Abstract
At early developmental stages, correlated neuronal activity is thought to exert a critical control on functional and structural refinement of synaptic connections. In the hippocampus, between postnatal day 2 (P2) and P6, network-driven giant depolarizing potentials (GDPs) are generated by the synergistic action of glutamate and GABA, which is depolarizing and excitatory. Here the rising phase of GDPs was used to trigger Schaffer collateral stimulation in such a way that synchronized network activity was coincident with presynaptic activation of afferent input. This procedure produced a persistent increase in spontaneous and evoked α-amino-3-hydroxy-5-methyl-4-isoxadepropionic acid-mediated glutamatergic currents, an effect that required calcium influx through postsynaptic L-type calcium channels. No potentiation was observed when a delay of 3 sec was introduced between GDPs and afferent stimulation. Pairing-induced potentiation was prevented by scavengers of endogenous BDNF or tropomyosin-related kinase receptor B (TrkB) receptor antagonists. Blocking TrkB receptors in the postsynaptic cell did not prevent the effects of pairing, suggesting that BDNF, possibly secreted from the postsynaptic cell during GDPs, acts on TrkB receptors localized on presynaptic neurons. Application of exogenous BDNF mimicked the effects of pairing on synaptic transmission. In addition, pairing-induced synaptic potentiation was blocked by ERK inhibitors, suggesting that BDNF activates the MAPK/ERK cascade, which may lead to transcriptional regulation and new protein synthesis in the postsynaptic neuron. These results support the hypothesis that, during a critical period of postnatal development, GABAA-mediated GDPs are instrumental in tuning excitatory synaptic connections and provide insights into the molecular mechanisms involved in this process.
Keywords: development, giant depolarizing potential, excitatory postsynaptic current, synaptic pairing, TrkB receptors
Spontaneously occurring neuronal oscillations constitute a hallmark of developmental networks (1). In the immature hippocampus, giant depolarizing potentials (GDPs) represent a primordial form of synchrony between neurons, which precedes more organized forms of activity, such as the theta and gamma rhythms (2). These events, which are characterized by recurrent membrane depolarization with superimposed fast-action potentials separated by long and variable intervals of several seconds, are generated when the synaptic traffic and cell firing within the network increase to a threshold level (3). GDPs are synaptic in origin and involve the action of both glutamate and GABA, which, during a restricted period of postnatal development, is depolarizing and excitatory (4–6). The depolarizing action of GABA during GDPs results in the activation of voltage-dependent calcium channels and N-methyl-d-aspartate receptors (7). GDPs also can be recorded in vivo in rat pups, in which they occur during immobility periods, sleep, and feeding (8). GDP-associated calcium waves are thought to be crucial for the structural refinement of the neuronal connectivity and the establishment of the adult neuronal circuit during a critical period of synapse formation (9, 10). Rewiring would involve electrical activity and the cooperative and competitive interactions between converging inputs.
As suggested by the Hebb postulate for associative learning (11), correlated spiking of pre- and postsynaptic neurons can result in strengthening or weakening of synapses depending on the temporal order of spiking. GDPs are therefore ideal for allowing associative modifications of coincident signals. We previously demonstrated that at mossy fiber–CA3 connections, which during the first postnatal week are mainly GABAergic (12), pairing GDPs with afferent stimulation induced a persistent increase in synaptic efficacy, which is usually restricted to the activated synapse (13).
In the present work, we examined whether excitatory glutamatergic synapses can be modified in an associative way by pairing GABAA-mediated GDPs with Schaffer collateral stimulation. Our findings suggest that “pairing” persistently enhances synaptic efficacy at these synapses, an effect that depends on the rise of calcium in the postsynaptic cell. In addition, compared with our previous work (13), in the present study, we identified the intracellular signaling pathway mediating pairing-induced potentiation, which involves the activation of BDNF and the ERK pathway.
Results
Pairing GDPs with Schaffer Collateral Stimulation Enhances Synaptic Efficacy at CA3–CA1 Synapses.
Whole-cell recordings in current-clamp mode from 170 CA1 pyramidal neurons in hippocampal slices from P2- to P6-old rats revealed the presence of GDPs (Fig. 1B). In accord with a previous work from CA3 pyramidal cells, we used a pairing procedure to correlate GDPs with Schaffer collateral activation (Fig. 1A). For this purpose, the rising phase of GDPs (which occurred at the frequency of 0.069 ± 0.012 Hz; n = 40) was used to trigger Schaffer collateral stimulation in stratum radiatum in such a way that synchronized network activity was coincident with presynaptic activation of the afferent input (see Fig. 1B). Before pairing, minimal stimulation of afferent fibers (at 0.05 Hz) evoked in CA1 principal cells held at −53 mV (corresponding to EGABA) synaptic currents intermingled with response failures. Synaptic currents were mediated by α-amino-3-hydroxy-5-methyl-4-isoxadepropionic acid (AMPA) receptors because they were readily blocked by 20 μM 6,7-dinitroquinoxaline-2,3-dione (n = 6; data not shown).
Fig. 1.
