Abstract
The incidence of yeast infections has increased in the recent decades, with Candida albicans still being the most common cause of infections. However, infections caused by less common yeasts have been widely reported in recent years. Based on the internal transcribed spacer 1 (ITS 1) and ITS 2 sequences of the rRNA genes, an oligonucleotide array was developed to identify 77 species of clinically relevant yeasts belonging to 16 genera. The ITS regions were amplified by PCR with a pair of fungus-specific primers, followed by hybridization of the digoxigenin-labeled PCR product to a panel of oligonucleotide probes immobilized on a nylon membrane for species identification. A collection of 452 yeast strains (419 target and 33 nontarget strains) was tested, and a sensitivity of 100% and a specificity of 97% were obtained by the array. The detection limit of the array was 10 pg of yeast genomic DNA per assay. In conclusion, yeast identification by the present method is highly reliable and can be used as an alternative to the conventional identification methods. The whole procedure can be finished within 24 h, starting from isolated colonies.
Fungal infections have increased in incidence in recent decades, often as a result of advanced medical treatments and the increase in the number of immunocompromised patients. Candida albicans is still the most frequent cause of fungal infections. However, the use of broad-spectrum antibiotics and antifungal agents for prophylaxis has led to a shift in the epidemiology and etiology of Candida and non-Candida yeast species infections (36, 37). Infections caused by non-Candida albicans and other less-common emerging yeasts, such as Cryptococcus, Pichia, Rhodotorula, Saccharomyces, and Trichosporon, have been widely reported in recent years (8, 12, 13, 19, 31, 33, 34, 45). The identification of yeast pathogens with this increasing diversity by conventional methods may be difficult and sometimes inconclusive (6). The introduction of reliable methods that have the potential to identify a wide and taxonomically diverse array of opportunistic yeasts is imperative, since some emerging or less common species may have quite different susceptibilities to antifungal agents (16, 41, 43).
Commercially available yeast identification systems, such as the Vitek Yeast Biochemical Card (bioMérieux Vitek, Taipei, Taiwan), API 20C (bioMérieux), and API ID32C (bioMérieux), are convenient to use. However, an incubation period of 24 to 48 h is normally required before biochemical reactions can be interpreted (14). In addition to the biochemical tests contained in these two kits, supplementary tests are occasionally required before a final identification can be obtained. While these commercial products are effective for the identification of commonly encountered yeasts, their application is somewhat more limited for the identification of less frequently recovered taxa (11, 40). These limitations are probably attributable, in part, to the databases currently employed in the profile indexes. Misidentifications of some species by commercial kits have been reported (7, 11, 28, 29, 40), and even the well-known and medically important yeast Candida glabrata has been misidentified by phenotypic methods (7).
DNA-based methods used to identify a variety of yeasts have been developed (4). These molecular methods include length polymorphism analysis of the internal transcribed spacer (ITS) regions of the rRNA gene (2, 26, 49), restriction fragment length polymorphism analysis (28, 47), probe hybridization (7, 10, 38), and DNA sequencing (6, 15, 17, 24, 39). Although these methods have been proved to be accurate, a common limitation of them is that only a limited number of species can be analyzed. Microarray platforms that can simultaneously analyze hundreds or thousands of targets may have the potential to identify a wide spectrum of yeasts with high sensitivity and specificity. In the past few years, DNA array technology has been used to identify a variety of yeasts and molds (20, 21, 25). However, less than 10 yeast species were included in the arrays in studies by Huang et al. (21) and Leinberger et al. (25).
In this study, an oligonucleotide array targeting the fungal ITS regions was developed to identify 77 yeast species (16 genera) of clinical importance. Instead of the detection of fluorescence intensity after hybridization, colorimetric detection was used in this study, and nylon membranes instead of glass slides were used as the solid supports for oligonucleotide probes.
MATERIALS AND METHODS
Yeast strains.
A total of 419 target strains, representing 44 Candida species (275 strains) and 33 non-Candida species (144 strains), were used for identification by the array (Table 1). Among these strains, 309 were reference (or type) strains and 110 were clinical isolates. Reference strains were obtained from the American Type Culture Collection (ATCC, Manassas, VA), the Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan), and Centraalbureau voor Schimmelcultures (CBS, Utrecht, The Netherlands). Clinical isolates were obtained from the Mycology Reference Centre, Department of Microbiology, Leeds Teaching Hospitals Trust (Leeds, United Kingdom), the Laboratory of Parasitology and Mycology of Angers University Hospital (Angers, France), and the National Cheng Kung University Medical Center (Tainan, Taiwan). Isolates were identified to the species level based on traditional criteria (18) or with the API ID 32C system. In addition, a total of 33 nontarget strains (33 species) were used for specificity testing of the array (data not shown).
TABLE 1.
Reference strains and clinical isolates used in this study
| Species (teleomorph)a | Reference strain(s)b | Clinical isolate(s) | Total no. of strains |
|---|---|---|---|
| Candida spp. | |||
| Candida albicans | BCRC 20511, 20512T, 20513, 20518, 20519, 21538, 22063, CBS 2718, 2730, 5990, 6431, 6589 | 343, 1009, 2514, 3321, 3434, 3623, 3653, 4252, 4339, ATTC 66390, LMA 938838, 962507, 499010350, RB 1325, 1326, 1331 | 28 |
| Candida boidinii | BCRC 20464T, 20472, 21432, 21483, 21757 | 5 | |
| Candida cacaoi (Yamadazyma farinosa) | BCRC 21368T, 21682, 21881 | 3 | |
| Candida cantarelli | BCRC 21613T, CBS 5383, 5445, 5654 | 4 | |
| Candida catenulata | BCRC 21507, 22316T, CBS 564, 565, 1904 | 5 | |
| Candida chodatii (Pichia burtonii) | BCRC 22087T, 22012 | 2 | |
| Candida colliculosa (Torulaspora delbrueckii) | BCRC 21429, 22074T, CBS 158, 6991 | 4 | |
| Candida dattila (Lachancea thermotolerans) | BCRC 22043, CBS 1877, 2803, 2860, 2907 | 5 | |
| Candida dubliniensis | CBS 2747, 7987, 7988, 8500, 8501 | RB 1168, 1271, 1306 | 8 |
| Candida famata (Debaryomyces hansenii) | BCRC 22304, 22712, CBS 1791, 1792, 1795T | 5 | |
| Candida freyschussii | BCRC 21555T, CBS 2161 | 2 | |
| Candida glabrata | BCRC 20586T, CBS 860, 861, 2175, 7307 | 1762, 9796, 9787, LMA 901085, 905756, 945574, RB 1284, 1295, 1324 | 14 |
