Abstract
She4p/Dim1p, a member of the UNC-45/CRO1/She4p (UCS) domain-containing protein family, is required for endocytosis, polarization of actin cytoskeleton, and polarization of ASH1 mRNA in Saccharomyces cerevisiae. We show herein that She4p/Dim1p is involved in endocytosis and actin polarization through interactions with the type I myosins Myo3p and Myo5p. Two-hybrid and biochemical experiments showed that She4p/Dim1p interacts with the motor domain of Myo3/5p through its UCS domain. She4p/Dim1p was required for Myo5p localization to cortical patch-like structures. Using random mutagenesis of the motor region of MYO5, we identified four independent dominant point mutations that suppress the temperature-sensitive growth phenotype of the she4/dim1 null mutant. All of the amino acid substitutions caused by these mutations, V164I, N168I, N209S, and K377M, could suppress the defects of endocytosis and actin polarization of the she4/dim1 mutant as well. She4p/Dim1p also showed two-hybrid interactions with the motor domain of a type II myosin Myo1p and type V myosins Myo2p and Myo4p, and was required for proper localization of Myo4p, which regulates polarization of ASH1 mRNA. Our results suggest that She4p/Dim1p is required for structural integrity or regulation of the motor domain of unconventional myosins.
INTRODUCTION
Endocytosis, the internalization of portions of the plasma membrane along with extracellular fluid, is a universal process performed by all eukaryotic cells. In the budding yeast, S. cerevisiae, genetic analysis has identified a link between endocytosis and the actin cytoskeleton (Geli and Riezman, 1998; Wendland et al., 1998). During bud growth, yeast cells show polarized organization of two actin filament-containing structures: cortical actin patches and actin cables (Adams and Pringle, 1984; Kilmartin and Adams, 1984). The formation or reorganization of cortical actin patches is regulated by cortical patch-like protein structures that include Sla1p, Sla2p, Abp1p, Sac6p (fimbrin), Las17p/Bee1p (a homolog of mammalian Wiskott-Aldrich syndrome protein), Vrp1p (verprolin, a homolog of mammalian Wiskott-Aldrich syndrome protein-interacting protein), Myo3p/Myo5p (type I myosins), Rvs167p (amphiphysin), and proteins of the Arp2/3 complex (Pruyne and Bretscher, 2000). These proteins are involved in the uptake step of endocytosis through actin cytoskeleton regulation (Geli and Riezman, 1998; Wendland et al., 1998).
The DIM1 gene was isolated in a fluorescence-activated cell sorting-based screen using the fluorescent lipophilic dye FM4-64 to search for factors involved in membrane lipid endocytosis (Wendland et al., 1996). Molecular cloning revealed that DIM1 was identical to SHE4 (Wendland et al., 1996; see below). At 38°C, she4 null mutation (she4Δ) results in a two- to threefold reduction in the kinetics of mating pheromone internalization relative to that observed in she4Δ cells at 26°C or in wild-type cells (Wendland et al., 1996). Like many endocytosis mutants, she4Δ cells show a temperature-sensitive growth defect and a depolarized distribution of actin patches (Wendland et al., 1996; Wendland et al., 1998).
The SHE4 gene was also identified in an independent screen for factors required for Swi5p-dependent HO expression. In the same screen, four other genes were identified: MYO4/SHE1 (type V myosin), SHE2, SHE3, and BNI1/SHE5 (Jansen et al., 1996). The SHE genes are required for polarized distribution, to daughter cells, of ASH1 mRNA, which codes for Ash1p, the transcriptional repressor of the HO gene (Sil and Herskowitz, 1996; Long et al., 1997; Takizawa et al., 1997). The asymmetric localization of ASH1 mRNA to daughter cells results in the preferential accumulation of Ash1p in daughter cell nuclei and the selective expression of the HO endonuclease in mother cells (Bobola et al., 1996; Sil and Herskowitz, 1996; Long et al., 1997). Myo4p/She1p, She2p, and She3p are proposed to form a complex with ASH1 mRNA and to be translocated to the bud by the motor activity of Myo4p/She1p (Munchow et al., 1999; Bohl et al., 2000; Long et al., 2000; Takizawa and Vale, 2000). SHE4 has been shown to be required both for endocytosis and for polarization of ASH1 mRNA distribution, but the molecular mechanisms by which it influences these events have not been well understood.
The C-terminal half of She4p has significant similarity to Caenorhabditis elegans UNC-45, Podospora anserina CRO1, and Schizosaccharomyces pombe Rng3p (Barral et al., 1998; Berteaux-Lecellier et al., 1998; Wong et al., 2000). This C-terminal conserved region is referred to as the UNC-45/CRO1/She4p (USC) domain, and these proteins form the UCS protein family. UNC-45 is specifically expressed in muscle tissues and colocalizes with a specific isoform of myosin heavy chain, where it is proposed to play a role in the assembly of skeletal muscle myosin (Venolia and Waterston, 1990; Barral et al., 1998; Ao and Pilgrim, 2000). Recently, it was reported that UNC-45 acts as a molecular chaperone for the muscle myosin motor (Barral et al., 2002). CRO1 is essential for the transition between the syncytial and cellular stages (Berteaux-Lecellier et al., 1998). Rng3p is required for cytokinesis, and rng3 genetically interacts with type II myosin, myo2 (Balasubramanian et al., 1998; Wong et al., 2000).
Myosins are molecular motors that convert the energy of ATP hydrolysis into mechanical work, in the form of translocation along actin filaments. They constitute a large super-family of proteins implicated in diverse cellular functions (Mooseker and Cheney, 1995; Sellers, 1999). Budding yeast contains a total of five myosins from three types: two type I myosins (MYO3 and MYO5), one type II myosin (MYO1), and two type V myosins (MYO2 and MYO4) (Brown, 1997). Conventional (type II) myosins were the first type to be described. They include muscle myosins and similar myosins from nonmuscle cells. They have a two-headed structure and self-associate to form filaments. MYO1 is implicated in actin-ring formation during cytokinesis and is thought to deliver components required for cell separation to the septum (Watts et al., 1987; Rodriguez and Paterson, 1990; Bi et al., 1998; Lippincott and Li, 1998). Unconventional myosins are either two-headed or single-headed, and, in contrast with conventional myosins, do not seem to form filaments. MYO3 and MYO5 have been shown to play an important role in endocytosis and polarized assembly of cortical actin patches (Geli and Riezman, 1996; Goodson et al., 1996). Although deletion of either MYO3 or MYO5 does not result in an obvious growth phenotype, a double knockout is synthetically lethal or nearly so, suggesting functional redundancy between these genes (Geli and Riezman, 1996; Goodson et al., 1996). MYO2 is thought to be required for polarized growth and transport of certain secretory vesicles along actin cables from the mother to the bud (Johnston et al., 1991; Schott et al., 2002). MYO4/SHE1 controls the segregation of ASH1 mRNA as stated above.
In this article, we report that defects in endocytosis and actin cytoskeletal polarization in she4 mutant are caused by dysfunctions of Myo3/5p. The UCS domain of She4p physically interacts with the motor domain of Myo3/5p, and novel dominant point mutations in the motor region of MYO5 can bypass the requirement of She4p. She4p also interacts with Myo1p, Myo2p, and Myo4p in two-hybrid assays and is required for proper localization of Myo4p. Our results suggest that the UCS proteins play an important role for proper function of the unconventional, in addition to the conventional, myosins.
