Abstract
The development of vascular disease has its origins in an initial insult to the vessel wall by biological or mechanical factors. The disruption of homeostatic mechanisms leads to alteration of the original architecture of the vessel and its biological responsiveness, contributing to acute or chronic diseases such as stroke, hypertension and atherosclerosis. Endothelial dysfunction, macrophage infiltration of the vessel wall, and proliferation and migration of smooth muscle cells all involve different types of reactive oxygen species, produced by various vessel wall components. Although basic science and animal research have clearly established the role of reactive oxygen species in the progression of vascular disease, the failure of clinical trials with antioxidant compounds has underscored the need for better antioxidant therapies and a more thorough understanding of the role of reactive oxygen species in cardiovascular physiology and pathology.
The fundamental processes underlying the development of vascular diseases such as atherosclerosis, restenosis and hypertensive vascular remodeling have their origins in an initial insult to the vessel wall. Such an insult may arise from mechanical disruption that occurs during percutaneous coronary angioplasty or at regions of oscillatory shear stress, or it can result from biological causes, such as hypercholesterolemia, excess free radicals, diabetes, increased concentrations of plasma homocysteine, or infectious agents. Injury promotes disruption of the homeostatic mechanisms of the endothelial protective barrier, changing its natural properties, increasing its adhesiveness to leukocytes and altering its permeability. This endothelial dysfunction also leads to the formation of vasoactive molecules, which in turn induce inflammatory genes, inactivate nitric oxide (NO•) and its protective functions, and activate matrix metalloproteinases, leading to extracellular matrix remodeling, and increased smooth muscle cell (VSMC) growth, migration and proliferation. Each of these processes contributes to thickening of the vessel wall or the formation of lesions that can lead to hypertension, acute myocardial infarction and stroke.
Reactive Oxygen Species and Vascular Injury
In the past decade a staggering amount of evidence implicates a common denominator, reactive oxygen species (ROS), in the development of most cardiovascular diseases (Figure 1). Superoxide (O2•−) and hydrogen peroxide (H2O2) are two of the most biologically important ROS in the cardiovascular system, and are produced in vascular cells by a number of oxidases, including the NADPH oxidases (Nox) and xanthine oxidase, lipoxygenases, cytochrome p450, uncoupling of the mitochondrial respiratory chain, and uncoupling of endothelial nitric oxide synthase (eNOS) (Figure 2). Production is counterbalanced by antioxidant enzymes such as superoxide dismutases (SOD), catalase, glutathione peroxidase, thioredoxins and peroxiredoxins.
Figure 1.
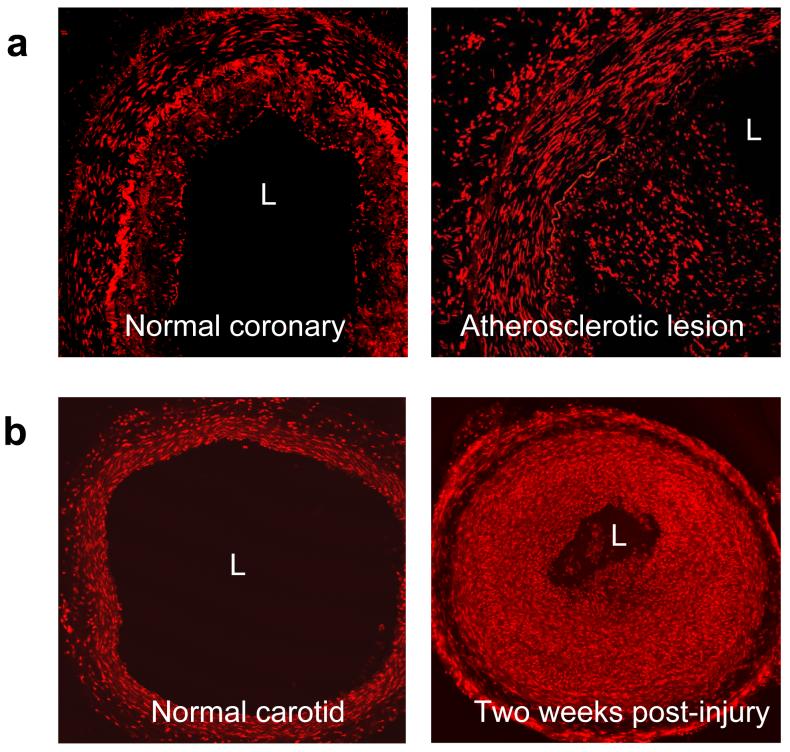
ROS production in vascular injury. a.Dihydroethidium (DHE) staining for superoxide of a stage II atherosclerotic lesion with signs of intimal hyperplasia (left) and stage V atherosclerotic where a complex plaque is already present with substantial intimal hyperplasia and partial occlusion of the arterial lumen (right). The red fluorescence shows intense superoxide production in all layers of the arterial wall. L=lumen. (Photograph courtesy of D. Sorescu). b.Superoxide production in rat carotid arteries after balloon injury. The arteries were balloon-injured and stained with dihydroethidium (DHE). Shown are a normal carotid and a carotid harvested 15 days after balloon injury. L=lumen. Modified from (Szocs et al. 2002).
