Abstract
Objectives
To define optical coherence tomographic (OCT) criteria for the diagnosis of a lamellar macular hole, and to increase understanding of lamellar hole pathogenesis by examining fine anatomic features using ultrahigh-resolution optical coherence tomography (UHR OCT).
Design
Retrospective observational case series.
Participants
Nineteen eyes of 18 patients with lamellar holes were imaged with UHR OCT between 2002 and 2004.
Methods
A UHR OCT system was developed for use in the ophthalmology clinic. All 6 UHR OCT images for each eye imaged were examined. Lamellar holes were diagnosed based on a characteristic OCT appearance. Criteria for the OCT diagnosis of a lamellar hole were as follows: (1) irregular foveal contour; (2) break in the inner fovea; (3) intraretinal split; and (4) intact foveal photoreceptors. From 1205 eyes of 664 patients imaged with UHR OCT, and retrospectively reviewed, 19 eyes of 18 patients were diagnosed with a lamellar hole based on these criteria. All 19 eyes were also imaged with standard resolution OCT. Their charts were retrospectively reviewed.
Main Outcome Measures
Standard and ultrahigh-resolution OCT images.
Results
On chart review, clinical diagnosis of a lamellar hole was made in only 7 of 19 eyes (37%). Twelve of 19 eyes (63%) had an epiretinal membrane (ERM) on clinical examination. Ten of 19 eyes (53%) had a posterior vitreous detachment. On UHR OCT, 17 of 19 eyes (89%) had ERMs. Eleven ERMs had an unusual thick appearance on UHR OCT. Due to poor visual acuity, 4 eyes underwent vitrectomy. Only 1 of 4 surgeries (25%) was visually and anatomically successful. Another eye improved visually, but a lamellar hole persisted. One eye progressed to a full-thickness macular hole preoperatively, which reopened after surgery. One eye developed a full-thickness hole postoperatively.
Conclusions
The diagnosis of a lamellar hole can be made based on OCT criteria, which could be applied to both standard and ultrahigh-resolution OCT. The increased resolution of UHR OCT sheds light on the pathogenesis of the lamellar hole. Epiretinal membranes were visualized on UHR OCT in the majority of eyes. Many ERMs had an unusual thick appearance on UHR OCT, which may represent either trapped vitreous or posterior hyaloid, and may help stabilize retinal anatomy. Conversely, ERM contraction may play a role in lamellar hole formation. Vitrectomy surgery was anatomically and visually successful in only 1 of 4 patients, suggesting caution when performing vitrectomy on lamellar holes.
The term lamellar macular hole was originally suggested by Gass1 in 1975, when he identified a macular lesion resulting from cystoid macular edema. Since then, the term lamellar hole has been used to describe an abortive process in full-thickness macular hole formation, in which, clinically, the patient has relatively preserved visual acuity, usually 20/40 or better, and the macula contains a stable, round, and well-circumscribed reddish lesion.2–8 Recently, optical coherence tomography (OCT) evaluation has become beneficial in the diagnosis of lamellar holes, as it is able to visualize retinal anatomy with near microscopic resolution. Optical coherence tomographic studies have shown that lamellar holes have a thin fovea with avulsion of inner layers of the macula.2–5,9,10 However, reports of lamellar holes on OCT are rare, and the specific definition, pathogenesis, and surgical recommendations for this macular condition remain unclear.
Optical coherence tomography is an imaging method capable of high resolution that has become clinically useful for the evaluation of retinal anatomy.11,12 It assists in understanding the definition, pathogenesis, and progression of a wide variety of retinal diseases.13–18 OCT has proven beneficial in visualizing the vitreoretinal interface, as details of the relation of the posterior hyaloid to the retina can be seen.5,19–22 Optical coherence tomographic imaging of the vitreoretinal interface has aided in distinguishing similar, yet distinct conditions, such as vitreomacular traction syndrome, full-thickness macular holes, macular pseudohole, and lamellar macular hole.2–5,9,13,17,19,21,23,24
With the aid of OCT imaging, vitreomacular traction has been implicated in the pathogenesis of vitreomacular traction syndrome, full-thickness macular hole, and lamellar hole.2–5,13,19,20,25–27 In vitreomacular traction syndrome, the posterior hyaloid partially separates from the retina, but remains attached at the fovea, causing cystoid macular edema.13,19,25 Optical coherence tomography has also been used to document the progression of vitreomacular traction to full-thickness macular holes. The earliest stage in macular hole formation seems to be vitreomacular traction (i.e., the vitreous partially separates from the macula while remaining attached at the fovea). This subsequently causes a dehiscence of the umbo and formation of a macular hole.4,5,20,26,27 Similar vitreomacular traction has been implicated in lamellar macular hole formation.2–5 However, it is not known why full-thickness macular holes occur in some patients and lamellar holes, in others.
Our group recently developed a new generation of ultrahigh-resolution optical coherence tomography (UHR OCT) technology capable of axial image resolutions of ~3 μm in the human eye.28,29 Enhanced imaging capabilities of UHR OCT improve the visualization of the intraretinal architecture. Our group previously reported a series of macular holes imaged with UHR OCT, including one case of a lamellar hole.10 Here we present a series of eyes with lamellar holes imaged with both standard resolution OCT and UHR OCT. Our goals are to clarify the definition of the lamellar macular hole via OCT, to examine fine anatomical features of lamellar holes using UHR OCT, and to strengthen the understanding of lamellar hole pathogenesis using UHR OCT.
Materials and Methods
The principle of OCT imaging has been described in detail in previous publications.11,12 The axial resolution in OCT imaging is inversely proportional to the bandwidth of the light source used for imaging. Stratus OCT (Carl Zeiss Meditec, Inc, Dublin, CA) uses a super luminescent diode light source that generates ~25 nm of bandwidth at ~800 nm center wavelength, and is capable of axial imaging resolution of ~10 μm. Stratus OCT images of the macula uses standard scans of 2 mm axial depth and 6 mm in the transverse direction. The StratusOCT images have ~10 μm axial and 20 μm transverse resolution in tissue, and consisted of 1024 axial pixels and 512 transverse pixels (i.e., a total of 524 288 pixels).