MATERIALS AND METHODS
Strains, Media, and Plasmids
The yeast strains used in this study are listed in Table 1. Isolation of temperature-sensitive myo5 tail mutants will be described elsewhere. The she4, myo3, myo5, bni1, and cla4 disruption; SHE4, MYO1, and MYO4 chromosomal green fluorescent protein (GFP) tagging; and MYO5 chromosomal TAP tagging were carried out using polymerase chain reaction (PCR)-based methods as described previously (Longtine et al., 1998; Rigaut et al., 1999). The sla2 disruption and MYO2 GFP-tagged strains were constructed on our strain background as follows. The regions containing the disruption or tagging marker and the flanking sequences were PCR amplified using either DDY546 (sla2Δ1::URA3) or YJC1431 (MYO2-GFP::HIS3) genomic DNA as a template. The resulting DNA fragment was then introduced into YEF473. Unless otherwise specified, strains were grown in YPDA-rich medium (1% yeast extract [Difco, Detroit, MI], 2% bacto-peptone [Difco], 2% glucose, and 0.01% adenine). Strains carrying plasmids were selected in synthetic medium (SD) containing the required nutritional supplements (Sherman, 1991). When indicated, 0.5% casamino acids (Difco) were added to SD medium without uracil (SDA-Ura). The plasmids used in this study are listed in Table 2. Schemes for the construction of plasmids and the sequences of PCR primers are available upon request. Escherichia coli strains DH5α and XL1-Blue were used for the construction and amplification of plasmids. Yeast transformations were performed using the lithium acetate method (Elble, 1992; Agatep et al., 1998).
Table 1.
The yeast strains used in this study
| Straina | Genotype | Origin/Reference |
|---|---|---|
| L40 | MATalys2-801 his3Δ-200 trp1-901 leu2-3,112 ade2 LYS2::(lexAop)4-HIS3 URA3::(lexAop)8-lacZ | Hollenberg et al., 1995 |
| TAT7b | MATalys2-801 his3Δ-200 trp1-901 leu2-3,112 ade2 LYS2::(lexAop)4-HIS3 ura3::(lexAop)8-lacZ | Imamura et al., 1997 |
| YKT550b | MATalys2-801 his3Δ-200 trp1-901 leu2-3,112 ade2 LYS2::(lexAop)4-HIS3 URA3::(lexAop)8-lacZ she4Δ::KanMX6 | This study |
| YEF473 | MATa/ α lys2-801/lys2-801ura3-52/ura3-52 his3Δ-200/his3Δ-200 trp1Δ-63/trp1Δ-63 leu2Δ-1/leu2Δ-1 | Longtine et al., 1998 |
| YKT159 | MATamyo3Δ::TRP1 MYO5-EGFP::KanMX6 | This study |
| YKT288 | MATamyo3Δ::TRP1 MYO5-EGFP::KanMX6 she4Δ::HIS3MX6 | This study |
| YKT114 | MATamyo3Δ::TRP1 myo5Δ::HIS3MX6 | This study |
| YKT274 | MATa/ α SHE4-EGFP::KanMX6/SHE4 | This study |
| YKT275 | MATashe4Δ::HIS3MX6 | This study |
| YKT276 | MATα she4Δ::HIS3MX6 | This study |
| DDY322 | MATα ura3-52 his3Δ-200 leu2-3,112 abp1Δ::LEU2 | A gift from Dr. Drubin |
| DDY1520 | MATaura3-52 his3Δ-200 trp1Δ-63 leu2Δ-1 aip1Δ::URA3 | A gift from Dr. Drubin |
| YMW211U | MATalys2-801 ura3-52 his3Δ-200 trp1Δ-63 leu2-1 arp2-1(H330L)::URA3 | Madania et al., 1999 |
| YMW221U | MATalys2-801 ura3-52 his3Δ-200 trp1Δ-63 leu2-1 arp2-2(G19D)::URA3 | Madania et al., 1999 |
| YKT382 | MATabni1Δ::HIS3MX6 | This study |
| YKT388 | MATacla4Δ::KanMX6 | This study |
| DDY1226 | MATα lys2-801 ura3-52 his3Δ-200 trp1Δ-63 leu2-3,112 cof1-22::LEU2 | A gift from Dr. Drubin |
| DDY1024 | MATalys2-801 ura3-52 his3Δ-200 leu2-3,112 ade2-101 ade3 pfy1-116::LEU2 | A gift from Dr. Drubin |
| DDY949 | MATaura3-52 his3Δ-200 trp1-1 leu2-3,112 rvs167Δ::TRP1 | A gift from Dr. Drubin |
| DDY318 | MATα lys2-801 ura3-52 his3Δ-200 trp1Δ-63 leu2Δ-1 sac6Δ::LEU2 | A gift from Dr. Drubin |
| YKT130 | MATavrp1Δ::LEU2 | Mochida et al., 2002 |
| YKT218 | MATasla1Δ::KanMX6 | Mochida et al., 2002 |
| DDY546 | MATα lys2-801 ura3-52 his3Δ-200 leu2-3,112 sla2Δ1::URA3 | A gift from Dr. Drubin |
| YKT186 | MATasla2Δ1::URA3 | This study |
| YKT91 | MATamyo3Δ::TRP1 myo5-1::KanMX6 | This study |
| YKT93 | MATamyo3Δ::TRP1 myo5-218::KanMX6 | This study |
| YKT111 | MATamyo3Δ::TRP1 myo5-360::KanMX6 | This study |
| YKT110 | MATamyo3Δ::TRP1 MYO5::KanMX6 | This study |
| YKT323 | MATaMYO5-TAP::TRP1 | This study |
| YKT640 | MATaMYO1-GFP::KanMX6 | This study |
| YKT641 | MATaMYO1-GFP::KanMX6 she4Δ::HIS3MX6 | This study |
| YJC1431 | MATα leu2 ura3 his3Δ-200 MYO2-GFP::HIS3 | A gift from Dr. Cooper |
| YKT520 | MATaMYO2-GFP::HIS3 | This study |
| YKT524 | MATaMYO2-GFP::HIS3 she4Δ::TRP1 | This study |
| YKT544 | MATaMYO4-GFP::HIS3MX6 | This study |
| YKT543 | MATaMYO4-GFP::HIS3MX6 she4Δ::TRP1 | This study |
YKT strains, except for YKT550, are in YEF473 background. For YKT strains, only relevant genotypes are described
These strains are derivatives of L40
Table 2.