Pairing GDPs with Schaffer collateral stimulation persistently enhances synaptic efficacy at CA3–CA1 connections. (A) Diagram of the hippocampus showing a CA1 pyramidal neuron receiving a synaptic input from a CA3 principal cell. The stimulating electrode (stim) was positioned in stratum radiatum. (B) GDPs recorded from a CA1 cell in current-clamp mode. Below the trace, two GDPs are shown on an expanded time scale. Note the absence of spikes riding on the top of GDPs because of a block of the sodium channel with intracellular QX-314. The rising phase of GDPs (between the dashed lines) was used to trigger synaptic stimulation (stim). (C) (Upper) Nine superimposed individual responses (successes and failures) evoked by Schaffer collateral stimulation and obtained in control and 20 min after pairing. (Lower) Average of 19 trials (successes and failures) obtained in the same experimental conditions. (D) Each bar represents the mean peak amplitude of synaptic responses (including failures; n = 8) and PPR (n = 8) obtained before (open bars) and 20 min after (filled bars) pairing. *, P < 0.05; **, P < 0.01. (E) Consecutive traces from the same neuron described in C showing sEPSCs obtained in control and 20 min after pairing. (F) Cumulative distribution of interevent interval (IEI) (Left) and amplitude (Right) for the cell shown in E before (thin line) and after (thick line) pairing. (G) Summary plots showing the mean frequency (Left) and amplitude (Right) of sEPSCs obtained before and after pairing (arrows indicate time 0; n = 14).
After a control period of 7–10 min, the patch was switched from voltage- to current-clamp mode, and Schaffer collateral responses were paired for 7 min with GDPs. In this period, the mean number of GDPs was 26.1 ± 3.4. As illustrated in Fig. 1 C and D, the pairing procedure produced a strong and persistent potentiation of Schaffer collateral-mediated synaptic currents (from 8.2 ± 1.6 to 17.6 ± 4.1 pA, 20 min after pairing; P < 0.01; n = 8) that was associated with a significant increase in the number of successes (from 15.3 ± 4.1% to 37.8 ± 5.5%; P < 0.01). In double-pulse experiments (n = 8), pairing-induced long-term potentiation (LTP) was accompanied with a significant reduction of the paired-pulse ratio (PPR) (from 5.7 ± 1.6 to 2.1 ± 0.7 pA; P < 0.05) (Fig. 1D) and a significant increase in the inverse squared value of the coefficient of variation of response amplitude (from 0.08 ± 0.03 to 0.94 ± 0.2 pA; P < 0.05; n = 8), suggesting that an increased probability of glutamate release accounted for LTP expression.
One interesting question is whether the pairing procedure may also affect AMPA-mediated spontaneous excitatory postsynaptic currents (sEPSCs). Therefore, sEPSCs were analyzed in the same cells used for the evoked responses. At immature CA3–CA1 synapses, the amplitude distribution of sEPSCs matches closely that of miniature events recorded in the presence of tetrodotoxin because these synapses bear only a single functional release site (14, 15). sEPSCs occurred at the frequency of 0.11 ± 0.03 Hz and had a mean amplitude of 14.3 ± 2.4 pA (n = 21). They were reversibly blocked by 20 μM 6,7-dinitroquinoxaline-2,3-dione, indicating that they were mediated by glutamate acting on AMPA receptors (n = 10; data not shown). Pairing GDPs with afferent stimulation (same cell shown in Fig. 1C) significantly and persistently (for at least 20 min) enhanced the frequency but not the amplitude of individual events (Fig. 1 E and F). On average, 20 min after pairing, the frequency of sEPSCs was 71% higher than in controls (it changed from 0.07 ± 0.02 to 0.12 ± 0.02 Hz; n = 14; P < 0.01) (Fig. 1G). This effect was not associated with significant changes in amplitude of individual events (15.2 ± 2.1 and 14.4 ± 3.2 pA before and after pairing, respectively; P > 0.5) (Fig. 1G). The increase in frequency, but not in amplitude, of sEPSCs suggests a presynaptic site of action.
In the absence of pairing, no significant changes in synaptic efficacy could be detected. “No pairing” consisted of switching the patch for 7 min from voltage- to current-clamp mode. In this period, GDPs occurred randomly in the absence of afferent stimulation. In these cells, the mean peak amplitude currents were 7.9 ± 2.1 and 7.1 ± 1.9 pA in control and 20 min after switching from current- to voltage-clamp conditions, respectively. Similarly, the success rate varied from 21.1 ± 5.8% to 18.4 ± 4.8% (n = 5; P > 0.5; data not shown), and the frequency of sEPSCs varied from 0.12 ± 0.02 to 0.11 ± 0.03 Hz; P > 0.5; n = 7).
To test the temporal specificity for LTP induction in 10 cells, a 3-sec delay between GDPs and synaptic stimulation was introduced. In agreement with a previous study (13), we found that with this delay the pairing procedure failed to induce synaptic potentiation. Before and after pairing, the amplitude of the evoked responses was not significantly different (6.4 ± 1.1 and 4.4 ± 0.9 pA, respectively; P > 0.5). In addition, no significant changes in the frequency (0.14 ± 0.06 and 0.13 ± 0.07 Hz; P > 0.5) or amplitude (9.9 ± 0.4 and 8.5 ± 0.2 pA, respectively) of sEPSCs were detected [supporting information (SI) Fig. 6].
