Abstract
Over a 2-year period (2003 to 2005) patients with community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) and community-acquired methicillin-susceptible Staphylococcus aureus (CA-MSSA) infections were prospectively identified. Patients infected with CA-MRSA (n = 102 patients) and CA-MSSA (n = 102 patients) had median ages of 46 and 53 years, respectively; the most common sites of infection in the two groups were skin/soft tissue (80 and 93%, respectively), respiratory tract (13 and 6%, respectively), and blood (4 and 1%, respectively). Fourteen percent of patients with CA-MRSA infections and 3% of patients with CA-MSSA infections had household contacts with similar infections (P < 0.01). Among the CA-MRSA isolates, the pulsed-field gel electrophoresis (PFGE) groups detected were USA300 (49%) and USA100 (13%), with 27 PFGE groups overall; 71% of the isolates were staphylococcal chromosome cassette mec (SCCmec) type IV, 29% were SCCmec type II, and 54% had the Panton-Valentine leucocidin (PVL) gene. Among the CA-MSSA isolates there were 33 PFGE groups, with isolates of the USA200 group comprising 11%, isolates of the USA600 group comprising 11%, isolates of the USA100 group comprising 10%, and isolates of the PVL type comprising 10%. Forty-six and 18% of the patients infected with CA-MRSA and CA-MSSA, respectively, were hospitalized (P < 0.001). Fifty percent of the patients received antibiotic therapy alone, 5% received surgery alone, 30% received antibiotics and surgery, 3% received other therapy, and 12% received no treatment. The median durations of antibiotic therapy were 12 and 10 days in the CA-MRSA- and CA-MSSA-infected patients, respectively; 48 and 56% of the patients in the two groups received adequate antimicrobial therapy, respectively (P < 0.001). The clinical success rates of the initial therapy in the two groups were 61 and 84%, respectively (P < 0.001); recurrences were more common in the CA-MRSA group (recurrences were detected in 18 and 6% of the patients in the two groups, respectively [P < 0.001]). CA-MRSA was an independent predictor of clinical failure in multivariate analysis (odds ratio, 3.4; 95% confidence interval, 1.7 to 6.9). In the community setting, the molecular characteristics of the S. aureus strains were heterogeneous. CA-MRSA infections were associated with a more adverse impact on outcome than CA-MSSA infections.
Methicillin-resistant Staphylococcus aureus (MRSA) is well recognized as a major cause of nosocomial infection worldwide. The risk factors for infection with these pathogens that are unique to the hospital population are well established (22). The emergence of MRSA as a cause of infection in the community in patients who have never been hospitalized and who have no other known risk factors for MRSA infection is a significant concern. Infections caused by these organisms have been described in earlier studies as having distinctive strain, virulence, and epidemiologic properties (8, 25, 34). Community-associated MRSA (CA-MRSA) strains differ from health care-associated S. aureus strains in that they are more frequently recovered from skin and soft tissue sources, with at least two clones, designated USA300 and USA400, that are widely and geographically disseminated having been described; these clones contain staphylococcal chromosome cassette mec (SCCmec) type IVa and produce the virulence factor Panton-Valentine leucocidin (PVL) (23, 28, 34). Although pediatric subjects first received worldwide attention for the acquisition of CA-MRSA that resulted in death (2), studies have also been conducted with adult subjects to determine the risk factors and prevalence of CA-MRSA and to identify the populations associated with outbreaks (2-5, 16). The risk factors or groups that have previously been described to be associated with CA-MRSA infection rather than health care-associated MRSA infection are younger age, sports team participation, men who have sex with men, incarceration, Alaska natives, and military recruits (2-5, 13, 14, 16, 19, 37). As the number of these infections has increased dramatically in the United States and elsewhere in the last few years in individuals who are not in these defined risk groups, additional information is urgently needed on the other risks involved and other distinctive epidemiologic features to better define the epidemiology and outcomes of CA-MRSA infections to better assist with the definition of effective treatment and control measures. The objectives of this prospective observational, controlled study were therefore to evaluate the molecular epidemiology of methicillin resistance, to determine the risk factors for methicillin resistance, and to evaluate the impact of CA-MRSA on the outcomes of patients with community-associated S. aureus infections.
(This research was partially presented in abstract form at the 5th International Conference on Emerging Infectious Diseases, Atlanta, GA, 2006, and the 16th European Congress on Clinical Microbiology and Infectious Disease, Nice, France, 2006.)
