Abstract
We experimentally manipulated nutrient input to a rocky intertidal community, using nutrient-diffusing flowerpots, to determine (i) whether nutrients limited intertidal productivity, (ii) how a large-scale oceanographic disturbance (an El Niño event) affected patterns of nutrient limitation, (iii) the relative impacts of molluscan grazers and nutrient limitation, and (iv) if responses to experimental nutrient addition among trophic levels were more consistent with prey-dependent or ratio-dependent food chain models. Nutrients measurably increased the abundance of micrograzers (amphipods and chironomid larvae), but not algal biomass, during the summer of an El Niño year. Nutrients had no effects in two non-El Niño years and during the autumn of an El Niño year. Adding nutrients did not affect food chain stability as assessed by temporal variation in algal biomass and micrograzer abundance. Large molluscan grazers caused large reductions in micrograzers and smaller reductions in algae, indicating consistent consumer effects. The results demonstrate that in this intertidal community, nutrient limitation can occur under conditions of nutrient stress, that top-down grazing effects are typically stronger than bottom-up nutrient effects, and that prey-dependent models are more appropriate than ratio-dependent models.
To understand and predict the consequences of environmental variation, it is becoming increasingly apparent that ecologists must learn how to link ecosystem-level processes (e.g., variation in nutrients or productivity) to community-level dynamics (changes in populations of interacting species). Dynamic models of interactions among species at multiple trophic levels provide a means to link ecosystem and community processes by incorporating nutrient levels as explicit parameters in equations describing the dynamics of producers at the bottom of the food web (1, 2, 3, 4). Additionally, these models can account for the interactions among consumers and their resources that numerous experiments have shown to be important in natural communities (e.g., refs. 5, 6, 7, 8, 9). The predictions of such models depend critically on the relationship between per-capita consumption rates and consumer density. For example, traditional models of consumer–resource interactions, termed “prey-dependent” models, have assumed that per-capita consumption rates increase as a function of prey density because predators encounter more prey over given time intervals. For simple food chains, these models predict that increasing ecosystem productivity should increase only the top trophic level and alternate trophic levels below it (1, 3, 4, 10, 11). Additionally, some forms of prey-dependent models predict decreasing stability with increasing productivity, referred to as the “paradox of enrichment,” which can increase extinction rates at high productivities (10). In contrast, recently proposed models (12, 13, 14) suggest that per-capita consumption rates are a function of the ratio of prey to predator numbers, which can be interpreted mechanistically as consumers sharing all available prey. These “ratio-dependent” models predict that increasing productivity causes proportional increases in all trophic levels, and that the “paradox of enrichment” never occurs. Determining which class of models is more applicable to natural communities requires field experiments manipulating productivity within a single trophic system because observational surveys of communities across productivity gradients can be confounded with shifts in species composition. Such shifts within and between trophic levels can alter the patterns predicted by prey-dependent models (15, 16, 17).
Rocky intertidal communities have proven extremely tractable for experimental investigations, and community-level interactions have been studied extensively within these habitats (reviewed in refs. 18, 19, 20, 21). With few exceptions (22, 23, 24, 25, 26), however, the effects of variation in nutrients and productivity have not been explored in these habitats. Nevertheless, large-scale ecosystem level changes have the potential to affect the dynamics of rocky intertidal communities (25). In particular, El Niño events along the Pacific coast of North America have the capacity to impose major changes on nearshore benthic dynamics. These events cause ocean temperatures to increase, which reduces nutrient mixing to the surface waters and therefore may reduce intertidal productivity (27). In this paper we report the results of experimental manipulations of nutrients in a rocky intertidal community to determine (i) whether nutrient variation affects populations of intertidal organisms, (ii) whether the degree of nutrient limitation varies with El Niño events, (iii) if predictions of prey-dependent or ratio-dependent models are more consistent with the responses of species to nutrient additions at different trophic levels, and (iv) the relationship between community-level and ecosystem-level processes in this community.
METHODS
We conducted our experiments in the mussel-dominated, middle intertidal zone on Tatoosh Island, Washington State (48°24′N, 124°44′W). Experiments manipulating nutrients and grazers were conducted at four times to assess the potential effects of an El Niño event: (i) from May 19 to August 28, 1992, a summer series during a strong El Niño event, (ii) from August 28 to November 20, 1992, a fall series during a strong El Niño event, (iii) from May 8 to September 17, 1993, a non-El Niño year with a dense recruitment of the acorn barnacle Balanus glandula, and (iv) from June 24 to August 13, 1994, a non-El Niño year.