Overall, these experiments clearly show that pairing with Schaffer collateral stimulation GDPs causes a persistent potentiation of the amplitude of evoked sEPSCs and the frequency of sEPSCs. Although otherwise stated in the following experiments, we focused only on pairing-induced long-term changes on sEPSCs on CA1 pyramidal cells, in which minimal stimulation of Schaffer collaterals evoked sEPSCs to the first or second stimulus.
Pairing-Induced Synaptic Potentiation Requires a Calcium Rise in the Postsynaptic Cell.
A common trigger for LTP is a postsynaptic rise in intracellular calcium concentration (16). To test whether a rise of postsynaptic calcium during GDPs is responsible for a pairing-induced increase in synaptic efficacy, cells were loaded with a 20 mM concentration of the calcium chelator 1,2-bis (2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA). In these cases, the pairing procedure failed to cause any persistent change in synaptic strength (n = 6; P > 0.1) (Fig. 2). A tendency toward depression was observed, although it did not reach a significant level (in the presence of BAPTA, the frequency of sEPSCs measured in control and 20 min after pairing was 0.08 ± 0.03 and 0.06 ± 0.03 Hz, whereas the amplitude was 13.1 ± 1.9 and 10.1 ± 3.9 pA before and after pairing, respectively). These experiments suggest that, at CA3–CA1 synapses, a pairing-induced persistent increase in the frequency of sEPSCs depends on a calcium rise in the postsynaptic cell.
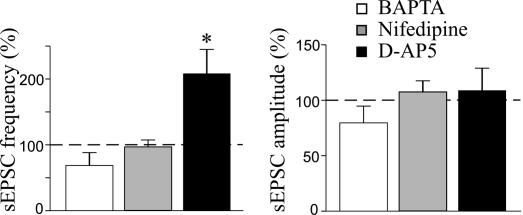
Fig. 2.
Pairing-induced potentiation requires a rise of intracellular calcium concentration in the postsynaptic cell via voltage-dependent calcium channels. Mean frequency (Left) and amplitude (Right) of sEPSCs expressed as a percentage of controls (dashed lines) obtained 20 min after pairing in the presence of 20 mM intracellular BAPTA (white bars; n = 6), 10 μM extracellular nifedipine (gray bars; n = 7), or 50 μM D-AP5 (black bars; n = 6). *, P < 0.05.
A rise of intracellular calcium may occur via voltage-dependent calcium channels or NMDA receptors (7, 17). To identify the source of calcium responsible for GDP-induced potentiation in the frequency of sEPSCs, additional experiments were performed in the presence of a 50 μM concentration of the NMDA receptor antagonist D-(−)-2-amino-5-phosphonopentaoic acid (D-AP5) (n = 6) or a 10 μM concentration of the voltage-dependent calcium channel blocker nifedipine (n = 7). D-AP5 failed to prevent pairing-induced persistent changes in the frequency of sEPSCs (208.1 ± 34.8% of control; P < 0.05), whereas nifedipine blocked the changes (96.9 ± 10.5% of control; P > 0.5) (Fig. 2). It is worth noting that nifedipine and D-AP5 did not modify the shape or amplitude of GDPs (data not shown). These results indicate that, early in postnatal life, a calcium rise through the voltage-dependent calcium channel is the trigger for activity-dependent changes in synaptic strength.
To mimic the effects of GDPs on synaptic potentiation, we repeatedly depolarized postsynaptic neurons with bursts of action or plateau potentials evoked in the presence of 20 μM D-AP5. However, as illustrated in SI Fig. 7, a postsynaptic calcium rise through theta bursts or plateau potentials failed to enhance synaptic efficacy.
Pairing-Induced Synaptic Potentiation Requires the Activation of BDNF and the ERK Pathway.