| Candida globosa (Citeromyces matritensis) | CBS 162T, 864 | 2 | |
| Candida guilliermondii (Pichia guilliermondii) | BCRC 20862, 21500T, 21549, 21559 | RB 1012, 1055, 1216 | 7 |
| Candida haemulonii | BCRC 21572T, CBS 6590, 7801, 7802 | 4 | |
| Candida holmii (Kazachstania exigua) | BCRC 21524T, 21999, 22000 | 3 | |
| Candida inconspicua | BCRC 21658T, CBS 990, 1735, 2833 | LMA 90289, RB 1226 | 6 |
| Candida intermedia (Kluyveromyces cellobiovorus) | BCRC 20863, 21250T, 21604, 22567 | 4 | |
| Candida kefyr (Kluyveromyces marxianus) | BCRC 20516, 20517, 21269, 21355, 22057T | LMA 911323, 938657, 938779, 944459, 947644, RB 1227 | 11 |
| Candida krusei (Issatchenkia orientalis) | BCRC 20514T, 21321, 21720, 21796, 22342 | 2283, 16462, LMA 911256, 945615, 948501, RB 1222, 1237, 1317 | 13 |
| Candida lambica (Pichia fermentans var. fermentans) | BCRC 21347, 22067T, 22068, 22090, 22091 | 5 | |
| Candida lipolytica (Yarrowia lipolytica) | BCRC 20864, 21541, 21542, 21596 | 4 | |
| Candida lusitaniae (Clavispora lusitaniae) | BCRC 20326, 21387T, 21740, CBS 7270 | LMA 932648, 947060, 947315, 948764, RB 1283, 1288, 1294 | 11 |
| Candida maltosa | BCRC 21327, 21482, 21614T | 3 | |
| Candida melibiosica | CBS 5814T, 6211 | 2 | |
| Candida membranifaciens | BCRC 21563, 22398T, 22399 | 3 | |
| Candida norvegensis (Pichia norvegensis) | BCRC 21851, 22096T, 22097, CBS 1911 | 4 | |
| Candida norvegica | BCRC 21616T, CBS 2670, 4027, 4737 | 4 | |
| Candida parapsilosis, genotype I | BCRC 20515T, 21253, 21544 | 240, 282, 308, 403, 1905, 2985, 3080, 3851, 7410, 9360, 9692, LMA 938558, 961299, RB 1318, 1320 | 18 |
| C. parapsilosis, genotype II (Candida orthopsilosis) | 770, 8053 | 2 | |
| Candida parapsilosis, genotype III (Candida metapsilosis) | BCRC 20865 | 43, 1833, 2304, C4-2 | 5 |
| Candida pelliculosa (Pichia anomala) | BCRC 20857, 20858, 21359, 21741, 22583T | 4731, LMA 892971, RB 766, 778, 913 | 10 |
| Candida pintolopesii (Kazachstania telluris) | BCRC 21439, 22003, 22239 | 3 | |
| Candida robusta (Saccharomyces cerevisiae) | BCRC 20263, 20270, 20271, 20405, 20490, 21447T | RB 1254, 1299 | 8 |
| Candida rugosa | BCRC 21356, 21709T | RB 1158 | 3 |
| Candida sake | BCRC 21621T, CBS 5690, 5740 | 3 | |
| Candida santamariae | BCRC 21562, 21617T, CBS 4261, 4515T | 4 | |
| Candida silvicola (Pichia holstii) | CBS 4069, 4140T, 4141 | 3 | |
| Candida sphaerica (Kluyveromyces lactis) | BCRC 21716, 22153, 22154, 22055, 22604 | 5 | |
| Candida steatolytica (Zygoascus hellenicus) | BCRC 21746T, 22232, CBS 7652 | 3 | |
| Candida tannotolerans (Vanderwaltozyma yarrowii) | BCRC 21747, 22822, CBS 2684, 8242 | 4 | |
| Candida tropicalis | BCRC 20520T, 20521, 21436, 21437, 21560 | 104, 1075, 2785, 4996, 8023, 8173, 8327, LMA 9077, 921810, 945762, RB 1298, 1330 | 17 |
| Candida utilis (Pichia jadinii) | BCRC 20260, 20325, 20334, 20860, 20928T, 21357 | 6 | |
| Candida valida (Pichia membranifaciens) | BCRC 22069T, 21399, 21441 | 3 | |
| Candida viswanathii | BCRC 21330T, 22554 | 2 | |
| Candida zeylanoides (Pichia dubia) | BCRC 21743T, 21749, 22396, CBS 947 | LMA 91304 | 5 |
| Non-Candida spp. | |||
| Arthroascus schoenii | BCRC 21401, 22503T, 22504, CBS 2556 | 4 | |
| Brettanomyces bruxellensis (Dekkera bruxellensis) | BCRC 20932, 21414T, 21440, 21518, 21519 | 5 | |
| Candida albidus | BCRC 20526, 21672T, 21860, CBS 969 | LMA 935479 | 5 |
| Cryptococcus curvatus | BCRC 21735, CBS 570, 2744, 8770 | 4 | |
| Cryptococcus laurentii | BCRC 20527T, 21997 | 2 | |
| Candida neoformans (Filobasidiella neoformans) | BCRC 20528T, 20532, 22241, 22873, 22874, 22875, CBS 883, 919, 1622, 6955, 6997 | 4439, 4889, 7241, LMA 94277, 925461, 935479, 957786, 959159, 49800123 | 20 |
| Cryptococcus uniguttulatus (Filobasidium uniguttulatum) | CBS 1727, 1730, 2770, 2994, 4257 | 5 | |
| Debaryomyces carsonii | BCRC 21529, 22098T, CBS 4409, 5254 | 4 | |
| Debaryomyces etchellsii | BCRC 21479T, CBS 2012, 5519, 5603 | 4 | |
| Debaryomyces maramus | BCRC 21526, CBS 1958T | 2 | |
| Kloeckera apiculata (Hanseniaspora uvarum) | BCRC 20539, 21362, CBS 312, 314, 2582 | 5 | |
| Kloeckera apis (Hanseniaspora guilliermondii) | BCRC 22105, 22106, 22112T, CBS 4378 | 4 | |
| Kloeckera japonica (Hanseniaspora valbyensis) | CBS 281, 479T, 2590 | 3 | |
| Kluyveromyces delphensis (Nakaseomyces delphensis) | BCRC 22017, CBS 2170 | 2 | |
| Kodamaea ohmeri | BCRC 21349, 21592, 22178T, 22556, 22557 | 5 | |
| Lachancea cidri | BCRC 21728T, CBS 2950, 2951, 5666 | 4 | |
| Lachancea fermentati | BCRC 21433, 21760T, 22453 | 3 | |
| Lodderomyces elongisporus | BCRC 21390T, CBS 2606, 5912 | 3 | |
| Pichia spartinae | BCRC 22766T, CBS 6059, 6077, 6661 | 4 | |
| Rhodotorula glutinis (Rhodosporidium diobovatum) | BCRC 20576, 21418T | 2 | |
| Rhodotorula minuta | BCRC 22482, 22483, 22484, 22485 | 4 | |
| Rhodotorula mucilaginosa | BCRC 21442, 21667T, 21712, 21713, 21770 | 5 | |
| Saccharomyces kluyveri | BCRC 21498T, 21977, 22001, CBS 2861 | 4 | |
| Saccharomycopsis fibuligera | BCRC 20455, 21379, 21380, 21449, 21465, 21511T | 6 | |
| Sporobolomyces salmonicolor (Sporidiobolus salmonicolor) | CBS 490, 4474 | 2 | |
| Trichosporon aquatile | BCRC 22271T, 22272, CBS 5973, 5988 | 4 | |
| Trichosporon asahii | CBS 2479, 2936, 4829, 7631 | 4 | |
| Trichosporon cutaneum | BCRC 21675T, 22273 | LMA 94117, 94256, 931422, 931440 | 6 |
| Trichosporon inkin | BCRC 21503T, CBS 7613, 7629, 7655 | 4 | |
| Trichosporon pullulans | BCRC 22275, 22313, CBS 2543 | 3 | |
| Williopsis saturnus var. saturnus | BCRC 20463, 21360, 21659, 21692, 21765 | 5 | |
| Zygosaccharomyces bisporus | BCRC 21505T, 21725, 21726 | 3 | |
| Zygotorulaspora florentinus | BCRC 21648T, CBS 748, 6078, 6703 | 4 |
Anamorphic names are used in this study, and teleomorphic species names are given in parentheses. Some species were only given the names of teleomorphs since they have no anamorphic names.
A superscript “T” indicates type strains.
DNA extraction.
Yeasts were subcultured on Sabouraud dextrose agar and incubated at 28°C for 24 to 48 h. Colonies of yeast were suspended in saline, and the genomic DNA was extracted by using the blood and tissue genomic DNA extraction Miniprep system (Viogene, Taipei, Taiwan), as described previously (24), except that the lyticase digestion step was omitted.
Amplification of the ITS regions for hybridization.
Digoxigenin (DIG)-labeled amplicons for array hybridization were obtained by PCR using the fungus-specific universal primers ITS1 (5′-DIG-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-DIG-TCCTCCGCTTATTGATATGC-3′) (50). Each primer was labeled with a digoxigenin molecule at its 5′ end and was obtained from MdBio, Inc. (Taipei, Taiwan). The PCR products encompassed ITS 1, the 5.8S rRNA gene, ITS 2, and partial regions of the 18S and 28S rRNA genes. The reaction mixtures and thermocycling conditions used for PCR were described previously (24).
Design of oligonucleotide probes.
