Abstract
Following learning, a memory is fragile and undergoes a protein synthesis-dependent consolidation process in order to become stable. Established memories can again become transiently sensitive to disruption if reactivated and require another protein synthesis-dependent process, known as reconsolidation, in order to persist. Here, we show that, in the basolateral amygdala (BLA), protein synthesis is necessary for both consolidation and reconsolidation of inhibitory avoidance (IA) memory, while the expression of the transcription factor CCAAT enhancer binding protein β (C/EBPβ) is essential only for the reconsolidation process. Moreover, the critical roles of both protein synthesis and C/EBPβ following IA reactivation are temporally restricted, as they are necessary only for recent but not old IA memories. These results, together with previous findings showing that in the hippocampus both protein synthesis and C/EBPβ expression are required for consolidation but not reconsolidation of IA indicate that the stabilization process that takes place either after training or memory retrieval engages distinct neural circuits. Within these circuits, the C/EBPβ-dependent molecular pathway appears to be differentially recruited.
New memories are initially in a labile state and become long-lasting through a process known as consolidation, which transiently requires gene expression (Davis and Squire 1984; Kandel 2001; Dudai 2002). Established memories again become sensitive to disruption following reactivation, for example, by retrieval, and undergo a gene expression-dependent reconsolidation process in order to persist (Nader et al. 2000a; Sara 2000; Dudai and Eisenberg 2004; Alberini 2005).
Gene expression required for memory consolidation critically involves the activation of the cAMP-response element binding protein (CREB)–C/EBP gene expression pathway in specific brain regions (Bailey et al. 1996; Alberini 1999; Carew and Sutton 2001). In fact, in several types of memories across species, the disruption of either CREB or C/EBP family members in specific brain areas results in amnesia (Alberini et al. 1994; Yin and Tully 1996; Guzowski and McGaugh 1997; Silva et al. 1998; Kandel 2001; Taubenfeld et al. 2001a; Josselyn et al. 2004), which can be rescued if the knocked-down factor is re-expressed in a restricted number of cells (Han et al. 2007). Conversely, the overexpression of either factor results in long-term memory responses following training protocols that normally induce only short-term responses (Yin et al. 1995, but see Perazzona et al. 2004; Josselyn et al. 2001; Lee et al. 2001; Jasnow et al. 2005).
On the other hand, the nature of the genes and proteins as well as the underlying circuitry and temporal boundaries critical for the reconsolidation process are still poorly understood. The reconsolidation of contextual fear conditioning requires the role of CREB somewhere in the forebrain (Kida et al. 2002). In IA, the CREB-C/EBP pathway becomes activated and is required in the hippocampus during consolidation (Taubenfeld et al. 1999, 2001b), whereas C/EBPβ is dispensable within this region during reconsolidation (Taubenfeld et al. 2001a). This suggests that reconsolidation does not anatomically recapitulate the initial consolidation process (Dudai and Eisenberg 2004; Alberini 2005). However, because IA reconsolidation is disrupted by systemic inhibition of protein synthesis (Taubenfeld et al. 2001a; Milekic and Alberini 2002), brain regions outside the hippocampus must be engaged in the reconsolidation process. Evidence points to the amygdala as a candidate region; first, the BLA is critical for the formation and expression of fear memories in general, including IA (Munoz and Grossman 1981; Liang et al. 1982; Parent and McGaugh 1994; Ambrogi-Lorenzini et al. 1996, 1999; for review, see LeDoux 2000). Second, protein synthesis in the amygdala is required for IA memory consolidation (Berman et al. 1978), and, finally, amygdala protein synthesis is critical for the reconsolidation of cued fear conditioning (Nader et al. 2000b).
Additionally, in many other types of memories, including IA, across several species, the requirement for protein synthesis following memory reactivation has been found to be temporally graded (Litvin and Anokhin 2000; Milekic and Alberini 2002; Eisenberg and Dudai 2004; Suzuki et al. 2004; Boccia et al. 2006; Frankland et al. 2006).
To better understand the anatomical and temporal characteristics of the molecular mechanisms recruited in the amygdala during IA consolidation and reconsolidation, we investigated the requirements for protein synthesis and C/EBPβ over time in this region following either training or reactivation of both recent and old IA memories.
Results
Protein synthesis in the amygdala is required for both the consolidation and reconsolidation of IA
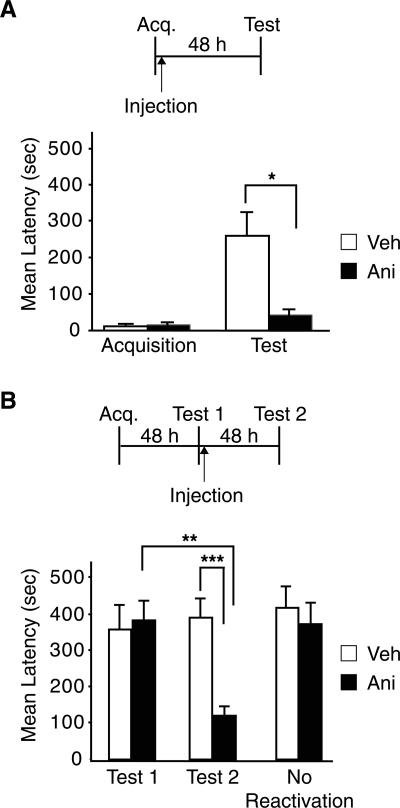
Previous work has shown that bilateral injections of cycloheximide into the amygdala immediately after training impair the retention of IA memory at subsequent tests (Berman et al. 1978). Here we tested whether anisomycin produces the same effect. Rats were bilaterally implanted with cannulae targeting the BLA. One week later, these rats were trained in IA and, immediately after, half of the rats were injected bilaterally with anisomycin, while the other half received the same volume of vehicle solution. All rats were re-tested 48 h later. As shown in Figure 1A, anisomycin significantly decreased the avoidance latencies (41.0 ± 13.6 sec) compared to vehicle (257.5 ± 63.0 sec; t-test; P < 0.05), confirming that protein synthesis is required in the BLA for IA memory consolidation.
Figure 1.
IA memory consolidation and reconsolidation require protein synthesis in the BLA. (A) IA memory consolidation requires protein synthesis in the BLA. Injection of anisomycin (Ani; n = 8) into the BLA immediately after training significantly impairs memory retention at 48 h compared to vehicle (Veh; n = 8; *P < 0.05). (B) IA memory reconsolidation requires protein synthesis in the BLA. Anisomycin (Ani; n = 8;) injected into the BLA immediately following reactivation (Test 1) significantly impairs memory retention at 96 h after training (Test 2) compared to vehicle (Veh; n = 7; ***P < 0.001) and compared to its Test 1 retention latency (**P < 0.01). Rats that did not undergo reactivation (No Reactivation) and were injected into the BLA with anisomycin had normal memory retention at 96 h after training. Temporal diagrams beside each graph illustrate injection, training, and testing time points. All values are expressed as mean latency ± SEM.