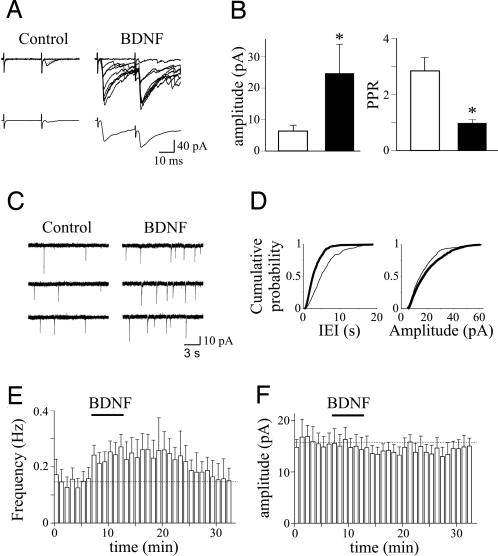
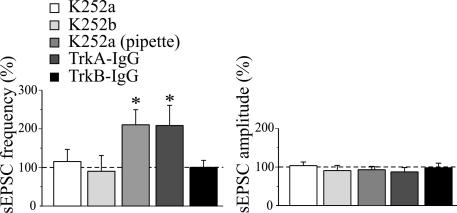
The present data clearly show that, although the induction of LTP is postsynaptic (dependent on the postsynaptic rise of calcium through GDPs), its expression is presynaptic, as suggested by the decrease in PPR and increase in frequency, but not in the amplitude, of sEPSCs. Therefore, the postsynaptic cell provides a transcellular retrograde signal to the presynaptic neuron. One attractive candidate is BDNF, which can be released in a calcium-dependent way by depolarization of the postsynaptic cell (18–20) and plays a crucial role in synaptic plasticity (21). BDNF acts on tropomyosin-related kinase receptor B (TrkB), and this interaction activates different signaling pathways. First, we proved that, at immature CA3–CA1 connections, BDNF is able to mimic the effects of pairing on synaptic strength. As illustrated in Fig. 3, bath application of 40 μg/ml BDNF produced a strong potentiation of Schaffer collateral-mediated synaptic currents (from 6.4 ± 1.8 in control to 24.8 ± 9.4 pA 10 min after BDNF; P < 0.05; n = 6) that was associated with a significant increase in the number of successes (from 23.6 ± 8.4% to 68.0 ± 10.1%; P < 0.05) and a decrease in PPR (from 2.9 ± 0.5 to 0.9 ± 0.1 pA; P < 0.05). In addition, BDNF enhanced the frequency of sEPSCs (from 0.14 ± 0.02 to 0.23 ± 0.02 pA; P < 0.01; n = 8) without altering their amplitude (from 15.9 ± 1.5 to 15.2 ± 2.1 pA; P > 0.1). These effects were prevented by bath application of 150 nM k-252a, a specific inhibitor of protein kinase coupled to Trk receptors (n = 3) (SI Fig. 8) (22). However, when k-252a was included in the patch pipette to block postsynaptic TrkB receptors (20), it failed to prevent BDNF-induced potentiation of sEPSCs (n = 6) (SI Fig. 8), indicating that BDNF enhances synaptic efficacy acting on presynaptic TrkB receptors. In a second set of experiments, the involvement of endogenous BDNF in pairing-induced synaptic potentiation was assessed with k-252a. Bath application of 150 nM k-252a fully prevented pairing-induced enhancement of the frequency of sEPSCs. Mean frequency values were 0.14 ± 0.03 and 0.16 ± 0.04 Hz before and after pairing, respectively (P > 0.5; n = 9) (Fig. 4), whereas mean amplitude values were 14.9 ± 1.1 and 15.7 ± 2.3 pA before and after pairing, respectively (P > 0.5) (Fig. 4). In the absence of pairing, k-252a alone did not modify the frequency and amplitude of sEPSCs (frequency values were 0.11 ± 0.03 vs. 0.12 ± 0.02 Hz and amplitude values were 14.2 ± 4.2 vs. 12.7 ± 3.4 pA in control and in the presence of k-252a, respectively). Similar results were obtained with 150 nM k-252b, which is a weaker inhibitor of protein kinase coupled to Trk receptors (23). On average, in six cells before and after pairing, the frequency values of sEPSCs were 0.08 ± 0.02 and 0.07 ± 0.02 Hz, respectively (P > 0.5), whereas the amplitude values were 15.9 ± 2.5 and 14.8 ± 1.4 pA, respectively (P > 0.5) (Fig. 4). To better assess the contribution of endogenous TrkB ligands to GDP-induced potentiation, we incubated the slices for at least 3 h with a soluble form of TrkB receptor (TrkB–IgG) engineered as an immunoadhesin to prevent TrkB activation (24, 25). In slices preincubated with 1 μg/ml TrkB–IgGs, the pairing procedure did not affect the frequency or amplitude of sEPSCs (mean frequency values were 0.08 ± 0.03 Hz in control and 0.08 ± 0.02 Hz after pairing; n = 6; P > 0.5; mean amplitude values were 18.9 ± 2.7 pA in control and 17.7 ± 2.2 pA after pairing; P > 0.5) (Fig. 4). TrkB–IgGs did not alter the frequency or amplitude of synaptic events (frequency values were 0.09 ± 0.02 vs. 0.08 ± 0.03 Hz and amplitude values were 15.8 ± 2.7 vs. 12.1 ± 1.6 pA in the absence or presence of TrkB–IgG, respectively). In contrast, when slices were incubated with TrkA–IgGs, which sequester endogenous nerve growth factor, but not endogenous BDNF (26), GDP pairing produced a persistent increase in the frequency of sEPSCs (Fig. 4). On average, 20 min after pairing, the frequency of sEPSCs was 107% higher than in control (mean frequency values were 0.07 ± 0.02 and 0.14 ± 0.03 Hz before and after pairing, respectively; n = 7; P < 0.05), whereas the amplitude was slightly reduced to 87.1 ± 9% of control (mean amplitude values were 16.3 ± 1.9 and 14.2 ± 1.6 pA before and after pairing, respectively; P > 0.5). Additionally, in the case of TrkA–IgGs, no changes in the frequency or amplitude of spontaneous synaptic activity were detected in the absence of pairing. Although BDNF is probably released from the postsynaptic cell during GDP-induced membrane depolarization (20), it probably acts on TrkB receptors present on presynaptic terminals because k-252a in the patch pipette was unable to block pairing-induced potentiation (n = 6) (Fig. 4). Altogether, these data show that endogenous TrkB, but not TrkA ligands, is required for pairing-induced persistent potentiation in the frequency of sEPSCs.
