Abstract
In a previous study, we found that human neural stem cells (HNSCs) exposed to high concentrations of secreted amyloid-precursor protein (sAPP) in vitro differentiated into mainly astrocytes, suggesting that pathological alterations in APP processing during neurodegenerative conditions such as Alzheimer's disease (AD) may prevent neuronal differentiation of HNSCs. Thus, successful neuroplacement therapy for AD may require regulating APP expression to favorable levels to enhance neuronal differentiation of HNSCs. Phenserine, a recently developed cholinesterase inhibitor (ChEI), has been reported to reduce APP levels in vitro and in vivo. In this study, we found reductions of APP and glial fibrillary acidic protein (GFAP) levels in the hippocampus of APP23 mice after 14 days treatment with (+)-phenserine (25 mg/kg) lacking ChEI activity. No significant change in APP gene expression was detected, suggesting that (+)-phenserine decreases APP levels and reactive astrocytes by posttranscription regulation. HNSCs transplanted into (+)-phenserine-treated APP23 mice followed by an additional 7 days of treatment with (+)-phenserine migrated and differentiated into neurons in the hippocampus and cortex after 6 weeks. Moreover, (+)-phenserine significantly increased neuronal differentiation of implanted HNSCs in hippocampal and cortical regions of APP23 mice and in the CA1 region of control mice. These results indicate that (+)-phenserine reduces APP protein in vivo and increases neuronal differentiation of HNSCs. Combination use of HNSC transplantation and treatment with drugs such as (+)-phenserine that modulate APP levels in the brain may be a useful tool for understanding mechanisms regulating stem cell migration and differentiation during neurodegenerative conditions in AD.
Keywords: amyloid precursor protein, transplantation, immunohistochemistry, neurogenesis, Alzheimer's disease
Transplantation of neural stem cells (NSCs) to the developing brain and in animal models of neurodegeneration has demonstrated that migration and differentiation of these cells is regulated primarily by environmental cues (1–4). Pathological changes that occur in neurodegenerative disorders such as Alzheimer's disease (AD) may profoundly affect the brain microenvironment, which may in turn affect the fate of NSCs.
The amyloid hypothesis, which postulates that β-amyloid (Aβ) neurotoxicity plays a causative role in AD, has dominated much of AD research (5) and the absence of a lethal phenotype in amyloid-precursor protein (APP) knockout mice (6) has detracted attention from the physiological functions of APP. Several studies have shown that APP is involved in regulating neurite outgrowth, cell proliferation, neuronal migration, and differentiation (7–10). APP expression is also increased after brain injury, and increased levels are observed in apoptotic cells (11, 12). Other studies report that Aβ inhibits NSC migration by increasing amyloid-associated cell death and by dysregulation of cellular calcium homeostasis (13, 14). These findings suggest that not only Aβ but that also altered APP processing during the course of AD may have effects on stem cell biology.
Previously, we showed that human NSCs (HNSCs) transplanted into aged rats differentiated into neural cells and could reverse age-associated cognitive impairment in these animals (3). This study demonstrated that the aged rat brain was capable of providing necessary environmental conditions for HNSCs to retain their multipotency and provided some evidence for the potential of stem cell replacement therapies to improve memory and cognitive deficits in AD. However, we recently found increased in vitro glial differentiation of HNSCs treated with high doses of secreted APP or transfected with wild-type APP (15). This finding suggests that stem cell replacement approaches would have reduced effectiveness in the AD brain, in which impaired APP metabolism would prevent or reduce neuronal differentiation of implanted cells. Therefore, we suggest that regulation of APP levels in the brain is necessary for implementing neuroplacement strategies.
(−)-Phenserine is a recently developed cholinesterase inhibitor (ChEI) currently in clinical trials for treatment of mild to moderate AD. Recent studies have reported that besides its ChEI activity, (−)-phenserine also lowers APP and Aβ levels in neuronal cells in culture and in rodents by translational regulation of APP protein synthesis (16–18). However, the doses at which (−)-phenserine decreases APP production in vitro are higher than those that elicit its ChEI activity in patients treated with the experimental drug. Typically, ChEIs have dose limitations and may cause undesirable side effects due to the excessive amounts of acetylcholine produced after treatment. Chirally pure (+)-phenserine lacks ChEI activity but has similar effects on APP production as its (−)-enantiomer (16). In this study, we measured the effects of (+)-phenserine (25 mg/kg) on full-length APP protein levels in an AD transgenic mouse model (APP23 mice) at 4–7 months of age. We also investigated whether (+)-phenserine-induced alterations of endogenous APP levels in these mice, which in turn could influence the migration and differentiation of transplanted HNSCs. Here we show a physiological function of APP in regulating HNSC migration and differentiation fate in vivo.
Results
Reduced APP Protein Expression After (+)-Phenserine Treatment.
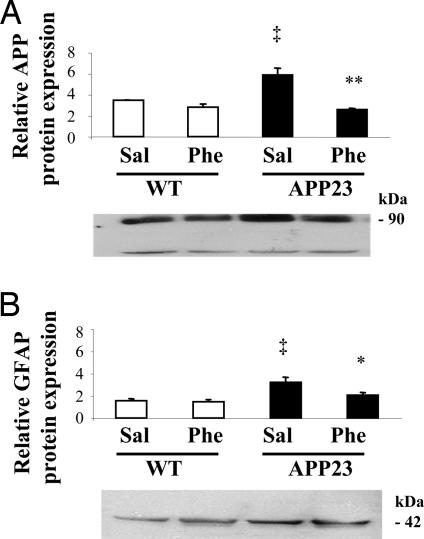
To investigate the effects of (+)-phenserine on full-length APP protein expression, Western blot analysis was performed on cortical and hippocampal tissues from APP23 mice treated with either (+)-phenserine (25 mg/kg i.p. per day for 14 days) or saline. APP23 mice showed significantly (P < 0.05) higher levels of APP (77% increase) compared with that of controls (Fig. 1A). After (+)-phenserine treatment, a significant decrease (38%) in APP protein expression was observed in the hippocampus of APP23 mice (P < 0.01) compared with saline treated mice (Fig. 1A). No significant change in APP protein expression was observed between (+)-phenserine treated and saline treated wild type mice (Fig. 1A). APP protein expression was also reduced in the cerebral cortices of APP23 and wild-type mice after (+)-phenserine treatment, but these reductions did not reach statistical significance (P > 0.05, data not shown).
