Abstract
The burgeoning field of vascular tissue engineering holds promise for the creation of a practical and successful small-diameter arterial bypass graft. Many creative combinations of autologous cells and scaffolds exist along with an equally long list of microenvironmental cues used to create a functional arterial conduit. This review outlines our work using abdominal wall fat as a source of autologous stem cells for vascular tissue engineering, focusing specifically on this stem cell’s availability and potency to differentiate into endothelial-like cells. In a series of 49 patients undergoing elective peripheral vascular surgery, an abundant quantity of adult stem cells was harvested from fat obtained via liposuction. The efficacy of the isolation did not appear influenced by advanced age, obesity, renal failure or vascular disease, although fat from diabetic patients yielded significantly less stem cells. Additionally, these adipose-derived stem cells acquired several morphological and molecular endothelial phenotypes when exposed to growth factors (ECGS, VEGF) and physiologic shear stress in vitro. Taken together, these studies suggest that fat appears to be a viable source of autologous stem cells for use in vascular tissue engineering.
Keywords: Tissue engineering, Arterial bypass, Stem cells, Adipose Tissue
Vascular therapies are rapidly evolving with a major emphasis on the endovascular approach to arterial disease. Despite this important trend, bypass of occluded arteries remains an important technique in the treatment of peripheral and cardiovascular disease. It is well known that the gold standard conduit for such procedures is autologous vascular tissue such as greater saphenous vein and internal mammary and radial artery. In the absence of suitable autologous tissues alternative conduits such as non-absorbable prosthetic grafts and cryopreserved vascular allografts are employed, but with significantly inferior results when used for small-diameter artery bypass.
Tissue engineering, defined as the combination of cells, scaffolding and signaling to form a biologically active tissue,1 offers potential alternatives to the problem of suboptimal bypass conduits. Save the pioneering work by Herring that introduced the concept seeding the luminal surface of non-absorbable prosthetic grafts to improve patency,2 this field largely began with Weinberg and Bell’s report of collagen tubes integrated within a Dacron mesh and seeded with endothelial cells.3 Significant progress and milestones have been achieved since, as outlined by multiple current and excellent reviews.1,4–7 As can be imagined, a myriad of cell and scaffold combinations have been investigated, including a vast number of in vitro signaling paradigms that foster graft functionality. These paradigms involve altering the cell/scaffold microenvironment to optimize luminal non-thrombogenicity, graft strength and compliance, and vasoactivity.
The use of autologous differentiated endothelial cells to line the lumen of a graft is hampered by the need to harvest a blood vessel from the patient and subsequently isolate and culture enough endothelial cells to seed the conduit confluently. Autologous stem cells, derived from the recipient’s tissues, represent an alternative source of cells. To date, most attempts to create tissue-engineered grafts using stem cells have employed either endothelial progenitor cells (EPCs) harvested from blood, or stem cells harvested from bone marrow.1,4–7 Both of these cell populations have shown remarkable potential to modulate into endothelial-like cells; however, given their origins within the bone marrow, cell availability may be severely hampered by advanced patient age and other co-morbidities associated with vascular disease.8
In an attempt to alleviate the problem of cell availability, we initiated efforts to isolate from subcutaneous fat a population of cells that can differentiate into endothelial-like cells. Autologous adipose tissue is harvested easily and in abundance from most patients using local anesthesia and a small liposuction cannula. Following enzymatic dispersion of the tissue specimen, mature adipocytes are separated from the stromal-vascular fraction (SVF) using centrifugation. After a brief period of culture (days), during which the adipose-derived stem cell (ASC) population adheres to the culture dish, an abundant amount of cells with homogenous surface markers are obtained.9,10 As a mesenchymal stem cell line, ASCs have been shown to differentiate into multiple cell lines including bone, fat, muscle, and cartilage,11 and more recently endothelial cells12–14 and smooth muscle cells.15
This review elucidates our work defining two critical characteristics of human stem cells isolated from adipose tissue: 1) availability in patient populations most likely to benefit from this technology, and 2) potency to differentiate into endothelial cells in response to in vitro microenvironmental cues. Within this framework, we emphasize the practical nature of using these cells to create a tissue-engineered bypass graft.
Availability of ASC
One of the most important characteristics of a cell type employed in tissue engineering is that it be readily and abundantly available in the specific patient population it is intended for use. In the case of small-artery bypass, the cell must be obtainable in the elderly population with multiple cardiovascular risk factors such a diabetes. If a particular cell type is quite rare, ex vivo amplification becomes necessary prior to graft creation, increasing both time and expense in an already costly manufacturing process.