MATERIALS AND METHODS
We performed a case-control study with a cohort of patients with community-onset S. aureus infection. The study was conducted at four teaching hospitals in the midwestern United States (William Beaumont Hospital, Royal Oak, MI; Henry Ford Hospital, Detroit, MI; Pittsburgh Veterans Administration Medical Center, Pittsburgh, PA; and Rush University Medical Center, Chicago, IL) from October 2003 to 2005. Institutional review board approval was obtained from all participating centers.
Subject participant identification.
Patients were prospectively identified from review of microbiology reports for clinical cultures positive for S. aureus. MRSA prevalence was determined from a review of the microbiology laboratory records over the period of study (4,060 patients). Eligible subjects were those with at least one sample from any site that was culture positive for S. aureus who met the following three criteria for community-associated S. aureus: (i) the sample for culture was obtained during an outpatient visit or within 48 h of hospitalization; (ii) the subject had not been admitted to a hospital, nursing home, or any other long-term care facility within the last 1 year; and (iii) the subject had no history within the past year of known risk factors for MRSA, including current intravenous drug use, surgery, dialysis, an indwelling catheter, or a percutaneous medical device. Patients gave their informed consent that anonymous data would be collected and entered into the database.
Randomly selected patients were included in study groups according to the results of in vitro testing for susceptibility to methicillin of the first isolate recovered from the patient, and informed consent was obtained. Each patient infected with CA-MRSA was matched with one control subject with a community-associated methicillin-susceptible S. aureus (CA-MSSA) infection from the same time period. The control subjects were not matched to the case patients by any clinical or demographic characteristics.
Clinical data collection.
Clinical data were collected from a review of medical records and patient interviews. Each participant was administered a questionnaire at the time of enrollment to collect information on demographics, medical history, antimicrobial use, hospitalization, comorbid conditions, and the outcomes of infection. Adequate antimicrobial therapy was defined as the receipt of an agent with in vitro activity against the isolated organism. Outcomes were assessed by investigators blinded to the infecting S. aureus strain characteristics and were defined according to the following scheme: cure, complete resolution of the infection after completion of the antibiotic treatment; failure, persistence of the infection and requirement of an additional intervention or a change in antibiotic therapy; relapse, resolution of the infection after treatment and the appearance of new symptoms within 2 weeks after the completion of antibiotic therapy; recurrence, development of an infection at the same site or a different site after 2 weeks of completion of treatment; and indeterminate, cases with unknown outcomes. Patients were hospitalized and managed at the discretion of the clinicians caring for the patient. Death was considered attributable to S. aureus infection if at least one of the following criteria were present: (i) cultures were positive for S. aureus at the time of death, (ii) death occurred before the resolution of the signs and symptoms of S. aureus infection, or (iii) death occurred within 2 weeks of the onset of the S. aureus infection without another explanation (21).
Microbiological data methods.
The initial identification of isolates and identification of their in vitro susceptibilities were determined by the clinical microbiology laboratories at each participating institution by automated dilution testing by the use of Clinical and Laboratory Standards Institute (formerly NCCLS) standards (1, 6, 29). In vitro susceptibility to clindamycin was determined by using the D-test (clindamycin induction) methodology. Molecular epidemiologic methods included comparison of pulsed-field gel electrophoresis (PFGE) patterns, PCR detection of the PVL gene, and SCCmec types (23, 33, 34). Data for all the S. aureus isolates were entered into a database by using the Gel Doc 2000 gel documentation system (Bio-Rad), and the PFGE patterns were compared by using Bionumerics software. Isolates were considered to be in the same PFGE group if they had greater than 80% similarity by using the Dice coefficient (23, 33, 34). Isolates were classified into PFGE groups by using the CDC classification system of McDougal and colleagues (23, 34).
Statistical analysis.
The methods of bivariate comparisons included the chi-square, Student's t, and Mann-Whitney U tests. Forward stepwise logistic regression was performed to evaluate the independent association of MRSA with risk factors and outcomes. A total of 102 patients were enrolled in each group to achieve a sample size expected to detect a 10% difference in outcomes (90% power, alpha value of 0.05) and to provide an adequate size for multivariate analysis of the risk factors. Data were analyzed with SPSS software (version 14.0; SPSS).
RESULTS
General.