Fig. 1.
Relative protein levels of total sAPP (22C11) (A) and GFAP (B) in the hippocampus of 6- to 8-month-old APP23 and nontransgenic mice that were treated with either saline or (+)-phenserine (25 mg/kg), respectively, for 14 days. *, P < 0.05 and **, P < 0.01 indicates significantly different from saline-treated (ANOVA). ‡, P < 0.05 indicates significantly different within saline-treated group (ANOVA). All values are expressed as mean ± SEM from three to four independent experiments.
Reduced Glial Fibrillary Acidic Protein (GFAP) Protein Expression After (+)-Phenserine Treatment.
A previous study reported that APP overexpression in APP23 mice is also associated with marked gliogenesis in the brains of these mice (19). Therefore we also measured the expression of GFAP in these mice and investigated whether treatment with (+)-phenserine could alter GFAP levels. We observed that APP23 mice had significantly (P < 0.05) higher (109%) GFAP protein expressed in the hippocampus compared with that of wild-type mice. After (+)-phenserine treatment (25 mg/kg i.p. per day for 14 days), a significant (P < 0.05) reduction (36%) in GFAP expression was measured in APP23 mice (Fig. 1B). No significant changes were observed between (+)-phenserine-treated and saline-treated wild-type mice (Fig. 1B). GFAP protein expression in the cerebral cortices of APP23 and wild-type mice was similar, and no significant change was observed after (+)-phenserine treatment (data not shown).
Effect of (+)-Phenserine on APP Gene Expression in APP23 Mice.
To investigate whether (+)-phenserine-induced reduction of APP protein expression in APP23 mice was mediated at the transcriptional level, quantitative real-time RT-PCR analysis was performed on cortical and hippocampal tissues from treated animals. However, no significant changes were observed in APP gene expression after (+)-phenserine treatment in both APP23 and wild-type mice (data not shown), indicating that (+)-phenserine reduces APP levels by posttranscriptional processing.
Effects of (+)-Phenserine on Glial Differentiation of Transplanted HNSCs in APP23 Mice.
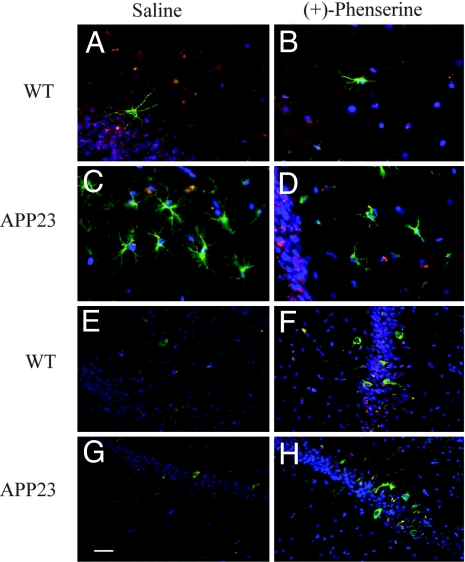
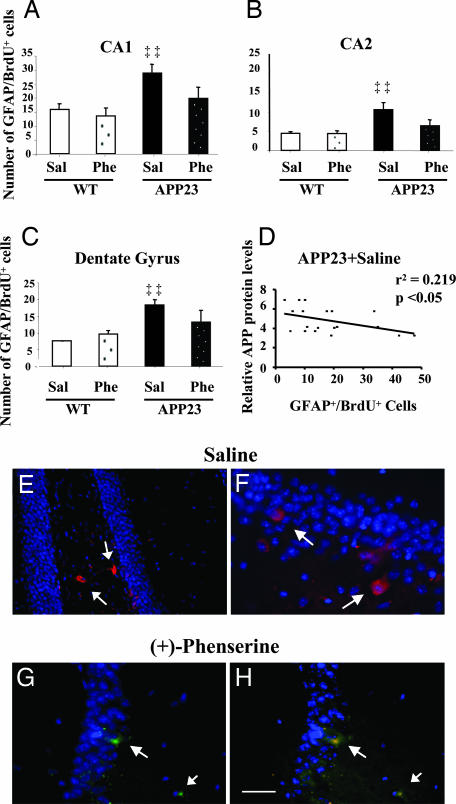
Six weeks after implantation, fluorescent immunohistochemistry was used to identify cells derived from transplanted HNSCs (BrdU-labeled) and to examine their differentiation into neural and glial cells. We also sought to determine whether the (+)-phenserine-induced effects on APP could influence the differentiation fate of transplanted HNSCs. Transplanted HNSCs survived in vivo, and an extensive number of cells exhibiting characteristic astroglial morphologies, and coexpressing BrdU with the astrocytic marker for human GFAP (GFAP+/BrdU+) were observed in the molecular and granule layers of the hippocampal CA1 region (Fig. 2 A–D). Typically, APP23 mice showed more pronounced immunoreactivity for GFAP+/BrdU+ compared with controls. Cells expressing GFAP+/BrdU+ in hippocampal regions were counted, and the results were expressed as the average number of GFAP+/BrdU+ cells per region for each treatment group (Fig. 3 A–C). APP23 mice showed significantly (P < 0.01) more GFAP+/BrdU+ double immunopositive cells compared with that of wild-type mice (Fig. 3 A–C). In addition, a significant correlation (P < 0.05; linear regression r = 0.47) between the number of GFAP+/BrdU+ double immunopositive cells and APP protein expression was demonstrated in the hippocampus of APP23 mice (Fig. 3D). After (+)-phenserine treatment, a marked reduction (ranging from 28% to 40%) in the number of GFAP+/BrdU+ double immunopositive cells was observed in hippocampal regions of APP23 mice (Fig. 3 A–C), indicating that (+)-phenserine reduces glial differentiation caused by APP overexpression. No significant differences in the number of GFAP+/BrdU+ double immunopositive cells were observed in the hippocampus of wild-type mice after (+)-phenserine treatment (Fig. 3 A–C). To eliminate the possibility that (+)-phenserine contributed to increased cell death rather than decreased glial differentiation of transplanted HNSCs, we measured caspase-3 immunoreactivity in brain sections from saline and phenserine treated APP23 mice. We detected a few apoptotic cells-derived from transplanted HNSCs in both the dentate gyrus and CA1 hippocampus of APP23 mice (Fig. 3 E–H), yet no significant difference in the number of apoptotic nuclei was detected in mice treated with (+)-phenserine compared with those who received saline. These results indicate that (+)-phenserine did not mediate any significant toxic effects on transplanted cells.
