Abstract
Multiple trials by the World Health Organization have established levonorgestrel as the gold standard in hormonal emergency contraception (EC). However, changes in menstrual patterns following EC have been observed, so we undertook this prospective study carried out to identify and determine the characteristics of these changes. Women requesting EC at any of two hospitals, one family planning unit and 12 pharmacies in Yaoundé, Cameroon were enrolled if they had a history of regular menstrual cycles over the previous 3 months and agreed to follow up until the end of the subsequent menstrual cycle. Pre-treatment menstrual patterns were compared with those of the EC treatment cycle and the cycle after EC. In a set of 232 participants (mean age of 25), we observed 34 (14.7%) cases of incident intermenstrual bleeding and statistically significant changes in menstrual cycle length, menstrual period length and menstrual appearance compared to baseline, patterns which differed according to whether EC was taken well before, close to or well after expected ovulation for that cycle. The majority of these changes disappeared in the following cycle. Levonorgestrel emergency contraception is associated with significant but transient changes in menstrual patterns in a significant proportion of users.
Keywords: Adolescent; Adult; Contraceptives, Postcoital; administration & dosage; Female; Humans; Levonorgestrel; administration & dosage; Menstrual Cycle; drug effects; Menstruation; drug effects; Prospective Studies; Regression Analysis; Time Factors; Uterine Hemorrhage; epidemiology
Introduction
A trial by the World Health Organization published in 1998 established levonorgestrel (0.75 mg twice, 12 hours apart) as the gold standard in hormonal emergency contraception (EC), and more recent studies have demonstrated that the regimen can be safely simplified to a single-dose administration (1.5 mg) with no difference in effectiveness and no increase in side effects.1, 2, 3 Results from these studies and others indicate that menstrual bleeding and cycle disturbance are among the most frequent side effects associated with EC treatment, with some 30% of users reporting bleeding within seven days of treatment and up to 13% experiencing more than 7 days’ delay in menses.2, 4 Approved product information for levonorgestrel EC recommends performing a pregnancy test in the event that users’ next menstrual period is five or more days late, but detailed information on menstrual cycle characteristics (ie dysmenorrhea, intermenstrual bleeding, modifications in cycle length, persistence of disruption in subsequent cycles) following levonorgestrel EC is scarce, providing health care providers little guidance for counseling on this particular subject. Changes in menstrual patterns following EC use can be a source of concern and confusion for users, some of whom tend to misinterpret the occurrence of menstrual bleeding as a sign that treatment has been effective.5 Indeed, in the same study, the most frequent questions asked by EC users were related to vaginal bleeding and menstrual disturbance.
We undertook the present study in order to obtain specific information on bleeding patterns following levonorgestrel EC use in an effort to provide guidance for health care providers and EC users.
Materials and Methods
We carried out a prospective observational study of the menstrual cycle characteristics of women who requested EC between April 2003 and March 2004 at any of two hospitals, one family planning center or twelve pharmacies in Yaoundé, Cameroon. We obtained ethical approval of the protocol from the Department of Gynecology and Obstetrics of the University of Yaounde I School of Medicine before beginning enrollment.
Women were eligible for the study if they requested EC within 72 hours of a single act of unprotected intercourse and had a history of regular menstrual cycles and regular menstrual periods in the previous three months (range of no more than two days in cycle length and one day in menstrual period duration). They were included if they provided informed consent for participation in the study and agreed to refrain from unprotected intercourse for the remainder of the menstrual cycle and the duration of the following cycle. Women were excluded from the study if they were on hormonal contraception, had attempted to use abortifacient products, had further acts of unprotected intercourse in the same menstrual cycle, were found to be pregnant at the time of emergency contraception use, or were breastfeeding. Women found to be pregnant at the time of follow-up were excluded from the analysis population.