Many of the original studies evaluating fat as a source of stem cells examined liposuction specimens obtained from young, healthy plastic surgery patients.9,11 The applicability of such studies in the “vascular” population is questionable as it is likely that age and co-morbidity adversely affects most cell populations. To address this issue, our group studied the availability of ASCs in 49 patients undergoing elective vascular surgical procedures (16; full manuscript in preparation). Each patient donated approximately 15gm of periumbilical fat obtained via liposuction during his or her vascular procedure. After enzymatic dispersion, positive selection for absorbance to culture dish plastic for one week and negative selection for CD31 and CD45 using magnetic beads, a homogenous population of ASCs was obtained. Florescence activated cell sorting (FACS) revealed these cells to be >98% positive for CD13, CD29 and CD90 (Figure 1), cell surface antigens reported by others to be common to ASCs.9
Figure 1. Morphological and molecular characterization of adipose-derived stem cells (ASCs).
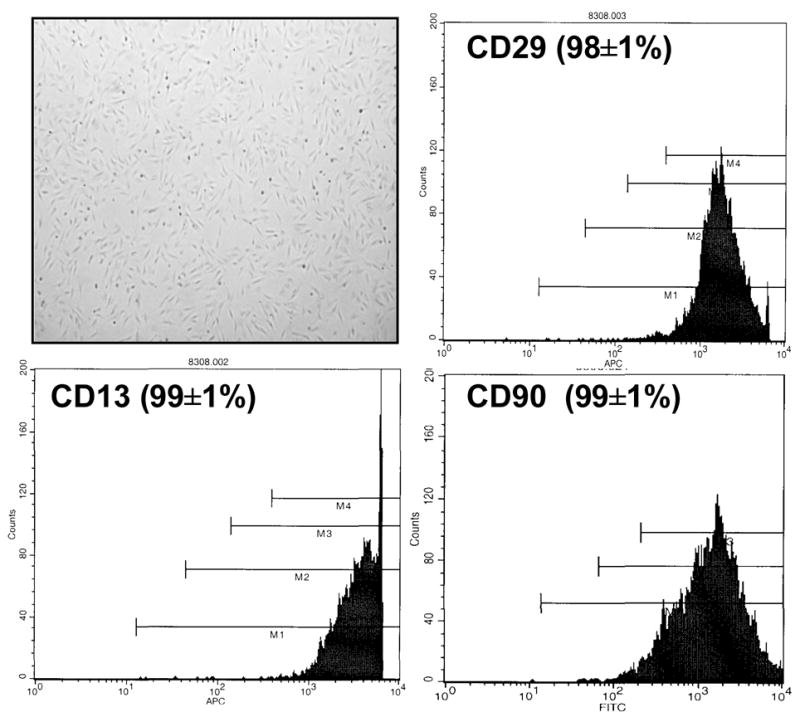
Inverted phase light micrograph (40x, unstained) of ASCs grown in culture demonstrating spindle-like morphology. After negative selection of cells for CD31 and CD45 using magnetic cell sorting, representative fluorescent activated cell sorting of ASCs grown in culture (passage four) demonstrate a homogeneous population of cells positive for CD13, 29 and 90 surface markers.
The number of stem cells per gram of adipose tissue harvested was analyzed with respect to patient age, gender, body mass index, and presence of diabetes, end-stage renal and peripheral vascular disease. The major finding of this study was that stem cells availability was not proven inferior for those of advanced age, obesity, renal failure or vascular disease (Table 1). The presence of diabetes did appear to adversely affect the quantity of stem cells harvested, but not to a point where stem cell harvest would be considered impractical in this patient population. Based on the number of cells obtained one week after harvest, an average of only 50–100gm of adipose tissue would be required to seed confluently the luminal surface of a 40cm x 6mm bypass graft. In sum, this study suggests that ASCs is a practical source of autologous mesenchymal adult stem cells that can be used for tissue engineering purposes.
Table 1. Availability of adipose-derived stem cells in patients undergoing elective vascular surgical procedures.
The number of stem cells harvested did not appear to be affected by patient age, obesity, renal failure or PVD.
| Patient Population | ASCs/gm of fat | P value |
|---|---|---|
| All patients (n=49) | 134,167 | |
| Age>70 | 156,350 | N.S. |
| BMI >30 | 169,044 | N.S. |
| Diabetics | 87,086 | P<.05 |
| Renal failure | 141,156 | N.S. |
| PVD | 148,384 | N.S. |
BMI=body mass index; PVD=peripheral vascular disease; N.S.=not significant
Potency of ASC
The ability to acquire characteristics of cells resident within vascular tissue represents another important aspect of the stem cell’s usefulness in tissue engineering. Recent evidence suggests that ASCs have the ability to transform into cells with endothelial12–14 and smooth muscle15 phenotypes.
