Abstract
Transmissible spongiform encephalopathy strains can be differentiated by their behavior in bioassays and by molecular analyses of the disease-associated prion protein (PrP) in a posttranslationally transformed conformation (PrPSc). Until recently, isolates from cases of bovine spongiform encephalopathy (BSE) appeared to be very homogeneous. However, a limited number of atypical BSE isolates have recently been identified upon analyses of the disease-associated proteinase K (PK) resistance-associated moiety of PrPSc (PrPres), suggesting the existence of at least two additional BSE PrPres variants. These are defined here as the H type and the L type, according to the higher and lower positions of the nonglycosylated PrPres band in Western blots, respectively, compared to the position of the band in classical BSE (C-type) isolates. These molecular PrPres variants, which originated from six different European countries, were investigated together. In addition to the migration properties and glycosylation profiles (glycoprofiles), the H- and L-type isolates exhibited enhanced PK sensitivities at pH 8 compared to those of the C-type isolates. Moreover, H-type BSE isolates exhibited differences in the binding of antibodies specific for N- and more C-terminal PrP regions and principally contained two aglycosylated PrPres moieties which can both be glycosylated and which is thus indicative of the existence of two PrPres populations or intermediate cleavage sites. These properties appear to be consistent within each BSE type and independent of the geographical origin, suggesting the existence of different BSE strains in cattle. The choice of three antibodies and the application of two pHs during the digestion of brain homogenates provide practical and diverse tools for the discriminative detection of these three molecular BSE types and might assist with the recognition of other variants.
Prion diseases, or transmissible spongiform encephalopathies (TSEs), are a group of lethal and slow infectious diseases that are unique by the involvement of an agent that contains a host protein, the prion protein (PrP), in a posttranslationally transformed conformation (PrPSc) and that does not seem to contain any conventional form of nucleic acid (30, 46). PrP is essential in disease development since without its presence no infection has been shown to occur and during infection it usually deposits as PrPSc in nervous system tissues, which results in a disease with fatal consequences (9, 13, 19, 47, 51). PrPSc is partially resistant to proteinase K (PK), and the PK resistance-associated moiety is defined by the term PrPres.
As in microbial infections, prion diseases are subject to strain variations. In sheep and goats, in which scrapie has been known to occur for centuries, transmissions to mice and other experimental animals have revealed the occurrence of at least 20 different strain-dependent variations, including different vacuolar lesion patterns in the brain, different disease incubation times, different molecular characteristics of PrPres, different distributions of PrPSc in the brain, and different behavioral patterns in infected animals (5, 6, 11, 12, 26, 33, 50, 56). It is highly probable that the strain-dependent variations in PrPres are due to conformational variations of PrPSc, which, notably, determine the extent of degradation by PK (5, 6, 20, 43, 50, 55, 57).
Following the recognition of bovine spongiform encephalopathy (BSE) in cattle and the BSE epidemic in the United Kingdom (59, 60), serious health concerns prevailed because of the uncertainty about the potential risk of BSE to human health when bovine materials were used for medical purposes or served as food. A striking phenomenon in the BSE epidemic was the homogeneity of the agent, leading to the conclusion that only one strain of TSE was involved, as established in experimental inbred mouse models (11, 29). These observations were further substantiated when transgenic mice expressing either bovine or murine PrP in multiple copy numbers were challenged with BSE and the strain characteristics in all the mice were the same (15, 18, 52). However, the possibility of the presence of a mixture of strains among BSE and scrapie isolates has also been suggested (1, 2, 38).
More than 180,000 cases of BSE have been reported within the United Kingdom by passive surveillance since the beginning of the epidemic. In the European Union, since the year 2001, all slaughter cattle aged 30 months or older and all fallen stock animals older than age 24 months must be rapidly tested for BSE (24). This has led to the detection of over 5,000 BSE cases outside the United Kingdom. On the basis of the results available from diagnostic and limited bioassay studies, the cases from this active surveillance are expected to be of the same BSE type as the type detected in the United Kingdom (16, 22, 37). However, rare variants of BSE have now also been detected as a consequence of this active surveillance in cattle (Bos taurus) (8, 14, 17, 23, 45, 49, 61) and in a miniature zebu (Bos indicus) (53). The isolates from these cases showed by Western blot (WB) analysis a PrPres profile that deviated from that of the classical BSE cases (named the C type), in which the PrPres bands migrated to a higher (H type) or a lower (L type) position (8, 17). Both atypical forms, but especially the L type, were further characterized by a smaller proportion of diglycosylated PrPres compared to the proportion in C-type BSE isolates. The histological features seen in the initial report of the two L-type cases were peculiar because of the prominent involvement of the forebrain; in contrast, in C-type BSE, the brain stem is primarily affected. Moreover, PrPSc was deposited in unusual amyloid plaques, which led to the name bovine amyloidotic spongiform encephalopathy (17). Other reports describing PrPres from cattle with atypical profiles have appeared; these include both an L-type case and an H-type case in Germany and single unclassified cases in each of Poland, the United States, Japan, and Belgium (10, 14, 23, 45, 49, 61). Recent reports have shown that such atypical H- and L-type isolates are transmissible to mice and clearly differ from C-type BSE isolates, with unique incubation periods, PrPres profiles, and histological lesions (3, 4, 7, 14).
This study analyzed together 17 aberrant BSE isolates from six European countries with the purpose of studying in depth the behavior, comparability, and homogeneity of their molecular properties by the use of WB analysis. All BSE isolates could be differentiated into three groups, i.e., the L, C, or H type, by using the previously published criteria of apparent molecular mass and glycoprofile, while other criteria for their discrimination were also defined, such as sensitivity to PK, whether an additional nonglycosylated PrPres band was present, and antibody-dependent molecular patterns.
MATERIALS AND METHODS
Animals and tissues.
In this collaborative European network study of ruminant TSE strains, 9 brain stem samples from C-type BSE cases and 17 brain stem samples from atypical BSE cases from six different participating national reference laboratories were studied. Among the atypical samples, 6 were classified as being of the H type and 11 were classified as being of the L type, on the basis of their migration positions and the glycoprofile of PrPres, as published previously (8, 17). Data on these cases are presented in Table 1 and include the PrPres classification, the country of origin, the age and health status of the animal, and the test used for the initial diagnosis. The tissues had been stored at −20°C or lower.
TABLE 1.
