Abstract
The role of the gastrin peptide hormones (G17, G34) and their precursors (progastrins, PG; gly-extended gastrin, G-gly), in gastrointestinal (GI) cancers has been extensively reviewed in recent years (1–3). A possible important role of progastrin peptides in colon carcinogenesis has become evident from experiments with transgenic mouse models (1;3). It is now known that growth stimulatory and co-carcinogenic effects of gastrin/PG peptides are mediated by both proliferative and anti-apoptotic effects of the peptides on target cells (4;5). Several receptor subtypes have been described that mediate growth effects of gastrin peptides (1;2). Recently, we identified Annexin II as a high affinity binding protein for gastrin/PG peptides (6). Importantly, the expression of Annexin II was required for mediating growth stimulatory effects of gastrin and PG peptides on intestinal epithelial and colon cancer cells (6), suggesting that Annexin II may represent the elusive novel receptor for gastrin/PG peptides. The importance of this finding in relation to the structure and function of Annexin II, especially in GI cancers, is described below. Since this surprising finding represents a new front in our understanding of the mechanisms involved in mediating growth effects of gastrin/PG peptides in GI cancers, our current understanding of the role of Annexin II in proliferation and metastasis of cancer cells is additionally reviewed.
Keywords: Annexin-II, Progastrin, Tenascin-C, Colon Cancer, GI cancers, signalling
Annexin II: Introduction to Structure and Function
At least 20 members of the Annexin family have been described to date, all of which share a conserved core domain (Mr 30-40K), which binds membrane associated phospholipids. In addition the Annexin proteins contain a variable amino terminal ‘tail’ domain (Mr 3-6K), which is responsible for the specialized functions of the various Annexin proteins (7;8).
ANX II is a multi-functional protein (7), which binds acid phospholipids and actin with significant affinity (9). ANX-II is cleaved by chymotrypsin into a 33 KDa C-terminal core domain and a 3 KDa N-terminal domain of 30 amino acids. The first 14 residues of the N-terminal domain contains a high affinity binding site for S100A10, a Ca2+ binding protein (Mr 11K, Fig 1) (10). Majority of ANX-II is tightly associated with p11 forming an ANX-II2/p112 heterotetramer (AIIt). The relative amounts of heterotetrameric vs monomeric ANX-II vary from ~100% AIIt in intestinal epithelium to ~50% monomeric in fibroblasts (11).
Figure 1. Diagrammatic representation of the structure of Annexin II.

The known phosphorylation sites in the N-terminal end of the Annexin II molecule are shown (not to scale), based on literature presented in the text.
ANX II is expressed abundantly in rejuvenating cells (fibroblasts, endothelial cells, epithelial cells of the lung and GI tract) (12), but not by quiescent cells such as platelets, skeletal and smooth muscle myocytes, hepatocytes, etc. (12). High affinity binding proteins for Progastrins (PG) (including the recently identified protein, ANX II) are present on colonic and intestinal epithelial cells (1;13;14) and colon cancer cells (6), but are absent in the brain or liver (unpublished data from our laboratory), which agrees with the reported expression of ANX II in proliferating cells (12;15).
Annexin II is a growth regulated gene (16;17), and serum stimulated up-regulation of Annexin II expression is characteristic of early response genes (16). Annexin II was found to be necessary for cell proliferation by subtractive hybridization between primary human keratinocytes and a head-and-neck squamous cell carcinoma cell line (17). Annexin II expression is cell cycle regulated in CHO and HeLa cells (15). Early on it was reported that Annexin II may be involved in chromosomal DNA replication, based on evidence from experiments in cell-free extracts of Xenopus (15;18;19). ANX II expression is increased in many human cancers (12;20–22), and down-regulation of its expression in HeLa cells, 293 cells, IEC and human colon cancer cells results in the loss of proliferation (6;19). All these findings suggest that ANX II may be a key regulator of DNA synthesis and cell proliferation.
Many physiological roles have been proposed for Annexin II, including signal transduction, membrane fusion, cell adhesion, DNA synthesis and cell proliferation. Cellular functions of ANX II are regulated by post-translational modifications and cellular localization. The heterotetramer is mainly associated with the cytoskeleton (23), while the monomer is found in the cytoplasm and the nucleus (24;25). The monomeric form is also associated with the outer leaflet of the cellular membranes (26).
1. Effect of Post-translational modifications on functions of Annexin II
The post-translational modifications described to date include, 1) acetylation at Ser1, reported to be necessary for binding p11 (27), 2) proteolytic removal of the N-terminal domain, which abolishes binding to lipid rafts in smooth muscle cells (27), and 3) phosphorylation on Tyr23 (28), Ser11 and Ser25 (the latter being the primary phosphorylation site) (29;30), which may be required for binding phospholipids and F-actin (9;31;32) (Diagrammatically presented in Fig 1). In another paper, however, the authors reported that N-terminal acetylation of p36 Annexin II protein was not necessary for binding p11, and that N-terminal acetylation did not affect the conformational stability of AIIt or any other activity of AIIt (33). ANX II is a major substrate for phosphorylation by pp60vsrc (34). Residues Tyr 23 and Serines 11 and 25 are phosphorylated in cells by c-Src and PKC, respectively (29), after activation of either insulin receptor (R) (35), IGF1-R (36), or platelet derived growth factor-R (37). ANX II may thus function as a second messenger for transduction of extracellular signaling pathways. PG/gastrins bind membrane associated ANX II (6), which perhaps leads to co-localization and activation of ANX II and c-Src kinase. The latter possibility is highlighted by the fact that PG/gastrins activate c-Src kinase in target cells (38–40). ANX II may thus provide the link between the ligands, such as PG, plasma membrane and cytoskeleton, and play a role in cytomorphology and cell growth, as previously speculated (41).
The C-terminal core domain also plays an important role in the functions of Annexin II. The core domain comprises the intracellular binding sites for Ca2+, phospholipids and F-actin (9;32), and Ca2++ plays a major role in regulating the association of Annexin II with membranes and cytoskeleton (9;31;34).
More recently, poly/multi-ubiquitin conjugates of Annexin II were described in the Triton X-100 resistant cytoskeleton fractions, suggesting that the ubiquitinated Annexin II may facilitate binding with actin filaments (42). It is now known that degradation by proteasomes requires a polyUb-chain with a minimum of four Ub molecules linked to Lys48 moiety (43). However polyUb-chains branching at Lys29 and Lys63 target the proteins to non-proteasome-dependent cellular processes such as DNA repair, regulation of gene expression, apoptosis, and subnuclear trafficking (44;45). Since AnxII is an actin binding protein associated with lipid rafts (9;31), it has been suggested that binding of AnxII to the actin cytoskeleton makes it a substrate for Ub ligases associated with the cytoskeleton network which perhaps allows Annexin II to facilitate several other cellular functions (42), as described below.
2. Annexin II, associated with the extra-cellular surface of cells, functions as a high-affinity receptor for ligands/proteins
Monomeric and heterotetrameric Annexin II are frequently present on the extra cellular surface of endothelial cells and tumor cells, wherein ANX II has been described as high affinity binding receptor for plasminogen, tissue plasminogen activator (tPA) and plasmin (46), or as a high affinity receptor for the extra cellular matrix protein tenascin-C (47). More recently we reported that membrane associated ANX II functions as a high affinity receptor protein for PG/gastrins (6). At the same time, the S100A10 subunit of AIIt, rather than ANX II, has also been shown to bind tPA and plasminogen (48). It remains to be determined if S100A10 similarly binds gastrin/PG-like peptides. ANX II contributes to 40–50% of the plasminogen and t-PA binding sites on endothelial cells (49). We similarly reported that ANX II contributes to ~50% of the PG/gastrin binding sites on intestinal epithelial (IEC) cells (6).
Besides the above ligand associations, extracellular Annexin II is believed to participate in a number of processes localized to the cell surface. These include tumor cell interaction with hepatic sinusoidal endothelial cells (26), endothelial cell and glioma cell adhesion to matrix via tenascin C (47;50), trafficking of IgG in the placenta (51) and docking of cytomegalovirus on the surface of endothelial cells (52;53).
3A. Annexin-II is a high affinity receptor (binding) protein for progastrin/gastrin peptides
We recently made the unexpected discovery that Annexin-II represents the previously reported (54) 33–36 KDa receptor protein for gastrin/progastrin (6). This discovery was facilitated by the solubilization of the native binding proteins from cellular membranes, and the identification of the 33–36 KDa protein as ANX II by SELDI-TOF MS and MALDI-TOF MS (6). A physical association (binding) of PG with ANX II, in situ, was confirmed in colon cancer cells known to express autocrine PG, using co-immunoprecipitation assays (6). The monomeric 36 KDa ANX II was confirmed as a high affinity binding protein for PG-like peptides in an in vitro binding assay, strongly suggesting that ANX II can function as a membrane receptor for PG/G17 peptides (6) Importantly, we and others have demonstrated that PG does not bind CCK2R, while gastrins bind CCK2R with high affinity (1) (as diagrammatically presented in Fig 2). We further confirmed that ANX II expression is required for mediating ~50% of the growth effects of exogenous PG on intestinal (IEC) cells, and >80% of the growth effects of autocrine gastrins on gastrin-dependent human colon cancer cells (6). It is, however, possible that other proteins, besides ANX II, may be required for mediating 100% of the growth factor effects of PG/gastrins, since PG/gastrins were specifically bound to other, as yet, unknown proteins contained within the heterogeneous band of 42–48 KDa proteins (6), which may also contribute to the growth factor effects of PG/gastrins. In recent papers, Ahmed et al reported the importance of N and C terminal sequences in the gastrin-Gly molecule for conferring high and low affinity binding to novel receptors on colon cancer cells, respectively; these binding sites were distinct from CCK1R and CCK2 R (55;56). Both the low and high affinity binding sites mediated the growth effects of gastrin-like molecules on colon cancer cells (56), suggesting that different receptor sub-types, other than CCK1R and CCK2 R, may be involved in mediating growth factor effects of gastrins/progastrins. Based on our studies (6), it is possible, that the high affinity binding receptor proteins identified by Ahmed et al (6;55;56) may be ANX II. In summary, the findings to-date suggest that the biological effects of progastrin/gastrin like peptides are mediated via the novel PG-R like binding sites, including Annexin II (as diagrammatically presented in Fig 2).