The plasmids used in this study
| Plasmid | Characteristics/Reference |
|---|---|
| pBTM116 | DBDLexAa, TRP1, 2 μm (Bartel et al., 1993) |
| pKT1286 (pBTM116-SHE4) | DBDLexA-SHE4 (full length), TRP1, 2 μm |
| pKT1287 [pBTM116-SHE4 (1-356)] | DBDLexA-SHE4 (1-356 aa), TRP1, 2 μm |
| pBTM116-HA | DBDLexA-HA, TRP1, 2 μm (Imamura et al., 1997) |
| pKT1295 [pBTM116-HA-SHE4 (357-789)] | DBDLexA-HA-SHE4 (357-789 aa), TRP1, 2 μm |
| pGAD-C1, -C2, and -C3 | ADGAL4a, LEU2, 2 μm (James et al., 1996) |
| pKT1279 [pGAD-MYO3 (471-814)] | ADGAL4-MYO3 (471-814 aa), LEU2, 2 μm; isolated in this study from the yeast genomic library Y2HL-C1 provided by Dr. James |
| pKT1280 [pGAD-MYO5 (493-739)] | ADGAL4-MYO5 (493-739 aa), LEU2, 2 μm; isolated in this study from the yeast genomic library Y2HL-C3 provided by Dr. James |
| pKT1281 [pGAD-MYO3 (471-718)] | ADGAL4-MYO3 (471-718 aa), LEU2, 2 μm |
| pKT1282 [pGAD-MYO3 (471-671)] | ADGAL4-MYO3 (471-671 aa), LEU2, 2 μm |
| pKT1285 [pGAD-MYO3 (471-572)] | ADGAL4-MYO3 (471-572 aa), LEU2, 2 μm |
| pKT1284 [pGAD-MYO3 (555-671)] | ADGAL4-MYO3 (555-671 aa), LEU2, 2 μm |
| pKT1006 (pBTM116-HA-MYO3motor) | DBDLexA-HA-MYO3 motor (1-718 aa), TRP1, 2 μm |
| pACTII-HK | ADGAL4-HA, LEU2, 2 μm (Ozaki et al., 1996) |
| pACTII-HK-ACT1 | ADGAL4-HA-ACT1, LEU2, 2 μm (K. Ozaki-Kuroda and Y. Takai, unpublished) |
| pRS315 | LEU2, CEN6 (Sikorski and Hieter, 1989) |
| pKT1004 (pRS315-MYO5) | MYO5, LEU2, CEN6 |
| pKT1235 (pRS315-MYO5-GFP) | MYO5-GFP, LEU2, CEN6 |
| pKT1403 (pRS315-myo5-1-GFP) | myo5-1-GFP, LEU2, CEN6 |
| YEplac195 | URA3, 2 μm (Gietz and Sugino, 1988) |
| pKT1294 (YEplac195-SHE4) | SHE4, URA3, 2 μm |
| pKT1330 (YEplac195-MYO5) | MYO5, URA3, 2 μm |
| pKT1331 (YEplac195-MYO5Δmotor) | MYO5Δmotor, URA3, 2 μm |
| pKT1332 [YEplac195-MYO5 (V1641)] | MYO5 (with V1641 substitution), URA3, 2 μm |
| pKT1333 [YEplac195-MYO5 (N1681)] | MYO5 (with N1681 substitution), URA3, 2 μm |
| pKT1334 [YEplac195-MYO5 (N209S)] | MYO5 (with N209S substitution), URA3, 2 μm |
| pKT1335 [YEplac195-MYO5 (K377M)] | MYO5 (with K377M substitution), URA3, 2 μm |
| pRS316 | URA3, CEN6 (Sikorski and Hieter, 1989) |
| pKT1290 (pRS316-SHE4) | SHE4, URA3, CEN6 |
| pGEX-4T-1 | GST (Amersham Biosciences) |
| pKT1308 (pGEX-4T-1-SHE4) | GST-SHE4 |
| pKT1386 [pGAD-MYO1 (549-695)] | ADGAL4-MYO1 (549-695 aa), LEU2, 2 μm |
| pKT1387 [pGAD-MYO1 (549-648)] | ADGAL4-MYO1 (549-648 aa), LEU2, 2 μm |
| pKT1388 [pGAD-MYO2 (530-683)] | ADGAL4-MYO2 (530-683 aa), LEU2, 2 μm |
| pKT1389 [pGAD-MYO2 (530-636)] | ADGAL4-MYO2 (530-636 aa), LEU2, 2 μm |
| pKT1390 [pGAD-MYO4 (531-678)] | ADGAL4-MYO4 (531-678 aa), LEU2, 2 μm |
| pKT1391 [pGAD-MYO4 (531-631)] | ADGAL4-MYO4 (531-631 aa), LEU2, 2 μm |
DBDLexA is the DNA-binding domain of LexA. ADGAL4 is the transcriptional activating domain of Gal4p
Two-Hybrid Screening
Two-hybrid screening was performed as described previously (Bartel et al., 1993). L40 cells, carrying pKT1286, was independently transformed with three yeast genomic DNA libraries (Y2HL-C1, -C2, and -C3) (James et al., 1996), incubated in SD-Trp-Leu medium overnight, and then plated on SD-Trp-Leu-His plates supplemented with 1 mM 3-aminotriazole. Approximately 1.2 × 105 transformants were screened for each library. After incubation for several days at 30°C, 120, 1, and 120 colonies were picked up from the Y2HL-C1, -C2, and -C3 transformants, respectively. From these clones, 13 plasmids were isolated and reintroduced into L40 cells carrying pKT1286 to retest for growth on SD-Trp-Leu-His + 1 mM 3-aminotriazole plates. All 13 clones showed positive interactions and were then sequenced. Type I myosins, MYO3 (six clones) and MYO5 (six clones), and a transcriptional factor, ABF1 (1 clone), were obtained. Because most of clones analyzed encoded type I myosins, other clones were not analyzed further. Quantification of β-galactosidase activity was performed using o-nitrophenyl β-d-galactopyranoside as a substrate (Guarente, 1983). β-Galactosidase activity is expressed in Miller units (Miller, 1972).
Microscopic Observations
Microscopic observations were performed as described previously (Mochida et al., 2002) with some modifications. Briefly, to visualize GFP and the actin cytoskeleton simultaneously, exponentially growing cells were fixed with 3.7% formaldehyde (Wako Pure Chemicals, Osaka, Japan). Fixed cells were stained with 1 μM tetramethylrhodamine isothiocyanate (TRITC)-phalloidin (Sigma-Aldrich, St. Louis, MO). To visualize GFP-tagged proteins, cells were grown to early logarithmic phase, harvested, and resuspended in SD medium, and then cells were observed. To quantify actin cytoskeletal polarity, the number of actin patches in the mother cell of randomly selected small- to medium-budded cells was counted by focusing up and down through the mother cell. Small to medium buds were identified to be no >60% the size of the mother cell. Cells were scored as “polarized” when there were no more than three patches in the mother cell. Observations were performed on an ECLIPSE E800 microscope with either the appropriate fluorescence filter sets or differential interference contrast optics (Nikon Instec, Tokyo, Japan). Observations are based on at least 100 cells viewed. The images presented in this article were acquired using a cooled charge-coupled device camera (C4742-95-12NR; Hamamatsu Photonics, Hamamatsu, Japan) and AQUACOSMOS software (Hamamatsu Photonics).
Isolation of MYO5 Motor Mutants
To isolate mutations in MYO5 that could bypass the temperature-sensitive growth defect of she4Δ cells, we randomly mutagenized the motor region of MYO5 by a PCR-based method (Cadwell and Joyce, 1992). The mutagenic PCR conditions were as follows: 1 μl of plasmid pKT1004 (0.1 μg/μl) as a template, 1 μl of the forward (5′-TTTTGCCAATGGTGGCAGG-3′; 533 base pairs upstream of the start codon) and reverse (5′-TGTTCTGCATTCCTGGTG-3′; 3044 base pairs downstream of the start codon) primers (100 μM each), 10 μl of PCR buffer (10× stock, Mg2+ free), 10 μl of MgCl2 (10× stock, 25 mM), 10 μl of mutagenic dNTPs mixture (10× stock: 2 mM dATP, 2 mM dGTP, 10 mM dCTP, and 10 mM dTTP), 1 μl of MnCl2 (50 mM), 1 μl (5 U/μl) of Taq (Sigma-Aldrich), and 65 μl of double distilled H2O. PCR was carried out as follows: 94°C, 4 min → 94°C, 1 min; 45°C, 1 min; 72°C, 4 min for 30 cycles → 72°C, 10 min → 4°C. The ∼3.6-kbp PCR-amplified fragment was cleaned by ethanol precipitation. The mutagenized PCR products were mixed with an equal amount of pKT1331 plasmid, which was linearized by restriction enzyme digestion. This mixture of DNA was introduced into strain YKT275 to allow homologous recombination between the PCR products and the linearized plasmid, and the transformants were plated on YPDA. After incubation at 37°C for 2 d, the resulting colonies were recovered on SDA-Ura plates at 25°C and then rechecked for growth on YPDA plate at 37°C. From 3.6 × 105 initial transformants, 122 mutants were isolated. Of these, 10 plasmids were rescued and reintroduced into YKT275 to confirm the suppressor phenotype. These plasmids were sequenced. Each plasmid contained more than two mutations. The mutations responsible for suppressor activity were determined by site-directed mutagenesis of pKT1330 by using a QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA).
Fluid-Phase Endocytosis
Lucifer yellow-carbohydrazide (LY; Sigma-Aldrich) accumulation analysis was performed as described previously (Dulic et al., 1991). Lucifer yellow uptake was carried out for 30 min at 25°C. Samples were observed by fluorescence microscopy as described above.