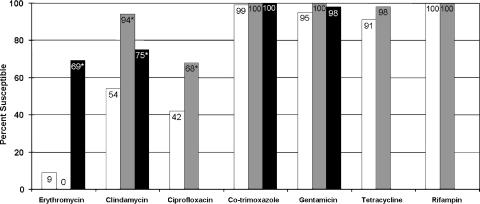
Over the study period, the prevalence of MRSA among community-associated S. aureus infections was 26.7%. There were 102 patients with CA-MRSA infections and 102 patients with CA-MSSA infections. The demographic characteristics and underlying conditions of these patients are summarized in Table 1. The skin was the most common site of infection for both groups of patients: CA-MRSA, 80%; CA-MSSA, 93%. Other infection sites included the respiratory tract (13% for CA-MRSA-infected patients and 6% for CA-MSSA-infected patients), blood (4% for CA-MRSA-infected patients and 1% for CA-MSSA-infected patients), and urine (3% for CA-MRSA-infected patients). The results of in vitro susceptibility testing are presented in Fig. 1.
TABLE 1.
Demographic characteristics of patients with community-acquired S. aureus infections
| Characteristic | CA-MRSA (n = 102) | CA-MSSA (n = 102) | P |
|---|---|---|---|
| Median (range) age (yr) | 46 (3-88) | 53 (3-82) | |
| Percentage of patients with the following characteristics: | |||
| Male gender | 58 | 57 | |
| Location when sample for culture was collected | <0.001 | ||
| Emergency department | 15 | 16 | |
| Outpatient clinic | 58 | 77 | |
| Hospital Within 48 h of hospitalization | 27 | 8 | |
| Multiple outpatient visits | 60 | 65 | |
| Multiple emergency visits | 22 | 24 | |
| Contact with a health care employee | 19 | 21 | |
| Contact with a hospitalized patient | 30 | 33 | |
| Men who have sex with men | 5 | 0 | 0.007 |
| Incarceration | 3 | 0 | |
| Prior staphylococcal infection | 27 | 21 | |
| Spider bite | 12 | 17 | |
| Any pet | 50 | 53 | |
| Gardening activity | 15 | 28 | 0.026 |
| Sports participation | 18 | 30 | 0.049 |
| Health club contact | 14 | 0 | <0.001 |
| Travel | 45 | 50 | |
| Cardiovascular disease | 14 | 13 | |
| Diabetes | 14 | 22 | |
| Chronic skin disease | 7 | 9 | |
| Renal disease | 4 | 1 | |
| Influenza | 3 | 5 | |
| Pulmonary disease | 19 | 4 | 0.001 |
| Malignancy | 6 | 4 | |
| Human immunodeficiency virus infection | 4 | 0 | |
| Neurological disease | 3 | 3 | |
| Concurrent infection | 19 | 14 | |
| No. of antibacterial classes used in past 1 yr | 0.038 | ||
| None | 46 | 54 | |
| One | 40 | 42 | |
| Two | 10 | 4 | |
| Three or more | 5 | 0 |
FIG. 1.
In vitro antimicrobial susceptibility of community-associated S. aureus. White bars, CA-MRSA (n = 102); gray bars, USA300 subset of CA-MRSA (n = 49); black bars, CA-MSSA (n = 102). The susceptibilities of the CA-MSSA isolates to ciprofloxacin, tetracycline, and rifampin were not tested. *, P < 0.05 compared with the results for CA-MRSA.
Molecular epidemiology.
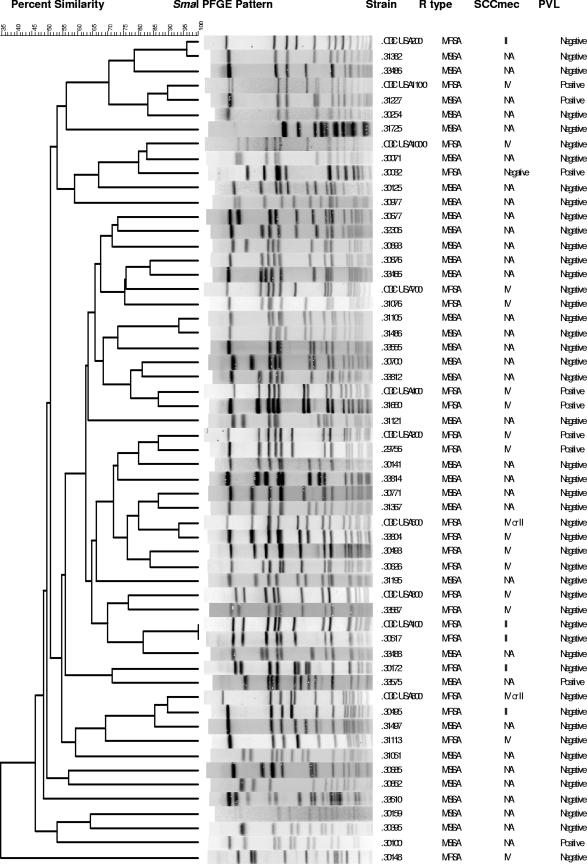
The results of PFGE dendrogram analysis are shown in Fig. 2. Overall, 27 different PFGE groups were identified in the CA-MRSA group and 33 PFGE groups were identified in the CA-MSSA group (Table 2). The predominant strain types associated with CA-MRSA infection were USA300 from 49 patients and USA100 from 13 patients. The most common CA-MSSA PFGE strain types were USA200 in 11 patients, USA600 in 11 patients, and USA100 in 10 patients. USA300 and USA400 strains were each found in two patients with CA-MSSA infections. Other PFGE groups included 23 CA-MRSA strain types and 25 CA-MSSA strain types that did not correspond to the USA types previously described in the literature. All USA300 and USA400 MRSA isolates were SCCmec type IVa. Among the other 20 MRSA isolates that were SCCmec type IV, 7 were SCCmec type IVa, 1 was type IVb, and the rest were not type IVa to IVc. The PVL gene was present in 54% of the CA-MRSA isolates and 10% of the CA-MSSA isolates. No specific clone was associated with gardening.
