Abstract
Ribulose-1,5-bisphosphate carboxylase/oxygenase (EC 4.1.1.39) is the key photosynthetic enzyme that catalyzes the first step of CO2 fixation. The chloroplast-localized holoenzyme of plants and green algae contains eight nuclear-encoded small subunits and eight chloroplast-encoded large subunits. Although much has been learned about the enzyme active site that resides within each large subunit, it has been difficult to assess the role of eukaryotic small subunits in holoenzyme function and expression. Small subunits are coded by a family of genes, precluding genetic screening or nuclear transformation approaches for the recovery of small-subunit mutants. In this study, the two small-subunit genes of the green alga Chlamydomonas reinhardtii were eliminated during random insertional mutagenesis. The photosynthesis-deficient deletion mutant can be complemented with either of the two wild-type small-subunit genes or with a chimeric gene that contains features of both. Thus, either small subunit is sufficient for holoenzyme assembly and function. In the absence of small subunits, expression of chloroplast-encoded large subunits appears to be inhibited at the level of translation.
Keywords: Chlamydomonas reinhardtii, chloroplast, insertional mutagenesis, photosynthesis, protein engineering
Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco, EC 4.1.1.39) is conceivably the most abundant and most important protein on earth. It enables atmospheric carbon to be captured into the biosphere by carboxylating ribulose 1,5-bisphosphate in the first step of photosynthesis. O2 competes with CO2 at the same active site, and substrate oxygenation initiates a fruitless photorespiratory pathway. If carboxylation could be increased or oxygenation decreased, a substantial increase in photosynthetic productivity would be realized (for reviews, see refs. 1 and 2).
Most Rubisco holoenzymes are composed of eight large and eight small subunits. In green plants, the 55-kDa large subunits are coded by a single rbcL chloroplast gene, and x-ray crystallography has revealed that each large subunit contains an α/β-barrel active site (for review, see ref. 3). Much has been learned about the contribution of large-subunit structure to holoenzyme function by employing chloroplast genetic methods in the green alga Chlamydomonas reinhardtii (1, 4, 5, 6), or by studying directed-mutant prokaryotic enzymes expressed in Escherichia coli (2, 7, 8). In contrast, little is known about the contribution of the small subunit to Rubisco structure and function.
In green plants, small subunits are coded by a family of two or more rbcS nuclear genes, synthesized as 20-kDa precursors in the cytoplasm, and processed to 15 kDa during transport into the chloroplast (for review, see ref. 9). Members of a family of rbcS genes are differentially expressed during plant development (10, 11), and the amino-acid sequences of the mature small subunits can differ at several residues (12). Because there are multiple rbcS genes, it has been difficult to determine whether small subunits might contribute to the enhanced catalytic efficiency of eukaryotic enzymes or whether small-subunit diversity may have some functional significance (13, 14). Rubisco from Rhodospirillum rubrum, which is the prokaryotic enzyme used most extensively for directed mutagenesis studies (4), lacks small subunits. Directed mutagenesis of cyanobacterial small subunits, expressed and assembled with cyanobacterial large subunits in E. coli, has shown that small subunits can influence holoenzyme stability and the rate of carboxylation (15, 16, 17, 18). However, such studies cannot assess the significance of the structural and functional differences between prokaryotic and eukaryotic Rubisco holoenzymes. No rbcS mutant has yet been recovered by genetic screening, and the eukaryotic holoenzyme cannot be expressed in E. coli (19, 20). Studies of the eukaryotic small subunit have been limited to assembly with cyanobacterial large subunits (21, 22) or transport of directed-mutant subunits into isolated chloroplasts (23, 24).
In this study, a C. reinhardtii mutant was recovered that lacks both of the rbcS genes. This photosynthesis-deficient mutant can be maintained due to the fact that C. reinhardtii is able to survive in the absence of photosynthesis when supplied with acetate (25). Because the deletion mutant can be rescued by transformation with a single rbcS gene, it will now be possible to address questions of small-subunit function within the chloroplast Rubisco holoenzyme.
MATERIALS AND METHODS
Strains and Culture Conditions.