In Vitro Binding Assay of Myo5p-TAP with GST-She4p
Recombinant She4p was expressed as a GST-fusion protein in E. coli DH5α and purified with glutathione Sepharose beads (Amersham Biosciences, Piscataway, NJ), according to the manufacturer's instructions. Preparation of Myo5p-TAP was carried out as described previously (Rigaut et al., 1999) with some modifications. Briefly, YKT323 cells were grown at 25°C in 1000 ml of YPDA medium to OD600 = 2.0 and lysed by two passages in a French pressure cell at 1000 psi. After dialysis, the extracts were applied to IgG Sepharose 6 Fast Flow (Amersham Biosciences). After washing with IPP150 (10 mM Tris-Cl pH 8.0, 150 mM NaCl, 0.1% Nonidet P-40), the immobilized Myo5p-TAP was used in an in vitro binding assay. According to the densitometry of SYPRO orange (Molecular Probes, Eugene, OR)-stained bands on SDS-PAGE gel by using LAS-1000 luminescent image analyzer (Fuji Photo Film, Tokyo, Japan), ∼1.5 μg of Myo5p-TAP was bound to 10 μl of IgG Sepharose. SYPRO orange staining was carried out according to manufacturer's instructions. Binary mixtures were prepared by adding GST-She4p (final 0.25 μM) to 10 μl of the Myo5p-TAP-bound IgG Sepharose, which was suspended in 100 μl of IPP150. GST-She4p was added to unbound IgG Sepharose as a negative control. Mixtures were incubated for 30 min at 4 or 30°C and then on ice for 10 min, before being washed intensively with IPP150. Protein complexes were subjected to SDS-PAGE, followed by staining with SYPRO orange. The amount of GST-She4p bound to Myo5p-TAP was quantified by densitometry as described above.
RESULTS
Type I Myosins, Myo3p/Myo5p Interact with She4p
To identify proteins that bind She4p and that thereby might be involved in endocytosis, regulation of the actin cytoskeleton, or development of cell polarity, we performed a two-hybrid screen by using full-length She4p as bait. Multiple clones that encode the type I myosins, Myo3p/Myo5p, were identified. One of these encoded Myo5p amino acids 493–739, and another encoded Myo3p amino acids 471–814 (Figure 1A). Interestingly, these She4p-interacting regions overlapped with the respective motor domains. Because Myo3/5p are involved in the uptake step of endocytosis and polarization of the actin cytoskeleton (Geli and Riezman, 1996; Goodson et al., 1996), we focused further analysis on the interaction between She4p and Myo3/5p.
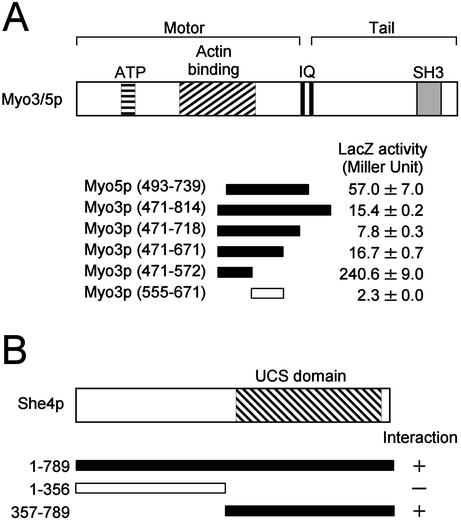
Figure 1.
Two-hybrid interactions between She4p and Myo3/5p. DNA fragments encoding various regions of She4p and Myo3/5p were cloned into pBTM (LexA DNA-binding domain plasmids) and pGAD (GAL4 transcriptional activating domain plasmids) vectors, respectively. The resultant plasmids were introduced into L40 cells. The interactions between She4p and Myo3/5p were examined qualitatively by histidine auxotrophy and quantitatively by β-galactosidase activity assay. The numbers in parentheses represent amino acid positions. Closed bars and open bars stand for His+ and His-, respectively. β-galactosidase (LacZ) activity represents the average and SD for two independent transformants. (A) Interactions between She4p (full length) and various regions of Myo3/5p. ATP, putative ATP-binding domain; IQ, IQ motifs; SH3, Src homology 3 domain. (B) Interactions between various regions of She4p and Myo3p (471–572).
We sought to determine the region of Myo3p required for interaction with She4p. Testing various truncated fragments of Myo3p for the interaction with full-length She4p, we found that a segment consisting of amino acids 471–572 of Myo3p, which is included in the motor domain, was sufficient for this interaction (Figure 1A). Chicken skeletal muscle myosin (type II) has been well studied and its three-dimensional structure has been resolved (Rayment et al., 1993a). Recently, the three-dimensional structure of Dictyostelium discoideum myosin-IE revealed that the core structural elements and topology of the type I myosin motor are essentially identical to those of the type II (Kollmar et al., 2002). When the amino acid sequence of the Myo3p motor domain is aligned with that of chicken skeletal muscle myosin, the She4p-interacting segment of Myo3p corresponds to amino acids 527–631 of chicken skeletal muscle myosin (Cope and Hodge, 2000), a segment that contains three of the four putative actin contact surfaces (Sellers, 1999; see Figure 8A). Two of these (residues 529–558 and 567–578 of chicken skeletal muscle myosin) are located within the lower 50-kDa subdomain and the other (residues 626–647 of chicken skeletal muscle myosin) is referred to loop2 (Sellers, 1999). Thus, the She4p-interacting segment is in the motor domain and overlaps with putative Myo3p actin-binding regions.
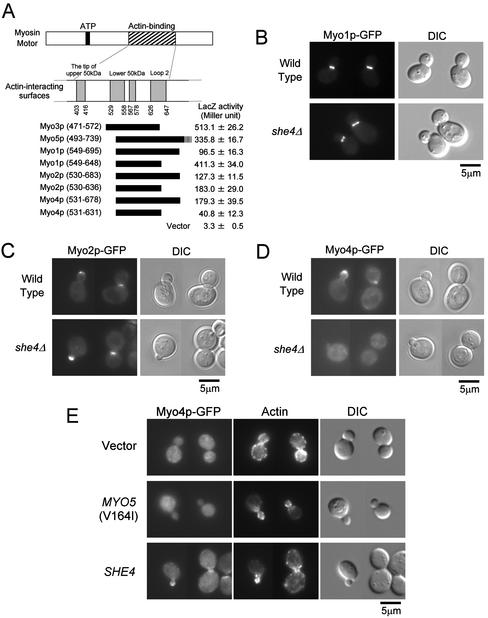
Figure 8.
She4p interacts with type II and type V myosins and is required for proper localization of Myo4p. (A) Two-hybrid interactions between She4p and myosins. DNA fragments encoding Myo1p, Myo2p, and Myo4p were cloned into pGAD vectors. The resultant plasmids were introduced into L40 cells carrying pKT1295. The interaction between the UCS domain of She4p and myosins were examined quantitatively by β-galactosidase activity assay. The myosin motor regions used in the experiment were represented schematically. The numbers below actin-binding surfaces and in parentheses represent amino acid positions in chicken skeletal muscle myosin and each yeast myosin, respectively. β-Galactosidase (LacZ) activity represents the average and SD for three independent transformants. (B) Localization of Myo1p-GFP in she4Δ cells. Myo1p-GFP–expressing cells of YKT640 (wild-type) and YKT641 (she4Δ) were exponentially grown at 25°C. Myo1p-GFP was visualized using a GFP band pass filter (Myo1p-GFP). The same exposure and processing parameters were used for comparison. Bar, 5 μm. (C) Localization of Myo2p-GFP in she4Δ cells. YKT520 (wild-type) and YKT524 (she4Δ) were observed as described in B. Bar, 5 μm. (D) Localization of Myo4p-GFP in she4Δ cells. YKT544 (wild-type) and YKT543 (she4Δ) were observed as described in B. Bar, 5 μm. (E) Delocalization of Myo4p-GFP in she4Δ mutant is not due to depolarization of the actin cytoskeleton. Myo4p-GFP–expressing cells, YKT543, were transformed with YEplac195 (vector), pKT1332 [MYO5 (V164I)], or pKT1290 (SHE4). Transformants were exponentially grown in SDA-Ura at 25°C, fixed, and stained with TRITC-phalloidin. Localization of Myo4p-GFP and F-actin was visualized with a GFP band pass filter (Myo4p-GFP) and a TRITC filter (actin), respectively. For each observation, the same exposure and processing parameters were used. Bar, 5 μm.
