Abstract
Pediatric Oncology Group (POG) protocol 9201 enrolled children with lesser-risk B-lineage acute lymphoblastic leukemia (ALL) defined by age (1-9), white blood cell count (WBC) less than 50 × 109/L (50 000/μL), DNA findings of trisomies 4 and 10 (or DNA index > 1.16), and lack of overt central nervous system (CNS) leukemia. After vincristine, prednisone, and asparaginase induction, 650 of 653 eligible patients attained remission (3 induction deaths) and received 6 courses of intravenous methotrexate (1 g/m2) with daily mercaptopurine. Weekly intramuscular methotrexate was added during maintenance; pulses of vincristine and prednisone were administered with periodic intrathecal chemotherapy. Treatment duration was 2.5 years. No alkylators, epipodophylotoxins, anthracyclines, or radiation were given. The 6-year event-free survival (EFS) was 86.6% with overall survival (OS) of 97.2%. Patients with less than 5% marrow blasts on induction day 15 had superior EFS. A difference not reaching conventional statistical significance (P = .068) was noted for superior outcomes in patients with trisomies of chromosomes 4 and 10 versus those lacking double trisomies. Sex, ethnicity, CNS status, and WBC were not predictive. This indicates the great majority of children with lesser-risk B-lineage ALL are curable without agents with substantial late effects.
Introduction
B-lineage acute lymphoblastic leukemia (ALL) is the most common childhood malignancy in industrialized countries.1 Multiple different treatments have produced cures for a majority of children with ALL.2,3 A wide variety of prognostic factors have been used to separate patients into those of lesser risk and higher risk. The Pediatric Oncology Group (POG) previously recognized patients with “favorable” age and initial white blood cell count (WBC) by National Cancer Institute common risk group criteria4 whose blasts have an elevated DNA index or trisomy of both chromosomes 4 and 10 in their leukemic cells to have an exceptionally good prognosis when treated with antimetabolite therapy,5 a finding supported by other studies.6,7 Because of the potential short- and long-term burden of therapy, POG 9201 was designed to extend this observation to a larger set of patients, attempting to clearly define a group of patients with an excellent prognosis even when treated with less-intensive treatment.
Patients, materials, and methods
Patients
The POG 9201 protocol opened as a limited institution pilot study in June 1992 and as a nonrandomized single-arm phase 3 group-wide study in November 1994. The study met accrual goals and closed to new patient enrollment in November 1999. Patients eligible for enrollment were diagnosed with B-lineage ALL (confirmed by a central POG laboratory), aged 1 to 9 years, and had an initial WBC less than 50 × 109/L (50 000/μL). Evidence of trisomies 4 and 10 was required if cytogenetics were abnormal (informative); it was assumed that “normal” cytogenetic studies might reflect lack of cell growth or division in the sample, and demonstration of a DNA index more than 1.16 was allowed as a surrogate marker; DNA index was determined by a POG reference laboratory and all cytogenetics were either determined centrally or centrally reviewed by one of the authors (A.J.C.). Fluorescence in situ hybridization (FISH), to determine trisomies of chromosomes 4 and 10, was performed on all samples by another central POG reference laboratory (M.J.P.); these results were not used in determining eligibility for the study but are used in analysis of outcomes. Initial cerebrospinal fluid (CSF) either had no leukemic cells (CNS1) or, if blasts were present, had a total WBC less than 5 (CNS2). All grossly traumatic initial spinal taps in patients with circulating peripheral blasts were included in the CNS2 category even if no blasts were noted in the CSF itself provided the CSF WBC was less than 5. Patients with initial CNS3 status or testicular leukemia were excluded. During the years 1997 to 1998, when the POG had open studies for all patients with B-lineage ALL, this study accrued approximately 20% (276/1374) of protocol registrations for patients older than 1 year. Informed consent for ALL classification studies and registration on POG induction therapy was obtained from legal guardians of all patients prior to the initiation of treatment. A separate consent for POG 9201 was required at the end of induction. All informed consent documents were approved by the local institutional review boards of all institutions entering patients on this protocol, followed then-current POG guidelines, and complied with the Declaration of Helsinki. This study (POG-9201) is registered with the National Cancer Institute (http://www.cancer.gov/clinicaltrials).
Between June 1992 and November 1999, a total of 658 patients were enrolled and initiated induction on the POG 9400 classification study as described in this report. Two patients were biologically ineligible due to cytogenetic findings and 3 others were administratively ineligible due to improper timing and/or signing of informed consent. Three patients died prior to completing induction (2 from overwhelming sepsis present at the time of diagnosis and the third from unclear cause), and an additional patient developed biopsy-proven glomerulonephritis during induction and was not registered on 9201 for treatment. Thus 653 of the 656 patients who were biologically eligible attained remission at the end of induction treatment, with the only failures being 3 induction deaths. Excluding patients not registered for treatment on this protocol, the remaining 649 patients, all of whom attained remission at the end of induction, are included in this report. All data received by the statistical office and/or study coordinator as of April 29, 2004, were included in the analyses. Data through end of treatment or first event were available for all patients with the exception of 4 who were removed from protocol therapy: 2 due to family relocations, 1 due to uncontrolled emesis, and 1 due to parental preference for care from a local physician. These patients were censored at that time point. Fifty-six percent of patients were male, with 68.6% white, 17% Hispanic, 6.6% African American, and 7.8% other races. Median age at enrollment was 3.9 years (range: 1.0-9.9 years). Fifty-four patients had CNS2 involvement or a traumatic spinal tap (red blood cell count 10 or more) at diagnosis. Seventy-three percent of patients had a WBC less than 10 × 109/L (10 000/μL).
