Abstract
The linkage of sister chromatids after DNA replication ensures the faithful inheritance of chromosomes by daughter cells. In budding yeast, the establishment of sister chromatid cohesion requires Ctf8, Dcc1, and Ctf18, a homologue of the p140 subunit of the replication factor C (RFC). In this report we demonstrate that in 293T cells, Flag-tagged Ctf18 forms a seven-subunit cohesion-RFC complex comprised of Ctf18, Dcc1, Ctf8, RFCp40, RFCp38, RFCp37, and RFCp36 (Ctf18-RFC). We demonstrate that a stoichiometric heteroheptameric Ctf18-RFC complex can be assembled by coexpressing the seven proteins in baculovirus-infected insect cells. In addition, the two other stable subcomplexes were formed, which include a pentameric complex comprised of Ctf18, RFCp40, RFCp38, RFCp37, and RFCp36 and a dimeric Dcc1-Ctf8. Both the five- and seven-subunit Ctf18-RFC complexes bind to single-stranded and primed DNAs and possess weak ATPase activity that is stimulated by the addition of primed DNA and proliferating cell nuclear antigen (PCNA). These complexes catalyzed the ATP-dependent loading of PCNA onto primed and gapped DNA but not onto double-stranded nicked or single-stranded circular DNAs. Consistent with these observations, both Ctf18-RFC complexes substituted for the replicative RFC in the PCNA-dependent DNA polymerase δ-catalyzed DNA replication reaction. These results support a model in which sister chromatid cohesion is linked to DNA replication.
Faithful inheritance of a complete set of the chromosome complement by daughter cells is essential for cell survival (1-4). In eukaryotes, newly synthesized sister chromatid DNAs are linked together physically by the cohesin complex (called cohesion) from the time they are replicated until their distribution between daughter cells in anaphase (1, 5-7). In budding yeast, the cohesin complex, composed of the four proteins Scc1/Rad21, Scc3, SMC1, and SMC3, is loaded onto chromatin during the G1/S transition and leads to the association of sister chromatids after DNA replication. Mutations in any one of these subunits result in the precocious separation of sister chromatids before cohesion is severed in anaphase and ultimately leads to cell death. Recent studies have revealed important insights into the cohesin structure and have shown that the severance of cohesion is mediated by separase, a protease that cleaves Scc1 (1, 5, 8-17). The steps leading to cohesion between sister chromatids, however, remain unknown. Chromosome-loss assays have identified a number of genes required for cohesion of sister chromatids. Based on their putative roles, cohesion genes have been characterized as either deposition or establishment factors (18). The deposition factors such as Scc2 and Scc4, which interact and must function during S phase, are required for the loading of cohesin onto DNA but are not part of the cohesin complex (19). Establishment factors, which include Ctf7/Eco1/Eso1, Ctf4/Pob1, Ctf18/Chl12, Dcc1, and Ctf8, are essential for cohesion and have some role in DNA replication (20-27). A number of studies support the notion that cohesion is linked closely to DNA replication. In yeast, the establishment factors interact genetically with a number of genes essential for DNA replication (ref. 21 and references therein). For example, Ctf18 interacts genetically with genes encoding DNA polymerases (Pols) δ and α and cdc7 kinase and physically interacts with proliferating cell nuclear antigen (PCNA). Also, ctf7 interacts genetically with pcn (18, 23), and Ctf4 was shown to physically interact with Pol α (28). Functional establishment factors as well as the cohesin subunits are required during S phase.
Ctf18, Dcc1, and Ctf8 form a complex with the four small subunits of replication factor C (RFC) in yeast (21, 22, 29-31). RFC is a five-subunit complex (RFC1-5) that is involved in the loading of the homotrimeric clamp PCNA onto DNA that confers processivity to Pol δ and ε during DNA replication (reviewed in ref. 32). All RFC subunits possess sequence similarities [RFC boxes (33)], and four of the five subunits have NTP-binding sites. A second alternative RFC derivative was shown recently to play a role in the DNA-damage replication checkpoint response (34, 35). In this case, the RFC1(p140) subunit is replaced by Rad17, resulting in the formation of the pentameric Rad17-RFC, which specifically loads the heterotrimeric Rad9-Rad1-Hus1 (9-1-1) clamp complex onto DNA (36, 37). The third alternative RFC complex, in which the RFC1 subunit is replaced to form the heteroheptameric Ctf18-RFC complex, is required for sister chromatid cohesion (21, 22, 30, 31). The clamp for Ctf18-RFC is likely to be PCNA because Ctf18 was identified as a PCNA-interacting protein in PCNA proteomic experiments (38). In all three RFC derivatives, the small RFC2-5 subunits are associated with a distinct larger subunit containing RFC boxes.
To examine the function of the human Ctf18-Dcc1-Ctf8-RFC complex, we cloned, overexpressed, and purified this complex from 293T and baculovirus-infected insect cells. The purified Ctf18-RFC complex is a weak DNA-dependent ATPase that binds to single-stranded and primer/template DNAs. Consistent with the observation that Ctf18-RFC interacts physically with PCNA, in the presence of ATP, Ctf18-RFC loads PCNA onto primed and gapped circular DNAs but not onto nicked or single-stranded circular DNA. Furthermore, Ctf18-RFC substituted for the replicative RFC in supporting DNA synthesis catalyzed by Pol δ in a PCNA-dependent reaction, suggesting that the cohesion RFC is a bona fide PCNA clamp loader.
Materials and Methods
Plasmids and Proteins. hCtf18 (GenBank accession no. XM027413) and hDcc1 (GenBank accession no. BC001316) cDNAs were amplified from IMAGE clones 4040321 and 3454505, respectively. The previously reported hCtf8 cDNA (GenBank accession no. 301014) was amplified from a HeLa cDNA library. These cDNAs were subcloned into pET28a (Novagen) (for bacterial expression), pFastBac1 (Invitrogen) (for baculovirus expression of untagged proteins), pFastBacHtb-Flag2 (Invitrogen) (for baculovirus expression of His6-Flag2 fusion proteins), and pIRESpuro2-His6-Flag2 (CLONTECH) (for human cell expression). All plasmids were sequenced to verify that mutations were not introduced during PCR and cloning. The baculoviruses were generated according to manufacturer instructions.