The myosin-binding segment of She4p was also determined by the yeast two-hybrid system. Truncated fragments of She4p were fused to the LexA DNA-binding domain and were examined for interaction with Myo3p. She4p was found to bind Myo3/5p through its conserved UCS domain (Figure 1B).
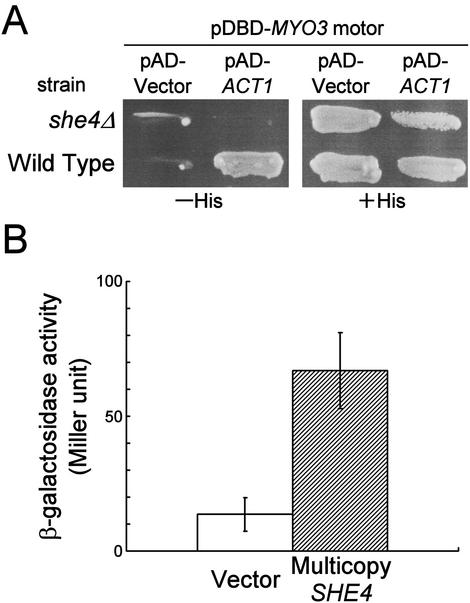
She4p Has Positive Effects on the Interaction between the Myo3p Motor and Act1p
Because She4p-interacting segment of Myo3/5p motor domain overlaps with its actin-binding region, we examined whether She4p affects the myosin motor-actin interaction. The two-hybrid system potentially detects the interaction with F-actin: some F-actin–binding proteins such as Sac6p (fimbrin) give positive interaction with Act1p (yeast actin) in the two-hybrid system (Amberg, unpublished data). We could detect the two-hybrid interaction between the Myo3p motor domain and Act1p, and observed a strong inhibition of reporter transcription in the she4Δ strain (Figure 2A). This effect was specific for the Myo3p motor, because the interaction between Act1p and other actin-binding proteins such as Pfy1p (profilin) and Bni1p/She5p (formin) could be detected in the she4Δ reporter strain (our unpublished data). On the other hand, overexpression of SHE4 in the wild-type reporter strain enhanced reporter transcription (Figure 2B).
Figure 2.
Effects of SHE4 disruption or overexpression on the two-hybrid interactions between Myo3p motor and Act1p. (A) L40 (wild-type) and YKT550 (she4Δ) cells were transformed with pKT1006 (pDBD-MYO3 motor) and either pACTII-HK-ACT1 (pAD-ACT1) or pACTII-HK (pAD-Vector), and the interaction between the motor domain of Myo3p and Act1p was examined qualitatively by histidine auxotrophy. (B) TAT7 cells containing pKT1006 were transformed simultaneously with pACTII-HK-ACT1 and either pKT1294 (multicopy SHE4) or YEplac195 (vector). The interaction between the motor domain of Myo3p and Act1p was examined quantitatively by β-galactosidase activity assay. Bar graph shows the average and SD for five independent transformants.
She4p Is Required for Proper Localization of Myo5p
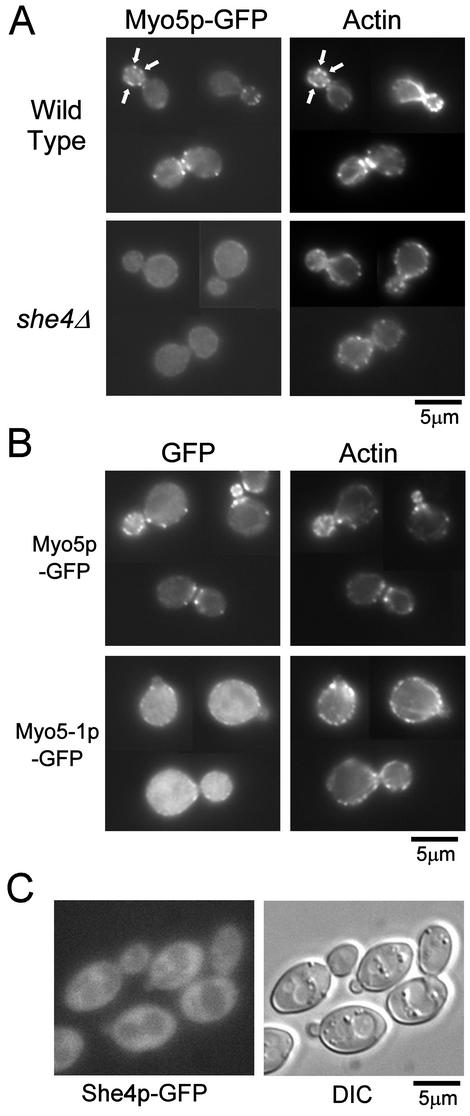
Myo3/5p shows highly polarized localization to patch-like structures enriched in the bud and the cell division site, and these patches partially colocalize with actin cortical patches (Goodson et al., 1996; Anderson et al., 1998). Given the possible role of She4p in proper Myo3/5p function, we questioned whether She4p was required for localization of Myo3/5p to the actin patches. To address this issue, we analyzed the localization pattern of GFP-tagged Myo5p (Myo5p-GFP), expressed under the control of its own promoter in she4Δ cells. Disruption of SHE4 was not associated with significant changes in expression level of Myo5p-GFP as determined by immunoblot analysis by using an anti-GFP antibody (our unpublished data). In wild-type cells, Myo5p-GFP was localized to patch-like structure predominantly existing in the bud and cell division site, and these Myo5p-patches colocalized with cortical actin patches as reported previously (Goodson et al., 1996; Anderson et al., 1998; Figure 3A). In >99% of she4Δ cells, the cortical actin patches lost their polarity (Wendland et al., 1996; Figures 3A and 6C), and Myo5p-GFP diffused throughout the cytosol (Figure 3A). These results indicate that the assembly and/or maintenance of Myo5p in patch-like structures require She4p. Because She4p interacts with the motor domain of Myo3/5p, we examined whether the motor domain is required for proper localization of Myo5p. The temperature-sensitive myo5-1 mutant has the E472K amino acid substitution, which mimics the E511K substitution of myo2-66 (Geli and Riezman, 1996). These glutamic acid residues are located in the putative actin-interacting region within the motor domain and, consistent with this, Myo2–66p shows a decreased affinity to actin filaments even in the absence of ATP (Reck-Peterson et al., 2001). Because the She4p-interacting segment of Myo3/5p (amino acids 471–572 in Myo3p) is near this glutamic acid residue, we determined the localization of GFP-tagged Myo5-1p at elevated temperature. In >90% of cells, mainly cytosolic staining and minor cortical punctate staining was observed, and cortical actin patches lost their polarity (Figure 3B). This localization pattern of Myo5-1p-GFP was very similar to that of Myo5p-GFP in she4Δ cells. Interestingly, similar results have been reported in Candida albicans: amino acid substitution at a putative phosphorylation site by a PAK-like kinase in the motor domain of type I myosin causes its mainly cytosolic localization and depolarization of cortical actin patches (Oberholzer et al., 2002). Our results suggest that mislocalization of Myo5p and concomitant depolarization of cortical actin patches in she4Δ cells are caused by dysfunction of the motor domain of Myo3/5p.