Our group developed a prototype UHR OCT system capable of performing studies in the ophthalmology clinic.10 A specially designed femtosecond, titanium:sapphire laser was used as the light source for UHR OCT imaging.30 The femtosecond laser generates ~125 nm of bandwidth at ~815 nm center wavelength, and the UHR OCT system is capable of axial imaging resolution of ~3 μm. The UHR OCT image uses scans with a 1.5 mm axial depth and 6 mm in the transverse direction. The UHR OCT images have ~3 μm axial and 15 to 20 μm transverse resolution in tissue and consisted of 3000 axial and 600 transverse pixels (total = 1 800 000 pixels). The prototype UHR OCT clinical ophthalmic system has been described in detail in previous studies.10
Optical coherence tomographic imaging is performed within well-established, safe retinal exposure limits set by the American National Standards Institute. The American National Standards Institute standard for safe retinal exposure accounts for wavelength, duration, and multiple exposures of the same spot on the retina.31 For this study, UHR OCT imaging was performed using the same incident optical power as Stratus OCT, as reported by our group in previous UHR OCT studies.10
The standard Stratus OCT imaging protocol was followed with both OCT systems in order to enable a direct comparison of the resulting images. Six radial macular scans of 6 mm length each were acquired at angles separated by 30° intervals. After OCT imaging was completed, all Stratus OCT and UHR OCT images were corrected for axial motion using standard re-registration algorithms. These algorithms have been used in all of the previous prototype and commercial OCT systems.32
Imaging was performed using our UHR OCT prototype in the ophthalmology clinic of the New England Eye Center at Tufts–New England Medical Center. The study was approved by the institutional review board committees of both the Tufts–New England Medical Center and the Massachussetts Institute of Technology, and is compliant with the Health Insurance Portability and Accountability Act of 1996. Data from each patient was kept in a database accessible only to researchers authorized by the patient, as required under the Health Insurance Portability and Accountability Act, who were approved by the Tufts and/or the Massachussetts Institute of Technology institutional review boards. Written informed consent for use of the images was obtained from all of the subjects in this study before UHR OCT imaging was performed.
One thousand two hundred five eyes of 664 patients were imaged with UHR OCT at New England Eye Center between November 2002 and September 2004. All 6 images for each eye were retrospectively evaluated. Lamellar holes were diagnosed based on UHR OCT appearance. Criteria for diagnosis of lamellar hole were as follows: (1) an irregular foveal contour; (2) a break in the inner fovea; (3) separation of the inner from the outer foveal retinal layers, leading to an intraretinal split; (4) absence of a full thickness foveal defect with intact photoreceptors posterior to the area of foveal dehiscence.
A lamellar hole likely caused by epiretinal membrane contraction is shown in Figure 1. Of note, this example may be categorized by clinicians as a macular pseudohole on fundus examination, as it is a stable macular lesion resembling a full thickness hole that is surrounded by epiretinal membrane. However, on OCT, it is more specifically a lamellar hole secondary to epiretinal membrane contraction, meeting the OCT criteria for a lamellar hole as previously defined.
Figure 1.
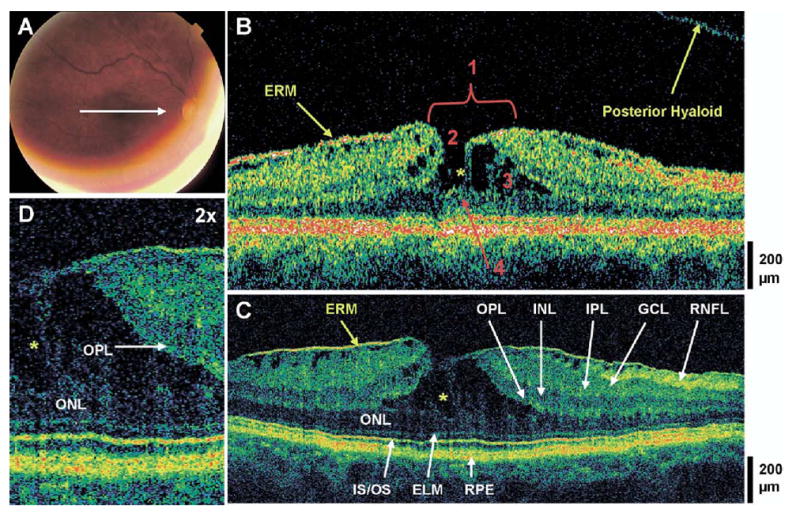
Lamellar hole and typical epiretinal membrane (ERM). The patient is a 69-year-old woman who presented with a best-corrected visual acuity of 20/40 in the right eye. Dilated fundus examination revealed an ERM in the macula, with a small sharply-circumscribed red lesion at the fovea, which was believed to be a pseudohole. A, Fundus photograph depicting lamellar hole and the direction of optical coherence tomographic (OCT) scans. B, Stratus OCT image demonstrates all 4 criteria for the diagnosis of a lamellar hole, which are labeled 1, an irregular foveal contour; 2, break in the inner fovea; 3, separation of the inner from the outer foveal retinal layers; and 4, absence of a full thickness foveal defect with intact foveal photoreceptors. The posterior hyaloid is detached from the macula, and an ERM of typical appearance is visible. C, Ultrahigh-resolution optical coherence tomographic (UHR OCT) image, which also shows intraretinal separation occurring between the outer plexiform layer (OPL) and the outer nuclear layer (ONL). The foveal photoreceptor layers are intact below the area of foveal dehiscence (i.e., the ONL, the inner/outer segment junction [IS/OS], and the external limiting membrane [ELM]). Other retinal layers and the retinal pigment epithelium (RPE) are labeled as follows: retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), and inner nuclear layer (INL). D, Magnification (×2) of UHR OCT image shows strands of tissue spanning between the separated ONL and OPL (yellow asterisks), and the intraretinal split.
All eyes examined with UHR OCT between 2002 and 2004 that fit the previously described criteria were included in this report. Based on this criteria, 19 eyes of 18 patients were found to have a lamellar macular hole on UHR OCT, and their charts were reviewed. Best-corrected visual acuity was measured using standard Snellen eye charts. To calculate mean visual acuity, Snellen acuities were converted to the logarithm of the minimum angle of resolution scale, and the mean logarithm of the minimum angle of resolution visual acuity was calculated, and was then converted back to the Snellen scale. Ocular history, full ocular examination, Stratus OCT, and fundus photographs were recorded. Eight of 18 patients were men, and 10 were women. Mean age was 64 years (range, 38–80). Eleven of 19 eyes were the right eye.
