Abstract
During cell stress, Saccharomyces cerevisiae increases the synthesis of chitin and glucans to strengthen and repair the cell wall. In this study, we show that under conditions of cell stress, the steady-state localization of chitin synthase III (Chs3p) shifts from internal stores (chitosomes) to the plasma membrane (PM). This redistribution occurs rapidly and requires the activators of the cell wall stress response signaling pathway, the G protein Rho1p, and the protein kinase Pkc1p, but not the cell integrity response mitogen-activated protein kinase cascade. Furthermore, expression of activated forms of Rho1p or Pkc1p, in the absence of cell stress, is sufficient to redistribute Chs3p to the PM. In cells deficient for both the clathrin adaptor complex 1 and Chs6p, where Chs3p is transported to the PM by an alternative bypass pathway, cell wall stress did not cause mobilization of Chs3p, suggesting that Rho1p/Pkc1p regulate Chs3p exit from the trans-Golgi network. The mobilization of an intracellular reservoir of Chs3p presents a novel opportunity to investigate the genetic basis of regulated vesicular traffic.
All cells react to environmental stresses through signal transduction pathways that ultimately activate gene transcription programs required to cope with cellular damage. In the yeast Saccharomyces cerevisiae, a range of insults, including heat stress, hypoosmotic shock, and physical damage, induce the activation of the yeast isozyme of the protein kinase C Pkc1p, which is essential for the maintenance of cell wall integrity (1). Cell wall stress is initially perceived through the actions of cell surface membrane proteins (Wsc1-4p and Mid2p) (2-4). Wsc1p and Mid2p interact directly with the guanine nucleotide exchange factor Rom2p (5) and facilitate the exchange of GDP for GTP in the G-protein Rho1p (6). Rho1p-GTP is recruited to membranes, activates Pkc1p (7), and initiates a mitogen-activated protein kinase (MAPK) cascade that regulates a variety of cellular responses. The Rho1p/Pkc1p-controled MAPK cascade comprises the MAP/Erk kinase (MEK) kinase Bck1, the MEKs Mkk1p and Mkk2p and the MAPK Slt2p/Mpk1p (1). Ultimately, Slt2p/Mpk1p regulates the activity of transcription factors and chromatin-binding proteins that modulate the transcription of many genes involved in cell wall biosynthesis (8).
Among the cellular responses that occur on cell wall stress is an increase in the levels of β-linked glucans and chitin. This increase in cell wall polysaccharide is the result of both an increase in the Slt2p-dependent expression of genes encoding β1-3 glucan synthases (Fks1p and Fks2p) and, to a lesser extent, of chitin synthase III (Chs3p) (8-10). However, there is increasing evidence that the posttranscriptional control of cell wall repair also plays a significant role in maintaining cell integrity. For example, Rho1p-GTP is an allosteric activator of Fks1p (11), and activation of Pkc1p during cell wall stress leads to a depolarized distribution of Fks1p from sites of active growth, presumably to repair cell wall damage (12). Similarly, the activity and localization of Chs3p is posttranslationally regulated. Chs3p requires accessory factors for exit from the endoplasmic reticulum (ER) (Chs7p) (13), the trans-Golgi (TGN) network (Chs5p and Chs6p) (14, 15), and for enzymatic activity (Chs4p) (16). Furthermore, yeast cells regulate the content of cellular chitin. The yeast cell wall is composed of ≈2% chitin, but in mutants defective in cell wall assembly, chitin levels increase to >20% of total cell wall polymers even though Chs3p levels do not vary significantly (17-19). At steady state, a significant proportion (≈50-70%) of Chs3p is maintained in a internal reservoir in the TGN/early endosome (14, 20). We investigated whether Chs3p might also be regulated posttranslationally through its cellular localization and provide evidence that the internal pools of Chs3p represent a storage compartment that permits the rapid mobilization of this cell wall repair enzyme during cell damage. We show that activation of Rho1p and Pkc1p, but not the downstream targets of the Bck1p-dependent MAPK cascade, is necessary and sufficient to regulate the exit of Chs3p from internal compartments during cell wall stress.
Materials and Methods
Yeast Strains, Growth Conditions, and Plasmids. Yeast cells (Table 1) were grown in YPD broth (1% yeast extract/2% peptone/2% glucose) or in complete synthetic medium (CSM) dropout mixes (Q-biogene, Carlsbad, CA). For galactose expression plasmids, yeast strains were grown in CSM supplemented with 2% raffinose and induction was initiated by addition of galactose to 0.5%. Sensitivity to hypoosmotic media was assessed by growth on half-strength YPD agar (1%) plates at 37°C for 3-5 days. Heat stress was induced by growing yeast cells at 24°C and harvesting the cells by centrifugation followed by resuspension in media prewarmed to either 38°C (heat stress) or 24°C (control). The PMET25::CHS3 strain was constructed by integration into YPH499 of AgeI-digested pRS306 bearing the MET25 promoter fused to the 5′ end of CHS3. For imaging of Chs3-GFP during heat stress, we transformed YPH499 cells with pCHS3-GFP and pRS315-CHS7 (21), grew the transformants in CSM supplemented with 20 μg/ml adenine to OD600 0.3-0.6, and subjected to heat stress as described above.
