Abstract
Cytokines have progressively come to serve as indicators for the presence or severity of a disease. But accurate measurement of cytokine levels can be deterred by lack of proper handling and storage of the samples. In this study, we attempted to measure the effect of snap-freezing and refrigeration at the time of collection on cervical mucous. Luminex analyses of the frozen and refrigerated pairs exhibited no significant differences in levels for 7 out of the 10 cytokines measured simultaneously. The cytokines TNF-α, IFN-γ and IL-1β, were significantly different between the pairs with the refrigerated samples showing higher levels for each of these cytokines. The results suggest that refrigeration of mucous samples immediately after collection would allow for better conservation of the cytokines in cervical mucous.
Keywords: Cervical mucous, Cytokines, Luminex
1. Introduction
Intercellular mediators like cytokines, that modulate immune function, serve as precursors to effector mechanisms that are selected and propagated in an immune response. Therefore they are also indicators of the type of immune response elicited and their levels in biologic fluids can be used in monitoring and characterizing disease [1].
Cytokines are known to have a short half-life in-vivo and are also subject to rapid degradation in-vitro following sample collection if appropriate storage and handling procedures are not adopted. In several cytokine studies, these same factors involving sample collection, processing and storage have been shown to be critical for achieving accurate and reproducible results [2–4]. But, most of these published reports on cytokine stability and storage are related to serum or plasma samples [5–8]. Several studies of cytokines in cervical mucous have been performed to investigate the local immune response to human papillomavirus (HPV), in hopes of determining immune responses associated with clearance or disease progression. To date, because of uncertainties of the stability of cytokines in cervical mucous, studies have relied on snap freezing at the time of collection. For ease of implementation into clinical settings, simply refrigerating the sample until transferring to the lab for long-term storage and assay would have significant advantages [9,10]. In the present study, the effect of refrigeration or immediate freezing on the levels of ten cytokines in cervical mucosal samples was determined using Luminex xMap technology. Data generated from this study helped determine the appropriate conditions or storage of patient samples to aid in preservation of the cytokines for future assays.
2. Methods
2.1. Sample collection
Samples were collected from 35 women attending urban public health hospital colposcopy clinics who were enrolled as part of an ongoing study of cervical neoplasia [11]. After visualization of the cervix, two Weck-Cel sponges (Xomed Surgical Products, Jacksonville, FL) were placed into the cervical os for a minimum of 1 minute, or until the sponges appeared saturated. Each sponge was placed in a labeled microcentrifuge tube; one was placed on wet ice and refrigerated in the clinic, while the other was immediately snap-frozen on dry ice. Specimens were transferred to the laboratory within eight hours of collection and then stored at −80°C until further analysis.
2.2. Protein extraction from Weck-Cel
We used the protein extraction protocol described [12]. Briefly, the sponges were equilibrated in 300 μl of extraction buffer consisting of PBS (pH 7.0), 0.25M NaCl and 10% fetal calf serum, for 30 min at 4°C. The diluted samples were then separated from the sponge using a Spin-X filter unit (Corning Inc., Acton, MA) by centrifugation at 16, 000 X g for 15 min at 4°C. The sponge was then washed immediately with an additional 300 μl of extraction buffer and collected as before. Total protein content was measured using the Coomasie Plus™ kit (Pierce technologies) as per the manufacturer’s protocol.
2.3. Multiple cytokine testing using Bio-Plex™ protein array system
The cytokine concentrations were determined using the Bio-Plex protein array system and Bio-Plex multiple-cytokine assay kits (Bio-Rad, Hercules, CA) as per manufacturer’s protocols. The Bio-Plex 10-plex kits were used to determine the concentration of 10 cytokines in each sample being tested [IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, GM-CSF, IFN-gamma, TNF-alpha, and IL-1beta]. A wide range (1.95 pg/ml-32000 pg/ml) of assay standards re-suspended in standard diluent was used to plot the standard curves for the 10 cytokines. All reagents needed for the assays were provided in the kits. Calibration of the instrument was performed daily, along with the monthly-recommended system validation. The mucosal samples were diluted 1:2 in Bio-Plex serum diluent prior to analysis and assayed in duplicate. The assay technique was specific and sensitive for simultaneous detection of multiple cytokines with a coefficient of variation ≤ 10%.