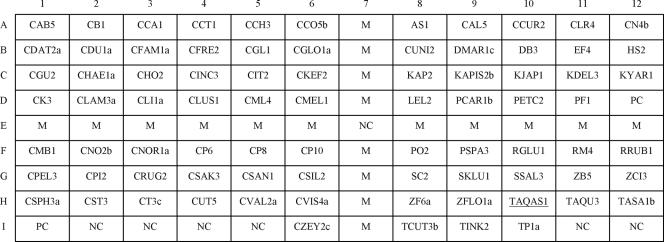
The oligonucleotide probes (16- to 30-mers) used for the identification of 77 yeast species are listed in Table 2. Probes were designed from either the ITS 1 or the ITS 2 region. The corresponding ITS sequences used for probe design were available in the GenBank database or determined in our previous studies (2, 24). Sequences extracted from GenBank (Table 2) were confirmed by at least one sequence from another reference strain of the same species in public databases. The alignment of multiple ITS sequences was made by using Discovery Studio Gene software (DS Gene, version 1.5; Accelrys, Inc., San Diego, CA). Based on the aligned sequences, areas displaying high intraspecies similarities and low interspecies similarities were used for probe synthesis. The melting temperature, GC content, and possible secondary structure of a designed probe were examined by using Vector NTI software (Invitrogen Corporation, Carlsbad, CA). In addition, the designed probes were checked for partial sequence homology with other microorganisms in public databases using the basic local alignment search tool (BLAST) algorithm. A total of 79 species-specific probes and one group-specific probe were used for fabrication of the oligonucleotide array (Table 2). In addition, a probe designed from a conserved sequence of the fungal 5.8S rRNA gene was used as a positive control probe (probe code, PC) (Table 2). Five to 17 bases of thymine were added to the 3′ or 5′ ends of probes that displayed weak hybridization signals after preliminary testing (1). Moreover, a digoxigenin-labeled bacterial universal primer 6R (5-DIG-GGGTTYCCCCRTTCRGAAAT-3, where Y is C or T and R is A or G) (probe code, M) was spotted on the array and used as a position marker (Fig. 1 and 2) (48). All probes were synthesized by MdBio, Inc. (Taipei, Taiwan).
TABLE 2.
Oligonucleotide probes used in this study
| Species (teleomorph) or control | Probe codea | Sequence (5′ to 3′)b | Length (bp) | Tm (°C)c | Locationd | GenBank accession no. |
|---|---|---|---|---|---|---|
| Candida albicans | CAB5 | TTATCAACTTGTCACACCAGATTATTACT(tttttt) | 29 | 53 | 102-130 (1) | AY207067 |
| Candida boidinii | CB1 | TAACTCTTTGGGAAAACTCTATACACTTTG | 30 | 56 | 81-110 (1) | AY936499 |
| Candida cacaoi | PF1 | TTTACAGTAGATAAATGCCGTTTGACTCTT | 30 | 58 | 80-109 (2) | AF218989 |
| Candida cantarelli | CCA1 | AGACTTCTCCCATACACTTGTGAACTTT | 28 | 56 | 26-53 (1) | AY936503 |
| Candida catenulata | CCT1 | AAAGTGATTGGTGTAGTATTACAGTTTACT | 30 | 52 | 44-73 (2) | AY493436 |
| Candida chodatii | CCH3 | AGCTCTTAGTTCAGTCCATTCGAAAAGT | 28 | 58 | 37-64 (2) | AY936510 |
| Candida colliculosa | CCO5b | ATTTTTCTGGCTTGGATGACTTTGT(tttttttt) | 25 | 56 | 83-107 (2) | AJ229075 |
| Candida dattila | CDAT2a | CAATTCGTAGTGGCGTTAGTA(tttttttttttttt) | 21 | 48 | 130-150 (2) | AY046207 |
| Candida dubliniensis | CDU1a | AAACTTGTCACGAGATTATT(ttttttttttttttt) | 20 | 41 | 108-127 (1) | AB049124 |
| Candida famata | CFAM1a | GGCCAGAGGTTTACTGAA(ttttttttttttttttt) | 18 | 45 | 107-124 (1) | AF336834 |
| Candida freyschussii | CFRE2 | GTAATGTCTAGGTTTACCAAATCATTGCGT | 30 | 53 | 87-116 (2) | AF218965 |
| Candida glabrata | CGL1 | TGGGAGTGTGCGTGGATCTCTCTATTCCAA | 30 | 68 | 177-206 (1) | AY207068 |
| Candida globosa | CGLO1a | TTCGTTTTAGGTGTTGGGCAGTA(tttttttttttt) | 23 | 55 | 13-35 (2) | AY936514 |
| Candida guilliermondii | CGU2 | GTGCTGTCGACCTCTCAATGT(ttttttttt) | 21 | 52 | 92-112 (2) | AY207076 |
| Candida haemulonii | CHAE1a | AATCAACCACCGTTAAGTTCAA(ttttttttttttt) | 22 | 51 | 32-53 (1) | AJ606467 |
| Candida holmii | CHO2 | GGTTGTTGCAGCTTATAGTTTTTGTGTAAT | 30 | 58 | 78-107 (2) | D89894 |
| Candida inconspicua | CINC3 | AGAGAGCGAACTATAAAACGCGC(ttttttt) | 23 | 56 | 71-93 (2) | AY936516 |
| Candida intermedia | CIT2 | GTTGTCGCAATACGTTACTTCAACTTTATT | 30 | 58 | 45-74 (2) | AF218968 |
| Candida kefyr | CKEF2 | AGTTTTCTATTTCTCATCC(ttttttttttttttt) | 19 | 37 | 88-106 (1) | AJ401700 |
| Candida krusei | CK3 | TGTGGAATATAGCATATAGTCGACAAGAGA | 30 | 57 | 54-83 (1) | AY207070 |
| Candida lambica | CLAM3a | TTCTTGGAGCGGWGCTCCAGA(tttttttttttttt) | 21 | 59 | 3-23 (2) | AF218969 |
| Candida lipolytica | CLI1a | CTCAATGATTACGTCATTTCACC(tttttttttttt) | 23 | 51 | 44-66 (2) | AF218983 |
| Candida lusitaniae | CLUS1 | TCAAACACGTTTACAGCACGACATTTC | 27 | 60 | 49-75 (2) | AY139788 |
| Candida maltosa | CML4 | TAGTAATGTACCGACGTAAACGACTTAGGT | 30 | 57 | 59-88 (2) | AY936522 |
| Candida melibiosica | CMEL1 | ATATCGCTCGCACTGTTTCTAAGCTAACA | 29 | 61 | 34-62 (2) | AY936524 |
| Candida membranifaciens | CMB1 | AACTGGGGCAGTAAATTTCTAGTAATTGG | 29 | 59 | 136-164 (2) | AJ606465 |
| Candida norvegensis | CNO2b | TGTCACCCAGAGAAAATCTCAAACGAG(tttttttt) | 27 | 61 | 65-91 (1) | AY139789 |
| Candida norvegica | CNOR1a | (ttttttt)TAGCCGGAGACTACAACCAAACTAATTT | 28 | 58 | 80-107 (1) | AY936525 |
| Candida parapsilosis | CP6 | TTCCACTCATTGGTACAAACTCCAAAACTT | 30 | 61 | 87-116 (2) | AY207079 |
| CP8 | TTTGGTAGGCCTTCTATATGGGGCCT(ttttttt) | 26 | 62 | 67-92 (1) | AY207072 | |
| CP10 | TTAACTGCGACCCTTTTCTTTCTACACA | 28 | 60 | 21-48 (1) | AY391849 | |
| Candida pelliculosa | CPEL3 | ATATTGACTTAGCAAGAGT(ttttttttttttttt) | 19 | 35 | 58-76 (2) | AF218991 |
| Candida pintolopesii | CPI2 | ACGTCTTCGTAGTAGGTTCTGCCAATT | 27 | 59 | 131-157 (2) | AJ223029 |
| Candida robusta | SC2 | TGTAAGTTTCTTTCTTGCTATTCCAAACGG | 30 | 61 | 135-164 (1) | Z95935 |
| Candida rugosa | CRUG2 | CGCGACCGTCTAAAACAGTTAAGCTTG | 27 | 62 | 49-75 (2) | AF218971 |
| Candida sake | CSAK3 | ACTTGCTTGCAAGAACACTAATAATTTA(ttttttt) | 28 | 54 | 39-66 (1) | AJ549822 |
| Candida santamariae | CSAN1 | GACCAGTAAAGTATTTG(tttttttttt) | 17 | 30 | 133-149 (2) | DQ066654 |