C. reinhardtii strains were maintained at 25°C in darkness on medium containing 10 mM sodium acetate and 1.5% Bacto agar (25). The cwdarg-7-8 mt− strain, which lacks a cell wall and requires arginine for growth (26), was grown with medium containing 0.5 mM arginine. It was designated as the wild type in this study. For experimental procedures, cells were grown in 50 or 500 ml of liquid medium on a rotary shaker at 120 rpm to a density of 2 × 106 cells per ml.
Plasmids and Genetic Engineering.
Plasmid pARG7.8, which contains the wild-type gene for argininosuccinate lyase (26), was used for the transformation of strain cwdarg-7-8 mt−. The 5-kb EcoRI fragment, containing rbcS1 (27), and the 7.9-kb HindIII–EcoRI fragment, containing rbcS2 (27), were cloned previously (28). These fragments were subcloned into pUC19 (29), creating plasmids pSS1 and pSS2, respectively. To create a chimeric rbcS gene, a 3-kb NsiI–EcoRI fragment that contains rbcS2 was subcloned into PstI/EcoRI-digested pUC19 to generate plasmid pNESS2. The 881-bp SacII fragment from pNESS2 (within the rbcS2 coding region) was then replaced by the corresponding 739-bp SacII fragment from pSS1. The resultant plasmid was named pNETASW.
Transformation and Insertional Mutagenesis.
Cells (4 × 107 in 0.4 ml of growth medium containing 5% polyethylene glycol) were transformed with pARG7.8 DNA (2–8 μg) or rbcS plasmid DNA (3 μg) by the glass bead vortexing method (30). The transformation mixture was then plated at a density of 1 × 107 cells per 100-mm Petri plate. pARG7.8 transformants were selected on acetate medium without arginine in darkness, and then replica-plated to minimal medium in the light to screen for acetate-requiring, photosynthesis-deficient mutants (25). Transformants obtained with rbcS plasmids were selected directly on minimal medium in the light (80 μE/m2/s).
Pulse Labeling, Gel Electrophoresis, and Western Blot Analysis.
Dark-grown, sulfate-starved cells were pulse-labeled with 35SO42− for 1 min and chased with 10 mM Na2SO4 for 60 min (31). Samples were extracted, equal amounts of radioactivity were subjected to SDS/PAGE with a gradient resolving gel of 7.5 to 15% acrylamide, and protein bands were visualized by fluorography (5). For Western blot analysis, total soluble proteins were extracted by sonication (32). The protein extracts were then fractionated by SDS/PAGE, transferred to nitrocellulose, and probed with rabbit anti-tobacco Rubisco immunoglobulin G (0.5 μg/ml) provided by Raymond Chollet (Department of Biochemistry, University of Nebraska, Lincoln, NE) (5, 28). Protein bands were visualized via enhanced chemiluminescence (Amersham) by using a secondary antibody conjugated to horseradish peroxidase (Bio-Rad) (5, 28).
PCR Amplification.
Total DNA was extracted (5), purified on CsCl gradients (33), and digested with HaeIII or EcoRI prior to initiating PCR reactions (34). PCR amplification of rbcL (35) was performed with oligonucleotide primers complementary to the known rbcL sequence (4, 5). For the amplification of rbcS1 and rbcS2 gene regions (27), oligonucleotide primers 5′-GCAGGATGTTCGAGACCTTC-3′ and 5′-CCTGCTTCTGGTTGTCGAAG-3′ were used. The reactions were performed for 31 cycles, each consisting of 1 min denaturation at 94°C, 1 min primer annealing at 57°C, and 3 min primer extension at 72°C.
DNA and RNA Hybridization.
Total DNA was digested with EcoRI and HindIII, separated on a 0.8% agarose gel, and blotted to nylon (33). The 1190-bp PstI fragment, corresponding to the 3′ end of rbcS2 (35), was nick-translated (33) with [32P]dCTP and used as a probe for both rbcS genes. Hybridization was performed in 5× SSPE (750 mM NaCl/50 mM NaH2PO4/5 mM EDTA, pH 7.4), 100 μg/ml denatured salmon sperm DNA at 65°C. Filters were washed in 0.1× SSPE/0.1% SDS at 65°C and subjected to autoradiography at −70°C. Total RNA was isolated, resolved in agarose-formaldehyde gel, and blotted to nylon membrane (36). A 32P-labeled PCR fragment of rbcL [bases 295-1245 relative to the 1428-bp coding region (35)] was used as a probe. Hybridization was performed in 6× SSPE, 50% formamide, 0.5% SDS, 100 μg/ml denatured salmon sperm DNA at 42°C. Filters were washed in 0.1× SSPE/0.1% SDS at 65°C and subjected to autoradiography at −70°C.