Data was obtained using Bio-Plex Manager software program (Bio-Rad) for standardization and standard curve acquisition followed by conversion to Excel™ (Microsoft Corporation, Seattle, WA) for further analysis. Optimal standard curve fits, either 4-parameter or 5-parameter, were selected for every assay run as well as for every cytokine within a given assay. Conversion of the optimal data to Excel ™ (Microsoft Corporation) facilitated the comparative analysis of cytokines between frozen and refrigerated patient samples.
2.4. Statistical Analysis
Statistical analysis of the data was performed using the Wilcoxon signed rank test to determine whether the median difference in parameter values between frozen and refrigerated mucosal samples was equal to 0. Statistical significance was determined if p-values were <0.05. All analyses were performed using the SAS software.
3. Results
3.1. Extraction of proteins from Weck-Cel sponge
The optimized protein extraction protocol from the Weck-Cel sponge was successfully applied to the 35 cervical mucus samples collected. The total protein content measured in these samples was similar for snap frozen (mean 4.41 ± 1.21 mg/ml; range 1.67–7.18 mg/ml) and refrigerated samples (mean 4.37 ± 1.08 mg/ml; range 1.62–6.27 mg/ml).
3.2. Comparison of Cytokine levels in refrigerated and frozen samples
The cytokine levels obtained from the frozen and refrigerated sample sets are shown in Fig. 1. Samples were run over the course of 4 months and frozen and refrigerated pairs were included in the same run. The intra-assay and inter-assay variabilities measured in 8 frozen and refrigerated samples was ≤ 10% in 8 out of the 10 cytokines. But, in the case of IFN-γ and IL-4, the interassay variability was 33% and 24% respectively. For several cytokines including, IL-2, IL-4, IL-6, IL-8, IL-12, IL-10 and GM-CSF, the data showed no significant differences in cytokine levels with a p-value of ≥ 0.3 (Table 1, Fig. 1). But, in the case of TNF-α, IFN-γ and IL-1β, there was a significant loss of cytokine in the samples that were frozen immediately after collection (p-value ≤ 0.05) (Table 1, Fig. 1).
Fig. 1.
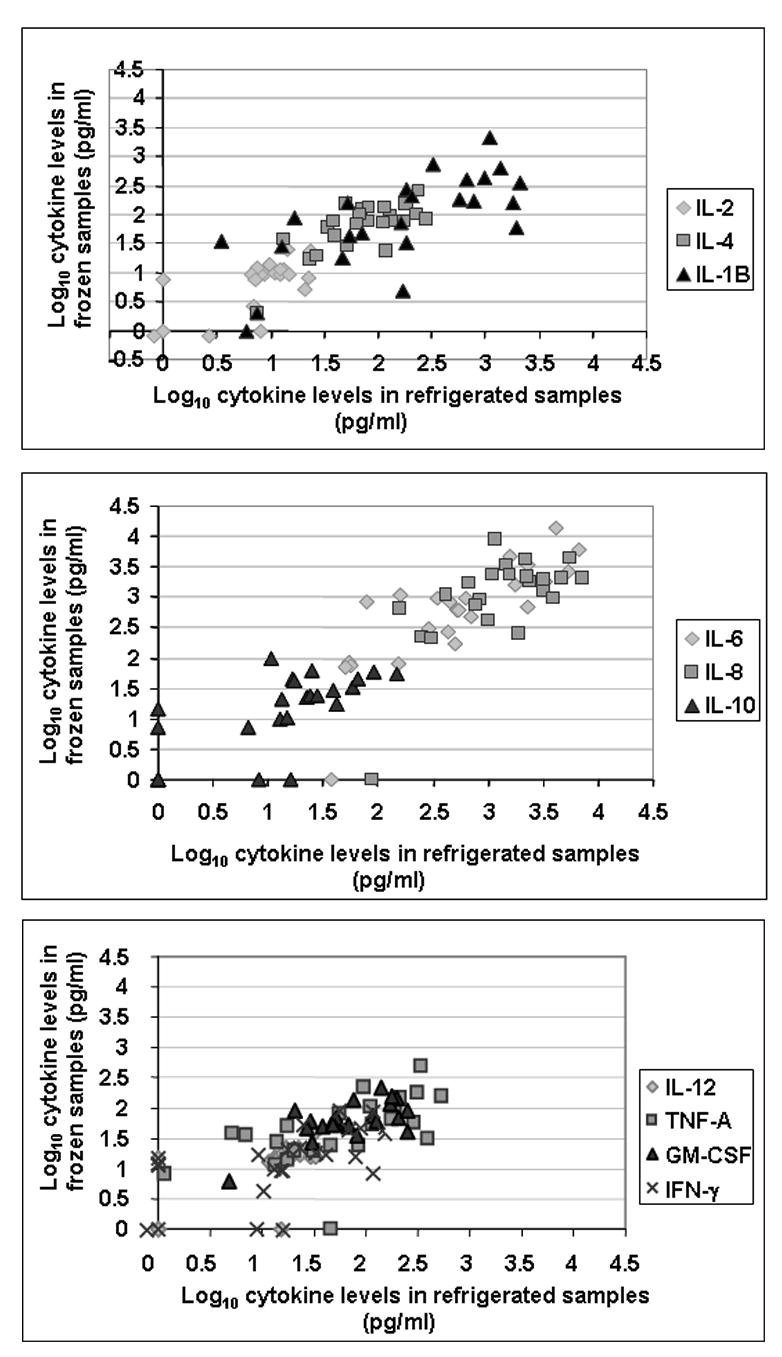
Scatter plots showing the correlation between frozen and refrigerated samples for ten cytokines as determined by the Bio-Plex assay.