Fig. 2.
Differentiation of HNSCs into astroglial cells and neuronal cells in vivo after treatment with either saline or (+)-phenserine (25 mg/kg). Representative fluorescent immunohistochemical images in the CA1 hippocampal region of 6- to 7-month-old APP23 and nontransgenic mice 6 weeks after HNSCs transplantation. Sections were double-immunofluorescence stained with GFAP (green) and BrdU (red) markers for astroglia cells and donor cells, respectively (A–D) or with neuronal marker β-III tubulin (green) and BrdU (red) (E–H). All nuclei were counterstained by DAPI (blue). (Scale bars: 20 μm.)
Fig. 3.
Transplanted HNSC expressing immunoreactivity for GFAP and BrdU after 6 weeks of differentiation in hippocampal regions of 6- to 7-month-old APP23 and nontransgenic mice that were treated with either saline or (+)-phenserine (25 mg/kg). All values are expressed as the mean ± SEM (n = 6–7 within each group) and were obtained by averaging counts of immunoreactive human-specific astroglial cells in the CA1 (A), CA2 (B), and dentate gyrus (C), measured bilaterally on four to six alternate sections for each mouse. ‡‡, P < 0.01 indicates a significant difference within the saline-treated group (ANOVA). (D) Correlation of APP protein levels with the number of GFAP+/BrdU+ cells in the hippocampus of 6- to 7-month-old APP23 mice that received saline only. Each point corresponds to average APP protein levels and the number of GFAP+/BrdU+ cells in the CA1, CA2, and dentate gyrus regions of each individual mouse. (Linear regression r = 0.47; P < 0.05). (E–H) Anti-caspase-3 staining of apoptotic cells-derived from transplanted HNSCs in the dentate gyrus of saline- (red) (E and F) and (+)-phenserine-treated APP23 mice (green) (G and H). Nuclei are stained with DAPI (blue); small arrows indicate apoptotic cell nuclei. (H) Colocalization of BrdU (red) and caspase-3 (green). (Scale bar: E, 10 μm; F, 100 μm.)
Effects of (+)-Phenserine on Neuronal Differentiation of Transplanted HNSCs in APP23 Transgenic Mice.
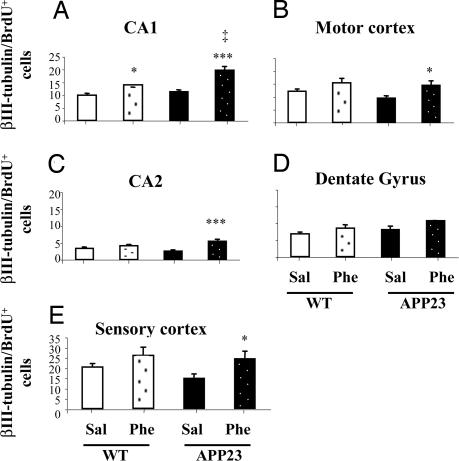
Examination of neuronal differentiation of transplanted HNSCs was performed in brain sections from APP23 and wild-type mice treated with (+)-phenserine or saline. The number of cells coexpressing BrdU with the neuronal marker for human β-III tubulin (β-III tubulin+/BrdU+) were counted (per square millimeter) in the molecular and granule layers of the hippocampal CA1 and CA2 and the dentate gyrus and in the pyramidal layers of the somatosensory and motor cortex. Transplanted cells that differentiated into β-III tubulin+/BrdU+ cells within the CA1 region had large pyramidal morphologies (Fig. 2 E–H), whereas those in the dentate granule layer displayed a small ovoid appearance characteristic for dentate granule neurons (data not shown). In the somatosensory and motor cortical regions, β-III tubulin+/BrdU+ cells exhibited both pyramidal and nonpyramidal morphologies (data not shown). To exclude the possibility that measured neuronal immunoreactivity was also detecting endogenous neurons in mouse that were not derived from the transplanted HNSCs, we stained, in parallel experiments, sections together with an antibody that specifically labels human nuclei. Similar results were obtained for human nuclei staining as with β-III tubulin and BrdU, thus verifying that differentiated cells were of human origin [supporting information (SI) Fig. 5]. We anticipated a reduced neuronal differentiation of transplanted HNSCs in saline-treated APP23 mice on the basis of earlier findings in vitro in which more glial differentiation of HNSCs was observed after treatment with secreted APP (sAPP) (23). However, no significant difference in the number of β-III tubulin+/BrdU+ double immunopositive cells was observed in hippocampal regions of APP23 mice compared with wild-type mice (Fig. 4A, C, and D), even though fewer β-III tubulin+/BrdU+ double immunopositive cells were detected in the motor and somatosensory cortex of APP23 mice in comparison with wild-type mice (Fig. 4 B and E). Interestingly, we observed a significant increase (ranging from 32% to 112%) in the number of β-III tubulin+/BrdU+ double immunopositive cells in the hippocampal CA1 and CA2 (P < 0.0001) and in the motor and the somatosensory cortex (P < 0.05) of APP23 mice after (+)-phenserine treatment compared with the number of cells found in APP23 mice treated with saline (Fig. 4 A–C and E). A significant (P < 0.05) increase (40%) in the number of β-III tubulin+/BrdU+ cells was observed only in the CA1 hippocampal region of wild-type mice treated with (+)-phenserine (Fig. 4A).