Purification of His6-Flag2-Ctf18 Complex from a Stable 293T Cell Line. Stably transfected 293T cells expressing a His2-Flag2-Ctf18 were grown in 7 liters of Joklik medium with penicillin, streptomycin, and 2.5 μg/ml puromycin to 90% confluence. The packed cells (25 ml) were washed with ice-cold PBS, lysed in 55 ml of lysis buffer [50 mM Tris·HCl, pH 7.5/0.15 M NaCl/10 mM β-glycerophosphate/10% glycerol/1% Tween 20/0.1% Nonidet P-40/1 mM sodium vanadate/1 mM sodium fluoride/proteinase inhibitors (2 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml anti-pain, and 0.1 mM benzamidine)], sonicated, and centrifuged at 4°C for 30 min at 43,500 × g. The supernatant (80 ml, 270 mg of protein) was incubated with 10 ml of Ni2+-agarose beads, preequilibrated with 50 mM Tris·HCl, pH 8.0/20 mM sodium phosphate, pH 8.0/0.15 M NaCl/proteinase inhibitors at 4°C overnight. The beads were packed onto a 20 × 2-cm Flex column (Kontes) and washed with 150 ml of wash buffer (100 mM sodium phosphate, pH 7.4/10% glycerol/0.15 M NaCl/proteinase inhibitors). Bound proteins were eluted with wash buffer plus 0.5 M imidazole. The imidazole-eluted fraction (10 ml, 42 mg of protein) was loaded onto a 1-ml SP-Sepharose column preequilibrated with buffer A (25 mM Hepes-KOH, pH 7.5/10 mM MgCl2/0.5 mM EDTA/10% glycerol/proteinase inhibitors) plus 50 mM KCl. Bound proteins were eluted with 25 ml of a 50 mM to 1 M KCl linear gradient in buffer A and 1 mM DTT, and the elution of Ctf18 and p37 (RFC5) was monitored by Western blotting with Flag and p37 antibodies, respectively. Both the His6-Flag2-Ctf18 and p37 peaked at ≈0.2 M KCl, and fractions containing both proteins were pooled (15 ml, 0.94 mg of protein). The pooled fraction then was incubated with 0.6 ml of Flag-M2 beads (Sigma), preequilibrated with buffer A plus 1 mM DTT/0.3 M NaCl/0.05% Nonidet P-40, overnight with constant rocking. The beads were washed three times with 1 ml of buffer A, and bound protein was eluted three times with 0.3 ml of buffer A containing 0.5 mg/ml Flag3 peptide for 45 min at 4°C with constant rocking (yielding 45 μg of protein).
Purification of Ctf18-Dcc1-Ctf8-RFC2-5 Complexes. Sf9 suspension cells were grown to 80% confluence in Grace's medium supplemented with 10% FBS. Sf9 cells (500 ml, 5 × 105 cells per ml) were infected with baculoviruses encoding His6-Flag2-Ctf18, Dcc1, Ctf8, and the RFC2-5 (His6-RFC5) subunits at a multiplicity of infection of 5, and the mixture was incubated for 72 h at 27°C with constant shaking (120 rpm). Cells were harvested by centrifugation at 600 × g at 4°C for 10 min, washed with 10 cell-pellet volumes of ice-cold PBS, and lysed in 10 ml of hypotonic buffer (50 mM Tris·HCl, pH 8.0/20 mM sodium phosphate, pH 8.0/1.5 mM MgCl2/0.5 mM PMSF/proteinase inhibitors) plus 10 mM KCl by Dounce homogenization (10 strokes), and the mixture was centrifuged at 4°C for 30 min at 2,400 × g. The NaCl concentration of the supernatant (cytosolic fraction) was adjusted to 0.42 M. The pellet was resuspended in 10 ml of hypotonic buffer containing 0.42 M NaCl. The resuspended pellet and cytosolic fraction were centrifuged at 4°C for 30 min at 43,500 × g. The nuclear and cytosolic fractions were combined (25 ml, 284 mg of protein) and mixed with 1.5 ml of Ni2+-agarose (Invitrogen) preequilibrated with 50 mM Tris·HCl, pH 8.0/20 mM sodium phosphate, pH 8.0/0.5 M NaCl/0.5 mM PMSF/proteinase inhibitors, and the suspension was rocked overnight at 4°C. The beads were washed three times with 15 ml of wash buffer (10 mM sodium phosphate, pH 7.4/10% glycerol/0.5 M NaCl/0.5 mM PMSF/proteinase inhibitors) and packed onto an 8 × 1.5-cm Flex column (Kontes). Bound proteins were eluted with 3 ml of wash buffer plus 0.5 M imidazole, yielding 4 mg of protein. An aliquot of the imidazole-eluted fraction (0.2 ml, 0.27 mg of protein) was layered onto a 5-ml 15-35% glycerol gradient containing 25 mM Tris·HCl (pH 7.5), 1 mM EDTA, 0.4 M NaCl, 1 mM DTT, 0.01% Nonidet P-40, and proteinase inhibitors and centrifuged at 250,000 × g for 20 h at 4°C, and fractions (0.12 ml) were collected from the bottom of the tube. The Ctf18-Dcc1-Ctf8-RFC2-5 complex, detected by Coomassie staining, sedimented between aldolase (7.4 s) and catalase (11.3 s). This procedure resulted in the isolation of 0.15 mg of the seven-subunit stoichiometric complex (in 0.36 ml). The five-subunit Ctf18-RFC2-5 and two-subunit Dcc1-Ctf8 complexes were overexpressed and purified in the same manner.
