Abstract
The cytoadhesion of Plasmodium falciparum-infected erythrocytes (IEs) to the endothelial cells lining the microvasculature, clogging the microvessels of various organs, is a key event in the pathogenesis of certain severe forms of malaria, such as cerebral malaria and pulmonary edema. Studies aiming to identify possible correlations between the severity of clinical cases and the presence of particular cytoadhesion phenotypes have been largely unsuccessful. One of the possible reasons for this failure is that some of the key receptors and/or mechanisms involved have yet to be identified. By combining IE selection, cell transfection, and adhesion inhibition assays, we identified a new cytoadhesion receptor, neural cell adhesion molecule (NCAM). NCAM is a member of the immunoglobulin superfamily and has nonpolysialylated and polysialylated isoforms, the latter being rare in adults. The nonpolysialylated form is present on the surfaces of endothelial cells in the microvessels of various organs in which IE sequestration occurs. We found that multiphenotypic IEs interacted with nonpolysialylated NCAM and with another, as yet unidentified receptor. These IEs also displayed cytoadhesion in flow conditions, presenting the unique ability to form adherent macroaggregates composed of hundreds of IEs. These features may act as virulence factors, increasing the capacity of IEs to clog microvessels via receptor synergy and macroaggregate formation, thereby facilitating the pathogenesis of severe forms of malaria.
Four species of Plasmodium infect humans, but only Plasmodium falciparum is able to escape the peripheral blood circulation by a phenomenon called sequestration. This sequestration results from the cytoadhesion of P. falciparum-infected erythrocytes (IEs) to endothelial cells or syncytiotrophoblasts, mainly at mature stages (trophozoites and schizonts), but also at the ring stage in some cases (25). Cytoadhesion is thought to play a key role in the pathogenesis of some severe forms of malaria (8), with dramatic outcomes when massive sequestration occurs in the microvasculature of organs such as the brain (cerebral malaria) or lung (pulmonary edema) (19, 24). Several host receptors on the surfaces of endothelial cells and syncytiotrophoblasts have been identified as potential mediators of IE cytoadhesion (7). However, none of these receptors has been clearly implicated in the pathogenesis of severe forms of malaria other than pregnancy-associated malaria, suggesting, among other hypotheses, that other as yet unidentified mechanisms and/or receptors may be involved.
NCAM (neural cell adhesion molecule) is a member of the immunoglobulin (Ig) superfamily and is encoded by a single gene extremely well conserved among vertebrates. Alternative mRNA splicing gives rise to three major isoforms of NCAM, with molecular masses of 120, 140, and 180 kDa, differing essentially in their cytoplasmic tails (13, 34). The extracellular part of NCAM contains five Ig-like domains and two fibronectin type III repeats. NCAM is the only carrier of a long unconventional linear carbohydrate polymer, polysialic acid (PSA) (17, 33), which consists of between 8 and more than 100 alpha-2-8-linked neuraminic acid units attached to the fifth Ig-like domain of the protein. The attachment of PSA to NCAM is regulated posttranslationally, and NCAM with a high PSA content (NCAMPSA+) is associated with changes to tissue morphogenesis during development, whereas poorly sialylated forms of NCAM (NCAMPSA−) predominate in postnatal tissues (12, 32). However, NCAMPSA+ persists in adult structures displaying ongoing neurogenesis and a high degree of remodeling, such as the hippocampus and olfactory bulb (29, 33). NCAM is constitutively expressed at the surfaces of various cells, including neurons, astrocytes, NK cells, microvascular endothelial cells from human brain (15) and dermis (21), and endovascular trophoblasts (5). It promotes axon growth, nerve branching and fasciculation, cell migration (17, 31, 33), and leukocyte and endothelial cell-cell adhesion (21) by homophilic and heterophilic interactions (10). The PSA moiety attenuates NCAM-mediated adhesion and modulates cell surface interactions.
We report here that the poorly polysialylated form of NCAM present in postnatal tissues is a receptor for IE cytoadhesion. IEs with the corresponding cytoadhesion phenotype can interact with purified and recombinant NCAMPSA−, cytoadhere to NCAMPSA−-transfected COS-7 cells, and interact with another, as yet unidentified receptor. NCAMPSA−-binding IEs also display a unique ability to form adherent macroaggregates under flow conditions.
MATERIALS AND METHODS
Cell cultures.
The FCR3 strain was cultured as previously described (35) in RPMI 1640 supplemented with bicarbonate, glutamine, 0.2% glucose, 50 μM hypoxanthine, 10 μg/ml gentamicin, and 0.25% AlbuMAX, containing O+ erythrocytes, in a candle jar at 37°C.
Clone 1D Saimiri brain microvascular endothelial (SBEC 1D) cells were obtained from Saimiri sciureus karyotype 14-7 animals and were maintained as previously described (14). These cells express CSA, CD36, intercellular adhesion molecule 1 (ICAM-1), and one or more unidentified receptors for IE cytoadhesion. Chinese hamster ovary (CHO) glycosylation mutant 745 (CHO-745) cells, which express neither heparan sulfate nor chondroitin sulfate (11), were cultured in culture medium without endothelial cell growth supplement.
Peripheral blood isolates.
Peripheral blood was collected from 22 patients attending consultations at the Albert Schweitzer Hospital in Lambaréné, Gabon. Written informed consent was obtained from the patients or their legal representatives. The study protocol was approved by the Ethics Committee of the International Foundation for the Albert Schweitzer Hospital. The ring stage IEs collected were allowed to mature in the culture conditions described above, and each isolate was then used in duplicate static cytoadhesion assays with CHO-745 cells, during the first cycle of maturation.
