Abstract
Drosophila melanogaster photoreceptor cells are capable of detecting single photons. This utmost sensitivity is critically dependent on the maintenance of an exceedingly low, dark, spontaneous activity of photoreceptor cells. However, the underlying mechanisms of this hallmark of phototransduction are not fully understood. An analysis of the Drosophila visual heterotrimeric (αβγ) Gq protein revealed that wild-type Drosophila flies have about a twofold excess of Gβ over Gα subunits of the visual Gq protein. Studies of Gβe mutants in which the excess of Gβ was genetically eliminated showed dramatic dark, spontaneous activity of the photoreceptor cells, whereas concurrent genetic reduction of the Gα subunit, which restored the excess of Gβ, abolished this effect. These results indicate that an excess of Gβ over Gα is a strategy used in vivo for the suppression of spontaneous activity, thereby yielding a high signal to noise ratio, which is characteristic of the photoreceptor light response. This mechanism could be relevant to the regulation of G protein signaling in general.
Introduction
Many signaling systems use heterotrimeric (αβγ) G proteins to relay signals from heptahelical receptors to downstream effectors. To accomplish signal transduction, G proteins act as conformational sensors of a guanine nucleotide, which is bound to the α subunit. G proteins that are charged with guanosine diphosphate (GDP) are in the inactive state, where the α and the βγ subunits are associated with each other. Receptor activation accelerates the exchange of bound GDP for free GTP (Cassel and Selinger, 1978) followed by the dissociation of active Gα-GTP from βγ subunits. Hydrolysis of the bound GTP by a GTPase reaction brings the Gα subunit back to the inactive state (Cassel et al., 1977), which is characterized by tightly bound GDP and a reassociation with the βγ complex. To ensure specificity, high effective concentrations, and speed of interaction, the G protein signaling components are usually attached to the membrane domain as peripheral membrane proteins.
Membrane attachment of heterotrimeric G proteins has been extensively investigated, and the effect of lipid modification on membrane localization has been addressed by several studies (Wedegaertner, 1998; Resh, 1999; Chen and Manning, 2001; Kosloff et al., 2002, 2003; Smotrys and Linder, 2004). All G protein α subunits (with the exception of transducin) are palmitoylated, and some are additionally modified by myristoylation. The α subunits of Gq/G11, including the Drosophila melanogaster eye–specific Gqα, as well as Gsα, G12α, and G13α are modified only by palmitoylation. The corresponding βγ subunits undergo isoprenylation of a cysteine residue at the so-called CAAX box of the γ subunit (for review see Wedegaertner, 1998; Resh, 1999; Chen and Manning, 2001). Plasma membrane attachment of the α subunits Gsα and Gqα is dependent on coexpression with the βγ subunits (Evanko et al., 2000, 2001). Furthermore, the βγ subunits, having only one membrane attachment signal on the γ subunit, are poorly targeted to the plasma membrane and require coexpression of the α subunit for efficient plasma membrane attachment (Evanko et al., 2001; Michaelson et al., 2002; Takida and Wedegaertner, 2003). Altogether, these studies led to a model of two membrane attachment signals that are needed for plasma membrane attachments and localization of heterotrimeric G protein subunits (Wedegaertner, 1998; Resh, 1999). It should be noted, however, that most of these studies have been performed by using various culture cells that were transfected with vectors yielding overexpressed proteins (usually the α and βγ subunits of the heterotrimeric G protein). This procedure is bound to cause distortion of the original stoichiometry of α and βγ subunits, which is difficult to control under these conditions. The extensively studied Drosophila visual system combined with the large repertoire of Drosophila visual mutants offer a unique opportunity to study in vivo the various roles of the βγ dimer, its cellular localization, and the functional consequences of altering α/βγ stoichiometry.
The Drosophila visual system is a specialized system that is composed of highly polarized and compartmentalized cells that sequester the phototransduction machinery in a specific signaling compartment called the rhabdomere (Minke and Hardie, 2000; Hardie and Raghu, 2001). This signaling compartment is functionally equivalent to the vertebrate rod photoreceptor outer segment, which also sequesters the phototransduction machinery in a specific cell compartment. Phototransduction in Drosophila is initiated upon the activation of rhodopsin by light and proceeds through a photoreceptor-specific Gq protein (Gqe; Scott et al., 1995), which, in turn, activates the phospholipase C enzyme effector (Devary et al., 1987). Upon activation, the eye-specific Gqα subunit (Gqαe) dissociates from the eye-specific βγ dimer (Gβγe) and translocates, at least in part, from the membrane to the cytosol (Kosloff et al., 2003; Cronin et al., 2004).
In this study, we show (by using a series of eye-specific Gβe hypomorph mutants) that the βγ dimer has a crucial role in both membrane attachment and rhabdomeral targeting of the α subunit that can account for the decreased light sensitivity previously observed in these mutants (Dolph et al., 1994). On the other hand, by using the almost null mutant for the eye-specific Gqα subunit Gαq 1, we found that the βγ dimer is dependent on the α subunit for membrane attachment but not for targeting to the rhabdomere, suggesting a role for the βγ dimer in targeting the heterotrimer to the photoreceptor signaling compartment (the rhabdomere). An analysis of the protein levels of Gqαe and Gβe subunits revealed a surprising twofold excess of the Gβe subunit over the Gqαe subunit. Mutants that eliminated this excess showed a dramatic increase in spontaneous activity of the phototransduction cascade. Conversely, double mutations that also reduced the level of Gqαe and, thereby, restored the excess of Gβe over Gqαe completely reversed this phenotype. Together, these results provide a significant insight into the strategy used by the photoreceptor cell in vivo to avoid spontaneous activity at the G protein level.
Results
The levels of Gqαe and Gβe subunits in Drosophila photoreceptors are maintained independently of one another
The α subunit of the heterotrimeric G protein and the tightly associated complex of βγ subunits undergo dissociation and reassociation during activation of the phototransduction cascade. Therefore, it is expected that these subunits would influence one another's level, localization, and function. Previous studies that addressed these questions used tagged subunits and heterologous expression in tissue culture cells. Qualitatively, it is now generally accepted that plasma membrane attachment of the α subunit requires coexpression of the βγ subunit complex (Degtyarev et al., 1994; Evanko et al., 2000, 2001), and, reciprocally, plasma membrane attachment of the βγ subunit complex requires coexpression of the α subunit (Michaelson et al., 2002; Takida and Wedegaertner, 2003). Although a great deal has been learned from these previous studies, little is known about the localization of G protein subunits in their natural environment and how the stoichiometry of these subunits affects the level, localization, and function of G protein subunits under physiological conditions. To test the effect of various subunits on the level of one another, we have used the Drosophila eye–specific Gβ subunit mutants (Gβe) that were described by Dolph et al. (1994) and the eye-specific Gqα subunit mutant (Gαq 1) that was described by Scott et al. (1995).