Fig. 3.
BDNF enhances synaptic efficacy at CA3–CA1 connections. (A) (Upper) Nine superimposed individual responses (successes and failures) evoked by Schaffer collateral stimulation and obtained in control and during bath application of 40 μg/ml BDNF. (Lower) Average of 19 trials (successes and failures) obtained in the same experimental conditions. (B) Each bar represents the mean peak amplitude of synaptic responses (including failures; n = 6) and PPR (n = 6) obtained in control (open bars) and during superfusion of BDNF (filled bars). *, P < 0.05. (C) Consecutive traces from the same cell shown in A illustrating sEPSCs obtained in control and during application of BDNF. (D) Cumulative distribution of interevent interval (IEI) (Left) and amplitude (Right) for the cell shown in C before (thin line) and after (thick line) pairing. (E) Summary plot showing the mean frequency (Left) and amplitude (Right) of sEPSCs obtained before and after pairing (n = 8).
Fig. 4.
Pairing-induced increase in frequency of sEPSCs requires the activation of TrkB receptors by BDNF. Mean frequency (Left) and amplitude (Right) of sEPSCs (normalized to prepairing control values; dashed lines) recorded before and 20 min after pairing in the presence of 150 nM K252a in the bath (n = 9), 150 nM K252b in the bath (n = 6), 150 nM K252a into the patch pipette (n = 6), 1 μg/ml TrkA–IgG (n = 5), and 1 μg/ml TrkB–IgG (n = 6). Note the increase in sEPSC frequency in the presence of TrkA–IgG.
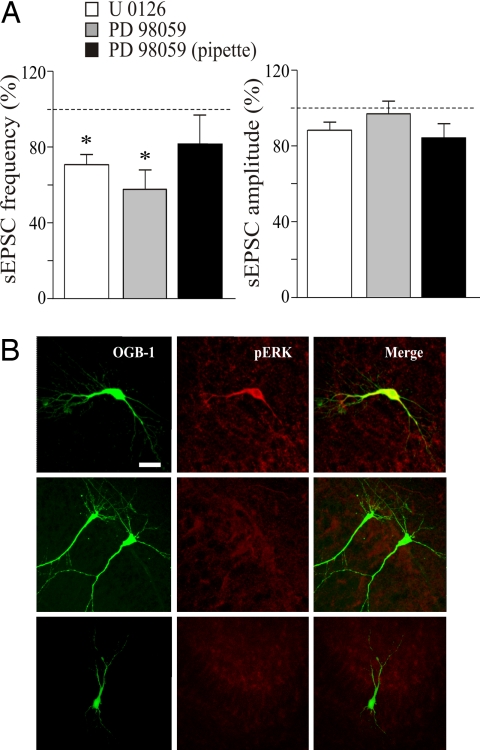
One of the most common signaling pathways activated by BDNF after binding to TrkB receptor is the ERK, a subfamily of MAPK (27). Upon phosphorylation, ERK is translocated into the nucleus, where it activates the transcription factor cAMP response element-binding protein, thus contributing to the persistent modifications in synaptic efficacy (28–31). To assess the role of ERK in synaptic potentiation, the pairing procedure was performed in the presence of the ERK inhibitors U0126 and PD98059. Bath application of 20 μM U0126 for 10 min did not modify the frequency and amplitude of sEPSCs, which remained at predrug levels (before and after U0126 application, the mean frequency values were 0.12 ± 0.02 and 0.11 ± 0.01 Hz, whereas the mean amplitude values were 14.9 ± 2.2 and 15.6 ± 1.5 pA; P > 0.5 for both frequency and amplitude; n = 6). However, after pairing, U0126 induced a persistent and significant reduction in the frequency of sEPSCs. On average, 20 min after pairing the frequency of sEPSCs was 71% of controls (frequency varied from 0.21 ± 0.04 to 0.15 ± 0.04 Hz before and after pairing, respectively; P < 0.05), whereas the amplitude was not significantly modified (16.6 ± 2.2 and 14.9 ± 0.9 pA before and after pairing, respectively; P > 0.5) (Fig. 5A). Similar effects were obtained with PD98059. Slices were preincubated for 10 min with 50 μM PD98059. Although PD98059 did not modify the frequency or amplitude of synaptic events (frequency values were 0.11 ± 0.02 and 0.09 ± 0.02 Hz and amplitude values were 14.9 ± 2.7 and 12.8 ± 1.8 pA before and after drug application, respectively; P > 0.5 for both frequency and amplitude), it prevented the potentiating effect of pairing and caused a significant reduction of the frequency of sEPSCs (mean frequency values were 0.15 ± 0.04 and 0.08 ± 0.02 Hz before and after pairing, respectively; 57% of controls; P < 0.05), but not in the amplitude (mean amplitude values were 15.2 ± 2.7 and 14.3 ± 3.1 pA before and after pairing, respectively; P > 0.5) of sEPSCs (n = 6) (Fig. 5A).
Fig. 5.