FIG. 2.
Dendrogram of CA-MRSA and CA-MSSA isolates showing PFGE results, SCCmec types, PVL presence, and resistance (R) type (the control strains used were from CDC [23]).
TABLE 2.
Molecular epidemiologic characteristics of CA-MRSA and CA-MSSA isolates
| Organism and USA type | No. of isolates | No. (%) of isolates with the following PVL gene result:
|
No. (%) of isolates of SCCmec type b:
|
||
|---|---|---|---|---|---|
| Positive | Negative | II | IV | ||
| CA-MSSA | |||||
| 100 | 10 | 1 | 9 | ||
| 200 | 11 | 1 | 10 | ||
| 300 | 2 | 2 | 0 | ||
| 400 | 2 | 0 | 2 | ||
| 500 | 1 | 0 | 1 | ||
| 600 | 11 | 0 | 11 | ||
| 700 | 5 | 0 | 5 | ||
| 1100 | 1 | 1 | 0 | ||
| Othera | 59 | 5 | 54 | ||
| Total | 102 | 10 (10) | 92 (90) | ||
| CA-MRSA | |||||
| 100 | 13 | 0 | 13 | 13 | 0 |
| 300 | 49 | 49 | 0 | 0 | 49 |
| 400 | 2 | 2 | 0 | 0 | 2 |
| 600 | 3 | 0 | 3 | 1 | 2 |
| Otherb | 35 | 4 | 31 | 16 | 18 |
| Total | 102 | 55 (54) | 47 (46) | 30 (29) | 71 (70) |
Other includes 23 strain types of CA-MRSA and 25 strain types of CA-MSSA.
One isolate was non-SCCmec I, II, III, or IV.
Risk factors and outcomes.
The characteristics associated with CA-MRSA infection by univariate comparisons included participation in a health club, the collection of samples for culture during the first 48 h of hospital admission (in comparison to sample collection as an outpatient), pulmonary disease, and prior use of antibacterial agents (Table 1). Gardening and sports participation were associated with CA-MSSA infection (Table 1). By multivariate regression analysis, pulmonary disease, prior use of antibacterial agents, and identification of the infection after hospital admission were associated with CA-MRSA infections, while participation in gardening predicted CA-MSSA infection (Table 3). In addition, 14 patients with CA-MRSA infections reported that members of their households had similar infections, and 3 of those with CA-MSSA infections reported household contacts with similar infections (P < 0.01). Of the patients with CA-MRSA infections, 80% received antimicrobial therapy, whereas 64% of those with CA-MSSA infections received antimicrobial therapy (P = 0.018). Patients in both groups received a wide variety of antibacterial agents; however, the patients with CA-MRSA infections tended to be less likely to receive adequate initial antibacterial therapy (48% and 56% for the CA-MRSA and CA-MSSA groups, respectively; P was not significant). Beta-lactams were the most common initial antimicrobial therapy in both the CA-MRSA and the CA-MSSA groups (29% and 35%, respectively), followed by vancomycin (28% and 7%, respectively).
TABLE 3.