We then tested whether protein synthesis in the amygdala is also required for IA reconsolidation (Fig. 1B). BLA-implanted rats underwent IA training followed by memory testing 48 h later (Test 1). This test reactivated the memory. Immediately after Test 1, half of the rats were injected with anisomycin and the other half with vehicle solution. All rats were re-tested 48 h after Test 1 (Test 2). As shown in Figure 1B, at Test 2, the rats that received anisomycin had impaired retentions compared to vehicle-injected controls (Veh: 388.7 ± 49.2 sec; Ani: 118.9 ± 20.2 sec), while, as expected, at Test 1 the animals had similar latencies (Veh: 356.1 ± 64.9 sec; Ani: 381.7 ± 52.3 sec). A two-way ANOVA that compared the latencies of the groups across treatment and test, revealed a significant effect of treatment (F(1,26) = 46.9; P < 0.0001), test (F(1,26) = 41.6; P < 0.0001) and a treatment × test interaction (F(1,26) = 68.5; P < 0.0001). A Bonferroni post-hoc test revealed that there was a significant difference in the avoidance latencies between vehicle and anisomycin-treated rats at Test 2 (P < 0.001) and between the Test 2 and Test 1 of anisomycin-injected rats (P < 0.01). To confirm that the impairment was not due to nonspecific effects of the drug or interference with the original consolidation process, anisomycin or vehicle was injected bilaterally into the BLA 48 h after training in the absence of Test 1. When memory retention was tested 48 h later, no difference between anisomycin and vehicle treatment was found, and both groups of animals had strong and comparable avoidance latencies (Veh: 415.0 ± 58.3 sec; Ani: 359.1 ± 55.1 sec).
Thus, protein synthesis in the amygdala is required for both the consolidation and reconsolidation of IA memory.
C/EBPβ is required in the amygdala for the reconsolidation, but not the consolidation, of IA
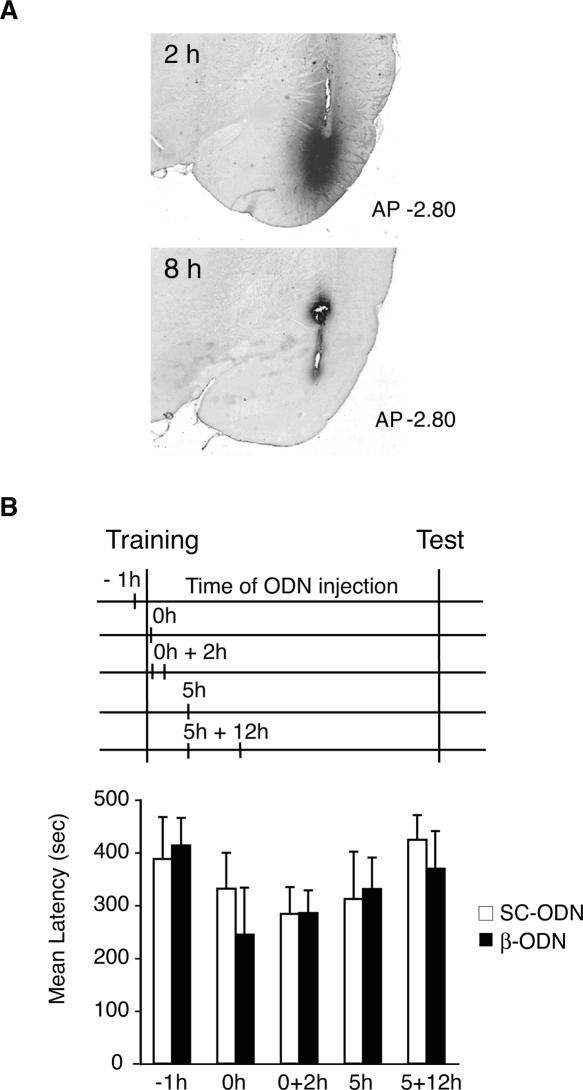
To establish the functional requirement of C/EBPβ in the amygdala during the consolidation and/or reconsolidation of IA memory, we tested the effect of C/EBPβ antisense oligodeoxinucleotide (ODN)-mediated knockdown on memory retention. Toward this end, we used an antisense ODN previously established to efficiently knock down the expression of C/EBPβ (β-ODN) in the brain as well as in other cell types (Pall et al. 1997; Taubenfeld et al. 2001a; Athos et al. 2002). We first determined the diffusion and stability of the injected β-ODN into the BLA. Rats were bilaterally injected into the BLA with biotinylated β-ODN and euthanized either 2 or 8 h after the injection. As depicted in Figure 2A, β-ODN was mainly confined to the BLA, although some minor diffusion into medial amygdala or piriform cortex was also observed in some cases. The stability of β-ODN in the BLA was relatively short; in fact, the ODN was clearly detected at 2 h but not at 8 h after injection (Fig. 2A). These results are consistent with those previously describing the stability of β-ODN and other antisense ODNs injected in the hippocampus (Guzowski and McGaugh 1997; Guzowski et al. 2000; Taubenfeld et al. 2001a).
Figure 2.
C/EBPβ in the BLA is not required for IA consolidation. (A) Biotinylated β-ODN diffusion and stability after injection into the BLA. Representative brain sections at −2.8 mm from bregma of animals injected with biotinylated β-ODN and euthanized 2 h or 8 h after injection. (B) C/EBPβ is not required in the BLA for IA memory consolidation. Training, testing, and injection time points are illustrated in a temporal diagram. β-ODN or SC-ODN was injected bilaterally into the BLA at the following time points: 1 h before training (−1 h, n = 7/group), immediately after (0 h, SC-ODN n = 10, β-ODN n = 8), 0 + 2 h (0 + 2 h, n = 12/group), 5 h (5 h, n = 8/group), or 5 + 12 h (n = 8/group) after training. No significant difference in latency was observed among β-ODN- and SC-ODN-treated rats at any of the injection time points. All values are expressed as mean latency ± SEM.
