Scholar]
- 33.Gao, L.-Y., B. J. Stone, J. K. Brieland, and Y. Abu Kwaik. 1998. Different fates of Legionella pneumophila pmi and mil mutants within macrophages and alveolar epithelial cells. Microb. Pathog. 25:291-306. [DOI] [PubMed] [Google Scholar]
- 34.Garsin, D. A., C. D. Sifri, E. Mylonakis, X. Qin, K. V. Singh, B. E. Murray, S. B. Calderwood, and F. M. Ausubel. 2001. A simple model host for identifying Gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 98:10892-10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giles, S. S., I. Batinić-Haberle, J. R. Perfect, and G. M. Cox. 2005. Cryptococcus neoformans mitochondrial superoxide dismutase: an essential link between antioxidant function and high-temperature growth. Eukaryot. Cell 4:46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogan, P. G., L. Chen, J. Nardone, and A. Rao. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17:2205-2232. [DOI] [PubMed] [Google Scholar]
- 37.Idnurm, A., Y.-S. Bahn, K. Nielsen, X. Lin, J. A. Fraser, and J. Heitman. 2005. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 3:753-764. [DOI] [PubMed] [Google Scholar]
- 38.Idnurm, A., J. L. Reedy, J. C. Nussbaum, and J. Heitman. 2004. Cryptococcus neoformans virulence gene discovery through insertional mutagenesis. Eukaryot. Cell 3:420-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karababa, M., E. Valentino, G. Pardini, A. T. Coste, J. Bille, and D. Sanglard. 2006. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 59:1429-1451. [DOI] [PubMed] [Google Scholar]
- 40.Kojima, K., Y.-S. Bahn, and J. Heitman. 2006. Calcineurin, Mpk1, and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology 152:591-604. [DOI] [PubMed] [Google Scholar]
- 41.Kraus, P. R., and J. Heitman. 2003. Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem. Biophys. Res. Commun. 311:1151-1157. [DOI] [PubMed] [Google Scholar]
- 42.Kraus, P. R., C. B. Nichols, and J. Heitman. 2005. Calcium and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryot. Cell 4:1079-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levitz, S. M. 2001. Does amoeboid reasoning explain the evolution and maintenance of virulence factors in Cryptococcus neoformans? Proc. Natl. Acad. Sci. USA 98:14760-14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, M., P. Du, G. Heinrich, G. M. Cox, and A. Gelli. 2006. Cch1 mediates calcium entry in Cryptococcus neoformans and is essential in low-calcium environments. Eukaryot. Cell 5:1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loftus, B. J., E. Fung, P. Roncaglia, D. Rowley, P. Amedeo, D. Bruno, J. Vamathevan, M. Miranda, I. J. Anderson, J. A. Fraser, J. E. Allen, I. E. Bosdet, M. R. Brent, R. Chiu, T. L. Doering, M. J. Donlin, C. A. D'Souza, D. S. Fox, V. Grinberg, J. Fu, M. Fukushima, B. J. Haas, J. C. Huang, G. Janbon, S. J. M. Jones, H. L. Koo, M. I. Krzywinski, J. K. Kwon-Chung, K. B. Lengeler, R. Maiti, M. A. Marra, R. E. Marra, C. A. Mathewson, T. G. Mitchell, M. Pertea, F. R. Riggs, S. L. Salzberg, J. E. Schein, A. Shvartsbeyn, H. Shin, M. Shumway, C. A. Specht, B. B. Suh, A. Tenney, T. R. Utterback, B. L. Wickes, J. R. Wortman, N. H. Wye, J. W. Kronstad, J. K. Lodge, J. Heitman, R. W. Davis, C. M. Fraser, and R. W. Hyman. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacLennan, D. H. 2000. Ca2+ signalling and muscle disease. Eur. J. Biochem. 267:5291-5297. [DOI] [PubMed] [Google Scholar]
- 47.Missall, T. A., and J. K. Lodge. 2005. Function of the thioredoxin proteins in Cryptococcus neoformans during stress or virulence and regulation by putative transcriptional modulators. Mol. Microbiol. 57:847-858. [DOI] [PubMed] [Google Scholar]
- 48.Missall, T. A., M. E. Pusateri, and J. K. Lodge. 2004. Thiol peroxidase is critical for virulence and resistance to nitric oxide and peroxide in the fungal pathogen, Cryptococcus neoformans. Mol. Microbiol. 51:1447-1458. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell, T. G., and J. R. Perfect. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mylonakis, E., F. M. Ausubel, J. R. Perfect, J. Heitman, and S. B. Calderwood. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. USA 99:15675-15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mylonakis, E., A. Idnurm, R. Moreno, J. El Khoury, J. B. Rottman, F. M. Ausubel, J. Heitman, and S. B. Calderwood. 2004. Cryptococcus neoformans Kin1 protein kinase homologue, identified through a Caenorhabditis elegans screen, promotes virulence in mammals. Mol. Microbiol. 54:407-419. [DOI] [PubMed] [Google Scholar]