To manipulate nutrients experimentally, we used a modified nutrient-diffusing flowerpot technique (28, 29). We examined the food chain on 15-cm diameter × 11.5-cm high clay flower pots turned upside down and cemented to the rock. Prior to installation, the pots were filled with agar (20 g of agar per liter of water) and sealed with silicon cement both around the edges of a plastic Petri dish fitted in the wide top of the pot and around the edges of a serum vial stopper fitted in the bottom drain hole. To manipulate nutrients, the agar in half of the pots was dissolved with 85 g of NaNO3 per liter of water and 138 g of NaH2PO4 per liter of water, to produce a 10 N:1 P mixture (0.5 M N:0.05 M P). When the pot is immersed in water, this technique produces a nutrient time-release capsule, allowing nitrate and phosphate to diffuse slowly through the agar and clay to be released to the algae that established on the outside of the flower pot after installation (releases of 6.7% of stored N and 3.7% of stored P per day were reported in ref. 28 for 8.8-cm × 8-cm pots). To insure that the nutrient treatments were maintained over time, we used a syringe to inject 20 ml of nutrient solution (170 g of NaNO3 per liter and 276 g of NaH2PO4 per liter) through the serum stopper into the center of the agar at 2-week intervals. The other half of the pots, which had no supplemental nutrients dissolved in their agar, served as controls.
To compare the effects of grazers with those of nutrients, we manipulated molluscan grazers (primarily the limpets Lottia pelta and Lottia digitalis and the chiton Katharina tunicata) by painting a ring of copper-based antifouling paint around the cement rim external to the base of the pots to deter molluscan grazers from invading the enclosed area (30, 31). Pots with antifouling paint were compared with those without antifouling paint to assess the effects of molluscan grazers. The experimental series conducted in the summer of 1992 did not include the treatment with molluscan grazers. The antifouling paint did not prevent the colonization of micrograzers, mostly (96.8% of grazers) larval Chironomidae (Telmatogeton spp.) but also some gammarid amphipods (2.8%) and a few isopods (0.4%). Consequently, the food chain on the flower pots consisted of two levels: algal producers (primarily diatoms, but also some ephemeral macro-algae, including Porphyra and Ulva) and micrograzers.
The treatments were deployed in a randomized block design, with each combination of grazer/nutrient manipulations placed in each of eight wave-exposed sites scattered around the periphery of Tatoosh Island. Blocks were separated from their nearest neighbors by 100–300 m of shore. The pots were placed in cleared areas within the mussel (Mytilus californianus) bed that dominates the middle-intertidal zone at this site. Because of wave action following installation of the pots, one nutrient pot was lost from the first series of experiments, a block of four pots was lost from the second series, and one nutrient pot was lost during the fourth series. We censused the pots twice during each experiment, once at the midpoint and once at the end. To census the pots, we scraped algae from the 39.4-cm2 top of the pot, hand-picking and counting all small grazing invertebrates from the sample, then drying the algae on pre-tared filter paper for 24 hr at 70°C and weighing it. Because the molluscan grazer manipulations affected barnacle cover, which in turn affected algal cover, algae and midges were sampled only from that portion of area not covered by barnacles in 1993. The resulting estimates of algal mass and grazer abundance were corrected for differences in area sampled in the different treatments, using a uniform grid of points, spaced at 1 cm, to estimate the percent cover of barnacles and barnacle-free area on the tops of the pots. The average algal mass and grazer number per unit area from each experiment were analyzed by blocked two-way analyses of variance. We also used two-way analysis of variance to examine the effects that nutrient addition had on community stability by comparing the temporal variance (variance between the midpoint and final sampling dates for each pot) and the coefficient of temporal variation (temporal variance × 100/mean) in the algal biomass and micrograzer abundance. Higher variance would indicate less stability in the sense of the tendency of the algal-grazer system to return and/or remain at an equilibrium point.
RESULTS
Nutrient addition affected the food chain on the pots in the summer of 1992, the El Niño year, but had no statistically significant effect in experiments conducted at other times (Fig. 1). Furthermore, increasing nutrients affected only the abundance of micrograzers, increasing their densities by 30%, but had no effect on overall levels of algal biomass. Although nutrient addition caused changes in mean micrograzer abundance in 1992, it had no discernible effect on the stability of the food chain. The temporal variance and the temporal coefficient of variation in both algae and grazers between census dates did not differ between nutrient treatments (Table 1).