The traditional cell used to impart a non-thrombogenic luminal surface upon a vascular graft is the endothelial cell. Hence, our group has focused on the microenvironmental cues that might differentiate ASCs towards endothelial cells.17 Amongst a host of factors, we have examined the effects of chemical (growth factors) and physical (shear force) stimuli on ASCs obtained from patients undergoing elective vascular surgical procedures. In practice, the stem cells would be exposed to the growth factors immediately following isolation and throughout the seeding (graft creation) process. The application of shear force could also be applied during the seeding process, taking advantage of its ability to promote cell attachment and retention, and would continue in vivo after graft implantation.
In these experiments, the specific endothelial phenotypes examined included: 1) morphological changes in response to shear force (alignment with flow) and seeding on Matrigel (cord formation) and 2) expression of endothelial proteins (endothelial nitric oxide synthase—eNOS, von Willebrand’s Factor—vWF, and CD31). The undifferentiated stem cells in vitro did not demonstrate any of these endothelial phenotypes. After three weeks of culture in media containing endothelial cell growth supplement (Figure 2), the ASCs re-aligned with shear force and formed cords in Matrigel. Expression of eNOS, vWF and CD31 was not demonstrated by either PCR or Western blot. The continued exposure of the cells in culture to physiological shear force (12 dynes), however, did result in expression of CD31 message and protein.
Figure 2. Differentiation of ASCs in response to endothelial cell growth supplement (ECGS) and shear stress.
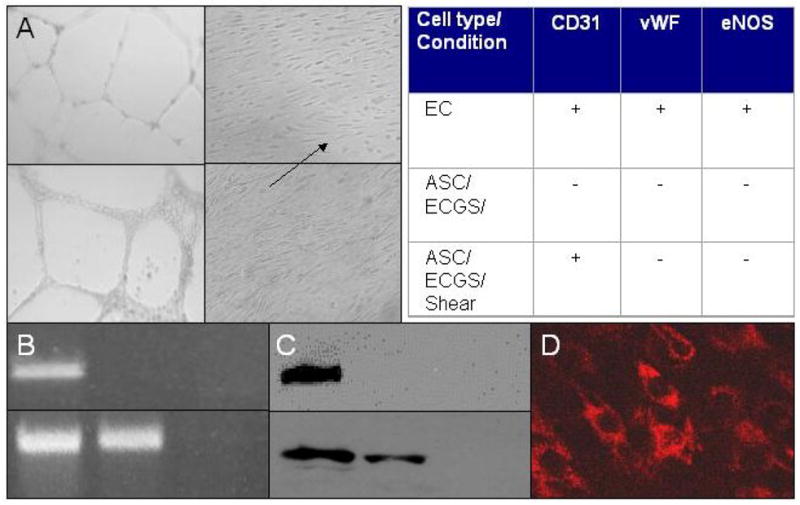
A. Morphological studies. Inverted phase light micrographs (40x, unstained) of human endothelial cells (upper two panels) forming cords on Matrigel (left) and aligning in the direction of shear stress (right). Similar findings are noted in human ASCs cultured in ECGS for a minimum of three weeks (lower two panels). B. Molecular studies (CD31 message). RT-PCR detecting CD31 message in human endothelial (left lane), ASCs (middle lane) and smooth muscle (right lane) cells before (upper row) and after (lower row) exposure to 12dynes shear for four days. ASCs grown in ECGS and exposed to shear express CD31 message, an endothelial cell phenotype marker. C. Molecular studies (CD31 protein). In studies analogous to those in B, Western blot for CD31 protein demonstrates that ASCs cultured in ECGS and exposed to shear stress express CD31 at the protein level. D. Immunohistochemical study (CD31). Laser confocal micrograph (40x) of ASCs grown in ECGS, exposed to shear stress and stained with human CD31 Mab confirm the presence of CD31 protein within the differentiated ASCs. Table. Summary of molecular studies of ASCs grown in ECGS and exposed to shear stress. Neither vWF nor eNOS was demonstrated by RT-PCR or Western blot.