Selection by panning.
Two protocols were used for panning on purified proteins. We coated 3-cm plastic petri dishes (Falcon, France) by overnight incubation at 4°C with NCAM purified from embryonic chicken brain (Chemicon International; AG265) at a concentration of 10 μg/ml in phosphate-buffered saline (PBS), pH 7.2, as previously described for chondroitin-4-sulfate (3, 9). The PSA moiety was then digested by incubation with 1 U/ml neuraminidase (Sigma; N-3001) in PBS for 2 h at 37°C. The remaining free sites on the dishes were saturated by incubation for 1 h with 2% bovine serum albumin (BSA) in PBS at 37°C. The plates were gently and quickly rinsed once with cytoadhesion medium and were then incubated with the IEs. In some experiments, H300 was added to the assay mixture at the same time as the IEs. This coating procedure was also used for assays of adhesion to recombinant NCAM-Fc (22). Panning was also performed on NCAM-coated tosyl-activated Dynabeads M-450 (no. 140.04; Dynal ASA, Oslo, Norway), according to the manufacturer's instructions (5 μg of NCAM per 107 Dynabeads). The IEs were mixed with the NCAM-Dynabeads at a ratio of 1:10 IEs to beads and incubated for 1 h at 37°C, with gentle agitation. A Dynal magnetic particle concentrator (MPC) was used to select IEs bound to the NCAM-Dynabeads, and the unbound IEs were removed by repeated washes. Fresh erythrocytes were then added to the IEs attached to the NCAM-Dynabeads, and the parasites were allowed to reinvade overnight. The NCAM-Dynabeads were then removed from the IE culture with the Dynal MPC.
Transfection of COS-7 cells.
COS-7 cells were transfected using Lipofectamine reagent (Invitrogen, France) complexed with the full-length NCAM-140 sequence fused to the green fluorescent protein sequence in the PEGFP-N1 expression vector (Invitrogen, France). Transfected cells were maintained at 37°C in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum until assays were carried out 48 h later. In parallel, and in the same transfection and culture conditions, the native PEGFP-N1 expression vector was expressed in COS-7 cells for control experiments.
Immunocytochemistry for NCAM and PSA.
Cell lines (CHO-745 and SBEC 1D) were cultured on 12-mm coverslips. Briefly, cells were fixed by incubation with 4% formaldehyde for 10 min, rinsed twice with PBS, and blocked by incubation with 5% BSA for 30 min. Anti-PSA (mouse monoclonal IgM; 1 μg/ml) (30) and anti-NCAM H300 (rabbit polyclonal IgG; Chemicon; 1 μg/ml) antibodies were incubated overnight with the cells at 4°C. Secondary antibodies Cy3-conjugated donkey anti-mouse IgM (0.7 μg/ml) and fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (0.7 μg/ml), purchased from Jackson Immunoresearch Laboratories Inc., were incubated with the cells for 1 h at 25°C. Cells were rinsed twice in 0.1 M PBS, mounted in antifading (Mowiol; Calbiochem) mounting medium, and covered with a coverslip. Digital images were collected using an LSM 510 scanning confocal microscope (Zeiss, Germany) equipped with a Kr/Ar laser and monitored with Axiovision 4.0 image acquisition software.
Immunoblot assay.
Cell lines were left untreated or treated overnight with endoneuraminidase (0.8 U/ml) and were homogenized in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA) supplemented with a protease inhibitor cocktail (Roche). Total protein levels were quantified using a modified Bradford assay (Bio-Rad protein assay). We then mixed 30 μg of protein with Laemmli loading buffer (18) and subjected this sample to sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 7.5% acrylamide gel. The separated proteins were transferred to a nitrocellulose membrane. The membrane was then incubated at room temperature for 30 min in Tris-buffered saline supplemented with 0.1% Tween 20 (TBS-T) and 5% (wt/vol) nonfat dry milk and probed overnight at 4°C with a rabbit anti-NCAM antibody (0.7 μg/ml; Chemicon Europe, United Kingdom). A rabbit antiactin antibody (1:5,000 dilution; Chemicon Europe, United Kingdom) was used as a protein loading control. The membranes were washed twice for 30 min each with TBS-T and incubated with the secondary antibody, horseradish peroxidase-conjugated goat anti-rabbit IgG (1:8,000 dilution; Jackson Immunoresearch, PA). Membranes were washed twice, for 30 min each, with TBS-T, and the bands were visualized using the Super Signal West Pico chemiluminescence substrate kit (Pierce, Rockford, IL).
Cytoadhesion and inhibition assays. (i) Static cytoadhesion assays.