Fig. 4.
Transplanted HNSC expressing immunoreactivity for neuronal marker β-III tubulin and BrdU after 6 weeks of differentiation in hippocampal and cortical regions of 6- to 7-month-old APP23 and nontransgenic mice that were treated with either saline or (+)-phenserine (25 mg/kg). All values are expressed as mean ± SEM. n = 6–7 within each group and were obtained by averaging counts of immunoreactive human-specific neuronal cells in the CA1, CA2, dentate gyrus (A, C, and D), and motor and sensory cortex (B and E), measured bilaterally on four to six alternate sections for each mouse. *, P < 0.05 and ***, P < 0.0001 indicates a significant difference from saline-treated (ANOVA). ‡, P < 0.05 indicates a significant difference within (+)-phenserine-treated groups (ANOVA).
Discussion
An understanding of the basic function of factors and signals that regulate HNSC biology in normal or diseased brain is still in its infancy. Several studies have shown that APP expression is up-regulated during development of the CNS, coinciding with a peak in neuronal differentiation (20, 21). Increased APP levels are also observed after brain damage (22, 23). Both of these events involve migration and differentiation of NSCs, suggesting that APP may also play an important physiological function in regulating stem cell biology. In a recent study, we demonstrated that treatment with recombinant sAPP promoted migration and differentiation of HNSCs in culture, and 22C11 antibody-mediated neutralization of sAPP in media inhibited these effects dose dependently (15). We also reported that HNSCs transplanted into APP knockout mice showed less migration and differentiation compared with wild-type mice (15). On the basis of these observations, we suggest that APP may be acting as a signaling factor in migration and differentiation of HNSCs.
The AD brain is characterized by accumulation of intracellular neurofibrillary tangles and extracellular Aβ deposits generated from proteolytic cleavage of APP (24). In addition, a severe impairment of cholinergic neurotransmission is observed in AD patients because of a pronounced loss of basal forebrain cholinergic neurons projecting to hippocampal and cortical regions. The resulting deficits in these regions correlate with the memory and cognitive impairment manifested clinically (25, 26). To date, the most effective treatment for AD is with ChEIs that stimulate an increase in levels of the neurotransmitter acetylcholine (27). Several of these drugs have been shown to affect APP processing and to lower Aβ in cell culture through mechanism(s) that are independent from their activities as ChEIs (28–30). The ChEI (−)-phenserine is currently being tested in clinical trials for the symptomatic treatment of mild to moderate AD, and its positive enantiomeric form, (+)-phenserine, has been found to significantly reduce APP and Aβ in both neuronal cell lines in culture and in animals by regulating APP protein synthesis (16, 18). As a consequence of its apparent lack of ChEI activity, (+)-phenserine may be administered in vivo in relatively high doses without adverse effects (31), and the compound is currently in clinical trials for AD treatment.
In the present study, we examined the effects of (+)-phenserine on APP protein expression, and the migration and differentiation of transplanted HNSCs in APP23 transgenic mice. To study the effects on APP and HNSC differentiation in APP23 mice, (+)-phenserine treatment and subsequent transplantation of HNSCs were performed in 3- to 4-month-old mice, which is before the onset of AD-like pathology. APP23 mice can express a 7-fold overexpression of mutated human APP751 in the brain, with Aβ plaque-like deposits that begin to appear in the hippocampus and neocortex from 6 months of age, and increased deposition is observed with age (19). Here we showed that (+)-phenserine significantly reduced APP as well as GFAP protein expression in the hippocampus of APP23 transgenic mice. (+)-Phenserine suppressed APP protein expression without altering APP gene expression, indicating the involvement of a posttranscriptional regulatory mechanism. Our findings are in agreement with earlier studies that showed that ChEIs, such as tacrine and (−)-phenserine, induced similar reductions in levels of both secreted and cellular APP in neuronal cells in culture (16, 32–33). A dramatic increase of APP in cholinergic projection areas has been demonstrated in a study using rats with forebrain cholinergic lesions (17). Further findings from this study showed that phenserine could reverse this effect and additionally reduce APP production in naïve animals (17). In our study, we also found a reduced glial differentiation of transplanted HNSCs in hippocampal regions of (+)-phenserine treated APP23 mice. In regions such as the CA1 hippocampus, glial differentiation of HNSCs was decreased by >50% in the APP23 mice after treatment with (+)-phenserine, which corresponded with a shift from a 2:1 to 1:1 ratio in the number of transplanted cells differentiating into a glial versus a neuronal lineage (SI Table 1).