The UHR OCT images of normal macula have been presented in prior studies. The retinal nerve fiber layer (RNFL), ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer (OPL), outer nuclear layer (ONL), external limiting membrane, inner/outer segment junction, and retinal pigment epithelium can be distinguished on UHR OCT. In this study, we identified the retinal layers in concordance with these prior reports.10,28,29,33,34
Results
Mean best-corrected visual acuity (BCVA) for patients with a lamellar hole was 20/43 (range, 20/20 to counting fingers at 8 feet). However, only 2 eyes had a BCVA of less than 20/100. One of these eyes had undergone vitrectomy surgery for a full thickness macular hole several months prior to imaging, and had a lamellar hole at the time of UHR OCT imaging. The other of these 2 eyes had decreased vision after a blunt trauma 20 years previously. Another patient had already had a pars plana vitrectomy for a macula-off retinal detachment 7 years prior to imaging; the BCVA in this patient was 20/30 at the time of UHR OCT imaging.
Mean refractive error was −2.55 diopters (D) (range, +1.50 D to −9.00 D). On initial clinical examination, only 7 of 19 (37%) eyes were initially diagnosed with a lamellar hole. Other initial diagnoses included macular pseudohole (4 eyes, 21%), epiretinal membranes (ERMs) with or without cystoid macular edema (5 eyes, 26%), full-thickness macular hole (2 eyes, 11%), and cystoid macular edema alone (1 eye, 5%). Of the 19 eyes, 12 eyes (63%) were reported to have an ERM on dilated fundus examination. Seven eyes (37%) had a posterior vitreous detachment (PVD) visible on examination.
For this study, the diagnosis of lamellar hole was made based on UHR OCT appearance, as defined in the “Materials and Methods” section. However, Stratus OCT was also able to demonstrate the 4 OCT criteria for lamellar hole diagnosis in all 19 eyes. The UHR OCT revealed additional structural details. On UHR OCT of all patients, the intraretinal split occurred either between the high backscattering OPL and the low backscattering ONL, or within the OPL. Additional findings seen on UHR OCT were ERMs, vitreomacular traction, and a visibly detached posterior hyaloid above the macula. The ERM was defined as a highly reflective band immediately anterior to the retina, as defined in previous OCT studies.3,18,24,35
Figure 1 shows a lamellar hole with a typical OCT appearance of the ERM. Seventeen of 19 eyes (89%) had ERMs on UHR OCT; all ERMs seen on fundus examination were visualized with UHR OCT. Eleven of these ERMs had an unusual appearance on UHR OCT; a less reflective area was present between the RNFL and the inner border of the ERM (Fig 2). In one case, this thickened ERM appeared continuous with the posterior hyaloid (Fig 3). In 6 of 11 eyes with these thick membranes, no PVD was apparent on biomicroscopy or OCT.
Figure 2.
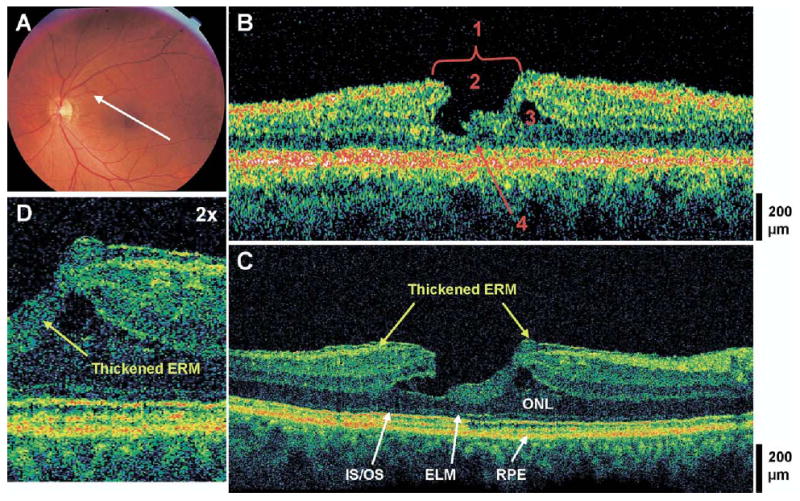
Lamellar hole and thick epiretinal membrane. The patient is a 65-year-old woman who presented with a best-corrected visual acuity of 20/30 in both eyes. She was diagnosed with bilateral lamellar holes 4 years prior. Dilated fundus examination revealed a mild epiretinal membrane (ERM) with a small sharply-circumscribed red lesion in the macula of the left eye. A, Fundus photograph depicting lamellar hole and the direction of the optical coherence tomographic (OCT) scans. B, Stratus OCT image meeting all 4 criteria for the diagnosis of the lamellar hole which are labeled as follows: 1, an irregular foveal contour; 2, break in the inner fovea; 3, separation of the inner from the outer foveal retinal layers; and 4, absence of a full thickness foveal defect with intact foveal photoreceptors. C, The corresponding ultrahigh-resolution optical coherence tomographic (UHR OCT) image, which also shows a thick ERM of moderate reflectivity. The photoreceptors (outer nuclear layer [ONL], the inner/outer segment junction [IS/OS], and the external limiting membrane [ELM]) are intact across the macula. D, Magnification (×2) of the UHR OCT image shows the thick ERM of moderate reflectivity dipping posteriorly into the area of foveal dehiscence. RPE = retinal pigment epithelium.
Figure 3.
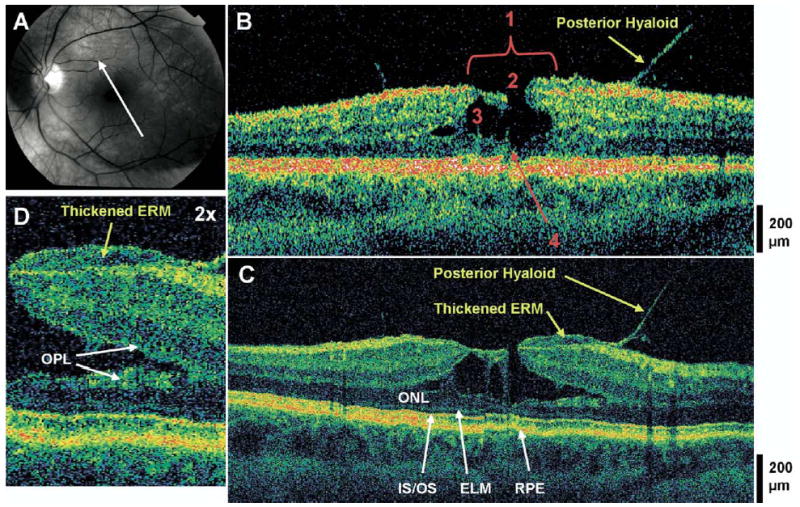
Lamellar hole, thick epiretinal membrane, and vitreomacular traction. The patient is a 46-year-old myopic woman (−7.25 diopters) who presented with a best-corrected visual acuity of 20/40 in the left eye. Dilated fundus examination revealed a prominent posterior hyaloid. There was a mild epiretinal membrane (ERM) with a small sharply-circumscribed red lesion in the fovea, which was believed to be a full-thickness macular hole with surrounding macular thickening. A, Red-free fundus photograph depicting the lamellar hole and the direction of the optical coherence tomographic (OCT) scans. B, Stratus OCT image meeting all 4 criteria for the diagnosis of lamellar hole, which are labeled as follows: 1, an irregular foveal contour; 2, break in the inner fovea; 3, separation of the inner from the outer foveal retinal layers; and 4, absence of a full thickness foveal defect with intact foveal photoreceptors. Vitreomacular traction is apparent. C, The corresponding UHR OCT image also shows a thick ERM of moderate reflectivity. This membrane appears continuous with the partially-separated posterior hyaloid. The photoreceptors (i.e., the outer nuclear layer [ONL], the inner/outer segment junction [IS/OS], and the external limiting membrane [ELM]) are intact across the macula. D, Magnification (×2) of UHR OCT image shows the thick ERM as it extends to the edge of the foveal break. In this case, the intraretinal split appears to be within the outer plexiform layer (OPL). RPE = retinal pigment epithelium.