Data on the participants’ menstrual cycle characteristics were collected at inclusion and at two follow-up contacts scheduled directly after each of the next two menstrual periods. At inclusion, the principal investigator collected relevant information on each participant’s gynecological and obstetrical history and dispensed levonorgestrel-only EC (NorLevo®, HRA Pharma) free of charge. EC was administered on admission to the study according to the current standard of care as two 0.75 mg tablets in a single intake,6 and each participant was provided a bleeding questionnaire to be brought back at the follow-up visits with instructions to record information on any intermenstrual or menstrual bleeding (dates, intensity, appearance) between EC and the follow-up visits.
At inclusion, women were asked about their typical menstrual cycle (over the past three cycles), which provided their baseline characteristics. Two follow-up visits were scheduled: one at the end of the EC cycle (treatment cycle) and the other at the end of the next cycle (post-EC cycle). We used self-reported data for baseline cycle length (described above) and defined cycle length for the treatment and post-EC cycles as the interval from the onset of each respective menstrual period to the day before the onset of the next respective period. We considered as having dysmenorrhea at baseline any participant who declared to have this symptom always or sometimes in her previous three cycles. We defined intermenstrual bleeding following EC intake as any blood loss occurring in an interval from 0 to 7 days following EC intake and followed by menstrual-like bleeding in an interval of 7 to 20 days after the onset of intermenstrual bleeding. We considered any episode of bleeding in the EC cycle not meeting the definition of intermenstrual bleeding to be a menstrual period. The expected day of ovulation was calculated based on baseline cycle length minus 13 days.7
Data were entered using EPI INFO® 2000 and analyzed using STATA® version 6.0. For each participant, we first described baseline cycle characteristics (average over the past three cycles) and the circumstances surrounding EC treatment (reason, cycle day, delay between intercourse and EC). We then compared cycle characteristics (including cycle length and duration of menstrual period) between the baseline cycle and the cycle in which EC was taken (n=232) to evaluate the changes associated with EC. Finally, we compared cycle characteristics between the baseline cycle and the post-EC cycle (n=132) to evaluate whether these changes persisted. To do so, we used paired tests (chi-squared or Student according to whether the comparison dealt with proportions or means). In order to control for differences in EC effects according to the time in the menstrual cycle in which intake occurred, we stratified our sample into three groups based on the day of EC intake relative to expected ovulation: pre-ovulatory (EC intake 3 or more days before expected ovulation), peri-ovulatory (EC intake no more than 2 days before or after expected ovulation), and post-ovulatory (EC intake 3 or more days after expected ovulation).
Since paired tests were performed, these comparisons were automatically adjusted for the women’s baseline characteristics. However, we were interested in testing whether baseline characteristics played a role in changes after EC, for instance whether these changes were different according to women’s age or baseline cycle length. To answer these questions, we used mixed-effects regression models (logistic or linear) including interaction terms between baseline characteristics and EC or post-EC cycle characteristics.8
Results
Study population
A total of 344 women were included in the study and received EC treatment, and 235 returned for the first follow-up visit, of whom three were excluded because they were found to be pregnant (EC failure rate of 1.3%), comprising an analysis population of 232. A further 100 women did not return for the second follow-up visit. Loss to follow-up was not correlated with demographic or menstrual cycle characteristics but was positively associated with contraceptive use and varied according to profession (with a lower lost-to-follow-up rate among students) (Table 1). Women requested EC on average 23.7 hours (range 2–72 hours) after mid-cycle intercourse (mean cycle day of intercourse: 14.4 ± 4), and nearly all of them (97%) took EC between days 8 and 21 of the onset of their last menstrual period.
Table 1.