Figure 2.
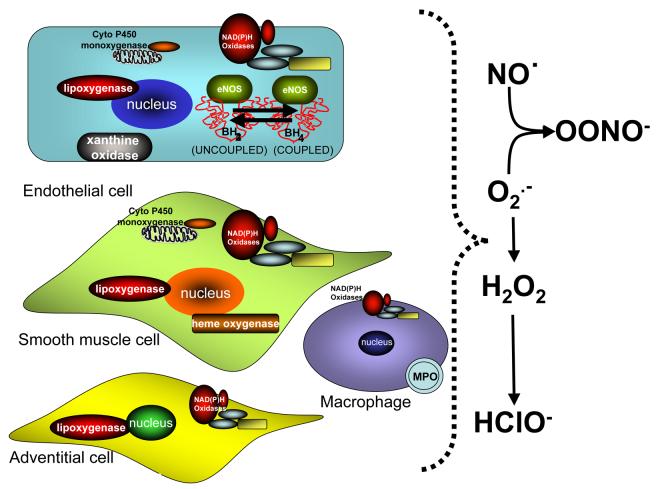
Sources of ROS in vascular cells. All components of the vascular wall produce ROS. Enzymatic sources include NADPH oxidases, lipoxygenases, heme oxygenase, xanthine oxidase, uncoupled eNOS, uncoupled mitochondrial electron transport and myeloperoxidase.
Another important ROS is NO•, produced by eNOS and inducible NOS (iNOS). Superoxide inactivates NO• and counteracts its vasodilatory and anti-inflammatory effects. The interaction of O2•− and NO• generates peroxynitrite (ONOO•), which has injurious effects on vascular cells. In addition, ONOO• oxidizes tetrahydrobiopterin thereby leading to eNOS uncoupling and diminished NO• production.
The importance of ROS in vascular injury lies in the fact that their production is positively regulated by many of the cytokines whose expression is increased after injury, and also by oscillatory shear stress and mechanical disruption (Taylor 1999). Whereas low levels of ROS are necessary for normal vascular function, excess production or impaired ROS removal, in a proinflammatory environment, regulates virtually all of the cellular responses to injury, including monocyte adhesion; platelet aggregation; inflammatory gene induction; VSMC apoptosis, proliferation and migration; matrix degradation and impaired endothelium-dependent relaxation.
Endothelial Dysfunction
Vascular injury begins with endothelial dysfunction and activation. The induction of adhesion molecules such as E- and P-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intercellular cell adhesion molecule-1 (ICAM-1), is an early marker of atherosclerosis. Their expression attracts, arrests and facilitates the transmigration of leukocytes. Cytokines like interleukins, tumor necrosis factor-α (TNFα), angiotensin II (Ang II), and vascular endothelial growth factor (VEGF) all induce the expression of adhesion molecules in a ROS-dependent fashion. Another important stimulant of adhesion molecules is fluid shear stress, which exerts both proinflammatory and protective effects, depending on the type of shear. Disturbed flow conditions which occur at branch points or curvatures enhance ICAM-1 and VCAM-1 expression (Walpola et al. 1995), whereas laminar shear stress prevents apoptosis and monocyte adhesion by producing NO• and inducing expression of antioxidant genes (Berk et al. 2001). Importantly, oscillatory shear stress leads to continuous O2•− production in a NADPH oxidase-dependent manner (Hwang et al. 2003), resulting in nuclear factor-κB (NF-κB)-mediated monocyte adhesion. Recent work has shown that bone morphogenic protein-4 (BMP-4) plays a significant role in the response to oscillatory shear stress by stimulating ROS from NADPH oxidases and regulating the expression of ICAM-1 (Sorescu et al. 2004).
The enhanced ROS production at sites of injury has multiple consequences for endothelial function. As noted, increased adhesion molecule expression attracts monocytes, which transmigrate and become macrophages and produce much higher amounts of ROS. ROS can also promote vascular leakage, which is important in ischemia–reperfusion-induced vascular injury and the induction of inflammatory responses. In addition, the combination of inactivation of NO• by O2•− and increased production of the endothelial hyperpolarizing factor H2O2 leads to impaired vasodilation (Bauersachs et al. 1996). The balance between endothelial proliferation and apoptosis is also altered by ROS, which can lead to excessive angiogenesis (Khatri et al. 2004) or loss of endothelial cells (Lin et al. 2004). All of these consequences contribute to the pathophysiology of the response to injury.