Vitreomacular traction was observed on OCT in 2 eyes (Figs 3, 4). The posterior hyaloid was detached from the macula and visible on OCT in 3 eyes (Figs 1, 5), suggesting a recent detachment of the posterior hyaloid from the macula. Therefore, adding these 3 eyes to the 7 eyes with PVD seen on biomicroscopic examination, the total number of patients with PVD was 10 (53%). All eyes without PVD had an ERM on examination and/or OCT.
Figure 4.
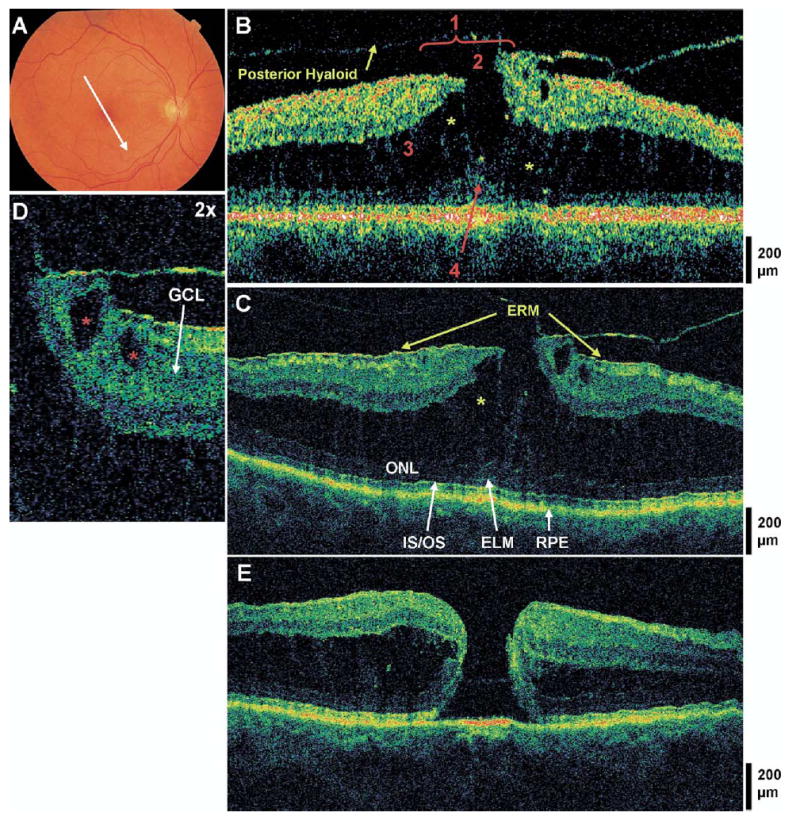
Lamellar hole, typical epiretinal membrane, and vitreomacular traction. The patient is a 61-year-old woman who presented with a best-corrected visual acuity of 20/50 in the right eye. Dilated fundus examination showed an epiretinal membrane (ERM) with diffuse cystoid macular edema. A, Fundus photograph depicting the lamellar hole and direction of the optical coherence tomographic (OCT) scans. B, Stratus OCT image meeting all 4 criteria for the diagnosis of lamellar hole, which are labeled as follows: 1, an irregular foveal contour; 2, break in the inner fovea; 3, separation of the inner from the outer foveal retinal layers; and 4, absence of a full thickness foveal defect with intact foveal photoreceptors. Vitreomacular traction is apparent. A large amount of intraretinal fluid is spitting the inner and outer retina, with multiple strands of tissue spanning between the split layers (yellow asterisks). C, The corresponding ultrahigh-resolution optical coherence tomographic (UHR OCT) image, which also shows a thin ERM anterior to the macula not detected by Stratus OCT. The outer nuclear layer (ONL), the inner/outer segment junction (IS/OS), and the external limiting membrane (ELM) are intact across the macula. D, Magnification (×2) of the UHR OCT image. Two small cysts are seen within the ganglion cell layer (GCL) (red asterisks). E, Postoperative UHR OCT image. The patient underwent a pars plana vitrectomy with peeling of the posterior hyaloid from the fovea, air/fluid exchange, and sulfur hexafluoride 20% injection. The patient subsequently developed a full-thickness macular hole.
Figure 5.