- 52.Mylonakis, E., R. Moreno, J. B. El Khoury, A. Idnurm, J. Heitman, S. B. Calderwood, F. M. Ausubel, and A. Diener. 2005. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 73:3842-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen, K., G. M. Cox, P. Wang, D. L. Toffaletti, J. R. Perfect, and J. Heitman. 2003. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and α isolates. Infect. Immun. 71:4831-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ochiai, N., M. Fujimura, M. Oshima, T. Motoyama, A. Ichiishi, H. Yamada-Okabe, and I. Yamaguchi. 2002. Effects of iprodione and fludioxonil on glycerol synthesis and hyphal development in Candida albicans. Biosci. Biotechnol. Biochem. 66:2209-2215. [DOI] [PubMed] [Google Scholar]
- 55.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. Perfect, and J. Heitman. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Onyewu, C., N. A. Afshari, and J. Heitman. 2006. Calcineurin promotes infection of the cornea by Candida albicans and can be targeted to enhance fluconazole therapy. Antimicrob. Agents Chemother. 50:3963-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perfect, J. R., S. D. Lang, and D. T. Durack. 1980. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am. J. Pathol. 62:177-194. [PMC free article] [PubMed] [Google Scholar]
- 58.Ralph, P., J. Prichard, and M. Cohn. 1975. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J. Immunol. 114:898-905. [PubMed] [Google Scholar]
- 59.Rodriguez, N. M., R. Docampo, H.-G. Lu, and D. A. Scott. 2002. Overexpression of the Leishmania amazonensis Ca2+-ATPase gene lmaa1 enhances virulence. Cell Microbiol. 4:117-126. [DOI] [PubMed] [Google Scholar]
- 60.Rowbotham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959-976. [DOI] [PubMed] [Google Scholar]
- 62.Sivakumaran, S., P. Bridge, and P. Roberts. 2002. Genetic relatedness among Filobasidiella species. Mycopathologia 156:157-162. [DOI] [PubMed] [Google Scholar]
- 63.Steenbergen, J. N., and A. Casadevall. 2003. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microbes Infect. 5:667-675. [DOI] [PubMed] [Google Scholar]
- 64.Steenbergen, J. N., J. D. Nosanchuk, S. D. Malliaris, and A. Casadevall. 2003. Cryptococcus neoformans virulence is enhanced after growth in the genetically malleable host Dictyostelium discoideum. Infect. Immun. 71:4862-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 98:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, Y. G. Asfaw, T. C. Sauer, L. K. Najvar, W. R. Kirkpatrick, T. F. Patterson, D. K. Benjamin, Jr., J. Heitman, and J. R. Perfect. 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suh, S.-O., J. V. McHugh, D. D. Pollock, and M. Blackwell. 2005. The beetle gut: a hyperdiverse source of novel yeasts. Mycol. Res. 109:261-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sumi, M., K. Kiuchi, T. Ishikawa, A. Ishii, M. Hagiwara, T. Nagatsu, and H. Hidaka. 1991. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem. Biophys. Res. Commun. 181:968-975. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki, C., and Y.-I. Shimma. 1999. P-type ATPase spf1 mutants show a novel resistance mechanism for the killer toxin SMKT. Mol. Microbiol. 32:813-823. [DOI] [PubMed] [Google Scholar]
- 70.Swanson, M. S., and B. K. Hammer. 2000. Legionella pneumophila pathogenesis: a fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 54:567-613. [DOI] [PubMed] [Google Scholar]
- 71.Tang, R. J., J. Breger, A. Idnurm, K. J. Gerik, J. K. Lodge, J. Heitman, S. B. Calderwood, and E. Mylonakis. 2005. Cryptococcus neoformans gene involved in mammalian pathogenesis identified by a Caenorhabditis elegans progeny-based approach. Infect. Immun. 73:8219-8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walton, F. J., A. Idnurm, and J. Heitman. 2005. Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol. Microbiol. 57:1381-1396. [DOI] [PubMed] [Google Scholar]
- 73.Wang, P., M. E. Cardenas, G. M. Cox, J. R. Perfect, and J. Heitman. 2001. Two cyclophilin A homologs with shared and distinct functions important for growth and virulence of Cryptococcus neoformans. EMBO Rep. 2:511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wold, L. E., K. Dutta, M. M. Mason, J. Ren, S. E. Cala, M. L. Schwanke, and A. J. Davidoff. 2005. Impaired SERCA function contributes to cardiomyocyte dysfunction in insulin resistant rats. J. Mol. Cell Cardiol. 39:297-307. [DOI] [PubMed] [Google Scholar]
- 75.Wu, Z., F. Liang, B. Hong, J. C. Young, M. R. Sussman, J. F. Harper, and H. Sze. 2002. An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol. 130:128-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang, Y., R. Lamm, C. Pillonel, S. Lam, and J.-R. Xu. 2002. Osmoregulation and fungicide resistance: the Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 68:532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]