BSE isolates used and their classificationa
| PrPres type and case no. | Countryb | Age (yr) | Health status | Initial diagnostic assayc |
|---|---|---|---|---|
| C type | ||||
| NL5 | NL | 5 | Clinical | I |
| NL6 | NL | 6 | Clinical | I |
| NL15 | NL | 9 | Clinical | P |
| NL16 | NL | 6 | Clinical | P |
| NL17 | NL | 6 | Clinical | P |
| NL20 | NL | 5 | Slaughter | P |
| NL36 | NL | 8 | Emergency | P |
| 288/02 | IT | 8 | Downer | P |
| R126/03 | GE | 13 | Slaughter | T |
| H type | ||||
| NL22 | NL | 13 | Slaughter | P |
| 03-0440 | FR | 15 | Fallen stock | P |
| 03-1928 | FR | 8 | Slaughter | P |
| 45 | PL | 10 | Slaughter | T |
| Mar-2006 | SW | 12 | Fallen stock | T |
| R152/04 | GE | 13 | Slaughter | T |
| L type | ||||
| NL47 | NL | 9 | Slaughter | P |
| NL58 | NL | 12 | Slaughter | P |
| 02-2528 | FR | 8 | Fallen stock | P |
| 10-88 | IT | 15 | Slaughter | P |
| 2 | PL | 13 | Slaughter | E |
| 15 | PL | 9 | Slaughter | T |
| 17 | PL | 13 | Emergency | T |
| 28 | PL | 11 | Slaughter | T |
| 31 | PL | 13 | Fallen stock | T |
| 43 | PL | 12 | Fallen stock | T |
| R172/02 | GE | 15 | Slaughter | T |
For all samples brain stem tissue was used.
NL, The Netherlands; IT, Italy; GE, Germany; FR, France; PL, Poland; SW, Sweden.
I, immunohistochemistry; P, Prionics Check WB assay; T, Bio-Rad TeSeE enzyme-linked immunosorbent assay; E, Enfer enzyme-linked immunosorbent assay.
Tissue treatments.
Ten percent homogenates were prepared by homogenization in lysis buffer consisting of 0.5% Triton X-100 and 0.5% sodium deoxycholate in phosphate-buffered saline (138 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 2.8 mM KH2PO4, pH 7.2). The homogenizations were performed for 45 s at 23,000 rpm in regular Prypcon vials with a MediFASTH homogenizer (Consul AR SA; Villeneuve, Switzerland). Coarse sediment was removed by centrifugation at 425 × g for 5 min at ambient temperature. Usually, digestions of 100 μl of homogenates were performed with PK (30 U/mg; 124568; Merck, Darmstadt, Germany) at 50 μg/ml for 60 min at 37°C; and the reactions were stopped by the successive addition of 10 μl of a solution of 3-mg/ml Pefabloc (Pefabloc SC; Roche, Almere, The Netherlands) in water and 100 μl of 20% (wt/vol) sucrose, 0.282 M Tris base, 0.212 M Tris-HCl, 4% (wt/vol) sodium dodecyl sulfate (SDS), 1.0 mM EDTA, 0.038% (wt/vol) bromophenol blue, and 4% (vol/vol) β-mercaptoethanol (2× sample buffer) with heating for 5 min at 95°C. For studies on the effect of pH, temperature, and enzyme concentration, phosphate- and Tris-based buffers with pHs ranging from 6.5 to 8.0 were prepared for adjustment of the pH during digestion with PK. The phosphate buffers were prepared by titrating 200 mM KH2PO4 solution with 200 mM Na2HPO4 solution until the desired pH was reached. The Tris buffers were prepared by titrating 200 mM Tris solution with 4 M HCl solution until the desired pH was reached. An equal volume of pH adjustment buffer was added to 50 μl of homogenate to obtain the desired pH. After the addition of PK to the phosphate-buffered saline in order to reach concentrations that varied from 5.6 μg/ml to 450 μg/ml, digestion was performed either for 40 min at 50°C or for 60 min at 37°C. Before and after digestion with PK, the pH of the sample was measured with a pH microelectrode (Biotrode; Hamilton). The reaction was stopped by the successive addition of 10 μl of 3 to 30 mg/ml Pefabloc and 100 μl of 2× sample buffer with heating for 5 min at 95°C. For a number of experiments, a precipitation step was introduced after Pefabloc was added to remove tissue matrix components and, eventually, to concentrate PrPres; the sample was mixed with 100 μl of 1-propanol, vortexed, and centrifuged at 21,000 × g for 5 min in a microcentrifuge (5417R; Eppendorf) at room temperature. The pellet was dissolved in sample buffer (2× sample buffer diluted with an equal volume of water) to the desired volume and heated. By dissolving the pellets in a volume smaller than that used at the start, a concentration step of at least 10-fold was achieved.
Deglycosylation by treatment with PNGase F.
Deglycosylation with 30 U of peptide N-glycosidase F (PNGase F; New England Biolabs) was performed as described previously (35).
SDS-PAGE, Western blotting, and immunochemical development.
Ten microliters of each sample was applied to each well for SDS-polyacrylamide gel electrophoresis (PAGE). Electrophoresis was performed for 40 min at 200 V in an XCell SureLock Mini-Cell gel electrophoresis system (Invitrogen, Breda, The Netherlands) with either 10 or 17 wells precast to 1 mm with 12% Bis-Tris NuPAGE gels and morpholinepropanesulfonic acid (MOPS) (or morpholineethanesulfonic acid [MES]) running buffer with antioxidant. A mixture of the commercially available markers MagicMark XP and SeeBlue Prestained (Invitrogen) was used as a reference for apparent molecular mass estimations. The subsequent WB procedures (electrotransfer, immunostaining, and the development of luminescence with CDP-Star as the substrate for alkaline phosphatase) were performed as described previously (35). The results were recorded on Hyperfilm ECL photographic film (Amersham, Buckinghamshire, United Kingdom), usually with exposure times of between 1 and 15 min. For quantification, the films were digitized with a film scanner (ScanMaker III; Microtek). The resulting image files were analyzed with quantitative software (Gel-Pro analyzer; Media Cybernetics), and the integrated optical densities and apparent molecular masses of the bands were exported to a spreadsheet for further calculation. For calculation of the glycoprofiles for each lane, a baseline was set from the beginning to the end of the peak profile, and the start and the end points of the peaks were determined (overlapping peaks were split in the valley). Only the three bands with molecular masses ranging from 30 to 16 kDa were used to calculate the glycoprofile, for which each band was expressed as a percentage of the summed integrated optical densities of the three bands in the triplet.
Antibodies and antibody binding specificities.
The monoclonal antibodies (MAbs) used in this study, all of which have suitable affinities for bovine PrP, have the following epitope specificities, as mapped by Pepscan analysis (27, 35, 58): MAb SAF32 is specific for the bovine PrP region 62/70/78/86QPHGGGW68/76/84/92 (25) and was obtained from SPI-BIO (Montigny le Bretonneux, France); antibodies 12B2, 9A2, and L42 are specific for101WGQGG105, 110WNK112, and 156YEDRYY161, respectively, and were described previously (31, 35); MAb 6H4 is specific for 156YEDRYYREN164 and was purchased from Prionics AG, Zürich, Switzerland (34); and MAb 94B4 (58) could be mapped to 198HTVTTTTK205.
Statistical analyses.
One-way analyses of variance were carried out to establish whether variations between groups of data were larger than expected; if so, subsequent differences between pairs of groups were considered significant if the probability of a nondifference was <0.05 in multiple-comparisons tests, according to the Student-Newman-Keuls test. The software used for these calculations was Instat Biostatistics from Graph-Pad Software, San Diego, CA.