Table 1.
Mean, median and p-values determined by the Wilcoxon signed rank test for the different cytokines in cervical mucus samples that were either refrigerated or frozen
| Cytokines | Difference between Frozen and Refrigerated samplesa |
Significance (p-value) | |
|---|---|---|---|
| Mean value | Median value | ||
| IL-1β | −298.45 | −25.02 | 0.01 |
| IL-2 | −0.32 | 0.0 | 0.89 |
| IL-4 | −12.93 | −3.5 | 0.36 |
| IL-6 | 391.63 | 18.64 | 0.31 |
| IL-8 | −359.38 | −82.91 | 0.29 |
| IL-10 | −2.20 | −0.0 | 0.85 |
| IL-12 | −0.47 | 0.0 | 0.94 |
| IFN-γ | −13.060 | −4.68 | 0.03 |
| TNF-α | −43.01 | −15.36 | 0.02 |
| GM-CSF | −16.35 | −1.86 | 0.30 |
The difference in cytokine levels between refrigerated and frozen samples is considered the variable in Wilcoxon signed rank test.
4. Discussion
This study was conducted to determine if snap-freezing or refrigeration of cervical mucous samples at the time of collection would affect measured cytokine levels. We found that only TNF-α, IFN-γ and IL-1β, exhibited significant differences in measured levels with refrigerated mucosal samples having higher levels of these cytokines as compared to their snap-frozen samples.
Not much is known as to how the biological structure of the cytokine is affected in these situations, but several reports on serum and plasma cytokine levels have indicated that most cytokines are prone to degradation if blood samples are not processed correctly and stored at −70°C. TNF-α, measured in serum or plasma is commonly cited as very susceptible to changes in collection and storage methods [13–15], and seems to possess the similar vulnerability in mucous samples. The levels of the cytokines TNF-α, IFN-γ and IL-1β, measured in our sample set were well above the detection limits of our assay, and therefore the differences seen were not assay artifacts. The results also imply that the time (8 h) by which the refrigerated samples were transferred to freezers did not affect cytokine levels, therefore making it very convenient for collection of these samples when conducting large clinical studies where storage facilities are not readily accessible.
Thus, in this study we have shown that refrigeration of mucous samples instead of snap-freezing them immediately after collection would allow for better conservation of the cytokines in cervical mucous. Cytokines exist in complex tertiary and sometimes quarternary structures that could be susceptible to structural damage when exposed to improper handling and storage conditions, and therefore it is critical to maintain an optimized protocol for collection, handling, storage and assay of the samples that are to be used for cytokine measurement.
This study has several limitations. As both the refrigerated and snap frozen samples were stored prior to extraction and testing, we were unable to measure the impact of storage. To conserve reagents we needed to perform the Luminex assay on batched samples, so immediate extraction and testing was not possible in our hands, nor is it likely to be possible in most epidemiologic studies. In addition, the number of samples tested was small, and the differences in TNF-α, IFN-γ and IL-1β, between refrigerated and snap frozen samples, while statistically significant, may not be biologically significant.
Acknowledgments
Supported in part by the National Cancer Institute’s Early Detection Research Network (EDRN), Interagency Agreement Y1-CN-0101-01.