Figure 1.
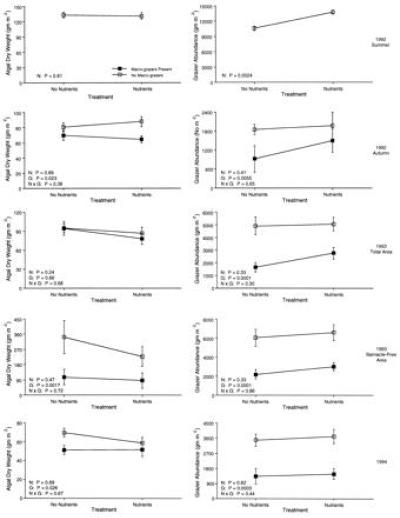
Mean (±SEM) algal ash free dry biomass (Left) and grazer abundance (Right) in different treatments manipulating molluscan grazers and nutrients. Top pair of panels, experiments from summer during 1992 El Niño; second pair, experiments from autumn during 1992 El Niño; third pair, experiments from summer 1993, a non-El Niño year with high barnacle settlement, expressed on the basis of total area; fourth pair, experiments from summer of 1993, expressed on the basis of barnacle-free area; bottom pair, experiments from 1994, a non-El Niño year. The lower left corner of each graph shows the probabilities of an effect of nutrients (N), grazers (G), and their interaction (N × G) from ANOVA.
Table 1.
Effects of increasing productivity on the temporal variance and coefficient of variation of algae and micrograzers in the first series of experiments
| Trophic group | Treatment (n) | Temporal
variance
|
Coefficient of variation
|
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | P | Mean | SD | P | ||
| Algae | Control (8) | 5.33 | 5.58 | 0.407 | 22.02 | 12.52 | 0.478 |
| + nutrients (7) | 16.50 | 37.06 | 29.28 | 23.16 | |||
| Micrograzers | Control (8) | 4.3 × 105 | 5.1 × 105 | 0.268 | 88.17 | 42.98 | 0.879 |
| + nutrients (7) | 8.4 × 105 | 8.6 × 105 | 84.92 | 37.09 | |||
In contrast to nutrient addition, the manipulation of molluscan grazers exhibited consistent effects on the food chain in all experiments in which it was examined. Excluding molluscan grazers caused significant increases both in algal biomass (25–194%) and in micrograzer abundance (66–154%; Fig. 1). No interactions were detected between grazer and nutrient treatments. Removing molluscan grazers had significant effects on barnacle abundance in 1993: the percentage of area covered by barnacles in the absence of molluscan grazers (62%) averaged three times higher than in the presence of molluscan grazers (21%; Fig. 2). Nutrient treatment had no effect on the percentage of area covered by barnacles.
Figure 2.
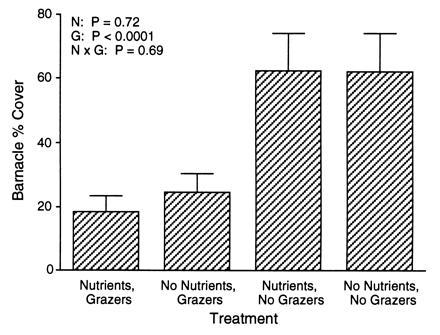
Mean (+SEM) percent area covered by barnacles (B. glandula) in different nutrient and grazer treatments from experiments conducted in the summer of 1993.
DISCUSSION
Our experiment demonstrates that, under some conditions, enhanced nutrients are capable of affecting intertidal populations at Tatoosh Island. Nutrient effects were detected only in experiments conducted during an El Niño year. Nutrient limitation is more likely during an El Niño event because warmer surface water inhibits upwelling of nutrient-rich deeper water (27). Even during an El Niño event, however, nutrients were not consistently limiting. During the 1992 autumn experiment, we detected no nutrient effects, suggesting that nutrient limitation in summer switched to light limitation later in autumn, when there was approximately 27% less daylight available. Our data suggest that El Niño events can affect patterns of nutrient limitation and that productivity in any given system is not necessarily limited consistently by a single factor as a consequence of intra- and interannual variations in resource regimes.