Vascular Cell Apoptosis
Apoptosis of vascular cells is an early event in both inflammatory-mediated and mechanical injury. In general, apoptosis has been associated with higher levels of ROS than those that support proliferation (see below) (Deshpande et al. 2002), such as those produced by macrophages and mechanical injury. Release of cytokines such as TNF-α following sepsis, ischemia reperfusion or shock contributes to endothelial apoptosis. Recently, it has been shown that TNF-α induced endothelial apoptosis is attenuated by N-acetyl-cysteine (NAC), implicating ROS in the response (Xia et al. 2006). Specifically, ROS regulate caspase-3 activation. Furthermore, O2•− production by activated macrophages triggers a calcium-dependent, inositol trisphosphate-linked apoptotic cascade in endothelial cells (Madesh et al. 2005). In contrast to endothelial death, VSMC apoptosis occurs mainly after mechanical injury. VSMC apoptosis is evident 30-90 minutes after balloon injury in rat carotid arteries, and is attenuated by treatment with the antioxidants NAC or pyrrolidine dithiocarbamate (PDTC) (Pollman et al. 1999). The apoptotic response appears to be mediated by activation of c-jun N-terminal kinase (JNK). Furthermore, in vitro studies have shown that H2O2 can induce apoptosis through a protein kinase C pathway, which is antagonized by Akt and heme oxygenase-1 (Brunt et al. 2006).
Vascular Smooth Muscle Cell Migration
After injury, the artery is repaired by cells that migrate from adjacent normal tissue. This is accomplished by a poorly defined mechanism; however, potent chemotactic factors for VSMC include basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β) and Ang II. Sundaresan et al. (1995) showed that vascular smooth muscle cell (VSMC) chemotaxis is inhibited by catalase. Recently, Weber et al. (2004) confirmed these results and showed that Src, phosphoinositide-dependent kinase-1 (PDK-1) and p21-activated kinase (PAK-1) are important mediators of this H2O2-dependent migration. Multiple sources of ROS have been implicated in migration. The novel Nox inhibitor VAS2870 attenuates PDGF-induced migration (Ten Freyhaus et al. 2006). Furthermore, in vivo studies have shown that remodeling is delayed in p47phox−/− mice and is completely abolished in mice lacking eNOS, suggesting that early remodeling requires NADPH oxidase-derived ROS, while late remodeling is more dependent on ONOO• generated by eNOS (Castier et al. 2005).
In addition to direct regulation of migration-related signaling, ROS also influence VSMC migration via induction of monocyte chemotactic protein-1 (MCP-1). MCP-1 expression is redox-sensitive, and involves ROS-mediated activation of extracellular signal-activated kinases (ERK1/2). It may have a dual role in both VSMC migration and matrix remodeling. In addition to promoting migration, MCP-1 also increases proinflammatory responses (Lo et al. 2005), leading to a vicious cycle of ROS production, VSMC migration and ERK1/2 activation.
Another important mechanism by which ROS mediate migration of VSMCs is via regulation of the breakdown of extracellular matrix. Matrix degradation is accomplished in part by the secretion of matrix metalloproteinases (MMPs), whose expression and activity are ROS-dependent. MMPs have been shown to be present at injury sites and their inhibitors are known to block VSMC migration both in vivo and in vitro after injury. Genetic deletion of either MMP-2 or MMP-9 reduces VSMC migration (Johnson et al. 2004). In a flow-dependent model of injury created by an A-V fistula, Castier et al. (2005) showed that MMP-9, but not MMP-2, is increased, resulting in outward remodeling. Since MMP-9 has been found to be elevated in acute coronary syndromes and because apoE/MMP-9 double knockout mice have larger atherosclerotic plaques, MMP-9 may be involved in the recruitment of VSMCs after plaque rupture (Johnson et al. 2005). Of importance, the activity of both MMP-2 and MMP-9 is regulated by ROS produced by VSMCs, endothelium and macrophages. ROS directly oxidize the catalytic center of these enzymes, resulting in either activation (low concentrations) or inactivation (high concentrations) (Rajagopalan et al. 1996). Inhibition of MMP-2 and MMP-9 activity can be achieved by overexpression of eNOS (Gurjar et al. 1999), and MMP-2 activity is increased by H2O2 and ONOO• (Rajagopalan et al. 1996). Not only do ROS regulate MMP activity, but they also modify MMP expression. Both MMP-2 and MMP-9 are induced by ROS (Luchtefeld et al. 2005). Expression of another MMP, MMP-1, which is important in collagen degradation, is increased by Ang II stimulation through NF–κB and activating protein-1 (AP-1) (Browatzki et al. 2005). TNFα stimulation has similar effects (Browatzki et al. 2005). Moreover, MMP-7, a MMP with high degradative ability, is also activated by ROS,specifically hypochloric acid (HOCl−) (Fu et al. 2001).