Pairing-induced increase in synaptic efficacy requires the activation of the ERK pathway. (A) Mean frequency (Left) and amplitude (Right) of sEPSCs (normalized to prepairing control values; dashed lines) recorded before and 20 min after pairing in the presence of the following ERK inhibitors: 20 μM U0126 (n = 5), 50 μM PD98059 added to the extracellular medium (n = 6), and 50 μM PD98059 added to the intrapipette solution (n = 5). *, P < 0.05. (B) (Left and Center) Immunofluorescence images showing different CA1 pyramidal cells stained with Oregon green BAPTA 488–1 (OGB-1) (Left), with polyclonal antibodies recognizing the doubly phosphorylated form of ERK1/2 (pERK) (Center). (Right) Merged images. Pairing GDPs with Schaffer collateral stimulation induced ERK phosphorylation in the recorded neuron (Top), whereas no ERK phosphorylation was present in the absence of pairing (Middle) or when pairing was performed in the presence of a 20 μM concentration of the ERK inhibitor U0126 (Bottom). (Scale bar: 20 μm.)
To test whether ERK activation occurred into the postsynaptic cell, PD98059 was applied directly into the patch pipette (32). Additionally, in this condition, 50 μM PD98059 prevented the effects of ERK upon paired-induced potentiation of the frequency of sEPSCs (mean frequency values were 0.09 ± 0.03 and 0.07 ± 0.02 Hz before and after pairing, respectively; P > 0.1; mean amplitude values were 16.1 ± 1.3 and 13.8 ± 1.9 pA before and after pairing, respectively; P > 0.1; n = 5) (Fig. 5A).
The involvement of ERK in GDP-induced changes in synaptic efficacy was further validated by immunocytochemical experiments, which demonstrated that the pairing procedure was able to induce ERK phosphorylation (n = 5), an effect that was prevented by 20 μM U0126 (n = 4) (Fig. 5B). In the absence of pairing, no ERK phosphorylation was observed (Fig. 5B). These data suggest that the persistent changes in frequency of sEPSCs after GDP pairing require the activation of the ERK pathway. ERK phosphorylation also was prevented by the TrkB receptor inhibitor k-252a in the patch pipette (n = 4) or in the bath (n = 3), suggesting that, in the present experiments, ERK phosphorylation was triggered by BDNF (SI Fig. 9).
Discussion
The present data clearly show that, during the first week of postnatal life, correlated presynaptic (Schaffer collateral) and postsynaptic (GDPs) activity induced a persistent enhancement of synaptic efficacy at glutamatergic CA3–CA1 connections. This outcome required a transient rise of calcium in the postsynaptic cell and the involvement of BDNF and the ERK pathway.
The pairing procedure not only produced a strong potentiation of the sEPSCs evoked by the Schaffer collateral stimulation but also persistently enhanced the frequency of spontaneous glutamatergic events.
In CA3 pyramidal neurons, evidence has been provided that, during a restricted period of postnatal development, long-term changes in GABAergic synaptic transmission can be induced by repetitive depolarizing pulses (25, 33, 34). Although in these studies the conditioning protocol used for LTP induction could have been of physiological relevance, in the present case, the persistent enhancement of glutamatergic transmission at CA3–CA1 synapses depended on a calcium rise through a voltage-dependent calcium channel activated by the depolarizing action of GABA during GDPs. Interestingly, when the afferent stimulation was paired with theta bursts or plateau potentials in the postsynaptic cell to mimic GDPs, it failed to persistently enhance the frequency of sEPSCs. When paired with afferent stimulation, plateau potentials only briefly enhanced the frequency of sEPSCs, which declined to a control level a few minutes after pairing. In the adult hippocampus, repetitive voltage pulses to the postsynaptic cell have been shown to induce a short-lasting potentiation of excitatory synaptic transmission (35). However, unlike the present case, transient changes in synaptic strength observed in the adult primarily depended on postsynaptic mechanisms because membrane depolarization not only enhanced the frequency of miniature events but also the amplitude of responses to exogenously applied AMPA. Whatever the mechanisms, our results indicate that the way by which calcium enters into the cell is crucial for pairing-induced potentiation. We cannot exclude, however, that in the present study GDPs may concomitantly stimulate presynaptic terminals to further strengthen synaptic currents. This result may, at least in part, explain why depolarizing the postsynaptic cell with theta bursts or plateau potentials failed to persistently enhance synaptic strength.