Figure 2. Hypothetical model depicting relative binding of progastrin (PG), G17 (gastrin 1–17) and CCK8 (cholecystokinin octapeptide) to CCK2R and PG-R binding sites (annexin II) and resultant biological effects.
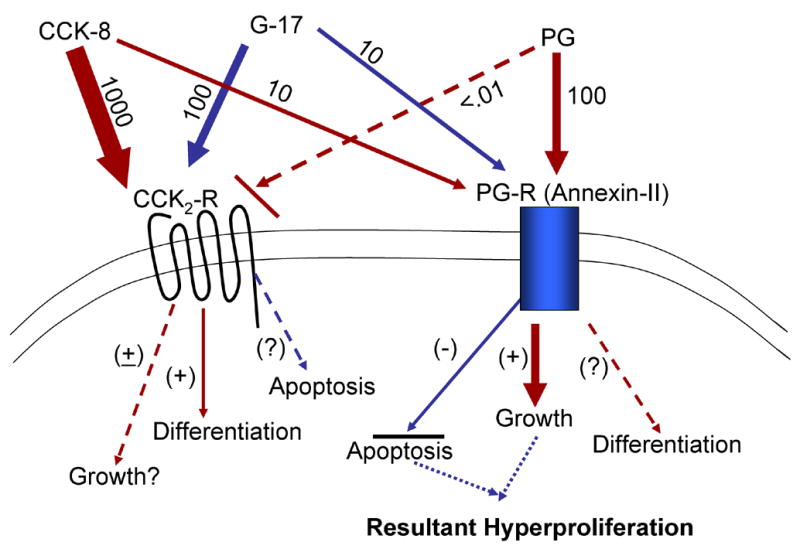
This hypothetical model was developed based on the findings of several investigators including our laboratory as described in the text.
The 36 KDa ANX II protein, bound to radio-labeled TNfnA-D, was generally seen as a doublet band in SDS-PAGE (47). In freshly prepared membrane extracts, the authors frequently saw a single band of 36 KDa; two lower molecular weight bands appeared at later times (47). The authors concluded that since ANX II has two protease cleavage sites near the NH2 terminus, it likely produces 2–3 closely spaced bands on SDS-PAGE on binding with the ligand such as TN-C (47). We similarly reported the presence of a wide band of 32–36 KDa proteins cross linked to radiolabeled gastrins, all of which were identified as ANX II by SELDI-TOF-MS (6). However, in vitro experiments, in the absence of cell associated proteases, only a single band of 36 KDa ANX II co-immunoprecipitated with PG (6).
3B. Binding of Tenascin-C with Annexin II: Role in Colorectal Cancers
Tenascin-C (TN-C) is a large extracellular matrix (ECM) protein expressed in tissues of the immune system, including the bone marrow (57), thymus (58), and the T cell areas of secondary lymphoid organs (59). Tenascin-C is also expressed at other sites in the adult, including tumors and healing wounds, and in restricted patterns in the developing embryo (60). TN-C expression is up-regulated during pathological circumstances like wound healing and tumorigenesis, and proliferative effects of TN-C have been reported on 3T3 fibroblasts (61), endothelial cells (50), and bone marrow mononuclear cells (62). Each subunit of TN-C is built of 14½ epidermal growth factor (EGF)-like repeats, followed by either 8 or 15 fibronectin-type III (TNfn) repeats due to alternative splicing (wherein an additional fragment representing the splice variant, TNfnA-D is expressed), and a fibrinogen-like C-terminal knob (discussed in (62)]. Erickson and co-investigators demonstrated that TNfnA-D binds Annexin II as a cell surface receptor when expressed as a soluble protein or as part of native TN-C (47). Erickson and colleagues investigated the binding of soluble tenascin-C (TN-C) to several cell lines using a radio-ligand binding assay, and demonstrated specific binding of TN-C with human glioma cells and aortic endothelial cells (47). Importantly, the alternatively spliced segment (TNfnA-D) inhibited the binding of native TN-C strongly and also bound the glioma and endothelial cells (47). The authors discovered that the cell surface receptor for TNfnA-D was a 35-KDa protein, identified as Annexin II (47). Purified Annexin II demonstrated binding to both TNfnA-D and TN-C at nM concentrations (47). Based on these results the investigators concluded that Annexin II is an extracellular receptor for the alternatively spliced segment of TN-C and may mediate cellular responses to soluble TN-C that contains the spliced fragment (47).
Overexpression of ANX II and TN-C proteins was measured in a high % of CRC, and appeared to correlate with tumor size, depth of invasion and pTNM stage (63). Interestingly, treatment of human astrocytoma U373 cells with gastrin resulted in overexpression of TN-C and S100A6 (64). Since c-Jun and NF-κB synergistically transactivate TN-C promoter in primary rat embryo fibroblasts (65), gastrins may activate TN-C promoter in glioblastoma cells via the same signaling molecules known to be activated in response to PG/gastrins in other cells (40;66).
Both PG and TNfnA-D/TN-C bind ANX-II at 0.1->1.0nM concentrations (6;47). Our studies further suggest that PG binds ANX II at the TN-C binding site, either with a significantly higher binding affinity, or equivalent affinity; however, only 60% of the binding sites appear to be common suggesting that either PG binds other proteins besides ANX II or it binds additional binding sites on ANX II that are not displaced by TN-C (6).
Radio-labeled TN-C or TNfnA-D bind 48 and 130 KDa proteins besides the 36 KDa ANX II protein on the cell surface of cells, suggesting that TN-C may bind other cytoskeletal proteins, when associated with ANX II (47). We similarly found that radio labeled gastrins were cross linked to not only the 36 KDa ANX II proteins but also to a heterogeneous band of 42–48 KDa proteins; non-specific binding to 72–80 KDa proteins was also identified (6). The non-specifically bound 72–80 KDa proteins may represent the low affinity (Kd=1μM) CCKC proteins (67). The 42–48 KDa proteins may represent cytoskeleton proteins which may become tightly associated with ANX II on binding the PG/gastrin ligands, as described for TN-C (47). Peptides related to the gastrin family have in fact been reported to induce the assembly of actin stress fibers (1), mediated by specific signaling cascades (68); the signaling cascades are likely initiated by binding to ANX II, which may result in the co-localization of PG and ANX II with several kinases and cytoskeletal proteins, as discussed below.
The large TN-C splice variant, containing TNfnA-D, are generally expressed in tissues undergoing active cellular migration or tissue remodeling (47). Transformed fibroblasts and fetal lung tissues express significantly higher TN-C RNA with the A-D domains than normal cells (69). Similarly TN-C variant was absent in normal adult mouse skin but appeared in healing skin and disappeared after wound healing (70). A possible importance of the large unspliced tenascin C in the invasion and metastasis of oral squamous cell carcinoma was described (71). TN-C mRNA for the large spliced variant was similarly described to be prevalent in the majority of the thyroid cancer cell lines and thyroid cancers examined (72). The large unspliced TN-C variant containing the A–D domains is increasingly expressed at the invasive fronts of urinary bladder cancers (73), once again suggesting that the unspliced variant that binds Annexin II with a much higher binding affinity, plays an important role in the invasive behavior of cancer cells. A recent review article further describes the role of Tenascin C and its splice variants in cancer (74).
3C. Binding of ANX-II with t-PA, plasminogen, plasmin and other ligands: Role in metastasis
Annexin II is expressed as a peripheral component of the outer leaflet of the endothelial cell plasma membrane (49). Although it lacks a classical signal peptide, Annexin II is constitutively translocated to the endothelial cell surface within 16 hours of its biosynthesis where it appears to constitute 4–5% of the total cellular pool (reviewed in (49). On the endothelial cells, Annexin II has been described as a profibrinolytic co-receptor for tissue plasminogen activator (t-A) and plasminogen (49). Annexin II contributes to >40–50% of the plasminogen and t-PA binding sites on the surface of cultured endothelial cells, resulting in the enhancement of plasminogen generation (49). Interestingly Annexin II demonstrated a high binding affinity for t-PA (Kd = 48 nM) but appeared to be specific for t-PA and did not bind u-PA. The residues 7–12 in the tail domain of Annexin II have been implicated in the binding of t-PA to Annexin II (49).
While Hajjar and colleagues have demonstrated the presence of high affinity t-PA binding sites on Annexin II, Waisman and colleagues have demonstrated that S100A10 subunit of AIIt functions as the plasminogen regulatory subunit (48;75). The C-terminal lysine of this subunit participates in the stimulation of t-PA dependent plasminogen activation by AIIt (48;75;76). The authors demonstrated that S100A10 subunit binds t-PA (Kd = 0.45 μM), plasminogen (Kd = 1.81 μM), and plasmin (Kd = 0.36 μM) (48). As per Waisman and colleagues, the Annexin-II subunit does not bind t-PA or plasminogen but binds plasmin (Kd = 0.78 μM) (48).
The plasminogen activators, urokinase-type plasminogen activator (uPA) and tissue plasminogen activator (t-PA) are specific serine proteases that cleave plasminogen into plasmin. t-PA mediates mainly intravascular thrombolysis, whereas uPA is involved in pericellular proteolysis during cell migration, wound healing, and tissue remodeling. Unlike the t-PA receptor, the uPA receptor is a 55–60 KDa glycoprotein that is anchored in the cell membrane by a glycosylphosphatidylinositol moiety (77;78). Increased uPA activity has been shown to be correlated with tumor invasiveness and to be a prognostic indicator of disease recurrence and metastasis in many types of cancer (79).
Ma, et al demonstrated that Annexin II functions as a high affinity receptor for β2 glycoprotein I on human endothelial cells (80), and the authors suggest that binding of β2GPI in association with anti-phospholipid antibodies to Annexin II may inhibit plasmin generation, by perhaps compromising the binding of plasminogen and t-PA to AIIT molecules on the endothelial cells (81).