Treatment plan
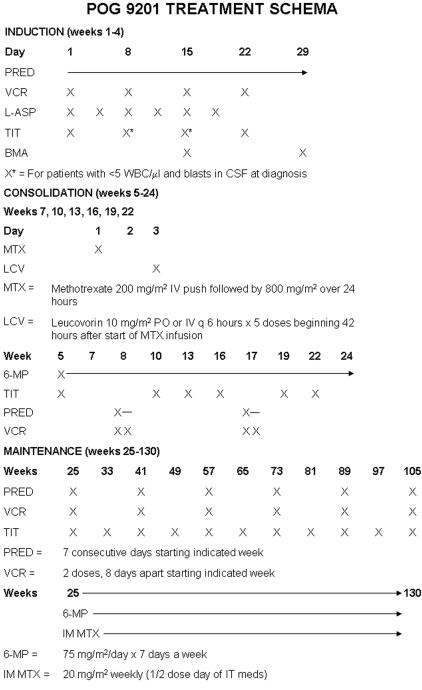
Initial induction therapy for all patients included vincristine (1.5 mg/m2 with 2.0 mg maximum dose) intravenously on days 1, 8, 15, and 22; prednisone 40 mg/m2 per day in 3 divided doses on days 1 to 28 and l-asparaginase 6000 international units/m2 intramuscularly on days 2, 5, 8, 12, 15, and 19. All patients received age-based intrathecal chemotherapy on days 1 and 15; patients with CNS2 status were treated identically to CNS1 patients except for also receiving IT chemotherapy on days 8 and 22 of induction. The initial protocol used triple intrathecal chemotherapy (methotrexate, hydrocortisone, and cytosine arabinoside). This was changed to methotrexate alone when therapeutic modifications were made to address CNS toxicity on companion protocols, although there was no indication of excess CNS toxicity in this study. The number of circulating blasts present on day 8 was recorded for the 368 patients who had circulating blasts present at the time of diagnosis. Percent residual blasts were determined by bone marrow morphology on days 15 and 29 of induction.
Consolidation therapy from weeks 5 to 25 included intravenous methotrexate 1 g/m2 as a 24-hour infusion at weeks 7, 10, 13, 16, 19, and 22 with delayed leucovorin rescue along with oral 6-mercaptopurine (6MP), 50 mg/m2 daily. Simultaneous intrathecal therapy was administered at weeks 10, 13, 16, 19, and 22. Pulses with 2 weekly doses of intravenous vincristine (1.5 mg/m2 with 2.0 mg maximum dose) and 7 days of oral prednisone (40 mg/m2 per day, in 3 divided doses taken with meals; maximum daily dose 60 mg) were given from weeks 8 to 9 and 16 to 17.
Continuation therapy from weeks 25 to 130 included oral 6MP 75 mg/m2 daily and IM methotrexate 20 mg/m2 weekly. Pulses of vincristine and prednisone, as in consolidation, were given at weeks 25 to 26, 41 to 42, 57 to 58, 73 to 74, 89 to 90, and 105 to 106. Intrathecal chemotherapy was given at week 25 and initially every 8 weeks through week 105, which was later modified (due to toxicity on companion protocols) to every 12 weeks to week 109. The original treatment schema is shown in Figure 1; as noted above, some changes were made in intrathecal therapy for consistency with alterations in companion studies felt to have excess toxicity. Patients with initial CNS1 status thus received 14 to 20 intrathecal treatments. There was no indication of a difference in outcome or toxicity related to the changes in the intrathecal prophylaxis schedule or drugs. Patients received Pneumocystis prophylaxis with trimethoprim/sulfamethoxazole, pentamidine, or dapsone from attainment of remission until 6 months following the completion of therapy. A diagnostic lumbar puncture and bone marrow aspiration along with a physical examination and routine complete blood count (CBC) were required at the end of treatment (weeks 130-131). A routine testicular biopsy was not required. Diagnostic spinal taps were planned at 4, 8, and 12 months off treatment while no off-treatment bone marrow aspirates were required in the absence of a clinical suspicion of relapse.
Figure 1.
POG 9201 treatment schema.
Statistical analyses
This was a single-arm nonrandomized study. Event-free survival (EFS) and overall survival (OS) times were computed for all eligible patients on study. Time to an adverse event was defined as days from the date of diagnosis until first relapse, second malignancy, or death from any cause. Patients not experiencing an event were censored as of the date of last contact. The EFS and OS estimates were computed using the Kaplan-Meier method8 and standard errors of the estimates were determined according to Peto and Peto.9
Toxicity grading
Toxicity was graded according to Common Toxicity Criteria (CTC) version 2.0: grade 3 indicating severe; grade 4, unacceptable or life-threatening toxicity; and grade 5, lethal toxicity.10 All toxicities were reviewed and scored by the primary study coordinator. Grade 1 and 2 toxicities (mild and moderate) were generally not considered significant, but all grade 2 or greater neurotoxicity was recorded.
Results
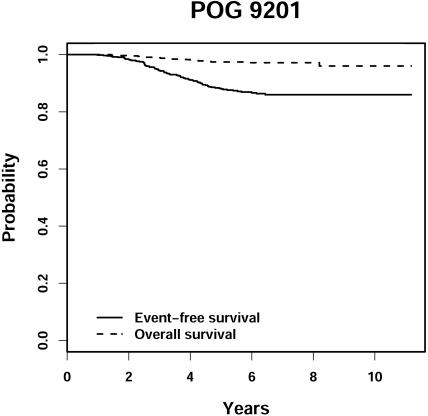
The 6-year EFS and OS were 86.6% ± 1.8% (± standard error) and 97.2% ± 0.87%, respectively (Figure 2). The highest risk for relapse was between 2 and 5 years from diagnosis.
Figure 2.
POG 9201 event-free and overall survival.