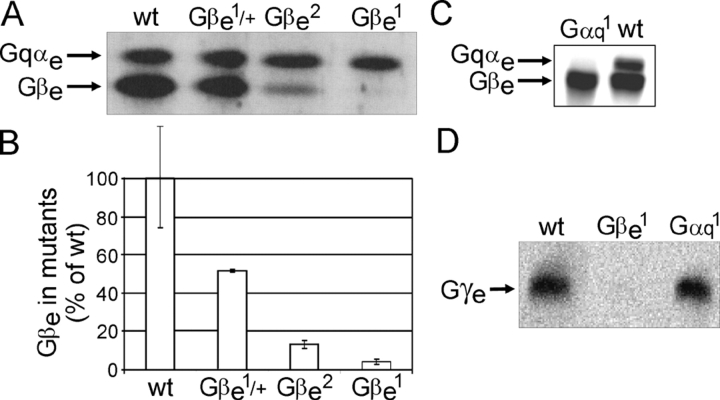
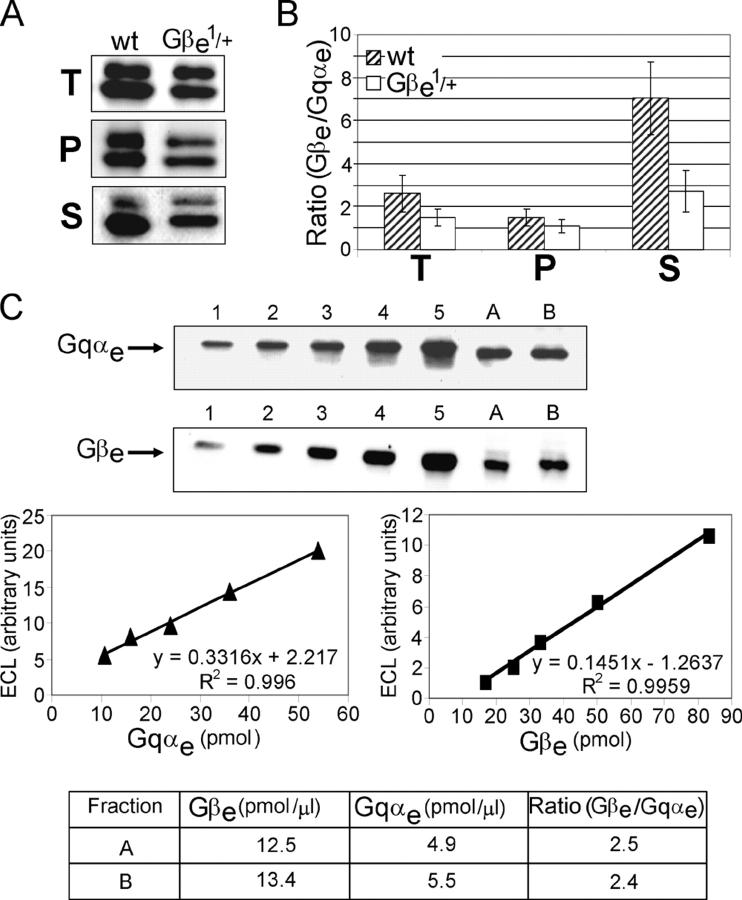
The hypomorph Gβe mutants Gβe 1, Gβe 2, and the heterozygote of the most severe mutant, Gβe 1/+, express the Gβe subunit protein at levels of 4, 13, and 50% of wild-type flies, respectively (Fig. 1, A and B). Despite the progressive decrease in the Gβe subunit level in these mutants, the level of the α subunit was undiminished and is the same level as in wild-type flies (Fig. 1 A). Similarly, in the Gαq 1 mutant, which expresses negligible levels of the α subunit, the level of the Gβe subunit was the same as in wild-type flies (Fig. 1 C). Although the levels of the Gβe subunit that we found in the Gβe 1 and Gβe 2 mutants were higher than those previously reported (Dolph et al., 1994), the progressive decrease of the Gβe subunit protein among these mutants was similar (Fig. 1 B). The eye-specific Gγe subunit, which forms an extremely tight complex with the Gβe subunit, completely disappeared in the severe Gβe 1 mutant but, like Gβe, was undiminished in the Gαq 1 mutant (Fig. 1 D). Therefore, we can conclude that the Gβe mutants are, in fact, βγ mutants and that the effects observed in Gβe mutants can be ascribed to a decrease in the level of the βγ subunit dimer without effecting the level of the α subunit.
Figure 1.
The levels of DGqe subunits in Gβe and Gαq1 mutants. (A) The levels of Gβe and Gqαe were determined for the three different dark-adapted Gβe mutants (Gβe 1, Gβe 2, and heterozygous Gβe 1/+) using Western blot analysis. Aliquots containing equivalent protein amounts of total head homogenates were separated on a 7.5–15% gel and were visualized with a mixture of Gqαe and Gβe antibodies at saturating concentrations. Each mutant has different levels of Gβe, whereas the level of Gqαe remains constant. (B) Quantification of Gβe levels in different Gβe mutants. The wild-type percentage level was set as 100%. Gβe levels in the heterozygous Gβe 1/+ mutant, the Gβe 2 mutant, and the most severe mutant, Gβe 1, are 50, 13, and 4%, respectively. Data represent mean values ± SEM (error bars) from seven independent experiments. (C) The levels of Gqαe and Gβe in dark-adapted Gαq 1 mutant and in wild-type flies were determined using Western blot analysis. The results show that Gβe levels are maintained independently of Gqαe. (D) Determination of Gγe levels in dark-adapted wild-type, Gβe 1, and Gαq 1 flies using Western blot analysis shows that the level of Gγe is completely dependent on Gβe but not on Gqαe.
The Gβγe subunits are essential for membrane attachment and targeting of the Gqαe subunit to the rhabdomere
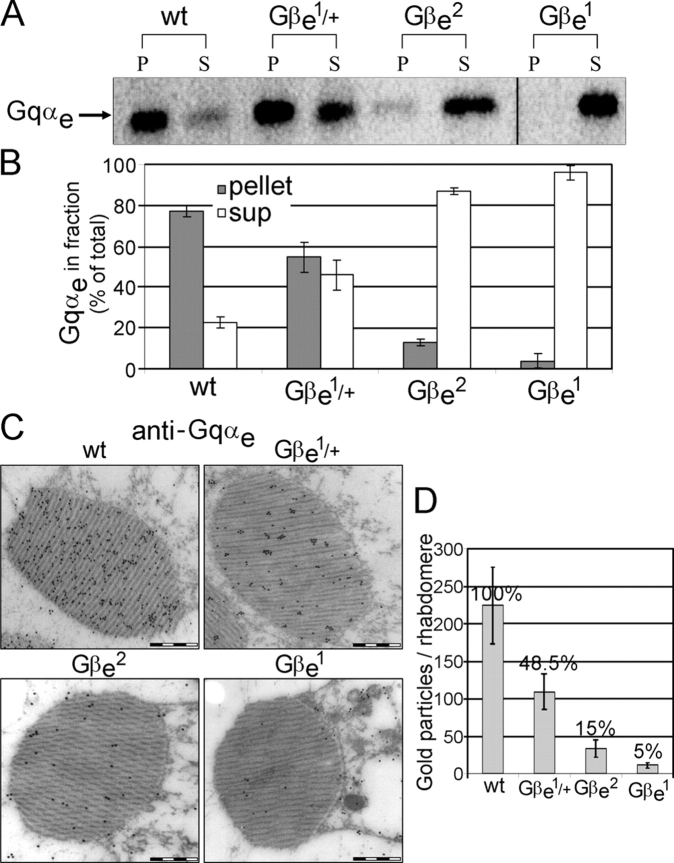
To understand how Gβe affects the localization of Gqαe, we extended our analysis to membrane attachment and targeting of the α subunit in Gβe mutants. As shown in Fig. 2, the low levels of βγ subunits in Gβe mutants cause a progressive decrease in the fraction of the α subunit that is attached to the membrane. Quantitatively, the decrease in membrane attachment of the α subunit is proportional to the percent decrease in the level of the β subunit.
Figure 2.
Gβe determines the membrane and rhabdomeral localization of Gqαe. (A) Western blot analysis shows the localization of Gqαe in the membrane (P, pellet) and in cytosol (S, supernatant) in dark-adapted Gβe mutants. (B) Gqαe distribution between the membrane and cytosol of dark-adapted Gβe mutants and of wild-type flies is represented by the percentage of Gqαe in each fraction (P and S) out of the total Gqαe amount (P + S) in each mutant. Data represent mean values ± SEM from five independent experiments. (C) Immunogold EM analysis of cross sections of a single rhabdomere using Gqαe antibodies that were applied to dark-adapted wild-type flies and Gβe mutants. Bars, 500 nm. (D) Number of gold particles in a cross section of a single rhabdomere. Each gold particle represents a Gqαe molecule. Data represent mean values ± SEM (error bars) from 20 different rhabdomeres for each mutant. Wild-type percentage level was set as 100%.
The molecules that participate in phototransduction, including the eye-specific DGqe subunits, are confined to a specific signaling compartment (the rhabdomere). Thus, we investigated how the decreased levels of βγ subunits affect the targeting of the α subunit to the signaling compartment. Using immunogold EM with antibodies against the eye-specific α subunit, we counted the gold particles in 20 cross sections of equal size from wild-type and mutant rhabdomeres. This analysis revealed that the quantity of the Gqαe subunit in the rhabdomere of different mutants corresponds with the level of the α subunit that is membrane attached (Fig. 2) and indicates that the βγ subunit complex controls both membrane attachment and rhabdomeral targeting of the α subunit.