Table 1. Strains and plasmids used in this study.
| Strain/plasmid | Genotype/description | Source or ref. |
|---|---|---|
| Strain | ||
| YPH499 | MATa ade2-101oc his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | Laboratory collection |
| YRV19 | MATa chs6Δ::HIS3 ade2-101oc his3-Δ200 leu2-Δ1 trp1 ura3-52 | 21 |
| YRV67 | MATa chs6Δ::HIS3 apl2A::TRP1 ade2-101oc his3-Δ200 leu2-Δ1 trp1 ura3-52 | 21 |
| YRV94 | YPH499 apm1Δ::URA3 | 21 |
| JCY485 | MATa chs3Δ::HIS3 ade2-101oc his3-Δ200 leu2-Δ1 lys2-801am trp1 ura3-52 | 20 |
| JK9-3da | MATa leu2-3.112 ura3-52 trp1 his4 rme1 HMLa | 12 |
| PA39-1b | JK9-3da wsc1Δ::KANMX | 12 |
| PA109-1c | JK9-3da bck1Δ::HIS3 | 12 |
| AS138-1b | JK9-3da rom2Δ::URA3 | 12 |
| NY1538 | MATa rho1-104 | W. Guo (University of Pennsylvania, Philadelphia) |
| JTY2792 | MATa pkc1Δ::LEU2 (YCp50 pkc1-2) | J. Thorner (University of California, Berkeley) |
| YRV152 | YPH499 chs3::pMET25::CHS3::URA3 | This study |
| Plasmid | ||
| pCHS3-GFP | pRS313 (CEN HIS3) with enhanced EGFP fused to the COOH terminus of Chs3p | 21 |
| pRS315-CHS7 | pRS315 (CEN LEU2) with CHS7 expressed from native promoter | 21 |
| pGAL-RHO1* | Expresses activated form of Rho1p (H68) | 12 |
| pGAL-PKC1* | Expresses activated form of Pkc1p | 12 |
| pGAL-BCK1* | Expresses BCK1-20 allele (constitutively active) | 12 |
Subcellular Fractionations and Sucrose Gradients. The analysis of organelles by differential centrifugation was performed as described (20). In brief, 5-10 OD600 of midlog cells were harvested by centrifugation and washed with ice-cold 20 mM NaN3/20 mM KF. Cells were digested with lyticase and the resulting spheroplasts coated with 1 mg/ml Con A and lysed by osmotic shock (lysis buffer: 10% sucrose in 20 mM triethanolamine, pH 7.2/1 mM EDTA/1 mM phenylmethylsulfonyl fluoride). Organelles were separated by centrifugation at 13,000 × g for 6 min. For the analysis of total membranes on density gradients, yeast cells (10-15 OD600) grown at 24°C or heat stressed at 38°C were placed on ice and washed with ice-cold 20 mM NaN3/20 mM KF. The cells were centrifuged, resuspended in 0.35 ml of lysis buffer supplemented with a 10× concentrate of protease inhibitor mixture (Boehringer Mannheim, Germany), and lysed by agitation with glass beads. Unlysed cells were removed by centrifugation (500 × g for 2 min). Total cell lysates (0.2 ml) were overlaid on a step sucrose/EDTA gradient [0.2 ml 55%/0.5 ml 45%/0.4 ml 30% sucrose (w/w) in 20 mM triethanolamine, pH 7.2/5 mM EDTA] and centrifuged at 55,000 rpm in a TLS55 rotor (Beckman) for 2.5 h. Fractions (0.2 ml) were collected manually from the top, solubilized in 1% SDS at 55°C for 10 min, and analyzed by SDS/PAGE and quantitative immunoblotting with polyclonal antibodies to Chs3p, Gas1p, Sec12p, Gda1p, Tlg1p (H. Pelham, Medical Research Council, London), or Pep12p (S. Emr, University of California at San Diego, La Jolla) and mouse mAbs to mammalian p44/42 (Cell Signal Technology, Beverly, MA) to detect phospho-Mpk1. Horseradish peroxidaseconjugated secondary antibodies and a Supersignal Femto-detection kit (Pierce) were used for immune detection of phospho Mpk1. For all other immunoblots, we used 35S-labeled anti-rabbit secondary antibodies (Amersham Pharmacia Biotech), as described in ref. 20.