Characteristics of participants and EC requests
| Participants followed-up at the two visits (n=132) | Participants lost or excluded between inclusion and 1st visit (n=112) | Participants lost or excluded between inclusion and 1st and 2nd visit (n=100) | |
|---|---|---|---|
| Age (y) | 24.1 (16–31) | 25.0 (16–36) | 26.8 (17–36) |
| Profession | |||
| Student | 93 (70.5%) | 71 (63.4%) | 35 (35.0%) |
| Paid employment | 20 (15.2%) | 18(16.1%) | 28 (28.0%) |
| Homemaker/none | 19 (16.4%) | 23 (20.6%) | 37 (37.0%) |
| Parity | |||
| 0 | 82(62.1%) | 57 (50.9%) | 50 (50.0%) |
| 1 | 41 (31.1%) | 35(31.3%) | 12 (12.0%) |
| ≥2 | 9 (6.8%) | 20 (17.9%) | 38 (38.0%) |
| Marital status | |||
| Single | 110(83.3%) | 91 (81.3%) | 85 (85.0%) |
| Married | 21 (15.9%) | 19 (17.0%) | 13 (13.0%) |
| Widow | 1 (0.8%) | 2(1.8%) | 2 (2.0%) |
| Use contraception | 40 (30.3%) | 53 (47.3%) | 59 (59.0%) |
| History of spontaneous or induced abortion | 57 (43.2%) | 50 (44.6%) | 41 (41.0%) |
| Baseline average cycle length (days) | 28.4 (23–33) | 28.5 (24–33) | 28.5 (26–33) |
| Baseline average menstrual period (days) | 4.0 (3–6) | 4.0 (3–7) | 4.0 (3–7) |
| Time from intercourse to EC (h) | 24.0 (4–65) | 24.0 (6–61) | 23.2 (5–72) |
| Cycle day of intercourse | 14.4 (7–22) | 14.3 (7–22) | 14.4 (7–21) |
| Cycle day of EC administration | 15.6 (8–22) | 15.5 (8–22) | 15.6 (7–24) |
Baseline cycle characteristics
Participants’ baseline menstrual cycle characteristics are presented in Table 2, stratified according to the time of EC intake in the cycle. Mean cycle length was of 28.4 days, with an overall range of 23 to 33 days, menstrual period duration was on average 4 days, and over half of participants reported dysmenorrhea (53.2%). Menstrual appearance was described as red (78.6%) or dark (17.1%) in color, with very few women reporting the presence of blood clots (3.1%) or a sticky appearance (1.1%), and one-fourth (26.5%) of women reported a history of pre-menstrual spotting (data not shown).
Table 2.
Menstrual cycle characteristics according to date of EC relative to expected ovulation: pre-ovulatory >2 days before ovulation, peri-ovulatory=from 2 days before to 2 days after ovulation, post-ovulatory >2 days after ovulation
| Pre-ovulatory EC (n=64 for EC cycle, n=32 for post-EC cycle) | Peri-ovulatory EC (n=90 for EC cycle, n=56 for post-EC cycle) | Post-ovulatory EC (n=78 for EC cycle, n=44 for post-EC cycle) | |
|---|---|---|---|
| Cycle length (mean days) | |||
| Baseline | 28.8 | 29.2 | 27.2 |
| EC cycle | 28.0† | 29.2 | 28.9† |
| Post-EC cycle | 28.5 | 29.3 | 27.3† |
| Menstrual period duration (mean days) | |||
| Baseline | 4.3 | 3.9 | 3.9 |
| EC cycle | 4.5 | 4.3† | 4.2† |
| Post-EC cycle | 4.4 | 4.4† | 4.3† |
| Dysmenorrhea (incidence) | |||
| Baseline | 0.43 | 0.56 | 0.59 |
| EC cycle | 0.28† | 0.60 | 0.42† |
| Post-EC cycle | 0.5 | 0.59* | 0.27* |
p<0.05 for comparison with baseline compared to baseline by paired t-test or McNemar’s paired chi-square test
p<0.01 for comparison with baseline compared to baseline by paired t-test or McNemar’s paired chi-square test
EC treatment cycle characteristics
Data on the characteristics of the EC treatment cycle are available for 232 women. Cycle length during the treatment cycle was shortened by two or more days for 49 (21%) participants and lengthened by two or more days for 57 (24%) participants (Figure 1). Cycle length was shortened by approximately one day for women who took EC in the pre-ovulatory phase of the cycle and was lengthened by close to two days for women who took EC in the post-ovulatory phase of the cycle, and these changes reached statistical significance (p<0.01) (Table 2). No difference in cycle length was observed for women who took EC right around the time of expected ovulation. Menstrual period duration did not change with pre-ovulatory EC intake but increased significantly (p<0.01) with peri- or post-ovulatory intake. Estimated blood loss was equal to baseline in 68.5% of participants (data not shown). Regression models showed that young women (under 20 years) and women with short cycle lengths at baseline (less than 26 days) experienced significantly lengthened cycles after EC treatment (Table 3).