3D. Receptor protein for Nts-1 (metastasin)
Besides binding p11, Annexin II was recently shown to be a receptor protein for S100A4 as well, also known as Nts-1 or metastasin (82). S100A4 is secreted by cancer cells and is detectable in the serum of cancer patients (83). The exogenous addition of S100A4 stimulates endothelial cell motility in vitro (84) as well as induces corneal neovascularization (83) and metastasis formation in vivo (85). S100A4 interacts with Annexin II on the surface of endothelial cells, similar to the interaction of p11, and either alone or in complex with Annexin II, S100A4 accelerates tPA-mediated conversion of plasminogen to plasmin, thus enhancing metastasis and motility.
While cell surface Annexin-II has been shown to facilitate binding of plasminogen to endothelial cells and macrophages (46;86), in tumor cells plasminogen binding and activation appears to be dependent primarily on the cell surface expression of S100A10 (87;88). Results of a recent study suggest that Annexin-II complex is involved in the formation of E-cadherin-based adherens junctions in epithelial cells (89). Cadherins directly bind β-catenin, which in turn binds α-catenin. α-Catenin then binds vinculin and α-actinin. Of these proteins, α-catenin, vinculin and α-actinin are actin filament (F-actin)-binding proteins (90). The association of E-cadherin with the actin cytoskeleton through these binding proteins strengthens the cell-cell adhesion activity of E-cadherin (90). Thus cellular localization of Annexin II may dictate its multiple functions in the various epithelial cell types.
4. Activation of kinases and signaling molecules in response to binding of Annexin-II with various ligands
4A
AIIt was identified as a receptor for plasmin-induced signaling in human peripheral monocytes, which involved the activation of nuclear factor (NF)- κB, JAK/STAT and p38 MAPK signaling cascades, leading to a full scale pro-inflammatory response (91). It is possible, that binding of PG to ANX II may mediate activation of a similar cascade of signaling molecules in target cells, since in preliminary studies we reported activation of NFκB and p38 MAPK in response to PG in cancer cells (66).
4B
In recent studies, a calcium dependent interaction between MEKK4 (MAPK/ERK kinase kinase) and Annexin II was observed when MEKK4 was immunoprecipitated from rat aortic smooth muscle cells treated with angiotensin II (92). MEKK4 is known to regulate MKK6 (MAPK kinase 6), which is an upstream kinase of the stress activated p38 MAP kinase (93–95). Additionally Pyk2 (proline-rich tyrosine kinase 2) phosphorylates MEKK4 in vitro and Pyk2-dependent phosphorylation further regulates MEKK4 dependent phosphorylation of MKK6 (92). Previously it has also been shown that p38 MAPK activity is important for regulation of cyclooxygenase II (COX II) gene transcription, which results in the production of pro-inflammatory prostaglandins and inflammation (96). It is therefore suggested that the binding of Annexin II with MEKK4 results in phosphorylation of MEKK4 by Pyk2, followed by activation of MKK6 and p38 MAPK resulting in up-regulation of COX II gene transcription (92).
4C
Interferon-γ dependent Tyr phosphorylation of MEKK4 via Pyk2 is also regulated by ANX II and SHP2 in keratinocytes (97). The membrane anchored ANX II may thus provide anchoring for the MEKK4 multimeric complex (97). SHP2 (Src-homology-II-domain-containing phosphatase) regulates Src family kinases by de-phosphorylating the upstream docking protein for the inhibitory c-Src kinase CSK, thus ensuring Src activation (98). The Pyk2-MEKK4 multi-protein complex also contains c-Src which is recruited to activate Pyk2, bound to ANX II and MEKK4 which may result in further phosphorylation of Pyk2 and ANX II, since ANX II is a physiological substrate for c-Src kinase (99). Activation of SHP2 is now believed to serve a dual role wherein, initially it activates c-Src (98) and Pyk2 (97) followed by dephosphorylating and inactivating MEKK4 and Pyk2. Since Src kinases are activated in response to PG (38–40), it is possible that PG binding to ANX II may provide a similar platform for multimeric complex formation of various kinases resulting in the observed activation of several signaling molecules in response to PG, including Src, PI3K/Akt, JAK2, STAT5/3, ERKs, p38 MAPK and NFκB (39;40;66;100) (as diagrammatically presented in Figure 3).
Figure 3. Hypothetical model describing possible mediatory role of annexin II, either in its monomeric p-36 form or tetrameric form, in mediating the growth factor and anti-apoptotic effects of progastrin (PG), in the presence or absence of tenascin C (TN-C).

The phosphorylation of molecules is presented as an inverted P, while the kinases that may be involved in mediating the effects, based on the literature presented in the text, are either presented diagrammatically (PKC, c-Src), or spelled out as such. Many of these are signaling molecules that are known to be involved in mediating the effects of Angiotensin via Annexin II, as described in the text.
5. Functions of ANX-II, localized to the inner surface of cellular membranes
ANX II is a prominent component of cholesterol-rich plasma membrane lipid rafts, located in the outer leaflet of cell membranes (101). The membrane rafts also contain caveolins, Src-related kinases, and transmembrane receptors such as CD44, suggesting that ANX II plays a prominent role in the organization of signal transduction related components (102). Membrane micro-domain organization of smooth muscle cells, stabilized by interaction with the underlying actin cytoskeleton, appears to be regulated by Annexin II (103). Annexin II is recruited to actin-rich membrane areas characterized by high cholesterol and phosphatidylinositol-(4,5)-biphosphate content (31;102;104;105). Membrane-bound ANX II present at the inner surface of plasma membranes, may thus serve as a platform for actin assembly (102;105). In spontaneously motile cells, Annexin II was concentrated in dynamic actin-rich protrusions (106). Based on their studies, the investigators concluded that Annexin II has an essential role in maintaining the plasticity of the dynamic membrane associated actin cytoskeleton, due to the direct interaction of Annexin II with polymerised and monomeric actin (106), which may play an equally important role in cancer cell invasion. In other words, Annexin II may provide a direct structural link between the growing ends of actin filaments and the plasma membrane. Depletion of Annexin II using SiRNA led to an accumulation of stress fibers and loss of protrusive and retractile activity (106).
Recombinant Annexin II was demonstrated to directly bind phosphatidylinositol 4,5-biphosphate (105). The affinity of Annexin II for binding PtdIns-4,5P2 was approximately KD ~5μM which is comparable with that reported for a variety of PtdIns-4,5P2 binding proteins. In vivo, Annexin II and PtdIns-4,5P2 were shown to co-localize at sites of pinosome formation. Binding of ANX II with PtdIns-4,5P2 results in actin-dependent macropinosome motility via the activation of PI3k and p38 MAPK (105). Since the activation of PI3k and p38 MAPK kinases mediate migratory effects of PG like peptides on target cells (100), it is possible that binding of PG with ANX II may similarly facilitate migration and invasion, via perhaps the interaction of ANX II with actin at the protrusive ends of the migratory cells, as described above. The latter possibility is further supported by the finding that down-regulation of Annexin II in the presence of Annexin II SiRNA resulted in the inhibition of the invasive properties of glioma cell lines in vitro (107), once again suggesting that the interaction of Annexin II with actin cytoskeleton, as well as its potential to bind invasion associated proteases, likely plays an important role in the invasion of cancer cells, especially in the presence of stimulatory ligands known to bind Annexin II (such as PG).
Recent reports suggest that both AIIT and Annexin-II have a modest selectivity for phosphatidylinositol-(4,5)-P2 over other phosphoinositides and that Lys279 and Lys281 are required for inducing the formation of 1 μ-sized PtdIns(4,5)P2 clusters which are stabilized by cholesterol (108). Annexin II and its ligand p11 are targeted to sites of PtdIns(4,5)P2 enrichment where F-actin accumulates (109). A Ca2+-independent binding of Annexin II to membranes has also been described for chromaffin granules (110), endosomes (111), and other biological membranes (112).
At mild acidic pH, Annexin II binds and aggregates membranes containing anionic phospholipids in the absence of Ca2+, while AIIt at mild acid PH undergoes conformational changes similar to those induced by Ca2+. Thus Annexin II appears to have access to the hydrophobic part of the cellular membranes at both acidic pH in the absence of Ca2+ and at neutral pH in the presence of Ca2+. This may explain our findings of a significant increase in the cross linking of gastrins to ANX II (~ 32–36 KDa) at slightly acidic pH (6).
6. Nuclear localization of ANX-II: Associated functions
Besides localization of Annexin-II to the outer and inner leaflets of the cellular membranes, AnxII monomer has also been shown to readily enter the nucleus, but it is rapidly exported due to a functional nuclear export signal (NES) sequence that closely overlaps the p11 binding region in the AnxII N-terminus (113). p11 binding to AnxII was shown to result in sequestration of the complex in the cytoplasmic compartment; phosphorylation appears to further affect the nucleocytoplasmic partitioning of AnxII (113). However, several reports suggest a role of monomeric AnxII in the nucleus, wherein it has been described as a part of a primer recognition protein complex that enhances DNA polymerase α activity in vitro (114). Annexin-II can also function as an RNA binding protein wherein it was shown to bind to the localization signal in the 3′ untranslated region of c-myc mRNA leading to its association with the cytoskeleton and perinuclear localization (115).
PKCε is considered a fully oncogenic PKC isoform and its translocation to the nucleus occurs when cells are stimulated with phorbol ester, arachidonic acid or serum (116). In a recent study, using MALDI-TOFF MS analysis, investigators identified the proteins associated with PKCε, when this molecule was translocated to the nucleus in 3T3 fibroblasts on treatment with phorbol esters (116;117). The investigators identified at least six proteins that were specifically associated with PKCε in the nucleus, including vimentin, β-actin and Annexin II (117). These results provide further evidence that monomeric Annexin II, that is perhaps phosphorylated at Ser11 or Ser25 in the nuclear export signal domain (118), in association with specific kinases such as PKCε, may play a role in DNA replication and cell proliferation as suggested previously by several investigators (discussed above).