Cytoadhesion and cytoadhesion inhibition assays were performed on SBEC 1D, CHO-745, and COS-7 cells, as previously described (26). The following inhibitors of cytoadhesion were used: soluble chondroitin sulfate A (CSA) at a concentration of 100 μg/ml (Fluka; 27042), H300 anti-NCAM rabbit polyclonal antibody at a concentration of 20 μg/ml (Santa Cruz Biotechnology, Inc.; sc-10735), goat anti-NCAM serum at a dilution of 1/10 (gift from Marie-Claude Amoureux, AbCys S.A., Paris, France), 123C3 anti-NCAM monoclonal antibody at a concentration of 100 μg/ml (Abcam; ab8077), and FA6-152 anti-CD36 monoclonal antibody at a concentration of 5 μg/ml (a gift from L. Edelman); alternatively, cells were subjected to prior treatment with chondroitinase ABC at a concentration of 1 U/ml (Fluka; 27038). Controls were performed with isotypic antibodies or nonimmune goat serum. Adherent IEs were counted under a light microscope in four randomly selected fields (each with an area of 0.2827 mm2), from two different wells for each condition, at a magnification of ×300 (Nikon; TMS). These fields were evenly distributed over the total area of the wells. Assays were performed, most at least in triplicate, on different days (unless otherwise stated), giving at least 12 values for each condition (8 in the few cases in which experiments were only duplicated), and the results are expressed as the numbers of adhering IEs (means ± standard deviations [SD]) per square millimeter of cell monolayer or as percentages of the control value. Mann-Whitney tests were used to evaluate the statistical significance of differences.
(ii) Flow-based cytoadhesion assays.
CHO-745 or SBEC 1D cell suspensions were allowed to settle on slides in petri dishes. After 2 h at 37°C, the cells adhered to the slide, and culture medium was added to cover the entire petri dish. The cells were then allowed to grow to confluence for 3 to 4 days, as described above. The slides were then mounted in a cell adhesion flow chamber, as previously described (1), and cytoadhesion was observed with an inverted microscope (Nikon; Eclipse TE 200) using the Plan Fluor ELWD 40/0.60 objective (Nikon). An aliquot of 1 ml of IE suspension, at 25% hematocrit and about 5% parasitemia in cytoadhesion medium (pH 7.2) containing 10% O+ serum (from a Caucasian donor who had never contracted malaria), was allowed to flow into the perfusion chamber (over a period of approximately 10 min) over the CHO-745 or SBEC 1D cell monolayer at 0.05 Pa. We then allowed cytoadhesion medium supplemented with 10% O+ serum to flow through the chamber for 10 min at 0.05 Pa to remove nonadherent IEs. The shear stress resistance of the cytoadherent IEs was measured after adhesion at 0.05 Pa, by gradually increasing the flow rate from 0.1 to 1.6 Pa and by flushing with cytoadhesion medium for 10 min for each shear stress. We also determined the ability of the IEs to adhere to the cells at various wall shear stresses. The IE suspensions were allowed to flow over the cells for 10 min at 0.1, 0.2, 0.4, and 0.8 Pa. The unbound IEs were washed at the same shear stress with cytoadhesion medium.
RESULTS
Interaction with purified and recombinant NCAM.
We investigated the capacity of FCR3 IEs to interact with NCAM purified from embryonic chicken brain, adsorbed onto plastic petri dishes, and treated with neuraminidase to mimic the weakly polysialylated form of NCAM found in postnatal endothelium. FCR3 IEs showed low levels of adhesion to NCAMPSA−, reaching only 6 ± 5 adherent IEs per mm2. The adherent IEs were cultured, and several additional rounds of panning were performed, using neuraminidase-treated NCAM-coated magnetic beads, to develop a subpopulation interacting specifically with NCAMPSA− (FCR3NCAMPSA−). After repeated panning, these IEs showed high levels of adhesion to plastic-adsorbed chicken brain NCAMPSA− (1,564 ± 591 adherent IEs/mm2) and recombinant NCAM-FcPSA− (1,510 ± 345 adherent IEs/mm2) and interacted with purified NCAMPSA−-coated magnetic beads and recombinant NCAM-FcPSA−-coated protein A-magnetic beads. The adhesion of FCR3NCAMPSA− IEs to adsorbed chicken brain NCAMPSA− was inhibited by 61% by the rabbit anti-NCAM polyclonal antibody H300 when this antibody was added to the assay at the same times as the IEs. The interaction with adsorbed chicken brain NCAMPSA+ (5 ± 9 adherent IEs/mm2) was identical to that for the control, in which BSA replaced NCAM, and was therefore not significant.
Cytoadhesion to CHO-745 and NCAM-transfected COS-7 cells.
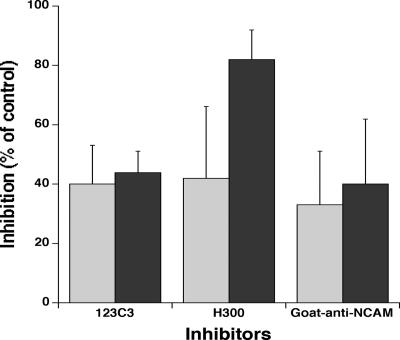
FCR3NCAMPSA− IEs adhered to CHO-745 cells at a level of 1,600 ± 493 IEs/mm2 of cell monolayer. This cytoadhesion was partially inhibited (about 40%) by a mouse anti-NCAM monoclonal antibody (123C3). Rabbit (H300) and goat anti-NCAM polyclonal antibodies gave similar results when coincubated with the cells during the adhesion assays (Fig. 1). However, the level of inhibition was strongly increased (82%) by incubating CHO-745 cells with H300 for 2 hours before adding the IEs (Fig. 1).
FIG. 1.