Figure 1.
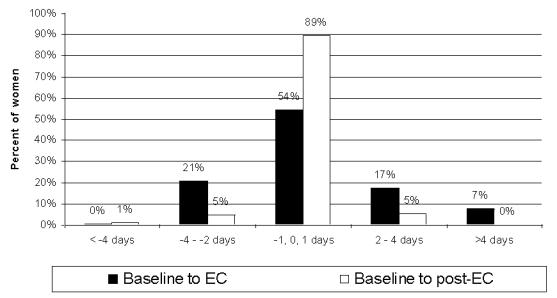
Change in menstrual cycle length (baseline compared to EC cycle and baseline compared to post-treatment cycle)
Table 3.
Variation in cycle length by age and baseline cycle length based on simple mixed-effects regression models including interaction terms between test variable and baseline cycle length (mean, 95% CI)
| EC treatment cycle compared to baseline (n=232) | Post-treatment cycle compared to baseline (n=132) | |||||
|---|---|---|---|---|---|---|
| n | Days | P | n | Days | P | |
| Age | ||||||
| <20 | 15 | 2.1 (0.9–3.4) | 0.001 | 13 | −0.7 (−1.4–0.06) | 0.07 |
| 20–25 | 85 | 0.4 (−0.03–0.8) | 0.07 | 60 | 0.2 (−0.1–0.5) | 0.18 |
| >25 | 132 | −0.02 (−0.5–0.4) | 0.94 | 59 | 0.1 (−0.3–0.5) | 0.59 |
| Baseline cycle length | ||||||
| <27 days | 46 | 2.3 (1.7–3.0) | < 0.001 | 36 | 0.9 (0.4–1.3) | < 0.001 |
| 27–29 days | 23 | 0.1 (−0.3–0.5) | 0.66 | 14 | 0.2 (−0.2–0.6) | 0.33 |
| >29 days | 85 | −0.5 (−1.0–0.01) | 0.05 | 56 | −0.5 (−0.8–0.1) | 0.01 |
Menstrual appearance during the EC treatment cycle was reported to be significantly darker and stickier than at baseline (p<0.001), but intra-individual changes in menstrual appearance were noticed in only 15% of cases (data not shown). The incidence of dysmenorrhea decreased (Table 2). During the EC treatment cycle, 34 (14.7%) participants experienced intermenstrual bleeding which lasted an average of 2.4 days (range 1–7) and was generally lighter in abundance than regular menstrual bleeding (89% of cases) but did not differ in appearance from baseline menses (data not shown). The cases of intermenstrual bleeding can be considered as incident as they occurred almost entirely (in 98% of cases) in participants who had not reported pre-menstrual spotting at baseline. Intermenstrual bleeding began as early as 8 hours after EC intake and on average on the fourth day thereafter and was significantly (p<0.01) more common for women who had intercourse early in the cycle, several days before their presumed date of ovulation.
Post-treatment cycle characteristics
Data on the post-treatment cycle are available for 132 women. Cycle length returned to baseline values in the post-treatment cycle for pre-ovulatory EC intake but remained slightly longer than baseline for post-ovulatory EC intake (Table 2, Figure 1). Although menstrual periods remained slightly longer than baseline for the peri- and post-ovulatory groups (Table 2), the range of values was notably tighter, with no participant reporting a menstrual period longer than six days (data not shown). Menstrual appearance also returned the profile reported at baseline (data not shown). The decrease in dysmenorrhea reported in the treatment cycle persisted in the post-treatment cycle (Table 2). Estimated blood loss was equal to baseline in 99% of participants (data not shown).