The shift to increased neuronal differentiation after (+)-phenserine treatment was most apparent in the CA2 region of APP23 mice, in which glial differentiation decreased by 36% (SI Table 2). However, (+)-phenserine treatment did not significantly affect neural differentiation of transplanted HNSCs in the dentate gyrus of either wild-type or APP23 mice. Adult neurogenesis typically occurs in the subventricular zone and the dentate gyrus of the hippocampus (34). Endogenous neuroregeneration in the dentate gyrus may therefore depend mainly on the stem cells that already reside in the subgranular zone of the dentate granule cell layer of the hippocampus (35), whereas endogenous stem cells residing in the subventricular zone may not migrate into the dentate gyrus (36). Thus, it is possible that exogenous HNSCs may not necessarily follow the same distribution pattern as endogenous stem cells.
It has been proposed that (+)-phenserine mediates a specific effect on human APP through translational regulation of protein synthesis (16, 18, 37). We would therefore expect APP levels to remain unaffected after (+)-phenserine treatment in the control mice, because these mice do not carry the human form of APP. However, we did observe an effect of (+)-phenserine on neuronal differentiation of transplanted HNSCs in wild-type mice in the present study, suggesting that other mechanisms exist. Earlier studies have implicated that APP exerts its effects on cell proliferation, growth, and differentiation by activating the MAP/ERK signaling pathway (38). Accordingly, a recent study in our group showed that APP is involved in promoting astrocytic differentiation of NT2-/D1 neural precursor cells induced by treatment with staurosporin, a protein kinase C inhibitor and inducer of cell differentiation. Staurosporin treatment increased sAPP in these cells, which led to activation of the Erk1/2 signaling pathway and increased astrocytic differentiation of the NT2-/D1 cells (39). To confirm APP involvement, APP expression was suppressed in these cells by using RNA interference methods, and this resulted in reduced GFAP expression (23). In another study, we showed that treatment of HNSCs in culture with sAPP was associated with an increased expression of genes related to the Notch and JAK/STAT-signaling cascades (15). These cascades are known to play a pivotal role in neuron–glia differentiation (40), and we suggest that it is possible that the reduction in glial differentiation of transplanted HNSCs in APP23 mice observed herein could be a consequence of (+)-phenserine-mediated inhibition of APP effect(s) on Notch and JAK/STAT pathways. Only a few studies up to date have investigated the cell fate of endogenous populations of stem cells in the adult brain in regards to APP overexpression and Aβ pathogenesis. One study demonstrated impaired neurogenesis in the dentate gyrus of transgenic mice expressing the Swedish double mutation (K595N, M596L) (14), whereas other studies measured increased neurogenesis both in the AD human postmortem brain (41) and in the brains of transgenic mice expressing the Swedish and Indiana APP (PDGF-APPSw,Ind) mutations (42). In the present investigation we have measured increased neurogenesis in the hippocampus and cortex of APP23 mice and in the CA1 hippocampal region of wild-type mice after (+)-phenserine treatment. It is possible that a discrepancy in the findings of both decreased (14) and increased (42) neurogenesis in AD transgenic mice and those presented here could be attributed to cell-intrinsic differences between endogenous and exogenous stem cells. Our current findings suggest that (+)-phenserine may stimulate increased neuronal differentiation or neurogenesis by a mechanism that may involve APP interaction(s) with other factors. To confirm these results, additional studies were performed in vitro on differentiating HNSCs treated with (+)-phenserine. Similar to our in vivo findings, we observed that (+)-phenserine suppressed APP and GFAP protein expression, and increased the number of neuronal cells in differentiated cell populations of HNSCs in vitro (SI Fig. 6).
A recent study by Jin et al. (43) demonstrated that the ChEIs tacrine, galanthamine and the NMDA receptor antagonist memantine, promote increased neurogenesis both in isolated cultures from cortical progenitor cells and in mice. The mechanisms through which these disparate drugs increase neurogenes is still unclear, yet the investigators suggested that a common mechanism, mediated through muscarinic receptor-coupled phosphoinositide signaling is involved (43). They reported that this effect could also be due to activation of cholinergic receptors that are expressed on neuronal progenitors and that these receptors in turn may stimulate neurogenic factors (43–45). Because (+)- phenserine does not possess ChEI activity, stimulation of neurogenesis may likely not be mediated through cholinergic receptors that may be expressed on the differentiated HNSCs. The exact mechanisms, with regards to which signaling pathway(s) are involved in mediating the (+)-phenserine-induced effects on APP in regulating stem cell migration and differentiation in vivo, are beyond the scope of our present study. Thus, future studies will be crucial for investigating the specific molecular mechanisms underlying this phenomena, as well as comparative studies for determining the efficacy of various doses of (+)-phenserine.
In conclusion, our present findings suggest that altered APP levels regulate NSC biology in the adult brain, and this may have serious implications for the pathophysiology of AD and other diseases involving dysregulation of APP metabolism such as Down's syndrome. High levels of APP in the brain may exhaust stem cell populations as a result of premature or increased glial differentiation. Further understanding of the mechanisms involved in regulating stem cell biology during neurodegeneration is needed, and a combination of augmentation of stem cell populations by transplantation and a pharmacological approach to regulate APP levels may aid future development of novel strategies for therapeutical interventions of these diseases.
Materials and Methods
HNSC Culture.
HNSCs originally isolated from 9-week-old fetal cortical tissue were purchased from BioWhittaker (Walkersville, MD), and the cells were expanded and passaged in serum-free culture media, as described in ref. 46. Briefly, HNSCs were cultured in DMEM/F12 (GIBCO, Burlington, ON, Canada) supplemented with 20 ng/ml EGF and 20 ng/ml basic fibroblast growth factor (bFGF) (R & D, Minneapolis, MN), B27 (1:50; GIBCO), 5 μg/ml heparin (Sigma, St. Louis, MO), and antibiotic-antimycotic mixture (1:100; GIBCO) in a humidified atmosphere of 5% CO2 at 37°C. Before transplantation, HNSCs were incubated with 3 μM BrdU (Sigma) for 48 h to label cell nuclei to distinguish them from the host cells.