Vascular Smooth Muscle Proliferation
VSMC proliferation also plays a central role in cardiovascular injury. The ROS dependency of the growth response has been investigated in detail. Originally, Sundaresan et al. (1995) showed that PDGF-induced DNA synthesis in cultured VSMCs is inhibited by catalase and NAC. This was confirmed by Kong et al. (2001). However, more recent work has cast doubt upon the conclusion that true PDGF-mediated proliferation is ROS-dependent. Rapamycin, a relatively new immunosuppressant that acts on the protein synthesis mediator mTOR, inhibits PDGF-induced proliferation without affecting ROS production (Park et al. 2005). Furthermore, although PDGF-induced migration is inhibited by the novel Nox inhibitor VAS2870, this inhibitor has no effect on DNA synthesis (Ten Freyhaus et al. 2006). Whereas these latter observations could be used to support the concept that both ROS-sensitive and ROS-insensitive signaling pathways mediate PDGF-induced proliferation, we have not been able to demonstrate a requirement for ROS in PDGF-induced growth in these cells (unpublished observations). However, other pro-proliferative agonists do appear to require ROS. Thrombin-induced proliferation is blocked by the flavin-containing oxidase inhibitor diphenylene iodonium (DPI) (Patterson et al. 1999), and catecholamine-induced VSMC proliferation can be blocked by NAC, the superoxide scavenger Tiron and DPI (Bleeke et al. 2004). Finally, urokinase plasminogen activator (UPA) promotes VSMC proliferation in a ROS-dependent manner, and has been to involve the NADPH oxidases Nox1 and Nox4 (Menshikov et al. 2006).
In vivo, although antioxidants inhibit neointimal formation, it is difficult to separate their effects on proliferation and migration, the latter of which is nearly universally found to be ROS-sensitive (see above). Removal of O2•− from the injury site by SOD mimetics reduces neointima formation after balloon injury (Muscoli et al. 2004). Similarly, in rabbits, adenoviral mediated gene transfer of SOD and catalase reduces restenosis after balloon angioplasty (Durand et al. 2005). Probucol, a lipid lowering agent with antioxidant properties, inhibits neointima formation in rabbits after balloon injury through heme oxygenase-1-dependent and -independent pathways (Deng et al. 2004). Neointima formation in rabbits was shown to be driven by adventitial and endothelial Nox2-derived O2•− in collared arteries (Paravicini et al. 2002). In rats, cuff injury neointima formation was also demonstrated to be dependent on O2•− (Ozumi et al. 2005).
The mechanisms by which ROS regulate proliferation are complex. Of the possible candidates, it appears that H2O2 is the ROS that mediates most growth-related signaling. Furthermore, the concentration of H2O2 is critically important for its effects on cells. High concentrations have been shown to be apoptotic, moderate doses cause cell arrest in the G1 phase, and low doses support proliferation (Baas et al. 1995,Deshpande et al. 2002). Finally, the intracellular location of H2O2 production is likely important, as ROS producing enzymes are found both in caveolae and focal adhesions (Hilenski et al. 2004).
H2O2 mediates its effects on proliferation by regulating the activation of specific signaling pathways. Thrombin stimulates a p47phox-regulated NADPH oxidase to produce ROS, which in turn activate the JAK/STAT pathway, required for growth (Patterson et al. 1999). In addition, thrombin stimulates ERK1/2, p38 mitogen activated protein kinase (p38MAPK) and JNK signaling, leading to induction of c-Fos and Jun-B, which combine to activate AP-1-mediated gene expression. Activation of all of these pathways is inhibited by NAC or DPI (Rao et al. 1999). Similar to the signaling pathways utilized by AngII, catecholamines stimulate VSMC proliferation by ROS-dependent transactivation of the EGF receptor (Zhang et al. 2004). Importantly, the trophic effect of catecholamines on vascular media derived from animal subjected to balloon injury 4 days earlier has been shown to be dependent on H2O2 (Bleeke et al. 2004). Interestingly, although the downstream signaling pathways activated by UPA to induce growth are unclear, induction of UPA receptor expression is redox-sensitive (Oka et al. 2000).
In vivo studies have shown that inactivation of ERK, p38MAPK or JNK prevents neointimal formation in balloon injured rat carotid arteries by inhibiting proliferation (Kim et al. 2003). The serine/threonine kinase Pim-1, which belongs to the family of calcium/calmodulin regulated kinases, has been shown to be induced by ROS, and is elevated after injury. In addition, Pim-1 regulates cell cycle progression and apoptosis via multiple pathways, including phosphorylation of the cell cycle phosphatase cdc25A, and c-Myb, a transcription factor that is essential for VSMC replication (Katakami et al. 2004).