Regression models (Table 3) confirmed that the overall return to normal profile was consistent across all age groups as well as for women with baseline cycle length of 27–29 days. Women with short (<27 days) or long (>29 days) baseline cycles had a persistent lengthening or shortening of their cycles, respectively, compared to baseline.
Discussion
In this study of levonorgestrel EC use in women with a history of regular menstrual cycles, we observed significant but transient changes after EC in menstrual cycle characteristics including cycle length, duration of menses, menstrual appearance and incidence of intermenstrual bleeding. Cycle characteristics returned largely to baseline values in the next complete menstrual cycle.
Inclusion in this study was based on menstrual cycle regularity defined as limited intra-individual variability in cycle length (+/− 2 days) and menstrual period duration (+/− 1 day). Were these inclusions criteria too strict? Epidemiological literature on menstrual cycle regularity is very poor. The menstrual cycle of a human being is characterized by a mean interval of 28 days between two menses.9 Significant natural variability in menstrual cycle length has been observed, even among women who believe they have “regular” cycles; indeed, in a recent study nearly half of women who claimed to have a consistent cycle length in fact experienced a cycle length range of +/− 7 days.10 However, in the same study, average cycle length was not more than two days different than the estimated cycle length in 62% of participants, suggesting that a majority of women are able to accurately describe their cycle length.
We set out to collect objective data on menstrual cycle characteristics, a task which proved possible for all parameters except one: menstrual appearance. Participants recorded their own impressions of menstrual appearance, and no blinded objective assessment was made of this parameter. We recognize that the changes observed in menstrual appearance (differences in color) between the baseline and EC cycles could in fact be the product of observation bias, in that participants expected their menses to appear different after EC treatment. Intra-individual changes were noticed in 15% of cases.
Being able to predict changes in cycle length after EC is very important so that women can be counseled and reassured about what to expect. In our study, taking EC early in the menstrual cycle (two or more days before expected ovulation) was associated with a shortened cycle length and incident intermenstrual bleeding, which is consistent with the fact that levonorgestrel administration in the early follicular phase is associated with a blunted LH peak, shortened luteal phase length and ovulation blockade.11,12,13
Taking EC later in the menstrual cycle (two or more days after expected ovulation), on the other hand, tended to prolong the menstrual cycle, as can be expected in light of the direct relationship that has been observed between circulating progestogen metabolite concentrations and luteal phase length.14 On the overall, EC treatment was associated with a significant but slight increase in menstrual cycle length, which reinforces the importance of current recommendations for pregnancy testing in the event that the onset of next menses is delayed by more than five days after EC intake (NorLevo product information).
We recorded 34 cases (14.7%) of incident intermenstrual bleeding within seven days of EC, which is similar to the rates of bleeding not related to menses seen in previous large-scale studies: 16% in a WHO study of a total of 1978 LNG EC users,2 and 16% in a Nigerian study of 544 LNG EC users.15 Progestogen-only regular contraceptive methods (oral pills, implants or progestogen-only releasing intra-uterine systems) are associated with frequent vaginal bleeding disturbances and breakthrough bleeding,16,17 and although the pathogenesis of such breakthrough bleeding remains ill-defined it appears to be mediated via progestogen-induced down regulation of sex steroid receptors and altered expression of local mediators that are implicated in menstruation and differentially expressed throughout the menstrual cycle.18,19 Further studies with histopathological endpoints may be needed to understand the mechanism of this effect.
The seemingly significant decrease in frequency of dysmenorrhoea is likely biased by our data collection methods, because we compared cumulative incidence over the three cycles before EC to incidence in single cycles. A study taking into account only women who always have dysmenorrhoea is needed to confirm our results.
A growing body of literature recommends co-prescription of EC with hormonal oral contraception (OC) in the event of OC compliance problems,6 but OC use induces artificial bleeding patterns that are completely different in their nature from natural cycle characteristics, so we chose to exclude OC users from our study. It is critical, however, for health care providers to be particularly sensitive to the question of bleeding patterns among OC users who miss their pills in light of the fact that continual OC use could mask an early pregnancy.