RESULTS
Brain materials were studied after digestion with PK at 37°C or 50°C in 10% homogenates and were subjected to SDS-PAGE and WB analysis either directly or after a further treatment with 1-propanol to concentrate PrPres while removing the bulk of the tissue matrix components. All investigations were focused on the molecular behavior of PrPres, determined by using conventional WB techniques. Temperature had no profound effect on the results. The alcohol treatment allowed almost 100% recovery of PrPres from all isolates. This pretreatment had no effect on the results obtained. Recently, we showed that antibodies to PrP can be categorized into groups A, B, and C, which are thought to bind in the bovine PrP regions from positions 62 to 107, 109 to 164, and 154 to 236, respectively (7). From these we selected for this study MAbs SAF32 and 12B2 as representatives for group A; MAbs 9A2, L42, and 6H4 as representatives for group B; and MAb 94B4 as a representative for group C.
Migration profile of atypical PrPres.
Samples from confirmed BSE cases from France, Germany, Italy, Poland, Sweden, and The Netherlands were grouped into the C, H, or L type according to the migration features observed in the participating laboratories relative to the migration features of C-type BSE isolates (Table 1). Indeed, the PrPres of the C-type isolates showed a triplet of non-, mono-, and diglycosylated bands; and a triplet banding pattern was also observed for the H-type and L-type isolates, but they had different migration positions when they were analyzed by using an antibody from group B (Fig. 1A). The apparent molecular masses of the non-, mono-, and diglycosylated bands of the H-type BSE isolates were about 1.4 kDa greater than those of the C-type BSE isolates (Fig. 1B). For L-type samples, the difference in the migration of the diglycosylated PrPres band was especially pronounced compared to the migration of the band for C-type samples, running at a position ∼0.7 kDa lower (Fig. 1B), while only a small difference of ∼0.3 kDa was observed for the non- and monoglycosylated bands. An additional band of ∼7 kDa was observed near the running front in all BSE types when antibodies from group A or B were used for detection; however, for the H-type BSE isolates, this band was more prominent (Fig. 1A, arrow).
FIG. 1.
Results of WB analysis of L-, C-, and H-type BSE isolates with group B antibody L42. (A) Lanes with L-, C-, and H-type material with tissue equivalents of 0.5, 0.2, and 0.2 mg, respectively, placed between molecular mass marker lanes; (B) apparent molecular masses of diglycosylated (circles), monoglycosylated (squares), and nonglycosylated (triangles) bands with standard deviations for L-type (n = 11), C-type (n = 9), and H-type (n = 6) isolates. Arrow, 7-kDa band.
Deglycosylation.
Deglycosylation by PNGase F treatment of L-, C-, and H-type BSE isolates resulted in the disappearance of di- and monoglycosylated bands and an increase in the nonglycosylated band when a group B-specific antibody was used (Fig. 2). For each BSE type, after PNGase F treatment the position of the nonglycosylated band was slightly higher than that of the lowest band for untreated samples (compare the lanes labeled + and − in Fig. 2). When the group C-specific antibody was used, the H-type samples showed an additional band at an apparent molecular mass of 10 kDa before digestion with PNGase F (Fig. 2B, arrow); upon digestion, this band had an increased signal and retained its migration position. Depending on whether the running conditions used MOPS or MES buffer for the SDS-PAGE Bis-Tris gels, the nonglycosylated bands in particular migrated to different relative mass positions, i.e., 10 and 12 kDa for the MOPS and MES buffers, respectively (data not shown).
FIG. 2.
Deglycosylation effect of PNGase F on BSE types. Applied tissue equivalents for L-, C- and H-type isolates: before deglycosylation, 0.4, 0.1, and 0.4 mg, respectively; after deglycosylation, 0.1, 0.025, and 0.1 mg, respectively; respectively. (A) Blot development with group B antibody 6H4; (B) blot development with group C antibody 94B4. The migration positions of molecular mass markers of 30 and 20 kDa are indicated by the bars on the left. The arrow points to the 10-kDa band, which appears in H-type BSE when the blot was developed with 94B4.
Resistance to PK digestion.
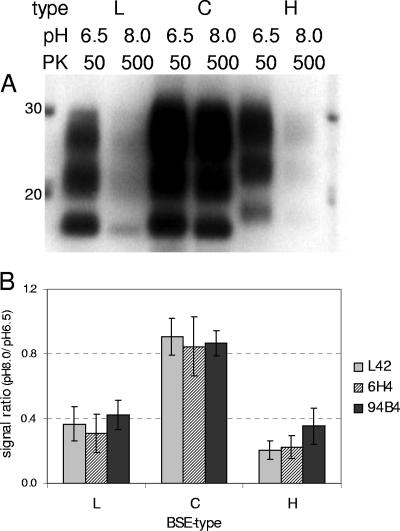
The levels of resistance of the isolates to PK were compared. At pHs above 7.0 or with increasing PK concentrations (20 to 500 μg/ml), the signal intensities of the L and H types decreased, with no change in the PrPres profile, although the C-type-related PrPres signal remained rather stable. This was most evident when the digestion was performed at two extreme conditions: mild (50 μg/ml PK and pH 6.5) or stringent (500 μg/ml PK and pH 8.0) at 37°C (Fig. 3A). Under mild conditions, the full-length PrP disappeared, while PrPSc was converted to PrPres in all individual BSE isolates, irrespective of the antibody used. Under stringent conditions, the signals of L- and H-type PrPress decreased more rapidly, while that of the C-type PrPres stayed nearly at the same level. The loss of signal between mild and stringent conditions was less than 20% for the C-type isolates, while it was more than 50% for both L- and H-type isolates (Fig. 3B).
FIG. 3.
Differentiation of L-, C- and H-type BSE isolates by susceptibility to PK digestion at 37°C under two conditions for pH and PK concentration: pH 6.5 and PK 50 μg/ml and pH 8.0 and PK 500 μg/ml. The tissue equivalent applied was 0.5 mg per lane. (A) Example of the results obtained with antibody L42; (B) resistance to PK digestion expressed as the integrated optical density ratio for the two digestion conditions with L42 and 6H4 (group B antibodies) and 94B4 (group C antibody).
Investigation of PrPres properties by using different PrP N-terminus-specific antibodies.