Results of multivariate analysis of risk factors for CA-MRSA infection
| Risk factor for CA-MRSA infection | Odds ratio | 95% confidence interval | P |
|---|---|---|---|
| Prior antibioticsa | 1.835 | 1.156-2.912 | 0.010 |
| Pulmonary disease | 4.975 | 1.558-15.871 | 0.007 |
| Identified after hospital admission (within 48 h) | 3.922 | 1.628-9.434 | 0.002 |
| Gardening (associated with CA-MSSA) | 0.432 | 0.201-.924 | 0.030 |
The odds ratio is per each additional antibiotic class.
Forty-six percent of patients with CA-MRSA infection and 18% of patients with CA-MSSA infection were hospitalized (P < 0.001). The cure rate at the end of therapy for the CA-MRSA patients was 61%, and that for the CA-MSSA patients was 84% (P < 0.001). The median durations of therapy were 12 days (range, 3 to 42 days) and 10 days (range, 5 to 84 days) for the two groups, respectively. Recurrence or relapse was also more common in the CA-MRSA group (18%, compared with 6% in the CA-MSSA group; P < 0.015). There was one infection-related death of a patient with a rapidly progressive necrotizing CA-MRSA pneumonia. By multivariate regression analysis by comparison of the organism, the receipt of active antimicrobial therapy (with in vitro activity against the organism isolated), and the patient's underlying illness (pulmonary disease), only the presence of CA-MRSA was found to be significantly associated with clinical failure (odds ratio, 3.4; 95% confidence interval, 1.7 to 6.9; P < 0.001). Furthermore, in a subset analysis of patients with CA-MRSA skin and soft tissue infections who received antimicrobial therapy (n = 65), the cure rates tended to be higher among patients receiving active antimicrobial therapy (61% versus 38% for patients not receiving active antimicrobial therapy; P was not significant), regardless of whether incision and drainage were performed.
DISCUSSION
Staphylococcus aureus is among the top two causes of nosocomial bloodstream infections in the United States, is the etiologic agent in 47% to 52% of these infections, and is responsible for as many as 50% of all nosocomial infections (22). The prevalence of community-associated S. aureus infection varies by geographic area, however, with the reported rates of methicillin resistance among S. aureus isolates being up to 70 percent (11, 26, 27, 32) and with a CA-MRSA disease incidence of 18.0 to 25.7 cases per 100,000 population in the United States (11). Despite an increasing number of reports of CA-MRSA infections, in earlier studies limited controlled information was available on their epidemiologies and outcomes (10, 18, 31). Earlier retrospective trials have the limitation of relying on the patients’ medical records for the known hospital-associated MRSA risk factors, but these may not have been captured in the patients’ records. Also, in some studies limited molecular information was available for assessment of the epidemiology relative to the molecular characteristics of the strain. The published prospective trials have mostly been surveillance programs conducted in the community outside of health care facilities. In those studies, the prevalence rate of MRSA was significantly lower than those reported from retrospective studies. Some studies were done with very select, otherwise healthy subjects at locations such as schools, day care centers, homeless shelters, and military bases. Therefore, it is difficult to fully understand the scope of the problem and its impact on the health care received by other populations.
The present study extends the findings of earlier studies by prospectively evaluating the epidemiology and the outcomes of patients with CA-MRSA infections compared to those of patients with CA-MSSA infections. This study was done in the midwestern United States, a geographic area where MRSA has been known to exist in the community and hospitals since the 1970s (7, 20, 30). In this study, we found, after a review of medical records and patient questionnaires, that over a 2-year period from 2003 to 2005 the overall prevalence of MRSA was 26.7% among patients with community-associated S. aureus infections and no previously identified risk factors for MRSA infection.
Patients with CA-MRSA and CA-MSSA infections were similar in age, and the sites of infection were similar between the two groups. In univariate comparisons we found a number of characteristics that are associated with CA-MRSA infections, including men who have sex with men, pulmonary disease, and health club attendance; gardening was a more common risk factor in patients with CA-MSSA infections. No predominant clone was associated with gardening. Although a history of “spider bite” is commonly cited by patients with CA-MRSA infections, the prevalence of a history of “insect or spider bite” was not significantly different overall between the two groups in our study. Prior antimicrobial use was common in both CA-MRSA-infected patients (54%) and CA-MSSA-infected patients (46%); however, patients with CA-MRSA infections were more likely to have received multiple classes of antimicrobial agents in the previous year.