These studies, along with the noted recent literature, suggested the potential of these stem cells to become endothelial-like. However, the lack of eNOS expression raised concern about the usefulness of ASCs to provide a graft with a non-thrombogenic lumen. Indeed a small pilot study examining the effect of seeding grafts with undifferentiated ASCs in a canine model suggested that the cells indeed did not provide the conduit with a non-thrombogenic lumen.18
Vascular endothelial growth factor (VEGF) is well-known and important stimulus of angiogenesis and endothelial progenitor cell (EPC) differentiation into endothelial cells. In studies similar to those just outlined, our group examined the effect VEGF on the differentiation of ASCs into endothelial cells.19 Following in vitro culture of the cells for a minimum of one week in endothelial growth media (EGM-2, Cambrex, East Rutherford, NJ) containing 10ug/ml VEGF and other growth factors, the ASCs again demonstrated re-alignment with flow and cord formation in Matrigel. Of greater significance is that message for eNOS, vWF and CD31 has now been detected (Table 2). This finding requires further confirmation with Western blotting but suggests that these stem cells may indeed be an appropriate cell for vascular graft creation.
Table 2. Differentiation of ASCs when cultured in media containing VEGF.
Stem cells grown in media containing VEGF (see text) for a minimum of one week express message for proteins specific for endothelial cells suggesting the ability of ASCs to differentiate towards endothelial lineage. Human endothelial (EC) and smooth muscle (SMC) cells served as positive and negative controls, respectively.
vWF=von Willebrand’s Factor; eNOS=endothelial nitric oxide; RT-PCR=reverse transcriptase-polymerase chain reaction; EGM2=endothelial cell growth media
Additional studies with ASCs
An important aspect of designing a vascular graft is creating a stable, non-thrombogenic luminal surface. Preliminary data above suggests that undifferentiated ASCs may not be inherently non-thrombogenic; therefore, successful use of these stem cells will likely require differentiation prior to implantation into the vascular system. While one potential advantage of stem cells over differentiated endothelial cells for use in graft creation lies in ease of harvest and availability, the time required for differentiation may offset this advantage.
One of the goals of our work using ASCs is to incorporate this differentiation requirement into the process of establishing a stable monolayer of cells on the graft lumen, thereby minimizing the time for graft creation. Herein, taking advantage of the ASCs’ responsiveness to shear, we have begun to examine the effect of flow conditioning on the retention of stem cells seeded onto the surface of our scaffold of choice—decellularized vein graft (references 20 and 21 characterize the in vitro and in vivo nature of this scaffold prior to seeding). Flow conditioning has been used by others to enhance the attachment of endothelial cells to vascular grafts.22 Following seeding and culture of the grafts under static conditions for up to four days, exposure of the stem cells to minimal amounts of shear force (three dynes for one hour) results in substantial loss of cells (DiMuzio, unpublished results 2006). In contrast, seeded stem cells that have be gradually exposed to increasing shear stress up to 12 dynes over three days remain fully adherent and demonstrate re-alignment with flow (Figure 3).
Figure 3. Attachment and retention of ASCs to vascular scaffolding.
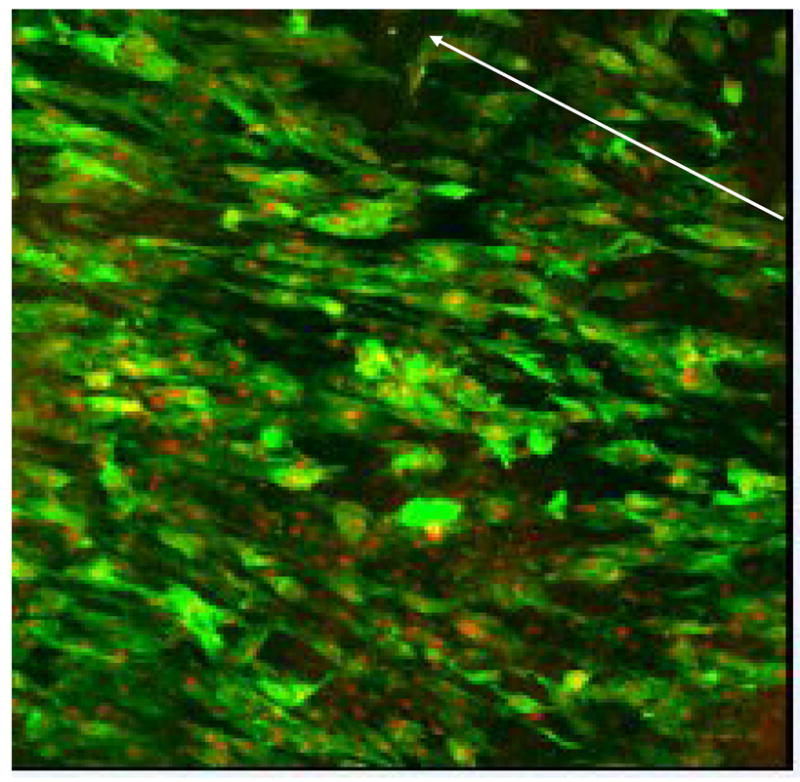
Laser confocal micrograph (100x, phalloidin and propidium iodide stain) of human stem cells seeded onto decellularized vein allograft. After 24h of static seeding, the graft was exposed to gradually increasing shear force (from 0 to 9 dynes) over 72h. The arrow indicates direction of flow. The stem cells aligned with flow and maintained a confluent monolayer of cells under physiological shear force.