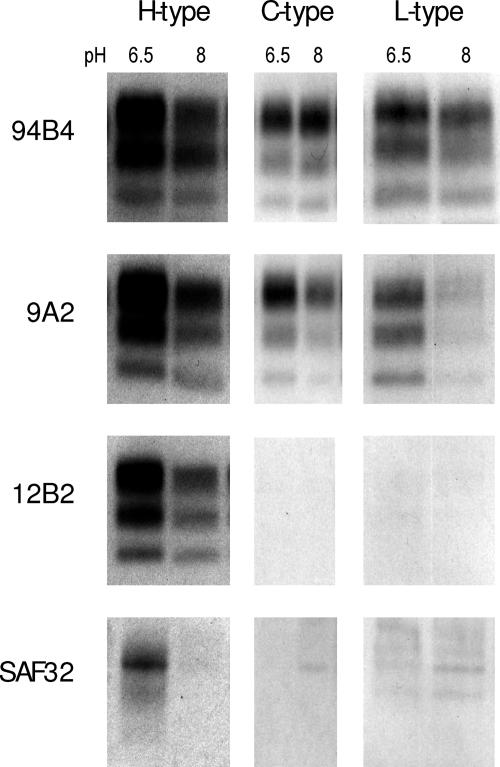
The differences in the molecular masses of the PrPres bands between the BSE types were further investigated with all samples and antibodies from group A and B that bind very close to the region around the PK cleavage site, that is, MAbs SAF32, 12B2, and 9A2. Core-specific antibody 94B4 from group C was also used as a reference for the retention of the part of PrPSc conferring the most PK resistance. This was tested with 50 μg/ml PK at pH 6.5 or 500 μg/ml PK at pH 8.0 (Fig. 4). After mild digestion at pH 6.5, MAb SAF32, directed against the octarepeats of PrP, detected only one fine band for H-type BSE in the region where the diglycosylated PrPres migrates and no signal for C- or L-type BSE. Antibody 12B2 bound to all bands of the PrPres triplet of the H type but not at all or hardly at all to the PrPres bands of the C type and not at all to the PrPres bands of the L type; MAb 9A2 detected the PrPres triplets of all BSE types.
FIG. 4.
N-terminal truncation of PrPres and differentiation of H-type BSE isolates from C- and L-type BSE isolates with antibody 12B2. WBs of H-, C-, and L-type BSE isolates after PK digestion at 37°C with 50 μg/ml PK and pH 6.5 and pH 8.0 are shown. The tissue equivalent applied was 0.25 mg per lane. The gels were developed with group A antibodies 12B2 and SAF32, group B antibody 9A2, and group C antibody 94B4, as indicated.
Glycoprofiles.
When the PrPres glycoprofiles were compared, the L type was distinctly different from the C type due to a lower proportion of the diglycosylated band, which was about 40% of the total amount for the L type but about 60% or more for the C type (Fig. 5). These data were further confirmed by statistical analyses (see Materials and Methods). The H type showed two different glycoprofiles, depending on the antibody used for detection. With group B antibodies L42 and 6H4, the glycoprofile of the H-type samples was indistinguishable from that of the C-type samples and clearly different from that of the L-type samples. With antibody 94B4, however, the PrPres profile was intermediate between that of the C type and the L type, which was due to a relatively lower percentage of the diglycosylated band and a higher percentage of the monoglycosylated band in the H type (Fig. 2 and Fig. 5A). In comparison to the percentage of the diglycosylated PrPres bands obtained with antibody L42, the use of antibody 6H4 resulted in a slightly higher percentage of the diglycosylated PrPres bands for the C and the H types (Fig. 5B and C, respectively).
FIG. 5.
Glycoprofiles of PrPres from L-, C-, and H-type BSE isolates. PK digestion was performed at 37°C with 50 μg/ml PK at pH 6.5. The tissue equivalent applied was 0.5 mg per lane. Development was with group C antibody 94B4 (A) and group B antibodies L42 (B) and 6H4 (C). Symbols: squares, C type; circles, H type; triangles, L type.
In the supplemental material for this report, the results for nearly all H-type and L-type cases from a single experiment are presented together to display the homogeneity in the properties of PrPres, mentioned above, that discriminated them from the C type.
DISCUSSION
Seventeen atypical BSE isolates were brought together for an in-depth study of the molecular and biochemical properties of PrPres, which represents the PK resistance-associated moiety of PrPSc. These isolates originated from a geographical region spanning a wide area in Europe and were designated the L or the H type. Four isolates were already reported (8, 14, 17); and 13 additional isolates are described here for the first time: 3 from The Netherlands, 7 from Poland, 2 from France, and 1 from Sweden. The ages of the animals from which both the H-type and the L-type isolates were recovered varied from 8 to 15 years. H- and L-type isolates displayed higher susceptibilities to PK digestion than C-type BSE isolates, and also, further discrimination procedures were unambiguous. For a robust discrimination, five major criteria for PrPres were established; and these five criteria (molecular mass, antibody binding affinity, digestion with PK at two conditions, glycoprofile determination, and whether one or two PrPres populations are present) can, in principle, be applied in a single WB analysis to whole homogenates.
Determination of the molecular mass differences in the PrPres bands has been the first basis for the discrimination of BSE types (8, 14, 17), but this needs some comment. C-type BSE isolates have a PrPres migration pattern intermediate between those of the H and the L types. The differences in apparent molecular masses between the C and the H types for each of the three PrPres bands averages 1.3 kDa, as was also noticed previously (8). However, the difference between the L type and the C type is only 0.3 kDa for the nonglycosylated and the monoglycosylated bands but about 0.8 kDa for the diglycosylated band. This was also evident from the report on the German (14) and Italian (17) cases. In practice this molecular mass discrimination, at least with only the nonglycosylated band, is therefore considered unsuitable for the definite recognition of L-type cases.
With respect to the nonglycosylated moiety in the PrPres triplet, H-type isolates again displayed a remarkable feature: the presence of two nonglycosylated fragments, one at 19.1 kDa and the second one at about 10 kDa. However, the 10-kDa band was detectable only with group C antibodies (with MAbs 94B4 and SAF84 having the highest affinities), which bind to PrP in the C-terminal region from positions 154 to 236 (7). If PNGase F was applied to PK-digested homogenates, the increased concentrations of these two deglycosylated PrPres bands indicate that both fragments are also present as glycosylated moieties. However, depending on whether SDS-PAGE in Bis-Tris gels used MOPS or MES buffer, the second nonglycosylated band in particular migrated to different relative mass positions, which were 10 and 12 kDa, respectively; and this variation might be attributed to the influence of the pH in the buffer on the physical behaviors of the peptides and on their SDS binding capacities in this small mass range, although the real effects involved are not fully understood. The appearance of potentially two PrPres populations of 19.1 kDa and 10 kDa in a single isolate has also recently been found in C75BL/6 mice infected with H-type isolates, which is evidence for the true strain-dependent nature of this molecular phenomenon (7). The occurrence of a mixed PrPres population is rather unique for TSE strains and suggests the existence of two PrPSc populations which differ in their susceptibilities to PK digestion and probably also in their conformation. Two PrPSc populations could occur either in one or in two different copolymers. Alternatively, the phenomenon of two PrPres populations could be the product of a single PrPSc conformer unique to the H type with two differentially sensitive PK cleavage sites. It remains to be determined whether this is an in situ phenomenon or whether it is caused by the PK treatment. Another interesting aspect of the deglycosylation experiments with PNGase F was the slightly slower rate of migration of the deglycosylated band of each BSE type after treatment with PNGase F compared to the rate for the nonglycosylated band in untreated samples (compare the lanes labeled + and − in Fig. 2). This phenomenon has already been described previously for PrPres derived from cattle and sheep with BSE (39, 58). While this difference in migration between the nonglycosylated and deglycosylated bands is not yet fully resolved, it might be a consequence of the formation of a negative charge which occurs when PNGase F transforms asparagine into aspartic acid during removal of N-linked carbohydrates. This creates a negative repulsion, which results in fewer dodecyl sulfate ions per protein molecule and, thus, in a lower net negative charge load (21, 44).