We then assessed whether C/EBPβ knockdown in the BLA following training affected memory retention. As depicted in Figure 2B, we injected β-ODN or a control scrambled ODN (SC-ODN) at different time points before and after training. These included: 1 h before training (−1 h), immediately after (0 h), and 5 h after training (5 h). Moreover, to investigate the effect of a more persistent treatment, we delivered two injections at either 0 and 2 h (0 + 2 h) or 5 and 12 h (5 + 12 h) after training. All rats were tested 48 h after training. As shown in Figure 2B, all groups exhibited strong memory retention, regardless of treatment or injection time point (−1 h: SC-ODN, 387.6 ± 79.6 sec; β-ODN, 413.3 ± 52.6 sec; 0 h: SC-ODN, 331.6 ± 67.9 sec; β-ODN, 244.5 ± 89.1 sec; 0 + 2 h: SC-ODN, 284.8 ± 50.3 sec; β-ODN, 285.5 ± 42.7 sec; 5 h: SC-ODN, 312.1 ± 89.4 sec; β-ODN, 331.0 ± 59.3 sec; [Tronel et al. 2005] 5 + 12 h: SC-ODN, 424.2 ± 46.8 sec; β-ODN, 369.6 ± 71.0 sec). A two-way ANOVA comparing animals across treatment and injection time points revealed no significant differences between the groups. Thus, while protein synthesis is necessary, C/EBPβ seems to be dispensable in the BLA for IA memory consolidation.
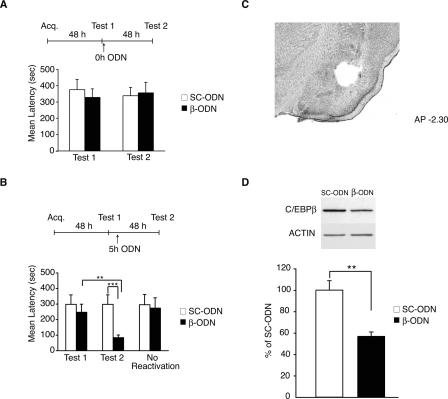
In the second set of experiments, we tested the functional role of C/EBPβ following memory reactivation (Fig. 3). Rats underwent IA training followed by testing for memory retention 48 h later (Test 1). Following this recall test, they received bilateral injections into the BLA of either β-ODN or control SC-ODN, either immediately after Test 1 (0 h, Fig. 3A) or 5 h later (5 h, Fig. 3B). All rats were re-tested 48 h after Test 1 (Test 2). As shown in Figure 3A, the injection of β-ODN at 0 h had no effect on memory retention at Test 2; both groups, which showed similar latencies at Test 1 (SC-ODN: 375.6 ± 63.0 sec; β-ODN: 328.5 ± 52.4 sec), maintained their retention levels at Test 2 (SC-ODN: 338.6 ± 50.6 sec; β-ODN: 356.4 ± 64.4 sec).
Figure 3.
C/EBPβ is required in the BLA for IA memory reconsolidation. (A) β-ODN injected in the BLA immediately after reactivation does not affect IA memory reconsolidation. Rats were injected bilaterally into the BLA with either β-ODN (n = 8) or SC-ODN (n = 8) immediately after retrieval (Test 1) at 48 h after training. When re-tested for memory retention 48 h later (Test 2, 96 h after training), all rats had similar latencies. (B) β-ODN-injected into the BLA 5 h after reactivation impairs IA memory. Rats injected with β-ODN (n = 11) 5 h after memory reactivation (Test1) had significantly lower retention latencies when retested 48 h later (Test 2, 96 h post-training) compared to rats injected with SC-ODN (n = 9, ***P < 0.001). Animals receiving β-ODN (n = 8) 48 h after training, but in the absence of reactivation (No Reactivation), had strong retention when tested at 96 h. These latencies were not significantly different from those of rats that received SC-ODN (n = 8). Temporal diagrams next to each graph depict injection, training, and testing time points. (C) Representative brain section of BLA tissue punches. (D) Densitometric analysis of Western blots (one representative sample per treatment is shown) of BLA punches extracts (n = 4/group) immunostained with anti-C/EBPβ. Data are expressed as mean % ± SEM of the SC-ODN (100%) control mean values. C/EBPβ values were normalized to those of actin.
In contrast, when ODNs were injected 5 h after Test 1 (Fig. 3B), β-ODN significantly impaired IA memory retention at Test 2. A two-way ANOVA revealed a significant effect of treatment (F(1,32) = 63.0; P < 0.0001), test (F(1,32) = 23.9; P < 0.0001), and a treatment × test interaction (F(1,32) = 24.0; P < 0.0001). A Bonferroni post hoc test indicated that the Test 2 retentions of β-ODN-injected animals (99.5 ± 22.2 sec) were significantly lower compared to those of SC-ODN-injected animals (325.0 ± 60.3 sec; P < 0.001; Fig. 3B) and to their Test 1 latencies (P < 0.01). As expected, all animals had similar latencies at Test 1 (SC-ODN: 324.9 ± 60.4 sec; β-ODN: 273.9 ± 53.9 sec). To determine whether the memory disruption caused by β-ODN was specific to reactivated memories rather than an interference of the consolidation process or to nonspecific effects of the treatment, groups of rats were injected with β-ODN or SC-ODN 53 h after training, but in the absence of Test 1. No significant difference in the mean avoidance latency was found between the group of animals that received β-ODN (273.9 ± 66.4 sec) and the control group, which was injected with SC-ODN (295.7 ± 66.6 sec). Also, no significant differences were found between these groups and their relative latencies at Test 1. To confirm that β-ODN treatment disrupts the expression of C/EBPβ in the amygdala, groups of rats were trained and then tested 48 h later (Test 1). Five hours following Test 1, one group received a bilateral injection of β-ODN, and the other was injected with SC-ODN. All rats were euthanized 12 h after Test 1, and tissue punches of BLAs from each rat were taken and lysed as described in Materials and Methods. A representative section showing the area typically dissected with BLA punches is depicted in Figure 3C. Quantitative Western blot analyses carried out on the extracts determined the concentration of C/EBPβ in each sample. As depicted in Figure 3D C/EBPβ expression was significantly disrupted in β-ODN-injected BLAs (56.8% ± 4.0%) compared to SC-ODN-injected controls (100% ± 8.9%; t-test; P < 0.01).
Together, these data show that C/EBPβ plays a critical role in the BLA following reactivation but not acquisition of IA.
Protein synthesis and C/EBPβ are required in the amygdala for the reconsolidation of recent but not old IA memories
Systemic injections of anisomycin have previously shown that there is a temporally graded requirement for protein synthesis after IA reactivation, which decreases as the age of the memory increases (Milekic and Alberini 2002).
Are the requirements for protein synthesis and C/EBPβ in the amygdala major contributors to this gradient? To address this question, we determined whether the dependence for either protein synthesis or C/EBPβ in the amygdala during IA reconsolidation follows the temporal profile observed with systemic injections. As we have shown above (Figs. 1B, 3B), IA memory reactivated by testing (Test 1) 2 d following training becomes labile and is disrupted by bilateral injections of either anisomycin or β-ODN. Here we tested the effect of blocking protein synthesis or C/EBPβ in the amygdala at the same time points following the reactivation of a 2-wk-old IA memory (Fig. 4).
