Abstract
Cotton rats (Sigmodon hispidus) are susceptible to the recently discovered human metapneumovirus (hMPV), an agent closely related to human respiratory syncytial virus. Since certain respiratory syncytial virus vaccines can induce enhanced disease upon viral challenge, we have done similar experiments with hMPV in cotton rats. Young adult cotton rats were vaccinated with a formalin-inactivated preparation of hMPV strain C-85473, or with a mock preparation of the vaccine on day 0 and again on day 28. All animals were challenged intranasally on day 49 with 107 TCID50 of the same hMPV strain. Animals were sacrificed on days 4, 7, and 10 post-challenge and lungs were removed for viral quantitation, histopathology, and cytokine mRNA expression analysis (interferon-gamma (IFN-γ) and interleukin-4 (IL-4)). Although the vaccinated animals showed almost complete protection from viral replication in the lungs (<102.0 TCID50 per gram), there was a dramatic increase in the lung pathology, particularly the interstitial pneumonitis and alveolitis with elevated serum neutralizing antibody titer prior to challenge. Cytokine profiles were distinctive from those observed during primary infection and re-infection. The data raise safety concerns for hMPV vaccine preparations.
Keywords: Human metapneumovirus, Cotton rat, Formalin vaccine enhancement
1. Introduction
Human metapneumovirus (hMPV), is a paramyxovirus, related by sequence to human respiratory syncytial virus (hRSV), and was first described in 2001 [25]. Like RSV, hMPV infects humans of all ages but causes more severe disease in infants, the elderly and the immunocompromised [3,6,9,17,25,26]. Like hRSV, hMPV can re-infect throughout life [29], although essentially all children have had a first infection by age 5. Its ability to co-infect with other respiratory infectious agents, including RSV and pneumococci [14,27], combined with its relatively slow replication in cell culture likely accounted for its ability to escape isolation until 2001. Various animal models have been utilized to study hMPV infection including mice, hamsters, and several species of primates [10,12,13,23]. Recent studies indicate that infection of cotton rats with hMPV is associated with efficient viral replication in the lung and reproducible pulmonary pathology [10,28,30]. The cotton rat has also been used extensively to study hRSV especially in the area of enhanced pulmonary disease caused by some vaccine candidates for hRSV [19-22].
The use of an early formalin-inactivated hRSV vaccine (FI-hMPV) together with natural environmental challenge with the virus resulted in severe pulmonary disease, hospitalization and two deaths among the immunized children [11]. We have described the lung tissues from the fatal cases in detail [20]. Evaluation of formalin-inactivated hRSV vaccine has been critical in studying the phenomenon of enhancement. In the present study, we have immunized cotton rats with formalin-inactivated hMPV vaccine, and subsequently challenged the animals with hMPV. We compared these animals to those immunized with mock vaccine, or those with a secondary infection with hMPV. We present data on viral replication, enhanced pulmonary histopathology, neutralizing antibody titers, and expression levels of (interferon-gamma (IFN-γ) and interleukin-4 (IL-4). This study raises safety concerns that should be addressed during the evaluation of potential hMPV vaccine candidates.
2. Materials and methods
2.1. Cell line and virus
LLC-MK2 cells were maintained in minimal essential medium (Gibco/BRL, Bethesda, MD) supplemented with 10% fetal bovine serum. The hMPV strain C-85473 is a clinical isolate passed six times on LLC-MK2. Viral infection used minimal essential medium supplemented with 0.2% glucose, 0.1% bovine serum albumin, 0.0002% trypsin, and 0.1% gentamicin (hMPV infection medium). Viral titers were determined by 10-fold serial dilutions of virus in 24-wells plates containing LLC-MK2 cells. Before infection, cells were washed twice with PBS to remove residual serum proteins that could inhibit trypsin activity. Infected plates were incubated at 37 °C with 5% CO2 and replenished with 1 μl of fresh trypsin (0.0002%) every other day. Endpoints used cytopathic effect on the cell monolayers. Virus titers were reported as Log10 50% tissue culture infectious dose per milliliter of culture supernatant or per gram of lung (Log10 TCID50/ml or Log10 TCID50/g) [10]. TCID50 was calculated by the method of Reed and Muench. The vaccine was prepared from a stock of hMPV with a titer of 108 TCID50/ml by making the solution 1:4000 in formalin, stirring vigorously, and incubating 72 h at 37 °C with daily vigorous stirring. Precipitation with alum has been omitted from the vaccine formulation. The vaccine was then kept at 4 °C for at least 30 min before use. The vaccine contained no replicating virus in cell culture. Mock vaccine was prepared similarly but used uninfected cell culture as the starting material.