In Vitro Transcription/Translation (IVT) Immunoprecipitation. Coupled IVT reactions and immunoprecipitation were performed as described (39). The rabbit antisera used for immunoprecipitations were raised against bacterially expressed hCtf18 (amino acids 1-243), hDcc1, and hCtf8 purified by nickel-chelate chromatography. The antiserum against the 37 RFC subunit has been described (39).
DNA Binding, ATPase, and Replication Assays. These assays were carried out as described (40-42). Specific additions are indicated in the figure legends.
Results
Isolation and Assembly of the Human Cohesion-RFC Complex. Ctf18, Dcc1, and Ctf8 are essential for the establishment of sister chromatid cohesion in yeast (21, 22, 30). BLAST searches identified homologues of ctf18, dcc1, and ctf8 present in yeast, plants, and mammals (21, 22). Sequence analyses revealed that human (h)Ctf18, hDcc1, and hCtf8 contain 975, 393, and 121 amino acids and possess molecular masses of 107, 45, and 14 kDa, respectively. Ctf18 is an RFC-like protein containing the conserved RFC boxes II-V and VII-VIII (30, 33). Unlike RFC1, the largest subunit of replicative RFC, Ctf18 lacks the ligase homology domain. Ctf18 proteins from different species share a high homology in their C-terminal 20-aa region, which may be important for their function. In contrast, sequence analyses of hDcc1 and hCtf8 did not reveal known conserved motifs.
To identify hCtf18-interacting proteins in vivo, we generated a human 293T cell line constitutively expressing doubly tagged Ctf18 and purified the Ctf18 complex by nickel-chelate, SPSepharose, and Flag-M2 affinity chromatographic steps. Components of the isolated hCtf18 complex were separated by SDS/PAGE and visualized by silver staining (Fig. 1A). As revealed by mass spectrometric and immunoblot analysis, hCtf18 was associated with Dcc1, Ctf8, and the four RFC2-5 subunits similar to that seen in yeast.
Fig. 1.
Isolation of Ctf18-RFC complexes. (A) Isolation of Ctf18 complexes from 293T cells. A silver-stained SDS/12% polyacrylamide gel of the Flag peptide eluted Ctf18 complex (0.46 μg of protein) isolated from stably transfected 293T cells as described in Materials and Methods is shown. All proteins indicated were identified by mass spectrometry with the exception of Ctf8, which was detected by Western blot analysis. (B) Characterization of Ctf complexes isolated from baculovirus-infected Sf9 cells. Shown is a Coomassie-stained SDS/12% polyacrylamide gel of purified Ctf complexes isolated by glycerol gradient centrifugation as described in Materials and Methods.(Left) Containing 2 μg of purified Ctf18-RFC(7S). (Center) Containing 1.2 μg of Ctf18-RFC(5S). (Right) Containing 6 μg of Dcc1-Ctf8 heterodimer.
We then produced the Ctf18-RFC complexes using the baculovirus expression system and purified this material by nickel-chelate affinity followed by glycerol gradient sedimentation. By using different combinations of the viruses to infect Sf9 cells, we purified three stable complexes: Ctf18-Dcc1-Ctf8-RFC2-5 [Ctf18-RFC(7S)], Ctf18-RFC2-5 [Ctf18-RFC(5S)], and the Dcc1-Ctf8 heterodimer (Fig. 1B). Electrophoretic analysis of the Coomassie-stained protein bands (Fig. 1B) indicated that the subunit composition of each complex was near stoichiometric: Ctf18-RFC(7S) contained the molar subunit ratio Ctf18 (1.0)/Dcc1 (1.3)/Ctf8 (1.1)/RFC2 + 3 (2.0)/RFC4 + 5 (2.2); the molar subunit ratio of Ctf18-RFC(5S) was Ctf18 (1)/RFC2 + 3 (2.0)/RFC4 + 5 (1.8); and the Dcc1-Ctf8 heterodimer was 1:1.2. All three complexes were monomeric in structure based on their Stokes radii and sedimentation constants (data not presented).
Order of Assembly of the Seven-Subunit Cohesion RFC. To gain insight into the assembly of the seven-subunit cohesion-RFC complex, we examined the interactions among the subunits expressed in rabbit reticulocyte lysates (IVT). As shown in Fig. 2A, hCtf18 but not hDcc1 or hCtf8 interacted with RFC2-5 to form the five-subunit complex. This association required RFC5(p38) (Fig. 2B, compare lane 3 with lane 6). Both hCtf18 and hDcc1 were required to form the seven-subunit hCtf18-RFC complex (Fig. 2C). Ctf8 specifically interacted with Ctf18 in the five-subunit complex, resulting in the production of an unstable six-subunit complex (Fig. 2C, lanes 1-3) that was stabilized by the addition of Dcc1 (Fig. 2C, lanes 10-12). Neither hCtf8 nor hDcc1 alone interacted with hCtf18 (Fig. 2D). However, formation of the stable hCtf18-hDcc1-hCtf8 heterotrimer and hDcc1-hCtf8 heterodimer were detected (Fig. 2D). The excess levels of Dcc1 and Ctf8 subunits present in the seven-subunit complex isolated with Ctf8 antibodies most likely reflect the presence of the Dcc1-Ctf8 dimer formed in the IVT reaction (Fig. 2C, lane 12). Conversion of the five-subunit Ctf18-RFC to the seven-subunit Ctf18-RFC by the addition of purified Dcc1-Ctf8 was not detected (data not shown), suggesting that Ctf8 and Dcc1 are added sequentially to Ctf18-RFC (2-5). It remains to be determined whether the five-subunit Ctf18-RFC and Dcc1-Ctf8 complexes exist in human cells.
Fig. 2.