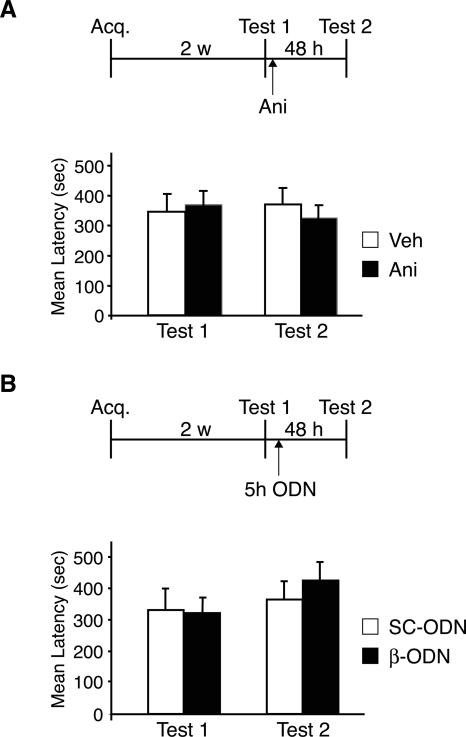
Figure 4.
Disruption of either protein synthesis or C/EBPβ in the BLA following reactivation does not affect the retention of a 2-wk-old IA memory. (A) Rats injected with anisomycin (Ani; n = 8) into the BLA immediately after reactivation, 2 wk after training (Test 1), maintained very strong latencies when retested 48 h later (Test 2) and were not significantly different from rats injected with vehicle solution (Veh, n = 8). (B) Rats injected with β-ODN (n = 9) into the BLA immediately after reactivation, 2 wk after training (Test 1), maintained very strong latencies when retested 48 h later (Test 2). These latencies were similar to the Test 2 latencies of rats injected with SC-ODN (n = 8). Temporal diagrams next to each graph depict injection, training, and testing time points. Values are expressed as mean latency ± SEM.
Rats underwent IA training followed by memory reactivation 2 wk later (Test 1). Immediately after Test 1, half of the animals received anisomycin injections into the BLA, while the other half were injected with vehicle solution. All rats were re-tested 48 h later (Test 2). As shown in Figure 4A, both groups showed similar Test 1 latencies (Veh: 349.8 ± 54.9 sec; Ani: 368.8 ± 40.9 sec) and maintained similar retentions at Test 2 (Veh: 370.2 ± 50.0 sec; Ani: 320.3 ± 42.6 sec), demonstrating that, as with systemic treatments, blocking protein synthesis in the BLA does not disrupt the reconsolidation of a 2-wk-old IA memory.
Similar results were found when C/EBPβ was knocked down (Fig. 4B). Rats underwent IA training and 2 wk later were tested to reactivate their memory (Test 1). All rats showed similar retentions (SC-ODN: 328.6 ± 66.5 sec; β-ODN: 317.5 ± 47.1 sec). Five hours after Test 1, half of the animals were injected bilaterally into the BLA with β-ODN and the other half with SC-ODN. The rats were re-tested 48 h later (Test 2). All rats showed an intact memory at Test 2. Moreover, the IA retentions of animals that received β-ODN (422.3 ± 56.2 sec) were also comparable to those that received SC-ODN control injections (362.2 ± 56.6 sec).
In summary, these data indicate that the requirement for protein synthesis and C/EBPβ in the amygdala after memory reactivation is limited to recent memories.
Discussion
In this study, we show that, in contrast to the hippocampus where protein synthesis is required for the consolidation but not reconsolidation of IA memory, amygdala protein synthesis plays an essential role during both processes. Moreover, only during reconsolidation is there a requirement for C/EBPβ in the amygdala. In agreement with our previous findings based on systemic inhibition of protein synthesis (Milekic and Alberini 2002), the requirement for protein synthesis and C/EBPβ in the amygdala during reconsolidation is limited to recent memories.
We conclude that the changes activated in the brain during both consolidation and reconsolidation of IA use similar molecular mechanisms (i.e., C/EBPβ) but engage distinct brain regions. Furthermore, the amygdala represents a critical area where a temporally limited requirement of both protein synthesis- and C/EBPβ-dependent events occurs during IA memory reconsolidation.
The role of the amygdala in memory reconsolidation
Inactivation studies have demonstrated that both the BLA and hippocampus play a critical role in IA memory consolidation (Munoz and Grossman 1981; Liang et al. 1982; Parent and McGaugh 1994; Ambrogi-Lorenzini et al. 1996, 1999). Conversely, during IA reconsolidation, protein synthesis is dispensable in the hippocampus (Taubenfeld et al. 2001a), but, as shown above, required in the amygdala. Why is protein synthesis required in the amygdala but not in the hippocampus during memory reconsolidation? Does the amygdala play a special role following memory reactivation?
A possible explanation is that the reactivation of IA memory induced during the testing trial mainly involves “cue”- and “fear”-related representations rather than “contextual” ones. If this is true, then the amygdala, a region that processes “cue–fear” associations, and not the hippocampus, which processes “contextual” associations, might become preferentially re-engaged during IA reconsolidation. This hypothesis is in agreement with our recent findings (Tronel et al. 2005) showing that the re-experience, in a novel context, of a single cue (light), which was originally presented during training, is sufficient to destabilize the retention of an IA memory. In fact, this memory is disrupted when C/EBPβ is knocked down in the amygdala, but not in the hippocampus.
Therefore, it is reasonable to hypothesize that the nature of the reactivation trial may predict which brain regions are actively engaged during reconsolidation. According to this logic, it follows that either different types of reactivation trials (e.g., reinforced vs. non-reinforced) or the reactivation of other types of memories, which rely on other memory systems, may recruit different brain areas during reconsolidation.
A second not mutually exclusive hypothesis suggests that the amygdala is more frequently engaged during the reconsolidation process of several types of memories, both appetitive and aversive, because of its general role in mediating the influence of emotional arousal on memory. In support of this hypothesis, although somewhat controversial, several authors have shown that the reconsolidation of memories created by either appetitive or aversively motivated learning requires protein synthesis or the function of specific molecules in the amygdala (Nader et al. 2000b; Koh and Bernstein 2003; Duvarci and Nader 2004; Wang et al. 2005; Bucherelli et al. 2006; Lee et al. 2006a, b; Lin et al. 2006; Tronson et al. 2006; but see Bahar et al. 2004; Hellemans et al. 2006; Milekic et al. 2006; Yim et al. 2006).