The reduced light sensitivity of Gβe mutants is caused by the mislocalization of Gqαe
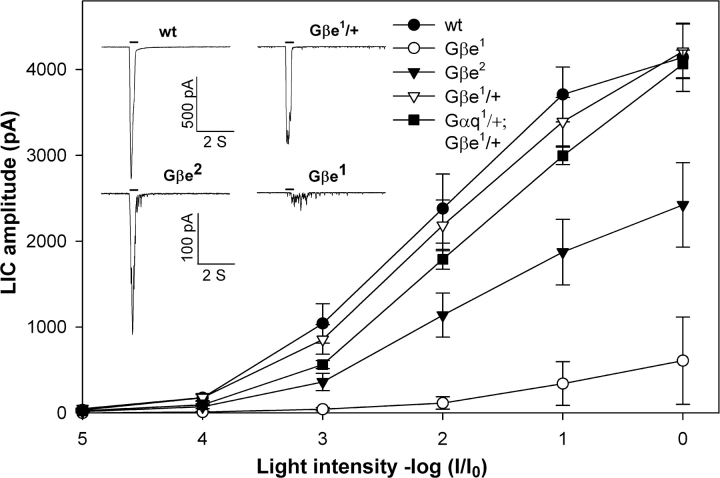
One of the major advantages of Drosophila for the study of phototransduction in vivo is the ability to examine the electrophysiological response in detail and characterize the phenotype that results from a decrease in a specific phototransduction component, which is caused by mutation. Two physiological phenotypes were observed for Gβe mutants (Dolph et al., 1994). The first phenotype was a dramatic loss of light sensitivity (reaching a decrease by two orders of magnitude in the Gβe 1 mutant), and the second phenotype was a slow termination of the light response. To address the possibility that the reduced sensitivity to light in Gβe mutants arises from a reduction in membrane-bound Gqαe, we reexamined the sensitivity to light in four Drosophila mutants with reduced levels of Gβe. Figs. 2 and 3 show a correlation between the level of membrane-bound Gqαe (Fig. 2) and the sensitivity of the response to light (Fig. 3) in which low levels of membrane-bound Gqαe correspond to low light sensitivity. The latter was accompanied by a modified waveform of the light-induced current (Fig. 3, inset). The fact that heterozygous Gβe 1/+ showed only a minor reduction in the sensitivity to light is consistent with previous results showing that 50% of Gqαe is sufficient to maintain normal sensitivity to light (Scott et al., 1995). Together, these results indicate that the loss of light sensitivity is caused by the effect of Gβe mutants on membrane attachment and targeting of the Gqαe subunit to the signaling compartment (the rhabdomere). Clearly, when rhodopsin and Gqαe are present in different cellular compartments, the Gqαe subunit cannot transfer signals from rhodopsin to the phospholipase C enzyme.
Figure 3.
Sensitivity to light in wild-type flies and Gβe mutants. Mean peak of the light-induced currents (LIC) in response to increasing intensities of orange light is plotted as a function of the light intensity. Gβe 1 mutants (open circle) showed a 2-log reduction in sensitivity to light, whereas Gβe 2 mutants (closed triangle) showed an ∼0.7 log reduction in sensitivity to light. Gβe 1/+ (open triangle) and Gαq 1/+;Gβe 1/+ (closed square) were not significantly different from wild-type flies (closed circle). Each curve represents a mean of >10 different experiments. Error bars represent SEM. Inset shows whole cell recordings of LIC from isolated ommatidia clamped at −70 mV. The maximal orange light intensity was attenuated by 2 log units in all of the traces. Note the different scales for the top and bottom traces.
Membrane localization of the Gβe subunit
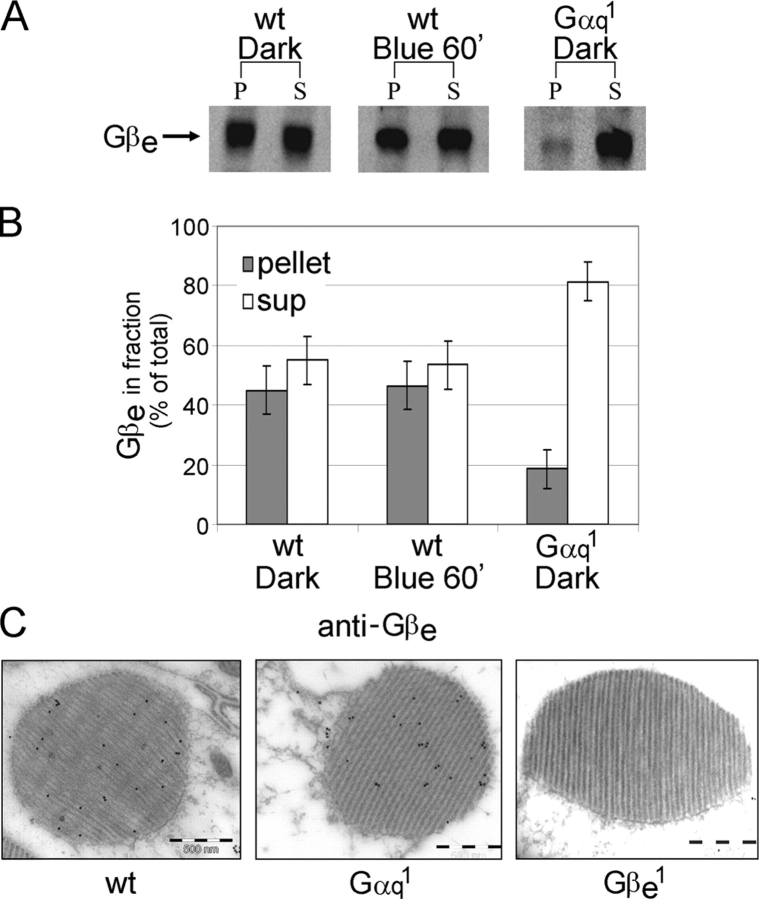
To examine the effect of Gqαe on the localization of Gβe, we measured the distribution of Gβe between the membrane and cytosol in wild-type and Gαq1 mutant flies. In contrast to the light-dependent translocation of Gqαe from the membrane to the cytosol (Kosloff et al., 2003), Gβe was about equally distributed between the membrane and the cytosol under both light and dark conditions (Fig. 4, A and B). A longer period of illumination for up to 4 h did not alter the Gβe distribution (not depicted). These results suggest that the βγ complex remains partly bound to the membrane even when the α subunit is translocated to the cytosol. Indeed, it has been shown that although rhodopsin–Gα interactions are reduced upon activation, rhodopsin–Gβγ interactions remain undiminished (Phillips and Cerione, 1992). Moreover, electrostatic calculations showed that upon dissociation from the Gα subunit, the β subunit of transducin exposed a prominent patch of basic residues that enhanced the membrane affinity of the βγ dimer by about an order of magnitude (Murray et al., 2001). However, it has also been shown in the rat visual system that Gβγ subunits translocate from the outer to the inner rod segment in response to light, albeit at a slower rate than the translocation of the α subunit (Sokolov et al., 2002).
Figure 4.
Membrane attachment and rhabdomeral targeting of Gβe. (A) Western blot analysis shows the localization of Gβe in membrane (P, pellet) and in cytosol (S, supernatant) of wild-type, dark-adapted, or illuminated flies and of dark-adapted Gαq 1 flies. Illumination was with blue light for 60 min. In wild-type flies, Gβe was about equally distributed between the membrane and the cytosol both under dark and light conditions. In Gαq 1 mutant flies, however, Gβe failed to reach the membrane and was mostly soluble. (B) Percentage of Gβe in fractions (P and S) out of the amount of total Gβe (P + S) of each treatment. Data represent mean values ± SEM (error bars) from 10 independent experiments. (C) Immunogold EM analysis of cross sections of a single rhabdomere from dark-adapted wild-type flies and Gαq 1 mutant flies using affinity-purified Gβe antibodies revealed that Gβe is targeted to the rhabdomere even in the near absence of Gqαe. Gβe 1 mutant flies were used as a control for the specificity of the antibodies. Bars, 500 nm.
The effect of Gqαe on the membrane attachment of Gβe was further studied using the Gαq1 mutant. In this mutant, which has a negligible level of Gqαe, the Gβe subunit is localized mainly to the cytosol (>80%; Fig. 4, A and B), suggesting that a newly synthesized Gβγ complex is dependent on the α subunit for membrane attachment. Failure of the βγ complex to bind by itself to the plasma membrane was previously observed in transfected cells (Evanko et al., 2001; Michaelson et al., 2002; Takida and Wedegaertner, 2003) and in Gα RNA interference of Caenorhabditis elegans embryos (Gotta and Ahringer, 2001). However, immunogold EM using specific antibodies against Gβe revealed that the βγ complex is targeted to the rhabdomere even in the near absence of the α subunit (Fig. 4 C) but apparently remains soluble within this compartment. This result indicates that the βγ complex is targeted to the rhabdomere independently of Gqαe but depends on the α subunit for tight membrane attachment. The presence of soluble Gβe in the rhabdomere can be a result of interactions with protein partners like phosducin (Sokolov et al., 2004) and regulators of G protein signaling proteins (Snow et al., 1998). Although homologues of these proteins are present in the Drosophila genome, their cellular localization in Drosophila photoreceptors are currently unknown. The cellular localization of the Gqe heterotrimer may be determined by the βγ complex. This finding is consistent with a previous report that ectopic targeting of the βγ complex to the mitochondria leads to mitochondrial localization of the Gzα subunit (Fishburn et al., 2000).