Animals.
APP23 mice, expressing the 751-aa human APP (hAPP751) with the Swedish double mutation (K670N, M671L) (47) were received as a gift from NovartisPharma (Basel, Switzerland) and were used to breed a colony of experimental animals by backcrossing to C57BL/6 mice. Mice were housed in standard cages with access to food and water ad libitum during a 12/12 h light/dark cycle. Genotypes were confirmed by PCR (48), and in all experiments wild-type littermates served as controls. All animal experimental procedures were carried out in compliance with National Institutes of Health Guidelines for Care and Use of Laboratory Animals and were approved by the Animal Research Committee (protocol 00-24) at the University of Central Florida.
(+)-Phenserine Treatment.
A total of 55 age- and sex-matched APP23 (n = 30) and wild-type (n = 25) mice (ages ranged from 4 to 7 months) were administered with either (+)-phenserine (25 mg/kg per day i.p.) or 0.9% saline for 14 consecutive days. Animals were subsequently divided into two groups that were either killed after 14 days of treatment (n = 17 APP23 and n = 13 wild-type, respectively) or received HSNCs transplanted into the lateral ventricle (n = 13 APP23 mice and n = 12 wild-type, respectively). (+)-Phenserine or saline injections were continued once a day for 1 week after a 2-day recovery from surgery. All animals were killed within 12 h of receiving the final injection by an overdose of a 1:1 mixture of 100 mg/kg ketamine and 20 mg/kg xylazine, followed by transcardial perfusion with PBS. Brains were removed and dissected into the hippocampus and cortex, and tissue samples were stored at −80°C until experiments were performed. The groups of transplanted animals were transcardially perfused with 4% paraformaldehyde (pH 7.4). Brains were removed, postfixed for 12 h, and cryoprotected in 20% sucrose in PBS overnight. Twenty-micrometer coronal brain sections were cut and processed for immunofluorescence.
Animal Surgery and Transplantation.
Anesthetized animals were mounted on a stereotaxic apparatus (ASI Instruments, Warren, MI). HNSCs (≈105 cells) were suspended in 10 μl of PBS and slowly injected into the right lateral ventricle of each mouse. Intraventricular injection minimizes disruption of brain tissue and may leverage endogenous signals (e.g., chemokines released by microglia in response to damage) that might affect stem cell migration. No immunosuppressant was used, and animals were monitored for body weight, swelling, and proper healing of the incision site.
Protein Isolation and Western Blot Analysis.
Dissected cortical and hippocampal tissues from (+)-phenserine and saline-treated animals were homogenized in ice-cold lysis buffer containing 1% Nonidet P-40, 150 mM NaCl, 50 mM Tris (pH 8.0), and protease inhibitor mixture (Roche, Indianapolis, IN). The homogenates were centrifuged and washed twice at 12,000 × g for 15 min at 4°C. Fifteen micrograms of protein was loaded per well, and proteins were separated by SDS/PAGE and then blotted onto PVDF membranes for 120 min at 30 V. For the detection of full-length APP and GFAP protein, membranes were incubated overnight with primary antibodies mouse monoclonal anti-Alzheimer precursor protein A4 (22C11) (1:1,000; Chemicon, Temecula, CA), rabbit anti-GFAP (1:1,000; Promega, Madison, WI), and polyclonal rabbit anti-β-actin (1:1,000; Cell Signaling Technology, Danvers, MA). After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (anti-mouse IgG and anti-rabbit IgG; Jackson Immunoresearch, West Grove, PA) for 1–2 h. Signals were visualized by incubation of membranes in ECL Plus reagents and exposure to Hyperperformance Chemiluminescence film (Amersham Biosciences, Buckinghamshire, U.K.). Films were scanned, and the optical density of each specific band relative to β-actin was analyzed by the public domain National Institutes of Health Image J software.
Real-Time RT-PCR Analysis.
Total RNA from hippocampal and cortical tissues from treated animals was extracted with TRIzol (Invitrogen) according to the manufacturer's protocol. cDNA synthesis was performed with 1 μg of total RNA and reagents from the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to kit instructions. Relative quantification with real-time PCR was determined using the MyiQ Real-Time PCR Detection System software (Bio-Rad), and reactions were performed in a thermal iCycler by using the Bio-Rad MyIQ SYBR Green Supermix as described in SI Table 3. The authenticity of the PCR products was verified by a melt-curve analysis.
Fluorescent Immunohistochemistry.
Free-floating coronal brain sections (20 μm) were denatured with 1 M HCl for 20 min and neutralized with PBS for 30 min at room temperature (RT) to increase the accessibility of the anti-BrdU antibody to the BrdU incorporated in the cell nuclei. The sections were then blocked in PBS containing 0.25% Triton X-100 and 3% normal donkey serum for 1 h and incubated with sheep polyclonal anti-BrdU (1:1,000; Abcam, Cambridge, MA), mouse anti-human nuclei (1:100; Chemicon), mouse IgG2b anti-human β-III tubulin, clone SDL3D10 (1:2,000; Sigma) and mouse anti-NeuN (1:1,000; Abcam), or rabbit IgG anti-human GFAP (1:500; Sigma) overnight at 4°C. For apoptosis measurements, sections were incubated with rabbit anti-active caspase-3 antibody (1:125; Promega, Madison, WI). Sections were incubated with corresponding secondary antibodies (1:500 to 1:1,000), conjugated with fluorescein (FITC) or rhodamine (TRITC) (Jackson Immunoresearch) for 2 h at RT. After final washes in PBS-T, sections were mounted and cover slipped with Vectashield with DAPI (Vector Laboratories, Burlingame, CA) for fluorescent microscopic analysis.