The role of C/EBPβ: Distinctive features between consolidation and reconsolidation
Our data imply that different molecular pathways are required in the amygdala during either the consolidation or reconsolidation of IA memory. These results are, to a certain extent, in line with previous reports showing that consolidation and reconsolidation may recruit distinct molecular mechanisms within a given brain region. For example, although contextual fear conditioning requires protein synthesis in the hippocampus during both processes (Debiec et al. 2002), antisense-mediated knockdown demonstrated that hippocampal brain-derived neurotrophic factor (BDNF) is critical for consolidation but not reconsolidation. Conversely, the latter but not the former is impaired when hippocampal expression of Zif268 is disrupted (Lee et al. 2004). However, as our data did not investigate double dissociable molecular mechanisms, they might simply suggest that, within a brain region, some molecular requirements may appear to be differentially engaged during either consolidation or reconsolidation. This distinctive engagement could be due, for example, to time and/or functional redundancy of protein families. Consistent with these possibilities, von Hertzen and Giese (2005) reported that some immediate early genes are differentially expressed in the hippocampus during consolidation or reconsolidation of contextual fear conditioning. Specifically, whereas serum- and glucocorticoid-induced kinase 1 is increased after both training and reactivation, nerve growth factor-inducible gene B is only induced following training.
Thus, on one hand, we could speculate that some cAMP-dependent responses in the amygdala are necessary after the reactivation but not acquisition of IA. Interestingly, this is in agreement with previous data showing that blocking noradrenergic receptors, which are known to be coupled to the cAMP-dependent pathway (McGaugh 2004) in the amygdala, disrupts reconsolidation but not consolidation of auditory fear conditioning (Debiec and LeDoux 2004). Alternatively, it is possible that the gene expression induced after training is more redundant than the one induced after memory reactivation (von Hertzen and Giese 2005) and therefore that C/EBPβ might play a role in the amygdala during consolidation, but, because of redundancy, its disruption might be functionally irrelevant. In agreement with this hypothesis, we have recently found that another member of the C/EBP family, C/EBPδ, is required in the amygdala during IA consolidation (data not shown).
In summary, our present results, in agreement with previous findings (Berman and Dudai 2001; Taubenfeld et al. 2001a; Hernandez et al. 2002; Tronel and Sara 2002; Bahar et al. 2004; Lee et al. 2004; Salinska et al. 2004; von Hertzen and Giese 2005), suggest that both the molecular mechanisms and the brain regions engaged during either consolidation or reconsolidation overlap only partially.
The temporally limited requirement of protein synthesis and C/EBPβ in the amygdala
The requirements of both protein synthesis and C/EBPβ underlying IA memory reconsolidation in the amygdala are temporally limited, as they are evident in recent but not old memories.
These results appear to disagree with conclusions proposed by Nader et al. (2000b) and Debiec et al. (2002), who reported that protein synthesis in the amygdala or hippocampus, respectively, is still required when a 2-wk-old or a 45-d-old auditory or contextual fear conditioning memory is reactivated. An explanation for these contrasting findings and conclusions has been suggested by other studies, which used different species and learning paradigms, including contextual fear conditioning in mice. These studies showed that there is a gradient of protein synthesis dependence after memory reactivation, which is a function of the strength and number of reactivations and can vary with the nature of the reactivation trial (Suzuki et al. 2004; Frankland et al. 2006). Thus, changes in the protocol of reactivation and/or training seem to reveal differences in the duration of the gradient of protein synthesis required for reconsolidation (Alberini 2007). Moreover, it should be kept in mind that, as seen for memory consolidation (Dudai 2004), the systems and cellular processes underlying reconsolidation are likely to differ among different learning paradigms. For example, IA differs from auditory and contextual fear conditioning in the involvement of amygdala and cortical areas during both acquisition and consolidation (Phillips and LeDoux 1995; Izquierdo et al. 1997; Zanatta et al. 1997; Wilensky et al. 2000). These differences likely reflect distinctions in the neuronal networks underlying each type of learning. In addition, lesion studies showed that the amygdala has an enduring role in the expression of contextual fear conditioning (Maren et al. 1996; Gale et al. 2004), while it is only temporarily required following IA training (Liang et al. 1982).
The temporal requirement for both protein synthesis and C/EBPβ in the amygdala parallels that observed following systemic inhibition of protein synthesis (Milekic and Alberini 2002). Although our data do not exclude the possibility that other regions might also be involved, these results suggest that the BLA is a primary or, perhaps, the only site where new protein synthesis is critical for IA reconsolidation.
Why is the gene-expression/protein synthesis-dependent process of reconsolidation time-dependent? One possibility is that, over time, an anatomical reorganization may result in a different memory “trace” that has become independent of protein synthesis following reactivation (Liang et al. 1982). A second possibility is that the trace may or may not change its anatomical organization over time. However, its physical substrates, previously labile, become stable and insensitive to protein synthesis inhibitors. For example, physical substrates of memory consolidation could lie within synaptic structural modifications (Bailey and Kandel 1993; Andersen and Soleng 1998; O’Malley et al. 1998, 2000; Engert and Bonhoeffer 1999; Geinisman et al. 2001; Steward and Worley 2002). If the stability or the number of modified synapses in the amygdala increases over time, and this process takes more than a week to complete, it is possible that the reactivation of a recent memory destabilizes most of these modifications. On the other hand, the reactivation of an older memory, at which point more and/or stronger synapses are associated with its trace, will not influence its stability.
How can we integrate these mechanistic explanations into a more systemic view of the memory process? New synapse formation and, therefore establishment of memory traces, may occur very rapidly, within minutes, yet the reconsolidation gradient may take weeks or perhaps months to complete. What is the overall effect of time on memory stability? Perhaps, in order to become a long-lasting memory, a single learning event requires its trace to be reactivated repeatedly, and the intensity and duration of the memory might be a function of the number of reactivations (Alberini 2007). Thus, the result of these reactivations, which can be either explicit or implicit and also occur during sleep, may be perpetuating the initial changes induced by learning until memory stability reaches a plateau (Alberini 2005).
Materials and Methods
Animals
Adult male, Long Evans rats weighing between 200 and 250 g were used in all experiments. Animals were individually housed and maintained on a 12 h/12 h light–dark cycle. All experiments were carried out during the light cycle. The rats were allowed ad libitum access to food and water. The animals were handled for 3 d before behavioral procedures. All protocols complied with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Mt. Sinai School of Medicine Animal Care Committees.