The Gβe subunit is present in excess over the Gqαe subunit
The presence of 80% of Gqαe in a membrane-bound form in wild-type dark-adapted flies (Fig. 2 B, left), whereas only 50% of Gβe is membrane bound (Fig. 4 B, left), raised the question of the stoichiometry of these two components. To determine the levels of the subunits in vivo, we performed immunoblot analysis with a mixture of Gqαe- and Gβe-specific antibodies at a concentration five times that required for their saturation. Furthermore, two different anti-Gβe antibodies that were raised against two different sequences of the Gβe protein gave similar results (see SDS-PAGE and immunoblotting). In wild-type flies, the amount of Gβe was ∼2.5 times higher than the amount of Gqαe (Fig. 5, A and B). To verify the excess of Gβe over Gqαe subunits in wild-type flies, which was determined by Western blotting, we calibrated the immunoblot with the use of purified recombinant Drosophila Gqαe and Gβe proteins. We determined the concentrations of the recombinant proteins spectrophotometrically by using calculated extinction coefficients of Gqαe = 42,350 cm−1 M−1 and Gβe = 60,000 cm−1 M−1 at 280 nm. This quantitative analysis of two samples of wild-type fly head homogenate again revealed an excess of Gβe over Gqαe of ∼2.5 times (Fig. 5 C).
Figure 5.
A physiological excess of Gβe over Gqαe that is abolished in heterozygous Gβe1/+ mutant flies. (A) Western blot analysis of Gqαe (top band) and Gβe (bottom band) in dark-adapted wild-type and heterozygous Gβe 1/+ flies shows an excess of Gβe over Gqαe in wild-type flies that is abolished in the Gβe 1/+ mutant. Western blots were performed with a mixture of Gqαe and Gβe antibodies. T, total amount in the cell; P, pellet (membrane); S, supernatant (cytosol). (B) The ratio between Gβe and Gqαe in different fractions. Data represent mean values ± SEM (error bars) from 10 independent experiments. (C) Two samples of head homogenates (indicated as A and B) were analyzed by Western blotting along with five samples containing various amounts of recombinant Drosophila Gqαe (top) and Gβe (bottom) standards (indicated as 1–5). Calibration curves were obtained by plotting the amount of ECL signal in each band against the amount of the recombinant protein in the standard. Because the volume of head homogenates that was applied on each gel was different in order to fit the linear range of each calibration curve, Gqαe and Gβe amounts in the head homogenates were calculated for 1 μl of homogenate. Gqαe and Gβe amounts in each homogenate and the ratio between them are represented in the table at the bottom. Data are representative of six independent experiments. The ratio between Gβe and Gqαe was determined as 2.6 ± 0.2.
Most of the excess Gβe was present in the cytosol, whereas the membrane-bound fraction contained both the α and β subunits in about equal amounts (Fig. 5, A and B). Therefore, in rhabdomere membranes, all of the Gαe molecules, which are in close proximity to rhodopsin, may be associated with the Gβe subunit. This finding also indicates that in the Drosophila photoreceptor cells, there is a soluble pool of free Gβe subunit in the rhabdomere. The localization of soluble Gβe in the signaling organelle, the rhabdomere (Fig. 4), could be functionally important.
The unexpected excess of Gβe over Gqαe was almost completely abolished in the Gβe 1 heterozygous mutant (Gβe 1/+). The ratio between Gβe and Gqαe in this mutant was ∼1:1. The decrease in Gβe levels of this mutant did not change the ratio between membrane-bound Gqαe and Gβe, which remained ∼1:1. In the soluble fraction, however, we found a large decrease of excess Gβe. Although the ratio between soluble Gβe and Gqαe in wild-type flies was ∼7:1, the ratio in the Gβe 1/+ mutant was reduced to ∼2.5:1 (Fig. 5, A and B).
Spontaneous activity of Gβe mutants
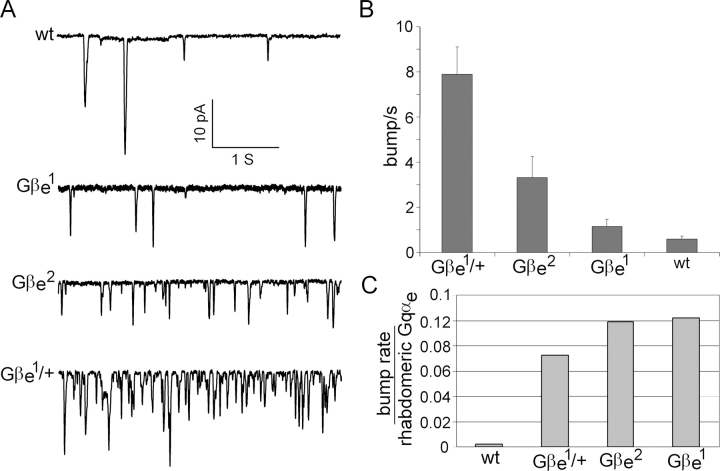
A new and striking phenotype of Gβe mutants was revealed in this study. Whole cell patch-clamp recording of dark-adapted mutant photoreceptor cells showed spontaneous, unitary, inward currents that were similar in shape to the single photon responses known as quantum bumps (Fig. 6; Henderson et al., 2000). The frequency of these spontaneous responses was different for the various Gβe mutants. For the Gβe 1 mutant, only a low frequency of spontaneous bumps was observed, which was not much different from the frequency of spontaneous bumps observed in wild-type flies. A higher frequency of spontaneous bumps was clearly noted for the Gβe 2 mutant, whereas the most dramatic increase in the frequency of spontaneous bumps was observed for the Gβe 1 heterozygous mutant (Gβe 1/+). The high frequency of spontaneous bumps in the heterozygous Gβe 1 mutant is surprising because this mutant has normal sensitivity to light in contrast to the Gβe 1 homozygote, which is the most severe mutant but has an almost normal frequency of spontaneous bumps (Figs. 3 and 6). This complex behavior can be explained by the decreased levels of Gqαe observed in the signaling compartment of these mutants (Fig. 2). Indeed, when the bump frequency was normalized to the number of rhabdomeral Gqαe, a similar bump frequency per rhabdomeral Gqαe was observed for all of the Gβe mutants, whereas the wild-type bump frequency remained much lower (Fig. 6 C).
Figure 6.
Spontaneous activation of the visual signaling cascade in Gβe mutants. (A) Whole cell recordings of light-induced currents from isolated ommatidia of dark-adapted Gβe mutants and wild-type flies clamped at −70 mV. Spontaneous bumps are observed in complete darkness at different rates in the various mutants. (B) Histogram plotting the bump frequency of various mutants. Data represent mean values ± SEM (error bars) from at least eight different experiments. The difference between the wild-type and Gβe 1 mutant is not statistically significant (P < 0.1). The statistics include bumps with amplitudes of >2.5 pA, which clearly exceeds the background noise. (C) The bump frequency of various Gβe mutants and of wild-type flies was divided by the number of rhabdomeral Gqαe of each mutant as determined by the immunogold labeling assay (Fig. 2 D).
To find out whether the high frequency of spontaneous activity is caused by activation of the G protein and not by the spontaneous activation of rhodopsin, we generated a heterozygous Gβe 1/+ mutant with highly decreased levels of rhodopsin. To reduce the rhodopsin level in Gβe 1/+ flies, we reduced the chromophore level by raising the flies on a carotenoid-deficient medium (Minke and Kirschfeld, 1979) for three generations (Gβe 1/+ Vit A−). The metarhodopsin potential (M potential) is a linear electrical manifestation of the level of rhodopsin in fly photoreceptors (Pak and Lidington, 1974; Minke and Kirschfeld, 1980). Fig. 7 A shows the amplitude of the M potential in Gβe 1/+ flies raised on standard medium (top) compared with Gβe 1/+ flies raised on carotenoid-deficient medium (bottom). The virtually complete elimination of M potential after carotenoid deprivation clearly shows that the rhodopsin level was largely reduced in these flies. This conclusion was further supported by measuring the sensitivity to light after carotenoid deprivation, which resulted in a reduction of ∼300-fold in sensitivity to light without a change in the distribution of Gqαe in carotenoid-deprived flies (not depicted). Fig. 7 shows that the high rate of spontaneous bumps, which is characteristic of Gβe 1/+ flies, was not significantly changed by reduced levels of rhodopsin. This indicates that the high frequency of spontaneous bumps in the Gβe 1/+ mutant does not arise from the spontaneous activation of rhodopsin in the dark.
Figure 7.