Order of assembly of the Ctf18-Dcc1-Ctf8-RFC2-5 complex. An IVT system containing the indicated expression vectors was used to synthesize labeled proteins. Products were immunoprecipitated with the antibody indicated above the lanes, and the precipitated material then was separated by SDS/PAGE and analyzed by autoradiography. (A) Interaction of Ctf18, Dcc1, or Ctf8 with RFC2-5. (B) Critical role of the RFC5(p38) subunit in the formation of the Ctf18-RFC2-5 complex. Flag-tagged Ctf18 complexes were adsorbed to Flag-M2 antibody beads. + and -, presence or absence, respectively, of the competing Flag peptide (1 mg) added in the immunoprecipitation step. (C) Formation of six- and seven-subunit Ctf18-RFC complexes. (D) Interactions among the Ctf18, Dcc1, and Ctf8 subunits. LO, load-on; PR, preimmune serum; D1, Dcc1-specific antibodies; C8, Ctf8-specific antibodies.
DNA-Binding Properties of the Seven- and Five-Subunit Ctf18-RFC Complexes. The DNA-binding properties of replicative RFC and checkpoint Rad17-RFC are similar (42, 43). In the presence of 175 mM NaCl, both complexes bind specifically to the 3′ end of a primed DNA, whereas at 50 mM NaCl they bind to single-stranded (ss) and double-stranded (ds) DNA as well. The DNA-binding properties of seven- and five-subunit Ctf18-RFC seem to be more salt-sensitive than the replicative RFC (data not presented). At 50 mM NaCl, the DNA-binding activity of replicative RFC was 3-fold higher than the seven-subunit Ctf18-RFC, whereas at 175 mM NaCl the DNA-binding activity of replicative RFC was ≈15- and 4-fold higher than the seven- and five-subunit Ctf18-RFC complexes, respectively. The specificity of the DNA-binding activities of the two Ctf18-RFC complexes were examined in the presence of 50 mM NaCl by measuring their binding to radiolabeled primed DNA in the presence of increasing levels of unlabeled ds-, ss-, or primed DNAs. As shown in Fig. 3, the binding of Ctf18-RFC to the labeled primed template was competed equally by unlabeled primed and ssDNAs, whereas dsDNA did not compete. Thus, Ctf18-RFC binds to ssDNA and most likely preferentially to a double-stranded/single-stranded junction structure present in primed DNA, properties similar to the other RFC clamp loaders.
Fig. 3.
Competition of the binding of the Ctf18-RFC(7S) to labeled primed DNA by unlabeled DNAs. Reaction mixtures (15 μl) containing 0.5 pmol of clamp loader, 100 fmol of 32P-labeled primed 50-bp dsDNA with a 50-nt ssDNA (5′ overhang), unlabeled DNAs as indicated, 25 mM Hepes (pH 7.5), 3 mM MgCl2, 1 mM DTT, and 0.1 mg/ml BSA were incubated for 30 min on ice. The DNA bound to the protein complex was quantified by a nitrocellulose filter-binding assay. The DNA substrate used has been described (40).
DNA-Dependent ATPase Activity. Similar to replicative RFC and checkpoint Rad17-RFC (36, 42), Ctf18-RFC(7S) possesses a weak DNA-dependent ATPase activity (Table 1). The ATPase activity of Ctf18-RFC was stimulated by ssM13 DNA and hexaprimed M13 DNA but not by nicked dsDNA or intact dsDNA (data not presented). The stimulation by primed DNA was greater than that observed with ssDNA, suggesting that Ctf18-RFC has a preference for primed DNA over ssDNA.
Table 1. ATPase activity of cohesion-RFC.
| Additions | pmol of Pi released per min per pmol of protein | Fold increase |
|---|---|---|
| None | 1.12 | 1 |
| ssM 13 | 2.54 | 2.27 |
| Hexaprimed M 13 | 4.67 | 4.17 |
| Hexaprimed M13 + hRPA | 2.62 | 2.34 |
| Nicked pBluescript | 1.20 | 1.07 |
| Hexaprimed M 13 + 9-1-1 | 1.02 | 0.91 |
| Hexaprimed M 13 + PCNA | 6.98 | 6.23 |
| Hexaprimed M 13 + PCNA + hRPA | 11.0 | 9.82 |
Reaction mixtures (20 μl) containing 25 mM Tris·HCl (pH 7.5), 2 mM DTT, 3 mM magnesium acetate, 200 μg/ml BSA, 50 μM ATP, and 500 fmol of Ctf18-Rfc(7S) were incubated at 37°C for 1 h in the presence of 100 fmol of DNA, 2 pmol of PCNA, or 20 pmol of hRPA where indicated. The amount of inorganic phosphate (Pi) released was measured as described (28).
Both PCNA and 9-1-1 clamps stimulate the DNA-dependent ATPase activity of their clamp-loading complexes (36, 42, 44). Similar experiments with Ctf18-RFC(7S) demonstrated that PCNA stimulated this activity only ≈1.5-fold, whereas 9-1-1 had no effect. Maximal stimulation of the DNA-dependent ATPase activity required the addition of human replication protein A (hRPA) and PCNA, leading to a 10-fold increase in the ATPase activity over that observed with the Ctf18-RFC(7S) complex alone. The ATPase activity of the five-subunit Ctf18-RFC was identical to that observed with the heptameric complex (data not presented). In the presence of øx ssDNA, the DNA-dependent ATPase activity of Ctf18-RFC(7S) was ≈25-fold lower than that found with the replicative RFC (2.66 and 64.9 pmol of Pi released per min per pmol of protein, respectively).