Inhibition of the cytoadhesion of FCR3NCAMPSA− IEs to CHO-745 cells by anti-NCAM antibodies. We assessed the ability of anti-NCAM 123C3 monoclonal antibody and H300 and goat anti-NCAM polyclonal antibodies to inhibit FCR3NCAMPSA− IE cytoadhesion to CHO-745 cells, when added to the cells concomitantly with the IEs (light gray bars) or after incubation of the antibodies for 2 hours with the CHO745 cells before the addition of IEs (dark gray bars). Values correspond to the means ± SD of two experiments for 123C3 and goat-anti-NCAM antibodies without prior incubation (eight values from two experiments performed on two different days) and at least three experiments for the other conditions.
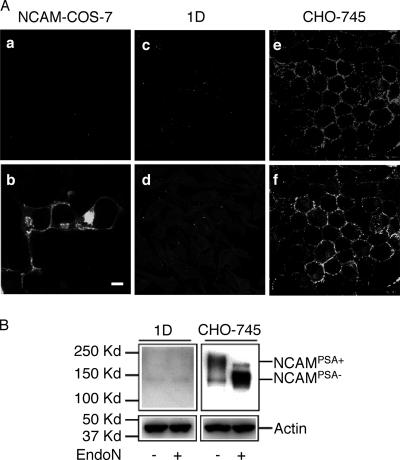
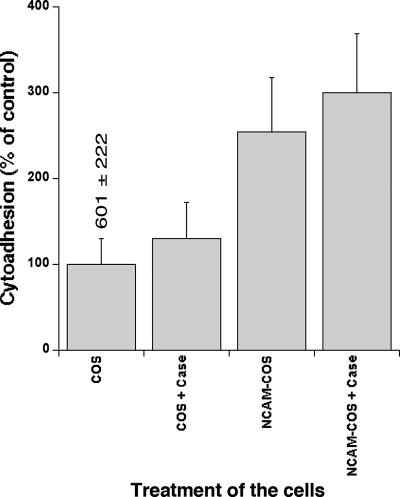
COS-7 cells are among the rare cell lines known to express absolutely no NCAM. COS-7 cells transfected with the full-length sequence of the NCAM140 gene, as demonstrated by expression of the green fluorescent protein reporter gene (70 to 90% of all COS-7 cells), expressed NCAM molecules without the PSA moiety at their surfaces (Fig. 2A). These NCAMPSA− molecules were also detected on the surfaces of CHO-745 cells, although the proportion of cells displaying expression was lower than that for NCAMPSA+ (Fig. 2B). FCR3NCAMPSA− IEs cytoadhered to mock-transfected COS-7 (Mock-COS-7) cells, with 601 ± 222 IEs/mm2 of cell monolayer (Fig. 3). Since CSA is the only known IE cytoadhesion receptor present on the surfaces of Mock-COS-7 cells, we tried to inhibit this cytoadhesion by prior treatment of the cells with chondroitinase ABC. The difference in cytoadhesion between the untreated cells and the chondroitinase ABC-treated cells was not statistically significant (P = 0.1153 for Mock-COS-7 cells and P = 0.1782 for NCAM-transfected COS-7 cells), indicating that FCR3NCAMPSA− IEs cytoadhered to Mock-COS-7 cells via an unidentified receptor. In contrast, chondroitinase ABC treatment inhibited 82% ± 7% of FCR3CSA cytoadhesion to COS-7 cells (control not shown). The transfection of COS-7 cells with NCAM increased the level of cytoadhesion by a factor of 2.5 to 3, indicating that FCR3NCAMPSA− IEs were able to use cellular NCAMPSA− for cytoadhesion. This was confirmed by the partial inhibition (54%, P = 0.0159) obtained with the anti-NCAM H300 antibody.
FIG. 2.
Immunocytochemistry of NCAM on NCAM-transfected COS-7, SBEC 1D, and CHO-745 cells. (A) Confocal images of NCAM-transfected COS-7 cells (a and b), SBEC 1D cells (c and d), and CHO-745 cells (e and f) immunostained with anti-PSA (a, c, and e) and anti-NCAM antibodies (b, d, and f). The absence of visible labeling of the SBEC 1D cells in confocal images is due to low levels of expression of the 140-kDa NCAM lacking the PSA chain, as revealed by overexposure of the immunoblot. Scale bar, 10 μm. (B) Immunoblot of PSA-NCAM and NCAM on SBEC 1D and CHO-745 cells and endoneuraminidase (EndoN) digestion profile. Note the shift in molecular size of CHO-745 NCAM induced by EndoN digestion.
FIG. 3.
Cytoadhesion of FCR3NCAMPSA− IEs to NCAM-transfected COS-7 cells. Controls were set up in which adhesion to Mock-COS-7 cells was assessed without (COS; considered as 100%) or with (COS + Case) prior treatment of the cells with 1 U/ml of chondroitinase ABC. The level of cytoadhesion of IEs per mm2 of the control cells is indicated. The cytoadhesion of IEs to NCAM-transfected COS-7 cells without (NCAM-COS) or with (NCAM-COS + Case) prior treatment of the cells with 1 U/ml of chondroitinase ABC is expressed as a percentage of control. Values correspond to the means ± SD of three experiments, except for COS + Case (two experiments).
Cytoadhesion to SBEC 1D.