Phosphoprotein Analysis. Cells were grown in YPD-low phosphate (LP) media (Q Biogene) to late log phase. The cells were harvested by centrifugation, and 5 OD600 units of cells were resuspended in 1 ml of fresh YPD-LP media at either 24°C or 38°C. Cells were pulse labeled with 25 μCi of 33PO4 (Amersham Pharmacia Biotech) for 1 h. Labeling was terminated with 20 mM NaN3/100 mM KF. Cells were harvested by centrifugation and lysed by agitation with glass beads in 0.3 ml of lysis buffer supplemented with phosphatase inhibitors (Sigma-Aldrich). Lysed cells (0.2 ml) were solubilized in 1% SDS at 55°C for 5 min and diluted to 1 ml in immunoprecipitation buffer (0.1% SDS/150 mM NaCl/50 mM Tris·HCl, pH 7.2/1% Triton X-100). The samples were split and proteins immunoprecipitated with antibodies against Chs3p and Pma1p (F. Portillo, Instituto de Investigaciones Biomedicas, Madrid) and protein A-Sepharose (Amersham Pharmacia Biotech).
Results and Discussion
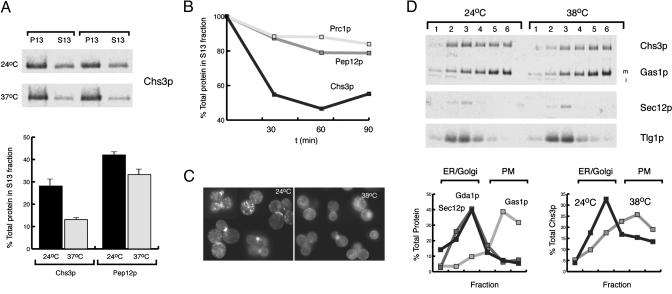
Heat Stress Alters the Subcellular Localization of Chs3p. Genetic studies in yeast have relied on temperature-sensitive alleles to uncover the role of essential genes in the secretory and endosomal pathways. However, sudden increases in temperature also have profound effects on the cells' internal architecture. During our studies of Chs3p traffic through the secretory pathway, we observed that the subcellular distribution of Chs3p was altered when yeast cells grown at 24°C were shifted to 37°C. To test the generality of this observation, we grew cells from diverse genetic backgrounds (YPH499, W303, JK9-3da) at 24°C in YPD and shifted the cells to 38°C for 1 h. The cells were converted to spheroplasts, lysed by osmotic shock, and total membranes were separated by differential centrifugation (13,000 × g for 6 min) to obtain a PM/ER fraction (P13) and a Golgi/endosome-rich fraction (S13). At both 24 and 38°C, Chs3p populates both fractions, but heat stress shifted the distribution of Chs3p from the S13 to the P13 fraction within 30 min, whereas the steady-state distribution of other TGN and endosomal markers was unaffected (Fig. 1 A and B). Because the P13 fraction consists of PM, ER, and some late Golgi membranes, we wished to further define the compartment in which Chs3p accumulated during heat stress. Wild-type cells were grown at 24°C, shifted to 38°C for 45 min, and lysed by mechanical disruption with glass beads. Total membranes were sedimented on a step sucrose/EDTA density gradient. At 24°C, ≈70% of Chs3p sedimented in fractions 1-3, which cofractionated with the ER marker Sec12p, the early endosomal marker Tlg1p, and the Golgi marker Gda1p, whereas the remaining ≈30% of Chs3p cofractionated with the PM marker Gas1p (Fig. 1C, fractions 4-6). After a shift to 38°C, Chs3p was redistributed to fractions 4-6 (≈65-75%), although the subcellular distribution of Sec12p, Gda1p, and Tlg1p was unaffected (Fig. 1C). These results indicate that during conditions of cell stress, Chs3p mainly cofractionates with the PM.
Fig. 1.
Chs3p is redistributed to the PM from internal compartments during heat stress. (A) Chs3p fractionation by differential centrifugation of membranes from cells incubated at 24 and 37°C (duplicate samples). Total membranes were separated by differential centrifugation (13,000×g for 6 min) into a PM/ER fraction (P13) and a Golgi/endosome-rich fraction (S13). (B) Kinetics of Chs3p depletion from S13 fractions during heat stress. The relative proportion of Chs3p, Pep12p, and carboxypeptidase Y (Prc1p) in the S13 fraction of YPH499 was determined after cells had been shifted to 38°C for 0, 30, 60, and 90 min. (C) Localization of Chs3-GFP during heat stress. YPH499 cells expressing pCHS3-GFP and pRS315-CHS7 were grown at 24°C and heat shocked as in A.(D) Subcellular fractionation of Chs3p on step sucrose/EDTA gradients. Cells were heat shocked as in A and lysed by agitation with glass beads, and the total membranes were separated on a step sucrose/EDTA gradient. The protein composition of fractions obtained from differential centrifugations and sucrose gradients were analyzed by SDS/PAGE and quantitative immunoblotting. {PM marker: Gas1p [m, mature form; i, immature form (ER)]; Golgi markers: Gda1p and Tlg1p; endosomal marker: Pep12p; ER marker: Sec12p}. Graphs show representative experiments (B and D) or the average of experiments performed in triplicate (A).