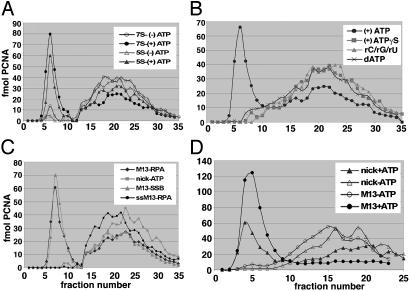
Loading of PCNA on Primed-M13 Templates. The stimulation of the DNA-dependent ATPase activity of Ctf18-RFC by PCNA suggested that the cohesion-RFC could load PCNA onto DNA. This notion was also supported by proteomic analyses that identified Ctf18 as a PCNA-interacting protein (38). Gel filtration experiments were carried out to determine whether Ctf18-RFC(7S and 5S) complexes loaded PCNA onto circular DNA structures. In this assay, free PCNA eluted in the included volume, whereas PCNA loaded onto DNA was recovered in the excluded volume. Fig. 4A shows that Ctf18-RFC(7S and 5S) loaded PCNA onto a hexaprimed M13 ssDNA in an ATP-dependent manner. PCNA loading specifically required ATP and its hydrolysis because it was not observed in the presence of ATPγS or in reactions with the other rNTPs or dATP in lieu of ATP (Fig. 4B). The rate of dATP hydrolysis by the Ctf18-RFC complexes was only 2-fold lower than that observed with ATP. Why dATP does not support PCNA loading remains unclear. Ctf18-RFC(7S) and Ctf18-RFC(5S) loaded PCNA onto primed and gapped circular DNA but not onto single-stranded circular DNA (Fig. 4C). Linearization of the gapped circular DNA after the loading of PCNA by Ctf18-RFC(7S) resulted in the removal of PCNA from the DNA, suggesting that the loaded PCNA was capable of sliding fully through DNA and was not physically associated with the clamp loader (data not presented). In contrast to replicative RFC, Ctf18-RFC did not load PCNA onto singly nicked circular DNA (Fig. 4 C and D). The presence of a ssDNA gap as short as 5 nucleotides in circular DNA was required for efficient PCNA loading by Ctf18-RFC (data not presented). ssDNA regions in gapped DNA coated with either Escherichia coli ssDNA-binding protein (SSB) or hRPA supported PCNA loading.
Fig. 4.
Loading of 32P-labeled PCNA onto indicated DNA templates by the five- and seven-subunit Ctf18 complexes. Reactions (50 μl) contained 150 fmol of indicated DNA substrate, 0.6 pmol of 32P-labeled PCNA, 0.2 pmol of PCNA clamp loader, 5.71 μg of hRPA, 40 mM Tris·HCl (pH 7.8), 10 mM magnesium acetate, 200 μg/ml BSA, and 50 mM NaCl. After 10 min at 37°C, the mixtures were loaded immediately onto a 5-ml Bio-Rad A-15 column equilibrated with the reaction buffer containing 150 mM NaCl. The distribution of the [32P]PCNA in eluted fractions was monitored by Cerenkov counting. (A) Loading of PCNA onto hexaprimed M13 ssDNA by the five- and seven-subunit Ctf18-RFC complex in the presence and absence of ATP. (B)Influence of NTPs on the loading of PCNA onto hexaprimed M13 DNA by Ctf18-RFC(7S). (C) Loading of PCNA by Ctf18-RFC(7S) on RPA-coated hexaprimed M13 DNA (M13-RPA), E. coli SSB-coated hexaprimed M13 DNA (M13-SSB), RPA-coated M13 DNA (ssM13-RPA), and nicked pBluescript (nick-ATP). (D) Loading of PCNA by RFC on RPA-coated hexaprimed M13 DNA (M13) or nicked pBluescript (nick) in the presence and absence of ATP.
Because replicative RFC can load as well as unload PCNA on DNA (42), we examined whether Ctf18-RFC(7S) did the same. For this purpose, PCNA was loaded onto a nicked circular DNA template with RFC, and the PCNA-DNA complex was separated from free PCNA. Incubation of the PCNA-DNA complex with either Ctf18-RFC(7S) or replicative RFC in the presence of ATP released PCNA from the template (data not shown).
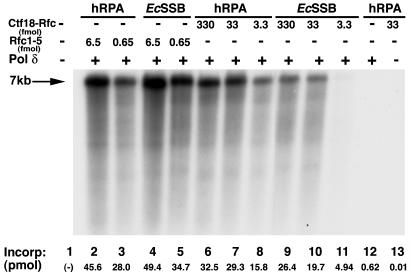
Ctf18-Dcc1-Ctf8-RFC (2-5) Supports Polδ Activity. During DNA replication, RFC binds to the 3′ end of the nascent DNA and loads PCNA onto DNA (reviewed in ref. 32). Loaded PCNA then is recognized by either Pol δ or Pol ε, which then elongates the primer. In the absence of the clamp/clamp loader system, these polymerases are incapable of elongating low levels of primed DNA templates. Because Ctf18-RFC loaded PCNA on primed templates, the cohesion clamp loader should also support Pol δ activity. Fig. 5 shows that Ctf18-RFC, similar to the replicative RFC, supported the Pol δ-catalyzed elongation of a singly primed DNA. This reaction depends on a clamp loader (lane 12), Polδ (lane 13), as well as PCNA and SSB (41). On a molar basis, the replicative RFC was 7-fold more active than the Ctf18-RFC(7S) (compare lane 3 with 8) and 60-fold more active than Ctf18-RFC(5S) (data not presented). Furthermore, unlike RFC, hRPA stimulated the level of DNA synthesized in the presence of the cohesion RFC to a greater extent than E. coli SSB (compare lanes 9-11 with lanes 6-8). The ability of the replicative RFC to support Pol δ activity more efficiently than the Ctf18 complexes is consistent with the differences noted in the PCNA loading onto primed circular DNAs and the DNA-dependent ATPase activities of these complexes.
Fig. 5.
Cohesion RFC supports the Pol δ-catalyze elongation of primed DNA. Reaction mixtures were as described (41) and contained 10 fmol of singly primed M13 ssDNA coated with 2.5 pmol of hRPA or E. coli SSB, 0.2 pmol of hPCNA, 100 fmol of hPol δ, and indicated levels of RFC or Ctf18 RFC. After 30 min at 37°C, aliquots of reaction mixtures were subjected to alkaline agarose electrophoresis and used to measure DNA synthesis. The nucleotide incorporation in each reaction is indicated below each lane. The position of fully extended DNA chains is indicated by the 7-kb marker.