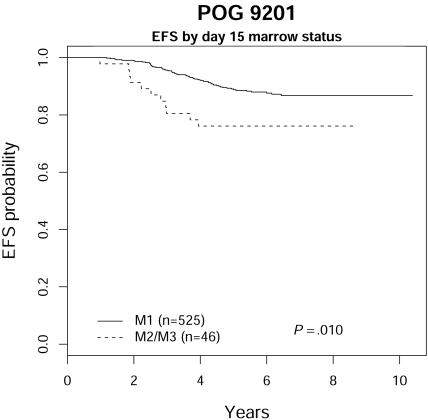
Data were available from day-15 marrow aspirates on 571 patients (others did not have marrow aspirations or the quality was inadequate for interpretation). Patients with 5% or fewer blasts in day-15 marrows (n = 525) had superior EFS (87.6% ± 2.0%) compared with those (n = 46) with more than 5% blasts (76.1% ± 9.3%) with a P value of .010 as shown in Figure 3. This supports multiple prior observations regarding the prognostic value of early response, whether measured by peripheral blasts on day-8 or day-15 bone marrow aspiration.11,12 In this study, only 9 patients had more than 100 blasts/μL at day 8, too few to allow meaningful statistical analysis, although 2 of these 9 patients have relapsed.
Figure 3.
POG 9201 EFS by day-15 marrow status.
There were no statistically significant differences in EFS based upon sex, CNS status, ethnicity, or initial WBC value (< 10 ×109/L versus 10-50 × 109/L [< 10 000 μ/L versus 10 000–50 000 μ/L]). The great majority of patients on this study were CNS1 (595/649 or 91.7%). They had a 6-year EFS of 87.5% ± 1.8% compared with 76.8% ± 8.3% for those who were either CNS2 or had traumatic CSF at diagnosis (n = 54). With this small sample size, results favoring CNS1 patients did not reach standard criteria for statistical significance in EFS (P = .072). Separate analysis of the 28 patients with conventional CNS2 disease (blasts present with red cell count < 10) demonstrated 6-year EFS of 79.6%, providing no indication of this group having a worse outcome than the entire 54 patients recognized with some measure of CNS involvement. Likewise, comparison of EFS (P = .54) between white and all other races showed no significant differences.
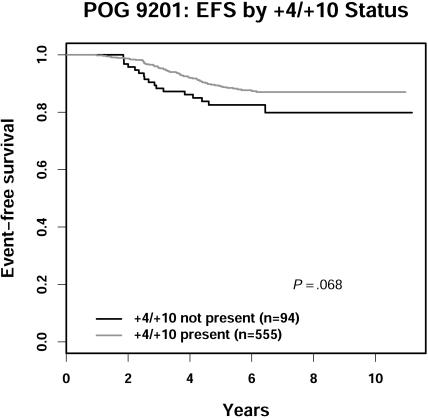
Patients with proven trisomies of chromosomes 4 and 10 (by cytogenetics or FISH) showed a nonsignificant trend toward better EFS compared with those lacking the double trisomies by both techniques (6-year EFS of 87.4% vs 82.5%, P = .068) (Figure 4). The difference in overall survival (97.7% vs 94.5%) was not significant (P = .11). Further, the “most favorable” subset of patients (n = 416) who had trisomies 4 and 10, an M1 marrow on day 15, and were CNS1 at diagnosis had a 6-year EFS of 88.9% ± 1.9% and an OS of 97.7% ± 0.9%, demonstrating that this fails to select patients with only good outcomes.
Figure 4.
POG 9201 EFS by trisomy +4/+10 status.
Salvage therapy and outcomes
Of the 79 relapses on this study, 13 occurred on treatment (3 CNS, 9 marrow, and 1 marrow plus CNS), and 10 were identified via end of treatment evaluation (6 CNS, 3 marrow, and 1 testicular). The 56 other relapses were noted between 2 to 46 months after end of treatment; 2 occurred in patients who had been removed from protocol therapy.
Outcomes for relapsed patients are summarized below, grouped by site of relapse.
Isolated testicular relapse (n = 7).
Isolated testicular relapse was the initial event for 7 patients, all overt on physical examination, and confirmed by biopsy. A single relapse was noted at the end of treatment; 5 between 3 to 8 months off treatment and 1 at 14 months off treatment. These patients included 6 retreated with systemic chemotherapy and testicular radiation; all are alive and well in second remission, off treatment 16 to 54 months. The other patient refused conventional treatment and tried alternative medications for 6 months until he had a marrow relapse. This patient is currently in remission on treatment. Since all were late testicular relapses, they would be expected to have an excellent salvage rate.13
Isolated CNS relapse (n = 12).
There were 12 patients with isolated CNS relapse as their initial event of whom 3 were diagnosed on treatment at weeks 84, 97, and 97. Routine end of treatment LPs identified 6 relapses. The other 3 were identified on routine LPs per protocol 5 to 13 months off treatment. A single patient had headaches for a week prior to the end of treatment LP, while all others were asymptomatic at the time of CNS relapse.
There was one death from brain herniation shortly after an end of treatment CNS relapse (not the patient with headaches). All others had second systemic treatment including cranial or craniospinal radiation with one having a second CNS relapse. This patient is well 11 months following a matched unrelated transplantation. All other patients are alive and well from 1 to 48 months off second treatment. This is in accord with anticipated salvage rates after late (> 18 months after diagnosis) isolated CNS relapse.14
Combined CNS and testicular relapse (n = 1).
A single patient had a CNS and testicular relapse 4 months off therapy and is in second remission 2 years off retreatment with chemotherapy and radiotherapy.
Other extramedullary relapse sites (n = 4).