To independently confirm that Chs3p was redistributed to the PM after heat shock, we expressed CHS3-GFP (21) in wild-type cells and followed the localization of Chs3-GFP before and after a shift to 38°C (Fig. 1D). In agreement with the differential centrifugation and sucrose fractionation experiments, we observed a partial redistribution of Chs3-GFP from internal compartments to the cell surface. Interestingly, during heat stress, Chs3-GFP at the PM was no longer preferentially associated with the bud-neck region of budding cells but showed a depolarized localization. Similar observations have been made in fks1Δ cells, where Chs3p and Chs4p become delocalized throughout the PM and no longer associate with the septin ring (17, 22).
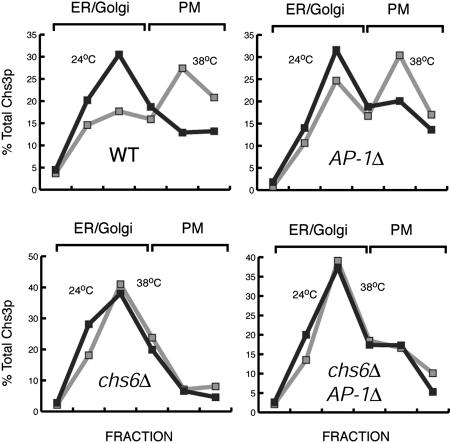
The Regulation of Chs3p Transport to the PM During Heat Stress Occurs at the Level of Chs3p Exit from the TGN. The shift in the localization of Chs3p to the PM at high temperatures could be the result of enhanced anterograde transport from the TGN/endosomal system or, given that heat stress leads to a transient repolarization of the actin cytoskeleton (12), the result of a partial inhibition of endocytosis. To address these possibilities, we examined the heat stress-induced relocalization of Chs3p in chs6Δ clathrin adaptor protein complex-1 (AP-1)Δ cells. In chs6 mutants, Chs3p fails to be incorporated into PM-bound transport vesicles from the TGN and accumulates in the TGN/endosomal system (15, 21). Mutations in the AP-1 (Apl2p, Apl4p, Aps1p, and Apm1) (23) disrupt the transport cycle required to retain Chs3p in this intracellular pool (21). As a result, Chs3p is able to bypass the Chs6p-dependent block and ultimately reaches the PM by an alternative route, possibly from an early endosome. However, chs6Δ AP-1Δ mutants maintain intracellular stores of Chs3p presumably by a backup recycling pathway from late endosomes (21). If the redistribution of Chs3p during heat stress was because of the inhibition of endocytosis, we would expect Chs3p to redistribute from internal stores to the PM in chs6Δ AP-1Δ mutants. As shown in Fig. 2, heat stress failed to mobilize Chs3p to the PM in chs6Δ and did not induce additional PM localization in chs6Δ AP-1Δ cells. Conversely, AP-1Δ mutants showed normal redistribution of Chs3p to the PM during heat stress (Fig. 2), suggesting that this response is not acting through AP-1. Similarly, chs5Δ AP-1Δ mutants, which also bypass the TGN to PM transport of Chs3p (21), did not accumulate additional Chs3p at the PM during heat stress (data not shown). Therefore, it appears that Chs3p exit from the TGN is the target of this regulated transport step in a process that requires Chs5p and Chs6p, but not AP-1.
Fig. 2.
Heat stress regulates Chs3p exit from the TGN. YPH499 (WT), YRV19 (chs6Δ), YRV94 (AP-1Δ), and YRV67 (chs6Δ AP-1Δ) cells were grown at 24°C, harvested by centrifugation, and resuspended in YPD at 24 or 38°C for 1 h. Cells were processed as in Fig. 1C.In chs6Δ AP-1Δ, Chs3p is transported to the PM from an endosomal compartment, rather than the TGN, and fails to accumulate at the PM during heat stress.
We surveyed other stresses to determine their impact on the steady-state distribution of Chs3p. Treatment with 0.01% SDS or 25 μg/ml chitin-binding dye calcofluor white also affected the localization of Chs3p, leading to a 30-40% increase in the levels of Chs3p at the PM (data not shown). These results suggest that the regulated accumulation of Chs3p at the PM constitutes a general response to cell wall damage.