7. Role of ANX-II in cellular transformation and cancers
A link between AnxII and cell transformation was first suggested by the identification of AnxII as a major v-src phosphorylation substrate in transformed fibroblasts (119). Subsequently, AnxII expression has been found to be up-regulated in several types of spontaneous neoplasms, such as pancreatic carcinoma (21), high-grade glioma (120), and in fibroblasts transformed by viral oncogenes such as v-H-ras, v-src and v-mos (121), and in cells treated with mitogenic or trophic factors such as EGF, FGF, NGF or TGFβ1 (16).
Overexpression of Annexin II has been reported in brain, breast, lung, hematologic, liver, pancreatic, gastric and colonic malignant tumors (122). Other than prostate cancers (118;123;124), wherein the expression of Annexin II was reported to be negatively associated with the progression of the metastatic disease, in almost all other cancers, expression of Annexin II is positively associated with malignant progression (reviewed in (125). Recent reports further confirm a selective expression of Annexin II by invasive breast cancers, and the important role of Annexin II towards tumor invasion and progression of breast cancer cells (126). The positive association of ANX-II with malignant progression may be due to several factors as discussed above. In addition, co-localization of ANX-II with Cathepsin B may also contribute to malignant progression of cancer cells as described below. The cysteine protease Cathepsin B is up-regulated in a variety of tumors at the invasive edge, and degrades extracellular matrix proteins such as collagen IV and laminin and activates precursor forms of urokinase plasminogen activator (uPA), initiating an extracellular proteolytic cascade (reviewed in (125). Pro-Cathepsin B interacts with Annexin II heterotetramer (AIIt) on the surface of tumor cells (reviewed in (125). The AIIt binding site for Cathepsin B differs from that for either plasminogen/plasmin or t-PA. AIIt interacts with other ECM proteins, e.g., collagen I and tenascin-C, forming a structural link between the tumor cell surface and the extracellular matrix (reviewed in (125). Thus binding to AIIt is believed to colocalize proteases and their substrates on the tumor cell surface which likely facilitates: 1) activation of precursor forms of proteases and initiation of proteolytic cascades; and 2) selective degradation of extracellular matrix proteins (reviewed in (125). Thus the recruitment of proteases to specific regions on the cell surface by AIIt, serves the function of a ‘proteolytic center’ which may enhance tumor cell detachment, invasion and motility (reviewed in (125).
7A. Role of ANX-II in GI cancers
Pancreatic Cancer
Annexin II expression was reported to be limited to proliferating ductular adenocarcinoma, and co-localized with cells expressing PCNA (21). In normal pancreas, Annexin II expression was not detected in ductular cells and there was no expression in acinar or islet cells (21). Based on these findings, the investigators concluded that Annexin II plays a role in cellular proliferation of pancreatic cancers. In a Syrian hamster model of pancreatic cancer, enhanced levels of annexins, especially Annexin II, were measured in pancreatic carcinoma cells and in the intra-pancreatic allografts of pancreatic carcinoma cells, compared to that in the normal pancreas (127). Importantly, the staining intensity of Annexin II was significantly higher in the proliferating regions of the primary tumors and in the metastatic foci (127). In pancreatic cancer cell lines, t-PA and Annexin II were reported to co-immunoprecipitate; co-localization of t-PA with Annexin II was confirmed by confocal microscopy (128). Importantly, disruption of t-PA/Annexin II interaction by a specific hexapeptide, significantly decreased the invasive capacity of the pancreatic cancer cells in vitro (128). Based on these results the investigators concluded that t-PA specifically binds Annexin II on the extracellular membrane of pancreatic cancer cells, resulting in activation of plasmin and tumor cell invasion (128). As described above, Tenascin C is a component of extra-cellular matrix that characterizes solid tumors, and cell surface ANX II is a high affinity receptor, especially for the large TNC splice variant. The expression of the large TNC transcript in pancreatic cancers but not in normal pancreas was confirmed in a recent study (129). Importantly, the expression of both the large TNC transcript and cell surface Annexin II was progressively increased at different stages of pancreatic carcinogenesis from low grade pancreatic intra-epithelial neoplasia to pancreatic ductile adenocarcinoma, wherein the pancreatic stellate cells were identified as the source of TNC, while Annexin II was expressed on the cell surface of pancreatic cancer epithelial cells (129). The authors observed a progressive switch from cytoplasmic to cell surface expression of Annexin II during the different phases of pancreatic carcinogenesis (129). The authors speculated that the simultaneous overexpression of the large splice variant of TNC and Annexin II in the invasive pancreatic tumors suggests a role of the two molecules in invasion.
Gastric cancers
Annexin II was identified as one of the many genes, up-regulated in gastric carcinomas (130). It was more strongly expressed on cellular membranes than in the cytoplasm of tumor cells in primary gastric carcinoma tissues (130). Annexin II was overexpressed in advanced gastric carcinomas and was believed to contribute to the progression of gastric carcinoma disease. Up-regulation of Annexin II was confirmed in gastric cancer cells infected with H. pylori (131). The authors used SELDI-TOF MS to identify biomarkers from H. pylori infected gastric cancer cells and discovered Annexin II as one of the proteins that was significantly up-regulated (131).
Annexin II is expressed in several human gastric carcinoma cell lines, and is expressed more strongly on the cellular membranes than in the cytoplasm of the primary gastric carcinoma tissues examined (130). The expression of Annexin II correlated with the expression of c-erbB-2 in gastric cancers, and patients with Annexin II expression had a poorer prognosis (130). H. pylori infection is known to result in mucosal gastritis and an increased risk of ulceration and gastric cancer (132). In a recent study, normal and gastric cancer cells were infected with H. pylori and differential expression of genes and proteins measured (133). H. pylori infection consistently up-regulated Annexin II, S100A7 and the Rho-GTP protein and significantly altered the location of E-cadherin from plasma membrane to intra-cellular vesicles (133).. Based on the results, the authors suggested that H. pylori infection results in destabilizing epithelial cell adherence via the up-regulation of specific membrane associated proteins such as Annexin II and S100A7, which may play a role in increasing the risk of gastric carcinogenesis (133).
Colorectal cancer
The expression of Annexin II and Tenascin-C was measured in human colorectal carcinoma cell lines and 105 primary colorectal carcinomas (63). Overexpression of Annexin II and Tenascin-C proteins was measured in 29.5% and 49.5%, respectively, of colorectal carcinomas, and appeared to be correlated with tumor size, depth of invasion and pTNM stage (63). Based on their data, the investigators suggested that since both Annexin II and Tenascin-C are over-expressed in advanced colorectal carcinomas, these proteins may play an important role in the progression and metastatic spread of colorectal carcinomas. Up-regulation of Annexin-II and its ligand tenascin-C, has been observed in the carcinomas of the colon, stomach, breast, and lung. The expression levels have been reported to be associated with the prognosis, differentiation and stage of colorectal and gastric carcinomas (63;130). Several investigators have demonstrated that the expression of tenascin-C/Annexin II complex correlates with a loss of focal cell adhesion, increased cellular proliferation and increased migration of cells (134).
A specific role of Annexin II in the proliferation of human colon cancer cells was further demonstrated in a study with butyrate. Butyrate induced cell differentiation and growth arrest of several colon cancer cell lines was associated with increasing levels of Annexin A1 and Annexin A5, but a significant decrease in the relative levels of Annexin-II, suggesting that Annexin-II plays a role in proliferation but not differentiation (135). Butyrate is the preferred energy source and is generally required for the maintenance of normal colonic epithelial cell development, and is believed to mediate the protective effect of dietary fiber in colon carcinogenesis (136). Butyrate has been shown to be protective against inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease (137). Butyrate has therefore been considered important for therapeutic purposes to suppress colonic inflammation and treatment of colorectal cancer (discussed in (137).
In a colorectal cancer cell line, COLO 222 which does not express Annexin-II, down-regulation of S100A10 (p11) resulted in a significant loss in the plasminogen binding, plasmin generation and a complete loss in plasminogen dependent cellular invasiveness (88). It thus appears that in some colorectal cancer cells, rather than Annexin-II, S100A10, plays a similar role and co-localizes with uPAR resulting in the activation of plasminogen (88).
7B. Role of Annexin II in other cancers
Medullary thyroid carcinoma
Using the method of differential display, differentially expressed transcripts were identified in medullary thyroid carcinomas (MTCs) that were not positive for the mutant RET onocogene (134). Annexin-II was identified as one of the transcripts overexpressed in the C-cell carcinomas, while its expression was absent in the normal thyroid (134). The authors reported co-localization of ANX-II with the RET receptor complex within caveolae of the cell membranes of C-cells, and the authors concluded that Annexin-II may promote malignant growth, tumor invasion and metastasis (134). Since up-regulation of Annexin-II in MTCs was not associated with mutation of the RET proto-oncogene, the authors speculated that expression of tenascin-C/Annexin II complex may be linked to additional genetic alterations within the tumors, correlating with a poor prognosis of the patients (134).
ANX-II expression in β-cell lymphomas, small cell lung cancers and oxidative stress induced renal cell carcinomas
In human B-cell lymphoma cell lines, a phosphorylated form of Annexin II is believed to be important for cell survival and proliferation (138). Similarly, elevated expression of Annexin II was reported in a multi-drug resistant small cell lung cancer cell line (20). Expression of Annexin II in conventional renal cell carcinomas correlated with Fuhrman grade and clinical outcome (139). In grade I tumors only a weak membranous staining was seen in immunopositive cells, while in grades II and III tumors, Annexin II was identified in the cytoplasm and cell membranes of tumor cells (139). In a rodent model of renal cancer, ferric nitrilotriacetate induces oxidative renal damage leading to high incidence of renal cell carcinoma (140). Differential display analysis of renal cell carcinomas revealed elevated expression of Annexin II (141). Annexin II was phosphorylated at serine and tyrosine residues in the renal cell carcinomas and coimmunoprecipitated with phosphorylated actin (22). Based on their findings, the investigators suggested that Anx-II may be regulated by redox status, and that phosphorylated ANX-II may play an important role in the proliferation and metastasis of oxidative stress-induced renal cell carcinomas (22).