In the present experiments, the involvement of BDNF in GDP-induced synaptic potentiation was demonstrated by the observation that scavengers of endogenous BDNF or blockers of protein kinase coupled to Trk receptors were able to prevent pairing-induced persistent enhancement of the frequency of sEPSCs. The present results do not indicate whether endogenous BDNF was released from the pre- or postsynaptic cell. However, evidence was recently provided that a moderate postsynaptic depolarization can elicit the release of BDNF from the postsynaptic cell in a calcium-dependent way (20). Once released, BDNF would affect TrkB receptors localized on both pre- and postsynaptic membranes. Although at the presynaptic level BDNF-induced activation of TrkB receptors would enhance transmitter release (20, 36, 37), at the postsynaptic level it would activate fast dendritic calcium transients (38) and different intracellular signaling pathways. One of the most common signaling pathways activated by BDNF is the MAPK/ERK cascade (28, 39). Activation of ERK would lead to transcriptional regulation and new protein synthesis (27) required for the enduring forms of synaptic plasticity (31). As in the late phase of LTP (31), in the present case the activation of ERK was targeted to the postsynaptic neuron as demonstrated by electrophysiological and immunocytochemical experiments. However, we cannot exclude a concomitant activation of ERK in the presynaptic neuron. The postsynaptic action of ERK may further support structural changes (triggered by BDNF activation of TrkB receptors), such as dendritic growth and increase in spine density (30, 40). The ERK pathway also can be activated by calcium entry by NMDA receptors (41). However, this event is unlikely because pairing-induced potentiation was independent of NMDA receptor activation. Therefore, BDNF can act presynaptically to alter the probability of glutamate release but also postsynaptically to produce the morphological modifications necessary for the formation of new synapses and the refinement of the adult neuronal hippocampal circuit.
In conclusion, our data demonstrate that network-driven GABAergic oscillations such as GDPs are essential for the functional maturation of glutamatergic synapses.
Materials and Methods
Slice Preparation.
Experiments were performed on hippocampal slices from P2–P6 Wistar rats as previously described (13). Briefly, animals were decapitated after being anesthetized with an i.p. injection of 2 g/kg urethane. The brain was quickly removed from the skull and placed in ice-cold artificial cerebrospinal fluid containing 130 mM NaCl, 3.5 mM KCl, 1.2 mM NaH2PO4, 25 mM NaHCO3, 1.3 mM MgCl2, 2 mM CaCl2, and 25 mM glucose saturated with 95% O2 and 5% CO2 (pH 7.3–7.4). Transverse hippocampal slices (400 μm thick) were cut with a vibratome and stored at room temperature in a holding bath containing the same solution. After a recovery period of at least 1 h, an individual slice was transferred to the recording chamber, where it was continuously superfused with oxygenated artificial cerebrospinal fluid at a rate of 2 to 3 ml/min at 33–34°C.
Electrophysiological Recordings.
Electrophysiological experiments were performed with CA1 pyramidal cells by using the whole-cell configuration of the patch-clamp technique in current- or voltage-clamp mode. Synaptic responses were evoked at 0.05 Hz by minimal stimulation of the Schaffer collateral. They were recorded in voltage-clamp conditions from a holding potential of −53 mV (EGABA). In most cases, paired stimuli were applied at 50-msec intervals. Patch electrodes were pulled from Borosilicate glass capillaries (Hingelberg, Malsfeld, Germany). They had a resistance of 4 to 6 MΩ when filled with an intracellular solution containing 135 mM K-gluconate, 20 mM KCl, 10 mM Hepes, 4 mM MgATP, 0.3 mM GTP, 0.5 mM EGTA, and 5 mM QX-314. In some experiments, recordings were performed with patch pipettes containing 20 mM BAPTA (Sigma–Aldrich, Milan, Italy). When 20 mM BAPTA was added to the pipette solution, K-gluconate was reduced from 135 to 115 mM. For plateau potential experiments, K-gluconate was substituted with CsMeSO4. In some experiments, cells were visualized with 100 μM Oregon green 488 BAPTA-1 (Molecular Probes, Eugene, OR).
Recordings were made with a patch-clamp amplifier (Axopatch 200A; Axon Instruments, Foster City, CA). Series resistance compensation was used only for current-clamp recordings. The stability of the patch was checked by repetitively monitoring the input and series resistance during the experiment. Cells exhibiting >20% changes in series resistance were excluded from the analysis. The following drugs were used: D-AP5 and 6,7-dinitroquinoxaline-2,3-dione (Tocris Cookson Ltd., Bristol, U.K.); nifedipine and BDNF (Sigma–Aldrich); and k-252a, k-252b, U0126, and PD 98059 (Calbiochem, La Jolla, CA). Immunoadhesins TrkA–IgG and TrkB–IgG were gifts from A. Cattaneo (International School for Advanced Studies).
All drugs except D-AP5 and BDNF were dissolved in DMSO. The final concentration of DMSO in the bathing solution was 0.1%. At this concentration, DMSO alone did not modify the shape or kinetics of synaptic currents. Drugs were applied in the bath by a three-way tap system by changing the superfusion solution to one differing only in its content of drugs. The ratio of flow rate to bath volume ensured complete exchange within 2 min.
Immunofluorescence Staining.
Hippocampal slices with paired or unpaired neurons were fixed overnight in 4% paraformaldehyde in 0.1 M phosphate buffer. After 2 h of preincubation in basic buffer, primary antibodies to doubly phosphorylated ERK1/2 (Cell Signaling Technology, Danvers, MA) were applied at a 1:200 ratio overnight at 4°C. After washing, the resulting immune complexes were visualized with Alexa Fluor 594-labeled goat anti-rabbit antibody (Invitrogen, Carlsbad, CA). Slices were imaged with the DM-IRE2 (Leica, Wetzlar, Germany) confocal system by using sequential dual-channel recording of the Oregon green-injected cells.
Data Acquisition and Analysis.