Less common extramedullary relapse occurred in 4 cases. The first was an extradural, lymphomatous mass (with flow cytometry identical to the original ALL); this patient is more than a year off treatment in second remission. Another patient had a cortical bone relapse 29 months off therapy with 5% marrow blasts marking like the original ALL and is in second remission on chemotherapy 13 months after relapse. The third patient relapsed in a preauricular lymph node 32 months off treatment and is in second remission after 3 months of treatment. The final patient had an orbital relapse 7 months off therapy, received alternative treatments, had a marrow relapse, and died after a transplantation.
Extramedullary relapses with abnormal marrows (n = 3).
There was one testicular relapse 2 months off treatment with 7% marrow blasts. This patient was retreated with chemotherapy and testicular radiation, remaining on treatment 23 months after relapse. Another patient had CNS disease on a routine LP 9 months off treatment and 8% marrow blasts. This patient had a marrow transplantation, relapsed 21 months later, and had a second transplantation. He is free of disease 46 months after second transplantation. The third patient had an ovarian relapse 22 months off treatment with 9% blasts in the marrow and is off treatment, 44 months after relapse.
Bone marrow ± other sites (n = 50).
There were 39 patients with isolated marrow relapse; 9 on treatment and 3 identified at end of treatment evaluation. Of these 12, 9 have died, 4 prior to and 5 after transplantation; 3 survivors are 32 to 56 months after transplantation.
The 27 patients experiencing a posttreatment isolated marrow relapse did so 9 to 38 months off treatment. In this group, 11 had transplantations with 3 deaths, 1 in relapse, and 7 in remission 17 to 60 months after transplantation. There were 16 treated with intensive chemotherapy and 15 are in second remission 10 to 66 months after relapse, while 1 is on treatment in third remission after a CNS relapse.
Marrow relapse was combined with extramedullary relapse in 11 patients, 8 having CNS relapse and 1 each CNS and testicular, testicular, and scalp relapses. One patient relapsed at week 61 and died after transplantation, while the others, relapsing 4 to 46 months off treatment remain alive 1 to 39 months after relapse, 2 after transplantations.
Relapses in patients previously removed from study (n = 2)
A patient off study for spinal myelopathy at week 23 received alternative chemotherapy and had a testicular relapse; he is 14 months off second treatment. Another patient transferred to a noncooperative group physician at week 69, received unknown treatment, and died after marrow relapse. Both were counted as failing at relapse.
Toxicities
The therapy was generally well tolerated. While 560 (86.3%) of the patients had at least one episode of grade 4 hematologic toxicity after induction, there were no episodes of fatal sepsis. The most common nonhematologic toxicity was elevated transaminases with 340 (52%) of patients having at least one episode of grade 3 or 4 toxicity. All of these were reversible and no patient was removed from protocol therapy or had therapy withheld for an excessive period of time as a result.
Neurotoxicity
Acute neurotoxicity was noted in 57 patients (8.8%) during treatment. The great majority were minor, although 2 patients developed major paralysis. The first had clinical findings compatible with Guillain-Barré syndrome shortly after bacterial sepsis. This patient developed spinal myelopathy and has stable paraplegia with a neurogenic bladder. The other patient developed acute quadriplegia after week 73 intrathecal medications as did 2 patients on other studies treated on the same day at the same institution; no additional intrathecal medications were given and the patient remains in first remission. Extensive investigation failed to identify a cause (anonymous by request, oral personal communication, October 8, 1997).
Seizures occurred in 14 (2.2%) patients with 4 during induction, 2 having scans indicating CNS microthrombi, likely related to asparaginase. Of the remaining 10 seizures, 2 were attributed to infection: 1 with bacterial sepsis and 1 with erlichiosis in blood and CSF. Another was felt to be a simple febrile convulsion and one a new onset seizure disorder (focal seizure by electroencephalogram (EEG) 6 weeks after most recent intrathecal medication with normal magnetic resonance imaging [MRI]). The remaining 6 (one with neurofibromatosis) had seizures within 10 days of intrathecal medications with no other risk factors identified.
The relatively low seizure frequency may have been due to the fact that when intrathecal medications were given 3 weeks apart during consolidation, 5 of 6 were administered concurrently with intravenous methotrexate and followed by intravenous fluids and leucovorin rescue, unlike some other reported studies with a higher incidence of seizures in which more frequent intrathecal medications were administered, cranial radiation was given, and/or no intrathecal medications were immediately followed by leucovorin rescue.15–17 This also differs from the seizure frequency on companion POG studies that separated intravenous methotrexate and intrathecal chemotherapy and provided no leucovorin rescue after any intrathecal medications.18 A currently open Children's Oncology Group study is examining this issue in greater detail through studies of neurologic outcomes of patients on differing studies.
Grade 2 or 3 headaches were reported in 5 patients, while 4 had transient motor weakness, aphasia, ataxia, and/or visual complaints; all of these 9 resolved.
There were 31 patients with motor or peripheral nerve toxicities related to vincristine, 6 of no clinical significance. Peripheral neurotoxicity at least transiently impacting normal activity occurred in 25, of whom 3 were proven to have Charcot-Marie Tooth (CMT) disease.19 Of these patients, 18 had changes in vincristine dosing, and 1 patient (with CMT) received no vincristine after induction. All patients with CMT required physical therapy; 2 had long-lasting disabilities.
Deaths in remission
There were 2 deaths in remission, both due to varicella shortly after the week-73 to -74 prednisone pulse. Neither patient was neutropenic or lymphopenic at the time and both were treated with acyclovir within 24 hours of hospital admission. A detailed review of all 110 cases of varicella on this protocol found that more severe infections were seen in patients who received prednisone near the time of the varicella infection.20
Second malignancies
Myelodysplasia characterized by monosomy 7 developed in 2 patients; in neither case was this cytogenetic finding observed at original diagnosis. While this is unusual as a side effect of similar treatments, it has been reported.21 Both patients have had marrow transplantations and are in remission. It is noteworthy that one of these patients had a sibling develop ALL followed by monosomy 7 (Paul Bowman, MD, Cook Children's Hospital, Fort Worth, TX, personal communication, November 5, 2003), which may imply a genetic link22 even though monosomy 7 was not identified at ALL diagnosis.