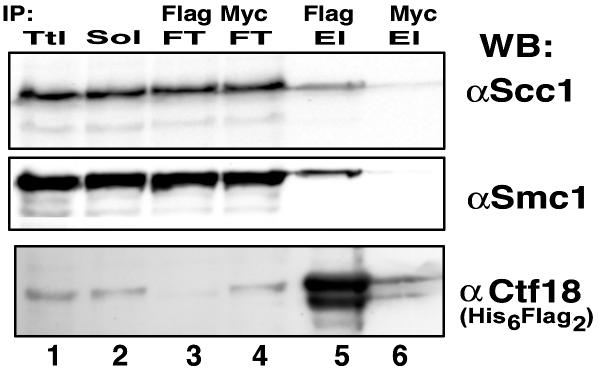
Ctf18 Interacts with Cohesin in Vivo. ctf18, dcc1, and ctf8 were identified as genes essential for sister chromatid cohesion, and cohesin subunits (scc1, smc1, and smc3) were identified as suppressors of Δctf8 mutations in yeast (22). For these reasons we examined whether Ctf18 interacted with cohesin subunits Scc1 and SMC1. For this purpose, sonicated and DNase I-treated whole-cell extracts were prepared from Flag2-Ctf18 stably transfected 293T cells. The Flag2-Ctf18 was immunoprecipitated with Flag beads and bound proteins recovered by peptide elution. Western blot analysis of the eluted material was carried out with hScc1, hSMC1, and hCtf18 antibodies (Fig. 6). Both SMC1 and Scc1 were detected in the material eluted from the Flag beads (lane 5) but not from eluates of proteins immunoprecipitated with the Myc beads (lane 6, negative control). Because Scc1 and SMC1 are components of the cohesin complex, this finding suggests that the Ctf18 complex may interact with the cohesin complex in vivo. However, this interaction may be weak or indirect because the further purification of the isolated Ctf18 complex resulted in the loss of the cohesin subunits (data not presented). These preliminary observations suggest that Ctf18 may play a role in the establishment of cohesion by interacting directly with the cohesin complex.
Fig. 6.
Coimmunoprecipitation of Scc1 and SMC1 with Ctf18 from stably transfected 293T cells. The cell pellet (8 ml) was lysed in 25 ml of lysis buffer as described in Materials and Methods. The mixture was sonicated, digested with 250 μg of pancreatic DNase I overnight on ice, and then centrifuged at 34,500 × g for 30 min at 4°C. An aliquot of the supernatant (12.5 ml, 125 mg of protein) was incubated with 200 μl of preequilibrated Flag beads or Myc beads (negative control) at 4°C overnight. Beads then were washed six times with 1 ml of PBS containing 0.05% Nonidet P-40, 1 mM DTT, and proteinase inhibitors, and the bound Flag-tagged Ctf18 complex was eluted with 1 mg/ml Flag3 peptide (Flag El) or with 1 mg/ml Myc peptide as indicated. The headings Ttl, Sol, and FT refer to total lysate, load-on (solubilized material), and material that did not bind (flow-through), respectively. Approximately 30 μg of protein of the Ttl, Sol, and FT fractions and 0.5 μg of the material eluted with the Flag peptide from Flag beads (Flag-El) and Myc beads (Myc-El) were loaded onto a SDS/12% PAGE followed by Western blotting with the antibodies specific for Ctf18, SMC1, and Scc1 (45).
Discussion
In yeast, ctf18, dcc1, and ctf8 are essential for chromatid cohesion. These and other genes involved in cohesion are ubiquitous among different species, reflecting the conserved role of this process in cell proliferation. We have cloned, overexpressed, and purified the human cohesion RFC complex and showed that it formed a heteroheptameric complex in vivo. Reconstitution of the Ctf18 complex in baculovirus-infected insect cells resulted in the isolation of two other stable subcomplexes: a pentameric Ctf18-RFC2-5 and a dimeric Dcc1-Ctf8 complex. IVT experiments suggest that the Ctf18-RFC2-5 complex is converted to the seven-subunit form stepwise by the addition of Ctf8 followed by the binding of Dcc1. Surprisingly, the three-subunit Ctf18-Dcc1-Ctf8 complex was detected only in IVT reactions and not in baculovirus-infected insect cells. The reasons for this discrepancy are unclear. Similar to the replicative RFC, the five- and seven-subunit Ctf18-RFC complexes load PCNA onto primed DNA templates. Although Ctf18-RFC might also interact with other unknown clamps, Ctf18 proteomic experiments with 293T and HeLa cells did not reveal novel potential clamp protein partners, although Western blot analysis detected the presence of PCNA (data not presented). Consistent with our observation that Ctf18-RFC is a PCNA clamp loader, Ctf18-RFC supported extensive elongation of singly primed M13 in reactions containing Pol δ and PCNA. The seven-subunit Ctf18-RFC complex was much more effective in supporting the Polδ replication reaction than the five-subunit complex. The reasons for this discrepancy are unclear because both complexes were equally active in the loading of PCNA onto DNA and exhibited identical DNA-dependent ATPase activity.
Ctf18-RFC is the third RFC-like complex to be isolated. In contrast to Rad17-RFC, which specifically loads the 9-1-1 complex onto DNA, both the replicative and cohesion RFCs load PCNA onto DNA. Because the replicative RFC is nearly 10-fold more effective in loading PCNA than the cohesion RFC, the replicative RFC probably plays the dominant cellular role in loading PCNA onto DNA. In keeping with this notion, the protein subunits unique to the cohesion clamp loader (Ctf18, Dcc1, and Ctf8) are not essential in Saccharomyces cerevisiae (21, 22, 30). Although cells devoid of these subunits are viable, they display marked genome instability and extensive chromosome loss. Thus, it is likely that Ctf18-RFC is recruited preferentially to sites in DNA associated with the cohesin complex to ensure efficient replication through such regions. Other proteins essential for cohesion such as Ctf7 and Ctf4 may aid in the recruitment of Ctf18-RFC. Ctf7/Eco1 has been reported to interact physically with all three RFC complexes (18) and genetically with Ctf18 and PCNA (23, 25). Ctf4, which interacts genetically with Ctf18 and Dna2 (21, 28, 46), seems to be a DNA-binding protein that interacts directly with Pol α (27, 28), suggesting that cohesion RFC may have a role in replication, particularly on the lagging strand.