7C. Role of ANX-II in association with PG/gastrins and TN-C in carcinogenesis
ANX II expression is up-regulated in fibroblasts transformed by v-H-ras, v-src and v-mos (121), and in cells treated with mitogenic or trophic factors such as EGF, FGF, NGF or TGFβ1 (16). Up-regulation of ANX II was also discovered in gastric epithelial cells infected with H. pylori (131). At the same time, H. pylori infection of gastric mucosa and growth factors such as EGF and TGFβ1 have been reported to increase the expression of gastrin gene, directly or indirectly (1;3;142). These findings suggest the intriguing possibility that the direct (EGF, TGFβ1, oncogenic K-ras) and indirect (H. pylori) growth effects of these agents may be magnified by an up-regulation of both the gastrin gene (PG) and its receptor, ANX II. Growth factors such as TGF-β1 and specific cytokines have also been recently shown to up-regulate the expression of TN-C by stellate cells associated with tumors (129). It is thus possible that surface associated Annexin II can bind growth stimulatory ligands such as PG and TN-C at different phases of carcinogenesis, besides binding a whole host of other molecules (such as TPA, plasminogen, plasmin, β-actin, kinases, etc.), which perhaps leads to enhanced growth and metastasis, suggesting an important role of Annexin II during different phases of carcinogenesis.
Based on the literature described above, it appears likely that the splice variant of TN-C may facilitate cell migration/invasion and tissue remodeling possibly through binding to ANX II with high affinity (47). Based on our studies so far, we hypothesize that in the absence of the expression of the splice variant form of TN-C, PG likely binds ANX II with a significantly higher binding affinity and dictates the functions of ANX II towards cellular proliferation and progression of the disease. However, on expression of the TN-C splice variant, which may occur during advanced stages of the CRC disease, the splice variant may bind ANX II with equivalent affinity and co-contribute to the functions of ANX II along with PG. Future studies in this field should help to dissect the relative contribution of ANX II, splice variant of TN-C and autocrine PG in the proliferation and metastasis of CRC cells.
Acknowledgments
I would like to thank Cheryl Simmons, Lynette Durant and Pat Gazzoli for their secretarial support and work on this article. These studies were supported by funds from grant #s R01CA097959 and RO1CA114264 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Rengifo-Cam W, Singh P. Role of progastrins and gastrins and their receptors in GI and pancreatic cancers: targets for treatment. Curr Pharm Des. 2004;10(19):2345–2358. doi: 10.2174/1381612043383999. [DOI] [PubMed] [Google Scholar]
- 2.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86(3):805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 3.Ferrand A, Wang TC. Gastrin and cancer: a review. Cancer Lett. 2006;238(1):15–29. doi: 10.1016/j.canlet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Wu H, Rao GN, Dai B, Singh P. Autocrine gastrins in colon cancer cells Up-regulate cytochrome c oxidase Vb and down-regulate efflux of cytochrome c and activation of caspase-3. J Biol Chem. 2000;275(42):32491–32498. doi: 10.1074/jbc.M002458200. [DOI] [PubMed] [Google Scholar]
- 5.Wu H, Owlia A, Singh P. Precursor peptide progastrin(1–80) reduces apoptosis of intestinal epithelial cells and upregulates cytochrome c oxidase Vb levels and synthesis of ATP. Am J Physiol Gastrointest Liver Physiol. 2003;285(6):G1097–G1110. doi: 10.1152/ajpgi.00216.2003. [DOI] [PubMed] [Google Scholar]
- 6.Singh P, Wu H, Clark C, Owlia A. Annexin II binds progastrin and gastrin-like peptides, and mediates growth factor effects of autocrine and exogenous gastrins on colon cancer and intestinal epithelial cells. Oncogene. 2006 doi: 10.1038/sj.onc.1209798. [DOI] [PubMed] [Google Scholar]
- 7.Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197(1):63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 8.Swairjo MA, Seaton BA. Annexin structure and membrane interactions: a molecular perspective. Annu Rev Biophys Biomol Struct. 1994;23:193–213. doi: 10.1146/annurev.bb.23.060194.001205. [DOI] [PubMed] [Google Scholar]
- 9.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82(2):331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 10.Waisman DM. Annexin II tetramer: structure and function. Mol Cell Biochem. 1995;149–150:301–322. doi: 10.1007/BF01076592. [DOI] [PubMed] [Google Scholar]
- 11.Gerke V. Tyrosine protein kinase substrate p36: a member of the annexin family of Ca2+/phospholipid-binding proteins. Cell Motil Cytoskeleton. 1989;14(4):449–454. doi: 10.1002/cm.970140402. [DOI] [PubMed] [Google Scholar]
- 12.Frohlich M, Motte P, Galvin K, Takahashi H, Wands J, Ozturk M. Enhanced expression of the protein kinase substrate p36 in human hepatocellular carcinoma. Mol Cell Biol. 1990;10(6):3216–3223. doi: 10.1128/mcb.10.6.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh P, Lu X, Cobb S, Miller BT, Tarasova N, Varro A, et al. Progastrin1–80 stimulates growth of intestinal epithelial cells in vitro via high-affinity binding sites. Am J Physiol Gastrointest Liver Physiol. 2003;284(2):G328–G339. doi: 10.1152/ajpgi.00351.2002. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin GS, Hollande F, Yang Z, Karelina Y, Paterson A, Strang R, et al. Biologically active recombinant human progastrin(6–80) contains a tightly bound calcium ion. J Biol Chem. 2001;276(11):7791–7796. doi: 10.1074/jbc.M009985200. [DOI] [PubMed] [Google Scholar]
- 15.Chiang Y, Schneiderman MH, Vishwanatha JK. Annexin II expression is regulated during mammalian cell cycle. Cancer Res. 1993;53(24):6017–6021. [PubMed] [Google Scholar]
- 16.Keutzer JC, Hirschhorn RR. The growth-regulated gene 1B6 is identified as the heavy chain of calpactin I. Exp Cell Res. 1990;188(1):153–159. doi: 10.1016/0014-4827(90)90291-h. [DOI] [PubMed] [Google Scholar]
- 17.Vellucci VF, Germino FJ, Reiss M. Cloning of putative growth regulatory genes from primary human keratinocytes by subtractive hybridization. Gene. 1995;166(2):213–220. doi: 10.1016/0378-1119(95)00543-9. [DOI] [PubMed] [Google Scholar]
- 18.Vishwanatha JK, Kumble S. Involvement of annexin II in DNA replication: evidence from cell-free extracts of Xenopus eggs. J Cell Sci. 1993;105 (Pt 2):533–540. doi: 10.1242/jcs.105.2.533. [DOI] [PubMed] [Google Scholar]
- 19.Chiang Y, Rizzino A, Sibenaller ZA, Wold MS, Vishwanatha JK. Specific down-regulation of annexin II expression in human cells interferes with cell proliferation. Mol Cell Biochem. 1999;199(1–2):139–147. doi: 10.1023/a:1006942128672. [DOI] [PubMed] [Google Scholar]
- 20.Cole SPC, Pinkoski MJ, Bhardwaj G, Deeley RG. Elevated Expression of Annexin-Ii (Lipocortin-Ii, P36) in A Multidrug Resistant Small-Cell Lung-Cancer Cell-Line. British Journal of Cancer. 1992;65(4):498–502. doi: 10.1038/bjc.1992.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vishwanatha JK, Chiang Y, Kumble KD, Hollingsworth MA, Pour PM. Enhanced expression of annexin II in human pancreatic carcinoma cells and primary pancreatic cancers. Carcinogenesis. 1993;14(12):2575–2579. doi: 10.1093/carcin/14.12.2575. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Akatsuka S, Ozeki M, Shirase T, Hiai H, Toyokuni S. Redox regulation of annexin 2 and its implications for oxidative stress-induced renal carcinogenesis and metastasis. Oncogene. 2004;23(22):3980–3989. doi: 10.1038/sj.onc.1207555. [DOI] [PubMed] [Google Scholar]
- 23.Zokas L, Glenney JR., Jr The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J Cell Biol. 1987;105(5):2111–2121. doi: 10.1083/jcb.105.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumble KD, Vishwanatha JK. Immunoelectron Microscopic Analysis of the Intracellular-Distribution of Primer Recognition Proteins, Annexin-2 and Phosphoglycerate Kinase, in Normal and Transformed-Cells. Journal of Cell Science. 1991;99:751–758. doi: 10.1242/jcs.99.4.751. [DOI] [PubMed] [Google Scholar]
- 25.Vishwanatha JK, Jindal HK, Davis RG. The role of primer recognition proteins in DNA replication: association with nuclear matrix in HeLa cells. J Cell Sci. 1992;101(Pt 1):25–34. doi: 10.1242/jcs.101.1.25. [DOI] [PubMed] [Google Scholar]
- 26.Tressler RJ, Updyke TV, Yeatman T, Nicolson GL. Extracellular annexin II is associated with divalent cation-dependent tumor cell-endothelial cell adhesion of metastatic RAW117 large-cell lymphoma cells. J Cell Biochem. 1993;53(3):265–276. doi: 10.1002/jcb.240530311. [DOI] [PubMed] [Google Scholar]
- 27.Konig J, Prenen J, Nilius B, Gerke V. The annexin II-p11 complex is involved in regulated exocytosis in bovine pulmonary artery endothelial cells. J Biol Chem. 1998;273(31):19679–19684. doi: 10.1074/jbc.273.31.19679. [DOI] [PubMed] [Google Scholar]