This study demonstrates that strains of S. aureus from patients documented to have community-associated infections had more molecular diversity than that described in earlier studies. In earlier investigations of clusters of CA-MRSA infections among people in U.S. prisons, athletes, military recruits, and children, isolates had indistinguishable PFGE patterns, clearly showing that a single highly stable strain is widely disseminated across the United States (34). This strain has been designated USA300-0114 and is exclusively SCCmec type IVa. The isolate is resistant only to beta-lactams and erythromycin, although plasmid-mediated resistance markers, such as markers for resistance to tetracycline and clindamycin, mediated by tet(K), and ermC, respectively, are starting to appear. In the present study we found that there was variability in the presence of the PVL gene, the SCCmec types, and the PFGE types of isolates from patients infected with CA-MRSA and CA-MSSA. Of 102 CA-MRSA isolates, 49% were USA300 and 13% were USA100. USA400 was uncommon. There were 27 PFGE strain types overall, including 23 CA-MRSA strain types not previously described in the CDC USA strain classification system described by McDougal and colleagues (23, 34). SCCmec type IV was the most common and was found in 71% of the isolates, followed by SCCmec type II in 29% of the isolates. Fifty-four percent of the isolates were positive for the PVL gene. Among the CA-MSSA strains there were 33 PFGE groups (25 previously undescribed groups); the most common PFGE groups were USA200 in 11% of the isolates, USA600 in 11%, and USA100 in 10%. USA300 strains were found in two patients with MSSA infections, and USA400 strains were found in two patients with MSSA infections. The PVL gene was present in 10% of the CA-MSSA strains. The undescribed PFGE groups were not believed to be subtypes of strains described earlier, as they differed from the other strains by greater than 20% by use of the Dice coefficient. These data differ from those from earlier studies that showed that only two CA-MRSA clones exist in the community (34, 35). In this study, we excluded injection drug users or others with known MRSA risk factors. These data therefore suggest that in a geographic area where MRSA has been known to be common for years, MRSA strains of a clonal origin formerly believed to be present only in injection drug users or individuals with health care contacts are also associated with community-acquired illness in some patients. These findings suggest that predominant nosocomial clones have somehow found their way into the community. The data from this study also suggest some similarities of CA-MSSA with CA-MRSA, since some isolates had clonal similarities by PFGE group and the presence of the PVL gene, supporting the findings of earlier studies that CA-MRSA may have an ancestral origin from MSSA isolates in the community.
The natural reservoir for CA-MRSA and its degree of transmissibility, including the risks and prevalence of transmission in the household setting, are undetermined. Also undetermined are the properties of virulent strains. A variety of virulence factors have been identified in these strains, with some studies showing that the presence of the PVL gene is a virulence factor (12, 17, 35) but others not showing this (36). A number of recent studies have reported a variety of serious infections, including necrotizing fasciitis, pneumonia, and death, as a result of infection with USA300 and USA400 strains (12, 15, 24). In a study of 812 U.S. Army soldiers (9), 3% of the participants were colonized with CA-MRSA; of that 3%, 38% developed soft tissue infection during a 10-week study period. In contrast, 28% were colonized with MSSA. Of the MSSA-colonized participants in the study, 3% developed infections during the study period (P < 0.001), suggesting that CA-MRSA is more virulent than MSSA. Importantly, in the present study, 14% of patients with CA-MRSA infections reported that members of their households had similar infections and 3% of those with CA-MSSA infections reported that household contacts had similar infections. In this study, 46% of patients infected with CA-MRSA were hospitalized for the management of the infection, whereas only 18% of those infected with CA-MSSA were hospitalized for the management of the infection (P < 0.001). There was one death, which occurred in a patient with CA-MRSA infection and necrotizing pneumonia who failed therapy with vancomycin.
Earlier studies have shown that many patients with CA-MRSA skin and soft tissue infections do not receive initial therapy with in vitro activity against MRSA (11, 26, 28); however, the receipt of inactive therapy does not always appear to have an adverse effect on outcome. In the present study, patients infected with CA-MRSA were more likely to be treated with antimicrobial therapy than those infected with CA-MSSA. Both groups were treated with a wide variety of agents, and patients with CA-MRSA infections were less likely to receive adequate initial therapy. Although it was not the primary end point of this study, it was observed that patients with CA-MRSA infections receiving active antimicrobial therapy were more likely to achieve clinical cure than those receiving inactive therapy. Importantly, regardless of the infection type, underlying illnesses, and antimicrobial therapy, CA-MRSA infections were found to be independently associated with worse outcomes.