| Candida silvicola | CSIL2 | ATACTCGGGTTTTAGGCTTGAGTTTGCTT | 29 | 62 | 33-61 (2) | AY936530 |
| Candida sphaerica | CSPH3a | ATACTCGTTTTTCGGGTTAA(ttttttttttttttt) | 20 | 46 | 33-52 (2) | AY046213 |
| Candida steatolytica | CST3 | TTAAGCACAATTTTCTGAAATACATTGGTG | 30 | 59 | 60-89 (2) | AY936532 |
| Candida tannotolerans | KYAR1 | AGTTCGCTTTCCCAGAGATGACAAA | 25 | 59 | 201-225 (1) | AY046183 |
| Candida tropicalis | CT3c | ACTCATTTTAAGCGACTTAG(tttttttttt) | 20 | 41 | 69-88 (2) | AY207080 |
| Candida utilis | CUT5 | CAACTCGTTATTTTCCAGACAGACT(tttttttttt) | 25 | 53 | 119-143 (2) | AY936536 |
| Candida valida | CVAL2a | AAGAAACGTTGCGGACGAAGCG(ttttttttttttt) | 22 | 62 | 72-93 (2) | DQ104722 |
| Candida viswanathii | CVIS4a | CTTGTGCAGTCGGCTCACCA(tttttttttt) | 20 | 57 | 99-118 (2) | AY139792 |
| Candida zeylanoides | CZEY2c | GAGCAGTATAGTATTTG(ttttttttttttt) | 17 | 27 | 133-149 (2) | AF218976 |
| Arthroascus schoenii | AS1 | ATGCTTCCCTTACCTTGTTAAGTAGCTTTA | 30 | 58 | 80-109 (2) | AY936498 |
| Brettanomyces bruxellensis | DB3 | CGAGGGTGTTTTCTTCAAAGGGAAG | 25 | 60 | 33-57 (2) | AF043503 |
| Cryptococcus albidus | CAL5 | CTAAAGACCGCTTTCTAATCCATTGATCT | 29 | 59 | 172-200 (2) | AY382336 |
| Cryptococcus curvatus | CCUR2 | AGTGAATTTAACATTTGTCTTCTGGCG | 27 | 58 | 77-103 (2) | AF410467 |
| Cryptococcus laurentii | CLR4 | ACCTCTGTGAACTGTGGACC(ttttttttttttttt) | 20 | 48 | 36-55 (1) | AF335935 |
| Cryptococcus neoformans | CN4b | TTTTATTACCTGTTGGACTTGGATT(tttttttttt) | 25 | 53 | 11-35 (2) | AY207082 |
| Cryptococcus uniguttulatus | CUNI2 | CTGGACTTGTCTATATGACTGGTTTGA | 27 | 55 | 94-120 (2) | AY382334 |
| Debaryomyces carsonii | PCAR1b | TTGCTTTGGCTTGTCTCTAGA(tttttttttttttt) | 21 | 50 | 79-99 (1) | AB054097 |
| Debaryomyces etchellsii | PETC2 | TACTGGATAGTACTGTTATGGCTTCTTCA | 29 | 55 | 83-111 (2) | AJ586528 |
| Debaryomyces maramus | DMAR1c | GGCTAGAGACTTACTGAA(tttttttttttt) | 18 | 35 | 107-124 (1) | AB053102 |
| Kloeckera apiculata | KAP2 | ATTGGAGACTGTTTCAG(ttttttttttttttt) | 17 | 35 | 61-77 (2) | AY046200 |
| Kloeckera apis | KAPIS2b | GTATTTATGAATTTATTC(ttttttttttttttttt) | 18 | 28 | 130-147 (2) | AJ512427 |
| Kloeckera japonica | KJAP1 | CAGTCAACTACTACACACAG(ttttttttttttttt) | 20 | 36 | 138-157 (1) | AJ512434 |
| Kluyveromyces delphensis | KDEL3 | TAAGTTTGTTGTGGGATGCTAATTCCTTT | 29 | 60 | 168-196 (2) | AY198400 |
| Kodamaea ohmeri | PO2 | GACGACAGTACTCTACAAAACGGTACC | 27 | 56 | 44-70 (2) | AF219004 |
| Lachancea cidri | ZCI3 | CAACTCGTAGGGGCTTA(tttttttttt) | 17 | 43 | 131-147 (2) | AY046205 |
| Lachancea fermentati | ZF6a | TGAGTGGACGCTACAAAG(ttttttttttttttttt) | 18 | 44 | 146-163 (2) | AY046206 |
| Lodderomyces elongisporus | LEL2 | AACCACTCCATTGTGCTTAATAAAAAGC | 28 | 58 | 89-116 (2) | AY391848 |
| Pichia spartinae | PSPA3 | AATACAGCGCACTCGACAATCA(tttttttt) | 22 | 55 | 86-107 (2) | AF423028 |
| Rhodotorula glutinis | RGLU1 | TAGTGAATCTGGTGGTGCTTG(ttttttttt) | 21 | 50 | 23-43 (2) | AF444539 |
| Rhodotorula minuta | RM4 | GATTATGGTTGTCTGTCGGCGTAATT | 26 | 59 | 45-70 (2) | AF444620 |
| Rhodotorula mucilaginosa | RRUB1 | TAATGATTGAAGAGGTGTTTGG(tttttttt) | 22 | 49 | 23-44 (2) | AF444541 |
| Saccharomyces kluyveri | SKLU1 | TGTTAACGGTTGTCTCTT(ttttttttttttt) | 18 | 39 | 73-90 (1) | AB037405 |
| Saccharomycopsis fibuligera | EF4 | GATTGAGTTTTCCATATATTTGCTTAAGGA | 30 | 57 | 76-105 (2) | AF218988 |
| Sporobolomyces salmonicolor | SSAL3 | GCCTTCGGGTTACTGAGC(ttttttttttttttttt) | 18 | 50 | 153-170 (2) | AF387784 |
| Trichosporon aquatile/ Trichosporon asahii/ Trichosporon inkin | TAQAS1e | TTGACATTAATGTCTGGTG(tttttttttttttttt) | 19 | 40 | 85-103 (2) | AF410475 |
| Trichosporon aquatile | TAQU3 | TTGGGCGTTGCGATCT(tttttttttt) | 16 | 50 | 38-53 (2) | AF410475 |
| Trichosporon asahii | TASA1b | ATATCCACTTACACCTGT(ttttttttttttttttt) | 18 | 35 | 27-44 (1) | AY055381 |
| Trichosporon cutaneum | TCUT3b | TCGGTCAATTGATTTTACAA(ttttttttttttttt) | 20 | 46 | 60-79 (1) | AF335957 |
| Trichosporon inkin | TINK2 | TTGACATTCATGTCTGG(ttttttttttttttt) | 17 | 37 | 84-100 (2) | AF218981 |
| Trichosporon pullulans | TP1a | TCCAGGCTATCATTTCATACAAACT(tttttttttt) | 25 | 53 | 92-116 (1) | AF444418 |
| Williopsis saturnus var. saturnus | HS2 | AGCCCAAACCTTACACACTGTGATTAGTTT | 30 | 61 | 40-69 (1) | Z93875 |
| Zygosaccharomyces bisporus | ZB5 | AACTGAGGTGGGTGATAGAAATATCGAAC | 29 | 59 | 186-214 (2) | AJ229176 |
| Zygotorulaspora florentinus | ZFLO1a | CTCTGTAACATGGGAGTTAGC(tttttttttttt) | 21 | 46 | 36-56 (2) | AY046168 |
| Positive control | PCf | GCATCGATGAAGAACGCAGC(ttttttttt) | 20 | 56 | 5.8S rRNA gene | EF134625 |
Oligonucleotide probes are arranged on the array as indicated in Fig. 1.
Five to 17 additional bases of thymine, indicated by “(t),” were added to the 5′ or 3′ end of the probe. Underlined nucleotides indicate a single mismatch base that was incorporated into the probe.
Tm, melting temperature.
The location of probe is indicated by the nucleotide number of either ITS 1 or ITS 2; the numbers (1 or 2) in parentheses indicate the ITS region from which the probe was designed.
A group-specific probe shared by three species (Trichosporon aquatile, Trichosporon asahii, and Trichosporon inkin).
The positive control probe was designed from a conserved region of the 5.8S rRNA gene.
FIG. 1.
Layout of oligonucleotide probes on the array (0.75 by 0.9 cm). The positive control probe PC (located at D12 and I1) was designed from a conserved region of the fungal 5.8S rRNA gene. Probe NC (located at E7, I2 to I5, I11, and I12) was a negative control (tracking dye only). Probe M (located at E1 to E12 and at A7 to I7, except E7) was a DIG-labeled bacterial universal primer and was used as a position marker. The group-specific probe TAQAS1 (located at H10) is underlined. The corresponding sequences of all probes are listed in Table 2.