RESULTS
Insertional Mutagenesis.
C. reinhardtii can be stably and efficiently transformed by simply agitating cells and DNA with glass beads (30). Because the DNA integrates into the nuclear genome at apparently random loci, the transforming DNA can act as an insertional mutagen to disrupt functional genes (37, 38). We initially decided to use this method to physically mark alleles of mutant nuclear genes that affect Rubisco expression, stability, and catalysis (28, 36, 39). A dark-grown cwdarg-7-8 mt− strain, which lacks a cell wall and requires arginine for growth (26), was transformed with plasmid pARG7.8, which contains the wild-type argininosuccinate lyase gene (26). Transformants were selected on acetate medium (without arginine) in darkness at a frequency of 2 × 10−5 cells, and then replica-plated to minimal medium in the light to screen for acetate-requiring, photosynthesis-deficient mutants (25). About 1.5% of the transformants required acetate for growth. This recovery frequency for acetate-requiring mutants is about 7 times greater than that reported for previous insertional-mutagenesis experiments performed in the light (37). It is well known that C. reinhardtii photosynthesis-deficient mutants can be killed or selected against on acetate medium in the light (25, 40). About 80% of our acetate-requiring insertional mutants were light-sensitive, and could be maintained only in darkness.
Rubisco Small-Subunit Mutant.
When the acetate-requiring insertional mutants were screened by SDS/PAGE and Western blot analysis, one of the light-sensitive strains, named T60-3, was found to lack Rubisco large and small subunits. This is a common attribute of a number of Rubisco mutants that have primary defects in large-subunit synthesis or assembly (5, 6, 31, 36). However, 35SO42−-labeling experiments indicated that little or no synthesis of either subunit occurred during a 1-min pulse (Fig. 1A). Because no Rubisco-deficient mutant had previously been found to lack small-subunit synthesis (see ref. 1), we thought that T60-3 might have a primary defect related to the small subunit. Genetic analysis has not been possible for the T60-3 mutant because it lacks flagella. This flagellar defect may have arisen spontaneously, or it may be the result of insertional mutagenesis (38). Nonetheless, we could not discriminate between a mutation within the chloroplast rbcL gene or a mutation in a nuclear gene by simply performing a genetic cross. Instead, the presence of an intact rbcL gene was verified by PCR (data not shown) and RNA hybridization (Fig. 1B). We then transformed the T60-3 mutant with either rbcS1 or rbcS2 (plasmids pSS1 or pSS2, respectively) to see whether photosynthesis-competent transformants could be selected on minimal medium in the light. The rbcS1 and rbcS2 plasmids were able to complement the T60-3 mutation, producing photosynthesis-competent colonies at frequencies of 1.4 × 10−5 and 2.9 × 10−6, respectively. These transformants have near wild-type phenotypes when maintained on minimal medium in the light. No spontaneous, photosynthesis-competent revertant of T60-3 has been observed after plating more than 5 × 108 cells on minimal medium in the light.
Figure 1.