The results of the present study extend the findings of earlier studies and show that patients with CA-MRSA infections are similar to patients with CA-MSSA infections in respect to their epidemiologic characteristics. Both CA-MRSA and CA-MSSA infections were more common among younger patients with prior antimicrobial exposure. The results of this study show that patients infected with CA-MRSA do not fall into the groups defined earlier, suggesting the presence of additional risks in the community that contribute to the acquisition of MRSA.
Acknowledgments
This study was supported in part by Centers for Disease Control and Prevention grant R01/CCR5234523452.
Footnotes
Published ahead of print on 28 March 2007.
REFERENCES
- 1.Bannerman, T. L. 2003. Staphylococcus, Micrococcus, and other catalase-positive cocci that grow aerobically, p. 384-404. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 2.Centers for Disease Control and Prevention. 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococus aureus-Minnesota and North Dakota, 1997-1999. JAMA 282:1123-1125. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2003. Methicillin-resistant Staphylococus aureus infections in correctional facilities—Georgia, California, and Texas, 2001-2003. Morb. Mortal. Wkly. Rep. 52:992-996. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2003. Methicillin-resistant Staphylococus aureus infections among competitive sports participants—Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000-2003. Morb. Mortal. Wkly. Rep. 52:793-795. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2004. Community-associated methicillin-resistant Staphylococus aureus infections in Pacific Islanders—Hawaii, 2001-2003. Morb. Mortal. Wkly. Rep. 53:767-770. [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2003. Performance standards for antimicrobial susceptibility testing. Fifteenth informational supplement. M-100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Crane, L. R., D. P. Levine, M. J. Zervos, and G. Cummings. 1986. Bacteremia in narcotic addicts in the Detroit Medical Center: epidemiology, risk factors and empiric therapy. Rev. Infect. Dis. 8:364-374. [DOI] [PubMed] [Google Scholar]
- 8.Diep, B. A., H. A. Carleton, R. F. Chang, G. F. Sensabaugh, and F. Perdreau-Remington. 2006. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 193:1495-1503. [DOI] [PubMed] [Google Scholar]
- 9.Ellis, M. W., D. R. Hospenthal, D. P. Dooley, P. J. Gray, and C. K. Murray. 2004. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin. Infect. Dis. 39:971-979. [DOI] [PubMed] [Google Scholar]
- 10.Frank, A. L., J. F. Marcinak, P. D. Mangat, J. T. Tjhio, S. Kelkar, P. C. Schreckenberget, and J. P. Quinn. 2002. Clindamycin treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr. Infect. Dis. 21:530-534. [DOI] [PubMed] [Google Scholar]
- 11.Fridkin, S. K., J. C. Hageman, M. Morrison, L. Thomson Sanza, K. Como-Sabetti, J. A. Jernigan, K. Harriman, L. H. Harrison, R. Lynfield, and M. M. Farley for the Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436-1444. [DOI] [PubMed] [Google Scholar]
- 12.Gillet, Y., B. Issartel, P. Vanhems, J.-C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotizing pneumonia in young immunocompetent patients. Lancet 359:753-759. [DOI] [PubMed] [Google Scholar]
- 13.Hageman, J. C., T. M. Uyeki, J. S. Francis, D. B. Jernigan, J. G. Wheeler, C. B. Bridges, S. J. Barenkamp, D. M. Sievrt, A. Srinivasan, M. C. Doherty, L. K. McDougal, G. E. Killgore, U. A. Lopatin, R. Coffman, J. K. MacDonald, S. K. McAllister, G. E. Fosheim, J. B. Patel, and L. C. McDonald. 2006. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003-04 influenza season. Emerg. Infect. Dis. 12:894-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold, B. C., L. C. Immergluck, M. C. Maranan, D. S. Lauderdale, R. E. Gaskin, S. Boyle-Vavra, C. D. Leitch, and R. S. Daum. 1998. Community-acquired methicillin-resistant Staphylococus aureus in children with no identified predisposing risk. JAMA 279:593-598. [DOI] [PubMed] [Google Scholar]
- 15.Huang, V., and M. J. Zervos. 2005. Methicillin-resistant Staphylococus aureus in the community—implications for clinicians. Infect. Dis. Clin. Pract. 13:93-95. [Google Scholar]
- 16.Kazakova, S. V., J. C. Hagerman, M. Matava, A. Srinivasan, L. Phelan, B. Garfinkel, T. Boo, S. McAllister, J. Anderson, B. Jensen, D. Dodson, D. Lonsway, L. K. McDougal, M. Arduino, V. J. Fraser, G. Killgore, F. C. Tenover, S. Cody, and D. B. Jernigan. 2005. A clone of methicillin-resistant Staphylococus aureus among professional football players. N. Engl. J. Med. 352:468-475. [DOI] [PubMed] [Google Scholar]