Our data on the glycoprofiles of the three different BSE types yield some further interesting information. A prominent aspect in L-type isolates is the small proportion of diglycosyl-PrPres, especially compared to that in C-type isolates and, to a lesser degree, also compared to that in H-type isolates. In C-type and H-type isolates, this fraction reaches values well above 55%, while in L-type isolates it remains below 55%. These differences were observed when detection antibodies that bind to the core region of the PrP-like group A, antibodies 9A2, L42, and 6H4, were applied. For the H type, the glycoprofile appeared, surprisingly, to depend also on the detection antibody applied: while core-specific antibodies like L42, 6H4, and 9A2 yielded features quite similar to those of C-type BSE isolates, with group C antibody 94B4 the glycoprofile was intermediate between those of C- and L-type BSE isolates due to the relatively large amount of staining in the position of the monoglycosylated band (Fig. 5A). A plausible explanation for this might be that C-terminus-specific antibodies like 94B4 react with two triple-banded PrPres populations, of which the second population migrates to a position 5 to 10 kDa lower than that where the first population migrates so that its diglycosylated band merges into the position of the monoglycosylated band of the PrPres of the first population (7). In this concept of two populations, group A and B antibodies like 12B2, 9A2, L42, and 6H4 can bind only to the first population; group C antibodies bind to both populations. These data further support the idea of the existence of a mixture of glycosylated PrPres populations, one of which is ∼1.3 kDa larger than that of the C type and can be bound by N-terminus-, core-, and C-terminus-specific antibodies, while the other is unusually short and consists of the region from approximately positions 163 to 242 of PrP which can be bound only by group C antibodies like 94B4 and SAF84. If PK cleaves near residue 163, the occurrence of an ∼7-kDa band, such as that which is most prominently present in H type, could represent a PrPSc fragment that is located at the N terminus of this cleavage site (7).
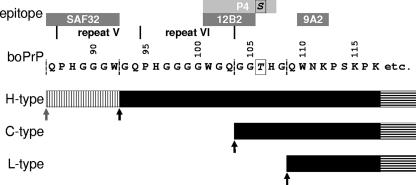
A difference in the length of the PrPres molecules in H-type isolates compared to the lengths in C- and L-type isolates was also clearly confirmed by using group A antibody 12B2, which binds to the N terminus of PrPres and which overtly detects H-type BSE isolates. Interestingly, SAF32, whose epitope (the octarepeat region) is more N-terminally located, also displays a limited affinity for binding to H-type PrPres if digestion is performed at pH 6.5, while group B antibody 9A2, in comparison to 12B2, which binds only 9 amino acid residues farther toward the C terminus of PrP does, fully binds to the PrPress of all three types of BSE, which is very similar to the signal obtained with antibodies like 94B4, L42, and 6H4. The epitope data and grouping of these antibodies (see Materials and Methods and Results) and the differences in the apparent molecular masses of the PrPres types point to major cleavage sites at approximately position 92 of bovine PrP for the H type, position 103 for the C type, and position 108 for the L type (Fig. 6).
FIG. 6.
Approximate interpretation of major PK cleavage sites in PrPSc for the three types of bovine BSE in relation to the molecular masses and the antibody specificities determined. The epitopes of the antibodies found to be reactive with the three different types of PrPres (the H, C, and L types) and the apparent molecular masses determined by SDS-PAGE for the nonglycosylated moieties in each type are depicted. etc., PrPres extends to the C terminus of mature PrP; black arrows, estimated major PK cleavage sites under standard conditions; vertical hatching, the residual binding site of antibody SAF32 in the H type that remains if PrPSc is cleaved at a minor cleavage site (gray arrow); boPrP, bovine PrP. Antibody P4 shows a high binding affinity if there is a serine at position 106, as in ovine and deer PrP, but not in bovine PrP; antibody 12B2 has a similarly high affinity to the same N-terminal amino acids as P4 but is not dependent on positions 106 and 107. The second population of PrPres discussed for H-type BSE isolates is not included in this illustration.
Cleavage of PrPSc by endoproteases like PK hydrolyzes the N terminus of PrPSc (6, 28, 32, 41, 42, 48). In C-type BSE isolates and under the conditions used here (50 μg PK/ml, 37°C, 10% brain homogenate in lysis buffer), this mainly occurs up to residue 109 of bovine PrP and, thus, between the epitopes of antibodies 12B2 and 9A2. This process, however, also depends on the pH during the reaction, the enzyme/substrate ratio, and also, possibly, the presence of denaturing agents (36, 40). For the BSE isolates studied here, it became clear that C-type BSE isolates are unusually resistant even under the harshest conditions of pH 8.0 and an enzyme concentration of 500 μg/ml. The H- and L-type isolates were more susceptible; however, some residual PrPres always remained, even under the most degradative conditions investigated. This resistance to PK and other subtilisin-like enzymes like keratinase has previously been noticed for scrapie isolate- and BSE isolate-infected brain homogenates, where only denaturation at temperatures well above 100°C allowed the full digestion of detectable PrP (36). For diagnostic purposes, even if the digestion conditions do not lead to a stable PrPres level, differences between BSE types can be reliably visualized by using two different PK cleavage conditions at 37°C: pH 6.5 with 50 μg PK/ml or pH 8.0 with 500 μg PK/ml.
This study serves the important goal of defining adequate tools for the discrimination of BSE types. Striking similarities in molecular properties were encountered for the samples of each BSE type when quite different treatments were applied before analysis by WB. The main features are summarized in Table 2. In practice, the following strategy for the determination of the BSE type (C, H, or L type) is proposed. A homogenate can be divided into two aliquots and titrated to obtain two conditions of digestion with PK: mild and stringent. After the PK digestion the digests are subjected to parallel electrophoresis and WB analysis with group A, B, and C antibodies (MAbs 12B2, L42, and 94B4, respectively) at established antibody concentrations and with similar film exposures. Under mild PK conditions, only the H type overtly binds to MAb 12B2 at an intensity similar to that at which it binds to MAbs L42 and 94B4. In the same blots, a stringent condition/mild condition signal ratio approximating 1 confirms the presence of the C type, while a much lower value is found for the L and H types. Simultaneously, the presence of the L type is confirmed under mild digestion conditions by its special glycoprofile, with approximately equal proportions of diglycosylated PrPres and monoglycosylated PrPres, while the C type shows a clearly higher proportion of the diglycosylated PrPres. The H-type character is further confirmed by subjecting PK digests (at pH 6.5 to 7.4) to digestion with PNGase F, which leads to the unique aspect of two deglycosylated PrPres bands when group C antibody is used.
TABLE 2.