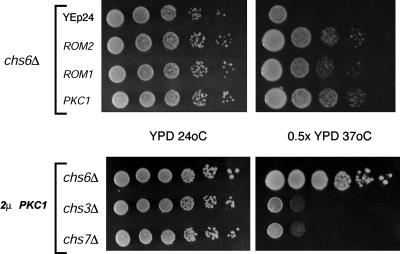
Overexpression of Regulators of the Cell Wall Integrity Signal Transduction Pathway Suppresses the Temperature-Sensitive Growth Phenotype of chs6Δ Cells. Cells deficient in Chs3p-dependent chitin synthesis lyse under hypoosmotic conditions at elevated temperatures (24). To understand the mechanism of Chs3p exit from the TGN, we performed a multicopy suppressor screen to identify genes that rescued the hypoosmotic growth deficiency of chs6Δ cells. chs6Δ cells were transformed with a YEp24-based yeast genomic library (25), and replica plated onto 0.5× YPD agar plates at 37°C. Plasmids encoding ROM1, ROM2, and PKC1 were isolated from colonies that grew under these conditions (Fig. 3). Rom1p and -2p are guanine nucleotide exchange factors for Rho1p (6), which in its GTP-bound state binds to and activates the protein kinase Pkc1p (7), leading to the transcription of genes involved in cell wall biosynthesis (Fig. 3B) (8). We considered the possibility that the suppression of the temperature sensitivity of chitin-deficient cells was the result of a compensatory increase in the synthesis of other cell wall components. If so, suppression would not depend on CHS3. However, unlike chs6Δ cells, neither chs3Δ nor chs7Δ mutants transformed with a 2μPKC1 plasmid grew in 0.5× YPD at 37°C (Fig. 3). Similar results were obtained with ROM1- and ROM2- containing plasmids (data not shown). Thus, suppression by activators of the cell wall integrity pathway requires the presence of Chs3p in post-ER compartments. Overexpression of PKC1 in chs6Δ cells restored ≈10% of wild-type levels of Chs3p transport to the PM (data not shown). This increase in Chs3p transport is sufficient to restore hypoosmotic resistance but not normal chitin levels, as assessed by sensitivity to calcofluor (data not shown), suggesting that Chs6p function cannot be fully substituted by overexpression of components of the cell wall integrity pathway.
Fig. 3.
Overexpression of activators of the cell wall integrity MAPK pathway suppresses the osmosensitivity of chs6Δ mutants in a Chs3p-dependent manner. 2μ plasmids containing ROM1, ROM2, and PKC1 restore growth to YRV19 (chs6Δ) cells at high temperatures under hypoosmotic conditions (0.5× YPD at 37°C). A 2μ PKC1 plasmid failed to suppress the hypoosmotic sensitivity of JCY485 (chs3Δ) and YRV4 (chs7Δ). (Upper) Two days of growth. (Lower) Three days of growth.
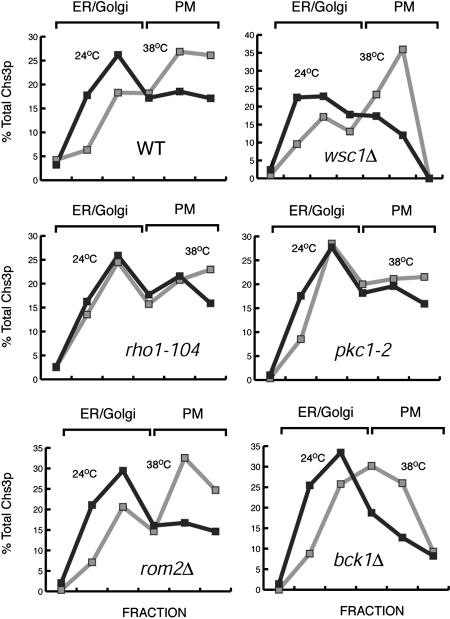
Rho1p and Pkc1p Are Necessary for the Heat Stress-Dependent Accumulation of Chs3p at the PM. Heat stress is a potent activator of Rho1p/Pkc1p and of the Bck1p/Mkk/Mpk kinase cascade (9). Therefore, we wanted to determine whether the heat stress-dependent redistribution of Chs3p to the PM required signal transduction through this pathway. We monitored the localization of Chs3p in yeast cells bearing either deletions or temperature-sensitive (ts) alleles of genes encoding components of the cell wall integrity pathway. Cells were grown at 24°C, shifted to 38°C, and total membranes were separated by centrifugation on step sucrose gradients. Cells bearing the ts allele rho1-104 or pkc1-2 did not display the stress-induced redistribution of Chs3p to the PM (Fig. 4). In contrast, cells bearing mutations in WSC1, ROM2, or BCK1 redistributed Chs3p to the PM during heat stress. Therefore, the heat stress-dependent redistribution of Chs3p required a functional Rho1p and Pkc1p but not the activators of these proteins or the activation of MAPK downstream effectors.
Fig. 4.
Rho1p and Pkc1p are required for the heat stress-dependent transport of Chs3p to the PM. JK9-3da (WT), PA39-1b (wsc1Δ), AS138-1b (rom2Δ), NY1538 (rho1-104), JTY2792 (pkc1-2), and PA109-1c (bck1Δ) cells were grown at 24°C, harvested by centrifugation, and resuspended in YPD at 24 or 38°C for 1 h. Total membranes were separated on step sucrose/EDTA gradients and the Chs3p levels determined as in Fig. 1C.