- 17.Labandeira-Rey, M., F. Couzon, S. Boisset, E. L. Brown, M. Bes, Y. Benito, E. M. Barbu, V. Vazquez, M. Hook, J. Etienne, F. Vandenesch, and M. G. Bowden. 2007. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315:1130-1133. [DOI] [PubMed] [Google Scholar]
- 18.Lee, M. C., A. M. Rios, M. F. Aten, A. Mejias, D. Cavuoti, G. H. McCracken, and R. D. Hardy. 2004. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr. Infect. Dis. J. 23:123-127. [DOI] [PubMed] [Google Scholar]
- 19.Lee, N. E., M. M. Taylor, E. Bancroft, P. J. Ruane, M. Morgan, L. McCoy, and P. A. Simon. 2005. Risk factors for community-associated methicillin-resistant Staphylococcus aureus skin infections among HIV-positive men who have sex with men. Clin. Infect. Dis. 40:1529-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine, D. P., L. R. Crane, and M. J. Zervos. 1986. Infectious endocarditis and bacteremia in narcotic addicts in the Detroit Medical Center: a prospective comparative study. Rev. Infect. Dis. 8:374-397. [DOI] [PubMed] [Google Scholar]
- 21.Lodise, T. P., P. S. McKinnon, L. Swiderski, and M. J. Rybak. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin. Infect. Dis. 36:1418-1423. [DOI] [PubMed] [Google Scholar]
- 22.Lowry, F. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 23.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, L. G., F. Perdreau-Remington, G. Reig, S. Mehdi, J. Perlroth, A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 25.Moellering, R. C. 2006. The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann. Intern. Med. 144:368-370. [DOI] [PubMed] [Google Scholar]
- 26.Moran, G. J., A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDougal, R. B. Carey, and D. A. Talan, for the EMERGEncy ID Net Study Group. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666-674. [DOI] [PubMed] [Google Scholar]
- 27.Morin, C. A., and J. L. Hadler. 2001. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J. Infect. Dis. 184:1029-1034. [DOI] [PubMed] [Google Scholar]
- 28.Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O'Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococus aureus infection. JAMA 290:2976-2984. [DOI] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, vol. 23, no. 2, 6th ed. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 30.Saravolatz, L. D., N. Markowitz, L. Arking, D. Pohlod, and E. Fisher. 1982. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann. Intern. Med. 96:11-16. [DOI] [PubMed] [Google Scholar]
- 31.Sattler, C. A., E. O. Mason, Jr., and S. L. Kaplan. 2002. Prospective comparison of risk factors and demographic and clinical characteristics of community-acquired, methicillin-resistant versus methicillin-susceptible Staphylococus aureus infection in children. Pediatric. Infect. Dis. J. 21:910-917. [DOI] [PubMed] [Google Scholar]
- 32.Shopsin, B., B. Mathema, J. Martinez, E. Ha, M. L. Campo, A. Fierman, K. Krasinski, J. Kornblum, P. Alcabes, M. Waddington, M. Riehman, and B. N. Kreiswirth. 2000. Prevalence of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in the community. J. Infect. Dis. 182:359-362. [DOI] [PubMed] [Google Scholar]
- 33.Singh, A., R. V. Goering, S. Simjee, S. L. Foley, and M. J. Zervos. 2006. Application of molecular techniques to the study of hospital infection. Clin. Microbiol. Rev. 19:512-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenover, F. C., L. K. McDougal, R. V. Goering, G. Killgore, S. J. Projan, J. B. Patel, and P. M. Dunman. 2006. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J. Clin. Microbiol. 44:108-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenesch, F., T. Naimi, M. C. Engright, et al. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voyich, J. M., M. Otto, B. Mathema, K. R. Braughton, A. R. Whitney, D. Welty, R. D. Long, D. W. Dorward, D. J. Gardner, G. Lina, B. N. Kreiswirth, and F. R. DeLeo. 2006. Is Palentine-Valentine leucocidin the major virulence determinant in community associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 194:1761-1770. [DOI] [PubMed] [Google Scholar]
- 37.Zinderman, C. E., B. Conner, M. A. Malakooti, J. E. LaMar, A. Armstrong, and B. K. Bohnker. 2004. Community-acquired methicillin-resistant Staphylococcus aureus among military recruits. Emerg. Infect. Dis. 10:941-944. [DOI] [PMC free article] [PubMed] [Google Scholar]