Microscopy and Analysis of Differentiation.
Cell migration and differentiation in transplanted mice (n = 6–7 mice in each group) were quantified by unbiased bilateral counts of the number of BrdU-positive cells expressing either the neuronal marker, β-III tubulin, or the glial marker, GFAP, in the molecular and granule layers of hippocampal CA1, CA2, and dentate gyrus, and motor and sensory regions of the cerebral cortex by using a Leica (Deerfield, IL) DMRB fluorescent microscope at ×400 magnification. Microscopic images were taken with an Axiocam digital camera (Zeiss, Oberkochen, Germany) mounted on the DMRB and processed using the QImaging with Q Capture software (QImaging, Burnaby, Canada). An average of four to six sections were counted for each animal. The numbers of transplanted cells counted in each section were averaged for each side so that the final numbers represented the mean neuron or astrocyte number per sampling area or per square millimeter.
Data and Statistical Analysis.
Data are presented as mean ± SEM of different experiments, and differences between groups were analyzed with one-way ANOVA followed by Bonferroni/Dunn and Scheffé post hoc comparison testing. Correlations between variables were determined by linear regression analysis (PRISM 3.0; GraphPad, San Diego, CA).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant AG 23472, a grant from BioFlorida, and Alzheimer Association Grant IIRG-03-5577. M.N. was supported by a grant from the Erik and Edith Fernströms Foundation (Sweden) and Swedish Medical Research Council Contract Grant 05817.
Abbreviations
- Aβ
β-amyloid
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- ChEI
cholinesterase inhibitor
- GFAP
glial fibrillary acidic protein
- HNSC
human neural stem cell
- sAPP
secreted APP.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705346104/DC1.
References
- 1.Sheen V, Macklis J. J Neurosci. 1995;15:8378–8392. doi: 10.1523/JNEUROSCI.15-12-08378.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brustle O, McKay R. Curr Opin Neurobiol. 1996;6:688–695. doi: 10.1016/s0959-4388(96)80104-8. [DOI] [PubMed] [Google Scholar]
- 3.Qu T, Brannen C, Kim H, Sugaya K. NeuroReport. 2001;12:1127–1132. doi: 10.1097/00001756-200105080-00016. [DOI] [PubMed] [Google Scholar]
- 4.Englund U, Fricker-Gates R, Lundberg C, Bjorklund A, Wictorin K. Exp Neurol. 2002;173:1–21. doi: 10.1006/exnr.2001.7750. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 6.Muller U, Cristina N, Li Z, Wolfer D, Lipp H, Rulicke T, Brandner S, Aguzzi A, Weissmann C. Cell. 1994;79:755–765. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 7.Salinero O, Moreno-Flores M, Wandosell F. J Neurosci Res. 2000;60:87–97. doi: 10.1002/(SICI)1097-4547(20000401)60:1<87::AID-JNR9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Caille I, Allinquant B, Dupont E, Bouillot C, Langer A, Muller U, Prochiantz A. Development (Cambridge, UK) 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 9.De Strooper B, Annaert W. J Cell Sci. 2000;113:1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- 10.Ando K, Oishi M, Takeda S, Iijima K, Isohara T, Nairn A, Kirino Y, Greengard P, Suzuki T. J Neurosci. 1999;19:4421–4427. doi: 10.1523/JNEUROSCI.19-11-04421.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koszyca B, Blumbergs P, Manavis J, Wainwright H, James R, Gilbert J, Jones N, Reilly P. J Neurotrauma. 1998;15:675–683. doi: 10.1089/neu.1998.15.675. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Wurtman R, Lee R. Brain Res. 2000;865:157–167. doi: 10.1016/s0006-8993(00)02183-1. [DOI] [PubMed] [Google Scholar]
- 13.Bondolfi L, Calhoun M, Ermini F, Kuhn H, Wiederhold K, Walker L, Staufenbiel M, Jucker M. J Neurosci. 2002;22:515–522. doi: 10.1523/JNEUROSCI.22-02-00515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haughey N, Nath A, Chan S, Borchard A, Rao M, Mattson M. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 15.Kwak YD, Brannen C, Qu T, Kim H, Dong X, Soba P, Majumdar A, Kaplan A, Beyreuther K, Sugaya K. Stem Cells Dev. 2006;15:381–389. doi: 10.1089/scd.2006.15.381. [DOI] [PubMed] [Google Scholar]
- 16.Shaw K, Utsuki T, Rogers J, Yu Q, Sambamurti K, Brossi A, Ge Y, Lahiri D, Greig N. Proc Natl Acad Sci USA. 2001;98:7605–7610. doi: 10.1073/pnas.131152998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haroutunian V, Greig N, Pei X, Utsuki T, Gluck R, Acevedo L, Davis KL, Wallace W. Brain Res Mol Brain Res. 1997;46:161–168. doi: 10.1016/s0169-328x(96)00297-5. [DOI] [PubMed] [Google Scholar]
- 18.Utsuki T, Yu Q, Davidson D, Chen D, Holloway H, Brossi A, Sambamurti K, Lahiri D, Greig N, Giordano T. J Pharmacol Exp Ther. 2006;318:855–862. doi: 10.1124/jpet.106.103309. [DOI] [PubMed] [Google Scholar]