Recently, leptin has been shown to have a proatherogenic effect in vitro promoting VSMC proliferation and migration. In vivo, leptin receptors are expressed in vascular cells and leptin signaling promotes atherosclerosis in mouse models (Park et al. 2001). Leptin has been shown to enhance the proliferation of human aortic VSMCs and MMP-2 expression, with concomitant increased generation of intracellular ROS and activation of PKC, ERK1/2 and NF-κB. Metformin inhibited the ROS production and PKC, ERK1/2 and NF-κB activation (Li et al. 2005). In addition, a different group showed that leptin induces hypertrophy in rat VSMCs via the p38MAPK pathway (Shin et al. 2005). These findings provide a basis for some of the smooth muscle abnormalities in diabetic vasculopathy.
Conclusions and Future Directions
Cell culture and animal studies have clearly shown a role for ROS in the progression of vascular disease. As noted above, in animal models, genetic manipulation of ROS-regulating enzymes or treatment with antioxidants have been effective in reducing the extent or worsening of vasculopathies. Some human antioxidant trials have been promising. For example, Duffy et al. (1999) showed in a randomized double blind trial that ascorbic acid supplementation can lower the blood pressure in otherwise healthy hypertensive patients. Another positive clinical study used a combination of antioxidants (NAC, Vitamin E (VitE) and Vitamin C (VitC)) to treat young (mean age 41) diabetic patients or patients with impaired glucose tolerance (IGT), and found a reduction of oxidative stress and inflammation after 15 days of treatment (Neri et al. 2005). The combination of VitE, fish oil, niacin and γ-oryzanol showed a beneficial effect in reducing oxidative stress and inflammatory markers in young dyslipidemic patients (Accinni et al. 2006).
However, overwhelmingly, clinical trials have failed to show a protective effect of antioxidants in humans. A meta-analysis of seven randomized VitE trials does not support a protective effect of VitE supplementation on the progression of cardiovascular disease or on clinical events in patients at high risk or with established disease (Vivekananthan et al. 2003). The reasons for the failure of these antioxidant trials is likely multifactorial. It is still very difficult to identify the subjects who have an imbalance between ROS production and antioxidant defenses and might benefit from antioxidant therapy. Furthermore, most patients enrolled in clinical trials have already established cardiovascular disease, and antioxidant intervention in these cases may be too late to be effective. In most animal studies, animals are treated with antioxidants prior to the initiation of disease. This suggests that antioxidant therapy may be more beneficial as a preventive measure, rather than a therapeutic tool. The lack of knowledge on the optimal dosage for the various antioxidants is also a handicap. More recently it has become apparent that the bioavailability and distribution of administered antioxidants may limit their usefulness. To be effective, antioxidants must reach the cellular compartment in which ROS are generated. For many vascular cells, this requires uptake into the cytoplasm or vesicles. Because VitE is lipid soluble, the levels of VitE in the plasma may not reflect its action in the vessel wall (Hodis et al. 2002), or its ability to penetrate to intracellular membranes. Although VitC is water-soluble and has access to intracellular compartments, it has a narrow absorption window with a maximum at 200 mg. An increase of oral administration can actually decrease its bioavailability (McGregor et al. 2006). A further complication is that all antioxidants can become oxidants in certain environments and at particular doses. For example, VitC in high doses increases DNA damage in healthy volunteers (Podmore et al. 1998), and VitE may act as a pro-oxidant in cigarette smokers (Weinberg et al. 2001).
The failure of classic antioxidants has led to a search for new, more effective compounds. The vasodilator activity of NO-donor phenols on rat aortic strips (Boschi et al. 2006), and the inhibition of proinflammatory gene expression by the new antioxidant AGI-1067 (Serebruany et al. 2006) are promising ex vivo results that require follow up. Attention has also shifted to dietary supplementation of antioxidants, on the theory that absorption, metabolism and bioavailability of these forms may not be mimicked by administration in the form of a pill (Rice-Evans 2001). However, it is clear from animal studies that individual ROS mediate specific pathophysiological responses in the vessel wall. Superoxide inactivates NO•, thus counteracting its vasodilatory, antiproliferative and anti-inflammatory effects. In contrast, H2O2 mediates VSMC apoptosis, proliferation and migration. Based on this analysis, the choice of antioxidant should depend opon the identity of the ROS responsible for the pathology. Thus, inhibition of H2O2 might be more effective in reducing neointima and plaque formation. When trying to scavenge ROS, it must be kept in mind that low levels of ROS are necessary for cell viability, so nonselective scavenging of ROS may be deleterious. A better approach might be to target those enzymes that produce excess ROS during disease initiation and progression. One target is the NADPH oxidases. The novel Nox inhibitor VAS2870 and the Nox2 peptide inhibitor gp91-dstat are examples of potential enzyme-targeted inhibitors, although their usefulness in patients remains to be determined. Another potential therapy is tetrahydrobiopterin (BH4), which can prevent eNOS uncoupling and restore NO• production. Finally, limited studies using allopurinol to inhibit xanthine oxidase have shown benefit in atherosclerosis and heart failure (Farquharson et al. 2002), but additional trials are needed. The final verdict on antioxidant treatment for vascular injury must therefore await the development of more effective antioxidants, and better biomarkers of oxidative stress.