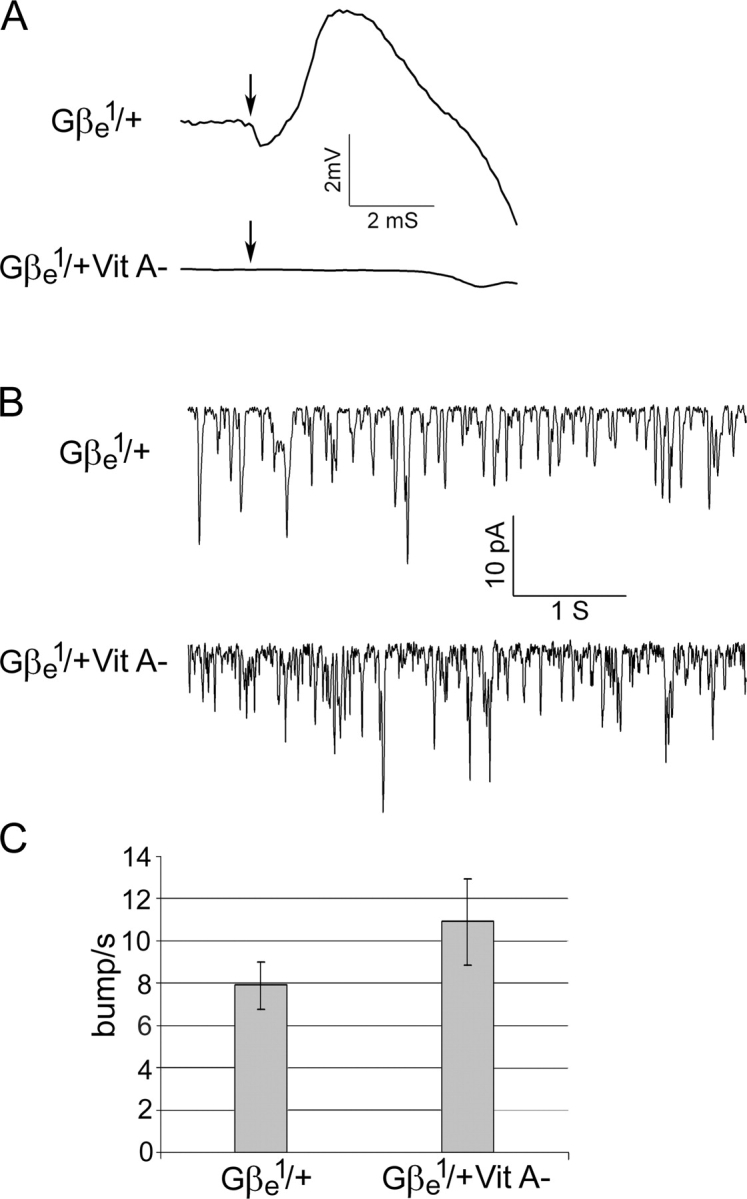
Rhodopsin is not essential for spontaneous bump production in the Gβe1/+ mutant. (A) ERG recordings of Gβe 1/+ and Gβe 1/+ Vit A− responses to a white flash, indicated by arrows. Gβe 1/+ flies that were raised on standard medium displayed M potential similar to that of wild-type flies, whereas the M potential of Gβe 1/+ Vit A− was abolished. Traces shown are means of 20 consecutive experiments. (B) Whole cell recordings of light-induced currents from isolated ommatidia of dark-adapted Gβe 1/+ mutants and Gβe 1/+ Vit A− flies clamped at −70 mV. A similar frequency of spontaneous bump was observed in flies reared under both conditions. (C) Histogram plotting the bump frequency of Gβe 1/+ and Gβe 1/+ Vit A− flies in the dark. Data represents mean values ± SEM (error bars) from eight different experiments. The difference between Gβe 1/+ and Gβe 1/+ Vit A− is not statistically significant (P < 0.1). The statistics include only bumps with an amplitude of >2.5 pA, which clearly exceeds the background noise.
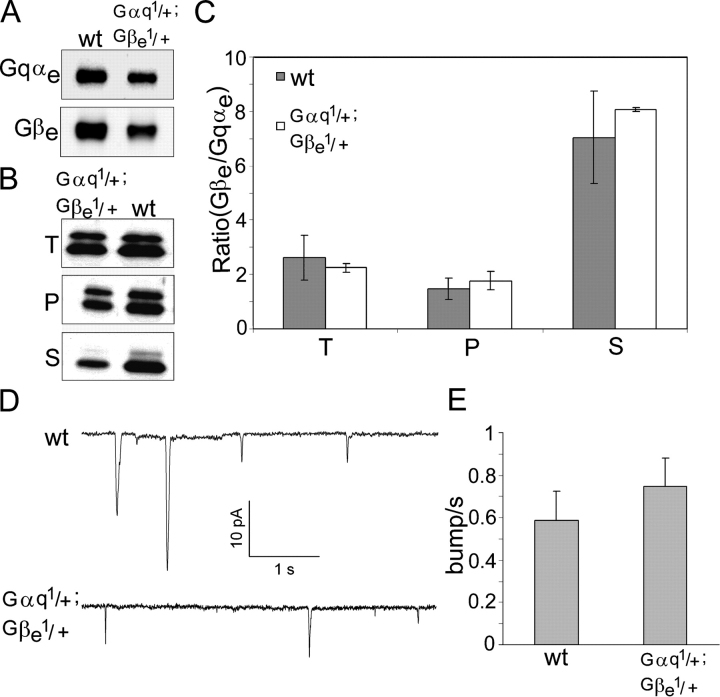
The excess of Gβe over Gqαe that was observed in wild-type flies is almost abolished in the Gβe 1/+ heterozygous mutant (Fig. 5); this finding raised the possibility that the excess in wild-type flies prevents the spontaneous activity of Gqαe observed in the Gβe 1/+ heterozygous mutant. To further test this hypothesis, we crossed the Gβe 1 mutant with the Gαq 1 mutant to generate a double mutant containing one copy of the Gqαe gene and one copy of the Gβe gene (Gαq 1/+;Gβe 1/+). The double mutant had about half the level of both Gqαe and Gβe as wild-type flies (Fig. 8 A), restoring the excess Gβe over Gqαe that was observed in wild-type flies (Fig. 8, B and C). This mutant showed almost normal sensitivity to light (Fig. 3) and no spontaneous activity in the dark (Fig. 8, D and E). This result strongly suggests that the excess of Gβe over Gqαe, rather than the absolute amount of the Gβe subunit, prevents the spontaneous activation of Gqαe in Drosophila photoreceptor cells.
Figure 8.
The Gβe excess over Gqαe is restored in a Gαq1/+;Gβe1/+ double mutant. This mutant has almost no spontaneous activity in the dark. (A) Gαq 1/+;Gβe 1/+ double mutants were generated by the crossing of Gβe 1 mutants with Gαq 1 mutants. Western blot analysis shows Gβe and Gqαe levels in dark-adapted wild type and in the Gαq 1/+;Gβe 1/+ double mutant. Quantification of the ECL signal shows that the Gqαe level was reduced to ∼50% and that the Gβe level was reduced to ∼60% of wild-type values. The experiment was repeated three times. (B) Western blot analysis of Gqαe (top band) and Gβe (bottom band) in dark-adapted wild-type and Gαq 1/+;Gβe 1/+ mutants shows that the excess of Gβe over Gqαe is restored in this mutant. Western blots were performed with a mixture of Gqαe and Gβe antibodies. T, total amount in the cell; P, pellet (membrane); S, supernatant (cytosol). (C) The ratio between Gβe and Gqαe in different fractions of the Gαq 1/+;Gβe 1/+ double mutant is very similar to that of wild-type flies. Data represent mean values ± SEM from five different experiments. Data from wild-type flies are the same as in Fig. 5 B and are shown here only for comparison. (D) Whole cell recordings of light-induced currents from isolated ommatidia of dark-adapted Gαq 1/+;Gβe 1/+ double mutants and of wild-type flies clamped at −70 mV. Data from wild-type flies are the same as in Fig. 6 A and are shown here only for comparison. (E) Histogram plotting the bump frequency of the Gαq 1/+;Gβe 1/+ double mutant compared with that of wild-type flies. Data represent mean values ± SEM (error bars) from at least eight different experiments. The difference between wild-type and Gqα1/+;Gβe 1/+ double mutants is not statistically significant (P < 0.1). The statistics include bumps with amplitudes of >2.5 pA, which clearly exceeds the background noise. Data of wild-type flies are the same as in Fig. 6 B and are shown here only for comparison (note the different bars of Figs. 6 B and 8 E).
Discussion
The decreased light sensitivity of Drosophila Gβe mutants
When Gβe mutants were first isolated (Dolph et al., 1994), it was reported that these mutations caused a dramatic decrease in the sensitivity to light, which was ascribed to participation of the β subunit in G protein–rhodopsin coupling. Our finding that the decrease in Gβe in Gβe mutants is accompanied by a proportional decrease in Gqα in the rhabdomeral compartment does not support the previously claimed catalytic effect of the β subunit on light sensitivity (Dolph et al., 1994). Rather, we conclude that the decrease in light sensitivity of these mutants is caused by the presence of rhodopsin and the major fraction of the G protein α subunit in two different cellular compartments. Clearly, when these two components are present in different cellular locations, the photo-excited rhodopsin is unable to catalyze the exchange of GDP that is bound to the Gqαe for free GTP, and the transduction process is prematurely terminated. The mechanism that underlies the decreased sensitivity to light in Gβe mutants, therefore, is a structural change in the localization of the Gqαe subunit.