Rho1p and Pkc1p serve multiple functions (e.g., establishment of a polarized exocyst, morphogenesis checkpoint controls, actin cytoskeleton organization, and activation of Fks1p) that are independent of the activation of the Mpk1 MAPK cascade and contribute to the ability of the cell to withstand cell wall damage (11, 12, 26). Thus, rho1 and pkc1 mutants display more severe growth defects than mutants in the Mpk1-MAPK cascade (1). The role of upstream effectors is less clear, because both Wsc1p and Rom2p have homologues with redundant and overlapping functions (4, 6).
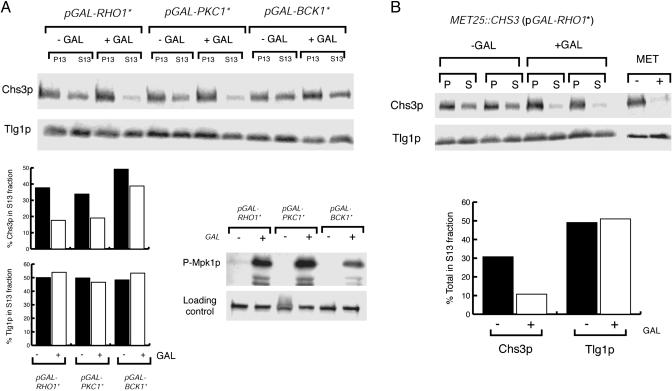
Expression of Constitutive Active Forms of Pkc1p and Rho1p but Not Bck1p Redistributes Chs3p from Internal Stores to the PM. Next, we asked whether activation of Rho1p and Pkc1p was sufficient to induce the mobilization of Chs3p. The localization of Chs3p in cells bearing constitutively active alleles of RHO1, PKC1, and BCK1 (12) expressed under the control of the GAL1 promoter was assessed by differential centrifugation of lysed spheroplasts. As a control for the galactose-dependent activation of the Mpk1p MAPK cascade, we monitored the accumulation of phosphorylated Mpk1p with a mammalian antiphospho p44/p42 MAPK antibody (Fig. 5A). Expression of PKC1* and RHO1* resulted in a 40-50% redistribution of Chs3p from the S13 fraction to the P13 fraction, whereas the distribution of the TGN/endosomal marker Tlg1p was unaffected (Fig. 5A). Fractionation of total membranes on step sucrose gradients confirmed the accumulation of Chs3p at the PM (data not shown). In contrast, expression of BCK1* did not significantly deplete the internal pools of Chs3p (Fig. 5A). Therefore, the redistribution of Chs3p does not require Mpk1p-dependent gene transcription and suggests that the transport of Chs3p is regulated posttranslationally. To test this hypothesis, we placed CHS3 under the control of the MET25 promoter and repressed the expression of CHS3 with 10 mM methionine before induction of GAL-RHO1* (Fig. 5B). Cells were then converted to spheroplasts, lysed, and total membranes separated by differential centrifugation. After the addition of galactose, existing Chs3p was depleted from the S13 fraction (45-50%), whereas fractionation of Tlg1p was unaffected. Also, as we had observed with heat stress, expression of PKC1* did not cause a significant (<10%) redistribution of Chs3p from the S13 to the P13 fraction in chs6 and chs6 apl2 mutants (data not shown). These results suggest that the activation of Rho1p and Pkc1p, but not the downstream effectors of the cell wall integrity MAPK cascade, is sufficient to redistribute preformed Chs3p from the TGN (Chs6p-dependent pathway) to the PM.
Fig. 5.
The expression of constitutively active forms of Rho1p and Pkc1p, but not Bck1p, redistributes Chs3p to the PM. (A) YPH499 cells were transformed with plasmids expressing activated forms of Rho1p, Pkc1p, and Bck1p under the control of the GAL1 promoter. The subcellular distribution of Chs3p and Tlg1p before and after the addition of 0.5% galactose (2 h) was determined by differential centrifugation and quantified as in Fig. 1 A. The activation of the Mpk1 MAPK was monitored with antiphospho-Mpk1p (p44/42). (B) Expression of RHO1* leads to the redistribution of preformed Chs3p. YRV204 (MET25::CHS3 pGAL-RHO1*) was grown in methionine-free medium and new Chs3p synthesis blocked by addition of 10 mM methionine for 30 min. Rho1p* was induced with 0.5% galactose for 2 h, and the distribution of Chs3p was determined by differential centrifugation (duplicate samples). (Right) Methionine-dependent expression of CHS3.