FIG. 2.
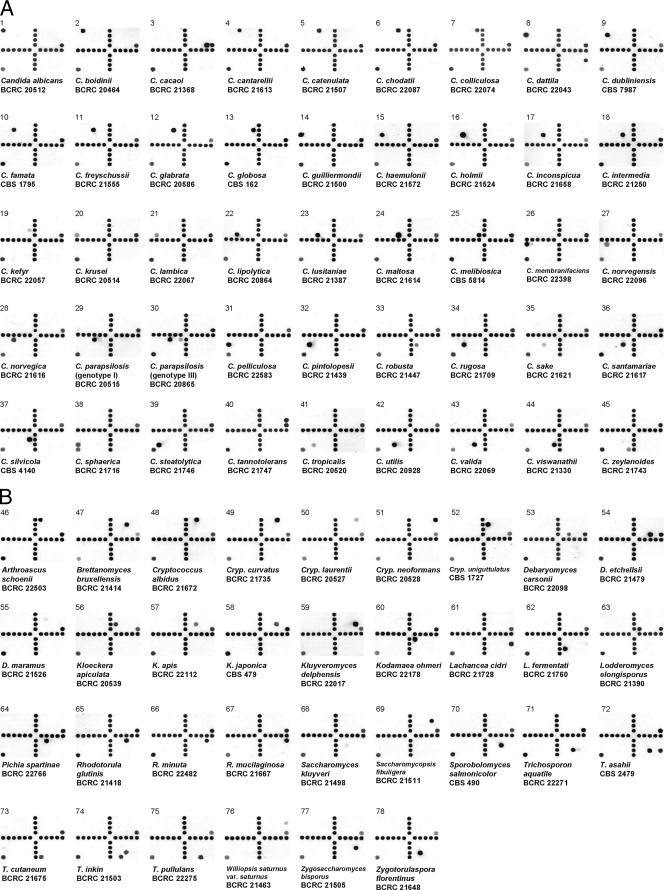
Hybridization results for 77 yeast species. Chips 1 to 45 (A) and 46 to 78 (B) are the hybridization patterns of Candida spp. (44 species) and non-Candida spp. (33 species), respectively. The chips are alphabetically arranged according to the species names. The corresponding probes hybridized on the arrays are indicated in Fig. 1, and the corresponding sequences of the hybridized probes are shown in Table 2. The positive control probe was located at D12 and I1 on each array.
Fabrication of oligonucleotide arrays.
The oligonucleotide probes were diluted 1:1 (final concentration, 10 μM) with a tracking dye solution composed of glycerol, dimethyl sulfoxide, sodium EDTA, and bromophenol blue (3). The probes were spotted onto a positively charged nylon membrane (Roche, Mannheim, Germany) by an Ezspot arrayer (model no. SR-A300; EZlife Technology, Taipei, Taiwan) using a 400-μm-diameter solid pin as described previously (48). The array (0.75 by 0.9 cm) contained 108 dots, including 80 dots for the identification of 77 yeast species (16 genera), 2 for positive controls (probe code, PC; final concentration, 5 μM), 7 for negative controls (probe code, NC; tracking dye only), and 19 for position markers (probe code, M; final concentration, 0.4 μM) (Fig. 1). The position markers formed a cross on the array after hybridization and helped to locate the hybridized probes (Fig. 1 and 2). Once all probes had been applied, the membrane was exposed to shortwave UV (Stratalinker 1800; Stratagene, La Jolla, CA) for 30 s.
Hybridization procedures.
Except where otherwise indicated, the hybridization procedures were carried out at room temperature (approximately 27°C) with a shaking speed of 60 rpm. Most reagents except buffers were obtained from the DIG nucleic acid detection kit (Roche). The hybridization procedures were the same as those described previously (20), except that the hybridization step was conducted at 50°C for 90 min. Unbound oligonucleotides on the array were removed by three washes (3 min each) in 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate (SDS). Each array was prehybridized at room temperature for 2 h with hybridization solution (5′ SSC, 1% [wt/vol] blocking reagent, 0.1% N-laurylsarcosine, and 0.02% SDS) in an individual well of a 24-well cell culture plate. The DIG-labeled PCR product amplified from an isolate was denatured at 95°C and immediately cooled on an ice bath. Ten microliters of denatured PCR products were diluted with 0.3 ml of hybridization solution and added to each well. Hybridization was carried out at 50°C for 90 min. After removal of the nonhybridized PCR products, the array was washed four times (5 min each) with 0.25× SSC-0.1% SDS, followed by incubation for 1 h with blocking solution (1% [wt/vol] blocking reagent dissolved in maleic acid buffer [0.1 M maleic acid, 0.15 M NaCl, pH 7.5]). After removal of the blocking solution, 0.3 ml of alkaline phosphatase-conjugated anti-DIG antibodies (diluted 1:2,500) was added to each well and incubated for 1 h. The array was washed three times (each 15 min) with washing solution (0.3% [vol/vol] Tween 20 in maleic acid buffer), followed by washing with detection buffer (0.1 M Tris-HCl, 0.15 M NaCl, pH 9.5) for 5 min. Finally, 0.3 ml of alkaline phosphatase substrates (nitroblue tetrazolium chloride-5-bromo-4-chloro-3-indolylphosphate) was added to each array and incubated at 37°C for 30 to 60 min without shaking. The hybridized array was washed three times with distilled water and air dried. The image of the hybridization pattern was captured and processed by a scanner (PowerLook 3000; UMAX, Taipei, Taiwan).
Definition of sensitivity and specificity.
A yeast strain was identified as one of the 77 target yeasts when the probe designed for the species and the positive control probes (Fig. 2) were hybridized. Sensitivity was defined as the number of target strains correctly identified (true positives) divided by the total number of target strains tested (30). Specificity was defined as the number of nontarget strains producing negative hybridization reactions (true negatives) divided by the total number of nontarget strains tested (30).
Analysis of discrepant strains.
For strains producing discrepant identification between the methods based on phenotypic characteristics and array hybridization, the D1-D2 region of the large-subunit RNA gene, ITS 1, and ITS 2 were amplified by PCR, sequenced, and then compared with sequences in public databases using the BLAST algorithm for species clarification. The fungus-specific, universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS2 (5′-GCTGCGTTCTTCATCGATGC-3′) were used to amplify the ITS 1 region, while the primer pair ITS3 (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) was used to amplify the ITS 2 region (50). The D1-D2 region was amplified by primers NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) (23). The conditions used for amplification of the ITS 1, ITS 2, and D1-D2 regions were the same as those for amplifying the whole ITS segment as described previously (24). PCR products were purified with a PCR-M cleanup kit (Viogene, Taipei, Taiwan) and sequenced on a model 377 sequencing system (Applied Biosystems, Taipei, Taiwan).
Limit of detection of the array.
Two strains (C. albicans BCRC 20512 and Kloeckera apis BCRC 22112) were used to determine the limit of detection of the oligonucleotide array. The DNAs of both strains were serially diluted 10-fold (10 ng/μl to 1 fg/μl) with a carrier DNA (1 ng/μl) extracted from a bacterium (Xanthobacter flavus BCRC 12271) by the boiling method (32). After PCR amplification of the diluted DNA, the amplicon was hybridized to the oligonucleotide array.
RESULTS
Oligonucleotide probe development.
Initially, one to five probes were designed for each of the 77 target species. Through a preliminary hybridization test, probes cross-reacting with heterologous species or producing weak hybridization signals with their homologous species were discarded. Finally, 80 probes (Table 2) were selected for the fabrication of the array. Under most conditions, a single probe was used to identify a species (Table 2). Among these probes, a group-specific probe (probe code, TAQAS1) was designed; the probe could hybridize with species of Trichosporon aquatile, Trichosporon asahii, and Trichosporon inkin. However, each of the three Trichosporon species had its own specific probe (Table 2).
Due to the presence of three genotypes among isolates of Candida parapsilosis (27, 42, 46), three probes (probe codes, CP6, CP8, and CP10) were designed to identify this frequently isolated species. Probe CP6 was shared by all three genotypes of C. parapsilosis; however, strains of genotypes II or III were able to hybridize with, in addition to probe CP6, an additional probe (CP8 or CP10). For example, C. parapsilosis BCRC 20515 (genotype I) hybridized only to probe CP6 (Fig. 2A). However C. parapsilosis BCRC 20865 (genotype III) hybridized to probes CP6 and CP10 (Fig. 2A), while clinical isolate C. parapsilosis 770 (genotype II) hybridized to probes CP6 and CP8 (data not shown). It was observed that the hybridization signals of probes CP8 and CP10 were relatively weaker compared to that of probe CP6.