Although nutrient addition did increase the abundance of intertidal organisms in the summer of 1992, there was no evidence of reduced stability with increased nutrients in this experiment, as determined by the lack of pattern in temporal fluctuations across nutrient treatments. This finding does not support the “Paradox of Enrichment” (10), which predicts reduced stability and ultimately reduced diversity with increasing productivity. The lack of such an effect could result for several reasons. For example, productivity levels may not have been unnaturally high in the nutrient addition treatments because the overall nutrient levels in the water column were lower during the El Niño event. The consumer satiation that leads to the paradox is expected to be a selective disadvantage over evolutionary time because consumers that do not become satiated can obtain extra energy to produce more offspring. Consumer satiation therefore may be unlikely over the natural range of variation in prey abundance (32, 33).
In contrast to nutrients, molluscan grazers consistently exerted strong direct and indirect effects on members of the intertidal community. Limpets and chitons significantly reduced algae by grazing (7, 30, 31, 34), and they also reduced barnacles either by “bulldozing” or by consuming newly settled barnacle larvae (34). Arthropod micrograzers decreased in the presence of macrograzers, apparently in response to direct and indirect effects: limpets consume both midges (31) and the algal food source of the midges. Algae also responded indirectly to molluscan grazers through changes in barnacle cover. Algae were absent or were present only as a small surface film on the tests of barnacles, possibly because the barnacles captured and ate settling algae before the algae could become established, because the barnacles dislodged established algae through the movements of their cirri, or because the barnacles provided a refuge for micrograzers from desiccation or grazers. Consequently, although algae in the 1993 experiment increased when grazers were excluded on a per-barnacle-free-area basis, they showed no grazer effects when examined on a total-area basis (Fig. 1). The consistent molluscan grazer effects, relative to nutrient effects, observed in this study in part justify the past focus of studies in rocky intertidal communities on interactions among species rather than the effects of nutrients (5, 7, 8, 18, 19, 20, 21, 34), although our experiments included only a limited number of taxa. The weak nutrient effects are also consistent with patterns observed with varying inputs of nutrient-rich guano in the San Juan Islands of Washington State, where the addition of nutrients appeared to have few positive direct effects on species (24), and with the weak responses observed by a variety of taxa on Tatoosh Island to the 1982–1983 El Niño event (35). There is, however, some evidence that large-scale patterns of nutrient availability can indirectly have important effects on intertidal communities in Oregon, by increasing phytoplankton populations fed upon by sessile animals such as mussels (25).
The pattern of responses to nutrients observed in our field experiments also bears upon models of food chains in ecology. When nutrients enhanced population sizes, they did so only for micrograzers, not algae. This pattern is predicted by prey-dependent models of a two-level food chain, where increasing algal productivity supports higher grazer abundance, which in turn prevents long-term increases in algae. Previous experiments have demonstrated that intertidal midges can control their algal resources (36). Patterns of response among trophic levels similar to those observed in our study have been reported in field experiments on food chains conducted in freshwater lakes (37), ponds (38), and rivers (3, 39). The results of these field experiments suggest that prey-dependent consumer resource interactions may form a reasonable foundation for integrating the effects of productivity and species interactions in communities with more web-like functional architectures (15, 16, 40).