Much remains to be accomplished to fully understand the role of ROS in cardiovascular physiology and pathology. Future work will help us to better elucidate the mechanisms that regulate enzymatic generation of ROS, the importance of subcellular compartmentalization of ROS, and the molecular effects of ROS on individual proteins. New transgenic and knockout animal models are needed to study in detail the effects of individual generators of ROS and to identify their effectors. New biomarkers of oxidative stress are needed to identify early the need for antioxidant therapy, and to assess the effectiveness of new antioxidants. As we begin to appreciate the myriad roles of ROS in the vasculature, we will be better able to target specific sources, locations, cell types and diseases therapeutically.
Figure 3.
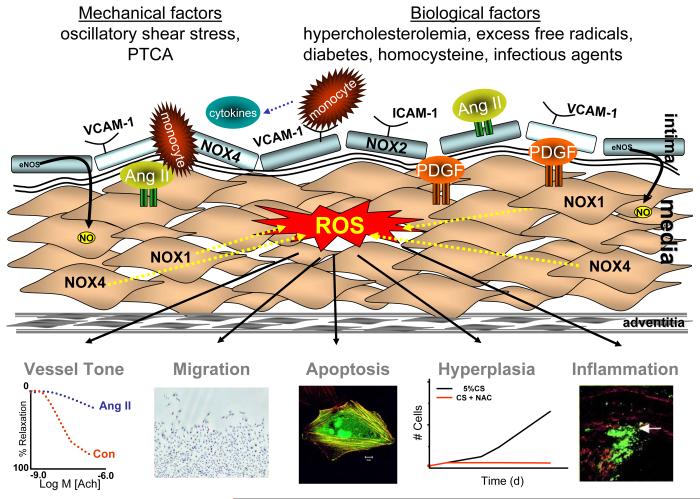
Role of ROS in the response to injury. Reactive oxygen species mediate many of the responses to vascular injury. Both mechanical factors and biological factors produced in response to injury can stimulate the production of reactive oxygen species in macrophages, endothelial cells, smooth muscle cells and adventitia. These ROS then impair vessel tone and promote an inflammatory response, and increase smooth muscle cell migration, proliferation and apoptosis. Figure depicting migration shows VSMCs migrating into a wound area stained with hematoxylin and eosin after stimulation with PDGF. Figure depicting inflammation shows the shoulder region of a plaque stained for macrophages. Adapted from (Sorescu et al. 2002) Figure depicting apoptosis is a GFP-transfected cell stained with phalloidin. (Courtesy of Alicia Lyle)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by NIH grants HL058000, HL038206, and HL058863.
References
- Accinni R, Rosina M, Bamonti F, et al. Effects of combined dietary supplementation on oxidative and inflammatory status in dyslipidemic subjects. Nutr Metab Cardiovasc Dis. 2006;16:121–127. doi: 10.1016/j.numecd.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Baas AS, Berk BC. Differential activation of mitogen-activated protein kinases by H2O2 and O2- in vascular smooth muscle cells. Circ Res. 1995;77:29–36. doi: 10.1161/01.res.77.1.29. [DOI] [PubMed] [Google Scholar]
- Bauersachs J, Popp R, Hecker M, et al. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–3347. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- Berk BC, Abe JI, Min W, et al. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann N Y Acad Sci. 2001;947:93–109. doi: 10.1111/j.1749-6632.2001.tb03932.x. discussion 109-111. [DOI] [PubMed] [Google Scholar]
- Bleeke T, Zhang H, Madamanchi N, et al. Catecholamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ Res. 2004;94:37–45. doi: 10.1161/01.RES.0000109412.80157.7D. [DOI] [PubMed] [Google Scholar]
- Boschi D, Tron GC, Lazzarato L, et al. NO-donor phenols: a new class of products endowed with antioxidant and vasodilator properties. J Med Chem. 2006;49:2886–2897. doi: 10.1021/jm0510530. [DOI] [PubMed] [Google Scholar]
- Browatzki M, Larsen D, Pfeiffer CA, et al. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res. 2005;42:415–423. doi: 10.1159/000087451. [DOI] [PubMed] [Google Scholar]
- Brunt KR, Fenrich KK, Kiani G, et al. Protection of human vascular smooth muscle cells from H2O2-induced apoptosis through functional codependence between HO-1 and AKT. Arterioscler Thromb Vasc Biol. 2006;26:2027–2034. doi: 10.1161/01.ATV.0000236204.37119.8d. [DOI] [PubMed] [Google Scholar]
- Castier Y, Brandes RP, Leseche G, et al. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- Deng YM, Wu BJ, Witting PK, et al. Probucol protects against smooth muscle cell proliferation by upregulating heme oxygenase-1. Circulation. 2004;110:1855–1860. doi: 10.1161/01.CIR.0000142610.10530.25. [DOI] [PubMed] [Google Scholar]
- Deshpande NN, Sorescu D, Seshiah P, et al. Mechanism of hydrogen peroxide-induced cell cycle arrest in vascular smooth muscle. Antioxid Redox Signal. 2002;4:845–854. doi: 10.1089/152308602760599007. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Gokce N, Holbrook M, et al. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048–2049. doi: 10.1016/s0140-6736(99)04410-4. [DOI] [PubMed] [Google Scholar]