Twelve probes were intentionally designed to incorporate one single base mismatch with their respective complementary target sequences (Table 2) to eliminate weak cross-hybridizations produced by nonhomologous species. These 12 probes were used to identify Candida colliculosa, Candida dattila, Candida globosa, Candida haemulonii, Candida lambica, Candida lipolytica, Candida tropicalis, Candida viswanathii, Candida zeylanoides, Debaryomyces maramus, Lachancea fermentati, and Trichosporon pullulans (Table 2). The 12 modified probes had no cross-hybridization with other species, but still displayed good hybridization signals with their respective target yeasts.
Identification of reference strains by the array.
A total of 342 reference strains, including 309 target strains (Table 1) and 33 nontarget strains (Table 3), were tested. Figure 2 shows the hybridization results of reference strains of different target species. All 309 target strains were correctly identified by the oligonucleotide array, producing a test sensitivity of 100%. Of the 33 nontarget reference strains (33 species), only one strain, Trichosporon ovoides CBS 7556, cross-hybridized to the group-specific probe (probe code, TAQAS1) shared by Trichosporon aquatile, Trichosporon asahii, and Trichosporon inkin. The remaining 32 strains did not produce any hybridization signals with probes on the array except the positive control probe. Therefore, the test specificity of the array was 97% (32/33).
TABLE 3.
Nontarget species used for specificity test in this study
| Species (teleomorph)a | Strainb |
|---|---|
| Acremonium strictum | BCRC 32239T |
| Aspergillus fumigatus (Sartorya fumigata) | BCRC 30099 |
| Aspergillus nidulans (Emericella nidulans) | BCRC 30100T |
| Candida kruisii | BCRC 21573T |
| Candida methanolovescens (Pichia minuta var. | |
| minuta) | BCRC 20476 |
| Candida mogii (Zygosaccharomyces rouxii) | BCRC 21506T |
| Cryptococcus daszewskae | CBS 5123 |
| Cryptococcus humicola | BCRC 21639T |
| Cryptococcus luteolus | BCRC 22372T |
| Debaryomyces polymorphus | BCRC 21478T |
| Exophiala jeanselmei | CBS 835.95 |
| Fusarium solani (Nectria haematococca) | ATCC 36031 |
| Geotrichum capitatum | CBS 327.86 |
| Microsporum canis (Arthroderma otae) | ATCC 10214 |
| Microsporum gypseum (Arthroderma gypseum) | CBS 161.69 |
| Mucor flavus | CBS 673.66 |
| Penicillium marneffei | CBS 334.59T |
| Pichia ciferrii | BCRC 22168T |
| Pichia henricii | BCRC 22170 |
| Pichia pastoris | BCRC 21531 |
| Rhizopus oryzae | BCRC 31107 |
| Saccharomycopsis capsularis | NRRL Y-17639 |
| Saccharomycopsis crataegensis | BCRC 22563 |
| Scedosporium prolificans | CBS 100390 |
| Sporobolomyces roseus var. roseus | BCRC 22375 |
| Torulopsis methanothermo (Pichia angusta) | BCRC 20467 |
| Trichosporon debeurmannianum | CBS 1896 |
| Trichosporon dermatis | CBS 2043 |
| Trichosporon jirovecii | CBS 6864T |
| Trichosporon mucoides | CBS 7625T |
| Trichosporon ovoides | CBS 7556T |
| Trichophyton rubrum | BCRC 32805 |
| Trichophyton verrucosum | ATCC 28203 |
Anamorphic names are used in this study, and teleomorphic species names are given in parentheses. Some species were only given the names of teleomorphs since they have no anamorphic names.
A superscript “T” indicates a type strain.
Identification of clinical isolates by the array.
A total of 110 clinical isolates, including 14 Candida species (96 strains) and 3 non-Candida species (15 strains), were analyzed by the array (Table 1). Of the 110 isolates, 98 were correctly identified and 12 produced discrepant identifications by phenotypic characteristics and array hybridization. Among the 12 isolates, 5 (Candida pelliculosa LMA 892971 and Trichosporon cutaneum LMA 94117, 94256, 931422, and 931440) were not identified by the oligonucleotide array. By sequencing the ITS 1, ITS 2, and D1-D2 regions of the five isolates, our previous study demonstrated that Candida pelliculosa LMA 892971 (Pichia anomala, teleomorph) was a misidentification of Pichia fabianii and that the four isolates of Trichosporon cutaneum were misidentifications of Trichosporon dermatis (24). Both ITS 2 and D1-D2 regions of Candida pelliculosa LMA 892971 had a sequence similarity of 100% with reference sequences (accession numbers AF335967 and AF335971) of Pichia fabianii in GenBank. Likewise, the four discrepant isolates of Trichosporon cutaneum (LMA 94117, 94256, 931422, and 931440) displayed a sequence similarity of 100% with reference sequences (accession number AF143557 in the ITS 1 and ITS 2 regions and accession number AF143555 in the D1-D2 region) of Trichosporon dermatis in GenBank (24). Since Pichia fabianii and Trichosporon dermatis were nontarget species in this study, their PCR products did not hybridize with any probes on the array except the positive control probe.
Of the remaining seven discrepant isolates, Candida dubliniensis RB 1168, Candida guilliermondii RB 1055, Candida inconspicua LMA 90289, Candida inconspicua RB 1226, Candida krusei RB 1237, Candida rugosa RB 1158, and Cryptococcus albidus LMA 935479 were identified as Candida albicans, Candida parapsilosis, Candida krusei, Candida glabrata, Pichia norvegensis, Saccharomyces cerevisiae, and Cryptococcus neoformans, respectively, by the array. The accuracy of identification of the seven discrepant isolates by hybridization was confirmed by sequence analysis of the ITS 1, ITS 2, and D1-D2 regions in our previous study (24). As the 12 discrepant clinical isolates (5 unidentified isolates and 7 misidentified isolates) were proved to be misidentified by phenotypic methods, the test sensitivity of clinical isolates by the array was 100% (105/105). If reference strains and clinical isolates were taken together, the overall test sensitivity of the array was also 100% (414/414).
Limit of detection of the array.
Serial 10-fold dilutions of DNA extracted from Candida albicans BCRC 20512 and Kloeckera apis BCRC 22112 were used to determine the limit of detection of the array. The present method was able to detect genomic DNA at a level of 10 pg per assay of both strains (data not shown).
DISCUSSION
In this study, an oligonucleotide array was developed to identify 77 species (16 genera) of medically relevant yeasts, including some less-common emerging species belonging to Cryptococcus, Pichia, Rhodotorula, Saccharomyces, and Trichosporon species. A test sensitivity of 100% (414/414) and a specificity of 97% (32/33) were obtained by the array. The prominent feature of the present method is that it replaces the various morphological and metabolic characteristics for yeast identification with a single standardized protocol encompassing DNA extraction, PCR amplification of the ITS regions, and the hybridization of PCR product to the array. The hybridized spot (blue color on a white background), having a diameter of 400 μm, could be recognized easily by the naked eye. The present method will be useful when the identification of yeast pathogens to the species level is necessary, and the current array has the potential to be extended by adding further oligonucleotides to it without significantly increasing cost or complexity.
The divergence in the ITS length and sequence among different species has been used for fungal identification (5, 9, 26, 49). Based on the length polymorphism of the ITS 2 region (237 to 429 bp), Chen et al. (5) were able to identify 92% of the clinical yeast isolates (34 species) by using the capillary electrophoresis technique. Recently, an oligonucleotide microarray based on the ITS 2 sequence was developed to identify 20 species of pathogenic fungi, including Aspergillus, Candida, Cryptococcus, Microsporum, Mucor, Trichophyton, and other genera (21). Our previous study also demonstrated that the ITS 2 region is a more discriminative target than the ITS 1 region for yeast identification (24). However, considering the factors (specificity, melting temperature, GC content, and secondary structure) that can influence the array performance (35), probes were designed from either the ITS 1 or the ITS 2 region in this study. Among the 80 probes listed in Table 2, 58 were designed from the ITS 2 region.