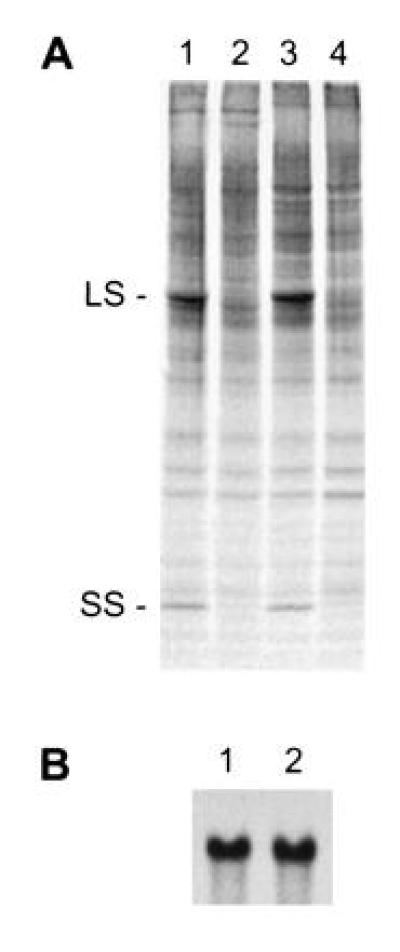
Mutant T60-3 fails to synthesize Rubisco large and small subunits. (A) Pulse labeling of soluble cell proteins in wild-type (lanes 1 and 3) and mutant T60-3 (lanes 2 and 4). Dark-grown cells were labeled with 35SO42− for 1 min (lanes 1 and 2) and chased with 10 mM Na2SO4 for 1 h (lanes 3 and 4). Equal amounts of radioactivity were fractionated with SDS/PAGE, and protein bands were visualized by fluorography. LS, large subunit; SS, small subunit. (B) Amount of rbcL mRNA in wild-type (lane 1) and mutant T60-3 (lane 2). Total RNA was isolated, resolved in an agarose-formaldehyde gel (10 μg of RNA per lane), and probed with a 32P-labeled PCR fragment of rbcL (bases 295–1245 relative to the 1428-bp coding region).
Deletion of the rbcS Locus.
Because the two rbcS genes are physically linked within the haploid genome of C. reinhardtii (Fig. 2), and insertional mutagenesis can be accompanied by deletions as large as 23 kb (38), we thought that both rbcS genes may have been deleted in the T60-3 mutant. To test this hypothesis, we attempted to PCR amplify the rbcS genes. Because the two rbcS genes share extensive homology (27), a single pair of oligonucleotides was used to amplify an internal region of both genes simultaneously (Fig. 2). The PCR products differ in size because intron 2 is larger in the rbcS2 gene. As shown in Fig. 3A, wild-type DNA produced 753- and 888-bp PCR products (from rbcS1 and rbcS2, respectively), but mutant T60-3 DNA failed to produce either product. When DNA from rbcS1 or rbcS2 transformants was PCR amplified, only the expected 753-bp (rbcS1) or 888-bp (rbcS2) product was observed (Fig. 3A). Although it appeared that mutant T60-3 lacked both rbcS genes and could be rescued by transformation with either rbcS1 or rbcS2, we were curious to know how much of the rbcS locus had been deleted. Therefore, DNA hybridization experiments were performed in which a single PstI restriction fragment from rbcS2 was used as a probe for both rbcS genes (Fig. 2). As shown in Fig. 3B, when HindIII/EcoRI-digested wild-type DNA was analyzed, the 32P-labeled probe detected a 1.5-kb HindIII–EcoRI fragment (containing the 3′ end of rbcS1) and a 7.9-kb HindIII–EcoRI fragment (containing the entire rbcS2 gene). When mutant T60-3 DNA was analyzed, neither fragment was observed (Fig. 3B), and the rbcS1 and rbcS2 transformants contained only the 1.5-kb or 7.9-kb HindIII–EcoRI fragment, respectively (Fig. 3B). The exact size of the deletion in the T60-3 mutant is not known, but DNA hybridization also failed to detect sequences within the 5′ flank of rbcS2 (Fig. 2) when a 2-kb HindIII–EcoRV fragment was used as a probe (data not shown). Thus, it is possible that the entire 13-kb rbcS locus (Fig. 2) is absent from mutant T60-3. We formally denote this mutation and mutant strain as rbcS-T60-3.
Figure 2.
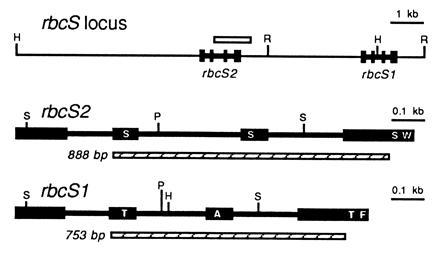
Organization of the rbcS locus in C. reinhardtii (27). The coding regions of the rbcS1 and rbcS2 genes are displayed as exons (solid boxes) connected by introns (thick lines). Both genes are oriented in a 5′ to 3′ direction. A PstI restriction fragment used as a 32P-labeled DNA probe is shown as an open box. PCR products, generated from both rbcS1 and rbcS2 with a single pair of oligonucleotides, are shown as hatched boxes. Restriction sites are indicated: R, EcoRI; H, HindIII; P, PstI; S, SacII. Each rbcS gene encodes a 185-residue polypeptide. The first 45 residues are removed during chloroplast import to form the 140-residue mature small subunits. Approximate locations of amino-acid differences between the two small subunits (residues 22, 47, 128, and 132) are marked with single-letter abbreviations within the exons: A, Ala; F, Phe; S, Ser; T, Thr; W, Trp.