We also examined how a decrease in the α subunit of the Gαq 1 mutant influences membrane attachment and targeting of the βγ subunits to the rhabdomere. In this case, the βγ dimer is soluble and not membrane attached but is still targeted to the rhabdomere. The presence of βγ in the rhabdomeral cytosol may be physiologically important for preventing spontaneous activity because the βγ subunits are in close proximity to the membrane-bound signaling molecules.
We have previously shown that the eye-specific Gqαe subunit translocates from rhabdomeral membranes to the cytosol in response to illumination (Kosloff et al., 2003). Gqαe behaves like many other Gα subunits, which demonstrate activity-dependent translocation from the membrane to the cytosol (for review see Resh, 1999; Chen and Manning, 2001; Smotrys and Linder, 2004). The Drosophila eye–specific βγ dimer behaves differently from the Gqαe subunit, as it does not show any significant change in its distribution even after prolonged illumination (Fig. 4). A possible reason for this result might be an interaction between the γ subunit of the βγ dimer and the photoactivated rhodopsin. Such an interaction has been reported for the transducin γ subunit and the active form of vertebrate rhodopsin (Kisselev and Downs, 2003). Both vertebrate and invertebrate photoreceptor cells contain high concentrations of rhodopsin, and even a weak interaction could be significant as a result of mass action. It should be noted, however, that studies in rat retina detected light-dependent movement of both the α and βγ subunits from the rod outer to inner segment, although the βγ subunits moved more slowly than the α subunit, suggesting that it might be caused by an interaction of the βγ complex with phosducin (Sokolov et al., 2002, 2004). The different behavior of Gβγ subunits in vertebrate and Drosophila might be caused by the difference in stability of the active rhodopsin in these two systems. Whereas vertebrate rhodopsin undergoes bleaching and inactivation, the activated rhodopsin of Drosophila is stable for hours (Minke and Selinger, 1996).
Spontaneous, dark photoreceptor activity in Gβe mutants
A functional hallmark of visual photoreceptors is utmost sensitivity of the capacity for single photon detection. This sensitivity is achieved by very high concentrations of the photoreceptor rhodopsin and its target G protein as well as by the large amplification that is generated during the phototransduction process. High sensitivity also depends on an exceedingly low spontaneous activity (low, dark noise) that sets the limit on the absolute sensitivity of this signaling system. Rhodopsin is the only G protein–coupled receptor that has covalently linked 11 cis-retinal that behaves like a “quasi” antagonist in the dark, preventing spontaneous activity. The visual G protein, however, needs special mechanisms to prevent spontaneous activation, but these mechanisms remain unknown.
The Gβγ subunits are known to bind to Gα-GDP switch regions, thereby stabilizing the binding of GDP and suppressing spontaneous receptor-independent activation (Itoh and Gilman, 1991; Lambright et al., 1996; Sondek et al., 1996; Preininger and Hamm, 2004). To find out whether this interaction is relevant to the Drosophila eye–specific Gq heterotrimer, whose three-dimensional structure has not been determined, we have constructed a homology model of the DGqe heterotrimer (αβγ) based on the crystal structure of transducin (unpublished data). It appears from the model that the Gβγe subunit complex directly contacts the switch I and switch II space regions of Gqαe as was previously reported for other G proteins. Therefore, it is conceivable that the Drosophila Gβγe complex prevents the spontaneous activation of Gqαe by binding to Gqαe switch regions. It should be pointed out, however, that the physiological consequences of this effect in vivo have not been described previously. Furthermore, the mechanism that suppresses the spontaneous activity of G proteins under physiological conditions is unknown. In this study, we report (Fig. 6) the observation of a high frequency of spontaneous activity in the heterozygous Gβe 1/+ mutant in which the level of the β subunit was decreased to 50% of its level in wild-type flies. Surprisingly, a further decrease in the level of Gβe in the Gβe 2 and Gβe 1 mutants did not increase the frequency of spontaneous activity but rather decreased the frequency. This is easily seen in the most severe mutant (Gβe 1), which has only 4% of Gβe, as the spontaneous activity is not much different from the low frequency in wild-type flies. This is probably the reason why the increase in spontaneous activity was not detected in the initial characterization of Gβe mutants (Dolph et al., 1994). These results indicate that the relationship between the level of Gβe and the spontaneous activity is not straightforward. We suggest that the observed spontaneous activity of β mutant photoreceptor cells is regulated by two opposite effects of the βγ dimer. On the one hand, the decrease in βγ levels leaves some Gα-GDP unassociated with βγ, and this free Gα-GDP undergoes spontaneous exchange of the bound GDP for free GTP, leading to spontaneous activity. On the other hand, the decreased level of βγ leads to a proportional decrease of Gα in the signaling compartment, resulting in a diminished ability to activate the phototransduction process. To test this notion, we normalized the observed rate of spontaneous activity to the number of Gqαe subunits in the rhabdomeres of Gβe mutants that lack excess Gβe over Gqαe. We found (Fig. 6 C) similar frequencies of normalized spontaneous activity for all of the Gβe mutants, which is consistent with a role of excess βγ over Gqαe in suppressing spontaneous activity.
The presence of excess Gβe over Gqαe in the Drosophila photoreceptor cell
One of the unexpected and novel findings of this study is the presence of the Drosophila eye–specific Gβe subunit in ∼2.5-fold excess over the Gqαe subunit. Because the levels of α and β subunit proteins are maintained independently of one another, unequal levels of these subunits are mechanistically possible. Our calibration curves using purified recombinant Gβe and Gqαe proteins (Fig. 5 C) verified the excess of Gβe over Gqαe subunits, which was determined by immunoblot analysis with a mixture of Gqαe and Gβe antibodies (Fig. 5 A). Furthermore, we have shown that as long as the two antibodies are maintained at saturating concentrations and determinations are performed in the same gel, levels of the α and β subunits are obtained that nicely fit the expected results from gene dosage effects (Fig. 5 A). Furthermore, according to the “two-signal model” for membrane attachment of peripheral membrane proteins, one expects to find equal amounts of membrane-bound Gqαe and Gβe subunits. In accord with this notion, although we found about twofold excess of total Gβe over Gqαe, an analysis of these subunits in the membrane-bound fraction gave a ratio of 1:1.
In the heterozygous Gβe 1/+ mutant, in which there is a reduction of 50% in the level of the β subunit, yielding a β/α ratio of ∼1, we found a dramatic increase in the spontaneous activity of photoreceptor cells (Figs. 5 and 6). The critical role of the excess of Gβe over Gqαe was revealed in the Gαq 1/+;Gβe 1/+ double heterozygous mutant, in which the rate of spontaneous activity was dramatically reduced by restoring the excess of Gβe over Gqαe. This indicates that the excess of Gβe, rather than its absolute amount, is important to maintain a low frequency of spontaneous activity. Furthermore, this mutant rules out the possibility that the spontaneous activity we observed was caused by side effects of the Gβe mutation. Altogether, this is the first demonstration of the strategy of excess βγ over the α subunit in vivo for the suppression of spontaneous activity at the G protein level.
Two possible mechanisms can explain how the excess of Gβe over Gqαe prevents spontaneous activity. One mechanism could be through participation of the soluble pool of rhabdomeral Gβe in accelerating the hydrolysis of Gqαe-GTP if spontaneous exchange occurs. This mechanism is currently under investigation. The second mechanism could be through the stabilization of Gqαe-GDP, thus preventing the exchange of bound GDP for free GTP. In an insightful, theoretical paper dealing with the spontaneous activity of G proteins by using thermodynamic model simulations, it was found that the concentration of β equal to that of α is barely sufficient to suppress spontaneous activity, whereas a twofold excess of βγ over the α subunit produces a large decrease in spontaneous activity (Onaran et al., 1993). Altogether, our in vivo studies point to the importance of βγ subunits as principle modulators of spontaneous activity and to the relevance of this strategy in vivo.
Materials and methods
Fly stocks
Drosophila of the following strains were used: wild-type, Oregon-R w (obtained from W.L. Pak, Purdue University, West Lafayette, IN); Gaq 1, a severe hypomorph for Gqαe (obtained from C.S. Zuker, University of California, San Diego, San Diego, CA; Scott et al., 1995); Gβe 1, a severe hypomorph mutant of eye-specific Gβe; and Gβe 2, a less severe hypomorph mutant of eye-specific Gβe (obtained from C.S. Zuker; Dolph et al., 1994).