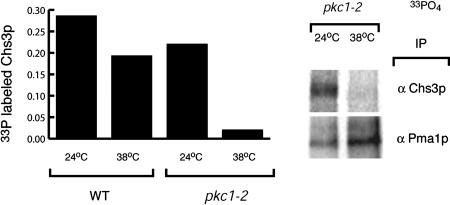
Pkc1p Is Required for the Phosphorylation of Chs3p. Previous observations from our laboratory indicated that Chs3p is a phosphoprotein (J. Chuang and R.S., unpublished results). We asked whether phosphorylation of Chs3p depended on Pkc1p. Wild-type and pkc1-2 cells were grown in low phosphate media and labeled with 33PO4 for 1 h at either 24 or 38°C. The cells were lysed in the presence of protein phosphatase inhibitors and the membranes solubilized in 1% SDS. Chs3p and the PM H+-ATPase Pma1p, a known phosphoprotein (27), were immunoprecipitated. At the permissive temperatures, both Pma1p and Chs3p were labeled by 33PO4 in both wild-type and pkc1-2 cells. On a shift to 38°C, we observed a >95% decrease in the efficiency of 33PO4 incorporation into Chs3p in pkc1-2 cells, whereas Pma1p was efficiently radiolabeled. In contrast, wild-type cells exhibited only a 20-25% decrease in the 33PO4 incorporation into Chs3p at 38°C (Fig. 6). These results indicate that the phosphorylation of Chs3p requires a functional Pkc1p. However, the levels of phospho-Chs3p did not correlate with its enhanced transport to the PM. The cells in these experiments were labeled with 33PO4 for 1 h to obtain a signal strong enough for reliable quantification. Thus, if Chs3p phosphorylation is regulated by heat stress, the regulation is not apparent at steady state, which does not rule out the possibility that phosphorylation regulates Chs3p transport, because phosphorylation maybe transient. For example, it is possible that Chs3p may be phosphorylated during exit from the TGN or ER and then dephosphorylated at the PM. These results suggest that Pkc1p-dependent phosphorylation of Chs3p can occur at the TGN/endosomal system, although it is not clear whether these modifications are sufficient to drive Chs3p transport to the PM.
Fig. 6.
Chs3p is a Pkc1p-dependent phosphoprotein. YPH499 and JTY2792 (pkc1-2) cells were grown in low-phosphate media and labeled with 33PO4 for 1 h at either 24 or 38°C. Chs3p and Pma1p were immunoprecipitated from SDS-solubilized lysates in the presence of phosphatase inhibitors and 33PO4 incorporation determined. The incorporation of 33PO4 into Chs3p shown was normalized to Pma1p labeling.
Our observations on the transport of Chs3p during cell stress reinforce the notion that signal transduction events can act to control multiple cellular events independently of gene transcription. Rho1p and Pkc1p process signals through a variety of integral membrane proteins, guanine nucleotide exchange factors, GTPase activating proteins, and lipid signaling molecules to regulate various cellular functions, including cell division (2, 28), the cytoskeleton (12), and, as shown in the present work, protein secretion. The translocation of Chs3p to the PM in response to signal transduction in yeast is analogous to what has been observed in mammalian cells for the translocation of the insulin-dependent glucose transporter GLUT4 to the PM. GLUT4 is normally retained at the TGN/endosomal system of adipocytes and muscle cells (29). On engagement of the insulin receptor, the mammalian Pkc1p homologue, PKCζ, is activated by insulin-dependent PI3 kinases and recruited to intracellular membranes (30). The overexpression of constitutively active forms of PKC mimic the effect of insulin on the transport of GLUT4 (31), indicating that downstream targets of PKC are sufficient to modulate the transport of proteins to the PM.
It is becoming increasingly apparent that yeast cells use posttranslational modification of cargo proteins in response to environmental cues to control their transit through the secretory pathway. Whether it is the conjugation of ubiquitin to the amino acid permease Gap1p to divert this protein to the vacuole during growth in nitrogen-rich sources (32) or the cell wall stress-dependent transport of Chs3p, yeast provides a powerful system to investigate how eukaryotic cells translate extracellular signals into differential vesicular transport.
Acknowledgments
We thank W. Guo, J. Thorner, P. Delley, M. Hall (University of Basel, Basel), and J. Chuang for providing strains and plasmids. We also thank E. Miller and M. Lee in our laboratory for comments on the manuscript. Work in the laboratory is supported by National Institutes of Health Grant GM26755 and funds from the Howard Hughes Medical Institute. R.H.V. was supported by Postdoctoral Fellowship DRG-1506 from the Damon Runyon Cancer Research Fund.
Abbreviations: ER, endoplasmic reticulum; MAPK, mitogen-activated protein kinase; PM, plasma membrane; TGN, trans-Golgi network; AP-1, clathrin adaptor protein complex 1; Chs3p, chitin synthase III.