We assessed the capacity of FCR3NCAMPSA− IEs to cytoadhere to SBEC 1D cells, which express NCAMPSA− at extremely low levels (Fig. 2). Surprisingly, strong cytoadhesion was observed (4,565 ± 2,985 IEs/mm2 of cell monolayer), which could not have been mediated by the almost undetectable quantity of NCAMPSA− present at the cell surface. We tried to identify the receptor involved in this cytoadhesion by carrying out inhibition assays. The cytoadhesion of FCR3NCAMPSA− IEs was not affected by prior treatment of the cells with chondroitinase ABC or by the presence of soluble CSA or H300 (data not shown). The inhibitory capacities of chondroitinase ABC and soluble CSA were controlled on the cytoadhesion of FCR3CSA IEs to SBEC 1D cells, giving 93% ± 8% and 94% ± 6% inhibition, respectively. Weak but significant inhibition of FCR3NCAMPSA− IEs was obtained with the anti-CD36 FA6-152 antibody (19% ± 13%; P = 0.0003). The inhibitory capacity of FA6-152 was controlled on the cytoadhesion of FCR3CD36 IEs to SBEC 1D cells at 83% ± 4%.
Cytoadhesion under flow conditions.
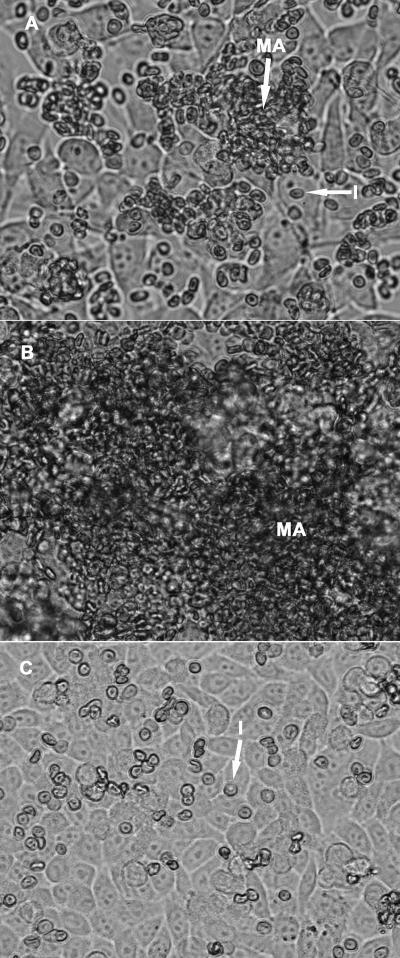
Flow-based cytoadhesion assays demonstrated that FCR3NCAMPSA− IEs were able to cytoadhere, at 0.05 Pa, to both CHO-745 cells, via NCAMPSA−, and SBEC 1D cells, via an unidentified receptor. This cytoadhesion could not be quantified due to high levels of IE aggregation. Cytoadherent aggregates were observed on both target cell types, some containing fewer than 10 IEs and others being larger than the microscope field and consisting of thousands of IEs (Fig. 4). These aggregates formed during the 10 min during which the IE suspension flowed through the observation chamber. Cytoadhesion occurred at wall shear stresses of up to 0.2 Pa. Adherent aggregates were susceptible to increases in wall shear stress and were pulled off the cells completely at 0.4 Pa. In contrast, individual cytoadherent IEs were more resistant to increases in shear stress, with many remaining attached to the cell at 1.6 Pa.
FIG. 4.
Cytoadhesion of FCR3NCAMPSA− IEs under flow conditions. FCR3NCAMPSA− IEs cytoadhered individually (I) or as macroaggregates (MA) to SBEC 1D (A and B) and CHO-745 (C) cells under flow conditions similar to those observed in microvessels (0.05 Pa; A and B). Macroaggregates of less than 100 IEs (A) or thousands of IEs (B) were observed up to a wall shear stress of 0.4 Pa, and numerous individual cytoadherent IEs resisted wall shear stresses of up to 1.6 Pa (C).
Isolates from patients bind to cellular NCAM.
All the isolates tested cytoadhered to CHO-745 cells, at levels ranging from 436 ± 171 to 2,713 ± 551 adherent IEs/mm2 of cell monolayer (Table 1). We cannot, of course, exclude the possibility that various proportions of the adherent IEs interacted with a receptor other than NCAMPSA−. We investigated the involvement of NCAM in this cytoadhesion by assessing the capacity of the H300 anti-NCAM antibody to inhibit the interaction when added to the cells together with the IEs. Cytoadhesion inhibition assays were carried out in duplicate, on the same day, to prevent the selection in culture of a particular adhesion phenotype. Only two isolates displayed no change in cytoadhesion in the presence of H300, revealing an interaction with molecules other than NCAM. Antibody decreased cytoadhesion by less than 10% for another two isolates. In 16 of the remaining 18 isolates, cytoadhesion was inhibited by more than 20%, indicating that NCAM-binding IEs were present in most isolates.
TABLE 1.