Consistent with the known biological function of the Ctf proteins, we detected the interaction of Ctf18-RFC with the subunits of the cohesin complex in 293T cells. Because the majority of the cellular SMC1 and Scc1 proteins are found as the cohesin complex (47), it is likely that the cohesion RFC interacted with the complete cohesin complex in cells. Further purification of the cohesin-Ctf18 complex from 293T cells, however, resulted in its dissociation, suggesting that they interact weakly and/or the interaction is mediated by other proteins that are lost on further purification. In contrast to our findings, it was reported that the cohesin subunits did not immunoprecipitate with endogenous Ctf18 (48), suggesting that overexpression of Ctf18 may be required to detect this interaction. Further studies with purified components are needed to determine whether the interaction between these two complexes is direct or indirect.
The physical and genetic interactions between the establishment factors and DNA replication proteins involved in Okazaki fragment synthesis and maturation have led to a model for cohesion establishment that may involve a more complicated process in lagging-strand synthesis and maturation (21, 49-51). The model proposes the occurrence of “polymerase switch” in which a replicative polymerase tethered to PCNA is displaced by DNA Pol σ (Trf4/Pol κ) to establish cohesion during the replication of the DNA region bound by cohesin. Recent reports suggest that Pol σ and its family of proteins are poly(A) polymerases rather than DNA polymerases (52, 53). These findings suggest that Trf4 mutants that cause cohesion defects may arise by an indirect consequence of altered protein expression, more in keeping with the multiple phenotypes observed with these mutations. Although a polymerase switch mechanism may occur, it is also possible that when a replication fork encounters a cohesin-DNA complex, the cohesion RFC is recruited in place of the replicative RFC at the lagging strand. This clamp-loader switch may be mediated by interactions between cohesin, cohesion RFC, and other establishment factors. Once the cohesion RFC binds to the primer end, it may recruit the establishment factors, which support sister chromatid pairing after DNA replication. Reconstitution of a DNA replication system that utilizes cohesin-DNA templates would contribute to the understanding of how cohesion is established during DNA replication.
Abbreviations: Pol, polymerase; PCNA, proliferating cell nuclear antigen; RFC, replication factor C; IVT, in vitro transcription/translation; h, human; ss, single-stranded; ds, double-stranded; RPA, replication protein A; SSB, ssDNA-binding protein; 9-1-1, Rad9-Hus1-Rad1.
References
- 1.Nasmyth, K. (2001) Annu. Rev. Genet. 35, 673-745. [DOI] [PubMed] [Google Scholar]
- 2.Cahill, D. P., Lengauer, C., Yu, J., Riggins, G. J., Willson, J. K., Markowitz, S. D., Kinzler, K. W. & Vogelstein, B. (1998) Nature 392, 300-303. [DOI] [PubMed] [Google Scholar]
- 3.Lengauer, C., Kinzler, K. W. & Vogelstein, B. (1997) Nature 386, 623-627. [DOI] [PubMed] [Google Scholar]
- 4.Wolstenholme, J. & Angell, R. R. (2000) Chromosoma 109, 435-438. [DOI] [PubMed] [Google Scholar]
- 5.Hirano, T. (2000) Annu. Rev. Biochem. 69, 115-144. [DOI] [PubMed] [Google Scholar]
- 6.Lee, J. Y. & Orr-Weaver, T. L. (2001) Annu. Rev. Cell Dev. Biol. 17, 753-777. [DOI] [PubMed] [Google Scholar]
- 7.Uhlmann, F. (2003) Curr. Biol. 13, R104-R114. [DOI] [PubMed] [Google Scholar]
- 8.Ciosk, R., Zachariae, W., Michaelis, C., Shevchenko, A., Mann, M. & Nasmyth, K. (1998) Cell 93, 1067-1076. [DOI] [PubMed] [Google Scholar]
- 9.Uhlmann, F., Lottspeich, F. & Nasmyth, K. (1999) Nature 400, 37-42. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal, R. & Cohen-Fix, O. (2002) Cell Cycle (Georgetown, Tex.) 1, 255-257. [PubMed] [Google Scholar]
- 11.Campbell, J. L. & Cohen-Fix, O. (2002) Trends Biochem. Sci. 27, 492-495. [DOI] [PubMed] [Google Scholar]
- 12.Cohen-Fix, O. (2001) Cell 106, 137-140. [DOI] [PubMed] [Google Scholar]
- 13.Gruber, S., Haering, C. H. & Nasmyth, K. (2003) Cell 112, 765-777. [DOI] [PubMed] [Google Scholar]
- 14.Michaelis, C., Ciosk, R. & Nasmyth, K. (1997) Cell 91, 35-45. [DOI] [PubMed] [Google Scholar]
- 15.Nasmyth, K. (1999) Trends Biochem. Sci. 24, 98-104. [DOI] [PubMed] [Google Scholar]
- 16.Nasmyth, K., Peters, J. M. & Uhlmann, F. (2000) Science 288, 1379-1385. [DOI] [PubMed] [Google Scholar]
- 17.Uhlmann, F. (2001) Curr. Opin. Cell Biol. 13, 754-761. [DOI] [PubMed] [Google Scholar]
- 18.Kenna, M. A. & Skibbens, R. V. (2003) Mol. Cell. Biol. 23, 2999-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciosk, R., Shirayama, M., Shevchenko, A., Tanaka, T., Toth, A. & Nasmyth, K. (2000) Mol. Cell 5, 243-254. [DOI] [PubMed] [Google Scholar]