Discussion
It is clear that patients with high hyperdiploidy by virtue of trisomies of chromosomes such as 4, 10, and 17 or 4, 10, and 18 potentially have a superior outcome.5,23,24 The United Kingdom group has reported a 5-year EFS of 86% for low-risk females and a 5-year overall survival of 96% for all patients aged 1 to 9 years with trisomies 4 and 18.25 However, some studies have shown that lesser-risk children with ALL may actually have results similar to those in standard- and higher-risk groups if their therapy is not sufficiently intense.26,27 It is therefore critical to maintain sufficient intensity to preserve the potentially excellent outcomes seen in this group of patients.
This study demonstrates that moderately-intensive antimetabolite-based chemotherapy produces better than 90% long-term survival in young children with lesser-risk ALL, comprising approximately 20% of childhood cases of B-lineage ALL as noted above. It is noteworthy that none of these children received radiation, anthracyclines or alkylating agents, dexamethasone, or topoisomerase inhibitors as part of their initial treatment protocol. Results from Holland, the Nordic group, and several United States studies have demonstrated 5-year EFS of approximately 85% in their lowest-risk groups when cranial radiation was omitted, further supporting this approach to CNS prophylaxis5,24,28–30 for these patients. A meta-analysis of worldwide trials including almost 3000 patients also showed equivalence of radiotherapy and long-term intrathe-cal therapy.31
Intravenous methotrexate and intrathecal prophylaxis have been associated with long-term neurocognitive dysfunction,17,32 though the degree to which any one child is affected is highly variable. Encouraging studies, describing populations of adults, treated for ALL during childhood, without cranial radiation, have found that the likelihood of being employed, married, and insured is comparable with that of the general population.33 The relatively low seizure frequency in this study may have been due to the fact that when intrathecal medications were given 3 weeks apart during consolidation, they were administered concurrently with intravenous methotrexate and thus were followed by intravenous fluids and leucovorin rescue as noted above.
Vincristine has been associated with long-term neuropathies, but the cumulative dose on this trial was low, as was the cumulative steroid dose. In this young population treated with prednisone rather than dexamethasone, there were no reports of avascular necrosis recorded on the off-therapy follow-up forms submitted by participating institutions.34
Thus it is likely that the great majority of the patients in this study will not only be long-term survivors but will do so with a minimum of significant late effects.
The issue of CNS2 status has been controversial with some noting this as an adverse prognostic feature35 and others finding these patients having outcomes identical to those of CNS1 patients.36,37 This may relate to the fact that patients with initial traumatic CSF have also been noted to have inferior outcomes37,38; they were thus grouped with CNS2 patients in this study. In these lesser-risk patients, with the addition of 2 intrathecal chemotherapy treatments during induction, there was no statistical difference between the CNS1 patients and those in the CNS2 group, as also seen in the St Jude Total Therapy XIIIB, which used a similar strategy.39 This finding remained true when only the 28 patients with CNS2 status as defined by Burger et al37 and Gajjar et al38 were considered. Whether the same result in this defined patient group would have been obtained without this slight intensification of therapy is unknown. The number of CNS2 patients was small enough that a substantial difference in outcome would have been required to reach statistical significance.
Many previous studies have found differences in outcome based upon ethnic group,40–42 though other studies have not confirmed this,43 and the issue when corrected for disease biology remains unclear.44 Males have historically been reported to have an inferior prognosis,45,46 although recent reports focused on lesser-risk patients have found no significant differences related to sex.39,47 Neither race nor sex was predictive of outcome in this study. There have been no deaths among the 44 African-Americans enrolled; the 6-year EFSs for males and females are 84.8% and 88.5%, respectively, with corresponding OSs of 96.4% and 98.2%. One assumes that this is due to the relative uniformity of this lesser-risk group of patients, defined by age, WBC at diagnosis, favorable cytogenetics, and the absence of CNS3 disease. Thus, these host and disease characteristics are the more critical determinants of outcome.
A critical challenge remains, to identify patients at higher risk of relapse who might potentially benefit from more aggressive therapy. Even among this group of lesser-risk patients, greater than 10% of the patients had an event of some type by years 7 to 8. The patients without trisomies 4 and 10 had EFS of about 80% and patients with more than 5% residual blasts on day-15 marrows had an EFS of about 75%. Patients with both of these unfavorable features (n = 6) had an EFS of 50%. Thus, an accurate assessment of blast cytogenetics and the use of early morphologic response to augment therapy may have improved the EFS and overall survival for the group as a whole, especially for those within this favorable group who are identified as having some higher-risk features.48 Whether the use of dexamethasone (instead of prednisone) or a delayed intensification phase would improve outcome is unknown. Some randomized studies, with excellent overall results, have demonstrated the superiority of dexamethasone,49,50 while other trials have not.51 A delayed intensification commonly improves outcome among standard-risk patients, but also adds anthracycline, an alkylating agent and an increased risk of infection.30
It should be noted that our study did not include patients with the TEL:AML112,21 translocation, which is now believed to also confer a generally favorable prognosis, except for slow molecular responders.52 These patients very rarely have hyperdiploidy and would largely represent a distinct group of patients from those who were eligible for this study.53 Whether those with this translocation would have similar outcomes with this therapy is addressed by a subsequent study, POG 9904.