Discrimination between BSE types on the basis of molecular properties of PrPres
| BSE type | Size differencea (kDa) | Binding to Ab 12B2 | Glycoprofileb (% diglyc) | No. of bands after deglycosylation with PNGase Fc | Proteolytic susceptibility at pH 8/pH 6.5d |
|---|---|---|---|---|---|
| C | Reference | No | >50 | 1 | >0.7 |
| H | +1.4 | Yes | Dual charactere | 2 | <0.6 |
| L | −0.3 | No | <50 | 1 | <0.6 |
Approximate difference from the value for the C type for the nonglycosylated band of the PrPres population in the 17- to 19-kDa region; the test was conducted with group B antibody 9A2, L42, or 6H4.
Percentage of diglycosylated (diglyc) fraction compared with that for the C type.
Data represent results obtained with group C antibodies, like 94B4 and SAF84, which bind to the C-terminal domain at positions 163 to 242 of bovine PrP.
See Fig. 3 for the method used to calculate the ratio.
Depending on the use of antibodies of groups A and B or of group C (Fig. 5).
The increasing number of recognized atypical BSE cases represents only a small fraction of the total BSE population. This might well reflect an increased awareness, since the early reports in 2004, of the existence of deviant phenotypes of BSE in laboratories involved in the identification of BSE isolates. In addition, this fraction occurs in older animals and might increase due to the increased age of animals with BSE at the time of detection as a result of the decrease in the level of the BSE epidemic linked to contaminated meat and bone meal. The high incidence of atypical cases in Poland might also have a relation to the higher numbers of aged animals under surveillance. Such cases, including C-type BSE, might also represent previously unnoticed sporadic forms of BSE. With this study and recent publications (8, 14, 17), better awareness and better possibilities for recognition are now available. Sporadic forms of BSE are likely to exist, since even countries with low levels of exposure or unlikely exposure to BSE, like Sweden, Austria, the United States, Japan, and Canada, have detected cases of BSE.
In routine BSE screening, atypical BSE cases (those of the H type and the L type) can, in theory, be missed if the PrPres signals are under the detection levels either due to the application of PK digestion conditions that are too harsh or due to the use of an unsuitable PrP N-terminus-specific antibody (L type). However, it is unlikely that many of these atypical cases have in fact been missed, since the isolates used in this study were detected or their presence was confirmed by the most frequently used commercial screening methods (Table 1). Another concern is the anatomical distribution of the PrPSc deposition in the brain if it differs from that of C-type BSE, in which the obex region is strongly involved in pathology and which is used for routine diagnosis (54). A recent description of an H-type isolate in a zebu showed that the distribution of lesions was similar to that observed in typical BSE (53). In contrast, the Italian L-type case studied here displayed only a comparably weak signal in the obex region, while the thalamus and olfactory bulb were the regions with the highest signals (17). This concern is supported by the fact that it still cannot be predicted whether these cases represent a risk to human health (10). If one or both of the atypical BSE types leads to a human health risk even higher than that presented by C-type BSE, it would be crucial to be able to reliably detect such cases in the bovine population and to differentiate them from C-type BSE.
These data, in any case, might contribute to our understanding of the origin of the BSE epidemic, which remains unresolved and which is also a concern for the future. It is possible that one of such atypical forms could have changed to C-type BSE and was the origin of BSE. It is just as possible that they could have coexisted with C-type BSE as sporadic forms while the epidemic was disguising sporadic cases.
Supplementary Material
Acknowledgments
This study was possible through funds to the group for Ruminant Strain Typing within the NeuroPrion European Network of Excellence (EC FOOD-CT-2004-506579); the Dutch LNV Ministry of Agriculture, Nature and Food Quality (project 8041889000, to J.P.M.L., A.D., and F.G.V.Z.); and other national funding sources.
We acknowledge Berndt Klingeborn, Lena Renström, and Maria Nöremark, SVA, for their involvement with the detection of the Swedish case. We thank Lourens Heres and Linda McPhee for helpful comments on the manuscript.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org.
Published ahead of print on 18 April 2007.
REFERENCES
- 1.Asante, E. A., J. M. Linehan, M. Desbruslais, S. Joiner, I. Gowland, A. L. Wood, J. Welch, A. F. Hill, S. E. Lloyd, J. D. Wadsworth, and J. Collinge. 2002. BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. EMBO J. 21:6358-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron, T., A. G. Biacabe, J. N. Arsac, S. Benestad, and M. H. Groschup. 13 November 2006, posting date. Atypical transmissible spongiform encephalopathies (TSEs) in ruminants. Vaccine. doi: 10.1016/j.vaccine. 2006.10.058. [DOI] [PubMed]
- 3.Baron, T. G., A. G. Biacabe, A. Bencsik, and J. P. Langeveld. 2006. Transmission of new bovine prion to mice. Emerg. Infect. Dis. 12:1125-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beringue, V., A. Bencsik, A. Le Dur, F. Reine, T. L. Lai, N. Chenais, G. Tilly, A. G. Biacabe, T. Baron, J. L. Vilotte, and H. Laude. 2006. Isolation from cattle of a prion strain distinct from that causing bovine spongiform encephalopathy. PLoS Pathog. 2:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessen, R. A., and R. F. Marsh. 1992. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J. Virol. 66:2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessen, R. A., and R. F. Marsh. 1994. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J. Virol. 68:7859-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biacabe, A.-G., J. G. Jacobs, A. Bencsik, J. P. M. Langeveld, and T. G. M. Baron. H-type bovine spongiform encephalopathy: complex molecular features and similarities with human prion diseases. Prion, in press. [DOI] [PMC free article] [PubMed]
- 8.Biacabe, A. G., J. L. Laplanche, S. Ryder, and T. Baron. 2004. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep. 5:110-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, H. R., N. L. Goller, R. D. Rudelli, G. S. Merz, G. C. Wolfe, H. M. Wisniewski, and N. K. Robakis. 1990. The mRNA encoding the scrapie agent protein is present in a variety of non-neuronal cells. Acta Neuropathol. (Berlin) 80:1-6. [DOI] [PubMed] [Google Scholar]