- 28.Glenney JR, Jr, Tack BF. Amino-terminal sequence of p36 and associated p10: identification of the site of tyrosine phosphorylation and homology with S-100. Proc Natl Acad Sci U S A. 1985;82(23):7884–7888. doi: 10.1073/pnas.82.23.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould KL, Woodgett JR, Isacke CM, Hunter T. The protein-tyrosine kinase substrate p36 is also a substrate for protein kinase C in vitro and in vivo. Mol Cell Biol. 1986;6(7):2738–2744. doi: 10.1128/mcb.6.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jost M, Gerke V. Mapping of a regulatory important site for protein kinase C phosphorylation in the N-terminal domain of annexin II. Biochimica et Biophysica Acta-Molecular Cell Research. 1996;1313(3):283–289. doi: 10.1016/0167-4889(96)00101-2. [DOI] [PubMed] [Google Scholar]
- 31.Rescher U, Gerke V. Annexins--unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117(Pt 13):2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- 32.Filipenko NR, Waisman DM. The C terminus of annexin II mediates binding to F-actin. J Biol Chem. 2001;276(7):5310–5315. doi: 10.1074/jbc.M009710200. [DOI] [PubMed] [Google Scholar]
- 33.Kang HM, Kassam G, Jarvis SE, Fitzpatrick SL, Waisman DM. Characterization of human recombinant annexin II tetramer purified from bacteria: Role of N-terminal acetylation. Biochemistry. 1997;36(8):2041–2050. doi: 10.1021/bi962569b. [DOI] [PubMed] [Google Scholar]
- 34.Gerke V, Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984;3(1):227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biener Y, Feinstein R, Mayak M, Kaburagi Y, Kadowaki T, Zick Y. Annexin II is a novel player in insulin signal transduction. Possible association between annexin II phosphorylation and insulin receptor internalization. J Biol Chem. 1996;271(46):29489–29496. doi: 10.1074/jbc.271.46.29489. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Chan JL, Zong CS, Wang LH. Effect of tyrosine mutations on the kinase activity and transforming potential of an oncogenic human insulin-like growth factor I receptor. J Biol Chem. 1996;271(1):160–167. doi: 10.1074/jbc.271.1.160. [DOI] [PubMed] [Google Scholar]
- 37.Brambilla R, Zippel R, Sturani E, Morello L, Peres A, Alberghina L. Characterization of the tyrosine phosphorylation of calpactin I (annexin II) induced by platelet-derived growth factor. Biochem J. 1991;278(Pt 2):447–452. doi: 10.1042/bj2780447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh P, Narayan S, Adiga RB. Phosphorylation of pp62 and pp54 src-like proteins in a rat intestinal cell line in response to gastrin. Am J Physiol. 1994;267(2 Pt 1):G235–G244. doi: 10.1152/ajpgi.1994.267.2.G235. [DOI] [PubMed] [Google Scholar]
- 39.Brown D, Yallampalli U, Owlia A, Singh P. pp60c-Src Kinase mediates growth effects of the full-length precursor progastrin1-80 peptide on rat intestinal epithelial cells, in vitro. Endocrinology. 2003;144(1):201–211. doi: 10.1210/en.2002-220501. [DOI] [PubMed] [Google Scholar]
- 40.Ferrand A, Bertrand C, Portolan G, Cui G, Carlson J, Pradayrol L, et al. Signaling pathways associated with colonic mucosa hyperproliferation in mice overexpressing gastrin precursors. Cancer Res. 2005;65(7):2770–2777. doi: 10.1158/0008-5472.CAN-04-0978. [DOI] [PubMed] [Google Scholar]
- 41.Cheney RE, Willard MB. Characterization of the interaction between calpactin I and fodrin (non-erythroid spectrin) J Biol Chem. 1989;264(30):18068–18075. [PubMed] [Google Scholar]
- 42.Lauvrak SU, Hollas H, Doskeland AP, Aukrust I, Flatmark T, Vedeler A. Ubiquitinated annexin A2 is enriched in the cytoskeleton fraction. FEBS Lett. 2005;579(1):203–206. doi: 10.1016/j.febslet.2004.11.076. [DOI] [PubMed] [Google Scholar]
- 43.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19(1):94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickart CM. Ubiquitin enters the new millennium. Mol Cell. 2001;8(3):499–504. doi: 10.1016/s1097-2765(01)00347-1. [DOI] [PubMed] [Google Scholar]
- 45.Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem. 2003;278(38):35857–35860. doi: 10.1074/jbc.R300018200. [DOI] [PubMed] [Google Scholar]
- 46.Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J Biol Chem. 1994;269(33):21191–21197. [PubMed] [Google Scholar]
- 47.Chung CY, Erickson HP. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-C. J Cell Biol. 1994;126(2):539–548. doi: 10.1083/jcb.126.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacLeod TJ, Kwon M, Filipenko NR, Waisman DM. Phospholipid-associated annexin A2-S100A10 heterotetramer and its subunits: characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin. J Biol Chem. 2003;278(28):25577–25584. doi: 10.1074/jbc.M301017200. [DOI] [PubMed] [Google Scholar]
- 49.Hajjar KA, Acharya SS. Annexin II and regulation of cell surface fibrinolysis. Ann N Y Acad Sci. 2000;902:265–271. doi: 10.1111/j.1749-6632.2000.tb06321.x. [DOI] [PubMed] [Google Scholar]
- 50.Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell. 1996;7(6):883–892. doi: 10.1091/mbc.7.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kristoffersen EK, Matre R. Surface annexin II on placental membranes of the fetomaternal interface. Am J Reprod Immunol. 1996;36(3):141–149. doi: 10.1111/j.1600-0897.1996.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 52.Wright JF, Kurosky A, Pryzdial EL, Wasi S. Host cellular annexin II is associated with cytomegalovirus particles isolated from cultured human fibroblasts. J Virol. 1995;69(8):4784–4791. doi: 10.1128/jvi.69.8.4784-4791.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bold S, Ohlin M, Garten W, Radsak K. Structural domains involved in human cytomegalovirus glycoprotein B-mediated cell-cell fusion. J Gen Virol. 1996;77(Pt 9):2297–2302. doi: 10.1099/0022-1317-77-9-2297. [DOI] [PubMed] [Google Scholar]
- 54.Chicone L, Narayan S, Townsend CM, Jr, Singh P. The presence of a 33-40 KDa gastrin binding protein on human and mouse colon cancer. Biochem Biophys Res Commun. 1989;164(1):512–519. doi: 10.1016/0006-291x(89)91749-x. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed S, Murphy RF, Lovas S. Importance of N- and C-terminal regions of gastrin-Gly for preferential binding to high and low affinity gastrin-Gly receptors. Peptides. 2005;26(7):1207–1212. doi: 10.1016/j.peptides.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed S, Budai B, Heredi-Szabo K, Farkas J, Toth G, Murphy RF, et al. High and low affinity receptors mediate growth effects of gastrin and gastrin-Gly on DLD-1 human colonic carcinoma cells. FEBS Lett. 2004;556(1–3):199–203. doi: 10.1016/s0014-5793(03)01408-x. [DOI] [PubMed] [Google Scholar]
- 57.Klein G, Beck S, Muller CA. Tenascin is a cytoadhesive extracellular matrix component of the human hematopoietic microenvironment. J Cell Biol. 1993;123(4):1027–1035. doi: 10.1083/jcb.123.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hemesath TJ, Stefansson K. Expression of tenascin in thymus and thymic nonlymphoid cells. J Immunol. 1994;152(2):422–428. [PubMed] [Google Scholar]
- 59.Chilosi M, Lestani M, Benedetti A, Montagna L, Pedron S, Scarpa A, et al. Constitutive expression of tenascin in T-dependent zones of human lymphoid tissues. Am J Pathol. 1993;143(5):1348–1355. [PMC free article] [PubMed] [Google Scholar]
- 60.Erickson HP, Bourdon MA. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- 61.End P, Panayotou G, Entwistle A, Waterfield MD, Chiquet M. Tenascin: a modulator of cell growth. Eur J Biochem. 1992;209(3):1041–1051. doi: 10.1111/j.1432-1033.1992.tb17380.x. [DOI] [PubMed] [Google Scholar]
- 62.Seiffert M, Beck SC, Schermutzki F, Muller CA, Erickson HP, Klein G. Mitogenic and adhesive effects of tenascin-C on human hematopoietic cells are mediated by various functional domains. Matrix Biol. 1998;17(1):47–63. doi: 10.1016/s0945-053x(98)90124-x. [DOI] [PubMed] [Google Scholar]
- 63.Emoto K, Yamada Y, Sawada H, Fujimoto H, Ueno M, Takayama T, et al. Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer. 2001;92(6):1419–1426. doi: 10.1002/1097-0142(20010915)92:6<1419::aid-cncr1465>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 64.Kucharczak J, Pannequin J, Camby I, Decaestecker C, Kiss R, Martinez J. Gastrin induces over-expression of genes involved in human U373 glioblastoma cell migration. Oncogene. 2001;20(48):7021–7028. doi: 10.1038/sj.onc.1204882. [DOI] [PubMed] [Google Scholar]
- 65.Mettouchi A, Cabon F, Montreau N, Dejong V, Vernier P, Gherzi R, et al. The c-Jun-induced transformation process involves complex regulation of tenascin-C expression. Mol Cell Biol. 1997;17(6):3202–3209. doi: 10.1128/mcb.17.6.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rengifo-Cam W, Singh P. Anti-apoptotic effects of progastrins are mediated by NfkP65 activation in AR42J pancreatic cancer cells. Gastroenterology. 2005;128(4):A485. [Google Scholar]
- 67.Zhang QX, Baldwin GS. Structures of the human cDNA and gene encoding the 78 kDa gastrin-binding protein and of a related pseudogene. Biochim Biophys Acta. 1994;1219(2):567–575. doi: 10.1016/0167-4781(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 68.Stepan VM, Tatewaki M, Matsushima M, Dickinson CJ, Del Valle J, Todisco A. Gastrin induces c-fos gene transcription via multiple signaling pathways. Am J Physiol. 1999;276(2 Pt 1):G415–G424. doi: 10.1152/ajpgi.1999.276.2.G415. [DOI] [PubMed] [Google Scholar]