The patients in our study had an overall 7- to 8-year survival of more than 95%. This is in accord with smaller prior studies of patients selected for favorable host and disease characteristics.5,24–26,28–30,39 Further improvements in outcome, without increasing the burden of care for all, will result from the identification of prognostic features, such as the presence of minimal residual disease, within this group, so that selective intensification can be applied. Since significant late toxicities were uncommon, great care must be taken in regard to any reduction of therapy.26,27,48 However, it is clear there is a subgroup of patients, identified in this study by the lack of trisomies of both chromosomes 4 and 10, slow initial response, or both, that has potential to benefit from further intensification of therapy. DNA microarrays, gene expression profiling, and other molecular measures of early response should provide more sensitive techniques for the identification of such slow responders.54,55 Conversely, we predict that rapid disappearance of minimal residual disease, measured by techniques more sensitive than light microscopy, would be associated with an even better outcome than reported here, although the patients selected for most favorable features by the techniques available in this study did not have an EFS significantly better than the entire population.
Supplementary Material
Acknowledgments
This work was supported by NIH grant U10CA98543. A.R.C. and Kathi Dale received support from NIH Grant U10CA30969 awarded to the Pediatric Oncology Group and COG Grant 98643. B.A.B. is a Georgia Cancer Coalition Distinguished Clinician. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm.
The authors thank Mary Opel from the COG Statistical Office in Gainesville, FL, whose efforts made possible the accurate collection of data for all patients. We also thank Kathi Dale whose help in organizing data for the first author was invaluable; Mike Nash of St Jude Children's Hospital for the determination of DNA index on all samples; and Dr Michael Borowitz of Johns Hopkins for confirmation of the lineage in all cases. Dr Naomi Winick made numerous suggestions that greatly improved the paper. Finally, we greatly appreciate the CRAs, RNs, PNPs, and MDs of the POG institutions participating in this study for timely data submission and rapid responses to queries from the study coordinators. The authors also appreciate the valuable assistance of Donna Correia and Roxanne Hernandez in the COG Publications Office.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Presented in abstract form at the American Society of Pediatric Hematology/Oncology meeting, Washington, DC, May 16, 2005.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.R.C. designed the study, reviewed all patient data in detail, and was the primary writer of the paper; P.L.M. was the secondary coordinator, answered queries when A.R.C. was absent, contributed to the design, and reviewed all amendments; S.B.L. and M.D. performed the primary data analyses; B.A.B. coordinated a companion protocol and worked closely with the first author regarding all the amendments and review of outcome data; J.K. coordinated a companion protocol and worked closely with the first author regarding all amendments and overall study design; J.P. coordinated the POG 9400 classification protocol to assure assignment of patients to the correct treatment study and reviewed questions regarding this study during the time of patient accrual; M.J.P. performed FISH analysis on all study patients, coordinated submission of results to the statistical office, and assisted in karyotype review; A.J.C. performed karyotypes on large numbers of patients and reviewed all karyotype results for each patient entered in the study; J.J.S. designed statistical aspects of the study and reviewed all the amendments; B.C., working with the first author on this matter, contributed to the study design, and as POG ALL Chair, oversaw all aspects of the study; he also contributed extensively to the final paper.
A complete list of Children's Oncology Group Study P9201 participating institutions is provided in Document S1, available on the Blood website; see the Supplemental Appendix link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Allen R. Chauvenet, Children's Oncology Group, 440 E Huntington Dr 300, Arcadia, CA 91006; e-mail: achauvenet@triad.rr.com.
References
- 1.Gurney JG, Severson RK, Davis S, Robison LL. Incidence of cancer in children in the United States: sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75:2186–2195. doi: 10.1002/1097-0142(19950415)75:8<2186::aid-cncr2820750825>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339:605–615. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Campana D, Evans WE. Childhood acute lymphoblastic leukaemia–current status and future perspectives. Lancet Oncol. 2001;2:597–607. doi: 10.1016/S1470-2045(01)00516-2. [DOI] [PubMed] [Google Scholar]
- 4.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 5.Harris MB, Shuster JJ, Carroll A, et al. Trisomy of leukemic cell chromosomes 4 and 10 identifies children with B-progenitor cell acute lymphoblastic leukemia with a very low risk of treatment failure: a Pediatric Oncology Group study. Blood. 1992;79:3316–3324. [PubMed] [Google Scholar]
- 6.Hann I, Vora A, Harrison G, et al. Determinants of outcome after intensified therapy of childhood lymphoblastic leukaemia: results from Medical Research Council United Kingdom acute lymphoblastic leukaemia XI protocol. Br J Haematol. 2001;113:103–114. doi: 10.1046/j.1365-2141.2001.02668.x. [DOI] [PubMed] [Google Scholar]