- 19.Sturchler-Pierrat C, Staufenbiel M. Ann NY Acad Sci. 2000;920:134–139. doi: 10.1111/j.1749-6632.2000.tb06915.x. [DOI] [PubMed] [Google Scholar]
- 20.Salbaum J, Ruddle F. J Exp Zool. 1994;269:116–127. doi: 10.1002/jez.1402690205. [DOI] [PubMed] [Google Scholar]
- 21.Trapp B, Hauer P. J Neurosci Res. 1994;37:538–550. doi: 10.1002/jnr.490370413. [DOI] [PubMed] [Google Scholar]
- 22.Kirazov E, Kirazov L, Bigl V, Schliebs R. Int J Dev Neurosci. 2001;19:287–296. doi: 10.1016/s0736-5748(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 23.Murakami N, Yamaki T, Iwamoto Y, Sakakibara T, Kobori N, Fushiki S, Ueda S. J Neurotrauma. 1998;15:993–1003. doi: 10.1089/neu.1998.15.993. [DOI] [PubMed] [Google Scholar]
- 24.Haass C, Hung A, Schlossmacher M, Oltersdorf T, Teplow D, Selkoe D. Ann NY Acad Sci. 1993;695:109–116. doi: 10.1111/j.1749-6632.1993.tb23037.x. [DOI] [PubMed] [Google Scholar]
- 25.Bierer L, Haroutunian V, Gabriel S, Knott P, Carlin L, Purohit D, Perl D, Schmeidler J, Kanof P, Davis K. J Neurochem. 1995;64:749–760. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- 26.Nordberg A. Biol Psychiatry. 2001;49:200–210. doi: 10.1016/s0006-3223(00)01125-2. [DOI] [PubMed] [Google Scholar]
- 27.Doody R, Stevens J, Beck C, Dubinsky R, Kaye J, Gwyther L, Mohs RC, Thal L, Whitehouse P, DeKosky S, Cummings J. Neurology. 2001;56:1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 28.Lahiri D, Farlow M, Nurnberger J, Jr, Greig N. Ann NY Acad Sci. 1997;826:416–421. doi: 10.1111/j.1749-6632.1997.tb48495.x. [DOI] [PubMed] [Google Scholar]
- 29.Pakaski M, Kasa P. Curr Drug Targets CNS Neurol Disord. 2003;2:163–171. doi: 10.2174/1568007033482869. [DOI] [PubMed] [Google Scholar]
- 30.Racchi M, Mazzucchelli M, Lenzken S, Porrello E, Lanni C, Govoni S. Chem Biol Interact. 2005;157–158:335–338. doi: 10.1016/j.cbi.2005.10.099. [DOI] [PubMed] [Google Scholar]
- 31.Greig N, Ruckle J, Comer P, Brownell L, Holloway H, Flanagan D, Jr, Canfield C, Burford R. Curr Alzheimer Res. 2005;2:483–492. doi: 10.2174/156720505774330564. [DOI] [PubMed] [Google Scholar]
- 32.Lahiri D, Lewis S, Farlow M. J Neurosci Res. 1994;37:777–787. doi: 10.1002/jnr.490370612. [DOI] [PubMed] [Google Scholar]
- 33.Lahiri D, Farlow M, Hintz N, Utsuki T, Greig N. Acta Neurol Scand Suppl. 2000;176:60–67. doi: 10.1034/j.1600-0404.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- 34.Gage FH. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 35.van Praag H, Schinder A, Christie B, Toni N, Palmer T, Gage F. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doetsch F, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maloney B, Ge YW, Greig N, Lahiri D. FASEB J. 2004;18:1288–1290. doi: 10.1096/fj.03-1703fje. [DOI] [PubMed] [Google Scholar]
- 38.Greenberg S, Koo E, Selkoe D, Qiu W, Kosik K. Proc Natl Acad Sci USA. 1994;91:7104–7108. doi: 10.1073/pnas.91.15.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwak YD, Choumkina E, Sugaya K. Biochem Biophys Res Commun. 2006;344:431–437. doi: 10.1016/j.bbrc.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 40.Fischer D, van Dijk R, Sluijs J, Nair S, Racchi M, Levelt CN, van Leeuwen F, Hol E. FASEB J. 2005;19:1451–1458. doi: 10.1096/fj.04-3395.com. [DOI] [PubMed] [Google Scholar]
- 41.Jin K, Peel A, Mao XO, Xie L, Cottrell B, Henshall D, Greenberg D. Proc Natl Acad Sci USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin K, Galvan V, Xie L, Mao X, Gorostiza O, Bredesen D, Greenberg D. Proc Natl Acad Sci USA. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin K, Xie L, Mao X, Greenberg D. Brain Res. 2006;1085:183–188. doi: 10.1016/j.brainres.2006.02.081. [DOI] [PubMed] [Google Scholar]
- 44.Atluri P, Fleck M, Shen Q, Mah S, Stadfelt D, Barnes W, Goderie S, Temple S, Schneider A. Dev Biol. 2001;240:143–156. doi: 10.1006/dbio.2001.0453. [DOI] [PubMed] [Google Scholar]
- 45.Ma W, Maric D, Li B, Hu Q, Andreadis J, Grant G, Liu Q, Shaffer K, Chang Y, Zhang L, Pancrazio J, Pant H, Stenger D, Barker J. Eur J Neurosci. 2000;12:1227–1240. doi: 10.1046/j.1460-9568.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 46.Brannen C, Sugaya K. NeuroReport. 2000;11:1123–1128. doi: 10.1097/00001756-200004070-00042. [DOI] [PubMed] [Google Scholar]
- 47.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold K, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti P, et al. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calhoun M, Burgermeister P, Phinney A, Stalder M, Tolnay M, Wiederhold K, Abramowski D, Sturchler-Pierrat C, Sommer B, Staufenbiel M, Jucker M. Proc Natl Acad Sci USA. 1999;96:14088–14093. doi: 10.1073/pnas.96.24.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.