In Cameroon, hormonal oral contraception has been available since 1980, and family planning efforts were first officially integrated into national public health policy in 1989; a study of the evolution of family planning in Cameroon between the years 1974 and 2000 showed an increase in the number of family planning clinics from 1 to 374 and in the number of users of modern contraceptive methods from 250 to over 1 million (D. Nouthe and E.R. Mbu, unpublished data, 2000). The overall prevalence of modern contraceptive methods, an estimated 7.5%, remains low compared to figures from developed countries but is among the highest in sub-Saharan Africa.20 A dedicated levonorgestrel-only EC product has been available since 2002 and is distributed in certain hospitals, family planning centers and pharmacies on a non-prescription basis. The participants in this study mirror the overall population of EC users in Cameroon, consisting for the majority of unmarried students and young professionals (NEC Nguepi, unpublished data, 1999).
Follow-up of our population was a challenge, probably related to the diversity of recruitment sites involved in the study (numerous pharmacies, hospitals, family planning center). Thirty percent of the follow-up data available were obtained by telephone, and in 24% of cases, participants returned only after a phone call. It is critical to evaluate the potential impact of the relatively high loss to follow up on our results. Women lost to follow-up were not different in terms of key traits that would likely have an impact on the results observed (demographics, baseline menstrual cycle characteristics). If participants who experienced incident bleeding irregularities were more prone to return for follow-up than others, our results would overestimate the effect of levonorgestrel EC on menstrual cycle characteristics. This does not seem likely in light of the fact that our results (incidence of intermenstrual bleeding, for example) are in line with published studies.2, 15 It is unlikely that women were lost to follow-up due to pregnancy, as pregnant women would have been in touch with the investigational site for pregnancy counseling and care; further, the EC failure rate observed is similar to that documented in published studies.1, 2, 4 Loss to follow-up was particularly high for the post-treatment cycle, but it seems reasonable to hypothesize that a number of participants did not feel the need to return for follow-up because their menstrual cycles had returned to normal by that time.
A study currently in press by Raymond and colleagues conducted at nearly the same time as ours in a population of American women found that when taken in the first 3 weeks of the menstrual cycle, levonorgestrel EC significantly shortened that cycle as compared both with the usual cycle length and with the cycle duration in a comparison group of similar women who had not taken EC.21 These results correspond to what we observed for women who took EC in the pre-ovulatory phase but are in contradiction with what we saw for EC taken in the peri-ovulatory or post-ovulatory phases. Even though our results differ from Raymond’s in terms of the direction of the impact of EC treatment in the second half of the menstrual cycle on cycle length, both studies indicate that any menstrual cycle changes produced by levonorgestrel EC appear to disappear in subsequent cycles.
The two main differences between the studies appear to lie in stratification techniques and definitions of bleeding parameters. To explore whether these difference could explain the seemingly contradictory results, we re-ran our analyses stratifying, as Raymond et al did, by cycle week of treatment (week 1 to 4) rather than by date of EC relative to expected ovulation. We were not able to reproduce Raymond et al’s results, and this approach to stratification produced highly imbalanced groups because 97% of the women in our study took EC in cycle week 2 or 3. In this analysis of our data, treatment in week 2 shortened the cycle whereas treatment in week 3 lengthened it, similar to what we observed for pre- and post-ovulatory treatment. What is missing from the week-wise stratification but visible in our approach is that women treated right around expected ovulation (in the peri-ovulatory phase, as we define it) do not experience significant changes in cycle length, whereas those treated 3 or more days before or after ovulation do.