2.2. Cotton rat studies
Inbred Sigmodon hispidus cotton rats were obtained from a colony maintained at Virion Systems, Inc. (Rockville, MD). Young adult animals weighing approximately 100 g were used for the experiment. The animals were housed in large polycarbonate cages and were fed a standard diet of rodent chow and water ad libitum. The colony was monitored for antibodies to paramyxoviruses and rodent viruses and none were found. All studies were conducted under applicable laws and guidelines and after approval from the Virion Systems, Inc. Animal Care and Use Committee.
One hundred and five cotton rats were pre-bled and immunized intramuscularly at 0.1 ml/100 g with FI-hMPV (undiluted) or with FI-hMPV diluted 1:10 or 1:100 in PBS. Additional animals were mock immunized with a vaccine prepared similarly from mock-infected cells. Additional groups consists of animals infected intranasally with 107 TCID50 hMPV strain C-85473 in a volume of 0.1 ml/100 g, and two unvaccinated groups for primary infection and uninfected group. On day 28, all immunized animals were bled and with the exception of the infected group, all FI-hMPV or FI-mock groups were boosted with the same preparations. On day 49, all animals were bled and challenged intranasally with 0.1 ml/100 g of 107 TCID50 wild-type hMPV strain C-85473 except for one uninfected control group. Five animals from each experimental group were exanguinated and the lungs were removed for viral quantitation, lung cytokine analysis, and pulmonary histopathology on days 4, 7, and 10 post-challenge.
2.3. Pulmonary histopathology
Lungs were removed, inflated with formalin to normal volume, and immersed in 10% buffered formalin. Fixed lungs were trimmed, embedded in paraffin, sectioned at 5-μm, and stained with hematoxylin-eosin. The histopathologic score evaluated four categories of lesions. Peribronchiolitis was inflammatory cells, primarily lymphocytes, surrounding a bronchiole. Perivasculitis was inflammatory cells, primarily lymphocytes surrounding small blood vessels. Interstitial pneumonitis was increased thickness of alveolar walls, often associated with an infiltration of neutrophils. Alveolitis was inflammatory cells, primarily neutrophils and macrophages within the alveolar spaces. This scoring is identical to our scoring of hRSV infection in the lungs of infected cotton rats [22], except that changes in large bronchi were not tabulated. Each histopathologic change was scored on a scale of 0 (no change) to 4 (maximum inflammation). Since the scoring was non-linear, statistical analysis was not appropriate.
2.4. Lung cytokine analysis
Total RNA was extracted from homogenized lung tissue using the RNeasy purification kit (QIAGEN). One micrograms of total RNA was used to prepare cDNA using oligo dT primers and Super Script II RT (Invitrogen). Cotton rat cytokine cDNA were PCR-amplified using the primers and conditions previously described [1,2]. Amplified products were quantified using the Southern Blot procedure as previously described [2]. The signal obtained for each analyzed gene was normalized to the level of β-actin (“housekeeping gene”) expressed in the corresponding organ. Cytokine levels were expressed as the geometric mean ± the standard error (S.E.M.) for all animals in a group at a given time. Differences among groups were evaluated by the Student's t-test of summary data.