- 69.Oyama F, Hirohashi S, Shimosato Y, Titani K, Sekiguchi K. Qualitative and quantitative changes of human tenascin expression in transformed lung fibroblast and lung tumor tissues: comparison with fibronectin. Cancer Res. 1991;51(18):4876–4881. [PubMed] [Google Scholar]
- 70.Chuong CM, Chen HM. Enhanced expression of neural cell adhesion molecules and tenascin (cytotactin) during wound healing. Am J Pathol. 1991;138(2):427–440. [PMC free article] [PubMed] [Google Scholar]
- 71.Franz M, Hansen T, Richter P, Borsi L, Bohmer FD, Hyckel P, et al. Complex formation of the laminin-5 gamma2 chain and large unspliced tenascin-C in oral squamous cell carcinoma in vitro and in situ: implications for sequential modulation of extracellular matrix in the invasive tumor front. Histochem Cell Biol. 2006;126(1):125–131. doi: 10.1007/s00418-005-0126-5. [DOI] [PubMed] [Google Scholar]
- 72.Tseleni-Balafouta S, Gakiopoulou H, Fanourakis G, Voutsinas G, Balafoutas D, Patsouris E. Tenascin-C protein expression and mRNA splice variants in thyroid carcinoma. Exp Mol Pathol. 2006;80(2):177–182. doi: 10.1016/j.yexmp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 73.Berndt A, Anger K, Richter P, Borsi L, Brack S, Silacci M, et al. Differential expression of tenascin-C splicing domains in urothelial carcinomas of the urinary bladder. J Cancer Res Clin Oncol. 2006;132(8):537–546. doi: 10.1007/s00432-006-0106-8. [DOI] [PubMed] [Google Scholar]
- 74.Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett. 2006:1–21. doi: 10.1016/j.canlet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 75.Fogg DK, Bridges DE, Cheung KK, Kassam G, Filipenko NR, Choi KS, et al. The p11 subunit of annexin II heterotetramer is regulated by basic carboxypeptidase. Biochemistry. 2002;41(15):4953–4961. doi: 10.1021/bi012045y. [DOI] [PubMed] [Google Scholar]
- 76.Kassam G, Le BH, Choi KS, Kang HM, Fitzpatrick SL, Louie P, et al. The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry. 1998;37(48):16958–16966. doi: 10.1021/bi981713l. [DOI] [PubMed] [Google Scholar]
- 77.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3(12):932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 78.Ploug M, Behrendt N, Lober D, Dano K. Protein structure and membrane anchorage of the cellular receptor for urokinase-type plasminogen activator. Semin Thromb Hemost. 1991;17(3):183–193. doi: 10.1055/s-2007-1002608. [DOI] [PubMed] [Google Scholar]
- 79.Duffy MJ. Urokinase-type plasminogen activator: a potent marker of metastatic potential in human cancers. Biochem Soc Trans. 2002;30(2):207–210. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 80.Ma K, Simantov R, Zhang JC, Silverstein R, Hajjar KA, McCrae KR. High affinity binding of beta 2-glycoprotein I to human endothelial cells is mediated by annexin II. J Biol Chem. 2000;275(20):15541–15548. doi: 10.1074/jbc.275.20.15541. [DOI] [PubMed] [Google Scholar]
- 81.Wolberg AS, Roubey RA. Annexin A2: better left alone. Blood. 2005;105(5):1845–1846. doi: 10.1182/blood-2004-12-4786. [DOI] [PubMed] [Google Scholar]
- 82.Semov A, Moreno MJ, Onichtchenko A, Abulrob A, Ball M, Ekiel I, et al. Metastasis-associated protein S100A4 induces angiogenesis through interaction with Annexin II and accelerated plasmin formation. J Biol Chem. 2005;280(21):20833–20841. doi: 10.1074/jbc.M412653200. [DOI] [PubMed] [Google Scholar]
- 83.Ambartsumian N, Klingelhofer J, Grigorian M, Christensen C, Kriajevska M, Tulchinsky E, et al. The metastasis-associated Mts1(S100A4) protein could act as an angiogenic factor. Oncogene. 2001;20(34):4685–4695. doi: 10.1038/sj.onc.1204636. [DOI] [PubMed] [Google Scholar]
- 84.Schmidt-Hansen B, Ornas D, Grigorian M, Klingelhofer J, Tulchinsky E, Lukanidin E, et al. Extracellular S100A4(mts1) stimulates invasive growth of mouse endothelial cells and modulates MMP-13 matrix metalloproteinase activity. Oncogene. 2004;23(32):5487–5495. doi: 10.1038/sj.onc.1207720. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt-Hansen B, Klingelhofer J, Grum-Schwensen B, Christensen A, Andresen S, Kruse C, et al. Functional significance of metastasis-inducing S100A4(Mts1) in tumor-stroma interplay. J Biol Chem. 2004;279(23):24498–24504. doi: 10.1074/jbc.M400441200. [DOI] [PubMed] [Google Scholar]
- 86.Falcone DJ, Borth W, Khan KMF, Hajjar KA. Plasminogen-mediated matrix invasion and degradation by macrophages is dependent on surface expression of annexin II. Blood. 2001;97(3):777–784. doi: 10.1182/blood.v97.3.777. [DOI] [PubMed] [Google Scholar]
- 87.Choi KS, Fogg DK, Yoon CS, Waisman DM. p11 regulates extracellular plasmin production and invasiveness of HT1080 fibrosarcoma cells. FASEB J. 2003;17(2):235–246. doi: 10.1096/fj.02-0697com. [DOI] [PubMed] [Google Scholar]
- 88.Zhang LB, Fogg DK, Waisman DM. RNA interference-mediated silencing of the S100A10 gene attenuates plasmin generation and invasiveness of Colo 222 colorectal cancer cells. Journal of Biological Chemistry. 2004;279(3):2053–2062. doi: 10.1074/jbc.M310357200. [DOI] [PubMed] [Google Scholar]
- 89.Yamada A, Irie K, Hirota T, Ooshio T, Fukuhara A, Takai Y. Involvement of the annexin II-S100A10 complex in the formation of E-cadherin-based adherens junctions in Madin-Darby canine kidney cells. J Biol Chem. 2005;280(7):6016–6027. doi: 10.1074/jbc.M408215200. [DOI] [PubMed] [Google Scholar]
- 90.Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol. 2001;13(5):600–603. doi: 10.1016/s0955-0674(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 91.Laumonnier Y, Syrovets T, Burysek L, Simmet T. Identification of the annexin A2 heterotetramer as a receptor for the plasmin-induced signaling in human peripheral monocytes. Blood. 2006;107(8):3342–3349. doi: 10.1182/blood-2005-07-2840. [DOI] [PubMed] [Google Scholar]
- 92.Derbyshire ZE, Halfter UM, Heimark RL, Sy TH, Vaillancourt RR. Angiotensin II stimulated transcription of cyclooxygenase II is regulated by a novel kinase cascade involving Pyk2, MEKK4 and annexin II. Mol Cell Biochem. 2005;271(1–2):77–90. doi: 10.1007/s11010-005-5386-9. [DOI] [PubMed] [Google Scholar]
- 93.Gerwins P, Blank JL, Johnson GL. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J Biol Chem. 1997;272(13):8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- 94.Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95(4):521–530. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- 95.Takekawa M, Posas F, Saito H. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 1997;16(16):4973–4982. doi: 10.1093/emboj/16.16.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rebsamen MC, Capoccia R, Vallotton MB, Lang U. Role of cyclooxygenase 2, p38 and p42/44 MAPK in the secretion of prostacyclin induced by epidermal growth factor, endothelin-1 and angiotensin II in rat ventricular cardiomyocytes. J Mol Cell Cardiol. 2003;35(1):81–89. doi: 10.1016/s0022-2828(02)00281-x. [DOI] [PubMed] [Google Scholar]
- 97.Halfter UM, Derbyshire ZE, Vaillancourt RR. Interferon-gamma-dependent tyrosine phosphorylation of MEKK4 via Pyk2 is regulated by annexin II and SHP2 in keratinocytes. Biochem J. 2005;388(Pt 1):17–28. doi: 10.1042/BJ20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang SQ, Yang W, Kontaridis MI, Bivona TG, Wen G, Araki T, et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell. 2004;13(3):341–355. doi: 10.1016/s1097-2765(04)00050-4. [DOI] [PubMed] [Google Scholar]
- 99.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383(6600):547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 100.Hollande F, Choquet A, Blanc EM, Lee DJ, Bali JP, Baldwin GS. Involvement of phosphatidylinositol 3-kinase and mitogen-activated protein kinases in glycine-extended gastrin-induced dissociation and migration of gastric epithelial cells. J Biol Chem. 2001;276(44):40402–40410. doi: 10.1074/jbc.M105090200. [DOI] [PubMed] [Google Scholar]
- 101.Zhao WQ, Waisman DM, Grimaldi M. Specific localization of the annexin II heterotetramer in brain lipid raft fractions and its changes in spatial learning. J Neurochem. 2004;90(3):609–620. doi: 10.1111/j.1471-4159.2004.02509.x. [DOI] [PubMed] [Google Scholar]
- 102.Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, et al. Analysis of CD44-containing lipid rafts: Recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol. 1999;146(4):843–854. doi: 10.1083/jcb.146.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Babiychuk EB, Draeger A. Annexins in cell membrane dynamics. Ca(2+)-regulated association of lipid microdomains. J Cell Biol. 2000;150(5):1113–1124. doi: 10.1083/jcb.150.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harder T, Kellner R, Parton RG, Gruenberg J. Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol Biol Cell. 1997;8(3):533–545. doi: 10.1091/mbc.8.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hayes MJ, Merrifield CJ, Shao D, Ayala-Sanmartin J, Schorey CD, Levine TP, et al. Annexin 2 binding to phosphatidylinositol 4,5-bisphosphate on endocytic vesicles is regulated by the stress response pathway. J Biol Chem. 2004;279(14):14157–14164. doi: 10.1074/jbc.M313025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hayes MJ, Shao D, Bailly M, Moss SE. Regulation of actin dynamics by annexin 2. EMBO J. 2006;25(9):1816–1826. doi: 10.1038/sj.emboj.7601078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tatenhorst L, Rescher U, Gerke V, Paulus W. Knockdown of annexin 2 decreases migration of human glioma cells in vitro. Neuropathol Appl Neurobiol. 2006;32(3):271–277. doi: 10.1111/j.1365-2990.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- 108.Gokhale NA, Abraham A, Digman MA, Gratton E, Cho W. Phosphoinositide specificity of and mechanism of lipid domain formation by annexin A2-p11 heterotetramer. J Biol Chem. 2005;280(52):42831–42840. doi: 10.1074/jbc.M508129200. [DOI] [PubMed] [Google Scholar]