Figure 3.
She4p is required for proper localization of Myo5p-GFP and F-actin. (A) Localization of Myo5p-GFP in she4Δ cells. Myo5p-GFP expressing cells, YKT159 (wild-type) and YKT288 (she4Δ) were grown at 25°C, fixed and stained with TRITC-phalloidin. Localization of Myo5p and F-actin was visualized with a GFP band pass filter (Myo5p-GFP) and a TRITC filter (actin). For each observation, the same exposure and processing parameters were used. Arrows indicate examples of colocalization of Myo5p-GFP and actin patches. (B) Localization of Myo5-1p-GFP. YKT114 cells were transformed with plasmids, pKT1235 (Myo5p-GFP) or pKT1403 (Myo5-1p-GFP). Cells were grown in SD-Leu liquid medium at 25°C, incubated at 37°C for 1 h, fixed, stained, and observed as described in A. (C) Localization of She4p-GFP. She4p-GFP–expressing cells, YKT274, were grown at 25°C, and She4p-GFP was visualized using a GFP band pass filter (She4p-GFP) or differential interference contrast optics (DIC). Bars, 5 μm.
Figure 6.
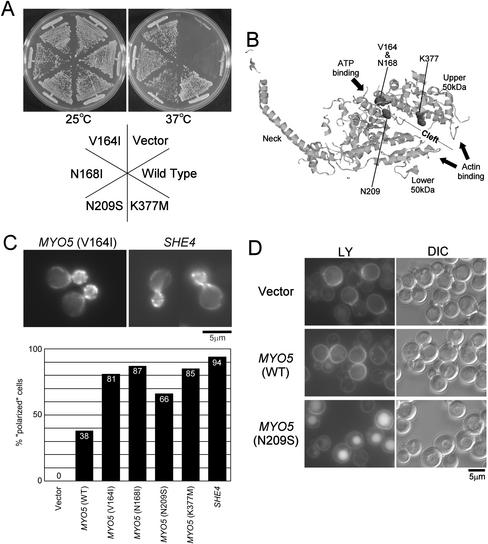
Novel dominant point mutations in the MYO5 motor region can bypass the requirement of She4p. (A) Effects on the temperature-sensitive growth defect of she4Δ cells. Plasmids pKT1332 (V164I), pKT1333 (N168I), pKT1334 (N209S), pKT1335 (K377M), pKT1330 (wild-type), and YEplac195 (vector) were introduced into YKT275 cells. Transformants were selected on SDA-Ura plates and streaked on YPDA plates. The plates were incubated for 2 d at 25°Cor37°C. (B) Three-dimensional structure of chicken skeletal muscle myosin S1 (the Protein Data Bank code, 2MYS). Residues corresponding to V164, N168, N209, and K377 of the yeast Myo5p (I222, N226, D264, and K431 of chicken skeletal muscle myosin S1, respectively; Cope and Hodge, 2000) are represented in space-filling format. These residues are located inside the cleft, which extends from the ATP-binding pocket to the actin-binding site and separate the upper and the lower 50-kDa subdomains. This picture was generated using RasMol (Sayle and Milner-White, 1995). (C) Effects on polarized localization of cortical actin patches in she4Δ cells. Plasmids YEplac195 (vector), pKT1330 [MYO5 (WT)], pKT1332 (V164I), pKT1333 (N168I), pKT1334 (N209S), pKT1335 (K377M), and pKT1290 (SHE4) were introduced into YKT275 cells. Transformants were grown in SDA-Ura liquid media at 25°C, fixed, and stained with TRITC-phalloidin. Top panels show localization of F-actin of she4Δ cells with plasmid pKT1332 [MYO5 (V164I)] or pKT1290 (SHE4). Bar, 5 μm. The bar graph shows the percentage of polarized cell (no more than three patches in the mother cell; see MATERIALS AND METHODS). (D) Effects on fluid-phase endocytosis defect of she4Δ cells. pKT1334 (N209S), pKT1330 (WT), and YEplac195 (vector) were introduced into YKT275. Cells grown in SDA-Ura at 25°C were incubated with lucifer yellow at 25°C for an additional 30 min. Accumulation of lucifer yellow in the vacuole was visualized using an fluorescein isothiocyanate band pass filter (LY). The same exposure and processing parameters were used for comparison. Bar, 5 μm.
We next examined the localization of chromosomally GFP-tagged She4p. This She4p-GFP is functional, because the SHE4-GFP strain demonstrated normal polarized localization of cortical actin patches and wild-type growth rate at 37°C (our unpublished data). In contrast to highly polarized localization of Myo5p-GFP, She4p-GFP showed diffuse cytosolic localization (Figure 3C). Similar result has been reported using indirect immunofluorescence study for Myc-tagged She4p (Takizawa and Vale, 2000). Thus, She4p does not seem to colocalize with Myo3/5p, suggesting that She4p and Myo3/5p do not form a stable complex. Rng3p, a UCS protein in S. pombe, also shows cytosolic localization (Wong et al., 2000).
Genetic Interactions between SHE4 and Actin-related Genes and MYO5
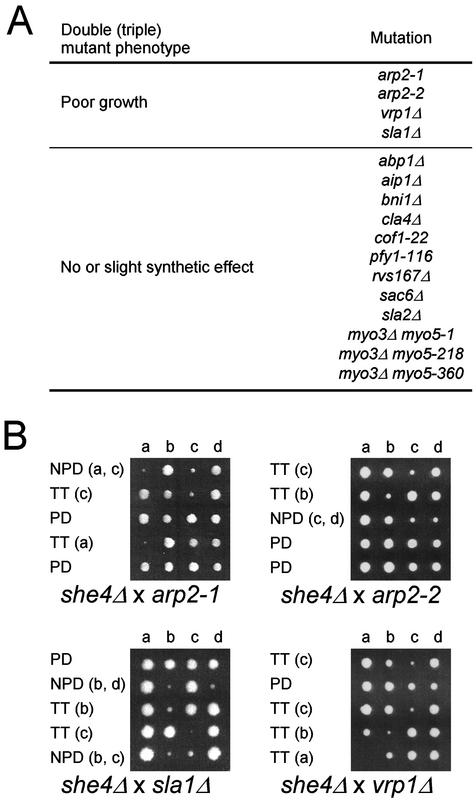
We examined the genetic interaction of the she4Δ mutation with mutations of genes involved in the regulation of the actin cytoskeleton, including abp1Δ, aip1Δ, arp2-1, arp2-2, bni1Δ, cla4Δ, cof1-22, pfy1-116, rvs167Δ, sac6Δ, vrp1Δ, sla1Δ, and sla2Δ. The she4Δ mutant was crossed with each mutant, and the resulting diploid was sporulated and dissected for tetrad analysis. The growth characteristics of the resulting double mutants were determined at 25°C. The vrp1Δ, arp2-1, arp2-2, and sla1Δ mutations exhibited a poor growth phenotype at 25°C when combined with the she4Δ mutation, whereas the other double mutants showed little or no reduced growth at 25°C (Figure 4). Vrp1p and the Arp2/3 complex physically interact with the Myo3/5p tail and are involved in activation of actin polymerization (Evangelista et al., 2000; Geli et al., 2000; Lechler et al., 2000). Consistently, it has been reported that both arp2-33 and arc40-40, mutations in genes encoding subunits of the Arp2/3 actin-nucleating complex, exhibited synthetic lethal interactions with she4Δ mutation in systematic genetic analysis (Tong et al., 2001). It is likely that impaired Myo3/5p motor function due to lack of She4p exacerbates the growth defects caused by impaired Myo3/5p-tail–dependent actin polymerization.
Figure 4.