2.5. Neutralizing antibody assay
HMPV-neutralizing antibody titers were determined by an end-point dilution assay [23]. Briefly, after centrifugation of blood samples, sera were collected and incubated at 56 °C for 30 min to inactivate non-specific inhibitors. Two-fold dilutions of sera were performed in quadruplates in hMPV infection medium, and then mixed with an equal volume of infection medium containing 500 TCID50 of hMPV strain C-85473. The antibody–virus mixtures were incubated at 37 °C for 2 h and then transferred into 24-well plates containing LLC-MK2 cells. Following an incubation of 5 h, the mixtures were removed and 500 μl of fresh infection medium was added to each well. Plates were incubated at 37 °C and 3 days later another 500 μl of infection medium were added with fresh trypsin. Neutralization titers, defined as the highest dilution of antibody at which culture wells were negative for infection, were determined on day 5. HMPV-neutralizing antibody titers were reported as mean reciprocal Log2 titers ± the standard error (S.E.M.). Differences among groups were evaluated by the Student's t-test of summary data.
3. Results
3.1. General observations
All animals appeared clinically normal throughout the experiment.
3.2. Viral quantitation
Animals vaccinated with the mock preparation (FI-mock), along with unvaccinated group showed robust hMPV replication in the lungs on day 4 as shown in Fig. 1. By day 7 post-infection, these animals had cleared the virus from the lungs. Cotton rats vaccinated with various dilutions of the FI-hMPV, as well as re-infected cotton rats showed undetectable levels of viral replication (detection limit of 2.0 Log10 TCID50/g of tissue).
Fig. 1.
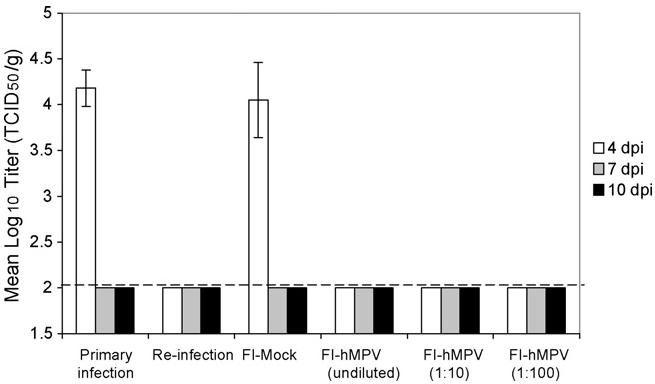
Human metapneumovirus titers in the lung of cotton rats challenged with 107 TCID50 homologous strain. Animals vaccinated with FI-hMPV (undiluted, 1:10, and 1:100) along with the previously intranasally infected animals were clear of any detectable virus. Only animals with a primary infection or those that received a mock vaccine had lung replication of the virus, and that only on day 4.
3.3. Cytokine levels
The expression of IFN-γ, a Th-1 type of cytokine, and IL-4, a Th-2 type of cytokine, were analyzed in the lungs of challenged animals. While IFN-γ was expressed by animals in all groups, elevated and prolonged expression of IL-4 was detected preferentially in hMPV-challenged animals vaccinated with FI-hMPV (Fig. 2). A modest decrease in the IFN-γ expression and an increase in IL-4 expression was noted in animals vaccinated with FI-hMPV when compared to animals vaccinated with mock or animals re-infected with hMPV.
Fig. 2.