Figure 3.
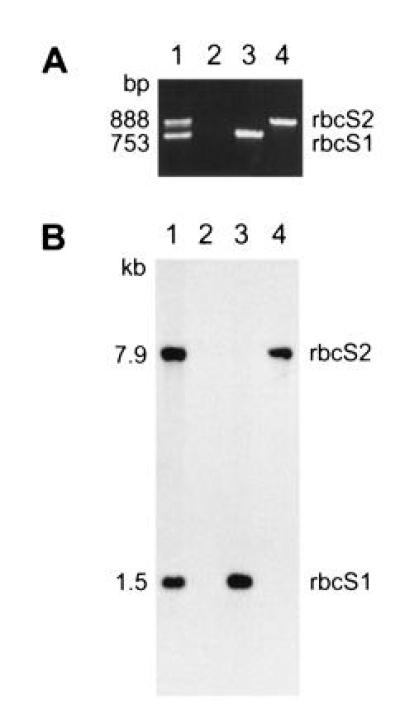
Mutant T60-3 lacks both rbcS genes, and can be transformed with either rbcS1 or rbcS2. (A) PCR amplification of rbcS gene regions from total DNA extracted from wild-type (lane 1), mutant T60-3 (lane 2), and rbcS1 and rbcS2 transformants of T60-3 (lanes 3 and 4, respectively). A single pair of oligonucleotides was used to amplify regions of rbcS1 and rbcS2 (753 and 888 bp, respectively) from the end of intron 1 to the middle of exon 4 (Fig. 2). (B) DNA hybridization analysis of EcoRI/HindIII-digested total DNA (5 μg per lane) extracted from wild-type (lane 1), mutant T60-3 (lane 2), and rbcS1 and rbcS2 transformants of T60-3 (lanes 3 and 4, respectively). The filter was probed with a 32P-labeled 1190-bp PstI fragment containing the 3′-coding region of rbcS2. The probe detects 1.5- and 7.9-kb fragments that contain the 3′ end of rbcS1 or the entire rbcS2 gene, respectively (Fig. 2).
Transformants That Contain rbcS1 or rbcS2.
Previous studies have indicated that the rbcS genes of C. reinhardtii are differentially expressed at the transcriptional level (27, 41). In particular, very little rbcS1 mRNA was found in dark-grown cells relative to the abundant rbcS2 mRNA (27). Clearly, either gene alone is sufficient for the production of Rubisco holoenzyme in light-grown cells, or we would not have been able to recover photosynthetic transformants on minimal medium in the light. However, if the expression of either gene was substantially inhibited in dark-grown cells, it might prove difficult to purify mutant holoenzymes in future genetic-engineering studies. As shown in Fig. 4, Western blot analysis revealed that rbcS1 and rbcS2 transformants synthesize appreciable levels of Rubisco when grown in darkness, further indicating that either rbcS gene is sufficient for the production of Rubisco holoenzyme.
Figure 4.
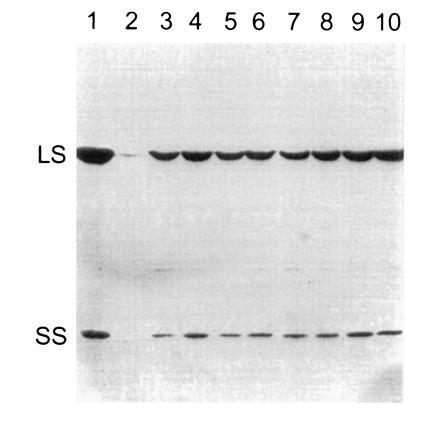
Western blot analysis of dark-grown rbcS transformants. Total soluble cell protein was extracted from wild-type (lane 1), mutant T60-3 (lane 2), and independent rbcS1 (lanes 3–6) and rbcS2 (lanes 7–10) transformants of T60-3. Each protein sample (30 μg) was fractionated with SDS/PAGE. The proteins were transferred to nitrocellulose and probed with rabbit antibody against tobacco Rubisco holoenzyme. The T60-3 mutant accumulates only a trace of large subunit. LS, large subunit; SS, small subunit.