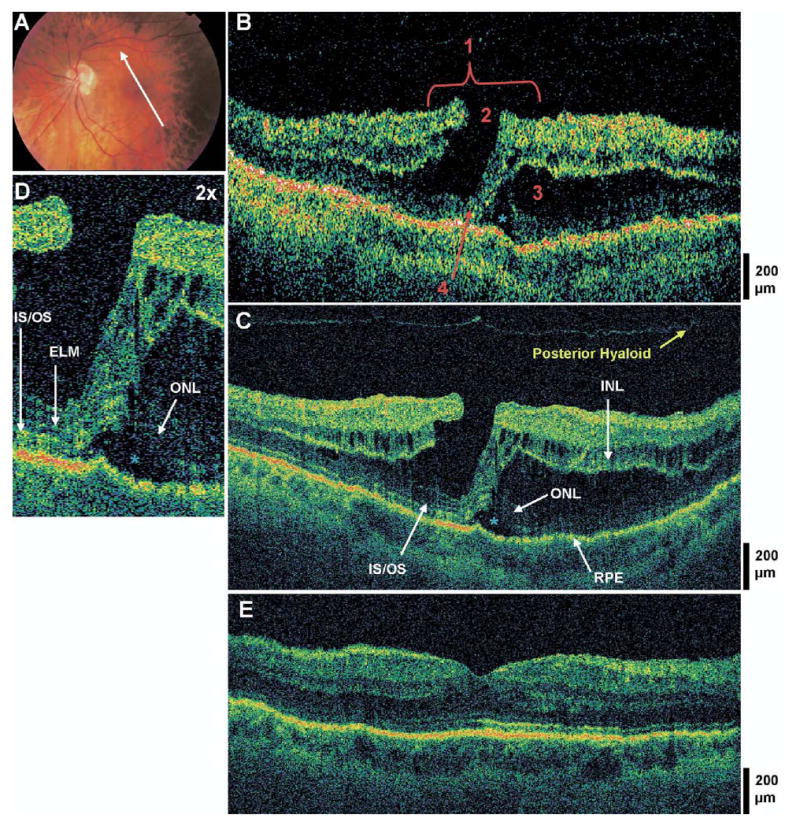
Lamellar hole with photoreceptor disruption and subfoveal fluid. The patient is a 57-year-old myopic woman (−4.75 diopters) who presented with a best-corrected visual acuity (BCVA) of 20/100 in the left eye. Dilated fundus examination revealed a sharply-circumscribed red lesion in the left macula, believed to be a pseudohole. A, Fundus photograph depicting lamellar hole and the direction of optical coherence tomographic (OCT) scans. B, Stratus OCT image meeting all 4 criteria for the diagnosis of lamellar hole, which are labeled as follows: 1, an irregular foveal contour; 2, break in the inner fovea; 3, separation of the inner from the outer foveal retinal layers; and 4, absence of a full thickness foveal defect with intact foveal photoreceptors. A large strand of tissue connects the inner to the outer retina at the fovea. C, The corresponding UHR OCT image, which also shows disruption of the inner/outer segment junction (IS/OS) junction and the external limiting membrane (ELM) at the fovea, but the outer nuclear layer (ONL) remains intact underneath the area of foveal dehiscence. A small pocket of subfoveal fluid is present (blue asterisk). Small cystic spaces are present within the inner nuclear layer (INL). D, Magnification (×2) of UHR OCT image, showing a subretinal fluid pocket and photoreceptor disruption. E, Postoperative UHR OCT image. The patient underwent a pars plana vitrectomy with peeling of the internal limiting membrane (ILM), air/fluid exchange, and sulfur hexafluoride 20% injection. Six months later, the hole was flat and closed on fundus examination and OCT, and BCVA had improved to 20/30 in the left eye.
Due to poor visual acuity, 4 eyes subsequently underwent pars plana vitrectomy, air/fluid exchange, and sulfur hexafluoride (20% to 25%) gas bubble injection. All 4 patients also had slight variations in their intraoperative procedures. One patient had peeling of an ERM intraoperatively. Two months postoperatively, this patient’s visual acuity improved from counting fingers at 8 feet to 20/125; however the lamellar hole remained open on fundus examination and OCT. The second patient developed a full-thickness macular hole after an initial UHR OCT evaluation showing a lamellar hole, and subsequently underwent intraoperative internal limiting membrane and posterior hyaloid peeling. Postoperatively, the full-thickness hole was closed initially, but was reopened several weeks later.
Preoperative and postoperative images from the third patient are shown in Figure 4. She underwent peeling of the posterior hyaloid for vitreomacular traction. The patient developed a full-thickness macular hole postoperatively. Preoperative and postoperative images for the fourth patient are shown in Figure 5. The patient underwent internal limiting membrane peeling intraoperatively. Six months postoperatively, the patient’s visual acuity had improved from 20/100 to 20/30, and the retina was flat and closed on fundus examination and OCT.
Discussion
Before OCT was available, the diagnosis of lamellar hole was based on certain characteristic history and examination findings. With the advent of widespread clinical use of OCT, there has been renewed interest in the diagnosis of lamellar macular hole.2–5,9,10 Optical coherence tomography is able to visualize the macular anatomy in high detail, and can distinguish the lamellar hole from similar, but distinct, macular conditions.3–5,9,10,13,17,19,21,23,24 Typically, lamellar holes have been regarded as the end result of an abortive process in full-thickness macular hole formation. Lamellar holes of this etiology have been shown to have a specific configuration on OCT. However, we have noted that an identical OCT can be found in a variety of etiological conditions. For this reason, we attempted to clarify the definition of lamellar macular hole on OCT, to examine fine anatomical features using UHR OCT imaging, and to increase the understanding of the lamellar hole pathogenesis. Distinguishing lamellar holes from other conditions is important, as each has different surgical implications. However, we are aware of only one other study that has shown a series of lamellar macular holes imaged with OCT.3
In the study by Haouchine et al,3 only 28% of lamellar holes diagnosed with OCT were diagnosed as lamellar holes on fundus examination. In our study, only 37% of lamellar holes on OCT were also diagnosed as such on initial fundus examination. This data suggests that OCT imaging is beneficial in the diagnosis of lamellar holes. It is therefore essential to develop OCT criteria to distinguish lamellar macular holes from similar macular conditions. The diagnosis can be made by qualitative image analysis of the 6 radial macular images taken using the standard OCT imaging protocol. We present 4 basic criteria for the OCT diagnosis of lamellar holes: (1) an irregular foveal contour; (2) a break in the inner fovea; (3) a dehiscence of the inner foveal retina from the outer retina; and (4) an absence of a full thickness foveal defect with intact foveal photoreceptors. The criteria can be applied to both standard and ultrahigh-resolution OCT.
These criteria differ slightly from findings in previous OCT and histopathologic reports of lamellar holes.3–5,9,36,37 One feature of lamellar holes common to patients from various studies was thinning of the fovea. The study by Haouchine et al3 also found that on average the surrounding perifoveal retina was of normal thickness. We attempt to define criteria for lamellar hole diagnosis that do not necessitate measurement of retinal thicknesses, and can instead be identified by close examination of an OCT image. The UHR OCT helps to visualize retinal anatomy specific to lamellar macular holes. In all 19 eyes, the separation of the inner from the outer retinal layers occurred either between the high backscattering OPL and the low backscattering ONL, or only within the OPL. Spanning this separation were often strands of retinal tissue, which may signify Mueller cell processes and/or photoreceptor axons (Henle fibers). Mueller cell processes are known to extend from the internal to the external limiting membrane, and photoreceptor axons originate from photoreceptor cell bodies in the ONL and extend into the inner retina.38 Mueller cell strands spanning the separation between the inner and outer retina may help to prevent dehiscence of the outer retinal layers.39
As reported by Haouchine et al,3 epiretinal membranes are common OCT findings in cases of lamellar holes; 62% of 29 cases in their study had ERM on OCT. In our study, UHR OCT found that 89% of patients had an ERM. The higher number in our study is likely due to increased resolution of the UHR OCT, which enhanced the ability to detect ERMs. Examples of this are seen in Figures 2, 3, and 4. The UHR OCT was also able to image all ERMs that were seen on dilated fundus examination.