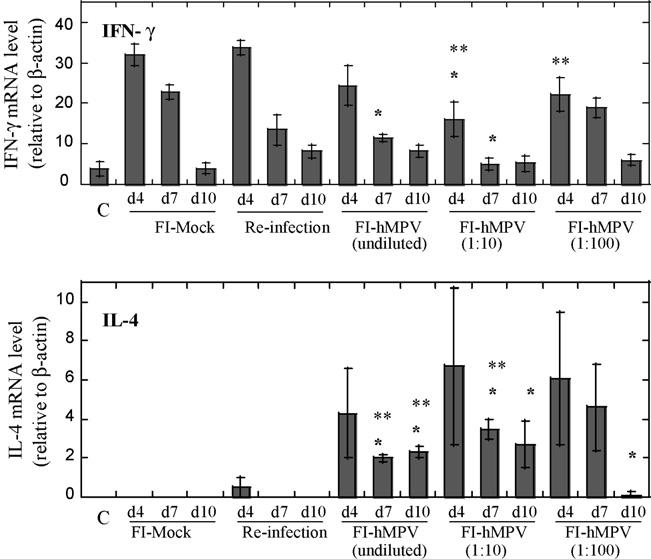
Pulmonary mRNA levels of cytokines IFN-γ and IL-4 after human metapneumovirus challenge with 107 TCID50 per animal. Animals were immunized on day 0 with FI-hMPV (undiluted, 1:10, and 1:100), live hMPV intranasal infection, or FI-mock as control. Uninfected control group is indicated as (C). All animals were re-immunized on day 28 except for the live hMPV infection group, and challenged with 107 TCID50 of homologous strain on day 49. Lung tissues were collected on days 4, 7, and 10 post-challenge. *P < 0.05; when FI-hMPV-vaccinated animals are compared to mock-vaccinated animals for each particular time point. **P < 0.05; when FI-hMPV-vaccinated animals are compared to re-infected animals for each particular time point.
3.4. Pulmonary histopathology
The lungs of cotton rats with a primary hMPV infection or immunized with FI-mock and then challenged with virus had a mild amount of peribronchiolitis and perivasculitis at day 4, which largely cleared by day 10. These animals had minimal to no interstitial pneumonitis and alveolitis (Fig. 3, top and bottom rows). Cotton rats infected with hMPV a second time had accentuated peribronchiolitis and perivasculitis, especially at day 4, and mild interstitial pneumonitis at day 4 only (Fig. 3, second row). Cotton rats that received FI-hMPV vaccine and were then challenged with wild-type virus showed accentuated peribronchiolitis at all time periods, and interstitial pneumonitis and alveolitis were present at all times (Fig. 3, third row). The degree of interstitial pneumonitits and alveolitis, the two key markers of enhancement is graphically shown in Fig. 4. The range, character and intensity of the lesions observed in the present hMPV experiment are indistinguishable from those seen with our prior hRSV vaccine enhancement experiments [22].
Fig. 3.

Pulmonary histopathology in hMPV challenged cotton rats vaccinated with FI-hMPV. Lung in the top row from previously untreated animals or the bottom row from animals that received mock vaccine shows lymphocytes around the bronchiole (peribronchiolitis) at day 4 that is cleared by day 10. The second row shows lung from an animal previously infected with metapneumovirus, and the peribronchiolitis is accentuated and continues through day 7, but largely cleared by day 10. Mild interstitial pneumonitis and alveolitis is present at day 4 only. The third row shows the lung from an animal immunized with formalin-inactivated vaccine and challenged. There is marked accentuation of peribronchiolitis and perivasculitis continuing through day 10, thickening of alveolar walls (interstitial pneumonitis), and a cellular infiltrate in alveoli (alveolitis).
Fig. 4.