- 20.Chestukhin, A., Pfeffer, C., Milligan, S., DeCaprio, J. A. & Pellman, D. (2003) Proc. Natl. Acad. Sci. USA 100, 4574-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna, J. S., Kroll, E. S., Lundblad, V. & Spencer, F. A. (2001) Mol. Cell. Biol. 21, 3144-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer, M. L., Gygi, S. P., Aebersold, R. & Hieter, P. (2001) Mol. Cell 7, 959-970. [DOI] [PubMed] [Google Scholar]
- 23.Skibbens, R. V., Corson, L. B., Koshland, D. & Hieter, P. (1999) Genes Dev. 13, 307-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka, K., Yonekawa, T., Kawasaki, Y., Kai, M., Furuya, K., Iwasaki, M., Murakami, H., Yanagida, M. & Okayama, H. (2000) Mol. Cell. Biol. 20, 3459-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toth, A., Ciosk, R., Uhlmann, F., Galova, M., Schleiffer, A. & Nasmyth, K. (1999) Genes Dev. 13, 320-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams, D. R. & McIntosh, J. R. (2002) Eukaryotic Cell 1, 758-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohler, A., Schmidt-Zachmann, M. S. & Franke, W. W. (1997) J. Cell Sci. 110, 1051-1062. [DOI] [PubMed] [Google Scholar]
- 28.Miles, J. & Formosa, T. (1992) Mol. Cell. Biol. 12, 5724-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naiki, T., Kondo, T., Nakada, D., Matsumoto, K. & Sugimoto, K. (2001) Mol. Cell. Biol. 21, 5838-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouprina, N., Kroll, E., Kirillov, A., Bannikov, V., Zakharyev, V. & Larionov, V. (1994) Genetics 138, 1067-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kouprina, N., Tsouladze, A., Koryabin, M., Hieter, P., Spencer, F. & Larionov, V. (1993) Yeast 9, 11-19. [DOI] [PubMed] [Google Scholar]
- 32.Waga, S. & Stillman, B. (1998) Annu. Rev. Biochem. 67, 721-751. [DOI] [PubMed] [Google Scholar]
- 33.Cullmann, G., Fien, K., Kobayashi, R. & Stillman, B. (1995) Mol. Cell. Biol. 15, 4661-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.al-Khodairy, F., Fotou, E., Sheldrick, K. S., Griffiths, D. J., Lehmann, A. R. & Carr, A. M. (1994) Mol. Biol. Cell 5, 147-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffiths, D. J., Barbet, N. C., McCready, S., Lehmann, A. R. & Carr, A. M. (1995) EMBO J. 14, 5812-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majka, J. & Burgers, P. M. (2003) Proc. Natl. Acad. Sci. USA 100, 2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bermudez, V. P., Lindsey-Boltz, L. A., Cesare, A. J., Maniwa, Y., Griffith, J. D., Hurwitz, J. & Sancar, A. (2003) Proc. Natl. Acad. Sci. USA 100, 1633-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohta, S., Shiomi, Y., Sugimoto, K., Obuse, C. & Tsurimoto, T. (2002) J. Biol. Chem. 277, 40362-40367. [DOI] [PubMed] [Google Scholar]
- 39.Uhlmann, F., Cai, J., Flores-Rozas, H., Dean, F. B., Finkelstein, J., O'Donnell, M. & Hurwitz, J. (1996) Proc. Natl. Acad. Sci. USA 93, 6521-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee, J. K. & Hurwitz, J. (2000) J. Biol. Chem. 275, 18871-18878. [DOI] [PubMed] [Google Scholar]
- 41.Zuo, S., Gibbs, E., Kelman, Z., Wang, T. S., O'Donnell, M., MacNeill, S. A. & Hurwitz, J. (1997) Proc. Natl. Acad. Sci. USA 94, 11244-11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai, J., Uhlmann, F., Gibbs, E., Flores-Rozas, H., Lee, C. G., Phillips, B., Finkelstein, J., Yao, N., O'Donnell, M. & Hurwitz, J. (1996) Proc. Natl. Acad. Sci. USA 93, 12896-12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsey-Boltz, L. A., Bermudez, V. P., Hurwitz, J. & Sancar, A. (2001) Proc. Natl. Acad. Sci. USA 98, 11236-11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellison, V. & Stillman, B. (1998) J. Biol. Chem. 273, 5979-5987. [DOI] [PubMed] [Google Scholar]
- 45.Gregson, H. C., Schmiesing, J. A., Kim, J.-S., Kobayashi, T., Zhou, S. & Yokomori, K. (2001) J. Biol. Chem. 276, 47575-47582. [DOI] [PubMed] [Google Scholar]
- 46.Formosa, T. & Nittis, T. (1999) Genetics 151, 1459-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haering, C. H., Lowe, J., Hochwagen, A. & Nasmyth, K. (2002) Mol. Cell 9, 773-788. [DOI] [PubMed] [Google Scholar]
- 48.Merkle, C. J., Karnitz, L. M., Henry-Sanchez, J. T. & Chen, J. (2003) J. Biol. Chem. [DOI] [PubMed]
- 49.Wang, Z. & Christman, M. F. (2001) Cell Biochem. Biophys. 35, 289-301. [DOI] [PubMed] [Google Scholar]
- 50.Wang, Z., Castano, I. B., De Las Penas, A., Adams, C. & Christman, M. F. (2000) Science 289, 774-779. [DOI] [PubMed] [Google Scholar]
- 51.Carson, D. R. & Christman, M. F. (2001) Proc. Natl. Acad. Sci. USA 98, 8270-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saitoh, S., Chabes, A., McDonald, W. H., Thelander, L., Yates, J. R. & Russell, P. (2002) Cell 109, 563-573. [DOI] [PubMed] [Google Scholar]
- 53.Read, R. L., Martinho, R. G., Wang, S. W., Carr, A. M. & Norbury, C. J. (2002) Proc. Natl. Acad. Sci. USA 99, 12079-12084. [DOI] [PMC free article] [PubMed] [Google Scholar]