Two distinct appearances of ERMs were observed on UHR OCT. One appearance was identical to previous reports of ERM on OCT (i.e., a thin highly-reflective line immediately anterior to and separate from the RNFL).3,18,24,35 However, in this study, the majority of ERMs on UHR OCT had an unusual appearance (i.e., a moderately-reflective material filled the space between the inner border of the ERM and the RNFL). In Figure 3, this membrane even appeared continuous with the posterior hyaloid.
It is unclear what this appearance of ERM on UHR OCT represents. One possibility is an ERM with trapped vitreous between it and the retina. An electron microscopic study by Foos40 has previously shown densely-packed vitreous fibrils between the ERM and the retina in some cases. The finding suggested that in these cases, the ERM grew anterior to the retina before the vitreous separated from the retina. Visualization of the thick ERMs of moderate reflectivity with UHR OCT, along with the finding that 89% of lamellar macular hole patients in this study had ERMs on UHR OCT, may suggest the implication of ERMs in the pathogenesis of the lamellar macular hole. It is believed that ERMs around full-thickness holes contract and lead to hole enlargement, and it is recommended to peel ERMs during vitrectomy for full-thickness macular hole repair.41–43 However, in the case of lamellar holes, it may be possible for an ERM to form before vitreous separation, thereby stabilizing the retina and preventing further foveal dehiscence and the formation of a full-thickness hole.
Another possibility is that this unusual thick membrane on UHR OCT represents posterior hyaloid or vitreous remnants attached to the macula. Figure 3 demonstrates a thick ERM that appears continuous with the partially-detached posterior hyaloid. Additionally, in 6 of 11 thick ERMs, no PVD was apparent on either biomicroscopy or OCT, suggesting that the posterior hyaloid remained attached to the retina in some of these patients. If the thick membrane is, in fact, posterior hyaloid, this may suggest that posterior hyaloid or vitreous remnants can actually stabilize retinal architecture, and prevent the formation of a full-thickness macular hole. Vitreous remnants have been shown by histopathology to remain attached to the fovea after vitreous detachment.44
Based on our proposed OCT definition of a lamellar macular hole, a variety of possible mechanisms for lamellar hole pathogenesis were suggested. Only 10 of 19 eyes (53%) had a PVD, on examination or on OCT, that would suggest an abortive process in the macular hole formation. However, the remaining 9 eyes without PVD all had ERMs, along with 8 of 10 of the eyes with PVD. Therefore, ERM contraction may play a role in lamellar hole formation in many patients, similar to the formation of a macular pseudohole. One patient had a lamellar hole after blunt trauma, and 2 patients also had lamellar holes after vitrectomy (1 for a macular hole and 1 for a retinal detachment). It seems clear that the pathogenesis of lamellar macular holes cannot simply be attributed to an abortive process in macular hole formation. Notably, Haouchine et al3 reported pseudo-opercula, suggestive of an aborted macular hole, in only 24% of patients.
In our study, due to the relatively small size of the UHR OCT database, we were able to evaluate all UHR OCT images taken during a 2-year period; therefore, a wide variety of patients was included in the initial image analysis. Also, lamellar hole diagnosis was based on strict OCT criteria, differing from prior OCT studies of lamellar holes. For these reasons, it is not surprising that there seems to be a wide variety of etiologies of the lamellar hole in our study. It seems that the configuration of the lamellar hole may not only be due to an abortive process of macular hole formation, or a consequence of macular edema as reported initially by Gass,1 but it may also be caused often by ERM contraction.
Vitrectomy surgery for macular holes has been proven successful.45–48 Vitrectomy for the treatment of macular pseudohole and/or vitreomacular traction is also indicated if the condition is visually significant.24,49–52 However, surgical treatment of lamellar holes has not been evaluated. In our study, 4 patients underwent vitrectomy surgery for poor visual acuity. Only one case was anatomically and visually successful. A second case had improvement in vision, but continued to have a persistent lamellar hole after surgery. Another case progressed to a full-thickness macular hole prior to surgery, which reopened after vitrectomy. The last case developed a full-thickness hole after vitrectomy and ERM peel. Although this is not enough data to make definitive conclusions, the experience of these 4 cases suggests caution when performing vitrectomy on lamellar holes.
Lamellar macular hole is regarded as a stable macular condition (one of our patients did progress to a full-thickness hole while being observed).3–9 Surgical peeling of the posterior hyaloid, ERM, or internal limiting membrane may destabilize the lamellar hole configuration, and may lead to the progression of a full-thickness hole. Alternatively, in certain cases in which the ERM is involved in formation of the lamellar hole, ERM peeling may be beneficial. A prospective study evaluating surgical treatment of lamellar macular holes must be performed to make any definitive conclusions.
Of note, it may be easy to confuse our definition of lamellar holes with that of macular pseudoholes. Macular pseudoholes have been defined as macular lesions that have the appearance of macular holes, but which do not have a loss of foveal tissue. The lesions are stable and visual acuity is relatively preserved. The term, macular pseudohole, has typically been used in the setting of a steepened foveal contour secondary to ERM contraction. To avoid confusion between macular pseudoholes and lamellar holes, we propose that the definition of macular pseudoholes be expanded to include any macular lesion that has the appearance of a macular hole, but in which there is not a full-thickness foveal defect, a definition which still retains the meaning of the word pseudohole. Our OCT definition of lamellar hole would then be a subcategory of macular pseudoholes in which there is a lamellar defect caused by separation of the inner from the outer retinal layers. Other authors may disagree with the criteria for lamellar hole diagnosis proposed in this article, and prefer different classification schemes for holes of various etiologies. We propose that the definition of lamellar holes be broadened, with specific morphologic OCT criteria as diagnostic features.