The two studies also differ in design with respect to definitions of intermenstrual bleeding versus menstrual bleeding. Raymond et al considered that intermenstrual bleeding could last no more than two consecutive days and that menstrual periods corresponded to episodes of at least 3 days of bleeding (either consecutive or separated only by 1 bleeding-free day) preceded by at least 2 days without bleeding. In our study, women who had episodes of bleeding 3 days long or more followed by menstrual-like bleeding 7 to 20 days later were considered to have had intermenstrual bleeding. In order to explore the impact of such difference in definition on the overall results, we re-ran our analyses after having reclassified women for whom our definitions diverged. In concrete terms, this meant re-categorizing intermenstrual bleeding as a menstrual period for 14 women in our study who reported episodes of intermenstrual bleeding that lasted 3 days or more. With this new classification, we re-ran our analyses, stratifying first by time of EC relative to ovulation, as in our study, and then by week of treatment, as per Raymond et al. The results revealed that reclassification of the 14 women in question with stratification according to our method did not reverse the statistically significant trends we have described: shortening of cycle length for pre-ovulatory treatment, no change in cycle length for peri-ovulatory treatment, and lengthening of cycle length for post-ovulatory treatment. When we stratified by cycle length of treatment, however, the results were different: no difference for week 1, significant shortening for week 2 and, curiously enough, lesser but still statistically significant shortening for week 3. Therefore, differences in definitions may indeed account for some of the differences between the two studies in cycle length patterns for women who took EC in the second half of the cycle. Nonetheless, in our sample, all of the women with episodes of intermenstrual bleeding according to our definition had full menstrual periods an average of 12 days later (range 7 to 16), for an overall EC cycle length of 29.6 days (range 25–35). Such a pattern has been seen in some studies to mechanistically be associated with an aborted LH peak and subsequent LH peak some 7 to 16 days later, followed by a normal rise in urinary pregnanediol.12 We therefore wonder about the clinical relevance of calling such episodes of bleeding menstrual periods to the extent that a risk of fecundation may persist for that cycle.
Finally, the two studies also differ in design with respect to inclusion criteria, methods of data collection, and presence comparison group. The inclusion criteria in our study in terms of regularity of cycle length and menstrual period duration were stricter than for Raymond et al, which may have produced a sample with cycle lengths more homogeneous, more robust and less susceptible to fluctuation than in the Raymond sample. Data in both studies were self-reported by the participants, but data collection tools were different: daily diary cards for Raymond et al versus bleeding questionnaires in our study. No external comparison group was available in our study, which is a weakness in comparison to Raymond et al, although intra-subject comparison to baseline in our study was based on average cycle characteristics over the three previous cycles before EC treatment rather than merely on “usual menstrual cycle length” as in Raymond et al. A more detailed comparison of data and analysis methods from the two studies may be necessary to understand the reasons behind the apparently inconsistent results. On the overall, however, the two studies are consistent in that they both indicate that any changes in bleeding patterns produced by levonorgestrel EC in the treatment cycle tend to disappear in the subsequent cycle.
In conclusion, this study is important in that it provides health care providers with clear data for counseling patients on intermenstrual bleeding (when, how long), cycle length and menstrual period characteristics after EC use. Even though our results confirm previous reports that levonorgestrel EC can indeed produce changes in menstrual cycle characteristics, patients and providers can be reassured that the changes experienced are transient and resolve in the next cycle.
Acknowledgments
The study’s field costs were financed by a grant from HRA Pharma, and the NorLevo treatments dispensed during the study were donated by HRA Pharma.
References
- 1.World Health Organization Task Force on Postovulatory Methods of Fertility Regulation. Randomized controlled trial of levonorgestel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428–38. [PubMed] [Google Scholar]
- 2.von Hertzen H, Piaggio G, Ding J, et al. Low doses of mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO randomised trial. Lancet. 2002;360:1803–10. doi: 10.1016/S0140-6736(02)11767-3. [DOI] [PubMed] [Google Scholar]
- 3.Cheng L, Gulmezoglu AM, Oel CJ, Piaggio G, Ezcurra E, Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2004;(3):CD001324. doi: 10.1002/14651858.CD001324.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Arowojolu AO, Okewole IA, Adenkule AO. Comparative evaluation of the effectivness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception. 2002;66:269–73. doi: 10.1016/s0010-7824(02)00337-2. [DOI] [PubMed] [Google Scholar]
- 5.Gainer E, Sollet C, Ulmann M, et al. Surfing on the morning after: analysis of an emergency contraception website. Contraception. 2003;67:195–99. doi: 10.1016/s0010-7824(02)00491-2. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Department of Reproductive Health and Research. Selected Practice Recommendations for Contraceptive Use. 2. Geneva: World Health Organization; 2004. [Google Scholar]
- 7.Trussell J, Rodríguez G, Ellertson C. New estimates of the effectiveness of the Yuzpe regimen of emergency contraception. Contraception. 1998;57(6):363–9. doi: 10.1016/s0010-7824(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 8.Brown H, Prescott R. Applied mixed models in medicine. New York: John Wiley & Sons; 1999. [Google Scholar]
- 9.Khan-Sabir N, Carr BR. Rebar RW, editor. The normal menstrual cycle and the control of ovulation. [Accessed August 28, 2005];Female reproductive endocrinology. Available at: http://www.endotext.org/female/female3/femaleframe3.htm.