Because the two rbcS genes encode slightly different proteins (27), we wondered whether the differences might have some functional significance. The mature rbcS2 gene product has Ser-22, Ser-47, Ser-128, and Trp-132, but the rbcS1 gene product has Thr-22, Ala-47, Thr-128, and Phe-132 (Fig. 2). To test whether these residues are involved in an essential structural interaction, a chimeric rbcS gene was created by replacing a SacII fragment from rbcS2 with the SacII fragment from rbcS1 (Fig. 2). This new gene, with flanking sequences from rbcS2, would encode a mature small-subunit protein containing Thr-22, Ala-47, Ser-128, and Trp-132. The chimeric gene was found to produce photosynthesis-competent transformants of rbcS-T60-3 at a frequency of 3.5 × 10−6 cells. Thus, the residues that differ between the two wild-type small subunits do not appear to play an essential role in holoenzyme assembly or function.
DISCUSSION
The rbcS-T60-3 photosynthesis-deficient mutant of C. reinhardtii lacks Rubisco holoenzyme (Fig. 1) due to the elimination of the rbcS gene family via random insertional mutagenesis. Because neither rbcS gene can be detected by PCR or DNA hybridization (Fig. 3), it is likely that the entire 13-kb rbcS locus has been deleted during the insertional event (Fig. 2). Consistent with this assessment, no photosynthetic-competent revertants can be recovered, but the rbcS-T60-3 mutant strain can be rescued by transformation with either of the two wild-type rbcS genes (Fig. 3). In fact, rbcS transformation occurs at frequencies comparable to those observed for other nuclear genes in C. reinhardtii (30, 37, 38). Because transformants that contain either rbcS gene express appreciable levels of Rubisco, even when grown in darkness (Fig. 4), it will now be possible to use directed and random mutagenesis to assess the role of the eukaryotic small subunit in holoenzyme structure and function.
Although the two small subunits differ at four residues (Fig. 2), transformation experiments showed that either rbcS gene is sufficient for the production of an active Rubisco holoenzyme. Thus, small-subunit heterogeneity is not required for holoenzyme function or assembly. The idea that both rbcS genes are functional in wild type accounts for the fact that no rbcS mutants have been recovered by genetic screening (see ref. 1). Such mutants would be recovered only if both rbcS genes received lethal mutations, the probability of which is the square of the recovery frequency for a single mutation. Because the rbcS1 and rbcS2 transformants accumulate less Rubisco than wild type (Fig. 4), it seems likely that multiple rbcS genes are necessary only for the production of more Rubisco holoenzyme.
The amino-acid differences between the two C. reinhardtii small subunits are generally conservative (Fig. 2), with the substituted residues having similar Van der Waals volumes. The most dramatic differences comprise the replacement of polar Ser-47 and Trp-132 residues in the rbcS2 gene product with hydrophobic Ala-47 and Phe-132 residues in the rbcS1 gene product. Nonetheless, a chimeric rbcS gene, encoding a small subunit with Ala-47 and Trp-132, is able to rescue the rbcS-T60-3 mutant. If the different residues contribute to unique structural interactions within each small subunit, such interactions are not essential for holoenzyme function or assembly. Detailed biochemical analysis will be required (and can now be performed) to determine whether differences in small-subunit structures produce differences in Rubisco catalysis (see ref. 14).