References
- 1.Heinisch, J. J., Lorberg, A., Schmitz, H. P. & Jacoby, J. J. (1999) Mol. Microbiol. 32, 671-680. [DOI] [PubMed] [Google Scholar]
- 2.Gray, J. V., Ogas, J. P., Kamada, Y., Stone, M., Levin, D. E. & Herskowitz, I. (1997) EMBO J. 16, 4924-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajavel, M., Philip, B., Buehrer, B. M., Errede, B. & Levin, D. E. (1999) Mol. Cell. Biol. 19, 3969-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verna, J., Lodder, A., Lee, K., Vagts, A. & Ballester, R. (1997) Proc. Natl. Acad. Sci. USA 94, 13804-13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philip, B. & Levin, D. E. (2001) Mol. Cell. Biol. 21, 271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bickle, M., Delley, P. A., Schmidt, A. & Hall, M. N. (1998) EMBO J. 17, 2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamada, Y., Qadota, H., Python, C. P., Anraku, Y., Ohya, Y. & Levin, D. E. (1996) J. Biol. Chem. 271, 9193-9196. [DOI] [PubMed] [Google Scholar]
- 8.Jung, U. S. & Levin, D. E. (1999) Mol. Microbiol. 34, 1049-1057. [DOI] [PubMed] [Google Scholar]
- 9.Kamada, Y., Jung, U. S., Piotrowski, J. & Levin, D. E. (1995) Genes Dev. 9, 1559-1571. [DOI] [PubMed] [Google Scholar]
- 10.Zhao, C., Jung, U. S., Garrett-Engele, P., Roe, T., Cyert, M. S. & Levin, D. E. (1998) Mol. Cell. Biol. 18, 1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qadota, H., Python, C. P., Inoue, S. B., Arisawa, M., Anraku, Y., Zheng, Y., Watanabe, T., Levin, D. E. & Ohya, Y. (1996) Science 272, 279-281. [DOI] [PubMed] [Google Scholar]
- 12.Delley, P. A. & Hall, M. N. (1999) J. Cell Biol. 147, 163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trilla, J. A., Duran, A. & Roncero, C. (1999) J. Cell Biol. 145, 1153-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos, B. & Snyder, M. (1997) J. Cell Biol. 136, 95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziman, M., Chuang, J. S., Tsung, M., Hamamoto, S. & Schekman, R. (1998) Mol. Biol. Cell 9, 1565-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trilla, J. A., Cos, T., Duran, A. & Roncero, C. (1997) Yeast 13, 795-807. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Rodriguez, L. J., Trilla, J. A., Castro, C., Valdivieso, M. H., Duran, A. & Roncero, C. (2000) FEBS Lett. 478, 84-88. [DOI] [PubMed] [Google Scholar]
- 18.Popolo, L., Gilardelli, D., Bonfante, P. & Vai, M. (1997) J. Bacteriol. 179, 463-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valdivieso, M. H., Ferrario, L., Vai, M., Duran, A. & Popolo, L. (2000) J. Bacteriol. 182, 4752-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuang, J. S. & Schekman, R. W. (1996) J. Cell Biol. 135, 597-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valdivia, R. H., Baggott, D., Chuang, J. S. & Schekman, R. W. (2002) Dev. Cell 2, 283-294. [DOI] [PubMed] [Google Scholar]
- 22.DeMarini, D. J., Adams, A. E., Fares, H., De Virgilio, C., Valle, G., Chuang, J. S. & Pringle, J. R. (1997) J. Cell Biol. 139, 75-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson, M. S. & Bonifacino, J. S. (2001) Curr. Opin. Cell Biol. 13, 444-453. [DOI] [PubMed] [Google Scholar]
- 24.Bulawa, C. E. (1992) Mol. Cell. Biol. 12, 1764-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson, M. & Botstein, D. (1982) Cell 28, 145-154. [DOI] [PubMed] [Google Scholar]
- 26.Harrison, J. C., Bardes, E. S., Ohya, Y. & Lew, D. J. (2001) Nat. Cell Biol. 3, 417-420. [DOI] [PubMed] [Google Scholar]
- 27.Portillo, F. & Mazon, M. J. (1985) FEBS Lett. 192, 95-98. [DOI] [PubMed] [Google Scholar]
- 28.Drgonova, J., Drgon, T., Roh, D. H. & Cabib, E. (1999) J. Cell Biol. 146, 373-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryant, N. J., Govers, R. & James, D. E. (2002) Nat. Rev. Mol. Cell Biol. 3, 267-277. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez, P., De Carcer, G., Sandoval, I. V., Moscat, J. & Diaz-Meco, M. T. (1998) Mol. Cell. Biol. 18, 3069-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braiman, L., Alt, A., Kuroki, T., Ohba, M., Bak, A., Tennenbaum, T. & Sampson, S. R. (2001) Mol. Cell. Biol. 21, 7852-7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helliwell, S. B., Losko, S. & Kaiser, C. A. (2001) J. Cell Biol. 153, 649-662. [DOI] [PMC free article] [PubMed] [Google Scholar]