Genetic interactions between SHE4 and actin-related genes and MYO5. (A) Summary of genetic interactions. Each mutant strain was crossed with she4Δ (YKT275 or YKT276), and resultant diploid cells were sporulated and dissected for tetrad analysis. The growth characteristics of the resulting double or triple mutants were determined at 25°C. The mutant strains used were YMW211U (arp2-1), YMW221U (arp2-2), YKT130 (vrp1Δ), YKT218 (sla1Δ), DDY322 (abp1Δ), DDY1520 (aip1Δ), YKT382 (bni1Δ), YKT388 (cla4Δ), DDY1226 (cof1-22), DDY1024 (pfy1-116), DDY949 (rvs167Δ), DDY318 (sac6Δ), YKT186 (sla2Δ), YKT91 (myo3Δ myo5-1), YKT93 (myo3Δ myo5-218), and YKT111 (myo3Δ myo5-360). (B) Genetic interactions of arp2-1, arp2-2, sla1Δ, and vrp1Δ with she4Δ mutation. Diploid cells obtained from an indicated cross were sporulated, dissected, and grown at 25°C for 3–4 d before being photographed. Colonies were then replica plated to determine the segregation of the marked mutant alleles. Tetrad genotype (TT, tetratype; PD, parental ditype; and NPD; nonparental ditype) is indicated, and the identity of the double mutant spore(s) is shown in parentheses.
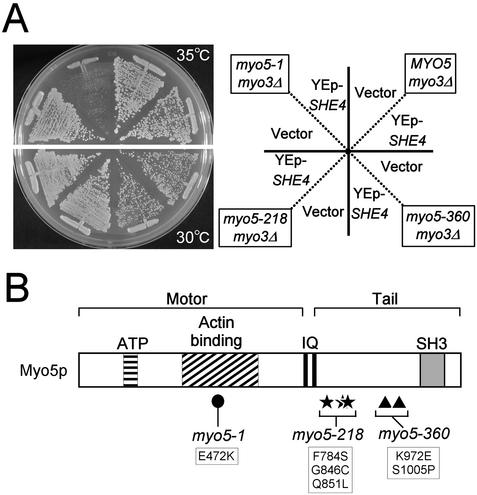
Because the myo5-1 mutation possesses its amino acid substitution (E472K) close to the She4p-interacting segment of the Myo3/5p (amino acids 471–572 in Myo3p), we checked for genetic interaction between she4Δ and myo5-1. However, the she4Δ myo3Δ myo5-1 triple mutant strain was viable and showed no obvious synthetic growth defect compared with a strain containing she4Δ single or myo3Δ myo5-1 double mutation (Figure 4A). Interestingly, overexpression of SHE4 inhibited the growth of myo5-1 cells at 35°C, a permissive temperature for the myo5-1 mutant (Figure 5). The SHE4 overexpression did not inhibit the growth of temperature-sensitive myo5-218 and myo5-360 cells with mutations in the tail domain (Figure 5), consistent with the result that She4p binds to Myo3/5p through its motor domain.
Figure 5.
SHE4 overexpression exacerbates the temperature-sensitive growth defect of the myo5 motor mutant. (A) YKT91 (myo5-1), YKT93 (myo5-218), YKT111 (myo5-360), and YKT110 (MYO5) cells were transformed with pKT1294 (YEp-SHE4) or YEplac195 (vector). Transformants were streaked on SDA-Ura plates and incubated for 3 d at 30°C (myo5-218 and myo5-360) or 35°C (myo5-1 and MYO5). (B) The domain structure of Myo5p and the amino acid substitution(s) of myo5-1 (closed circle), myo5-218 (stars), and myo5-360 (triangles) are depicted. ATP, putative ATP-binding domain; IQ, IQ motifs; SH3, Src homology 3 domain.
Novel Point Mutations in MYO5 Motor Bypass the Requirement of She4p
To further substantiate the role of She4p on Myo3/5p function, we attempted to isolate suppressor MYO5 mutants that bypass the requirement of She4p. We performed random mutagenesis on the motor region of MYO5 and screened for mutants that could suppress the temperature-sensitive growth defect of she4Δ cells. To enhance the suppression capability, the mutant Myo5ps were expressed from a multicopy plasmid. We identified four amino acid substitutions, V164I, N168I, N209S, and K377M, that could suppress temperature-sensitive growth of she4Δ cells (Figure 6A), and surprisingly each of these mutants were able to do so even when carried on low copy vectors (our unpublished data). Because the four mutant plasmids could support the growth of myo3Δ myo5Δ double mutant cells to an extent similar to that of wild-type at both 25 and 37°C (our unpublished data), these mutations are not likely to have a severe impact on the structure or function of the Myo5p motor. Interestingly, mapping of the locations of these amino acid substitutions on the three-dimensional structure of chicken skeletal muscle myosin (Rayment et al., 1993a) revealed that all substitutions locate inside the cleft separating the upper and the lower 50-kDa subdomains (Figure 6B). This cleft is proposed to open upon ATP-binding and close after hydrolysis, and the open and closed states are coupled with the weak and strong actin-binding states, respectively (Rayment et al., 1993b). These results suggest that a modulation of the actin-binding activities of Myo3/5p may be able to bypass the requirement of She4p for growth at elevated temperatures.
Because type I myosins are involved in polarization of the actin cytoskeleton and endocytosis (Geli and Riezman, 1996; Goodson et al., 1996), we examined effects of these mutated Myo5ps on actin cytoskeleton and endocytosis in the she4Δ mutant. To evaluate effects on distribution of the actin cytoskeleton, we counted the number of actin patches in the mother of small- to medium-budded cells. Whereas she4Δ cells with empty plasmid had completely depolarized actin patches (more than three patches in the mother cell; see MATERIALS AND METHODS), she4Δ cells with MYO5(V164I), MYO5(N168I), and MYO5(K377M) showed well-polarized distribution of actin patches at a similar level to cells with wild-type SHE4 (Figure 6C), indicating that mutations in the MYO5 motor region can suppress depolarization of actin cytoskeleton of she4Δ cells. Fluid-phase endocytosis was assessed by uptake of LY. she4Δ cells were defective in LY uptake at 25°C (Figure 6D), but all of the MYO5 mutants restored accumulation of LY in vacuoles (Figure 6D; our unpublished data). These results strongly suggest that defects of she4Δ cells in growth at elevated temperatures, actin polarization, and endocytosis are due to dysfunctions of type I myosins.
She4p Interacts with Myo5p in a Temperature-dependent Manner
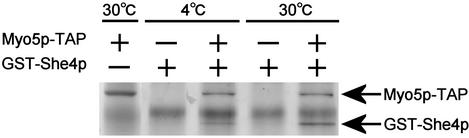
Our results strongly suggest that She4p plays an important role in the proper functioning of type I myosins through its interaction with their motor domains. It was reported that C. elegans UNC-45, which functions as a molecular chaperone for the muscle (type II) myosin motor, interacts with the myosin motor in a temperature-dependent manner (Barral et al., 2002). Elevated temperature is required in vitro for the well-studied chaperone, Hsp90 to form complexes with its substrates (Scherrer et al., 1990). Thus, we examined whether She4p interacts with Myo5p in a temperature-dependent manner. The interaction between TAP-tagged Myo5p (Myo5p-TAP), which was bound to IgG beads, and bacterially expressed full-length She4p fused to glutathione S-transferase (GST) was examined at 4 or 30°C. Although only a small amount of She4p was collected with Myo5p-TAP at 4°C, the yield was significantly increased at 30°C (Figure 7). The amount of GST-She4p bound to Myo5p-TAP at 30°C was 3.3 times greater than that at 4°C. These results are similar to those observed in the UNC-45-myosin binding experiment.
Figure 7.
She4p interacts with Myo5p in a temperature-dependent manner. Myo5p-TAP from YKT323 lysate was adsorbed to IgG Sepharose. GST-She4p was purified from bacterial lysate. IgG Sepharose with or without Myo5p-TAP was incubated with GST-She4p at 4 or 30°C. The IgG Sepharose was collected, and bound proteins were analyzed by SDS-PAGE and SYPRO orange staining. The result shown is a representative of four experiments.