- 10.Brown, P., L. M. McShane, G. Zanusso, and L. Detwiler. 2006. On the question of sporadic or atypical bovine spongiform encephalopathy and Creutzfeldt-Jakob disease. Emerg. Infect. Dis. 12:1816-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruce, M. E., A. Boyle, S. Cousens, I. McConnell, J. Foster, W. Goldmann, and H. Fraser. 2002. Strain characterization of natural sheep scrapie and comparison with BSE. J. Gen. Virol. 83:695-704. [DOI] [PubMed] [Google Scholar]
- 12.Bruce, M. E., and A. G. Dickinson. 1987. Biological evidence that scrapie agent has an independent genome. J. Gen. Virol. 68(Pt 1):79-89. [DOI] [PubMed] [Google Scholar]
- 13.Bueler, H., A. Aguzzi, A. Sailer, R. A. Greiner, P. Autenried, M. Aguet, and C. Weissmann. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339-1347. [DOI] [PubMed] [Google Scholar]
- 14.Buschmann, A., A. Gretzschel, A. G. Biacabe, K. Schiebel, C. Corona, C. Hoffmann, M. Eiden, T. Baron, C. Casalone, and M. H. Groschup. 2006. Atypical BSE in Germany—proof of transmissibility and biochemical characterization. Vet. Microbiol. 117:103-116. [DOI] [PubMed] [Google Scholar]
- 15.Buschmann, A., and M. H. Groschup. 2005. Highly bovine spongiform encephalopathy-sensitive transgenic mice confirm the essential restriction of infectivity to the nervous system in clinically diseased cattle. J. Infect. Dis. 192:934-942. [DOI] [PubMed] [Google Scholar]
- 16.Casalone, C., M. Caramelli, M. I. Crescio, Y. I. Spencer, and M. M. Simmons. 2006. BSE immunohistochemical patterns in the brainstem: a comparison between UK and Italian cases. Acta Neuropathol. (Berlin) 111:444-449. [DOI] [PubMed] [Google Scholar]
- 17.Casalone, C., G. Zanusso, P. Acutis, S. Ferrari, L. Capucci, F. Tagliavini, S. Monaco, and M. Caramelli. 2004. Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc. Natl. Acad. Sci. USA 101:3065-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castilla, J., A. Gutierrez Adan, A. Brun, B. Pintado, M. A. Ramirez, B. Parra, D. Doyle, M. Rogers, F. J. Salguero, C. Sanchez, J. M. Sanchez-Vizcaino, and J. M. Torres. 2003. Early detection of PrPres in BSE-infected bovine PrP transgenic mice. Arch. Virol. 148:677-691. [DOI] [PubMed] [Google Scholar]
- 19.Caughey, B., R. E. Race, and B. Chesebro. 1988. Detection of prion protein mRNA in normal and scrapie-infected tissues and cell lines. J. Gen. Virol. 69(Pt 3):711-716. [DOI] [PubMed] [Google Scholar]
- 20.Caughey, B., G. J. Raymond, and R. A. Bessen. 1998. Strain-dependent differences in beta-sheet conformations of abnormal prion protein. J. Biol. Chem. 273:32230-32235. [DOI] [PubMed] [Google Scholar]
- 21.Chu, F. K. 1986. Requirements of cleavage of high mannose oligosaccharides in glycoproteins by peptide N-glycosidase F. J. Biol. Chem. 261:172-177. [PubMed] [Google Scholar]
- 22.Debeer, S., T. Baron, and A. Bencsik. 2003. Neuropathological characterisation of French bovine spongiform encephalopathy cases. Histochem. Cell Biol. 120:513-521. [DOI] [PubMed] [Google Scholar]
- 23.De Bosschere, H., S. Roels, and E. Vanopdenbosch. 2004. Atypical case of bovine spongiform encephalopathy in an East-Flemish cow in Belgium. Int. J. Appl. Res. 2:52-54. [Google Scholar]
- 24.European Communities. 2001. Rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies (and amendments). European Parliament and the Council of the European Union, regulation (EC) no. 999/2001. Off. J. Eur. Communities, 31 May 2001. http://eur-lex.europa.eu/LexUriServ/site/en/oj/2001/l_147/l_14720010531en00010040.pdf.
- 25.Feraudet, C., N. Morel, S. Simon, H. Volland, Y. Frobert, C. Creminon, D. Vilette, S. Lehmann, and J. Grassi. 2005. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J. Biol. Chem. 280:11247-11258. [DOI] [PubMed] [Google Scholar]
- 26.Fraser, H., and A. G. Dickinson. 1968. The sequential development of the brain lesion of scrapie in three strains of mice. J. Comp. Pathol 78:301-311. [DOI] [PubMed] [Google Scholar]
- 27.Geysen, H. M., R. H. Meloen, and S. J. Barteling. 1984. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc. Natl. Acad. Sci. USA 81:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldmann, W., N. Hunter, T. Martin, M. Dawson, and J. Hope. 1991. Different forms of the bovine PrP gene have five or six copies of a short, G-C-rich element within the protein-coding exon. J. Gen. Virol. 72(Pt 1):201-204. [DOI] [PubMed] [Google Scholar]
- 29.Green, R., C. Horrocks, A. Wilkinson, S. A. Hawkins, and S. J. Ryder. 2005. Primary isolation of the bovine spongiform encephalopathy agent in mice: agent definition based on a review of 150 transmissions. J. Comp. Pathol 132:117-131. [DOI] [PubMed] [Google Scholar]
- 30.Griffith, J. S. 1967. Self-replication and scrapie. Nature 215:1043-1044. [DOI] [PubMed] [Google Scholar]
- 31.Harmeyer, S., E. Pfaff, and M. H. Groschup. 1998. Synthetic peptide vaccines yield monoclonal antibodies to cellular and pathological prion proteins of ruminants. J. Gen. Virol. 79(Pt 4):937-945. [DOI] [PubMed] [Google Scholar]
- 32.Hope, J., L. J. Morton, C. F. Farquhar, G. Multhaup, K. Beyreuther, and R. H. Kimberlin. 1986. The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J. 5:2591-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimberlin, R. H., S. Cole, and C. A. Walker. 1986. Transmissible mink encephalopathy (TME) in Chinese hamsters: identification of two strains of TME and comparisons with scrapie. Neuropathol. Appl. Neurobiol. 12:197-206. [DOI] [PubMed] [Google Scholar]
- 34.Korth, C., B. Stierli, P. Streit, M. Moser, O. Schaller, R. Fischer, W. Schulz-Schaeffer, H. Kretzschmar, A. Raeber, U. Braun, F. Ehrensperger, S. Hornemann, R. Glockshuber, R. Riek, M. Billeter, K. Wuthrich, and B. Oesch. 1997. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390:74-77. [DOI] [PubMed] [Google Scholar]
- 35.Langeveld, J. P., J. G. Jacobs, J. H. Erkens, A. Bossers, F. G. van Zijderveld, and L. J. van Keulen. 2006. Rapid and discriminatory diagnosis of scrapie and BSE in retro-pharyngeal lymph nodes of sheep. BMC Vet. Res. 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langeveld, J. P., J. J. Wang, D. F. Van de Wiel, G. C. Shih, G. J. Garssen, A. Bossers, and J. C. Shih. 2003. Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. J. Infect. Dis. 188:1782-1789. [DOI] [PubMed] [Google Scholar]