Assay of light-dependent Gβe localization
Assay for the light-dependent localization of Gβe was performed as described previously (Kosloff et al., 2003). In short, dark-adapted flies were subjected to illumination with activating blue light (18-W white light lamp with a 1-mm–thick wide band filter [Schott BG 28; Bes Optics] 12 cm away from the flies) for various durations at 22°C. Termination was performed by moving the flies to 4°C in the dark and promptly separating the fly heads. 10 flies were used for each time point.
Preparation of Drosophila head homogenate and fractionation
Heads were separated from 10 flies that were dark adapted overnight (except in Fig. 4) and homogenized in 1 ml isotonic homogenization buffer (20 mM Tris, pH 7.5, 120 mM KCl, 0.1 mM MgCl2, 0.1 mM PMSF, and 5 mM β-mercaptoethanol). Homogenate was either directly precipitated with 5% TCA or subjected to fractionation. Membranes and cytosol fractions were separated by centrifugation (15,800 g for 15 min at 4°C). The pellet was washed and centrifuged again, and the supernatants were combined. Ultracentrifugation at 150,000 g for 30 min did not change the distribution of α and β subunits between the fractions. The proteins were precipitated by 5% TCA, ran on SDS-PAGE, and subjected to quantification as described in SDS-PAGE and immunoblotting.
Preparation of recombinant Drosophila Gqαe and Gβe proteins
cDNA clones of Gqαe and Gβe genes were obtained from the Medical Research Council UK gene service. Gqαe cDNA was amplified and cloned into pQE-80 vector (QIAGEN) that contained an NH2-terminal 6× His tag and was expressed in Rosetta bacterial cells (Novagen). The recombinant (His)6-Gqαe protein was then purified on a Ni-Sepharose column (GE Healthcare) and eluted with a 20–250-nm imidazole gradient using fast protein liquid chromatography Akta explorer (GE Healthcare).
Gβe cDNA was amplified and cloned into pHis-parallel 1 (pET22) vector (obtained from P. Sheffield, University of Virginia, Charlottesville, VA) that contained an NH2-terminal 6× His tag and was expressed in HMS174 bacterial cells (Novagen). Purified recombinant (His)6-Gβe was extracted from inclusion bodies by applying 6 M guanidine HCl on a bacterial membrane extract that had been washed three times with 1% Triton X-100. Both proteins were ∼95% pure as determined by SDS-PAGE and Coomassie blue staining. Recombinant protein concentrations were determined spectrophotometrically by using a calculated molar extinction coefficient of 42,350 for Gqαe and 60,000 for Gβe at 280 nm.
SDS-PAGE and immunoblotting
Equal protein amounts that were determined by Bradford assay were loaded on the specified gel. For detection of the α or β subunits of DGqe, a 10% SDS-PAGE was used. To detect both subunits (α and β) on the same gel, proteins were separated on a gradient 7.5–15% SDS-PAGE. For the detection of Gγe, a 20% SDS-PAGE with 4 M urea was used. The urea was needed for separation of the γ subunit from the β subunit. Subsequent to SDS-PAGE separation, proteins were subjected to Western blot analysis using the specified antibodies.
Two different anti-Gβe polyclonal antibodies were made in rabbit as described previously (Palczewski et al., 1993). One antibody was made against a peptide from the COOH terminus of the protein (residues 333–346), and the other was made against a peptide from the NH2 terminus (residues 3–13).
For Gqαe detection, we used anti-Gqαe polyclonal antibodies that were previously made by us (Kosloff et al., 2003), and for Gγe detection, rabbit polyclonal antibodies that were directed against the Calliphora Gγe protein were used (obtained from A. Huber, University of Karlsruhe, Karlsruhe, Germany; Schulz et al., 1999).
To determine the ratio between Gqαe and Gβe subunits, we performed Western blot analysis using a mixture of anti-Gqαe and anti-Gβe each at a 1:1,000 dilution, which is five times higher than their saturating concentration. To rule out the possibility that the Gβe excess we observed is caused by the antibodies, we repeated these experiments with the two different Gβe antibodies and obtained the same results. To further ensure that the Gβe excess over Gqαe was not a result of the antibody concentrations, we repeated this procedure with a higher concentration of anti-Gqαe or with a twofold dilution (1:2,000) of anti-Gβe. In all of these cases, an excess of Gβe over Gqαe was observed.
Relative protein amounts on the same gel were determined by quantification of the ECL signal by using the Plus Gel system (LAS-1000; Fuji).
Immunogold EM
Immunogold EM was performed as described previously (Kosloff et al., 2003). All sections were made from flies that were dark adapted overnight. Sections were incubated with either Gqαe antibodies (dilution of 1:80) or Gβe affinity-purified antibodies (dilution of 1:20). Gβe antibodies were affinity purified by using Affi-Gel 10 gel (Bio-Rad Laboratories) according to the manufacturer's instructions for anhydrous coupling followed by elution with glycine-HCl, pH 2.5. The secondary antibody used was goat anti–rabbit conjugated to 18 nm of gold particles.
Sections were observed and photographed with a transmission electron microscope (Technai-12; Philips) equipped with a CCD camera (MegaView II; Soft Imaging System) and were visualized with analySIS 3.0 image processing software (Soft Imaging System).
Electroretinogram (ERG) and M potential
ERG recordings were performed on intact flies as described previously (Peretz et al., 1994). Orange light (OG-590 Schott edge filter; Bes Optics) from a Xenon high pressure lamp (operating at 50 W; model LPS 220; Photon Technology International) was delivered to the compound eye by an optic fiber and was attenuated by natural density filters. The maximal luminous intensity at the eye surface was 12.5 mW/cm2. M potential recordings were performed as described previously (Minke and Kirschfeld, 1980). In brief, an adapting light of maximal intensity 20-s blue light (Schott BG-28) from the Xenon high pressure lamp was delivered 1 min before each white test stimulus (70 jouls of photographic flash light).
Whole cell recording
Dissociated ommatidia were prepared from newly eclosed white-eyed adult flies (<1 h after eclosion; Hardie, 1991) that were maintained in a 12-h dark/12-h light cycle and kept in the dark 24 h before the experiment. Whole cell patch-clamp recordings were performed as previously described (Hardie and Minke, 1992). Signals were amplified with a patch-clamp amplifier (Axopatch 200B; Axon Instruments, Inc.), sampled at 2,000 Hz, and filtered below 1,000 Hz. The bath solution contained 120 mM NaCl, 5 mM KCl, 10 mM N-Tris buffer, pH 7.15, 4 mM MgSO4, and 1.5 mM CaCl2. The pipette solution contained 120 mM K gluconate, 2 mM MgSO4, 10 mM N-Tris buffer, pH 7.15, 4 mM MgATP, 0.4 mM Na2GTP, and 1 mM NAD+.
Transillumination of the halogen light source (100 W) was used as previously described (Peretz et al., 1994). The orange stimulating light (Schott OG-590) was applied via a condenser lens (Carl Zeiss MicroImaging, Inc.) and was attenuated by neutral density filters.
Acknowledgments
We thank R. Timberg and N. Feinstein from the Electron Microscopy unit (Life Sciences faculty at The Hebrew University) for help with EM; T. Danieli and M. Lebendiker (Wolfson Center for Applied Structural Biology) for help with expression and purification of the recombinant DGq subunits; C. Zuker and W.L. Pak for specific Drosophila mutants; and A. Huber for the Calliphora Gγe antibodies. We thank R. Korenberg, M. Kosloff, and Y. Litvak for carefully reading the manuscript and for helpful discussions.
This work was supported by grants from the National Institutes of Health (EY-03529 to B. Minke and Z. Selinger), the Israel Science Foundation (to Z. Selinger and B. Minke), the German-Israel Foundation (to B. Minke), and the Minerva Foundation (to B. Minke and Z. Selinger).
N. Elia and S. Frechter contributed equally to this paper.
Abbreviations used in this paper: ERG, electroretinogram; GDP, guanosine diphosphate.