- Durand E, Al Haj Zen A, Addad F, et al. Adenovirus-mediated gene transfer of superoxide dismutase and catalase decreases restenosis after balloon angioplasty. J Vasc Res. 2005;42:255–265. doi: 10.1159/000085658. [DOI] [PubMed] [Google Scholar]
- Farquharson CA, Butler R, Hill A, et al. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- Fu X, Kassim SY, Parks WC, et al. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- Gurjar MV, Sharma RV, Bhalla RC. eNOS gene transfer inhibits smooth muscle cell migration and MMP-2 and MMP-9 activity. Arterioscler Thromb Vasc Biol. 1999;19:2871–2877. doi: 10.1161/01.atv.19.12.2871. [DOI] [PubMed] [Google Scholar]
- Hilenski LL, Clempus RE, Quinn MT, et al. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, LaBree L, et al. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- Hwang J, Saha A, Boo YC, et al. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24:54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- Johnson JL, George SJ, Newby AC, et al. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005;102:15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakami N, Kaneto H, Hao H, et al. Role of pim-1 in smooth muscle cell proliferation. J Biol Chem. 2004;279:54742–54749. doi: 10.1074/jbc.M409140200. [DOI] [PubMed] [Google Scholar]
- Khatri JJ, Johnson C, Magid R, et al. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004;109:520–525. doi: 10.1161/01.CIR.0000109698.70638.2B. [DOI] [PubMed] [Google Scholar]
- Kim S, Iwao H. Stress and vascular responses: mitogen-activated protein kinases and activator protein-1 as promising therapeutic targets of vascular remodeling. J Pharmacol Sci. 2003;91:177–181. doi: 10.1254/jphs.91.177. [DOI] [PubMed] [Google Scholar]
- Kong G, Lee S, Kim KS. Inhibition of rac1 reduces PDGF-induced reactive oxygen species and proliferation in vascular smooth muscle cells. J Korean Med Sci. 2001;16:712–718. doi: 10.3346/jkms.2001.16.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Mamputu JC, Wiernsperger N, et al. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes. 2005;54:2227–2234. doi: 10.2337/diabetes.54.7.2227. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Shyue SK, Liu PL, et al. Adenovirus-mediated overexpression of catalase attenuates oxLDL-induced apoptosis in human aortic endothelial cells via AP-1 and C-Jun N-terminal kinase/extracellular signal-regulated kinase mitogen-activated protein kinase pathways. J Mol Cell Cardiol. 2004;36:129–139. doi: 10.1016/j.yjmcc.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Lo IC, Shih JM, Jiang MJ. Reactive oxygen species and ERK 1/2 mediate monocyte chemotactic protein-1-stimulated smooth muscle cell migration. J Biomed Sci. 2005;12:377–388. doi: 10.1007/s11373-005-1703-2. [DOI] [PubMed] [Google Scholar]
- Luchtefeld M, Grote K, Grothusen C, et al. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun. 2005;328:183–188. doi: 10.1016/j.bbrc.2004.12.152. [DOI] [PubMed] [Google Scholar]
- Madesh M, Hawkins BJ, Milovanova T, et al. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol. 2005;170:1079–1090. doi: 10.1083/jcb.200505022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor GP, Biesalski HK. Rationale and impact of vitamin C in clinical nutrition. Curr Opin Clin Nutr Metab Care. 2006;9:697–703. doi: 10.1097/01.mco.0000247478.79779.8f. [DOI] [PubMed] [Google Scholar]
- Menshikov M, Plekhanova O, Cai H, et al. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arterioscler Thromb Vasc Biol. 2006;26:801–807. doi: 10.1161/01.ATV.0000207277.27432.15. [DOI] [PubMed] [Google Scholar]
- Muscoli C, Sacco I, Alecce W, et al. The protective effect of superoxide dismutase mimetic M40401 on balloon injury-related neointima formation: role of the lectin-like oxidized low-density lipoprotein receptor-1. J Pharmacol Exp Ther. 2004;311:44–50. doi: 10.1124/jpet.104.068205. [DOI] [PubMed] [Google Scholar]
- Neri S, Signorelli SS, Torrisi B, et al. Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: a single-blind, 15-day clinical trial in patients with untreated type 2 diabetes, subjects with impaired glucose tolerance, and healthy controls. Clin Ther. 2005;27:1764–1773. doi: 10.1016/j.clinthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Oka H, Kugiyama K, Doi H, et al. Lysophosphatidylcholine induces urokinase-type plasminogen activator and its receptor in human macrophages partly through redox-sensitive pathway. Arterioscler Thromb Vasc Biol. 2000;20:244–250. doi: 10.1161/01.atv.20.1.244. [DOI] [PubMed] [Google Scholar]
- Ozumi K, Tasaki H, Takatsu H, et al. Extracellular superoxide dismutase overexpression reduces cuff-induced arterial neointimal formation. Atherosclerosis. 2005;181:55–62. doi: 10.1016/j.atherosclerosis.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Gulluyan LM, Dusting GJ, et al. Increased NADPH oxidase activity, gp91phox expression, and endothelium-dependent vasorelaxation during neointima formation in rabbits. Circ Res. 2002;91:54–61. doi: 10.1161/01.res.0000024106.81401.95. [DOI] [PubMed] [Google Scholar]
- Park HY, Kwon HM, Lim HJ, et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- Park J, Ha H, Ahn HJ, et al. Sirolimus inhibits platelet-derived growth factor-induced collagen synthesis in rat vascular smooth muscle cells. Transplant Proc. 2005;37:3459–3462. doi: 10.1016/j.transproceed.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Patterson C, Ruef J, Madamanchi NR, et al. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. J Biol Chem. 1999;274:19814–19822. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- Podmore ID, Griffiths HR, Herbert KE, et al. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392:559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- Pollman MJ, Hall JL, Gibbons GH. Determinants of vascular smooth muscle cell apoptosis after balloon angioplasty injury. Influence of redox state and cell phenotype. Circ Res. 1999;84:113–121. doi: 10.1161/01.res.84.1.113. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Meng XP, Ramasamy S, et al. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GN, Katki KA, Madamanchi NR, et al. JunB forms the majority of the AP-1 complex and is a target for redox regulation by receptor tyrosine kinase and G protein-coupled receptor agonists in smooth muscle cells. J Biol Chem. 1999;274:6003–6010. doi: 10.1074/jbc.274.9.6003. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- Serebruany V, Malinin A, Scott R. The in vitro effects of a novel vascular protectant, AGI-1067, on platelet aggregation and major receptor expression in subjects with multiple risk factors for vascular disease. J Cardiovasc Pharmacol Ther. 2006;11:191–196. doi: 10.1177/1074248406290598. [DOI] [PubMed] [Google Scholar]
- Shin HJ, Oh J, Kang SM, et al. Leptin induces hypertrophy via p38 mitogen-activated protein kinase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;329:18–24. doi: 10.1016/j.bbrc.2004.12.195. [DOI] [PubMed] [Google Scholar]
- Sorescu D, Weiss D, Lassegue B, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- Sorescu GP, Song H, Tressel SL, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, et al. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Szocs K, Lassegue B, Sorescu D, et al. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- Taylor WR. Hypertensive vascular disease and inflammation: mechanical and humoral mechanisms. Curr Hypertens Rep. 1999;1:96–101. doi: 10.1007/s11906-999-0079-5. [DOI] [PubMed] [Google Scholar]
- Ten Freyhaus H, Huntgeburth M, Wingler K, et al. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006 doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Vivekananthan DP, Penn MS, Sapp SK, et al. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- Walpola PL, Gotlieb AI, Cybulsky MI, et al. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol. 1995;15:2–10. doi: 10.1161/01.atv.15.1.2. [DOI] [PubMed] [Google Scholar]
- Weber DS, Taniyama Y, Rocic P, et al. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- Weinberg RB, VanderWerken BS, Anderson RA, et al. Pro-oxidant effect of vitamin E in cigarette smokers consuming a high polyunsaturated fat diet. Arterioscler Thromb Vasc Biol. 2001;21:1029–1033. doi: 10.1161/01.atv.21.6.1029. [DOI] [PubMed] [Google Scholar]
- Xia Z, Liu M, Wu Y, et al. N-acetylcysteine attenuates TNF-alpha-induced human vascular endothelial cell apoptosis and restores eNOS expression. Eur J Pharmacol. 2006;550:134–142. doi: 10.1016/j.ejphar.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chalothorn D, Jackson LF, et al. Transactivation of epidermal growth factor receptor mediates catecholamine-induced growth of vascular smooth muscle. Circ Res. 2004;95:989–997. doi: 10.1161/01.RES.0000147962.01036.bb. [DOI] [PubMed] [Google Scholar]