Previous 35SO42− pulse-labeling studies with rbcL chloroplast mutants have shown that, in the absence of large subunits, small subunits are synthesized at a normal rate, transported into the chloroplast, and then rapidly degraded (5, 6, 31). However, when small subunits are absent, due to the rbcS-T60-3 deletion (Fig. 3), large-subunits do not appear to be synthesized at a normal rate (Fig. 1A), even though a normal amount of rbcL mRNA is present (Fig. 1B). We doubt that the large subunits are degraded as fast as they are synthesized during pulse labeling because a normal rate of large-subunit synthesis has been observed in an rbcL nonsense mutant (31, 42), as well as in all rbcL missense mutants that have unstable holoenzymes (5, 6, 43). This raises the possibility that small-subunit expression controls Rubisco subunit stoichiometry by regulating large-subunit expression at the level of translation. A trace of large subunits does accumulate in the rbcS-T60-3 mutant (Fig. 4). Perhaps this indicates that large subunits are resistant to posttranslational degradation when in excess of small subunits (see ref. 5), necessitating translational control to achieve subunit stoichiometry. Previous studies with antisense rbcS tobacco plants showed that the level of rbcS mRNA determines the level of Rubisco holoenzyme without influencing the amount of rbcL mRNA (44). One of the antisense plants appeared to have decreased synthesis of the large subunit (44), indicating that translational control of large-subunit expression may also occur in higher plants. More recent analysis of the antisense rbcS mutants revealed that they have a reduction in rbcL mRNA associated with polysomes (45). This is particularly strong evidence for small-subunit control of rbcL mRNA translation. Further experiments will be necessary for elucidating the role of such posttranscriptional events in Rubisco large-subunit and holoenzyme expression.
Acknowledgments
This is paper no. 11412, Journal Series, Nebraska Agricultural Research Division. We thank Dr. R. Chollet for the Rubisco antibody. This work was supported by Grant 94-37306-0349 from the U.S. Department of Agriculture.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviation: Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase.
References
- 1.Spreitzer R J. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:411–434. [Google Scholar]
- 2.Hartman F C, Harpel M R. Annu Rev Biochem. 1994;63:197–234. doi: 10.1146/annurev.bi.63.070194.001213. [DOI] [PubMed] [Google Scholar]
- 3.Schneider G, Lindqvist Y, Brändén C I. Annu Rev Biophys Biomol Struct. 1992;21:119–143. doi: 10.1146/annurev.bb.21.060192.001003. [DOI] [PubMed] [Google Scholar]
- 4.Zhu G, Spreitzer R J. J Biol Chem. 1994;269:3952–3956. [PubMed] [Google Scholar]
- 5.Thow G, Zhu G, Spreitzer R J. Biochemistry. 1994;33:5109–5114. doi: 10.1021/bi00183a014. [DOI] [PubMed] [Google Scholar]
- 6.Spreitzer R J, Thow G, Zhu G. Plant Physiol. 1995;109:681–686. doi: 10.1104/pp.109.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson E M, Larimer F W, Hartman F C. Biochemistry. 1995;34:4531–4537. doi: 10.1021/bi00014a005. [DOI] [PubMed] [Google Scholar]
- 8.Harpel M R, Serpersu E H, Lamerdin J A, Huang Z H, Gage D A, Hartman F C. Biochemistry. 1995;34:11296–11306. doi: 10.1021/bi00035a039. [DOI] [PubMed] [Google Scholar]
- 9.Dean C, Pichersky E, Dunsmuir P. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:415–439. [Google Scholar]