Footnotes
“The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the funding agency.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gitika Panicker, Email: dhv1@cdc.gov.
Kristi S Meadows, Email: kts9@cdc.gov.
Daisy R Lee, Email: del5@cdc.gov.
Rosane Nisenbaum, Email: NisenbaumR@smh.toronto.on.ca.
Elizabeth R Unger, Email: EUnger@cdc.gov.
References
- 1.Poli G, Fauci AS. Cytokine modulation of HIV expression. Semin Immunol. 1993;5:165–73. doi: 10.1006/smim.1993.1020. [DOI] [PubMed] [Google Scholar]
- 2.Riches P, Gooding R, Millar BC, Rowbottom AW. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-alpha concentrations. J Immunol Methods. 1992;153:125–31. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 3.Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR. Measuring cytokine levels in blood. Importance of anticoagulants, processing, and storage conditions. J Immunol Methods. 1992;153:115–24. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- 4.Aziz N, Nishanian P, Mitsuyasu R, Detels R, Fahey JL. Variables that affect assays for plasma cytokines and soluble activation markers. Clin Diagn Lab Immunol. 1999;6:89–95. doi: 10.1128/cdli.6.1.89-95.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riches P, Gooding R, Millar BC, Rowbottom AW. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-alpha concentrations. J Immunol Methods. 1992;153:125–31. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 6.Aziz N, Nishanian P, Mitsuyasu R, Detels R, Fahey JL. Variables that affect assays for plasma cytokines and soluble activation markers. Clin Diagn Lab Immunol. 1999;6:89–95. doi: 10.1128/cdli.6.1.89-95.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR. Measuring cytokine levels in blood. Importance of anticoagulants, processing, and storage conditions. J Immunol Methods. 1992;153:115–24. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- 8.Flower L, Ahuja RH, Humphries SE, Mohamed-Ali V. Effects of sample handling on the stability of interleukin 6, tumour necrosis factor-alpha and leptin. Cytokine. 2000;12:1712–6. doi: 10.1006/cyto.2000.0764. [DOI] [PubMed] [Google Scholar]
- 9.Castle PE, Phillips TM, Hildesheim A, Herrero R, Bratti MC, Rodriguez AC, Morera LA, Pfeiffer R, Hutchinson ML, Pinto LA, Schiffman M. Immune profiling of plasma and cervical secretions using recycling immunoaffinity chromatography. Cancer Epidemiol Biomarkers Prev. 2003;12:1449–56. [PubMed] [Google Scholar]
- 10.Hildesheim A, McShane LM, Schiffman M, Bratti MC, Rodriguez AC, Herrero R, Morera LA, Cardenas F, Saxon L, Bowman FP, Crowley-Nowick PA. Cytokine and immunoglobulin concentrations in cervical secretions: reproducibility of the Weck-cel collection instrument and correlates of immune measures. J Immunol Methods. 1999;225:131–43. doi: 10.1016/s0022-1759(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 11.Rajeevan MS, Swan DC, Nisenbaum R, Lee DR, Vernon SD, Ruffin MT, Horowitz IR, Flowers LC, Kmak D, Tadros T, Birdsong G, Husain M, Srivastava S, Unger ER. Epidemiologic and viral factors associated with cervical neoplasia in HPV-16-positive women. Int J Cancer. 2005;115:114–20. doi: 10.1002/ijc.20894. [DOI] [PubMed] [Google Scholar]
- 12.Rohan LC, Edwards RP, Kelly LA, Colenello KA, Bowman FP, Crowley-Nowick PA. Optimization of the weck-Cel collection method for quantitation of cytokines in mucosal secretions. Clin Diagn Lab Immunol. 2000;7:45–8. doi: 10.1128/cdli.7.1.45-48.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Exley AR, Cohen J. Optimal collection of blood samples for the measurement of tumor necrosis factor alpha. Cytokine. 1990;2:353–6. doi: 10.1016/1043-4666(90)90065-2. [DOI] [PubMed] [Google Scholar]
- 14.Riches P, Gooding R, Millar BC, Rowbottom AW. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-alpha concentrations. J Immunol Methods. 1992;153:125–31. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 15.Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR. Measuring cytokine levels in blood. Importance of anticoagulants, processing, and storage conditions. J Immunol Methods. 1992;153:115–24. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]