- 7.Schrappe M, Reiter A, Zimmerman M, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981-1995. Leukemia. 2000;14:2286–2294. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Peto R, Peto J. Asymptotically efficient rank invariant test procedure. J Royal Stat Soc. 1972;135:185–198. [Google Scholar]
- 10.National Cancer Institute. Common Toxicity Criteria (CTC). [Accessed December 11, 2006]; Available at: http://ctep.cancer.gov/forms/CTCv204-30-992.pdf. [Google Scholar]
- 11.Ganyon PS, Desai AA, Bostrom BC, et al. Early response to therapy and outcome in childhood acute lymphoblastic leukemia. Cancer. 1997;80:1717–1726. doi: 10.1002/(sici)1097-0142(19971101)80:9<1717::aid-cncr4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Sandlund JT, Harrison PL, Rivera G, et al. Persistence of lymphoblasts on day 15 and days 22 to 25 of remission induction predicts a dismal treatment outcome in children with acute lymphoblastic leukemia. Blood. 2002;100:43–47. doi: 10.1182/blood.v100.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Wofford MM, Smith SD, Shuster JJ, et al. Treatment of occult or late overt testicular relapse in children with acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Clin Oncol. 1992;10:624–630. doi: 10.1200/JCO.1992.10.4.624. [DOI] [PubMed] [Google Scholar]
- 14.Ritchey AK, Pollock BH, Lauer SJ, et al. Improved survival of children with isolated CNS relapse of acute lymphoblastic leukemia: a pediatric oncology group study. J Clin Oncol. 1999;17:2745–3752. doi: 10.1200/JCO.1999.17.12.3745. [DOI] [PubMed] [Google Scholar]
- 15.Chessells JM, Cox TCS, Kendall B, et al. Neurotoxicity in lymphoblastic leukemia: comparison of oral and intramuscular methotrexate and two doses of radiation. Arch Dis Child. 1990;65:416–422. doi: 10.1136/adc.65.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winick NJ, Bowman WP, Kamen BA, et al. Unexpected acute neurologic toxicity in the treatment of children with acute lymphoblastic leukemia. J Natl Cancer Inst. 1992;84:252–256. doi: 10.1093/jnci/84.4.252. [DOI] [PubMed] [Google Scholar]
- 17.Mahoney DH, Shuster JJ, Nitschke R, et al. Acute neurotoxicity in children with B-precursor acute lymphocytic leukemia: an association with intermediate dose methotrexate and intrathecal triple therapy: a Pediatric Oncology Group study. J Clin Oncol. 1998;16:1712–1722. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 18.Rodes S, Bell BA, Abish S, et al. A report of the event-free survival (EFS) and neurotoxicity for children with newly diagnosed standard risk acute lymphoblastic leukemia (ALL) on Pediatric Oncology Group (POG) protocol 9405 [abstract]. Blood. 2005;106:259a. Abstract 882. [Google Scholar]
- 19.Chauvenet AR, Shashi V, Selsky C, et al. Vincristine-induced neuropathy as the initial presentation of Charcot-Marie-Tooth Disease in acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Pediatr Hematol Oncol. 2003;25:316–320. doi: 10.1097/00043426-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Hill G, Chauvenet AR, Lovato J, McLean TW. Recent steroid therapy increases severity of varicella infections in children with lesser risk acute lymphoblastic leukemia. Pediatrics. 2005;116:e525–e529. doi: 10.1542/peds.2005-0219. [DOI] [PubMed] [Google Scholar]
- 21.Aquino VM, Schneider NR, Sandler E. Secondary myelodysplasia with monosomy 7 arising after treatment for acute lymphoblastic leukemia in childhood. J Pediatr Hematol Oncol. 2001;23:48–50. doi: 10.1097/00043426-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Carroll WL, Morgan R, Glader BE. Childhood bone marrow monosomy 7 syndrome: a familial disorder? J Pediatr. 1985;107:578–580. doi: 10.1016/s0022-3476(85)80027-5. [DOI] [PubMed] [Google Scholar]
- 23.Sutcliffe MJ, Shuster JJ, Sather HN, et al. High concordance from independent studies by the Children's Cancer Group (CCG) and Pediatric Oncology Group (POG) associating favorable prognosis with combined trisomies 4, 10 and 17 in children with NCI standard-risk B-precursor acute lymphoblastic leukemia: a Children's Oncology Group (COG) initiative. Leukemia. 2005;19:734–740. doi: 10.1038/sj.leu.2403673. [DOI] [PubMed] [Google Scholar]
- 24.Heerema NA, Sather HN, Sensel MG, et al. Prognostic impact of trisomies of chromosomes 10, 17 and 5 among children with acute lymphoblastic leukemia and high hyperdiploidy (> 50 chromosomes). J Clin Oncol. 2000;18:1876–1887. doi: 10.1200/JCO.2000.18.9.1876. [DOI] [PubMed] [Google Scholar]
- 25.Moorman AV, Richards SM, Martineau M, et al. Outcome heterogeneity in childhood high-hyperdiploid acute lymphoblastic leukemia. Blood. 2003;102:2756–2762. doi: 10.1182/blood-2003-04-1128. [DOI] [PubMed] [Google Scholar]
- 26.Riehm H, Gadner H, Henze G, et al. Results and significance of six randomized trials in four consecutive ALL-BFM studies. Haematol Blood Transfus. 1990;33:439–450. doi: 10.1007/978-3-642-74643-7_81. [DOI] [PubMed] [Google Scholar]
- 27.Toyoda Y, Manabe A, Tsuchida M, et al. Six months of maintenance chemotherapy after intensified treatment for acute lymphoblastic leukemia of childhood. J Clin Oncol. 2000;18:1508–1516. doi: 10.1200/JCO.2000.18.7.1508. [DOI] [PubMed] [Google Scholar]
- 28.Kamps WA, Bokkerink JPM, Hakvoort-Cammel FGAJ, et al. BFM-oriented treatment for children with acute lymphoblastic leukemia without cranial radiation and treatment reduction for standard risk patients: results of DCLSSG protocol ALL-8 (1991-1996). Leukemia. 2002;16:1099–1111. doi: 10.1038/sj.leu.2402489. [DOI] [PubMed] [Google Scholar]