NCAM-mediated cytoadhesion of P. falciparum isolates
| Isolate | Parasitemia (%) | Cytoadhesiona (mean ± SD) | Inhibitionb (mean ± SD) |
|---|---|---|---|
| 1 | 1.4 | 579 ± 196 | 40 ± 12 |
| 2 | 1.5 | 1,766 ± 378 | 21 ± 10 |
| 3 | 4.7 | 2,713 ± 551 | 21 ± 7 |
| 4 | 1.9 | 1,500 ± 62 | 51 ± 9 |
| 5 | 2 | 1,754 ± 131 | 27 ± 12 |
| 6 | 7 | 2,085 ± 1,369 | 61 ± 27 |
| 7 | 7.6 | 1,424 ± 461 | 7 ± 24 |
| 8 | 7 | 708 ± 101 | 51 ± 33 |
| 9 | 7.6 | 1,896 ± 407 | 25 ± 3 |
| 10 | 5.6 | 1,044 ± 207 | 24 ± 15 |
| 11 | 1.2 | 2,494 ± 458 | 19 ± 15 |
| 12 | 1.2 | 485 ± 143 | 51 ± 4 |
| 13 | ?c | 776 ± 120 | 28 ± 13 |
| 14 | 1.5 | 543 ± 130 | 21 ± 17 |
| 15 | 2.7 | 454 ± 93 | 30 ± 14 |
| 16 | 1.3 | 525 ± 65 | 14 ± 11 |
| 17 | ? | 708 ± 112 | 27 ± 18 |
| 18 | 3.5 | 471 ± 138 | 0 |
| 19 | 2 | 436 ± 171 | 0 |
| 20 | 3.3 | 1,001 ± 208 | 22 ± 7 |
| 21 | 2 | 1,203 ± 299 | 22 ± 21 |
| 22 | 3.3 | 2,069 ± 454 | 8 ± 6 |
Number of cytoadherent infected erythrocytes per mm2 of CHO-745 cell monolayer.
Percent inhibition of cytoadhesion by the anti-NCAM H300 polyclonal antibody.
?, unknown.
DISCUSSION
We have shown that NCAMPSA− is a receptor for P. falciparum cytoadhesion. IEs are able to interact with NCAMPSA− purified from chickens, and a specific subpopulation can be developed by panning on this receptor. They do not interact with purified NCAMPSA+, probably because of steric hindrance or the strong negative charge of the PSA chain, but this does not exclude the possibility of NCAMPSA+ serving as a receptor for another IE subpopulation. IEs interacting with NCAMPSA− (IEsNCAMPSA−) interact with human recombinant NCAMPSA− and with cellular NCAMPSA− expressed at the surfaces of COS-7 cells after transfection with a construct encoding human NCAM140. The cytoadhesion of IEsNCAMPSA− to CHO-745 cells is inhibited by up to 80% by anti-NCAM antibodies, and IEsNCAMPSA− display cytoadhesion under flow conditions corresponding to those found in the microvasculature of organs in which sequestration occurs. Finally, IEs interacting with NCAM are present in most of the field isolates collected in Lambaréné (Gabon), although a precise determination of their proportion is complicated by the important variability observed in this preliminary study.
The extracellular domain of NCAM consists of five Ig-like and two fibronectin type III-like domains, identical in all three isoforms of the molecule. These domains display a high degree of amino acid conservation among vertebrates (87 to 95% identity between chickens, rats, cattle, and humans). This high degree of conservation makes it difficult to speculate about the site of interaction with the parasitic ligand PfEMP1. The distribution of NCAM in human tissues has been partially characterized. The presence of this molecule on the microvascular endothelium of brain and dermis, two target organs for sequestration, is well established (15, 21). NCAM has also been identified at the surfaces of several different types of cell, including endovascular trophoblasts, but seems to be absent from the surfaces of syncytiotrophoblasts (4, 5). Its presence in the microvasculature of other organs in which sequestration occurs remains to be investigated, together with the degree of polysialylation of the molecule on the endothelium. The dynamic characteristics of IENCAMPSA− cytoadhesion and NCAM expression on the microvascular endothelium suggest that this phenotype may be involved in the pathogenesis of cerebral malaria, at least.
The importance of the NCAMPSA−-binding phenotype in the development of severe malaria may not be exclusively dependent on the frequency of its presence in field isolates. Indeed, this phenotype has two distinctive features that might increase the cytoadhesion-linked virulence of IEsNCAMPSA−. First, IEsNCAMPSA− have a multiadhesion phenotype, displaying cytoadhesion to both Mock-COS-7 and SBEC 1D cells, both of which have very little, if any, NCAM at their surfaces. This cytoadhesion is not mediated by any of the known receptors present at the cell surface, as demonstrated by the failure of inhibition assays. Various multiadhesion phenotypes have already been described, associating CD36, thrombospondin, and ICAM-1 (2, 20, 36) or CD36 and platelet endothelial cell adhesion molecule 1/CD31 (6). A prevalence study on peripheral blood isolates provided evidence of a correlation between severe forms of malaria and the capacity of the corresponding isolates to bind to different receptors (16), although the authors did not investigate whether this was due to the presence of different monoadherence phenotypes in the isolates or to multiadherent IEs. As receptors such as CD36 and ICAM-1 act in synergy to increase IE cytoadhesion in flow conditions (20, 36), it seems likely that the severity of the disease depends at least in part on the capacity of IEs to interact with different receptors.