Data were stored on a disk after digitization with an A/D converter (Digidata 1322A; Axon Instruments, Union City, CA). Data were sampled at 20 kHz and filtered with a cutoff frequency of 2 kHz. Data acquisition was done by using pClamp 9 (Axon Instruments). sEPSCs and evoked EPSCs were analyzed offline with the Clampfit 9 program. sEPSCs were first collected by using the template function of Clampfit and then reviewed by visual inspection. The mean amplitude of sEPSCs was obtained by averaging successes and failures. PPR was calculated as the ratio between the mean amplitude of sEPSC2 and sEPSC1. The coefficient of variation of response amplitude was determined as the ratio between the standard deviation and the mean. Values are given as mean ± SEM. Significance of differences was assessed by Student's t test and Wilcoxon signed rank test (P < 0.05).
Supplementary Material
Acknowledgments
We thank Dr. Gian Michele Ratto for useful suggestions during the course of the experiments. This work was supported by Ministero Istruzione Universita' e Ricerca Grant MIUR-PRIN 2005 (to E.C.) and European Union Grant 503221 (to E.C.).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxadepropionic acid
- BAPTA
1,2-bis (2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- D-AP5
D-(−)-2-amino-5-phosphonopentaoic acid
- GDP
giant depolarizing potential
- LTP
long-term potentiation
- Pn
postnatal day n
- PPR
paired-pulse ratio
- sEPSC
spontaneous excitatory postsynaptic current
- TrkB
tropomyosin-related kinase receptor B.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704533104/DC1.
References
- 1.Ben-Ari Y. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 2.Buzsaki G, Draguhn A. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 3.Menendez de la Prida LM, Huberfeld G, Cohen I, Miles R. Neuron. 2006;49:131–142. doi: 10.1016/j.neuron.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherubini E, Gaiarsa JL, Ben-Ari Y. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Ari Y. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 7.Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- 8.Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsaki G. Science. 2002;296:2049–2052. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- 9.Garaschuk O, Linn J, Eilers J, Konnerth A. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- 10.Voigt T, Opitz T, de Lima AD. J Neurosci. 2005;25:4605–4615. doi: 10.1523/JNEUROSCI.3803-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebb D. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- 12.Safiulina VF, Fattorini G, Conti F, Cherubini E. J Neurosci. 2006;26:597–608. doi: 10.1523/JNEUROSCI.4493-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. Proc Natl Acad Sci USA. 2004;101:3967–3972. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsia AY, Malenka RC, Nicoll RA. J Neurophysiol. 1998;79:2013–2024. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- 15.Hanse E, Gustafsson B. J Physiol. 2001;531:467–480. doi: 10.1111/j.1469-7793.2001.0467i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malenka RC, Nicoll RA. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 17.Garaschuk O, Hanse E, Konnerth A. J Physiol. 1998;507:219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman LJ, Valverde J, Lim F, Geschwind MD, Federoff HJ, Geller AI, Hefti F. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 19.Lessmann V, Gottmann K, Malcangio M. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 20.Magby JP, Bi C, Chen ZY, Lee FS, Plummer MR. J Neurosci. 2006;26:13531–13536. doi: 10.1523/JNEUROSCI.4576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poo MM. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 22.Knusel B, Hefti F. J Neurochem. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T, Saito H, Matsuki N. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sermasi E, Margotti E, Cattaneo A, Domenici L. Eur J Neurosci. 2000;12:1411–1419. doi: 10.1046/j.1460-9568.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 25.Gubellini P, Ben-Ari Y, Gaiarsa JL. J Neurosci. 2005;25:5796–5802. doi: 10.1523/JNEUROSCI.0824-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pesavento E, Margotti E, Righi M, Cattaneo A, Domenici L. Neuron. 2000;25:165–175. doi: 10.1016/s0896-6273(00)80880-6. [DOI] [PubMed] [Google Scholar]
- 27.Sweatt JD. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso M, Medina JH, Pozzo-Miller L. Learn Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelleher RJ, III, Govindarajan A, Tonegawa S. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Shen MR, Chou CY, Browning JA, Wilkins RJ, Ellory JC. J Physiol. 2001;537:347–362. doi: 10.1111/j.1469-7793.2001.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caillard O, Ben-Ari Y, Gaiarsa JL. J Physiol. 1999;518:109–119. doi: 10.1111/j.1469-7793.1999.0109r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gubellini P, Ben-Ari Y, Gaiarsa JL. Eur J Neurosci. 2001;14:1937–1946. doi: 10.1046/j.0953-816x.2001.01823.x. [DOI] [PubMed] [Google Scholar]
- 35.Wyllie DJ, Manabe T, Nicoll RA. Neuron. 1994;12:127–138. doi: 10.1016/0896-6273(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 36.Lohof AM, Ip NY, Poo MM. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 37.Tao HW, Poo M. Proc Natl Acad Sci USA. 2001;98:11009–11015. doi: 10.1073/pnas.191351698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang SB, Stein V, Bonhoeffer T, Lohmann C. J Neurosci. 2007;27:1097–1105. doi: 10.1523/JNEUROSCI.3590-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang EJ, Reichardt LF. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 40.Segal RA, Greenberg ME. Ann Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 41.Hardingham GE, Arnold FJ, Bading H. Nat Neurosci. 2001;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.