Inhibitory avoidance (IA)
During IA training, the rats learned to associate a context with a mild electric footshock and avoid entering this context on subsequent exposures. The experiments were conducted as previously described (Milekic and Alberini 2002). Briefly, the IA training chamber consisted of a rectangular-shaped box with two compartments, a safe (lit) and a shock (dark) one, separated by a sliding door (Med-Associate ENV-010MC; Med-Associate). During training, each rat was placed in the safe compartment facing away from the door. After 10 sec, the door opened, allowing the rat access to the shock compartment, where a brief footshock (0.9 mA, 2 sec) was delivered. Latency to enter the shock compartment was taken as a measure of acquisition. Ten seconds after the footshock, rats returned to their home cages. Memory retention (Test) was tested at different time points after training by placing the animal in the safe compartment and recording the latency (in seconds) to enter the shock compartment. The footshock was not administered during the retention test, and testing concluded after 540 sec. Injections and testing were carried out under blind conditions. Statistical analyses were performed using either an ANOVA followed by a Bonferroni post hoc test or, for pairwise comparisons, a student t-test.
Implantation of cannulae
Experiments were performed as previously described (Tronel et al. 2005). Briefly, rats were anesthetized with ketamine (60 mg/kg, i.p.) and xylazine (7.5 mg/kg, i.p). Stainless steel cannulae (22 gauge) were stereotactically implanted bilaterally into the BLA (2.8 mm posterior to bregma, 5.3 mm lateral from midline, and 6.25 mm ventral). After surgery, rats were returned to their home cages for a 7-d recovery period before undergoing behavioral experiments.
Protein synthesis inhibitor injections
Anisomycin (Sigma) was dissolved in equimolar saline-HCL and adjusted to pH 7.2 with NaOH as described in Taubenfeld et al. (2001a). Rats received bilateral injections of 62.5 μg in 0.5 μL per side of anisomycin or vehicle at a rate of 0.4 μL/min (Nader et al. 2000b; Schafe and LeDoux 2000). The injection needle was left in place for 1 min following the injection to allow for complete dispersion of the solution.
Oligodeoxynucleotide (ODN) injections
The ODNs used in the present study have been employed in previous investigations (Taubenfeld et al. 2001a; Tronel et al. 2005). C/EBPβ antisense ODN (β-ODN; 5′-CCAGCAGGCGGTG CATGAAC-3′), scrambled ODN (SC-ODN; 5′-TCGGAGAC TAAGCGCGGCAC-3′), or vehicle (phosphate-buffered saline [PBS] at pH 7.4) was bilaterally injected in the BLA at 0.5 μL/side, 2 nmol/μL, and an infusion rate at 0.4 μL/min. The β-ODN is specific for a part of the C/EBPβ mRNA sequence that includes the translational start site. The SC-ODN, which serves as a control, does not show any homology with relevant sequences in the GenBank database and has the same base composition as the β-ODN, but in a randomized order. To increase stability, both ODNs were phosphorothioated on the three terminal bases on both 5′- and 3′-ends (Hooper et al. 1994; Widnell et al. 1996; Guzowski et al. 2000). The ODNs were RPC-purified and purchased from GeneLink. The ODNs, dissolved in PBS, were infused into the BLA through a 28-gauge injection needle, extending 1.5 mm beyond the tip of the guide cannula and connected via polyethylene tubing to a Hamilton syringe. The injection needle was left in place for 1 min following the injection to allow for complete dispersion of the solution.
To detect the diffusion and stability of the injected β-ODN, a biotinylated form of β-ODN was used. At the indicated times following injections, animals were perfused with 4% paraformaldehyde in PBS. Brains were post-fixed overnight in the same fixative with 30% sucrose. Forty-micrometer coronal sections were stained using HRP-conjugated avidin from the Immunopure ABC kit (Pierce). The staining was revealed by diaminobenzidene (Sigma) and enhanced with 0.012% of cobalt chloride and 0.01% nickel ammonium sulfate.
Histology
At the end of the behavioral experiments, rats were anesthetized with ketamine (60 mg/kg, i.p.) and xylazine (7.5 mg/kg, i.p.) and perfused intracardially with 10% PBS-buffered formalin. The brains were removed, post-fixed overnight in a 30% sucrose/formalin solution, and then cryo-protected in 30% sucrose/saline. To verify the location of the cannula implants, 40-μm coronal sections were cut through the targeted area, stained with cresyl violet, and examined under a light microscope. Rats with incorrect cannula placements were excluded from the analyses. In total, five animals were excluded from the entire study.
Western blot analyses
Rats were sacrificed at 12 h following IA reactivation, and their brains were rapidly removed and frozen. Amygdala punches were obtained with a neuro punch (19 gauge; Fine Science Tools) from frozen brains mounted on a sliding freezing microtome (Schafe et al. 2000; Lamprecht et al. 2002). The punches included the lateral and the basal nuclei and possibly portions of the lateral central nucleus and cortical tissue directly lateral to the external capsule. The samples were homogenized in cold lysis buffer with protease inhibitors (0.2 M NaCl, 5 mM EDTA, 10% glycerol, 2 mM NaPP, 100 mM HEPES, 2 mM sodium orthovanadate, 1 mM EGTA, 2 mM NaF, 0.5 mM PMSF, protease inhibitor cocktail 1:500 [Sigma], 1 μM microcystin, 1 mM benzamidine). Equal amounts of protein (5 μg) were resolved using 10% SDS-PAGE and analyzed by Western blots as previously described (Taubenfeld et al. 2001b). The primary antibodies were rabbit anti-C/EBPβ (Santa Cruz Biotechnology) and goat anti-actin antibody (I-19; Santa Cruz Biotechnology). The secondary antibody was HRP-labeled donkey anti-rabbit antibody (Amersham). Quantitative densitometric analysis was performed using NIH Image. C/EBPβ expression values were normalized to those of actin to account for variations in gel loading, and data were expressed as mean % ± SEM of the respective SC-ODN control group (100%) meanvalues. Statistical analyses were performed with an unpaired t-test.