Another particular feature of the NCAMPSA−-binding phenotype is its capacity for formation of adherent macroaggregates under flow conditions on CHO-745 cells as well as on SBEC 1D cells. At the wall shear stresses found in the microvasculature (between 0.05 and 0.2 Pa), individual IEsNCAMPSA− cytoadhere to the cell monolayer. Other IEsNCAMPSA− then stick to the adherent IEs, interacting with both the cells and the adherent IEs or exclusively with other IEs, forming within a few minutes aggregates ranging from a few IEs to thousands of IEs in size. These aggregates are gradually broken down by increasing wall shear stresses. In contrast, many of the IEs cytoadhering directly to cells resist increasing wall shear stresses up to 1.6 Pa. The profusion of adherent aggregates covering much of the cell monolayer makes it impossible to determine the number of IEs cytoadhering directly to the cells or the proportion of adherent IEs able to resist increasing wall shear stresses. An IE autoagglutination phenomenon has been reported (28). This agglutination, which occurs in suspension, is mediated by platelets through their surface glycoprotein CD36 and involves only CD36-binding IEs (23). The characteristics of the macroaggregation described here differ from those of autoagglutination. First, macroaggregation is initiated by the cytoadhesion of IEs to endothelial or CHO cells; it is never observed with IEs in suspension. Second, the IEs involved in macroaggregation do not interact with CD36, as shown by the absence of inhibition of cytoadhesion to SBEC 1D by the anti-CD36 antibody FA6-152. Finally, platelets cannot be involved in this phenomenon, as there were no platelets present in our cultures or binding assays. This characteristic of the NCAMPSA−-binding phenotype is unique among the cytoadhesion phenotypes studied under flow conditions. Indeed, none of the IEs binding to CSA, CD36, ICAM-1, vascular cell adhesion molecule 1, or P-selectin formed cytoadherent macroaggregates when tested in flow-based assays mimicking the blood circulation in microvessels (27, 36). We are currently investigating the mechanisms underlying this phenomenon. However, the characteristics of macroaggregate formation (speed and dependence on cell adhesion) suggest that the surfaces of IEs are modified following cytoadhesion, regardless of the receptor used, and that these modifications are mediated by changes in ligand conformation or the induction of expression of new parasitic molecules. This report therefore provides the first description of a phenomenon which, if confirmed in vivo, may worsen microvessel occlusion.
We have identified a new cytoadhesion phenotype involving the ability to interact with at least two receptors and a new mechanism of cytoadhesion likely to increase substantially the occlusion capacity of minor IE populations, particularly those interacting with rare receptors. The importance of this phenotype and this mechanism in the pathogenesis of severe forms of malaria remains to be determined in field studies. The nature of the parasitic ligand of IEsNCAMPSA− is currently under investigation, and further studies of the role of multiadhesion phenotypes, interphenotype synergies, and cytoadherent macroaggregate formation are clearly required to improve our understanding of severe forms of malaria.
Acknowledgments
We thank Nicolas Stephane for plasmid constructs, Marie-Claude Amoureux (from AbCys S.A., Paris) for the production of the goat anti-NCAM polyclonal antibody, and all the team of A. Schweitzer Hospital in Lambaréné (Gabon) for the field isolates collected.
This study was supported by a fellowship from the Fondation pour la Recherche Medicale to Valery Matarazzo and by grants from the Biology and Pathology Programs of the Malaria Parasite Network (BIOMALPAR); a Sixth Framework Program (FP6)-funded network of excellence; PAL+ 2002 from the Ministère de la Jeunesse, de l'Education et de la Recherche; and a grant and a postdoctoral fellowship (Michael Ramharter; no. 29202) from the Melinda and Bill Gates Foundation.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Avril, M., B. Traore, F. T. Costa, C. Lepolard, and J. Gysin. 2004. Placenta cryosections for study of the adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A in flow conditions. Microbes Infect. 6:249-255. [DOI] [PubMed] [Google Scholar]
- 2.Baruch, D. I., J. A. Gormely, C. Ma, R. J. Howard, and B. L. Pasloske. 1996. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 93:3497-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeson, J. G., W. Chai, S. J. Rogerson, A. M. Lawson, and G. V. Brown. 1998. Inhibition of binding of malaria-infected erythrocytes by a tetradecasaccharide fraction from chondroitin sulfate A. Infect. Immun. 66:3397-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankenship, T. N., and B. F. King. 1996. Macaque intra-arterial trophoblast and extravillous trophoblast of the cell columns and cytotrophoblastic shell express neural cell adhesion molecule (NCAM). Anat. Rec. 245:525-531. [DOI] [PubMed] [Google Scholar]
- 5.Burrows, T. D., A. King, and Y. W. Loke. 1994. Expression of adhesion molecules by endovascular trophoblast and decidual endothelial cells: implications for vascular invasion during implantation. Placenta 15:21-33. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Q., A. Heddini, A. Barragan, V. Fernandez, S. F. Pearce, and M. Wahlgren. 2000. The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J. Exp. Med. 192:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Q., M. Schlichtherle, and M. Wahlgren. 2000. Molecular aspects of severe malaria. Clin. Microbiol. Rev. 13:439-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chitnis, C., P. Sinnis, and L. Miller. 1999. The sporozoite, the merozoite and the infected red cell: parasite ligands and host receptors, p. 249-285. In M. Wahlgren and P. Perlmann (ed.), Malaria: molecular and clinical aspects. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 9.Cooke, B. M., S. J. Rogerson, G. V. Brown, and R. L. Coppel. 1996. Adhesion of malaria-infected red blood cells to chondroitin sulfate A under flow conditions. Blood 88:4040-4044. [PubMed] [Google Scholar]