In summary, we report a series of lamellar macular holes as visualized with the Stratus OCT and UHR OCT. Diagnosis of lamellar holes was based on 4 qualitative OCT criteria. Optical coherence tomography was beneficial in making the diagnosis of the lamellar macular hole. In addition, ERMs were visualized on UHR OCT in the majority of patients. Many of these membranes had an unusual thickened appearance of moderate reflectivity on UHR OCT, which may suggest either trapped vitreous between the ERM and the retina, or a thickened posterior hyaloid attached to the retina. The ERM and/or posterior hyaloid may help to stabilize retinal anatomy, and help to prevent the progression to a full-thickness macular hole. Conversely, the high number of ERMs in these patients suggests that ERM contraction may play a role in lamellar hole formation. Vitrectomy surgery was performed in 4 patients; 1 continued to have a lamellar hole after surgery, 1 developed a full-thickness hole that reopened after surgery, and 1 developed a full-thickness hole after surgery. These results suggest caution when performing vitrectomy on lamellar holes.
Footnotes
Presented in part at: American Academy of Ophthalmology Annual Meeting, November, 2005; Chicago, Illinois.
Supported in part by the National Institutes of Health, Bethesda, Maryland (contract nos.: RO1-EY11289-16, R01-EY13178, P30-EY13078); National Science Foundation, Arlington, Virginia (contract no.: ECS-0119452); Air Force Office of Scientific Research, Arlington, Virginia (contract no.: F49620-98-1-0139); Medical Free Electron Laser Program, Arlington, Virginia (contract no.: F49620-01-1-0186); and Carl Zeiss Meditec, Dublin, California.
Drs Fujimoto and Schuman receive royalties from intellectual property licensed by MIT to Carl Zeiss Meditec, and receive research support from Carl Zeiss Meditec.
References
- 1.Gass JD. Lamellar macular hole: a complication of cystoid macular edema after cataract extraction: a clinicopathologic case report. Trans Am Ophthalmol Soc. 1975;73:230–50. [PMC free article] [PubMed] [Google Scholar]
- 2.Tanner V, Chauhan DS, Jackson TL, Williamson TH. Optical coherence tomography of the vitreoretinal interface in macular hole formation. Br J Ophthalmol. 2001;85:1092–7. doi: 10.1136/bjo.85.9.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haouchine B, Massin P, Tadayoni R, et al. Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am J Ophthalmol. 2004;138:732–9. doi: 10.1016/j.ajo.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 4.Haouchine B, Massin P, Gaudric A. Foveal pseudocyst as the first step in macular hole formation: a prospective study by optical coherence tomography. Ophthalmology. 2001;108:15–22. doi: 10.1016/s0161-6420(00)00519-4. [DOI] [PubMed] [Google Scholar]
- 5.Gaudric A, Haouchine B, Massin P, et al. Macular hole formation: new data provided by optical coherence tomography. Arch Ophthalmol. 1999;117:744–51. doi: 10.1001/archopht.117.6.744. [DOI] [PubMed] [Google Scholar]
- 6.Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629–39. doi: 10.1001/archopht.1988.01060130683026. [DOI] [PubMed] [Google Scholar]
- 7.Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119:752–9. doi: 10.1016/s0002-9394(14)72781-3. [DOI] [PubMed] [Google Scholar]
- 8.Smiddy WE, Gass JD. Masquerades of macular holes. Ophthalmic Surg. 1995;26:16–24. [PubMed] [Google Scholar]
- 9.Takahashi H, Kishi S. Tomographic features of a lamellar macular hole formation and a lamellar hole that progressed to a full-thickness macular hole. Am J Ophthalmol. 2000;130:677–9. doi: 10.1016/s0002-9394(00)00626-7. [DOI] [PubMed] [Google Scholar]
- 10.Ko TH, Fujimoto JG, Duker JS, et al. Comparison of ultrahigh-and standard-resolution optical coherence tomography for imaging macular hole pathology and repair. Ophthalmology. 2004;111:2033–43. doi: 10.1016/j.ophtha.2004.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–32. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- 13.Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–29. doi: 10.1016/s0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 14.Hee MR, Baumal CR, Puliafito CA, et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology. 1996;103:1260–70. doi: 10.1016/s0161-6420(96)30512-5. [DOI] [PubMed] [Google Scholar]
- 15.Hee MR, Puliafito CA, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995;113:1019–29. doi: 10.1001/archopht.1995.01100080071031. [DOI] [PubMed] [Google Scholar]
- 16.Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of central serous chorioretinopathy. Am J Ophthalmol. 1995;120:65–74. doi: 10.1016/s0002-9394(14)73760-2. [DOI] [PubMed] [Google Scholar]
- 17.Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology. 1995;102:748–56. doi: 10.1016/s0161-6420(95)30959-1. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins JR, Puliafito CA, Hee MR, et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology. 1996;103:2142–51. doi: 10.1016/s0161-6420(96)30377-1. [DOI] [PubMed] [Google Scholar]
- 19.Munuera JM, Garcia-Layana A, Maldonado MJ, et al. Optical coherence tomography in successful surgery of vitreomacular traction syndrome [letter] Arch Ophthalmol. 1998;116:1388–9. [PubMed] [Google Scholar]
- 20.Chauhan DS, Antcliff RJ, Rai PA, et al. Papillofoveal traction in macular hole formation: the role of optical coherence tomography. Arch Ophthalmol. 2000;118:32–8. doi: 10.1001/archopht.118.1.32. [DOI] [PubMed] [Google Scholar]
- 21.Gallemore RP, Jumper JM, McCuen BW, II, et al. Diagnosis of vitreoretinal adhesions in macular disease with optical coherence tomography. Retina. 2000;20:115–20. [PubMed] [Google Scholar]
- 22.Uchino E, Uemura A, Ohba N. Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Arch Ophthalmol. 2001;119:1475–9. doi: 10.1001/archopht.119.10.1475. [DOI] [PubMed] [Google Scholar]