References
- Cassel, D., and Z. Selinger. 1978. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc. Natl. Acad. Sci. USA. 75:4155–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel, D., H. Levkovitz, and Z. Selinger. 1977. The regulatory GTPase cycle of turkey erythrocyte adenylate cyclase. J. Cyclic Nucleotide Res. 3:393–406. [PubMed] [Google Scholar]
- Chen, C.A., and D.R. Manning. 2001. Regulation of G proteins by covalent modification. Oncogene. 20:1643–1652. [DOI] [PubMed] [Google Scholar]
- Cronin, M.A., F. Diao, and S. Tsunoda. 2004. Light-dependent subcellular translocation of Gqalpha in Drosophila photoreceptors is facilitated by the photoreceptor-specific myosin III NINAC. J. Cell Sci. 117:4797–4806. [DOI] [PubMed] [Google Scholar]
- Degtyarev, M.Y., A.M. Spiegel, and T.L. Jones. 1994. Palmitoylation of a G protein αi subunit requires membrane localization not myristoylation. J. Biol. Chem. 269:30898–30903. [PubMed] [Google Scholar]
- Devary, O., O. Heichal, A. Blumenfeld, D. Cassel, E. Suss, S. Barash, C.T. Rubinstein, B. Minke, and Z. Selinger. 1987. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc. Natl. Acad. Sci. USA. 84:6939–6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph, P.J., H. Man-Son-Hing, S. Yarfitz, N.J. Colley, J.R. Deer, M. Spencer, J.B. Hurley, and C.S. Zuker. 1994. An eye-specific Gβ subunit essential for termination of the phototransduction cascade. Nature. 370:59–61. [DOI] [PubMed] [Google Scholar]
- Evanko, D.S., M.M. Thiyagarajan, and P.B. Wedegaertner. 2000. Interaction with Gβγ is required for membrane targeting and palmitoylation of Gαs and Gαq. J. Biol. Chem. 275:1327–1336. [DOI] [PubMed] [Google Scholar]
- Evanko, D.S., M.M. Thiyagarajan, D.P. Siderovski, and P.B. Wedegaertner. 2001. Gbeta gamma isoforms selectively rescue plasma membrane localization and palmitoylation of mutant Galphas and Galphaq. J. Biol. Chem. 276:23945–23953. [DOI] [PubMed] [Google Scholar]
- Fishburn, C.S., S.K. Pollitt, and H.R. Bourne. 2000. Localization of a peripheral membrane protein: Gbetagamma targets Galpha(Z). Proc. Natl. Acad. Sci. USA. 97:1085–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta, M., and J. Ahringer. 2001. Distinct roles for Galpha and Gbetagamma in regulating spindle position and orientation in Caenorhabditis elegans embryos. Nat. Cell Biol. 3:297–300. [DOI] [PubMed] [Google Scholar]
- Hardie, R.C. 1991. Voltage-sensitive potassium channels in Drosophila photoreceptors. J. Neurosci. 11:3079–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie, R.C., and B. Minke. 1992. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 8:643–651. [DOI] [PubMed] [Google Scholar]
- Hardie, R.C., and P. Raghu. 2001. Visual transduction in Drosophila. Nature. 413:186–193. [DOI] [PubMed] [Google Scholar]
- Henderson, S.R., H. Reuss, and R.C. Hardie. 2000. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+. J. Physiol. 524:179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, H., and A.G. Gilman. 1991. Expression and analysis of Gs alpha mutants with decreased ability to activate adenylylcyclase. J. Biol. Chem. 266:16226–16231. [PubMed] [Google Scholar]
- Kisselev, O.G., and M.A. Downs. 2003. Rhodopsin controls a conformational switch on the transducin gamma subunit. Structure (Camb). 11:367–373. [DOI] [PubMed] [Google Scholar]
- Kosloff, M., N. Elia, and Z. Selinger. 2002. Structural homology discloses a bifunctional structural motif at the N-termini of G alpha proteins. Biochemistry. 41:14518–14523. [DOI] [PubMed] [Google Scholar]
- Kosloff, M., N. Elia, T. Joel-Almagor, R. Timberg, T.D. Zars, D.R. Hyde, B. Minke, and Z. Selinger. 2003. Regulation of light-dependent Gqalpha translocation and morphological changes in fly photoreceptors. EMBO J. 22:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright, D.G., J. Sondek, A. Bohm, N.P. Skiba, H.E. Hamm, and P.B. Sigler. 1996. The 2.0 A crystal structure of a heterotrimeric G protein. Nature. 379:311–319. [DOI] [PubMed] [Google Scholar]
- Michaelson, D., I. Ahearn, M. Bergo, S. Young, and M. Philips. 2002. Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi. Mol. Biol. Cell. 13:3294–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke, B., and K. Kirschfeld. 1979. The contribution of a sensitizing pigment to the photosensitivity spectra of fly rhodopsin and metarhodopsin. J. Gen. Physiol. 73:517–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke, B., and K. Kirschfeld. 1980. Fast electrical potentials arising from activation of metarhodopsin in the fly. J. Gen. Physiol. 75:381–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke, B., and Z. Selinger. 1996. The roles of trp and calcium in regulating photoreceptor function in Drosophila. Curr. Opin. Neurobiol. 6:459–466. [DOI] [PubMed] [Google Scholar]
- Minke, B., and R.C. Hardie. 2000. Genetic dissection of Drosophila phototransduction. Molecular Mechanisms in Visual Transduction. D.G. Stavenga, W.J. DeGrip, and E.N. Pugh Jr., editors. Elsevier Science Publishing Co. Inc., NY. 449–525.
- Murray, D., S. McLaughlin, and B. Honig. 2001. The role of electrostatic interactions in the regulation of the membrane association of G protein beta gamma heterodimers. J. Biol. Chem. 276:45153–45159. [DOI] [PubMed] [Google Scholar]
- Onaran, H.O., T. Costa, and D. Rodbard. 1993. Beta gamma subunits of guanine nucleotide-binding proteins and regulation of spontaneous receptor activity: thermodynamic model for the interaction between receptors and guanine nucleotide-binding protein subunits. Mol. Pharmacol. 43:245–256. [PubMed] [Google Scholar]
- Pak, W.L., and K.J. Lidington. 1974. Fast electrical potential from a long-lived, long-wavelength photoproduct of fly visual pigment. J. Gen. Physiol. 63:740–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski, K., J. Buczylko, L. Lebioda, J.W. Crabb, and A.S. Polans. 1993. Identification of the N-terminal region in rhodopsin kinase involved in its interaction with rhodopsin. J. Biol. Chem. 268:6004–6013. [PubMed] [Google Scholar]
- Peretz, A., E. Suss-Toby, A. Rom-Glas, A. Arnon, R. Payne, and B. Minke. 1994. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 12:1257–1267. [DOI] [PubMed] [Google Scholar]
- Phillips, W.J., and R.A. Cerione. 1992. Rhodopsin/transducin interactions. I. Characterization of the binding of the transducin-beta gamma subunit complex to rhodopsin using fluorescence spectroscopy. J. Biol. Chem. 267:17032–17039. [PubMed] [Google Scholar]
- Preininger, A.M., and H.E. Hamm. 2004. G protein signaling: insights from new structures. Sci. STKE. 10.1126/stke.2182004re3. [DOI] [PubMed]
- Resh, M.D. 1999. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta. 1451:1–16. [DOI] [PubMed] [Google Scholar]
- Schulz, S., A. Huber, K. Schwab, and R. Paulsen. 1999. A novel Ggamma isolated from Drosophila constitutes a visual G protein gamma subunit of the fly compound eye. J. Biol. Chem. 274:37605–37610. [DOI] [PubMed] [Google Scholar]
- Scott, K., A. Becker, Y. Sun, R. Hardy, and C. Zuker. 1995. Gqα protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron. 15:919–927. [DOI] [PubMed] [Google Scholar]
- Smotrys, J.E., and M.E. Linder. 2004. Palmitoylation of intracellular signaling proteins: regulation and function. Annu. Rev. Biochem. 73:559–587. [DOI] [PubMed] [Google Scholar]
- Snow, B.E., A.M. Krumins, G.M. Brothers, S.F. Lee, M.A. Wall, S. Chung, J. Mangion, S. Arya, A.G. Gilman, and D.P. Siderovski. 1998. A G protein gamma subunit-like domain shared between RGS11 and other RGS proteins specifies binding to Gbeta5 subunits. Proc. Natl. Acad. Sci. USA. 95:13307–13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov, M., A.L. Lyubarsky, K.J. Strissel, A.B. Savchenko, V.I. Govardovskii, E.N. Pugh, and V.Y. Arshavsky. 2002. Massive light-driven translocation of transducin between the two major compartments of rod cells. A novel mechanism of light adaptation. Neuron. 34:95–106. [DOI] [PubMed] [Google Scholar]
- Sokolov, M., K.J. Strissel, I.B. Leskov, N.A. Michaud, V.I. Govardovskii, and V.Y. Arshavsky. 2004. Phosducin facilitates light-driven transducin translocation in rod photoreceptors. Evidence from the phosducin knockout mouse. J. Biol. Chem. 279:19149–19156. [DOI] [PubMed] [Google Scholar]
- Sondek, J., A. Bohm, D.G. Lambright, H.E. Hamm, and P.B. Sigler. 1996. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature. 379:369–374. [DOI] [PubMed] [Google Scholar]
- Takida, S., and P.B. Wedegaertner. 2003. Heterotrimer formation, together with isoprenylation, is required for plasma membrane targeting of Gβγ. J Biol Chem. 278:17284–17290. [DOI] [PubMed] [Google Scholar]
- Wedegaertner, P.B. 1998. Lipid modifications and membrane targeting of Gα. Biol. Signals Recept. 7:125–135. [DOI] [PubMed] [Google Scholar]