- 10.Cunningham, B. A., J. J. Hemperly, B. A. Murray, E. A. Prediger, R. Brackenbury, and G. M. Edelman. 1987. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science 236:799-806. [DOI] [PubMed] [Google Scholar]
- 11.Esko, J. D. 1991. Genetic analysis of proteoglycan structure, function and metabolism. Curr. Opin. Cell Biol. 3:805-816. [DOI] [PubMed] [Google Scholar]
- 12.Figarella-Branger, D., J. F. Pellissier, and G. Rougon. 1993. Cell adhesion molecules. Structure, role in metastatic processes and expression in human solid tumors. Ann. Pathol. 13:296-305. [PubMed] [Google Scholar]
- 13.Franceschini, I., K. Angata, E. Ong, A. Hong, P. Doherty, and M. Fukuda. 2001. Polysialyltransferase ST8Sia II (STX) polysialylates all of the major isoforms of NCAM and facilitates neurite outgrowth. Glycobiology 11:231-239. [DOI] [PubMed] [Google Scholar]
- 14.Gay, F., C. Robert, B. Pouvelle, S. Peyrol, A. Scherf, and J. Gysin. 1995. Isolation and characterization of brain microvascular endothelial cells from Saimiri monkeys. An in vitro model for sequestration of Plasmodium falciparum-infected erythrocytes. J. Immunol. Methods 184:15-28. [DOI] [PubMed] [Google Scholar]
- 15.Gingras, M. C., E. Roussel, J. M. Bruner, C. D. Branch, and R. P. Moser. 1995. Comparison of cell adhesion molecule expression between glioblastoma multiforme and autologous normal brain tissue. J. Neuroimmunol. 57:143-153. [DOI] [PubMed] [Google Scholar]
- 16.Heddini, A., F. Pettersson, O. Kai, J. Shafi, J. Obiero, Q. Chen, A. Barragan, M. Wahlgren, and K. Marsh. 2001. Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infect. Immun. 69:5849-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiss, J. Z., and G. Rougon. 1997. Cell biology of polysialic acid. Curr. Opin. Neurobiol. 7:640-646. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.MacPherson, G. G., M. J. Warrell, N. J. White, S. Looareesuwan, and D. A. Warrell. 1985. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am. J. Pathol. 119:385-401. [PMC free article] [PubMed] [Google Scholar]
- 20.McCormick, C. J., A. Craig, D. Roberts, C. I. Newbold, and A. R. Berendt. 1997. Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J. Clin. Investig. 100:2521-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizutani, H., W. Roswit, J. Hemperly, T. Lawley, C. Compton, R. Swerlick, and T. S. Kupper. 1994. Human dermal microvascular endothelial cells express the 140-kD isoform of neural cell adhesion molecule. Biochem. Biophys. Res. Commun. 203:686-693. [DOI] [PubMed] [Google Scholar]
- 22.Muller, D., Z. Djebbara-Hannas, P. Jourdain, L. Vutskits, P. Durbec, G. Rougon, and J. Z. Kiss. 2000. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid-neural cell adhesion molecule-deficient hippocampus. Proc. Natl. Acad. Sci. USA 97:4315-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pain, A., D. J. Ferguson, O. Kai, B. C. Urban, B. Lowe, K. Marsh, and D. J. Roberts. 2001. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc. Natl. Acad. Sci. USA 98:1805-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patnaik, J. K., B. S. Das, S. K. Mishra, S. Mohanty, S. K. Satpathy, and D. Mohanty. 1994. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am. J. Trop. Med. Hyg. 51:642-647. [PubMed] [Google Scholar]
- 25.Pouvelle, B., P. A. Buffet, C. Lepolard, A. Scherf, and J. Gysin. 2000. Cytoadhesion of Plasmodium falciparum ring-stage-infected erythrocytes. Nat. Med. 6:1264-1268. [DOI] [PubMed] [Google Scholar]
- 26.Pouvelle, B., T. Fusai, C. Lepolard, and J. Gysin. 1998. Biological and biochemical characteristics of cytoadhesion of Plasmodium falciparum-infected erythrocytes to chondroitin-4-sulfate. Infect. Immun. 66:4950-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pouvelle, B., B. Traore, P. A. Nogueira, B. Pradines, C. LePolard, and J. Gysin. 2003. Modeling of Plasmodium falciparum-infected erythrocyte cytoadhesion in microvascular conditions: chondroitin-4-sulfate binding, a competitive phenotype. J. Infect. Dis. 187:292-302. [DOI] [PubMed] [Google Scholar]
- 28.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rougon, G. 1993. Structure, metabolism and cell biology of polysialic acids. Eur. J. Cell Biol. 61:197-207. [PubMed] [Google Scholar]
- 30.Rougon, G., C. Dubois, N. Buckley, J. L. Magnani, and W. Zollinger. 1986. A monoclonal antibody against meningococcus group B polysaccharides distinguishes embryonic from adult N-CAM. J. Cell Biol. 103:2429-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutishauser, U. 1996. Polysialic acid and the regulation of cell interactions. Curr. Opin. Cell Biol. 8:679-684. [DOI] [PubMed] [Google Scholar]
- 32.Rutishauser, U., A. Acheson, A. K. Hall, D. M. Mann, and J. Sunshine. 1988. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science 240:53-57. [DOI] [PubMed] [Google Scholar]
- 33.Rutishauser, U., and L. Landmesser. 1996. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci. 19:422-427. [DOI] [PubMed] [Google Scholar]
- 34.Santoni, M. J., D. Barthels, G. Vopper, A. Boned, C. Goridis, and W. Wille. 1989. Differential exon usage involving an unusual splicing mechanism generates at least eight types of NCAM cDNA in mouse brain. EMBO J. 8:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 36.Udomsangpetch, R., P. H. Reinhardt, T. Schollaardt, J. F. Elliott, P. Kubes, and M. Ho. 1997. Promiscuity of clinical Plasmodium falciparum isolates for multiple adhesion molecules under flow conditions. J. Immunol. 158:4358-4364. [PubMed] [Google Scholar]