Summary
Adult autologous stem cells represent an important source of cells for vascular tissue engineering. Their usefulness is directly related to the availability of the cells in patient populations with vascular diseases as well as their ability to differentiate efficiently into vascular cells, particular those with endothelial phenotype. Recent work by our group and others suggest that stem cells derived from human adipose tissue appear to possess these important characteristics. Further work is necessary to optimize the microenvironmental cues important for the efficient differentiation of the cells and attachment onto vascular graft scaffolds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riha GM, Lin PH, Lumsden AB, Yao Q, Chen C. Application of stem cells for vascular tissue engineering. Tissue Engineering. 2005;11:1535–52. doi: 10.1089/ten.2005.11.1535. [DOI] [PubMed] [Google Scholar]
- 2.Herring MB, Dilley R, Jersild RA, Jr, Boxer L, Gardner A, Glover J. Seeding arterial prostheses with vascular endothelium. The nature of the lining. Ann Surg. 1979;190:84–90. doi: 10.1097/00000658-197907000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;87:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 4.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 5.Barrilleaux B, Phinney DG, Prockop Darwin, O’Connor KC. Review: Ex vivo engineering of living tissues with adult stem cells. Tissue Engineering. 2006;12:1–13. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- 6.Levenberg S. Engineering blood vessels from stem cells: recent advances and application. Current Opinion in Biotechnology. 2005;16:516–23. doi: 10.1016/j.copbio.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Sales KM, Salacinski HJ, Alobaid N, Mikhail M, Balakrishnan B, Sefalian AM. Advancing vascular tissue engineering: the role of stem cell technology. Trends in Biotechnology. 2005;9:461–7. doi: 10.1016/j.tibtech.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 9.Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–23. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Halvorsen YD, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–85. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 11.Zuk PA, Zhu M, Ashjian P, DeUgarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;12:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planat-Benard V, Silvestre J-S, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–63. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 13.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochemical and Biophysical Research Communications. 2005;332:370–9. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:12167–72. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMatteo CA, Golesorkhi N, Fischer LJ, Wrigley CW, McIlhenny SE, Tulenko TN, Shapiro I, Carabasi RA, Lombardi JV, Larson RA, DiMuzio P. Isolation of Adipose-Derived Stem Cells in Patients With Vascular Disease. Circulation Supplement II. 2006;114(18):446. [Google Scholar]
- 17.Fischer LJ, Lee AE, DiMatteo CA, Golesorkhi N, Wrigley CW, Galler A, Tulenko T, DiMuzio P. Role of chemical and physical stimuli in the differentiation of stem cells towards and endothelial phenotype. J Am Coll Surg. 2006;203(3S):S105. [Google Scholar]
- 18.Golesorkhi N, DiMatteo C, Grabo D, Fischer L, Tarola N, Shapiro I, Tulenko T, Carabasi RA, Larson R, Lombardi J, DiMuzio P. In vivo study of a tissue engineered graft seeded with autologous stem cells. JSR. 2006 doi: 10.2310/6670.2006.00058. In press. [DOI] [PubMed] [Google Scholar]
- 19.Mericli A, Tarola N, Fischer L, DiMatteo C, Golesorkhi N, Grabo D, McIlhenny S, Scully K, Tulenko T, Shapiro I, DiMuzio P. Growth-factor rich endothelilial cell media induces adipose-derived stem cells to acquire endothelial cell phenotype. JSR. 2006 In press. [Google Scholar]
- 20.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, Carabasi RA, DiMuzio P. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146–53. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 21.Martin ND, Schaner PJ, Tulenko TN, Shapiro IM, DiMatteo CA, Williams TK, Hager ES, DiMuzio P. In vivo behavior of decellularized vein allograft. J Surg Res. 2005;129:17–23. doi: 10.1016/j.jss.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Isenberg BC, Williams C, Tranquillo RT. Endothelialization and flow conditioning of fibrin-based media-equivalents. Ann Biomed Eng. 2006;34:971–85. doi: 10.1007/s10439-006-9101-0. [DOI] [PubMed] [Google Scholar]