- 109.Rescher U, Ruhe D, Ludwig C, Zobiack N, Gerke V. Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J Cell Sci. 2004;117(Pt 16):3473–3480. doi: 10.1242/jcs.01208. [DOI] [PubMed] [Google Scholar]
- 110.Ayala-Sanmartin J, Henry JP, Pradel LA. Cholesterol regulates membrane binding and aggregation by annexin 2 at submicromolar Ca(2+) concentration. Biochim Biophys Acta. 2001;1510(1–2):18–28. doi: 10.1016/s0005-2736(00)00262-5. [DOI] [PubMed] [Google Scholar]
- 111.Jost M, Zeuschner D, Seemann J, Weber K, Gerke V. Identification and characterization of a novel type of annexin-membrane interaction: Ca2+ is not required for the association of annexin II with early endosomes. J Cell Sci. 1997;110(Pt 2):221–228. doi: 10.1242/jcs.110.2.221. [DOI] [PubMed] [Google Scholar]
- 112.Liu L. Calcium-dependent self-association of annexin II: a possible implication in exocytosis. Cell Signal. 1999;11(5):317–324. doi: 10.1016/s0898-6568(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 113.Eberhard DA, Karns LR, VandenBerg SR, Creutz CE. Control of the nuclear-cytoplasmic partitioning of annexin II by a nuclear export signal and by p11 binding. J Cell Sci. 2001;114(Pt 17):3155–3166. doi: 10.1242/jcs.114.17.3155. [DOI] [PubMed] [Google Scholar]
- 114.Jindal HK, Chaney WG, Anderson CW, Davis RG, Vishwanatha JK. The Protein-Tyrosine Kinase Substrate, Calpactin-I Heavy-Chain (P36), Is Part of the Primer Recognition Protein Complex That Interacts with Dna Polymerase-Alpha. Journal of Biological Chemistry. 1991;266(8):5169–5176. [PubMed] [Google Scholar]
- 115.Mickleburgh I, Burtle B, Hollas H, Campbell G, Chrzanowska-Lightowlers Z, Vedeler A, et al. Annexin A2 binds to the localization signal in the 3′ untranslated region of c-myc mRNA. FEBS J. 2005;272(2):413–421. doi: 10.1111/j.1742-4658.2004.04481.x. [DOI] [PubMed] [Google Scholar]
- 116.Huang XP, Pi Y, Lokuta AJ, Greaser ML, Walker JW. Arachidonic acid stimulates protein kinase C-epsilon redistribution in heart cells. J Cell Sci. 1997;110(Pt 14):1625–1634. doi: 10.1242/jcs.110.14.1625. [DOI] [PubMed] [Google Scholar]
- 117.Xu TR, Rumsby MG. Phorbol ester-induced translocation of PKC epsilon to the nucleus in fibroblasts: identification of nuclear PKC epsilon-associating proteins. FEBS Lett. 2004;570(1–3):20–24. doi: 10.1016/j.febslet.2004.05.080. [DOI] [PubMed] [Google Scholar]
- 118.Liu J, Rothermund CA, Ayala-Sanmartin J, Vishwanatha JK. Nuclear annexin II negatively regulates growth of LNCaP cells and substitution of ser 11 and 25 to glu prevents nucleo-cytoplasmic shuttling of annexin II. BMC Biochem. 2003 Sep;4(1):10. doi: 10.1186/1471-2091-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Erickson E, Erickson RL. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus transforming gene product. Cell. 1980;21:829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- 120.Reeves SA, Chavez-Kappel C, Davis R, Rosenblum M, Israel MA. Developmental regulation of annexin II (Lipocortin 2) in human brain and expression in high grade glioma. Cancer Res. 1992;52(24):6871–6876. [PubMed] [Google Scholar]
- 121.Ozaki T, Sakiyama S. Molecular cloning of rat calpactin I heavy-chain cDNA whose expression is induced in v-src-transformed rat culture cell lines. Oncogene. 1993;8(6):1707–1710. [PubMed] [Google Scholar]
- 122.Harton JA, Ting JP. Class II transactivator: mastering the art of major histocompatibility complex expression. Mol Cell Biol. 2000;20(17):6185–6194. doi: 10.1128/mcb.20.17.6185-6194.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chetcuti A, Margan SH, Russell P, Mann S, Millar DS, Clark SJ, et al. Loss of Annexin II heavy and light chains in prostate cancer and its precursors. Cancer Research. 2001;61(17):6331–6334. [PubMed] [Google Scholar]
- 124.Liu JW, Shen JJ, Tanzillo-Swarts A, Bhatia B, Maldonado CM, Person MD, et al. Annexin II expression is reduced or lost in prostate cancer cells and its re-expression inhibits prostate cancer cell migration. Oncogene. 2003;22(10):1475–1485. doi: 10.1038/sj.onc.1206196. [DOI] [PubMed] [Google Scholar]
- 125.Mai J, Waisman DM, Sloane BF. Cell surface complex of cathepsin B/annexin II tetramer in malignant progression. Biochim Biophys Acta. 2000;1477(1–2):215–230. doi: 10.1016/s0167-4838(99)00274-5. [DOI] [PubMed] [Google Scholar]
- 126.Sharma MR, Koltowski L, Ownbey RT, Tuszynski GP, Sharma MC. Angiogenesis-associated protein annexin II in breast cancer: Selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp Mol Pathol. 2006 doi: 10.1016/j.yexmp.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 127.Kumble KD, Hirota M, Pour PM, Vishwanatha JK. Enhanced Levels of Annexins in Pancreatic-Carcinoma Cells of Syrian-Hamsters and Their Intrapancreatic Allografts. Cancer Research. 1992;52(1):163–167. [PubMed] [Google Scholar]
- 128.Diaz VM, Hurtado M, Thomson TM, Reventos J, Paciucci R. Specific interaction of tissue-type plasminogen activator (t-PA) with annexin II on the membrane of pancreatic cancer cells activates plasminogen and promotes invasion in vitro. Gut. 2004;53(7):993–1000. doi: 10.1136/gut.2003.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Esposito I, Penzel R, Chaib-Harrireche M, Barcena U, Bergmann F, Riedl S, et al. Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol. 2006;208(5):673–685. doi: 10.1002/path.1935. [DOI] [PubMed] [Google Scholar]
- 130.Emoto K, Sawada H, Yamada Y, Fujimoto H, Takahama Y, Ueno M, et al. Annexin II overexpression is correlated with poor prognosis in human gastric carcinoma. Anticancer Res. 2001;21(2B):1339–1345. [PubMed] [Google Scholar]
- 131.Das S, Sierra JC, Soman KV, Suarez G, Mohammad AA, Dang TA, et al. Differential protein expression profiles of gastric epithelial cells following Helicobacter pylori infection using ProteinChips. J Proteome Res. 2005;4(3):920–930. doi: 10.1021/pr050023i. [DOI] [PubMed] [Google Scholar]
- 132.Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Conlin VS, Curtis SB, Zhao Y, Moore ED, Smith VC, Meloche RM, et al. Helicobacter pylori infection targets adherens junction regulatory proteins and results in increased rates of migration in human gastric epithelial cells. Infect Immun. 2004;72(9):5181–5192. doi: 10.1128/IAI.72.9.5181-5192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Musholt TJ, Hanack J, Brehm C, von Wasielewski R, Musholt PB. Searching for non-RET molecular alterations in medullary thyroid carcinoma: expression analysis by mRNA differential display. World J Surg. 2005;29(4):472–482. doi: 10.1007/s00268-004-7748-y. [DOI] [PubMed] [Google Scholar]
- 135.Guzman-Aranguez A, Olmo N, Turnay J, Lecona E, Perez-Ramos P, Lopez dSI, et al. Differentiation of human colon adenocarcinoma cells alters the expression and intracellular localization of annexins A1, A2, and A5. J Cell Biochem. 2005;94(1):178–193. doi: 10.1002/jcb.20293. [DOI] [PubMed] [Google Scholar]
- 136.Augenlicht LH, Mariadason JM, Wilson A, Arango D, Yang W, Heerdt BG, et al. Short chain fatty acids and colon cancer. J Nutr. 2002;132(12):3804S–3808S. doi: 10.1093/jn/132.12.3804S. [DOI] [PubMed] [Google Scholar]
- 137.Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr Pharm Des. 2003;9(4):347–358. doi: 10.2174/1381612033391973. [DOI] [PubMed] [Google Scholar]
- 138.Chiang Y, Davis RG, Vishwanatha JK. Altered expressioin of annexin II in human B-cel lymphoma cell lines. Biochem Biophys Acta. 1996 Oct 11;1313(3):295–301. doi: 10.1016/0167-4889(96)00103-6. [DOI] [PubMed] [Google Scholar]
- 139.Zimmermann U, Woenckhaus C, Pietschmann S, Junker H, Maile S, Schultz K, et al. Expression of annexin II in conventional renal cell carcinoma is correlated with Fuhrman grade and clinical outcome. Virchows Arch. 2004;445(4):368–374. doi: 10.1007/s00428-004-1103-4. [DOI] [PubMed] [Google Scholar]
- 140.Toyokuni S, Okada S, Hamazaki S, Minamiyama Y, Yamada Y, Liang P, et al. Combined histochemical and biochemical analysis of sex hormone dependence of ferric nitrilotriacetate-induced renal lipid peroxidation in ddY mice. Cancer Res. 1990;50(17):5574–5580. [PubMed] [Google Scholar]
- 141.Tanaka T, Kondo S, Iwasa Y, Hiai H, Toyokuni S. Expression of stress-response and cell proliferation genes in renal cell carcinoma induced by oxidative stress. Am J Pathol. 2000;156(6):2149–2157. doi: 10.1016/S0002-9440(10)65085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lei S, Dubeykovskiy A, Chakladar A, Wojtukiewicz L, Wang TC. The murine gastrin promoter is synergistically activated by transforming growth factor-beta/Smad and Wnt signaling pathways. J Biol Chem. 2004;279(41):42492–42502. doi: 10.1074/jbc.M404025200. [DOI] [PubMed] [Google Scholar]