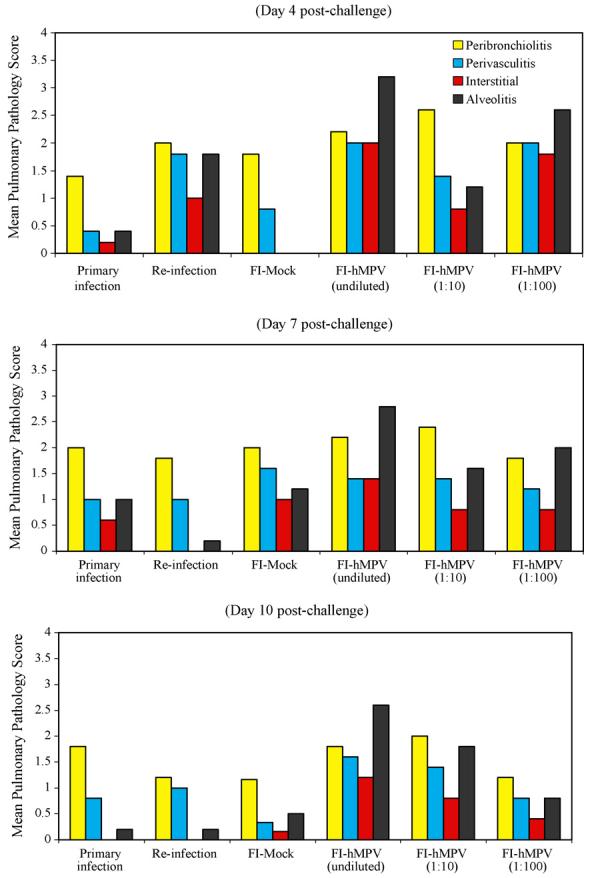
Exacerbation of pulmonary pathology in hMPV challenged cotton rats vaccinated with FI-hMPV. Medium power photomicrographs of cotton rat lungs at various days after challenge with human metapneumovirus. Each frame is centered on a bronchiole that is accompanied by a blood vessel. Pulmonary histopathology by days post-challenge (4, 7, and 10) with human metapneumovirus in cotton rats with a primary infection, reinfection, or that received mock or formalin-inactivated metapneumovirus vaccine before challenge. Pathology score from 0 to 4 scale in severity of infiltration of inflammatory cells using four parameters (Peribronchiolitis, Perivasculitis, Interstitial Pneumonitis, and Alveolitis).
3.5. Serum neutralizing antibody levels
HMPV-neutralizing antibody titers were determined by an end-point dilution assay using LLC-MK2 cells on sera samples collected on day 49 prior to hMPV challenge. All FI-hMPV vaccinated animals (undiluted, 1:10, and 1:100) showed increase levels of serum neutralizing antibody titers, 17.03, 26.44, and 4.54 Log2, respectively (Fig. 5). Animals infected with hMPV at 107 TCID50, by day 49 had serum neutralizing level of 10.46 Log2. No neutralizing titers were detected (<1.73 Log2) in the control animals (data not shown) or the FI-mock vaccinated animals.
Fig. 5.
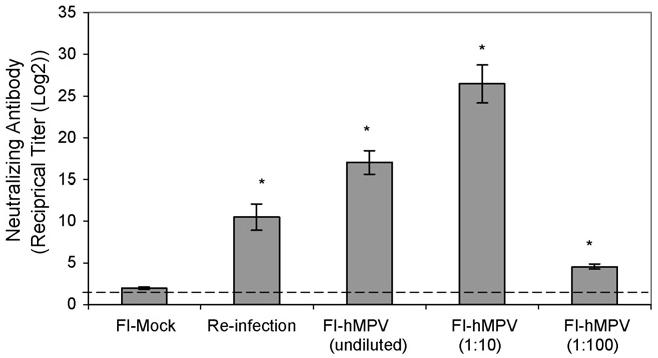
Levels of serum neutralizing antibody titers prior to hMPV challenge in the cotton rats on day 49. Reciprocal antibody titer in increments of Log2 shows increase levels in all FI-hMPV-vaccinated groups along with the re-infection group when comparing to mock vaccinated (primary infection) group (*P < 0.01).
4. Discussion
We show that hMPV infection increases the pulmonary lesions when the cotton rats are immunized and boosted with FI-hMPV and then challenged with homologous virus. Unlike the primarily or secondarily infected groups, the vaccinated groups had increased levels or accentuated interstitial pneumonitis and alveolitis. These two lesions appear to be specific for enhancement with hMPV, as is the case with hRSV [20,22]. Although there was a dramatic increase in lesions in cotton rats that were immunized with FI-hMPV before challenge, the vaccine was effective in preventing all viral replication in the lungs.