Acknowledgments
We thank Daniel Christoffel, Dillon Chen, Stephen Taubenfeld, and Peter Needle (Mount Sinai) for technical assistance, and Reginald Miller and the ACLL facility of Mount Sinai for technical support. This work was supported by the National Institute of Mental Health (R01 MH65635) and the Hirschl Trust Foundation to C.M.A.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.598307
References
- Alberini C.M. Genes to remember. J. Exp. Biol. 1999;202:2887–2891. doi: 10.1242/jeb.202.21.2887. [DOI] [PubMed] [Google Scholar]
- Alberini C.M. Mechanisms of memory stabilization: Are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Alberini C.M. Reconsolidation: The Samsara of memory consolidation. Debates Neurosci. 2007;1:17–24. [Google Scholar]
- Alberini C.M., Ghirardi M., Metz R., Kandel E.R. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Ambrogi-Lorenzini C.G., Baldi E., Bucherelli C., Sacchetti B., Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response: A tetrodotoxin functional inactivation study. Brain Res. 1996;730:32–39. doi: 10.1016/0006-8993(96)00427-1. [DOI] [PubMed] [Google Scholar]
- Ambrogi-Lorenzini C.G., Baldi E., Bucherelli C., Sacchetti B., Tassoni G. Neural topography and chronology of memory consolidation: A review of functional inactivation findings. Neurobiol. Learn. Mem. 1999;71:1–18. doi: 10.1006/nlme.1998.3865. [DOI] [PubMed] [Google Scholar]
- Andersen P., Soleng A.F. Long-term potentiation and spatial learning are both associated with the generation of new excitatory synapses. Brain Res. Brain Res. Rev. 1998;26:353–359. doi: 10.1016/s0165-0173(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Athos J., Impey S., Pineda V.V., Chen X., Storm D.R. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat. Neurosci. 2002;5:1119–1120. doi: 10.1038/nn951. [DOI] [PubMed] [Google Scholar]
- Bahar A., Dorfman N., Dudai Y. Amygdalar circuits required for either consolidation or extinction of taste aversion memory are not required for reconsolidation. Eur. J. Neurosci. 2004;19:1115–1118. doi: 10.1111/j.0953-816x.2004.03215.x. [DOI] [PubMed] [Google Scholar]
- Bailey C.H., Kandel E.R. Structural changes accompanying memory storage. Annu. Rev. Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Bailey C.H., Bartsch D., Kandel E.R. Toward a molecular definition of long-term memory storage. Proc. Natl. Acad. Sci. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman D.E., Dudai Y. Memory extinction, learning a new, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- Berman R.F., Kesner R.P., Partlow L.M. Passive avoidance impairment in rats following cycloheximide injection into the amygdala. Brain Res. 1978;158:171–188. doi: 10.1016/0006-8993(78)90013-6. [DOI] [PubMed] [Google Scholar]
- Boccia M.M., Blake M.G., Acosta G.B., Baratti C.M. Post-retrieval effects of icv infusions of hemicholinium in mice are dependent on the age of the original memory. Learn. Mem. 2006;13:376–381. doi: 10.1101/lm.150306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucherelli C., Baldi E., Mariottini C., Passani M.B., Blandina P. Aversive memory reactivation engages in the amygdala only some neurotransmitters involved in consolidation. Learn. Mem. 2006;13:426–430. doi: 10.1101/lm.326906. [DOI] [PubMed] [Google Scholar]
- Carew T.J., Sutton M.A. Molecular stepping stones in memory consolidation. Nat. Neurosci. 2001;4:769–771. doi: 10.1038/90458. [DOI] [PubMed] [Google Scholar]
- Davis H.P., Squire L.R. Protein synthesis and memory: A review. Psychol. Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Debiec J., LeDoux J.E. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Debiec J., LeDoux J.E., Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Molecular bases of long-term memories: A question of persistence. Curr. Opin. Neurobiol. 2002;12:211–216. doi: 10.1016/s0959-4388(02)00305-7. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Eisenberg M. Rites of passage of the engram: Reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Duvarci S., Nader K. Characterization of fear memory reconsolidation. J. Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M., Dudai Y. Reconsolidation of fresh, remote, and extinguished fear memory in Medaka: Old fears don’t die. Eur. J. Neurosci. 2004;20:3397–3403. doi: 10.1111/j.1460-9568.2004.03818.x. [DOI] [PubMed] [Google Scholar]
- Engert F., Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Frankland P.W., Ding H.K., Takahashi E., Suzuki A., Kida S., Silva A.J. Stability of recent and remote contextual fear memory. Learn. Mem. 2006;13:451–457. doi: 10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale G.D., Anagnostaras S.G., Godsil B.P., Mitchell S., Nozawa T., Sage J.R., Wiltgen B., Fanselow M.S. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J. Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y., Berry R.W., Disterhoft J.F., Power J.M., Van der Zee E.A. Associative learning elicits the formation of multiple-synapse boutons. J. Neurosci. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski J.F., McGaugh J.L. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc. Natl. Acad. Sci. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski J.F., Lyford G.L., Stevenson G.D., Houston F.P., McGaugh J.L., Worley P.F., Barnes C.A. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.H., Kushner S.A., Yiu A.P., Cole C.J., Matynia A., Brown R.A., Neve R.L., Guzowski J.F., Silva A.J., Josselyn S.A. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Hellemans K.G., Everitt B.J., Lee J.L. Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J. Neurosci. 2006;26:12694–12699. doi: 10.1523/JNEUROSCI.3101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez P.J., Sadeghian K., Kelley A.E. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nat. Neurosci. 2002;12:1327–1331. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- Hooper M.L., Chiasson B.J., Robertson H.A. Infusion into the brain of an antisense oligonucleotide to the immediate-early gene c-fos suppresses production of fos and produces a behavioral effect. Neuroscience. 1994;63:917–924. doi: 10.1016/0306-4522(94)90559-2. [DOI] [PubMed] [Google Scholar]
- Izquierdo I., Quillfeldt J.A., Zanatta M.S., Quevedo J., Schaeffer E., Schmitz P.K., Medina J.H. Sequential role of hippocampus and amygdala, entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. Eur. J. Neurosci. 1997;9:786–793. doi: 10.1111/j.1460-9568.1997.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Jasnow A.M., Shi C., Israel J.E., Davis M., Huhman K.L. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav. Neurosci. 2005;119:1125–1130. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Josselyn S.A., Shi C., Carlezon W.A., Neve R.L., Nestler E.J., Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J. Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn S.A., Kida S., Silva A.J. Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol. Learn. Mem. 2004;82:159–163. doi: 10.1016/j.nlm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Kandel E.R. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kida S., Josselyn S.A., de Ortiz S.P., Kogan J.H., Chevere I., Masushige S., Silva A.J. CREB required for the stability of new and reactivated fear memories. Nat. Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Koh M.T., Bernstein I.L. Inhibition of protein kinase A activity during conditioned taste aversion retrieval: Interference with extinction or reconsolidation of a memory? Neuroreport. 2003;14:405–407. doi: 10.1097/00001756-200303030-00021. [DOI] [PubMed] [Google Scholar]