- 29.Gustafson G, Schmiegelow K, Forestier E, et al. Improving outcome through two decades in childhood ALL in the Nordic countries: the impact of high-dose methotrexate in the reduction of central nervous system irradiation. Leukemia. 2000;14:2267–2275. doi: 10.1038/sj.leu.2401961. [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson RJ, Gaynon PS, Sather H, et al. Intensification of therapy for children with lower-risk acute lymphoblastic leukemia: long-term follow-up of patients treated on Children's Cancer Group trial 1881. J Clin Oncol. 2003;21:1790–1797. doi: 10.1200/JCO.2003.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Clarke M, Gaynon P, Hann I, et al. CNS-directed therapy for childhood acute lymphoblastic leukemia: Childhood ALL Collaborative Group Overview of 43 randomized trials. J Clin Oncol. 2003;21:1798–1809. doi: 10.1200/JCO.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 32.Hill JM, Kornblith AB, Jones D, et al. A comparative study of the long term psychosocial functioning of childhood acute lymphoblastic leukemia survivors treated by intrathecal methotrexate with or without cranial radiation. Cancer. 1998;82:208–218. [PubMed] [Google Scholar]
- 33.Pui C-H, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 34.Mattano LA, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report of the Children's Cancer Group. J Clin Oncol. 2000;18:3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 35.Mahmoud HH, Rivera GK, Hancock ML, et al. Low leukocyte counts with blast cells in cerebrospinal fluid of children with newly diagnosed acute lymphoblastic leukemia. N Engl J Med. 1993;329:314–319. doi: 10.1056/NEJM199307293290504. [DOI] [PubMed] [Google Scholar]
- 36.Gilchrist GS, Tubergen DG, Sather HN, et al. Low numbers of CSF blasts at diagnosis do not predict for the development of CNS leukemia in children with intermediate-risk acute lymphoblastic leukemia: a Children's Cancer Group report. J Clin Oncol. 1994;12:2594–2600. doi: 10.1200/JCO.1994.12.12.2594. [DOI] [PubMed] [Google Scholar]
- 37.Burger B, Zimmermann M, Mann G, et al. Diagnostic cerebrospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic lumbar puncture. J Clin Oncol. 2003;21:184–188. doi: 10.1200/JCO.2003.04.096. [DOI] [PubMed] [Google Scholar]
- 38.Gajjar A, Harrison P, Dandlund J, et al. Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood. 2000;96:3381–3384. [PubMed] [Google Scholar]
- 39.Pui C-H, Sandland JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 40.Bhatia S, Sather H, Heerema N, et al. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100:1957–1964. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 41.Pollock BH, DeBaun MR, Camitta BM, et al. Racial differences in the survival of childhood B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group study. Clin Oncol. 2000;18:813–823. doi: 10.1200/JCO.2000.18.4.813. [DOI] [PubMed] [Google Scholar]
- 42.Kadan-Lottick NS, Ness K, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290:2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 43.Pui C-H, Sunderland JT, Pei D, et al. Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA. 2003;290:2001–2007. doi: 10.1001/jama.290.15.2001. [DOI] [PubMed] [Google Scholar]
- 44.Carroll WL. Race and outcome in childhood acute lymphoblastic leukemia. JAMA. 2003;290:2061–2063. doi: 10.1001/jama.290.15.2061. [DOI] [PubMed] [Google Scholar]
- 45.Shuster JJ, Wacker P, Pullen J, et al. Prognostic significance of sex in childhood B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group Study. J Clin Oncol. 1998;16:2854–2863. doi: 10.1200/JCO.1998.16.8.2854. [DOI] [PubMed] [Google Scholar]
- 46.Pui C-H, Boyette JM, Relling MV, et al. Sex differences in prognosis for children with acute lymphoblastic leukemia. J Clin Oncol. 1999;17:818–824. doi: 10.1200/JCO.1999.17.3.818. [DOI] [PubMed] [Google Scholar]
- 47.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 48.Hunger SP, Winick NJ, Sather HN, Carroll WL. Therapy of low-risk subsets of childhood acute lymphoblastic leukemia: when do we say enough? Pediatr Blood Cancer. 2005;45:876–880. doi: 10.1002/pbc.20501. [DOI] [PubMed] [Google Scholar]
- 49.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood. 2003;101:3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell CD, Richards SM, Kinsey SE, et al. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL 97 randomized trial. Br J Haematol. 2005;129:734–745. doi: 10.1111/j.1365-2141.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 51.Igarashi S, Manabe A, Ohara A, et al. No advantage of dexamethasone over prednisolone for the outcome of standard- and intermediate-risk childhood acute lymphoblastic leukemia in the Tokyo Children's Cancer Study Group L95-14 protocol. J Clin Oncol. 2005;23:6489–6498. doi: 10.1200/JCO.2005.01.982. [DOI] [PubMed] [Google Scholar]
- 52.Madzo J, Zuna J, Muzikova K, et al. Slower molecular response to treatment predicts poor outcome in patients with TEL/AML1 positive acute lymphoblastic leukemia. Cancer. 2003;97:105–113. doi: 10.1002/cncr.11043. [DOI] [PubMed] [Google Scholar]
- 53.Rubnitz JE, Downing JR, Pui C-H, et al. TEL gene rearrangement in acute lymphoblastic leukemia: a new genetic marker with prognostic significance. J Clin Oncol. 1997;15:1150–1157. doi: 10.1200/JCO.1997.15.3.1150. [DOI] [PubMed] [Google Scholar]
- 54.Holleman A, Cheok MH, den Boer ML, et al. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351:533–542. doi: 10.1056/NEJMoa033513. [DOI] [PubMed] [Google Scholar]
- 55.Pui C-H, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 56.Chauvenet A, Martin P, Bell B, et al. Anti-metabolite therapy for lesser-risk B-lineage acute lymphoblastic leukemia of childhood: Pediatric Oncology Group (POG) Study 9201. Ped Blood Cancer. 2005;44:567. doi: 10.1182/blood-2006-12-061689. Abstract 1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chauvenet A, Martin P, Bell B, et al. Anti-metabolite therapy for lesser-risk b-lineage acute lymphoblastic leukemia of childhood: Pediatric Oncology Group (POG) Study 9201: International Society of Pediatric Oncology 2005. Ped Blood Cancer. 2005;45:421. Abstract O148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.