C. parapsilosis is a frequently isolated yeast pathogen. Strains of C. parapsilosis could be divided into three genotypes (27, 42). Recently, Tavanti et al. (46) proposed two new species (Candida orthopsilosis and Candida metapsilosis) to replace Candida parapsilosis genotypes II and III, respectively, and the species Candida parapsilosis is retained for genotype I isolates only. However, the new names are not widely used yet, as reflected in the culture lists of some prestigious culture collection centers, such as the ATCC and CBS. In this study, a common probe (CP6) was used to identify all genotypes of Candida parapsilosis, and two additional probes (CP8 and CP10) that can hybridize with genotypes II and III were constructed. For this reason, if only probe CP6 was hybridized, the test organism was identified as Candida parapsilosis (genotype I). If probe CP8 or CP10 was also hybridized, the strain would be Candida parapsilosis genotype II (Candida orthopsilosis) or III (Candida metapsilosis).
Trichosporon asahii, Trichosporon aquatile, and Trichosporon inkin are closely related species (44). Molecular phylogenetic trees based on both ITS sequences revealed that the three species and several other species form a distinct cluster among other Trichosporon species (44). A common probe (probe code, TAQAS1) was designed for the three Trichosporon species; however, each individual species can be differentiated from the other two species by its own specific probe (Table 2). Recently, a fatal case of sternal wound infection caused by Trichosporon inkin following aortic root surgery was reported (8). Trichosporon asahii can cause white piedra and onychomycosis in immunocompetent patients as well as various localized or disseminated infections in immunodeficient individuals. Fungemia caused by Trichosporon asahii was recently reported for a very low-birth-weight neonate (33).
It has been found that the addition of multiple thymine (or adenosine) bases to the 3′ (or 5′) ends of probes can improve the hybridization signal of oligonucleotide probes (1, 35). Although the mechanisms of adding low numbers (5 to 20) of thymine bases to a probe is not clear, it was proposed that this might decrease the steric hindrance between the probe and target DNA during hybridization or might increase the binding of probes to the nylon membrane (1, 35). In this study, five to 17 additional bases of thymine were added to the 3′ (or 5′) ends of some probes that displayed weak hybridization signals (Table 2). In our experience, the benefit of adding thymine bases to a probe is especially obvious for relatively short probes (16- to 20-mers).
Although the designed probes were carefully screened to avoid sequence homology with other microorganisms, many probes still cross-hybridized to nonhomologous species. To avoid cross-hybridization, 12 probes were intentionally designed to incorporate a mismatch base in each of them (Table 2). This strategy successfully eliminated nonspecific reactions, although the hybridization signals produced by the modified probes towards their target species decreased slightly. The incorporation of a mismatched base into a probe was based on the observations made previously by Ikuta et al. (22). Their results indicated that the G-T and G-A mismatches slightly destabilize a duplex, while the A-A, T-T, C-T, and C-A mismatches have significant destabilization effects. It was hoped that the incorporation of a mismatch in each of the 12 probes could eliminate nonspecific reaction, but at the same time still retain good hybridization signals toward their target yeasts with the result that sensitivity would not be sacrificed in the process of increasing specificity. This was successfully achieved in this study.
Commercially available identification kits, such as the API ID32C strip or Vitek YBC card, are commonly used for yeast identification in clinical laboratory. A recent study indicated that only 87% of clinical isolates were identified correctly to the species or genus level by the ID32C kit, with the remaining 13% isolates being either unidentified or misidentified (6). The most problematic species were Candida rugosa and Candida utilis; however, the two species were well distinguished by the present array (Fig. 2A). Candida rugosa is an emerging fungal pathogen and, along with Candida glabrata and Candida krusei, is a species of Candida with reduced susceptibility to the azole antifungals (37). In addition, strains of Candida inconspicua tend to be misidentified as Candida norvegensis by the ID32C panel (28), but both species were well differentiated by array hybridization (Fig. 2A).
In conclusion, the identification of clinically relevant yeasts by the present method is highly reliable and can be used as an accurate alternative to conventional identification methods. The method follows a common protocol that can be completed for isolated colonies within 24 h.
Acknowledgments
This project was supported by grants (NSC95-2323-B-006-007 and NSC95-2320-B-006-034) from the National Science Council, Taiwan, Republic of China.
Footnotes
Published ahead of print on 16 May 2007.
REFERENCES
- 1.Brown, T. J., and R. M. Anthony. 2000. The addition of low numbers of 3′ thymine bases can be used to improve the hybridization signal of oligonucleotides for use within arrays on nylon supports. J. Microbiol. Methods 42:203-207. [DOI] [PubMed] [Google Scholar]
- 2.Chang, H. C., S. N. Leaw, A. H. Huang, T. L. Wu, and T. C. Chang. 2001. Rapid identification of yeasts in positive blood cultures by a multiplex PCR method. J. Clin. Microbiol. 39:3466-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, C. C., L. J. Teng, S. K. Tsao, and T. C. Chang. 2005. Identification of clinically relevant viridans streptococci by an oligonucleotide array. J. Clin. Microbiol. 43:1515-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, S. C. A., C. L. Halliday, and W. Meyer. 2002. A review of nucleic acid-based diagnostic tests for systemic mycoses with an emphasis on polymerase chain reaction-based assays. Med. Mycol. 40:333-357. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y.-C., J. D. Eisner, M. M. Kattar, S. L. Rassoulian-Barrett, K. Lafe, S. L. Yarfitz, A. P. Limaye, and B. T. Cookson. 2000. Identification of medically important yeasts using PCR-based detection of DNA sequence polymorphisms in the internal transcribed spacer 2 region of the rRNA genes. J. Clin. Microbiol. 38:2302-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciardo, D. E., G. Schar, E. C. Bottger, M. Altwegg, and P. P. Bosshard. 2006. Internal transcribed spacer sequencing versus biochemical profiling for identification of medically important yeasts. J. Clin. Microbiol. 44:77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coignard, C., S. F. Hurst, L. E. Benjamin, M. E. Brandt, D. W. Warnock, and C. J. Morrison. 2004. Resolution of discrepant results for Candida species identification by using DNA probes. J. Clin. Microbiol. 42:858-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies, F., S. Logan, E. Johnson, and J. L. Klein. 2006. Sternal wound infection by Trichosporon inkin following cardiac surgery. J. Clin. Microbiol. 44:2657-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Baere, T., G. Claeys, D. Swinne, C. Massonet, G. Verschraegen, A. Muylaert, and M. Vaneechoutte. 2002. Identification of cultured isolates of clinically important yeast species using fluorescent fragment length analysis of the amplified internally transcribed rRNA spacer 2 region. BMC Microbiol. 2:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz, M. R., and J. W. Fell. 2004. High-throughput detection of pathogenic yeasts of the genus Trichosporon. J. Clin. Microbiol. 42:3696-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dooley, D. P., M. L. Beckius, and B. S. Jeffrey. 1994. Misidentification of clinical yeast isolates by using the updated Vitek Yeast Biochemical Card. J. Clin. Microbiol. 32:2289-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enache-Angoulvant, A., and C. Hennequin. 2005. Invasive Saccharomyces infection: a comprehensive review. Clin. Infect. Dis. 41:1559-1568. [DOI] [PubMed] [Google Scholar]
- 13.Fanci, R., and P. Pecile. 2005. Central venous catheter-related infection due to Candida membranaefaciens, a new opportunistic azole-resistant yeast in a cancer patient: a case report and a review of literature. Mycoses 48:357-359. [DOI] [PubMed] [Google Scholar]