- 10.Wanner L A, Gruissem W. Plant Cell. 1991;3:1289–1303. doi: 10.1105/tpc.3.12.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dedonder A, Rethy R, Fredericq H, Van Montagu M, Krebbers E. Plant Physiol. 1993;101:801–808. doi: 10.1104/pp.101.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meagher R B, Berry-Lowe S, Rice K. Genetics. 1989;123:845–863. doi: 10.1093/genetics/123.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider G, Knight S, Andersson I, Brändén C I, Lindqvist Y, Lundqvist T. EMBO J. 1990;9:2045–2050. doi: 10.1002/j.1460-2075.1990.tb07371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eilenberg H, Beer S, Gepstein S, Geva N, Tadmor O, Zilberstein A. Plant Physiol. 1991;95:298–304. doi: 10.1104/pp.95.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitchen J H, Knight S, Andersson I, Brändén C I, McIntosh L. Proc Natl Acad Sci USA. 1990;87:5768–5772. doi: 10.1073/pnas.87.15.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee B, Berka R M, Tabita F R. J Biol Chem. 1991;266:7417–7422. [PubMed] [Google Scholar]
- 17.Paul K, Morell M K, Andrews T J. Biochemistry. 1991;30:10019–10026. doi: 10.1021/bi00105a029. [DOI] [PubMed] [Google Scholar]
- 18.Read B A, Tabita F R. Biochemistry. 1992;31:519–525. doi: 10.1021/bi00117a031. [DOI] [PubMed] [Google Scholar]
- 19.Gatenby A A, van der Vies S M, Rothstein S J. Eur J Biochem. 1987;168:227–231. doi: 10.1111/j.1432-1033.1987.tb13409.x. [DOI] [PubMed] [Google Scholar]
- 20.Cloney L P, Bekkaoui D R, Hemmingsen S M. Plant Mol Biol. 1993;23:1285–1290. doi: 10.1007/BF00042362. [DOI] [PubMed] [Google Scholar]
- 21.Andrews T J, Lorimer G H. J Biol Chem. 1985;260:4632–4636. [PubMed] [Google Scholar]
- 22.Read B A, Tabita F R. Biochemistry. 1992;31:5553–5559. doi: 10.1021/bi00139a018. [DOI] [PubMed] [Google Scholar]
- 23.Flachmann R, Bohnert H J. J Biol Chem. 1992;267:10576–10582. [PubMed] [Google Scholar]
- 24.Archer E K, Keegstra K. Plant Mol Biol. 1993;23:1105–1115. doi: 10.1007/BF00042345. [DOI] [PubMed] [Google Scholar]
- 25.Spreitzer R J, Mets L. Plant Physiol. 1981;67:565–569. doi: 10.1104/pp.67.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gumpel N J, Rochaix J D, Purton S. Curr Genet. 1994;26:438–442. doi: 10.1007/BF00309931. [DOI] [PubMed] [Google Scholar]
- 27.Goldschmidt-Clermont M, Rahiré M. J Mol Biol. 1986;191:421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- 28.Gotor C, Hong S, Spreitzer R J. Planta. 1994;193:313–319. [Google Scholar]
- 29.Yanisch-Perron C, Vieira J, Messing J. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 30.Kindle K L. Proc Natl Acad Sci USA. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spreitzer R J, Goldschmidt-Clermont M, Rahiré M, Rochaix J D. Proc Natl Acad Sci USA. 1985;82:5460–5464. doi: 10.1073/pnas.82.16.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spreitzer R J, Chastain C J. Curr Genet. 1987;11:611–616. [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 34.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 35.Dron M, Rahiré M, Rochaix J D. J Mol Biol. 1982;162:775–793. doi: 10.1016/0022-2836(82)90547-2. [DOI] [PubMed] [Google Scholar]
- 36.Hong S, Spreitzer R J. Plant Physiol. 1994;106:673–678. doi: 10.1104/pp.106.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adam M, Lentz K E, Loppes R. FEMS Microbiol Lett. 1993;110:265–268. [Google Scholar]
- 38.Tam L W, Lefebvre P A. Genetics. 1993;135:375–384. doi: 10.1093/genetics/135.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Z, Green D, Westhoff C, Spreitzer R J. Arch Biochem Biophys. 1990;283:60–67. doi: 10.1016/0003-9861(90)90612-3. [DOI] [PubMed] [Google Scholar]
- 40.Spreitzer R J, Ogren W L. Plant Physiol. 1983;71:35–39. doi: 10.1104/pp.71.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldschmidt-Clermont G. Plant Mol Biol. 1986;6:13–21. doi: 10.1007/BF00021302. [DOI] [PubMed] [Google Scholar]
- 42.Zhang D, Spreitzer R J. Curr Genet. 1990;17:49–53. [Google Scholar]
- 43.Chen Z, Chastain C J, Al-Abed S R, Chollet R, Spreitzer R J. Proc Natl Acad Sci USA. 1988;85:4696–4699. doi: 10.1073/pnas.85.13.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodermel S R, Abbot M S, Bogorad L. Cell. 1988;56:673–681. doi: 10.1016/0092-8674(88)90226-7. [DOI] [PubMed] [Google Scholar]
- 45.Rodermel S, Haley J, Jiang C, Tsai C, Bogorad L. Proc Natl Acad Sci USA. 1996;93:3881–3885. doi: 10.1073/pnas.93.9.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]