- 10.Creinin MD, Kerveline S, Meyn LA. How regular is regular? An analysis of menstrual cycle regularity. Contraception. 2004;70:289–92. doi: 10.1016/j.contraception.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Gemzell-Danielsson K, Marions L. Mechanisms of action of mifepristone and levonorgestrel when used for emergency contraception. Hum Reprod Update. 2004;10(4):341–8. doi: 10.1093/humupd/dmh027. [DOI] [PubMed] [Google Scholar]
- 12.Hapangama D, Glasier AF, Baird DT. The effects of peri-ovulatory administration of levonorgestrel on the menstrual cycle. Contraception. 2001;63(3):123–9. doi: 10.1016/s0010-7824(01)00186-x. [DOI] [PubMed] [Google Scholar]
- 13.Durand M, del Carmen Cravioto M, Raymond EG, et al. On the mechanisms of action of short-term levonorgestrel administration in emergency contraception. Contraception. 2001;64(4):227–34. doi: 10.1016/s0010-7824(01)00250-5. [DOI] [PubMed] [Google Scholar]
- 14.Windham GC, Elkin E, Fenster L, et al. Ovarian hormones in premenopausal women: variation by demographic, reproductive and menstrual cycle characteristics. Epidemiology. 2002;13(6):675–84. doi: 10.1097/00001648-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Arowojolu AO, Okewole IA. Vaginal bleeding following the use of a single dose of 1.5mg levonorgestrel (LNG) for emergency contraception. West Afr J Med. 2004;23(3):191–3. doi: 10.4314/wajm.v23i3.28118. [DOI] [PubMed] [Google Scholar]
- 16.Anderson FD. Bleeding and Contraceptive Steroids. In: Alexander NJ, D’Arcangues C, editors. Steroid hormones and uterine bleeding. Washington, DC: AAAS Press; 1992. pp. 7–23. [Google Scholar]
- 17.Peers T, Stevens JE, Graham J, Davey A. Norplant implants in the UK: first year continuation and removals. Contraception. 1996;53:345–351. doi: 10.1016/0010-7824(96)00083-2. [DOI] [PubMed] [Google Scholar]
- 18.Critchley HOD, Jones RL, Lea RG, et al. Role of inflammatory mediators in human endometrium during progesterone withdrawal and early pregnancy. J Clin Endocrin Metab. 1999;84:240–8. doi: 10.1210/jcem.84.1.5380. [DOI] [PubMed] [Google Scholar]
- 19.Vincent AJ, Salamonsen LA. The role of matrix metalloproteinases and leukocytes in abnormal uterine bleeding associated with progestin-only contraceptives. Hum Reprod. 2000;15 (Suppl 3):135–143. doi: 10.1093/humrep/15.suppl_3.135. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein S, Ryan WA, editors. United Nations Population Fund. The State of World Population 2003. New York: United Nations Population Fund; 2003. [Google Scholar]
- 21.Raymond EG, Goldberg A, Trussell J, Hays M, Roach E, Taylor D. Bleeding patterns after use of levonorgestrel emergency contraceptive pills. Contraception. 2006;73(4) doi: 10.1016/j.contraception.2005.10.006. in press. [DOI] [PubMed] [Google Scholar]