- 37.Lezmi, S., A. Bencsik, and T. Baron. 2006. PET-blot analysis contributes to BSE strain recognition in C57BL/6 Mice. J. Histochem. Cytochem. 54:1087-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd, S. E., J. M. Linehan, M. Desbruslais, S. Joiner, J. Buckell, S. Brandner, J. D. Wadsworth, and J. Collinge. 2004. Characterization of two distinct prion strains derived from bovine spongiform encephalopathy transmissions to inbred mice. J. Gen. Virol. 85:2471-2478. [DOI] [PubMed] [Google Scholar]
- 39.Nonno, R., E. Esposito, G. Vaccari, M. Conte, S. Marcon, M. Di Bari, C. Ligios, G. Di Guardo, and U. Agrimi. 2003. Molecular analysis of cases of Italian sheep scrapie and comparison with cases of bovine spongiform encephalopathy (BSE) and experimental BSE in sheep. J. Clin. Microbiol. 41:4127-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Notari, S., S. Capellari, A. Giese, I. Westner, A. Baruzzi, B. Ghetti, P. Gambetti, H. A. Kretzschmar, and P. Parchi. 2004. Effects of different experimental conditions on the PrPSc core generated by protease digestion: implications for strain typing and molecular classification of CJD. J. Biol. Chem. 279:16797-16804. [DOI] [PubMed] [Google Scholar]
- 41.Oesch, B., D. Westaway, M. Walchli, M. P. McKinley, S. B. Kent, R. Aebersold, R. A. Barry, P. Tempst, D. B. Teplow, L. E. Hood, et al. 1985. A cellular gene encodes scrapie PrP 27-30 protein. Cell 40:735-746. [DOI] [PubMed] [Google Scholar]
- 42.Parchi, P., W. Zou, W. Wang, P. Brown, S. Capellari, B. Ghetti, N. Kopp, W. J. Schulz-Schaeffer, H. A. Kretzschmar, M. W. Head, J. W. Ironside, P. Gambetti, and S. G. Chen. 2000. Genetic influence on the structural variations of the abnormal prion protein. Proc. Natl. Acad. Sci. USA 97:10168-10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peretz, D., R. A. Williamson, G. Legname, Y. Matsunaga, J. Vergara, D. R. Burton, S. J. DeArmond, S. B. Prusiner, and M. R. Scott. 2002. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34:921-932. [DOI] [PubMed] [Google Scholar]
- 44.Plummer, T. H., Jr., and A. L. Tarentino. 1981. Facile cleavage of complex oligosaccharides from glycopeptides by almond emulsin peptide: N-glycosidase. J. Biol. Chem. 256:10243-10246. [PubMed] [Google Scholar]
- 45.Polak, M., W. Rozek, J. Rola, and J. F. Zmudzinski. 2004. Prion protein glycoforms from BSE cases in Poland. Bull. Vet. Inst. Pulawy 48:201-205. [Google Scholar]
- 46.Prusiner, S. B. 1982. Research on scrapie. Lancet ii:494-495. [DOI] [PubMed] [Google Scholar]
- 47.Prusiner, S. B., D. Groth, A. Serban, R. Koehler, D. Foster, M. Torchia, D. Burton, S. L. Yang, and S. J. DeArmond. 1993. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc. Natl. Acad. Sci. USA 90:10608-10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prusiner, S. B., D. F. Groth, D. C. Bolton, S. B. Kent, and L. E. Hood. 1984. Purification and structural studies of a major scrapie prion protein. Cell 38:127-134. [DOI] [PubMed] [Google Scholar]
- 49.Richt, J. A., R. A. Kunkle, D. Alt, E. M. Nicholson, A. N. Hamir, S. Czub, J. P. Kluge, A. J. Davis, and S. M. Hall. 2007. Identification and characterisation of two bovine spongiform encephalopathy cases diagnosed in the United States. J. Vet. Diagn. Investig. 19:142-154. [DOI] [PubMed] [Google Scholar]
- 50.Safar, J., H. Wille, V. Itri, D. Groth, H. Serban, M. Torchia, F. E. Cohen, and S. B. Prusiner. 1998. Eight prion strains have PrP(Sc) molecules with different conformations. Nat. Med. 4:1157-1165. [DOI] [PubMed] [Google Scholar]
- 51.Sailer, A., H. Bueler, M. Fischer, A. Aguzzi, and C. Weissmann. 1994. No propagation of prions in mice devoid of PrP. Cell 77:967-968. [DOI] [PubMed] [Google Scholar]
- 52.Scott, M. R., J. Safar, G. Telling, O. Nguyen, D. Groth, M. Torchia, R. Koehler, P. Tremblay, D. Walther, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 1997. Identification of a prion protein epitope modulating transmission of bovine spongiform encephalopathy prions to transgenic mice. Proc. Natl. Acad. Sci. USA 94:14279-14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seuberlich, T., C. Botteron, C. Wenker, V. Café-Marcal, A. Oevermann, B. Haase, T. Leeb, D. Heim, and A. Zurbriggen. 2006. Spongiform encephalopathy in a miniature zebu. Emerg. Infect. Dis. 12:1950-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons, M. M., P. Harris, M. Jeffrey, S. C. Meek, I. W. Blamire, and G. A. Wells. 1996. BSE in Great Britain: consistency of the neurohistopathological findings in two random annual samples of clinically suspect cases. Vet. Rec. 138:175-177. [DOI] [PubMed] [Google Scholar]
- 55.Spassov, S., M. Beekes, and D. Naumann. 2006. Structural differences between TSEs strains investigated by FT-IR spectroscopy. Biochim. Biophys. Acta 1760:1138-1149. [DOI] [PubMed] [Google Scholar]
- 56.Telling, G. C., P. Parchi, S. J. DeArmond, P. Cortelli, P. Montagna, R. Gabizon, J. Mastrianni, E. Lugaresi, P. Gambetti, and S. B. Prusiner. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079-2082. [DOI] [PubMed] [Google Scholar]
- 57.Thomzig, A., S. Spassov, M. Friedrich, D. Naumann, and M. Beekes. 2004. Discriminating scrapie and bovine spongiform encephalopathy isolates by infrared spectroscopy of pathological prion protein. J. Biol. Chem. 279:33847-33854. [DOI] [PubMed] [Google Scholar]
- 58.Thuring, C. M., J. H. Erkens, J. G. Jacobs, A. Bossers, L. J. Van Keulen, G. J. Garssen, F. G. Van Zijderveld, S. J. Ryder, M. H. Groschup, T. Sweeney, and J. P. Langeveld. 2004. Discrimination between scrapie and bovine spongiform encephalopathy in sheep by molecular size, immunoreactivity, and glycoprofile of prion protein. J. Clin. Microbiol. 42:972-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wells, G. A., A. C. Scott, C. T. Johnson, R. F. Gunning, R. D. Hancock, M. Jeffrey, M. Dawson, and R. Bradley. 1987. A novel progressive spongiform encephalopathy in cattle. Vet. Rec. 121:419-420. [DOI] [PubMed] [Google Scholar]
- 60.Wilesmith, J. W., G. A. Wells, M. P. Cranwell, and J. B. Ryan. 1988. Bovine spongiform encephalopathy: epidemiological studies. Vet. Rec. 123:638-644. [PubMed] [Google Scholar]
- 61.Yamakawa, Y., K. Hagiwara, K. Nohtomi, Y. Nakamura, M. Nishijima, Y. Higuchi, Y. Sato, and T. Sata. 2003. Atypical proteinase K-resistant prion protein (PrPres) observed in an apparently healthy 23-month-old Holstein steer. Jpn. J. Infect. Dis. 56:221-222. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.