- 23.Tsujikawa M, Ohji M, Fujikado T, et al. Differentiating full thickness macular holes from impending macular holes and macular pseudoholes. Br J Ophthalmol. 1997;81:117–22. doi: 10.1136/bjo.81.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massin P, Paques M, Masri H, et al. Visual outcome of surgery for epiretinal membranes with macular pseudoholes. Ophthalmology. 1999;106:580–5. doi: 10.1016/S0161-6420(99)90119-7. [DOI] [PubMed] [Google Scholar]
- 25.Uchino E, Uemura A, Doi N, Ohba N. Postsurgical evaluation of idiopathic vitreomacular traction syndrome by optical coherence tomography. Am J Ophthalmol. 2001;132:122–3. doi: 10.1016/s0002-9394(00)00957-0. [DOI] [PubMed] [Google Scholar]
- 26.Kishi S, Takahashi H. Three-dimensional observations of developing macular holes. Am J Ophthalmol. 2000;130:65–75. doi: 10.1016/s0002-9394(00)00383-4. [DOI] [PubMed] [Google Scholar]
- 27.Chan A, Duker JS, Schuman JS, Fujimoto JG. Stage 0 macular holes: observations by optical coherence tomography. Ophthalmology. 2004;111:2027–32. doi: 10.1016/j.ophtha.2004.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2003;121:695–706. doi: 10.1001/archopht.121.5.695. [DOI] [PubMed] [Google Scholar]
- 29.Drexler W, Morgner U, Ghanta RK, et al. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med. 2001;7:502–7. doi: 10.1038/86589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unterhuber A, Povazay B, Hermann B, et al. Compact, low-cost Ti:Al2O3 laser for in vivo ultrahigh-resolution optical coherence tomography. Opt Lett. 2003;28:905–7. doi: 10.1364/ol.28.000905. [DOI] [PubMed] [Google Scholar]
- 31.American National Standards Institute Safe Use of Lasers. ANSI Z136.1–1993. New York: American National Standards Institute; 1993. [Google Scholar]
- 32.Swanson EA, Izatt JA, Hee MR, et al. In-vivo retinal imaging by optical coherence tomography. Opt Lett. 1993;18:1864–6. doi: 10.1364/ol.18.001864. [DOI] [PubMed] [Google Scholar]
- 33.Anger EM, Unterhuber A, Hermann B, et al. Ultrahigh resolution optical coherence tomography of the monkey fovea. Identification of retinal sublayers by correlation with semithin histology sections. Exp Eye Res. 2004;78:1117–25. doi: 10.1016/j.exer.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Ergun E, Hermann B, Wirtitsch M, et al. Assessment of central visual function in Stargardt’s disease/fundus flavimaculatus with ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:310–6. doi: 10.1167/iovs.04-0212. [DOI] [PubMed] [Google Scholar]
- 35.Massin P, Allouch C, Haouchine B, et al. Optical coherence tomography of idiopathic macular epiretinal membranes before and after surgery. Am J Ophthalmol. 2000;130:732–9. doi: 10.1016/s0002-9394(00)00574-2. [DOI] [PubMed] [Google Scholar]
- 36.Guyer DR, de Bustros S, Diener-West M, Fine SL. Observations on patients with idiopathic macular holes and cysts. Arch Ophthalmol. 1992;110:1264–8. doi: 10.1001/archopht.1992.01080210082030. [DOI] [PubMed] [Google Scholar]
- 37.Frangieh GT, Green WR, Engel HM. A histopathologic study of macular cysts and holes. Retina. 1981;1:311–36. [PubMed] [Google Scholar]
- 38.Gass JD. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. 4. Vol. 1. St. Louis: Mosby; 1997. pp. 1–18. [Google Scholar]
- 39.Spaide RF. Closure of an outer lamellar macular hole by vitrectomy: hypothesis for one mechanism of macular hole formation. Retina. 2000;20:587–90. doi: 10.1097/00006982-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Foos RY. Vitreoretinal juncture—simple epiretinal membranes. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974;189:231–50. doi: 10.1007/BF02384852. [DOI] [PubMed] [Google Scholar]
- 41.Margherio RR, Margherio AR, Williams GA, et al. Effect of perifoveal tissue dissection in the management of acute idiopathic full-thickness macular holes. Arch Ophthalmol. 2000;118:495–8. doi: 10.1001/archopht.118.4.495. [DOI] [PubMed] [Google Scholar]
- 42.Yooh HS, Brooks HL, Jr, Capone A, Jr, et al. Ultrastructural features of tissue removed during idiopathic macular hole surgery. Am J Ophthalmol. 1996;122:67–75. doi: 10.1016/s0002-9394(14)71965-8. [DOI] [PubMed] [Google Scholar]
- 43.Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol. 2000;129:769–77. doi: 10.1016/s0002-9394(00)00358-5. [DOI] [PubMed] [Google Scholar]
- 44.Kishi S, Demaria C, Shimizu K. Vitreous cortex remnants at the fovea after spontaneous vitreous detachment. Int Ophthalmol. 1986;9:253–60. doi: 10.1007/BF00137539. [DOI] [PubMed] [Google Scholar]
- 45.Kim JW, Freeman WR, Azen SP, et al. Vitrectomy for Macular Hole Study Group. Prospective randomized trial of vitrectomy or observation for stage 2 macular holes. Am J Ophthalmol. 1996;121:605–14. doi: 10.1016/s0002-9394(14)70625-7. [DOI] [PubMed] [Google Scholar]
- 46.Freeman WR, Azen SP, Kim JW, et al. Vitrectomy for treatment of Macular Hole Study Group. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. Arch Ophthalmol. 1997;115:11–21. doi: 10.1001/archopht.1997.01100150013002. [DOI] [PubMed] [Google Scholar]
- 47.Wendel RT, Patel AC, Kelly NE, et al. Vitreous surgery for macular holes. Ophthalmology. 1993;100:1671–6. doi: 10.1016/s0161-6420(93)31419-3. [DOI] [PubMed] [Google Scholar]
- 48.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654–9. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 49.McDonald HR, Verre WP, Aaberg TM. Surgical management of idiopathic epiretinal membranes. Ophthalmology. 1986;93:978–83. doi: 10.1016/s0161-6420(86)33635-2. [DOI] [PubMed] [Google Scholar]
- 50.Rice TA, De Bustros S, Michels RG, et al. Prognostic factors in vitrectomy for epiretinal membranes of the macula. Ophthalmology. 1986;93:602–10. doi: 10.1016/s0161-6420(86)33689-3. [DOI] [PubMed] [Google Scholar]
- 51.McDonald HR, Johnson RN, Schatz H. Surgical results in the vitreomacular traction syndrome. Ophthalmology. 1994;101:1397–402. doi: 10.1016/s0161-6420(94)31158-4. discussion 1403. [DOI] [PubMed] [Google Scholar]
- 52.Margherio RR, Trese MT, Margherio AR, Cartright K. Surgical management of vitreomacular traction syndromes. Ophthalmology. 1989;96:1437–45. doi: 10.1016/s0161-6420(89)32711-4. [DOI] [PubMed] [Google Scholar]