Acknowledgments
We thank the Makah Tribal Council and the U.S. Coast Guard for allowing us to work on Tatoosh Island, J. Marks for useful discussions about the flowerpot technique, S. Gresens for midge identification, M. Decher for field assistance, and B. Menge for comments on the manuscript. The study was supported by National Science Foundation Grants BSR 91–00123, DEB 93–17980, OCE 91–15760, and OCE 92–21776.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- 1.Oksanen L, Fretwell S J, Arruda J, Niemela P. Am Nat. 1981;118:240–261. [Google Scholar]
- 2.DeAngelis D L. Dynamics of Nutrient Cycling and Food Webs. New York: Chapman & Hall; 1989. [Google Scholar]
- 3.Wootton J T, Power M E. Proc Natl Acad Sci USA. 1993;90:1384–1387. doi: 10.1073/pnas.90.4.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith F E. Eutrophication: Causes, Consequences, Correctives. Washington, DC: Natl. Acad. Press; 1969. pp. 631–645. [Google Scholar]
- 5.Paine R T. Am Nat. 1966;100:65–75. [Google Scholar]
- 6.Power M E. Science. 1990;250:811–814. doi: 10.1126/science.250.4982.811. [DOI] [PubMed] [Google Scholar]
- 7.Wootton J T. Ecology. 1992;74:981–991. [Google Scholar]
- 8.Wootton J T. Ecology. 1994;75:151–165. [Google Scholar]
- 9.Sih A, Crowley P, McPeek M, Petranka J, Strohmeier K. Annu Rev Ecol Syst. 1985;16:269–311. [Google Scholar]
- 10.Rosenzweig M L. Science. 1971;717:385–387. doi: 10.1126/science.171.3969.385. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig M L. Am Nat. 1973;107:275–294. [Google Scholar]
- 12.Arditi R, Ginzburg L R. J Theor Biol. 1989;139:311–326. [Google Scholar]
- 13.Getz W M. J Theor Biol. 1984;108:623–643. [Google Scholar]
- 14.Ginzburg L R, Akçakaya H R. Ecology. 1992;73:1536–1543. [Google Scholar]
- 15.Leibold M A. Am Nat. 1989;134:922–949. [Google Scholar]
- 16.Abrams P A. Am Nat. 1993;141:351–371. [Google Scholar]
- 17.Mittelbach G G, Osenberg C W, Leibold M A. In: Size Structured Populations: Ecology and Evolution. Ebenman B, Persson L, editors. Berlin: Springer; 1988. pp. 219–235. [Google Scholar]
- 18.Connell J H. Annu Rev Ecol Syst. 1972;3:169–192. [Google Scholar]
- 19.Paine R T. Acad Nat Sci Philadelphia Spec Publ. 1977;12:245–270. [Google Scholar]
- 20.Menge B A, Farrell T M. Adv Ecol Res. 1989;19:189–262. [Google Scholar]
- 21.Paine R T. Marine Rocky Shores and Community Ecology: An Experimentalist’s Perspective. Oldendorf/Luhe, Germany: Ecology Institute; 1994. [Google Scholar]
- 22.Bosman A L, Du Toit J T, Hockey P A R, Branch G M. Estuarine Coastal Shelf Sci. 1986;23:283–294. [Google Scholar]
- 23.Bosman A L, Hockey P A R. Mar Ecol Prog Ser. 1986;32:247–257. [Google Scholar]
- 24.Wootton J T. J Exp Mar Biol Ecol. 1991;151:139–153. [Google Scholar]
- 25.Menge B A, Daley B, Wheeler P A. In: Food Webs: Integration of Pattern and Dynamics. Polis G A, Winemiller K O, editors. New York: Chapman & Hall; 1995. pp. 258–274. [Google Scholar]
- 26.Bustamante R H, Branch G M, Eekhout S. Ecology. 1995;76:2314–2329. [Google Scholar]
- 27.Glynn P W. Annu Rev Ecol Syst. 1988;19:309–345. [Google Scholar]
- 28.Fairchild G W, Lowe R L, Richardson W B. Ecology. 1985;66:465–472. [Google Scholar]
- 29.Marks J C, Lowe R L. Can J Fish Aquat Sci. 1993;50:1270–1278. [Google Scholar]
- 30.Cubit J D. Ecology. 1984;65:1904–1971. [Google Scholar]
- 31.Robles C D. Oecologia. 1982;54:23–31. doi: 10.1007/BF00541103. [DOI] [PubMed] [Google Scholar]
- 32.Gilpin M E. Nature (London) 1975;254:137–139. [Google Scholar]
- 33.Schaffer W M, Rosenzweig M L. Theor Popul Biol. 1978;14:135–137. doi: 10.1016/0040-5809(78)90008-4. [DOI] [PubMed] [Google Scholar]
- 34.Dayton P K. Ecol Monogr. 1971;45:137–159. [Google Scholar]
- 35.Paine R T. Limnol Oceanogr. 1986;31:351–360. [Google Scholar]
- 36.Robles C D, Cubit J. Ecology. 1981;62:1536–1547. [Google Scholar]
- 37.Leibold M A. Oecologia. 1991;86:510–520. doi: 10.1007/BF00318317. [DOI] [PubMed] [Google Scholar]
- 38.Leibold M A, Wilbur H M. Nature (London) 1992;360:341–343. [Google Scholar]
- 39.Hill W R, Ryon M G, Schilling E M. Ecology. 1995;76:1297–1309. [Google Scholar]
- 40.Wootton J T, Parker M S, Power M E. Science. 1996;273:1558–1561. [Google Scholar]