- Lamprecht R., Farb C.R., LeDoux J.E. Fear memory formation involves p190 RhpGAP and ROCK proteins through a GRB2-mediated complex. Neuron. 2002;14:727–738. doi: 10.1016/s0896-6273(02)01047-4. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee J.A., Kim H.K., Kim K.H., Han J.H., Lee Y.S., Lim C.S., Chang D.J., Kubo T., Kaang B.K. Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses. Learn. Mem. 2001;8:220–226. doi: 10.1101/lm.40201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.L., Everitt B.J., Thomas K.L. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lee J.L., Milton A.L., Everitt B.J. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J. Neurosci. 2006a;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.L., Milton A.L., Everitt B.J. Reconsolidation and extinction of conditioned fear: Inhibition and potentiation. J. Neurosci. 2006b;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K.C., McGaugh J.L., Martinez J.L., Jensen R.A., Vasquez B.J., Messing R.B. Post-training amygdaloid lesions impair retention of an inhibitory avoidance response. Behav. Brain Res. 1982;4:237–249. doi: 10.1016/0166-4328(82)90002-x. [DOI] [PubMed] [Google Scholar]
- Lin H.C., Mao S.C., Gean P.W. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn. Mem. 2006;13:316–321. doi: 10.1101/lm.217006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin O.O., Anokhin K.V. Mechanisms of memory reorganization during retrieval of acquired behavioral experience in chicks: The effects of protein synthesis inhibition in the brain. Neurosci. Behav. Physiol. 2000;30:671–678. doi: 10.1023/a:1026698700139. [DOI] [PubMed] [Google Scholar]
- Maren S., Aharonov G., Fanselow M.S. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: Absence of a temporal gradient. Behav. Neurosci. 1996;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Milekic M.H., Alberini C.M. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Milekic M.H., Brown S.D., Castellini C., Alberini C.M. Persistent disruption of an established morphine conditioned place preference. J. Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz C., Grossman S.P. Spatial discrimination, reversal and active or passive avoidance learning in rats with KA-induced neuronal depletions in dorsal hippocampus. Brain Res. Bull. 1981;6:399–406. doi: 10.1016/s0361-9230(81)80010-x. [DOI] [PubMed] [Google Scholar]
- Nader K., Schafe G.E., LeDoux J.E. The labile nature of consolidation theory. Nat. Rev. Neurosci. 2000a;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Nader K., Schafe G.E., Le Doux J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000b;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- O’Malley A., O’Connell C., Regan C.M. Ultrastructural analysis reveals avoidance conditioning to induce a transient increase in hippocampal dentate spine density in the 6 hour post-training period of consolidation. Neuroscience. 1998;87:607–613. doi: 10.1016/s0306-4522(98)00178-x. [DOI] [PubMed] [Google Scholar]
- O’Malley A, Regan C.M. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99:229–232. doi: 10.1016/s0306-4522(00)00182-2. [DOI] [PubMed] [Google Scholar]
- Pall M., Hellberg P., Brannstrom M., Mikuni M., Peterson C.M., Sundfeldt K., Norden B., Hedin L., Enerback S. The transcription factor C/EBP-β and its role in ovarian function; evidence for direct involvement in the ovulatory process. EMBO J. 1997;16:5273–5279. doi: 10.1093/emboj/16.17.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent M.B., McGaugh J.L. Posttraining infusion of lidocaine into the amygdala basolateral complex impairs retention of inhibitory avoidance training. Brain Res. 1994;661:97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- Perazzona B., Isabel G., Preat T., Davis R.L. The role of cAMP response element-binding protein in Drosophila long-term memory. J. Neurosci. 2004;24:8823–8828. doi: 10.1523/JNEUROSCI.4542-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J. Neurosci. 1995;15:5308–5315. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinska E., Bourne R.C., Rose S.P. Reminder effects: The molecular cascade following a reminder in young chicks does not recapitulate that following training on a passive avoidance task. Eur. J. Neurosci. 2004;19:3042–3047. doi: 10.1111/j.0953-816X.2004.03407.x. [DOI] [PubMed] [Google Scholar]
- Sara S.J. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn. Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schafe G.E., LeDoux J.E. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J. Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe G.E., Atkins C.M., Swank M.W., Bauer E.P., Sweatt J.D., LeDoux J.E. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J. Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.J., Kogan J.H., Frankland P.W., Kida S. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Steward O., Worley P. Local synthesis of proteins at synaptic sites on dendrites: Role in synaptic plasticity and memory consolidation? Neurobiol. Learn. Mem. 2002;78:508–527. doi: 10.1006/nlme.2002.4102. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Josselyn S.A., Frankland P.W., Masushige S., Silva A.J., Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld S.M., Wiig K.A., Bear M.F., Alberini C.M. A molecular correlate of memory and amnesia in the hippocampus. Nat. Neurosci. 1999;2:309–310. doi: 10.1038/7217. [DOI] [PubMed] [Google Scholar]
- Taubenfeld S.M., Milekic M.H., Monti B., Alberini C.M. The consolidation of new but not reactivated memory requires hippocampal C/EBPβ. Nat. Neurosci. 2001a;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Taubenfeld S.M., Wiig K.A., Monti B., Dolan B., Pollonini G., Alberini C.M. Fornix-dependent induction of hippocampal CCAAT enhancer-binding protein β and δ co-localizes with phosphorylated cAMP response element-binding protein and accompanies long-term memory consolidation. J. Neurosci. 2001b;21:84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S., Sara S.J. Mapping of olfactory memory circuits: Region-specific c-fos activation after odor-reward associative learning or after its retrieval. Learn. Mem. 2002;3:105–111. doi: 10.1101/lm.47802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S., Milekic M.H., Alberini C.M. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 2005;3:1630–1638. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson N.C., Wiseman S.L., Olausson P., Taylor J.R. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat. Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- von Hertzen L.S., Giese K.P. Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J. Neurosci. 2005;25:1935–1942. doi: 10.1523/JNEUROSCI.4707-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.H., Ostlund S.B., Nader K., Balleine B.W. Consolidation and reconsolidation of incentive learning in the amygdala. J. Neurosci. 2005;25:830–835. doi: 10.1523/JNEUROSCI.4716-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell K.L., Self D.W., Lane S.B., Russell D.S., Vaidya V.A., Miserendino M.J., Rubin C.S., Duman R.S., Nestler E.J. Regulation of CREB expression: in vivo evidence for a functional role in morphine action in the nucleus accumbens. J. Pharmacol. Exp. Ther. 1996;276:306–315. [PubMed] [Google Scholar]
- Wilensky A.E., Schafe G.E., LeDoux J.E. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J. Neurosci. 2000;20:7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim A.J., Moraes C.R., Ferreira T.L., Oliveira M.G. Protein synthesis inhibition in the basolateral amygdala following retrieval does not impair expression of morphine-associated conditioned place preference. Behav. Brain Res. 2006;171:162–169. doi: 10.1016/j.bbr.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Yin J.C., Tully T. CREB and the formation of long-term memory. Curr. Opin. Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- Yin J.C., Del Vecchio M., Zhou H., Tully T. CREB as a memory modulator: Induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Zanatta M.S., Quillfeldt J.H., Schaeffer E., Schmitz P.K., Quevedo J., Medina J.H., Izquierdo I. Involvement of the hippocampus, amygdala, entorhinal cortex and posterior parietal cortex in memory consolidation. Braz. J. Med. Biol. Res. 1997;30:235–240. doi: 10.1590/s0100-879x1997000200012. [DOI] [PubMed] [Google Scholar]