Vaccine-enhanced disease has been seen in the human with two members of the Paramyxoviridae family, measles and hRSV [7,11]. Similar enhancement has been shown experimentally in cotton rats with hRSV [20,22], measles [18], parainfluenza 3 [16], and now hMPV. In all cases, the vaccines used were formalin-inactivated. However, enhancement was also found with the parainfluenza 3 vaccine inactivated by ultraviolet light [16]. Additionally, minor degrees of enhancement have been noted with a recombinant chimeric RSV FG glycoprotein and that effect was eliminated by a monophosphoral lipid A adjuvant which also reduces pathology of FI-RSV vaccine enhanced disease [19]. This adjuvant also had a dramatic effect in reducing both Th-1 and Th-2-type cytokines in a model of FI-RSV vaccine enhancement in cotton rats [4]. Enhancement was also noted when a purified hRSV F protein was used as a vaccine together with a CpG oligodeoxynucleotide adjuvant [21]. We believe that all Paramyxoviridae probably have the tendency to cause vaccine enhancement when non-replicating vaccines are used. At this time we have not developed a firm opinion as to the mechanism of vaccine induced enhancement with members of this virus family, but can conclude from these findings that inactivated hMPV vaccine candidates should be thoroughly screened for safety. Recent publication by Cseke et al. [5] shows no vaccine enhancement when cotton rats are immunized with either DNA plasmids expressing F-protein of hMPV or adjuvanted, soluble hMPV F protein lacking the transmembrane domain then challenged. This may suggest that the native form of F protein may not be involved in the mechanism of vaccine enhancement for hMPV. In the present experiments, although a dramatic increase in the pulmonary pathology was found with high levels of serum neutralizing antibody present, the lungs of the vaccinated groups had undetectable levels of virus whereas in primarily infected animals, and in hMPV-infected animals vaccinated with the mock preparation of vaccine replicating hMPV was recovered. The enhanced expression of IL-4 may be indication of a shift from a Th-1 to Th-2 response when vaccinated animals are challenged. Although the lesions of the hRSV enhancement are not fully replicated in the mouse model, analysis of that model suggests that an imbalanced immune response is involved in the enhancement [15]. We believe that the Paramyxoviridae vaccine-induced enhancement is not related to the antibody-dependent enhancement (ADE) of infectivity seen with dengue, Ebola, and some other viruses [8,24]. ADE tends to increase rather than reduce viral titers.
Acknowledgement
This work was supported by Virion Systems, Inc. corporate funds and by NIH Grant #AJ-057575 to JCGB.
References
- 1.Blanco JCG, Pletneva L, Boukhvalova M, Richardson JY, Harris KA, Prince GA. The cotton rat: an underutilized animal model for human infectious diseases can now be exploited using specific reagents to cytokines, chemokines, and interferons. J Interferon Cytokine Res. 2004;24:21–8. doi: 10.1089/107999004772719873. [DOI] [PubMed] [Google Scholar]
- 2.Blanco JCG, Richardson JY, Darnell MER, Rowzee A, Pletneva L, Porter DD, et al. Cytokine and chemokine gene expression after primary and secondary respiratory syncytial virus infection in cotton rats. J Infect Dis. 2002;185:1780–5. doi: 10.1086/340823. [DOI] [PubMed] [Google Scholar]
- 3.Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Côté S, et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–4. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 4.Boukhvalova MS, Prince GA, Soroush L, Harrigan DC, Vogel SN, Blanco JCG. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine. 2006;24:5027–35. doi: 10.1016/j.vaccine.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 5.Cseke G, Wright DW, Tollefson SJ, Johnson JE, Crowe JE, Williams JV. Human metapneumovirus fusion protein vaccines that are immunogenic and protective in cotton rats. J Virol. 2007;81:698–707. doi: 10.1128/JVI.00844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 7.Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967;202:1075–80. doi: 10.1001/jama.202.12.1075. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–67. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 9.Hamelin M-È, Abed Y, Boivin G. Human metapneumovirus: a new player among respiratory viruses. Clin Infect Dis. 2004;38:983–90. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamelin M-È, Yim K, Kuhn KH, Cragin RP, Boukhvalova M, Blanco JCG, et al. Pathogenesis of human metapneumovirus lung infection in BALB/c mice and cotton rats. J Virol. 2005;79:8894–903. doi: 10.1128/JVI.79.14.8894-8903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 12.Kuiken T, van den Hoogen BG, van Riel DAJ, Laman JD, van Amerongen G, Sprong L, et al. Experimental human metapneumovirus infection of cynomolgus macaques (Macaca fascicularis) results in virus replication in ciliated epithelial cells and pneumocytes with associated lesions throughout the respiratory tract. Am J Pathol. 2004;164:1893–900. doi: 10.1016/S0002-9440(10)63750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacPhail M, Schickli JH, Tang RS, Kaur J, Robinson C, Fouchier RAM, et al. Identification of small-animal and primate models for evaluation of vaccine candidates for human metapneumovirus (hMPV) and implications for hMPV vaccine design. J Gen Virol. 2004;85:1655–63. doi: 10.1099/vir.0.79805-0. [DOI] [PubMed] [Google Scholar]
- 14.Madhi SA, Ludewick H, Kuwanda L, van Niekerk N, Cutland C, Little T, et al. Pneumococcal coinfection with human metapneumovirus. J Infect Dis. 2006;193:1236–43. doi: 10.1086/503053. [DOI] [PubMed] [Google Scholar]
- 15.Openshaw PJM, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005;18:541–55. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottolini MG, Porter DD, Hemming VG, Prince GA. Enhanced pulmonary pathology in cotton rats upon challenge after immunization with inactivated parainfluenza virus 3 vaccines. Viral Immunol. 2000;13:231–6. doi: 10.1089/vim.2000.13.231. [DOI] [PubMed] [Google Scholar]
- 17.Peret TCT, Boivin G, Li Y, Couillard M, Humphrey C, Osterhaus ADME, et al. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002;185:1660–3. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polack FP, Auwaerter PG, Lee S-H, Nousari HC, Valsamakis A, Leiferman KM, et al. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat Med. 1999;5:629–34. doi: 10.1038/9473. [DOI] [PubMed] [Google Scholar]
- 19.Prince GA, Capiau C, Deschamps M, Fabry L, Garçon N, Gheysen D, et al. Efficacy and safety studies of a recombinant chimeric respiratory syncytial virus FG glycoprotein vaccine in cotton rats. J Virol. 2000;74:10287–92. doi: 10.1128/jvi.74.22.10287-10292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prince GA, Curtis SJ, Yim KC, Porter DD. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol. 2001;82:2881–8. doi: 10.1099/0022-1317-82-12-2881. [DOI] [PubMed] [Google Scholar]
- 21.Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol. 2003;77:13156–60. doi: 10.1128/JVI.77.24.13156-13160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prince GA, Prieels J-P, Slaoui M, Porter DD. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus) Lab Invest. 1999;79:1385–92. [PubMed] [Google Scholar]
- 23.Skiadopoulos MH, Biacchesi S, Buchholz UJ, Riggs JM, Surman SR, Amaro-Carambot E, et al. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J Virol. 2004;78:6927–37. doi: 10.1128/JVI.78.13.6927-6937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takada A, Watanabe S, Okazaki K, Kida H, Kawaoka Y. Infectivity-enhancing antibodies to Ebola virus glycoprotein. J Virol. 2001;75:2324–30. doi: 10.1128/JVI.75.5.2324-2330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RAM, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Hoogen BG, van Doornum GJ, Fockens JC, Cornelissen JJ, Beyer WEP, De Groot R, et al. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–7. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- 27.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. New Engl J Med. 2004;350:443–50. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams JV, Tollefson SJ, Johnson JE, Crowe JE., Jr The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. J Virol. 2005;79:10944–51. doi: 10.1128/JVI.79.17.10944-10951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams JV, Wang CK, Yang C-F, Tollefson SJ, House FH, Heck JM, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–095. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyde PR, Chetty SN, Jewell AM, Schoonover SL, Piedra PA. Development of a cotton rat-human metapneumovirus (hMPV) model for identifying and evaluating potential hMPV antivirals and vaccines. Antiviral Res. 2005;66:57–66. doi: 10.1016/j.antiviral.2004.12.009. [DOI] [PubMed] [Google Scholar]