- 14.Fenn, J. P., H. Segal, B. Barland, D. Denton, J. Whisenant, H. Chun, K. Christofferson, L. Hamilton, and K. Carroll. 1994. Comparison of updated Vitek Yeast Biochemical Card and API 20C yeast identification systems. J. Clin. Microbiol. 32:1184-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frutos, R. L., M. T. Fernández-Espinar, and A. Querol. 2004. Identification of species of the genus Candida by analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Antonie Leeuwenhoek 85:175-185. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Lopez, A., E. Mellado, J. L. Rodriguez-Tudela, and M. Cuenca-Estrella. 2005. Susceptibility profile of 29 clinical isolates of Rhodotorula spp. and literature review. J. Antimicrob. Chemother. 55:312-316. [DOI] [PubMed] [Google Scholar]
- 17.Gunn, S. R., X. T. Reveles, J. D. Hamlington, L. C. Sadkowski, T. L. Johnson-Pais, and J. H. Jorgensen. 2006. Use of DNA sequencing analysis to confirm fungemia due to Trichosporon dermatis in a pediatric patient. J. Clin. Microbiol. 44:1175-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazen, K. C., and S. A. Howell. 2003. Candida, Cryptococcus, and other yeasts of medical importance, p. 1693-1711. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 19.Holland, S. M., Y. R. Shea, and J. Kwon-Chung. 2004. Regarding “Trichosporon pullulans infection in 2 patients with chronic granulomatous disease.” J. Allergy Clin. Immunol. 114:205-206. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao, C. R., L. Huang, J. P. Bouchara, R. Barton, H. C. Li, and T. C. Chang. 2005. Identification of medically important molds by an oligonucleotide array. J. Clin. Microbiol. 3:3760-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, A., J. W. Li, Z. Q. Shen, X. W. Wang, and M. Jin. 2006. High-throughput identification of clinical pathogenic fungi by hybridization to an oligonucleotide microarray. J. Clin. Microbiol. 44:3299-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikuta, S., K. Takagi, R. B. Wallace, and K. Itakura. 1987. Dissociation kinetics of 19 base paired oligonucleotide-DNA duplexes containing different single mismatched base pairs. Nucleic Acids Res. 15:797-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtzman, C. P., and C. J. Robnett. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 35:1216-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leaw, S. N., H. C. Chang, H. F. Sun, R. Barton, J. P. Bouchara, and T. C. Chang. 2006. Identification of medically important yeast species by sequence analysis of the internal transcribed spacer regions. J. Clin. Microbiol. 44:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leinberger, D. M., U. Schumacher, I. B. Autenrieth, and T. T. Bachmann. 2005. Development of a DNA microarray for detection and identification of fungal pathogens involved in invasive mycoses. J. Clin. Microbiol. 43:4943-4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Y. L., S. N. Leaw, J. H. Chen, H. C. Chang, and T. C. Chang. 2003. Rapid identification of yeasts commonly found in positive blood cultures by amplification of the internal transcribed spacer regions 1 and 2. Eur. J. Clin. Microbiol. Infect. Dis. 22:693-696. [DOI] [PubMed] [Google Scholar]
- 27.Lin, D., L. Wu, M. G. Rinaldi, and P. F. Lehmann. 1995. Three distinct genotypes within Candida parapsilosis from clinical sources. J. Clin. Microbiol. 33:1815-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majoros, L., G. Karods, A. Belak, A. Maraz, L. Asztalos, E. Csanky, Z. Barta, and B. Szabo. 2003. Restriction enzyme analysis of ribosomal DNA shows that Candida inconspicua clinical isolates can be misidentified as Candida norvegensis with traditional diagnostic procedures. J. Clin. Microbiol. 41:5250-5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massonet, C., J. V. Eldere, M. Vaneechoutte, T. De Baere, J. Verhaegen, and K. Lagrou. 2004. Comparison of VITEK 2 with ITS2-fragment length polymorphism analysis for identification of yeast species. J. Clin. Microbiol. 42:2209-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClure, F. D. 1990. Design and analysis of qualitative collaborative studies: minimum collaborative program. J. Assoc. Off. Anal. Chem. 73:953-960. [PubMed] [Google Scholar]
- 31.Mele, G., M. Musci, C. Musto, L. D'Amato, A. Traficante, and N. Di Renzo. 2005. Pneumonia caused by Trichosporon pullulans in an autologous peripheral blood stem cell transplant recipient: possible misidentification. Bone Marrow Transpl. 35:1219-1220. [DOI] [PubMed] [Google Scholar]
- 32.Millar, B. C., X. Jiru, J. E. Moore, and J. A. Earle. 2000. A simple and sensitive method to extract bacterial, yeast and fungal DNA from blood culture material. J. Microbiol. Methods 42:139-147. [DOI] [PubMed] [Google Scholar]
- 33.Panagopoulou, P., J. Evdoridou, E. Bibashi, J. Filioti, D. Sofianou, G. Kremenopoulos, and E. Roilides. 2002. Trichosporon asahii: an unusual cause of invasive infection in neonates. Pediatr. Infect. Dis. J. 21:169-170. [DOI] [PubMed] [Google Scholar]
- 34.Pasqualotto, G. C., F. A. Copetti, C. F. Meneses, A. R. Machado, and A. L. Brunetto. 2005. Infection by Rhodotorula sp. in children receiving treatment for malignant diseases. J. Pediatr. Hematol. Oncol. 27:232-233. [DOI] [PubMed] [Google Scholar]
- 35.Peplies, J., F. O. Glockner, and R. Amann. 2003. Optimization strategies for DNA microarray-based detection of bacteria with 16S rRNA-targeting oligo nucleotide probes. Appl. Environ. Microbiol. 69:1397-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaller, M. A., and D. J. Diekema. 2004. Rare and emerging opportunistic fungal pathogens: concerns for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 42:4419-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaller, M. A., D. J. Diekema, A. L. Colombo, C. Kibbler, K. P. Ng, D. L. Gibbs, and V. A. Newell. 2006. Candida rugosa, an emerging fungal pathogen with resistance to azoles: geographic and temporal trends from the ARTEMIS DISK antifungal surveillance program. J. Clin. Microbiol. 44:3578-3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Playford, E. G., F. Kong, Y. Sun, H. Wang, C. Halliday, and T. C. Sorrell. 2006. Simultaneous detection and identification of Candida, Aspergillus, and Cryptococcus species by reverse line blot hybridization. J. Clin. Microbiol. 44:876-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pryce, T. M., S. Palladino, D. M. Price, D. J. Gardam, P. B. Campbell, K. J. Christiansen, and R. J. Murray. 2006. Rapid identification of fungal pathogens in BacT/ALERT, BACTEC, and BBL MGIT media using polymerase chain reaction and DNA sequencing of the internal transcribed spacer regions. Diagn. Microbiol. Infect. Dis. 54:289-297. [DOI] [PubMed] [Google Scholar]
- 40.Ramani, R., S. Gromadzki, D. H. Pincus, I. F. Salkin, and V. Chaturvedi. 1998. Efficacy of API 20C and ID 32C systems for identification of common and rare clinical yeast isolates. J. Clin. Microbiol. 36:3396-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodero, L., M. Cuenca-Estrella, S. Cordoba, P. Cahn, G. Davel, S. Kaufman, L. Guelfand, and J. L. Rodriguez-Tudela. 2002. Transient fungemia caused by an amphotericin B-resistant isolate of Candida haemulonii. J. Clin. Microbiol. 40:2266-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy, B., and S. A. Meyer. 1998. Confirmation of the distinct genotype groups within the form species Candida parapsilosis. J. Clin. Microbiol. 36:216-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serena, C., F. J. Pastor, M. Ortoneda, J. Capilla, N. Nolard, and J. Guarro. 2004. In vitro antifungal susceptibilities of uncommon basidiomycetous yeasts. Antimicrob. Agents Chemother. 48:2724-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugita, T., A. Nishikawa, R. Ikeda, and T. Shinoda. 1999. Identification of medically relevant Trichosporon species based on sequences of internal transcribed spacer regions and construction of a database for Trichosporon identification. J. Clin. Microbiol. 37:1985-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taj-Aldeen, S. J., S. H. Doiphode, and X. Y. Han. 2006. Kodamaea (Pichia) ohmeri fungaemia in a premature neonate. J. Med. Microbiol. 55:237-239. [DOI] [PubMed] [Google Scholar]
- 46.Tavanti, A., A. D. Davidson, N. A. Gow, M. C. Maiden, and F. C. Odds. 2005. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43:284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trost, A., B. Graf, J. Eucker, O. Sezer, K. Possinger, U. B. Gobel, and T. Adam. 2004. Identification of clinically relevant yeasts by PCR/RFLP. J. Microbiol. Methods 56:201-211. [DOI] [PubMed] [Google Scholar]
- 48.Tung, S. K., L. J. Teng, M. Vaneechoutte, H. M. Chen, and T. C. Chang. 2006. Array-based identification of species of the genera Abiotrophia, Enterococcus, Granulicatella, and Streptococcus. J. Clin. Microbiol. 44:4414-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turenne, C. Y., S. E. Sanche, D. J. Hoban, J. A. Karlowsky, and A. M. Kabani. 1999. Rapid identification of fungi by using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J. Clin. Microbiol. 37:1846-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gefland, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA.