Abstract
Homo-oligomerization of Bax (or Bak) has been hypothesized to be responsible for cell death through the mitochondria-dependent apoptosis pathway. However, partly due to a lack of structural information on the Bax homo-oligomerization and apoptosis inducing domain(s), this hypothesis has remained difficult to test. In this study, we identified a three-helix unit, comprised of the BH3 (helix 2) and BH1 domains (helix 4 and helix 5), as the homo-oligomerization domain of Bax. When targeted to mitochondria, this minimum oligomerization unit induced apoptosis in Bax−/−Bak−/− mouse embryonic fibroblasts (DKO). Strikingly, the central helix of Bax (helix 5), when replacing the corresponding helix (helix 5) of Bcl-xL, an anti-apoptotic Bcl-2 family protein structurally homologous to Bax, converted Bcl-xL into a Bax-like molecule capable of forming oligomers and causing apoptosis in the DKO cells. Finally, a series of systematic mutagenesis analyses revealed that homo-oligomerization is both necessary and sufficient for the apoptotic activity of Bax. These results suggest that active Bax causes mitochondrial damage through homo-oligomers of a three-helix functional unit.
Keywords: Bax, homo-oligomerization, apoptotic activity, BH3, Bcl-2 family
The Bcl-2 family proteins, characterized by the presence of at least one of the four Bcl-2 homology domains (BH1–4), are major regulators and effectors of the mitochondria-dependent pathway of apoptosis (Danial and Korsmeyer 2004). Functionally, the Bcl-2 family can be categorized into the anti-apoptotic and the proapoptotic groups. The anti-apoptotic family members—e.g., Bcl-2 and Bcl-xL—function to protect the integrity of the mitochondria. The proapoptotic family members can be further divided into two subgroups, the BH1–3 proapoptotic members (also called Bax-like or multidomain proapoptotic members)—i.e., Bax and Bak—and the BH3-only proteins, such as Bim, Bad, Bid, and Puma (Borner 2003). In response to apoptotic stimulation, select BH3-only molecules directly or indirectly activate the multidomain proapoptotic molecules, which in turn cause the permeabilization of the mitochondrial outer membrane and the release of cytochrome c and other apoptogenic factors (Willis and Adams 2005). When released into the cytoplasm, cytochrome c triggers the formation of the “apoptosome,” which is responsible for the proteolytic activation of the effector caspases and the demise of the cell (Jiang and Wang 2004).
The critical role of the BH1–3 proapoptotic molecules in the mitochondria-dependent pathway was demonstrated by both genetic and biochemical analyses. While mice deficient for either Bax or Bak showed minor phenotypes related to apoptosis, mice doubly deficient for Bak and Bax displayed severe defects in apoptosis, including an imbalance of hematopoietic cells and persistence of interdigital tissues known as webbing (Lindsten et al. 2000). More importantly, mouse embryonic fibroblasts (MEFs) from these mice showed almost complete resistance to mitochondria-dependent apoptosis induced by various stimuli, including UV, γ-irradiation, and serum starvation (Wei et al. 2001). In addition, these cells failed to respond to the BH3-only molecules to initiate apoptosis (Zong et al. 2001). On the other hand, biochemical studies using reconstituted systems have demonstrated that activated Bax or Bak can cause the release of cytochrome c from mitochondria (Jurgensmeier et al. 1998; Desagher et al. 1999; Saito et al. 2000; Wei et al. 2000). These results establish the role of Bax and Bak as the gateway to mitochondria-dependent apoptosis.
While Bak is a mitochondrial resident, Bax has been found in the cytoplasm or loosely attatched to mitochondria as a monomeric protein under normal conditions (Hsu et al. 1997; Hsu and Youle 1998). Upon apoptotic stimulation, Bax undergoes a conformational change and translocates to mitochondria via the mitochondrial targeting sequence at the C terminus (Wolter et al. 1997; Nechushtan et al. 1999). Once on mitochondria, both Bax and Bak were found to form homo-oligomers, which have been hypothesized to be responsible for cytochrome c release (Nechushtan et al. 2001).
Oligomerzation of Bax, as commonly detected by gel-filtration or cross-linking analyses, is associated with the permeabilization of mitochondrial membranes and the release of cytochrome c (Gross et al. 1998; Antonsson et al. 2001; Mikhailov et al. 2003). It has been demonstrated that nonionic detergents can induce homo-oligomer formation (Hsu and Youle 1997). These detergent-induced homo-oligomers of Bax have been shown to be able to induce mitochondrial membrane permeabilization (Antonsson et al. 2000; Kuwana et al. 2002). It is therefore postulated that homo-oligomers of Bax or Bak are responsible for permeabilization of the outer mitochondrial membrane and release of cytochrome c. However, since the mechanism of the homo-oligomerization process is poorly understood, the relationship between oligomerization and apoptotic activity of Bax remains uncertain (Borner 2003).
A number of proteins have been proposed to bind to and regulate Bax. Truncated Bid (tBid) has been proposed to directly interact with and activate Bax or Bak (Desagher et al. 1999; Wei et al. 2000; Kim et al. 2006). PUMA and some non-Bcl-2 family proteins, such as Bif-1, and p53, have also been suggested to function similarly to trigger activation of Bax (Cuddeback et al. 2001; Cartron et al. 2004; Chipuk et al. 2004). On the other hand, the anti-apoptotic Bcl-2 family proteins, their viral homologs (e.g., E1B 19K), and several non-Bcl-2 family proteins (e.g., Ku70 and humanin) have been reported to complex with Bax and thereby inhibit its mitochondrial translocation, homo-oligomerization, or apoptotic activity (Perez and White 2000; Sundararajan and White 2001; Guo et al. 2003; Sawada et al. 2003).
The overall structure of Bax bears strong resemblance to that of Bcl-xL, with a central hydrophobic helix (helix 5) surrounded by eight amphipathic α-helices (Suzuki et al. 2000). Similar to Bcl-xL, the BH1, BH2, and BH3 domains of Bax form a hydrophobic groove (Suzuki et al. 2000; Petros et al. 2004). However, while the hydrophobic groove of Bcl-xL has been found to receive the BH3 domain of Bad and Bak, the hydrophobic groove of Bax is occupied by its own C-terminal tail (CT) (helix 9), which is responsible for mitochondrial localization (Sattler et al. 1997; Petros et al. 2000; Suzuki et al. 2000). It is therefore hypothesized that Bax undergoes an extensive conformational change to disengage helix 9 and allow interactions with other BH3-only molecules (Suzuki et al. 2000). Of importance, helix 5 and helix 6 of both Bax and Bcl-xL form a hairpin structure, which has been termed the putative pore-forming domain, reminiscent of pore-forming bacterial toxins (Borner 2003). However, it is not obvious from the available structural information why Bax and Bcl-xL can perform opposite biological functions.
To understand how Bax forms homo-oligomers and exerts its apoptotic function, we carried out a structure–function analysis of Bax. We identified the long-sought-after Bax homo-oligomerization domain as well as the apoptosis-inducing domain of Bax. With the use of the Bax−/−Bak−/− MEF (DKO) cells and a series of extensive mutational analyses, the relationship between homo-oligomerization and the apoptotic activity of Bax was determined. Unexpectedly, our study also revealed a single structural element in Bax that can convert Bcl-xL into a BH1–3 proapoptotic molecule.
Results
Transfected GFP-Bax can be induced to form oligomers in Bax−/−Bak−/− MEFs (DKO)
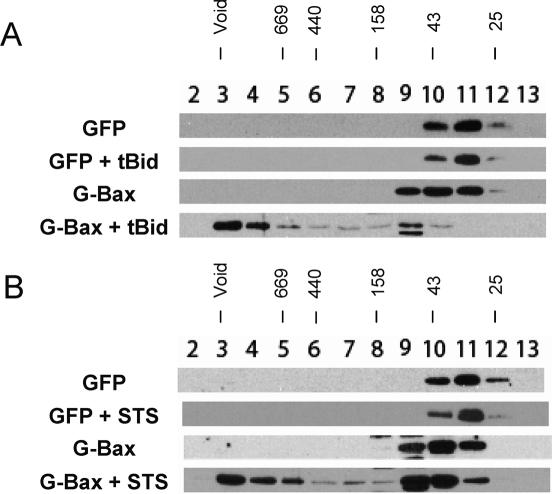
To study the mechanism of Bax homo-oligomerization, we transfected an expression plasmid encoding GFP-Bax (G-Bax) into Bax−/−Bak−/− MEFs (DKO) and examined oligomerization of the transfected Bax by gel-filtration analysis. Fractionation of cell extracts on a size-exclusion column in the presence of the zwitterionic detergent CHAPS has been commonly used to monitor the oligomerization of Bax (Antonsson et al. 2001). GFP expression plasmid was transfected and used as a negative control. As shown in Figure 1, 20 h after transfection, both GFP (27 kDa) and GFP-Bax (G-Bax, ∼50 kDa) can be detected in the gel-filtration analysis as monomers. Two strategies were tested to induce oligomerization of the transfected Bax. One is cotransfection with an expression plasmid for tBid, which is known to induce apoptosis through Bax and Bak (Wei et al. 2000, 2001; Zong et al. 2001), and the other is the addition of Staurosporine (STS), a general kinase inhibitor that presumably induces apoptosis through the mitochondrial pathway. As shown in Figure 1A, when expression plasmids for G-Bax and tBid were cotransfected, the majority of G-Bax migrated as high-molecular-weight complexes. Similarly, in Figure 1B, following transfection of the G-Bax or GFP plasmids, treatment of the cells with STS caused a significant portion of G-Bax, but not GFP, to shift to the high-molecular-weight fractions, indicating the formation of higher-order oligomers (Antonsson et al. 2001). These results indicate that both tBid and STS can induce homo-oligomerization of G-Bax. Since tBid cotransfection showed a consistently higher percentage of conversion from monomers to oligomers (Fig. 1), we chose tBid cotransfection as our strategy for further analysis of Bax homo-oligomerization.
Figure 1.
Homo-oligomerization of transfected Bax in Bax−/−Bak−/− MEFs (DKO). (A) Gel-filtration analysis of G-Bax in DKO cells with coexpression of tBid. DKO cells were transfected with the expression plasmids for the indicated proteins. Twenty hours after transfection, cells were harvested and lysed in 2% CHAPS. The cell extracts were analyzed on a Superdex 200 column. Each fraction was analyzed by Western blot using GFP antibody. Fraction numbers were specified. The molecular weights (in kilodaltons) for standard proteins were labeled above the fraction numbers. (B) Gel-filtration analysis of G-Bax in DKO cells treated with STS. DKO cells were transfected with the expression plasmids for the indicated proteins. Twenty hours after transfection, cells were treated with either DMSO or 1 μM STS. After an additional 8 h, cells were harvested and analyzed by gel filtration as described in A.
Identification of the oligomerization domain of Bax
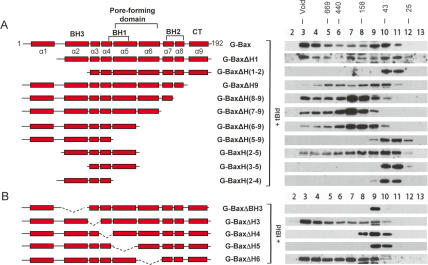
To locate the region of Bax responsible for oligomerization, a series of N-terminal and C-terminal deletion mutants of Bax were constructed as GFP fusion proteins, each of which was cotransfected with tBid expression plasmid into the DKO cells. Oligomerization of G-Bax and its truncation mutants was examined by gel-filtration analysis as described in Figure 1. As shown in Figure 2A, while deletion of helix 1 had minimum effect on oligomerization, the removal of both helix 1 and helix 2 (the BH3 domain) abolished oligomerization. Similarly, while the serial C-terminal truncation of helices 9, 8, 7, and 6 had little effect, a further deletion of helix 5 resulted in a complete loss of oligomerization. These results suggest that the central region of Bax encompassing helices 2–5 may play a critical role in oligomer formation. Strikingly, the mutant G-BaxH(2–5), which contains only helices 2–5, was able to form oligomers, indicating that helices 2–5 contain all the structural information necessary for homo-oligomerization. Interestingly, some deletion mutants that were unable to form high-molecular-weight homo-oligomers appeared to form monomers and dimers, as suggested by their migration on the size-exclusion column. However, the significance and mechanism of dimer formation remain to be studied.
Figure 2.
Mapping the oligomerization domain of Bax through N-terminal, C-terminal, and internal deletions. (A) Homo-oligomerization of GFP fusions of Bax and its N-terminal and C-terminal deletion mutants in the DKO cells. On the left, a schematic representation of wild-type Bax and the N-terminal and C-terminal truncation mutants. Each red rectangle represents a helix, which is numerically labeled (Suzuki et al. 2000). The lines outside of the rectangles represent nonhelical linker regions of Bax. Helix 9 is located within the CT. The indicated GFP fusion of Bax wild type and mutants was cotransfected with tBid expression plasmid into the DKO cells, and gel-filtration analyses were carried out and are shown on the right. (B) Mapping the oligomerization domain of Bax through internal deletion mutants. The amino acid sequences deleted in each construct are specified in Materials and Methods.
To further examine the role of the individual helices in Bax oligomerization, a series of internal deletions that remove individual helices from full-length Bax was constructed and tested for oligomerization in the DKO cells. As shown in Figure 2B, although the removal of helices 3 or 6 had little effect, the removal of helices 2, 4, or 5 individually abolished oligomer formation. These results suggest that the central region containing helices 2 (BH3), 4, and 5 functions as the homo-oligomerization domain responsible for oligomer formation of Bax.
Apoptotic activity of the Bax oligomerization domain in the DKO cells
Since homo-oligomerization has been hypothesized to be responsible for the apoptotic activity of Bax, we set out to examine the apoptotic activities of both full-length Bax and its oligomerization domain in the DKO cells. First, using Hoechst staining after transfection, we examined the apoptotic activities of the full-length G-Bax in the absence and presence of tBid cotransfection. Oligomerization was monitored by gel-filtration analysis as described in Figure 1. As shown in Figure 3B, while G-Bax alone displayed a modest level of apoptosis, cotransfection of G-Bax and tBid plasmids markedly enhanced the apoptotic activity, suggesting that G-Bax was activated by tBid in the DKO cells.
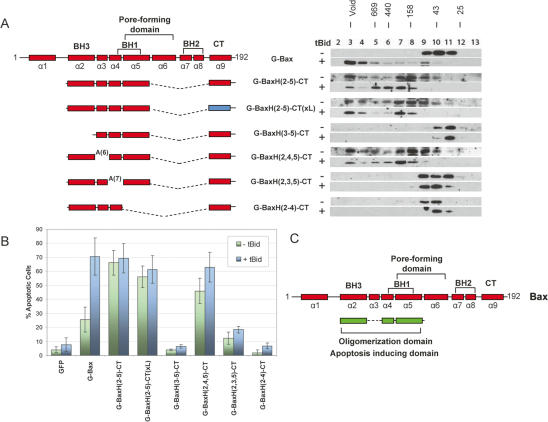
Figure 3.
The mitochondria-targeted oligomerization domain of Bax is capable of causing apoptosis in the DKO cells. (A) Gel-filtration analysis of the mitochondria-targeted oligomerization domain of Bax and its mutants in the DKO cells. Plasmids for the indicated Bax wild type and mutants were transfected individually with or without the tBid expression plasmid into the DKO cells. (B) Apoptotic activities of the mitochondria-targeted Bax oligomerization domain and its mutants in the DKO cells. The indicated expression plasmids were cotransfected with a GFP expression plasmid (pEGFPC3) into DKO cells. Twenty hours after transfection, cells were stained with Hoechst dye. The percentage of GFP-positive cells that underwent nuclear condensation and fragmentation were quantified. The results are the average of at least three independent transfections. (C) Summary of the locations of the oligomerization domain and the apoptosis domain identified in the present study. The rectangles represent α-helices of Bax. The green color indicates the domains identified in this study.
Second, we examined the apoptotic activity of the Bax oligomerization domain. Since mitochondrial localization has been shown to be required for Bax function, we attached the CT, a known mitochondria targeting sequence of Bax (Nechushtan et al. 1999), to the oligomerization domain. The resulting mutant, G-BaxH(2–5)-CT, was examined for oligomerization and apoptotic activity in the absence or presence of tBid cotransfection. As shown in Figure 3, unlike full-length G-Bax, G-BaxH(2–5)-CT formed oligomers and displayed strong apoptotic activities with or without tBid cotransfection. Similarly, when the CT of Bcl-xL, another mitochondrial targeting sequence (Kaufmann et al. 2003), was attached to the oligomerization domain, the resulting mutant G-BaxH(2–5)-CT(xL) also displayed oligomerization and apoptotic activities regardless of tBid cotransfection. These results indicate that the oligomerization domain of Bax, when targeted to the mitochondria, is fully capable of inducing apoptosis in the DKO cells. Of note, the constitutive oligomerization and apoptotic activity of the oligomerization domain suggested that helices of Bax outside the oligomerization domain might play an inhibitory role in Bax activation.
To further define the minimum oligomerization domain and the minimum region responsible for the apoptotic activity of Bax, a series of deletion and alanine substitution mutants were generated based on G-BaxH(2–5)-CT and examined for oligomerization and apoptosis. As expected, deletion of either helix 2 (BH3) or helix 5 completely abolished oligomerization and apoptotic activity in DKO cells (Fig. 3). To test the requirement for helix 3 or helix 4, we replaced the entire helix 3 or helix 4 with six or seven alanine residues, respectively (Fig. 3). While alanine replacement of helix 3 did not have any effect, alanine replacement of helix 4 greatly reduced both oligomerization and apoptotic activity (Fig. 3). The apoptotic activities of these mutants were also confirmed in a caspase assay (Supplementary Fig. S1). Thus, combined with the findings from the internal deletions of full-length Bax (Fig. 2B), we conclude that helices 2 (the BH3 domain), 4, and 5 constitute the minimum oligomerization domain that is also responsible for the apoptotic activity of Bax (Fig. 3C).
Bax helix 5 converts Bcl-xL into an activated Bax-like protein
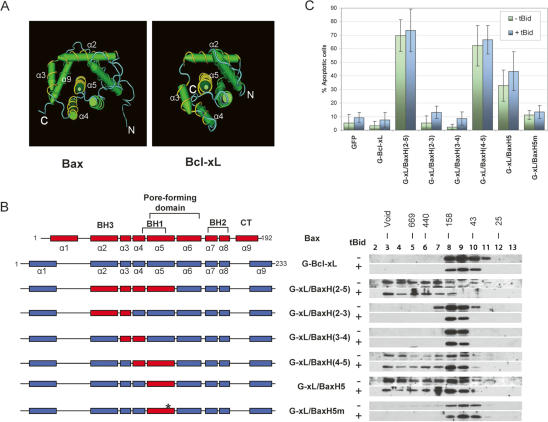
As a more stringent test for the role of helices 2, 4, and 5 as an oligomerization domain, a “domain-swapping” experiment between Bax and Bcl-xL was carried out. Based on the strong structural homology between Bax and Bcl-xL, especially in the helices 2–5 region, as illustrated in Figure 4A, we generated a Bcl-xL/Bax chimeric protein, replacing helices 2–5 of Bcl-xL with the corresponding region of Bax (Fig. 4B). Either wild-type Bcl-xL or the Bcl-xL/Bax chimera was transfected with or without an expression plasmid for tBid into the DKO cells and examined for oligomerization. As shown in Figure 4B, while G-Bcl-xL was unable to form high-molecular-weight homo-oligomers, the chimeric protein G-xL/BaxH(2–5) was fully capable of forming oligomers. To further pinpoint which helices confer oligomerization to Bcl-xL, we replaced one or two helices of Bcl-xL with the corresponding helices of Bax. As shown in Figure 4B, helices 2 and 3 as well as helices 3 and 4 of Bax failed to cause oligomerization to Bcl-xL. In contrast, Bax helices 4 and 5 caused Bcl-xL to form high-molecular-weight oligomers (Fig. 4B). More strikingly, Bax helix 5 alone, but not an alanine mutant of helix 5 (L113FYF116 to AAAA), was capable of converting Bcl-xL into a protein capable of homo-oligomerization (Fig. 4B). This result suggests that the BH3 domain of Bcl-xL can function in conjunction with Bax helix 5 to mediate homo-oligomerization. Indeed, as shown in Supplementary Figure S2, an alanine mutation in the Bcl-xL BH3 domain of G-xL/BaxH5 abolished the ability to form oligomers, indicating that the Bcl-xL BH3 functions similarly to Bax BH3.
Figure 4.
Identification of structural determinant for homo-oligomerization by domain swapping between Bcl-xL and Bax. (A) Structural homology between Bax and Bcl-xL. The structural representation for Bax (1F16) (Suzuki et al. 2000) and Bcl-xL (1MAZ) (Muchmore et al. 1996) were taken from the NCBI Chemical Database. The helices highlighted with yellow are helices 2–5. Helix 5 in both Bax and Bcl-xL is perpendicular to the page, pointing outward. (B) The homo-oligomerization of the Bcl-xL/Bax chimeric proteins in the DKO cells. Schematic representations of wild-type Bax, Bcl-xL, and the chimeric proteins are shown on the left, with Bax helices shown in red and Bcl-xL helices in blue. Plasmids for the indicated Bcl-xL/Bax chimeras were transfected individually with or without the tBid expression plasmid into the DKO cells. Gel-filtration and Western blot analyses were carried out as described in Figure 1 and Materials and Methods. The asterisk indicates the alanine mutation in helix 5. (C) The apoptotic activities of Bcl-xL and the Bcl-xL/Bax chimeric proteins in DKO cells. The percentage of apoptotic cells was quantified as described in Figure 3B and Materials and Methods.
Next, we examined the apoptotic activities of the chimeric proteins. Expression plamids for each of the chimeric proteins were transfected into the DKO cells in the presence or absence of tBid expression plamid. As shown in Figure 4C, while wild-type Bcl-xL, as well as G-xL/BaxH(2–3) and G-xL/BaxH(3–4), showed no apoptotic activities, all the chimeras with an intact Bax helix 5 (G-xL/BaxH5) demonstrated potent apoptotic activity in the absence and presence of tBid. As expected, the chimeric protein containing Bax helices 4 and 5 was found to be able to cause the release of cytochrome c from mitochondria in the DKO cells (Supplementary Fig. S3). In contrast, both the mutation in Bax helix 5 and the mutation in the Bcl-xL BH3 domain totally abolished apoptotic activity (Fig. 4C; Supplementary Fig. S2). It is worth noting that the apoptotic activity of the mutant G-xL/BaxH(4–5) is 20%–30% stronger than that of G-xL/BaxH5, suggesting that helix 4 of Bax may play a regulatory role on the apoptotic activity of Bax (Fig. 4C). These results established the unit of helices 2 (the BH3 domain), 4, and 5 of Bax as a bona fide oligomerization domain capable of causing apoptosis in the DKO cells when targeted to mitochondria. Furthermore, we conclude that Bax helix 5 is a major structural determinant for oligomerization, distinguishing between Bax and Bcl-xL.
Homo-oligomerization is sufficient for the apoptotic activity of Bax in the DKO cells
Next, we asked whether oligomerization is sufficient for the apoptotic activity of Bax. To answer this question, it is necessary to identify Bax mutants that spontaneously undergo homo-oligomerization. Such mutants should be constitutively active in inducing apoptosis in DKO cells if oligomerization is sufficient for apoptotic activity of Bax. We therefore sought to screen for Bax mutants that are able to spontaneously form oligomers in the DKO cells.
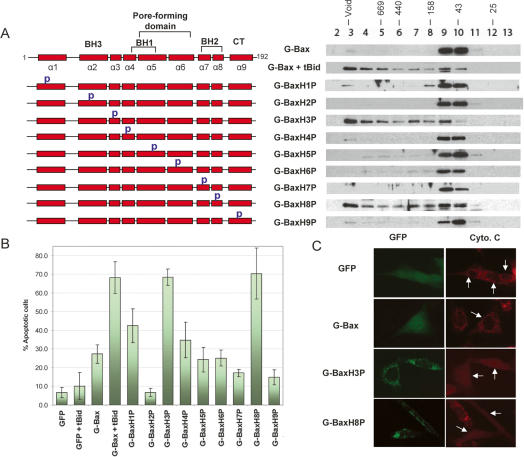
Since conformational changes are associated with Bax activation, we employed a “proline-scanning” mutagenesis strategy to perturb the Bax conformation and screen for Bax mutants that form oligomers without tBid cotransfection. A proline mutation, which is commonly used to introduce a turn in a helix, may be able to perturb the conformation of the monomeric Bax, which is composed of nine α-helices. Accordingly, as illustrated in Figure 5A, a total of nine single-proline mutants were generated in the context of full-length Bax, each with a single proline mutation located in the middle of a different α-helix. In order to test the effect of these proline mutations on oligomerization, each mutant was individually transfected into DKO cells in the absence of tBid expression plasmid. Comparable levels of the proline mutants were expressed in the DKO cells (Supplementary Fig. S7). As shown in Figure 5A, among the nine proline mutants, while most showed minimal oligomerization, G-BaxH3P and G-BaxH8P, which have proline mutation in helix 3 and helix 8, respectively, eluted primarily as high-molecular-weight oligomers. Moreover, both of these mutants constitutively localized to mitochondria (Supplementary Fig. S4). These results indicate that a conformational change may be able to cause mitochondrial translocation and oligomerization of Bax.
Figure 5.
Identification of constitutively active mutants of Bax. (A) Gel-filtration analysis of proline-scanning mutants of Bax in DKO cells. Schematic representation of the proline mutants of Bax are shown on the left. (p) The proline mutation. The amino acid residues for each proline mutant are specified in Materials and Methods. DKO cells were transfected with the expression plasmids of indicated wild type and proline mutants of Bax. All transfections in this figure were carried out in the absence of the tBid expression plasmid unless specified otherwise. (B) Apoptotic activities of the proline mutants of Bax in DKO cells. (C) Cytochrome c release from mitochondria induced by two proline mutants. Cells that were transfected with the indicated expression plasmids for GFP fusion proteins are shown on the left. Cells stained with cytochrome c antibody are shown on the right. Arrows point to the cells that express the indicated GFP fusion proteins.
We next examined the apoptotic activities of these mutants in DKO cells. As shown in Figure 5B, both G-BaxH3P and G-BaxH8P, which displayed spontaneous oligomerization, demonstrated strong apoptotic activities comparable to that displayed by the cotransfection of G-Bax and tBid expression plasmids. To examine the effects of these constitutively active mutants of Bax on the mitochondrial pathway of apoptosis, we examined the localization of cytochrome c after the transfection. As shown in Figure 5C, the expression of wild-type G-Bax, which is mainly localized to the cytoplasm in DKO cells, did not affect the mitochondrial localization of cytochrome c. In contrast, the expression of G-BaxH3P and G-BaxH8P induced a homogeneous distribution of cytochrome c throughout the cell, consistent with a release of cytochrome c from mitochondria. Taken together, these results strongly support the hypothesis that homo-oligomerization of Bax is sufficient for its apoptotic activity.
Homo-oligomerization is required for the apoptotic activity of Bax
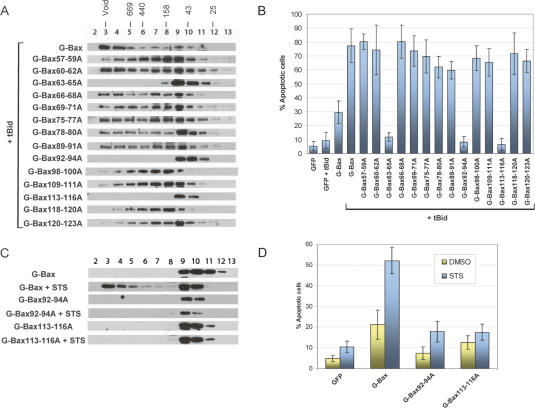
The identification of the oligomerization domain of Bax allowed us to formally test the requirement of homo-oligomerization for the apoptotic activity of Bax. A total of 14 alanine mutations that replace three or four residues with the same number of alanines were introduced into the oligomerization domain (helices 2–5) in the context of the full-length Bax (as illustrated in Supplementary Fig. S5). Each of these alanine-scanning mutants was transfected into the DKO cells in the presence of tBid expression plasmid. As shown in Figure 6A, while most did not have any effect, three mutations, located in helix 2 (G-Bax63–65A), helix 4 (G-Bax92–94A), and helix 5 (G-Bax113–116A), respectively, were found to be defective in oligomerization, strengthening our conclusion that these three helices are critical for homo-oligomerization. The apoptotic activities of the whole panel of alanine-scanning mutants were examined in DKO cells in the presence of the tBid-expressing plasmid. As shown in Figure 6B, while the majority of the mutants retained the apoptotic activity, the three mutants, G-Bax63–65A, G-Bax92–94A, and G-Bax113–116A, which are defective in homo-oligomerization, displayed a total loss of apoptotic activities. It is worth mentioning that the mutants were expressed at comparable levels in the DKO cells (Supplementary Fig. S7).
Figure 6.
Homo-oligomerization is required for the apoptotic activity of Bax. (A) The homo-oligomerization of Bax and its alanine-scanning mutants in DKO cells in the presence of tBid. The exact amino acid sequences of these mutations are illustrated in Supplementary Figure S5. DKO cells were transfected with the expression plasmids for the indicated alanine-scanning mutants in the presence of the tBid expression plasmid. Gel-filtration analysis was carried out as described in Figure 1. (B) The apoptotic activities of Bax and its alanine-scanning mutants in DKO cells in the presence of tBid. The apoptotic activities of these mutants were quantified as described in Figure 4 and Materials and Methods. (C) The homo-oligomerization of Bax and its alanine-scanning mutants in DKO cells in response to STS. DKO cells were transfected with the expression plasmids for the indicated G-Bax and its mutants. DMSO or STS (1 μM) was added to the cells 20 h after transfection. After 8 h, cells were harvested for gel-filtration analysis as described in Figure 1. (D) Apoptotic activities of Bax and alanine-scanning mutants in the DKO cells in response to STS. The apoptotic activities of these mutants were quantified as described in Figure 3 and Materials and Methods.
To exclude the possibility that the defect of these three alanine mutants may be due to their inability to interact with tBid, we tested if the proline mutation-induced activation of Bax can rescue their oligomerization and apoptotic activities. The proline mutation within helix 8, which caused activation of Bax (Fig. 5), was introduced to each of these three mutants. As shown in Supplementary Figure S6, when transfected into DKO cells in the absence of tBid expression plasmid, none of these compound mutations demonstrated oligomerization or apoptotic activity, indicating that the alanine mutations in these mutants indeed abolished the ability of Bax to form homo-oligomers.
To test the requirement of homo-oligomerization for the apoptotic activity of Bax in a different context, we examined the oligomerization and apoptotic activities of two of the oligomerization-defective alanine mutants, G-Bax92–94A and G-Bax113–116A, in response to STS. Expression plasmid for G-Bax, G-Bax92–94A and G-Bax113–116A were transfected into the DKO cells individually. Cells were treated with STS after transfection. As shown in Figure 6C,D, while the wild-type G-Bax displayed an induced oligomerization and apoptotic activity, none of the alanine mutations was able to form oligomers or cause apoptosis in the presence of STS. Taken together, these results indicate that oligomerization of Bax is required for its apoptotic activity.
Discussion
Bax and Bak constitute the gateway to mitochondria-dependent apoptosis (Wei et al. 2001). Although the solution structure of monomeric Bax provided critical insights into the regulation of Bax, the lack of structural information on the active form of Bax has been a major obstacle to our understanding of how these molecules exert their functions on mitochondria (Suzuki et al. 2000). In this study, with the aid of Bax−/−Bak−/− DKO cells, we dissected the Bax molecule and defined the structural requirements for homo-oligomerization as well as the apoptotic activity of Bax, thus providing insights into the active site of Bax. Through an extensive mutagenesis study, we also established the functional relationship between homo-oligomerization and apoptotic activity of Bax.
Although a three-dimensional (3D) structure is highly desirable to define the active form of Bax, three outstanding issues make solving the 3D structure technically challenging for this particular protein in its oligomeric form. (1) The extreme toxicity and insolubility of the active Bax mutants makes it difficult to obtain even small amounts of the active form of recombinant Bax (N. George and X. Luo, unpubl.). (2) Both in vivo and in vitro studies demonstrated that the oligomeric form of Bax exists as a heterogeneous mixture of Bax homo-oligomers of various sizes (Antonsson et al. 2001; Nechushtan et al. 2001). (3) Using detergents to induce oligomerization of the wild-type Bax also proves difficult for structural analysis. In their NMR study of the wild-type Bax, Suzuki et al. (2000) found that apart from a sharp conformational change induced by detergents, little structural information could be retrieved from the detergent-induced oligomers, presumably due to their large sizes.
The BH3 domain, helix 4, and the central helix (helix 5) as the oligomerization domain of Bax
The BH3 domain has been shown to be critical for both dimerization and apoptotic activity of Bax (Zha et al. 1996; Wang et al. 1998). However, it was not clear whether it directly participates in oligomerization or plays a regulatory role through sequences outside the oligomerization domain. Our results pointed out the role of the BH3 domain as an integral part of the oligomerization domain, thus providing new insights into its biochemical function. This conclusion, however, does not exclude the possibility that the BH3 domain may have other functions before or after the formation of the oligomers; for example, interacting with other mitochondrial proteins or mitochondrial lipids.
While both the BH3 domain and helix 4 are amphipathic, helix 5 is primarily hydrophobic. It can be speculated that the oligomers are held together primarily by hydrophobic interactions. Consistent with this prediction, the alanine mutations in helix 4 (G-Bax92–94A) and helix 5 (G-Bax113–116A), both of which abolished oligomerization and apoptotic activity, indeed replaced three and four bulky hydrophobic residues, respectively. Interestingly, the residues F92F93 from H4 and L113FYF116 from helix 5 are facing each other in close proximity in the monomeric Bax (Suzuki et al. 2000), raising the possibility that the interaction between these two groups may be critical for the formation of homo-oligomers. If this is true, it is then reasonable to speculate that the hairpin structure formed by helices 4 and 5 may be maintained in the homo-oligomers of Bax. However, the role of the helical conformation of helix 4 remains to be fully investigated. It is worth noting that one of the surprises from this study is that helix 6, which is part of the putative pore-forming domain of Bax (helices 5 and 6), was found to be not directly involved in oligomerization. However, helix 6 may play a role in the regulation of oligomerization or mitochondrial localization of Bax. Interestingly, we found that Bak helices 2–5 were also able to mediate oligomerization in the DKO cells (N. George, unpubl. data), suggesting that the role of helices 2–5 as the oligomerization domain may be conserved among the BH1–3 proapoptotic Bcl-2 family proteins.
It is worth mentioning that the X-ray structure of an inactive Bak homodimer was published recently. The identified dimer interface does not involve the regions we mapped as the homo-oligomerization domain (BH3 and BH1) of Bax and Bak (Moldoveanu et al. 2006).
The central helix (helix 5) of Bax as the major structural determinant for homo-oligomerization
Conversion of Bcl-xL into a proapoptotic molecule by splicing variation or proteolytic cleavage has been documented. The C-terminal portion of Bcl-xL (Bcl-xLΔN61), generated by caspase cleavage, was shown to be proapoptotic in Cos-1 cells (Clem et al. 1998). The Bcl-xL splice variant, Bcl-xS, was found to be a proapoptotic molecule in multiple cell types (Boise et al. 1993). However, it appears that the apoptotic activities of these Bcl-xL mutants are dependent on endogenous Bax or Bak. We found that Bcl-xLΔN61 was unable to form oligomers or induce apoptosis in DKO cells (N. George and X. Luo, unpubl.). Similarly, Bcl-xS was found to be unable to cause apoptosis in DKO cells, indicating that it is behaving as a BH3-only molecule (Lindenboim et al. 2005).
Our finding that Bax helix 5, but not helices 2, 3, or 4, can convert Bcl-xL into a Bax-like molecule indicated that the structural information that distinguishes Bcl-xL from Bax in terms of oligomer formation and apoptotic activity is coded within helix 5 (Fig. 4). On the other hand, the observation that Bax helix 5 can function together with other parts of Bcl-xL, most likely the BH3 domain and H4, to form oligomers and cause apoptosis in the DKO cells is in line with the strong structural homology between the two molecules. Intriguingly, the chimeric molecule with Bcl-xL BH3 and helix 3 replaced by those from Bax was found to behave as an anti-apoptotic molecule (N. George and X. Luo, unpubl.), suggesting that the BH3 of Bax is homologous to that of Bcl-xL in terms of anti-apoptotic function. However, when helices 2–5 or 4 and 5 of Bax were replaced by those from Bcl-xL, the resulting chimeric protein acted as a proapoptotic protein in HeLa cells, suggesting that more structural elements were required for the anti-apoptotic function of Bcl-xL (N. George and X. Luo, unpubl.). Since the amino acid sequences of helix 5 of Bax and Bcl-xL are homologous, it will not be surprising if only a point mutation in Bcl-xL helix 5 can convert Bcl-xL into a Bax-like molecule. More systematic mutational analysis is necessary to pinpoint the exact determinant for the apoptotic activity at the amino acid level.
The mechanism by which Bax causes damage to the mitochondrial outer membrane remains controversial. However, it has been found that Bax, but not Bcl-xL, can cause a decrease of the lifetime of planar phospholipid bilayer membranes (Basanez et al. 1999). Based on our findings, it can be speculated that the Bcl-xL/BaxH5 chimera behaves like Bax in this assay. If that is the case, it would argue that helix 5 of Bax may play a major role in destabilizing the phospholipid membranes.
Activation of Bax
Although a conformational change has been associated with the activation of Bax (Hsu and Youle 1998; Nechushtan et al. 1999), it has been unclear whether this change has a causal relationship with Bax activation. Our findings that proline mutation in either helix 3 or helix 8 can lead to oligomerization and strong apoptotic activity in DKO cells strongly support the notion that the conformational change is able to cause Bax activation (Fig. 5). It remains to be seen how the conformational change is maintained to effect full activation.
The observation that Bax was activated by a proline mutation in either helix 3 or helix 8 suggests that the monomeric Bax may be held in an inactive conformation by multiple helices under normal conditions. This hypothesis was supported by the constitutive apoptotic activities of Bax mutants that contain only the oligomerization domain and the C-terminal mitochondrial targeting sequence (Fig. 3). In addition, since the Bcl-xL/Bax fusion proteins were also found to be constitutively active (Fig. 4), it suggests that helices of Bcl-xL were unable to constrain the Bax oligomerization domain. These observations also suggest that Bax may be activated through perturbation at multiple sites. Thus, during apoptosis, multiple factors may serve as positive or negative regulators of Bax activation by either destabilizing or stabilizing different helices. In support of this hypothesis, tBid was shown to interact directly with Bax helix 1 to effect Bax activation (Cartron et al. 2004). Also consistent with this hypothesis, multiple proteins, including humanin, Ku70, p53, E1B 19K, Bcl-2, Bcl-xL, and others, have been found to affect the activation of Bax through interaction (Sundararajan and White 2001; Guo et al. 2003; Sawada et al. 2003; Chipuk et al. 2004). Thus, it is of interest to see if helices 3 and 8 might be able to directly interact with Bax modulators.
In this study, tBid was shown to activate the transfected full-length Bax in the DKO cells (Fig. 1). However, the mechanism of this activation, either by directly binding to Bax or by antagonizing the function of the anti-apoptotic Bcl-2 family proteins, remains a critical question that is being highly debated (Cheng et al. 2001; Kim et al. 2006; Willis et al. 2007).
Oligomerization of Bax is responsible for its apoptotic activity
The causal relationship between homo-oligomerization and the apoptotic activity of Bax has remained a hypothesis not readily testable due to the following difficulties. (1) The lack of knowledge of the oligomerization domain of Bax impeded systematic mutagenesis studies. (2) Bax−/− Bak−/− DKO cells, which are a critical tool for testing this hypothesis, have been generated only in recent years. (3) The rather nonspecific effects of nonionic detergents, which are known to cause conformational changes and oligomerization of Bax (Hsu and Youle 1997; Antonsson et al. 2001), contributed to the uncertainty. Bound by such limitations, especially the unavailability of the DKO cells, many studies argued against a requirement of homo-oligomerization for the apoptotic activity of Bax (for review, see Borner 2003).
The identification of the oligomerization domain as well as the use of the DKOs allowed us to perform extensive mutagenesis studies and ask whether the oligomerization is critical for the apoptotic activity of Bax. A strict correlation between oligomerization and apoptotic activity in the DKO cells clearly demonstrated the requirement of oligomerization for apoptotic activity of Bax (Figs. 3, 4, 6). Significantly, the minimum three-helix oligomerization unit was also found to be the minimum domain for apoptotic activity in the DKO cells (Fig. 3). The complete overlapping of these two minimum domains strongly supports the hypothesis that Bax effects mitochondrial damage or apoptosis through homo-oligomers. This conclusion was further supported by our finding that helix 5 of Bax conferred homo-oligomerization as well as apoptotic activity to the Bcl-xL/Bax chimera.
Using reconstituted systems with defined lipids that constitute the mitochondrial outer membrane, it has been clearly demonstrated that recombinant Bax together with tBid were able to release high-molecular-weight molecules trapped in liposomes, mimicking the release of cytochrome c from mitochondria (Kuwana et al. 2002). This cooperativity between Bax and tBid was also evident in our transfection experiments where cotransfection of Bax and tBid, but not either alone, resulted in apoptosis in DKO cells. However, it has been unclear whether tBid acts by directly activating Bax or by acting on the liposomes simultaneously with Bax to cause the observed changes (Kuwana et al. 2002). The identification of the spontaneously oligomerizing mutants, which can cause apoptosis in DKO cells independently of tBid (Fig. 5), demonstrated that oligomeric Bax is sufficient for apoptotic activity.
How the BH1–3 proapoptotic Bcl-2 family proteins—i.e., Bax and Bak—damage mitochondria and eventually kill cells remains one of the fundamental questions in the study of apoptosis. The structure–function analysis presented in this study allowed us to define the oligomerization domain and establish the causal relationship between homo-oligomer formation and apoptotic activity of Bax. It remains to be explored as to how the oligomers of the three-helix unit interact with mitochondrial lipids or proteins to cause the release of apoptogenic factors from mitochondria.
Materials and methods
Plasmid construction
The cDNAs of mouse Bax, human Bcl-xL, and human Bid, and their respective mutants were PCR-amplified using Taq polymerase with 23 cycles of 1 min at 55°C, 1 min at 94°C, and 1 min at 72°C. The forward and reverse primers contain an XhoI site and an EcoRI site, respectively. The PCR products were digested with XhoI and EcoRI and cloned into the XhoI–EcoRI-digested pEGFP-C3 plasmid (Clontec). Proline-scanning and alanine-scanning mutants were generated using standard site-directed mutagenesis with Pfu (Stratagene) followed by DpnI (New England BioLabs) digestion. Bcl-xL/Bax fusion protein plasmids were generated using the PCR “gene SOEing” method (Horton et al. 1990). G-BaxΔBH3 deleted residues 63–71, G-BaxΔH3 deleted residues 76–80, G-BaxΔH4 deleted residues 91–96, G-BaxΔH5 deleted residues 108–126, and G-BaxΔH6 deleted residues 130–146. G-BaxH(2–5)-CT was generated by ligating D53–K128 with T167–G192, and G-BaxH(2–5)-CT(xL) was generated by ligating Bax D53-K128 with R209-K233 of Bcl-xL. G-BaxH(2,4,5)-CT and G-BaxH(2,3,5)-CT were generated similarly to G-BaxH(2–5)-CT except that each residue between amino acids 76 and 80 and between amino acids 91 and 96, respectively, were changed to alanine. G-BaxH(3–5)-CT and G-BaxH(2–4)-CT were generated by ligating M74–K128 with T167–G192 and D53–N104 with T167–G192, respectively. G-xL/BaxH(2–5) was generated by replacing residues 80–158 of Bcl-xL with Bax 53–128. G-xL/BaxH(2–3) was generated by replacing residues 80–114 of Bcl-xL with Bax 53–83, G-xL/BaxH(3–4) was generated by replacing residues 101–135 of Bcl-xL with Bax 73–103, G-xL/BaxH(4–5) was generated by replacing residues 115–158 of Bcl-xL with Bax 84–128, and G-xL/BaxH5 was generated by replacing residues 136–158 of Bcl-xL with Bax 104–128. G-xL/BaxH5m was generated similarly to xL/BaxH5 except that Bax residues 113–116 were mutated to alanine. The following sites were mutated to proline in construction of the proline scan: M20 (H1P), L63 (H2P), Q77 (H3P), F93 (H4P), A117 (H5P), M137 (H6P), I152 (H7P), L161 (H8P), and G179 (H9P). Each construct was sequenced to ensure correct sequence in the coding region.
Cell culture
Simian virus 40 (SV40)-transformed Bax−/−Bak−/− MEFs (DKO MEFs, described in Wei et al. 2001) were maintained in DMEM supplemented with antibiotics and 10% fetal calf serum.
Transfection
The DKO MEFs were plated (at a density of 2.5 × 105 cells per 35-mm plate and 7 × 105 cells per 60-mm plate) and cultured for 20–24 h before transfection with the Effectene reagents (Qiagen) according to the manufacturer’s recommendation. For each transfection in a 60-mm plate, 100 ng of plasmid of interest were used and pcDNA3 was included in the mixture to maintain the total amount of DNA at 1.5 μg per transfection. Transfection in 35-mm dishes included 30 ng of plasmid of interest, 30 ng of pEGFP-C3 vector, and pcDNA3 to maintain total DNA at 0.7 μg per transfection. Where indicated, C-terminal Flag-tagged tBid was included at 120 ng per 60-mm plate transfection or 40 ng per 35-mm plate transfection. Twenty hours after transfection, cells were fixed for immunostaining, stained with Hoechst dye for quantification, or harvested for gel-filtration analysis.
Western blot and immunostaining
Protein samples were subjected to SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). The membranes were probed with GFP antibody (Santa Cruz Biotechnology) at 1:2000. Immunostaining was essentially performed as described (Luo and Sawadogo 1996). Where indicated, either TOM20 antibody (Santa Cruz Biotechnology) or cytochrome c antibody (PharMingen) was added to the paraformaldehyde-fixed cells at 1:200 dilutions. After washing in PBS, the cells were incubated with Texas Red-conjugated goat anti-mouse secondary antibody for cytochrome c and Alexa Fluor 594-conjugated goat anti-rabbit secondary antibody for TOM20 (Molecular Probes) at 1:500. GFP-positive and immunostained cells were photographed under a Nikon Eclipse 50i Fluorescence microscope.
Quantification of apoptotis by Hoechst staining
To quantify apoptosis according to nuclear morphology, cells were transfected with the plasmid of interest together with the GFP-expressing plasmid pEGFP-C3. Twenty hours after transfection, cells were stained with Hoechst 33342 at 1 μg/mL (Molecular Probes). Two different viewing areas were randomly chosen for each transfection. Pictures for both GFP and Hoechst staining were taken for each viewing area, which contained between 80 and 200 GFP-positive cells. The percentage of GFP-positive cells undergoing pycnotic nuclear condensation among all the GFP-positive cells was calculated for each viewing area. At least three independent transfection experiments were performed for each construct of interest.
Gel-filtration analysis for oligomerization after transfection
The DKO cells were transfected in the presence of 20 μM z-VAD. Whole-cell lysates were obtained 18–20 h after transfection by lysing the cells in lysis buffer (Buffer A with 2% CHAPS). The composition of Buffer A was 20 mM HEPES-KOH (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM dithiothreitol (DTT), and 0.1 mM PMSF, supplemented with protease inhibitors (5 mg/mL pepstatin A, 10 mg/mL leupeptin). The cell lysates were incubated for 1 h at 4°C with rotation before centrifugation 1 h at 100,000g for at 4°C. The supernatant, ∼1 mg of total protein as measured by Protein Assay (Bio-Rad), was loaded onto a FPLC Superdex 200 10/30 column equilibrated with 100 mM NaCl in Buffer A (with 1.8% CHAPS) and calibrated by the Calibration Kit provided by the manufacturer (Amersham Bioscience). Fractions were eluted at a rate of 0.5 mL/min in the same buffer. One-milliliter fractions were collected. Twenty microliters of each fraction were loaded onto SDS-PAGE and transferred to a nitrocellulose membrane after electrophoresis. Western blot analysis was performed to detect GFP fusion proteins.
Acknowledgments
We thank Gloria Borgstahl, Keith Johnson, Robert Lewis, and Robert Lahue for critical reading of the manuscript. We are eternally grateful for Dr. Stanley Korsmeyer, who provided the Bax−/−Bak−/− DKO MEFs. This work was supported by an NIH grant (GM76237), an AHA Scientist Development Grant (0335215N), as well as a pilot grant from the Nebraska Center for Cellular Signaling to X.L. N.M.G. was supported by the Eppley Institute training grant from NCI (CA009476) and a graduate fellowship from the University of Nebraska Medical Center.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1553607
References
- Antonsson B., Montessuit S., Lauper S., Eskes R., Martinou J.C., Montessuit S., Lauper S., Eskes R., Martinou J.C., Lauper S., Eskes R., Martinou J.C., Eskes R., Martinou J.C., Martinou J.C. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- Antonsson B., Montessuit S., Sanchez B., Martinou J.C., Montessuit S., Sanchez B., Martinou J.C., Sanchez B., Martinou J.C., Martinou J.C. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- Basanez G., Nechushtan A., Drozhinin O., Chanturiya A., Choe E., Tutt S., Wood K.A., Hsu Y., Zimmerberg J., Youle R.J., Nechushtan A., Drozhinin O., Chanturiya A., Choe E., Tutt S., Wood K.A., Hsu Y., Zimmerberg J., Youle R.J., Drozhinin O., Chanturiya A., Choe E., Tutt S., Wood K.A., Hsu Y., Zimmerberg J., Youle R.J., Chanturiya A., Choe E., Tutt S., Wood K.A., Hsu Y., Zimmerberg J., Youle R.J., Choe E., Tutt S., Wood K.A., Hsu Y., Zimmerberg J., Youle R.J., Tutt S., Wood K.A., Hsu Y., Zimmerberg J., Youle R.J., Wood K.A., Hsu Y., Zimmerberg J., Youle R.J., Hsu Y., Zimmerberg J., Youle R.J., Zimmerberg J., Youle R.J., Youle R.J. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise L.H., Gonzalez-Garcia M., Postema C.E., Ding L., Lindsten T., Turka L.A., Mao X., Nunez G., Thompson C.B., Gonzalez-Garcia M., Postema C.E., Ding L., Lindsten T., Turka L.A., Mao X., Nunez G., Thompson C.B., Postema C.E., Ding L., Lindsten T., Turka L.A., Mao X., Nunez G., Thompson C.B., Ding L., Lindsten T., Turka L.A., Mao X., Nunez G., Thompson C.B., Lindsten T., Turka L.A., Mao X., Nunez G., Thompson C.B., Turka L.A., Mao X., Nunez G., Thompson C.B., Mao X., Nunez G., Thompson C.B., Nunez G., Thompson C.B., Thompson C.B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Borner C. The Bcl-2 protein family: Sensors and checkpoints for life-or-death decisions. Mol. Immunol. 2003;39:615–647. doi: 10.1016/s0161-5890(02)00252-3. [DOI] [PubMed] [Google Scholar]
- Cartron P.F., Gallenne T., Bougras G., Gautier F., Manero F., Vusio P., Meflah K., Vallette F.M., Juin P., Gallenne T., Bougras G., Gautier F., Manero F., Vusio P., Meflah K., Vallette F.M., Juin P., Bougras G., Gautier F., Manero F., Vusio P., Meflah K., Vallette F.M., Juin P., Gautier F., Manero F., Vusio P., Meflah K., Vallette F.M., Juin P., Manero F., Vusio P., Meflah K., Vallette F.M., Juin P., Vusio P., Meflah K., Vallette F.M., Juin P., Meflah K., Vallette F.M., Juin P., Vallette F.M., Juin P., Juin P. The first α helix of Bax plays a necessary role in its ligand-Induced activation by the BH3-only proteins Bid and PUMA. Mol. Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Cheng E.H., Wei M.C., Weiler S., Flavell R.A., Mak T.W., Lindsten T., Korsmeyer S.J., Wei M.C., Weiler S., Flavell R.A., Mak T.W., Lindsten T., Korsmeyer S.J., Weiler S., Flavell R.A., Mak T.W., Lindsten T., Korsmeyer S.J., Flavell R.A., Mak T.W., Lindsten T., Korsmeyer S.J., Mak T.W., Lindsten T., Korsmeyer S.J., Lindsten T., Korsmeyer S.J., Korsmeyer S.J. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chipuk J.E., Kuwana T., Bouchier-Hayes L., Droin N.M., Newmeyer D.D., Schuler M., Green D.R., Kuwana T., Bouchier-Hayes L., Droin N.M., Newmeyer D.D., Schuler M., Green D.R., Bouchier-Hayes L., Droin N.M., Newmeyer D.D., Schuler M., Green D.R., Droin N.M., Newmeyer D.D., Schuler M., Green D.R., Newmeyer D.D., Schuler M., Green D.R., Schuler M., Green D.R., Green D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Clem R.J., Cheng E.H., Karp C.L., Kirsch D.G., Ueno K., Takahashi A., Kastan M.B., Griffin D.E., Earnshaw W.C., Veliuona M.A., Cheng E.H., Karp C.L., Kirsch D.G., Ueno K., Takahashi A., Kastan M.B., Griffin D.E., Earnshaw W.C., Veliuona M.A., Karp C.L., Kirsch D.G., Ueno K., Takahashi A., Kastan M.B., Griffin D.E., Earnshaw W.C., Veliuona M.A., Kirsch D.G., Ueno K., Takahashi A., Kastan M.B., Griffin D.E., Earnshaw W.C., Veliuona M.A., Ueno K., Takahashi A., Kastan M.B., Griffin D.E., Earnshaw W.C., Veliuona M.A., Takahashi A., Kastan M.B., Griffin D.E., Earnshaw W.C., Veliuona M.A., Kastan M.B., Griffin D.E., Earnshaw W.C., Veliuona M.A., Griffin D.E., Earnshaw W.C., Veliuona M.A., Earnshaw W.C., Veliuona M.A., Veliuona M.A., et al. Modulation of cell death by Bcl-XL through caspase interaction. Proc. Natl. Acad. Sci. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddeback S.M., Yamaguchi H., Komatsu K., Miyashita T., Yamada M., Wu C., Singh S., Wang H.G., Yamaguchi H., Komatsu K., Miyashita T., Yamada M., Wu C., Singh S., Wang H.G., Komatsu K., Miyashita T., Yamada M., Wu C., Singh S., Wang H.G., Miyashita T., Yamada M., Wu C., Singh S., Wang H.G., Yamada M., Wu C., Singh S., Wang H.G., Wu C., Singh S., Wang H.G., Singh S., Wang H.G., Wang H.G. Molecular cloning and characterization of Bif-1. A novel Src homology 3 domain-containing protein that associates with Bax. J. Biol. Chem. 2001;276:20559–20565. doi: 10.1074/jbc.M101527200. [DOI] [PubMed] [Google Scholar]
- Danial N.N., Korsmeyer S.J., Korsmeyer S.J. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Desagher S., Osen-Sand A., Nichols A., Eskes R., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J.C., Osen-Sand A., Nichols A., Eskes R., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J.C., Nichols A., Eskes R., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J.C., Eskes R., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J.C., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J.C., Lauper S., Maundrell K., Antonsson B., Martinou J.C., Maundrell K., Antonsson B., Martinou J.C., Antonsson B., Martinou J.C., Martinou J.C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A., Jockel J., Wei M.C., Korsmeyer S.J., Jockel J., Wei M.C., Korsmeyer S.J., Wei M.C., Korsmeyer S.J., Korsmeyer S.J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Zhai D., Cabezas E., Welsh K., Nouraini S., Satterthwait A.C., Reed J.C., Zhai D., Cabezas E., Welsh K., Nouraini S., Satterthwait A.C., Reed J.C., Cabezas E., Welsh K., Nouraini S., Satterthwait A.C., Reed J.C., Welsh K., Nouraini S., Satterthwait A.C., Reed J.C., Nouraini S., Satterthwait A.C., Reed J.C., Satterthwait A.C., Reed J.C., Reed J.C. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- Horton R.M., Cai Z.L., Ho S.N., Pease L.R., Cai Z.L., Ho S.N., Pease L.R., Ho S.N., Pease L.R., Pease L.R. Gene splicing by overlap extension: Tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- Hsu Y.T., Youle R.J., Youle R.J. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Hsu Y.T., Youle R.J., Youle R.J. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Hsu Y.T., Wolter K.G., Youle R.J., Wolter K.G., Youle R.J., Youle R.J. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang X., Wang X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- Jurgensmeier J.M., Xie Z., Deveraux Q., Ellerby L., Bredesen D., Reed J.C., Xie Z., Deveraux Q., Ellerby L., Bredesen D., Reed J.C., Deveraux Q., Ellerby L., Bredesen D., Reed J.C., Ellerby L., Bredesen D., Reed J.C., Bredesen D., Reed J.C., Reed J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T., Schlipf S., Sanz J., Neubert K., Stein R., Borner C., Schlipf S., Sanz J., Neubert K., Stein R., Borner C., Sanz J., Neubert K., Stein R., Borner C., Neubert K., Stein R., Borner C., Stein R., Borner C., Borner C. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J. Cell Biol. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Rafiuddin-Shah M., Tu H.C., Jeffers J.R., Zambetti G.P., Hsieh J.J., Cheng E.H., Rafiuddin-Shah M., Tu H.C., Jeffers J.R., Zambetti G.P., Hsieh J.J., Cheng E.H., Tu H.C., Jeffers J.R., Zambetti G.P., Hsieh J.J., Cheng E.H., Jeffers J.R., Zambetti G.P., Hsieh J.J., Cheng E.H., Zambetti G.P., Hsieh J.J., Cheng E.H., Hsieh J.J., Cheng E.H., Cheng E.H. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kuwana T., Mackey M.R., Perkins G., Ellisman M.H., Latterich M., Schneiter R., Green D.R., Newmeyer D.D., Mackey M.R., Perkins G., Ellisman M.H., Latterich M., Schneiter R., Green D.R., Newmeyer D.D., Perkins G., Ellisman M.H., Latterich M., Schneiter R., Green D.R., Newmeyer D.D., Ellisman M.H., Latterich M., Schneiter R., Green D.R., Newmeyer D.D., Latterich M., Schneiter R., Green D.R., Newmeyer D.D., Schneiter R., Green D.R., Newmeyer D.D., Green D.R., Newmeyer D.D., Newmeyer D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Lindenboim L., Kringel S., Braun T., Borner C., Stein R., Kringel S., Braun T., Borner C., Stein R., Braun T., Borner C., Stein R., Borner C., Stein R., Stein R. Bak but not Bax is essential for Bcl-xS-induced apoptosis. Cell Death Differ. 2005;12:713–723. doi: 10.1038/sj.cdd.4401638. [DOI] [PubMed] [Google Scholar]
- Lindsten T., Ross A.J., King A., Zong W.X., Rathmell J.C., Shiels H.A., Ulrich E., Waymire K.G., Mahar P., Frauwirth K., Ross A.J., King A., Zong W.X., Rathmell J.C., Shiels H.A., Ulrich E., Waymire K.G., Mahar P., Frauwirth K., King A., Zong W.X., Rathmell J.C., Shiels H.A., Ulrich E., Waymire K.G., Mahar P., Frauwirth K., Zong W.X., Rathmell J.C., Shiels H.A., Ulrich E., Waymire K.G., Mahar P., Frauwirth K., Rathmell J.C., Shiels H.A., Ulrich E., Waymire K.G., Mahar P., Frauwirth K., Shiels H.A., Ulrich E., Waymire K.G., Mahar P., Frauwirth K., Ulrich E., Waymire K.G., Mahar P., Frauwirth K., Waymire K.G., Mahar P., Frauwirth K., Mahar P., Frauwirth K., Frauwirth K., et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Sawadogo M., Sawadogo M. Functional domains of the transcription factor USF2: Atypical nuclear localization signals and context-dependent transcriptional activation domains. Mol. Cell. Biol. 1996;16:1367–1375. doi: 10.1128/mcb.16.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov V., Mikhailova M., Degenhardt K., Venkatachalam M.A., White E., Saikumar P., Mikhailova M., Degenhardt K., Venkatachalam M.A., White E., Saikumar P., Degenhardt K., Venkatachalam M.A., White E., Saikumar P., Venkatachalam M.A., White E., Saikumar P., White E., Saikumar P., Saikumar P. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J. Biol. Chem. 2003;278:5367–5376. doi: 10.1074/jbc.M203392200. [DOI] [PubMed] [Google Scholar]
- Moldoveanu T., Liu Q., Tocilj A., Watson M., Shore G., Gehring K., Liu Q., Tocilj A., Watson M., Shore G., Gehring K., Tocilj A., Watson M., Shore G., Gehring K., Watson M., Shore G., Gehring K., Shore G., Gehring K., Gehring K. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol. Cell. 2006;24:677–688. doi: 10.1016/j.molcel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Muchmore S.W., Sattler M., Liang H., Meadows R.P., Harlan J.E., Yoon H.S., Nettesheim D., Chang B.S., Thompson C.B., Wong S.L., Sattler M., Liang H., Meadows R.P., Harlan J.E., Yoon H.S., Nettesheim D., Chang B.S., Thompson C.B., Wong S.L., Liang H., Meadows R.P., Harlan J.E., Yoon H.S., Nettesheim D., Chang B.S., Thompson C.B., Wong S.L., Meadows R.P., Harlan J.E., Yoon H.S., Nettesheim D., Chang B.S., Thompson C.B., Wong S.L., Harlan J.E., Yoon H.S., Nettesheim D., Chang B.S., Thompson C.B., Wong S.L., Yoon H.S., Nettesheim D., Chang B.S., Thompson C.B., Wong S.L., Nettesheim D., Chang B.S., Thompson C.B., Wong S.L., Chang B.S., Thompson C.B., Wong S.L., Thompson C.B., Wong S.L., Wong S.L., et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Nechushtan A., Smith C.L., Hsu Y.T., Youle R.J., Smith C.L., Hsu Y.T., Youle R.J., Hsu Y.T., Youle R.J., Youle R.J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtan A., Smith C.L., Lamensdorf I., Yoon S.H., Youle R.J., Smith C.L., Lamensdorf I., Yoon S.H., Youle R.J., Lamensdorf I., Yoon S.H., Youle R.J., Yoon S.H., Youle R.J., Youle R.J. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 2001;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez D., White E., White E. TNF-α signals apoptosis through a bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol. Cell. 2000;6:53–63. [PubMed] [Google Scholar]
- Petros A.M., Nettesheim D.G., Wang Y., Olejniczak E.T., Meadows R.P., Mack J., Swift K., Matayoshi E.D., Zhang H., Thompson C.B., Nettesheim D.G., Wang Y., Olejniczak E.T., Meadows R.P., Mack J., Swift K., Matayoshi E.D., Zhang H., Thompson C.B., Wang Y., Olejniczak E.T., Meadows R.P., Mack J., Swift K., Matayoshi E.D., Zhang H., Thompson C.B., Olejniczak E.T., Meadows R.P., Mack J., Swift K., Matayoshi E.D., Zhang H., Thompson C.B., Meadows R.P., Mack J., Swift K., Matayoshi E.D., Zhang H., Thompson C.B., Mack J., Swift K., Matayoshi E.D., Zhang H., Thompson C.B., Swift K., Matayoshi E.D., Zhang H., Thompson C.B., Matayoshi E.D., Zhang H., Thompson C.B., Zhang H., Thompson C.B., Thompson C.B., et al. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 2000;9:2528–2534. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros A.M., Olejniczak E.T., Fesik S.W., Olejniczak E.T., Fesik S.W., Fesik S.W. Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Saito M., Korsmeyer S.J., Schlesinger P.H., Korsmeyer S.J., Schlesinger P.H., Schlesinger P.H. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat. Cell Biol. 2000;2:553–555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- Sattler M., Liang H., Nettesheim D., Meadows R.P., Harlan J.E., Eberstadt M., Yoon H.S., Shuker S.B., Chang B.S., Minn A.J., Liang H., Nettesheim D., Meadows R.P., Harlan J.E., Eberstadt M., Yoon H.S., Shuker S.B., Chang B.S., Minn A.J., Nettesheim D., Meadows R.P., Harlan J.E., Eberstadt M., Yoon H.S., Shuker S.B., Chang B.S., Minn A.J., Meadows R.P., Harlan J.E., Eberstadt M., Yoon H.S., Shuker S.B., Chang B.S., Minn A.J., Harlan J.E., Eberstadt M., Yoon H.S., Shuker S.B., Chang B.S., Minn A.J., Eberstadt M., Yoon H.S., Shuker S.B., Chang B.S., Minn A.J., Yoon H.S., Shuker S.B., Chang B.S., Minn A.J., Shuker S.B., Chang B.S., Minn A.J., Chang B.S., Minn A.J., Minn A.J., et al. Structure of Bcl-xL–Bak peptide complex: Recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- Sawada M., Sun W., Hayes P., Leskov K., Boothman D.A., Matsuyama S., Sun W., Hayes P., Leskov K., Boothman D.A., Matsuyama S., Hayes P., Leskov K., Boothman D.A., Matsuyama S., Leskov K., Boothman D.A., Matsuyama S., Boothman D.A., Matsuyama S., Matsuyama S. Ku70 suppresses the apoptotic translocation of Bax to mitochondria. Nat. Cell Biol. 2003;5:320–329. doi: 10.1038/ncb950. [DOI] [PubMed] [Google Scholar]
- Sundararajan R., White E., White E. E1B 19K blocks Bax oligomerization and tumor necrosis factor α-mediated apoptosis. J. Virol. 2001;75:7506–7516. doi: 10.1128/JVI.75.16.7506-7516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Youle R.J., Tjandra N., Youle R.J., Tjandra N., Tjandra N. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Wang K., Gross A., Waksman G., Korsmeyer S.J., Gross A., Waksman G., Korsmeyer S.J., Waksman G., Korsmeyer S.J., Korsmeyer S.J. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol. Cell. Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M.C., Lindsten T., Mootha V.K., Weiler S., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J., Lindsten T., Mootha V.K., Weiler S., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J., Mootha V.K., Weiler S., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J., Weiler S., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J., Ashiya M., Thompson C.B., Korsmeyer S.J., Thompson C.B., Korsmeyer S.J., Korsmeyer S.J. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes & Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei M.C., Zong W.X., Cheng E.H., Lindsten T., Panoutsakopoulou V., Ross A.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J., Zong W.X., Cheng E.H., Lindsten T., Panoutsakopoulou V., Ross A.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J., Cheng E.H., Lindsten T., Panoutsakopoulou V., Ross A.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J., Lindsten T., Panoutsakopoulou V., Ross A.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J., Panoutsakopoulou V., Ross A.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J., Ross A.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J., MacGregor G.R., Thompson C.B., Korsmeyer S.J., Thompson C.B., Korsmeyer S.J., Korsmeyer S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S.N., Adams J.M., Adams J.M. Life in the balance: How BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S.N., Fletcher J.I., Kaufmann T., van Delft M.F., Chen L., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., Fletcher J.I., Kaufmann T., van Delft M.F., Chen L., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., Kaufmann T., van Delft M.F., Chen L., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., van Delft M.F., Chen L., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., Chen L., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., Lee E.F., Fairlie W.D., Bouillet P., Fairlie W.D., Bouillet P., Bouillet P., et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Wolter K.G., Hsu Y.T., Smith C.L., Nechushtan A., Xi X.G., Youle R.J., Hsu Y.T., Smith C.L., Nechushtan A., Xi X.G., Youle R.J., Smith C.L., Nechushtan A., Xi X.G., Youle R.J., Nechushtan A., Xi X.G., Youle R.J., Xi X.G., Youle R.J., Youle R.J. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha H., Aime-Sempe C., Sato T., Reed J.C., Aime-Sempe C., Sato T., Reed J.C., Sato T., Reed J.C., Reed J.C. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- Zong W.X., Lindsten T., Ross A.J., MacGregor G.R., Thompson C.B., Lindsten T., Ross A.J., MacGregor G.R., Thompson C.B., Ross A.J., MacGregor G.R., Thompson C.B., MacGregor G.R., Thompson C.B., Thompson C.B. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes & Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]