She4p Also Interacts with Type II and Type V Myosins and Is Required for Proper Localization of Myo4p
Because the motor domains of the myosin family are highly conserved, it is possible that She4p interacts with and functions for other types of myosins. First, to test their interactions, we performed the two-hybrid assay between C-terminal half (UCS domain) of She4p and segments of Myo1p, Myo2p, and Myo4p, which are approximately equivalent to She4p-interacting segment of Myo3/5p. It was revealed that She4p also interacts with the other yeast myosins, Myo1p, Myo2p, and Myo4p (Figure 8A). Next, we examined whether She4p is required for localization of Myo1p, Myo2p, and Myo4p. Myo1p-GFP localized normally in she4Δ cells at the bud neck in >90% of budding cells (Bi et al., 1998; Lippincott and Li, 1998; Figure 8B). Myo2p-GFP normally localized in she4Δ cells at the bud tip in >90% of budding cells and at the bud neck in large-budded cells as in wild-type cells (Brockerhoff et al., 1994; Lillie and Brown, 1994; Karpova et al., 2000; Figure 8C). In contrast, Myo4p-GFP in >99% of she4Δ cells diffused uniformly throughout the cytosol (Figure 8D). In wild-type cells, Myo4p-GFP localized at the bud tip in >90% of budding cells and at the bud neck in large-budded cells as reported previously (Jansen et al., 1996; Munchow et al., 1999; Takizawa and Vale, 2000; Figure 8D). These results indicate that She4p also shows two-hybrid interactions with type V myosins and is required for the proper localization of Myo4p. The requirement of She4p for Myo4p localization is consistent with the hypothesis that “she” phenotype (Ash1p in the mother and daughter nuclei) of she4 mutant is due to dysfunction of Myo4p. However, another explanation is that delocalization of Myo4p results from depolarization of the actin cytoskeleton. To test this possibility, we analyzed localization of Myo4p-GFP in she4Δ cells, where a MYO5 suppressor mutant restored the actin cytoskeletal polarity. she4Δ cells (>99%) still showed delocalization of Myo4p-GFP, even when their actin cytoskeletal polarity was restored by a MYO5 suppressor mutant (Figure 8E). Thus, delocalization of Myo4p does not seem to be secondary effects of the depolarized actin cytoskeleton.
DISCUSSION
In this article, we show that the UCS domain of She4p physically interacts with the motor domain of type I myosins Myo3/5p. The isolation of MYO5 suppressor mutations that bypass the growth, endocytic, and actin cytoskeletal defects of the she4Δ mutant firmly established that She4p functions through the interactions with Myo3/5p.
The She4p-interacting segment of Myo3/5p overlaps with the putative actin-interacting segment, and the two-hybrid data suggest that She4p plays an important role in the motor-actin interaction. Consistent with this, colocalization of Myo5p and F-actin is disrupted in she4Δ cells. Moreover, dominant suppressor mutations in the MYO5 motor are located in a region that may affect the actin-binding activity of Myo3/5p. These results suggest that one possible function of She4p is to regulate the interaction between the Myo3/5p motor and F-actin. We attempted to examine the biochemical properties of Myo5p in she4Δ cells, but we were not able to perform an actin-pelleting assay for Myo5p using she4Δ cell lysate, because a significant amount of Myo5p was pelleted independently of F-actin (our unpublished data).
Because the interaction between Myo5p-TAP and GST-She4p is temperature dependent like that between myosin and UNC-45, which has an activity of molecular chaperone for myosin motor (Barral et al., 2002), She4p may also play a role as a molecular chaperone for Myo3/5p. Molecular chaperones promote productive protein folding by preventing off-pathway folding reactions, which lead to protein aggregation (Johnson and Craig, 1997). Actin-independent pelleting of Myo5p-TAP (stated above) could be interpreted as aggregation due to a loss of chaperone activity in the she4Δ mutant. Because the localization pattern of She4p is mainly cytosolic, She4p is likely to associate transiently with Myo3/5p or Myo4p, for example, immediately after myosin synthesis or after denaturation caused by stress. Because GST-She4p forms a complex with Myo5p-TAP in vitro, there might be a factor such as a cochaperone, which promotes efficient release of Myo5p form She4p. The idea that She4p functions as a molecular chaperone does not contradict our findings. The mutant Myo5ps, which bypass the requirement of She4p, may have the ability to fold by themselves, or may be more stable at elevated temperatures than wild-type Myo5p. The toxicity of SHE4 overexpression in myo5-1 strains could be explained if She4p interferes with actin–myosin motor interaction by binding to Myo5-1p at their actin-binding regions without correction of their incomplete three-dimensional structure. Consistently, although She4p demonstrated two-hybrid interactions with Myo5-1p, overexpression of SHE4 did not suppress the impaired two-hybrid interaction between Act1p and Myo3–1p (E472K), which possesses an amino acid substitution analogous to that of Myo5–1p (our unpublished data). Although we have not demonstrated that She4p functions as a molecular chaperone for Myo3/5p, if this is the case, She4p is not absolutely required for proper folding of Myo3/5p, because the growth defect of the she4Δ mutant was less severe than those of the myo3Δ myo5Δ double mutant (our unpublished data).
UNC-45 in C. elegans and Rng3p in S. pombe, well-studied UCS domain-containing proteins, are implicated in the functions of type II myosins. Unc-45 is proposed to act as a molecular chaperone for myosin motor (Barral et al., 2002). Rng3p colocalizes with a specific mutant protein of the type II myosin Myo2p (Wong et al., 2000), and we detected the interaction between Rng3p and the motor domain of Myo2p by the two-hybrid assay (our unpublished data). Consistent with this, we also showed that She4p interacts with a type II myosin, Myo1p. In addition, She4p interacted with type V myosins, Myo2p, and Myo4p in the two-hybrid system. Therefore, She4p interacts with all of the yeast myosins. However, the degree of requirement of She4p for their proper functioning varies between each myosin. Because she4 and myo4 mutant share the she phenotype and Myo4p is delocalized in the she4 mutant, She4p is most likely required for proper function of Myo4p. In contrast, She4p does not seem to be absolutely required for function of Myo2p, because she4Δ cells show normal polarized localization of Myo2p, and do not show any defects in polarized bud growth, which is regulated by Myo2p (our unpublished data). Similarly, she4Δ cells show normal bud-neck localization of Myo1p, and do not show any defects in cytokinesis, which is regulated by Myo1p (our unpublished data). There may be another type of functionally redundant protein that interacts with motor domain of Myo2p and Myo1p in S. cerevisiae. Based on the assumption that She4p functions as a molecular chaperone, another explanation is that Myo2p and Myo1p may have relatively high efficiency of self-folding.
Our results that She4p interacts with the motor domain of Myo4p and is required for proper localization of Myo4p strongly suggest that She4p also acts on Myo4p in a manner similar to the action of She4p on Myo3p and Myo5p. To further substantiate this possibility, we constructed mutant Myo4ps with N208I and K412M substitutions, which correspond to N168I and K377M in Myo5p, respectively. However, neither MYO4(N208I) nor MYO4(K412M) suppressed she phenotype of she4Δ cells (our unpublished data). This result may suggest that She4p possesses another function in polarized localization of ASH1 mRNA, in addition to correct folding or regulation of the motor domain of Myo4p. Interestingly, overexpression of the C-terminal half (UCS domain) of She4p was sufficient for suppression of temperature-sensitive growth defect of she4Δ cells, but full-length She4p was required for suppression of she phenotype (our unpublished data). The NH2-terminal half of She4p, which is less conserved than C-terminal UCS domain among the UCS proteins, may have this specific function in polarized localization of ASH1 mRNA.
Acknowledgments
We thank Dr. Ralf-Peter Jansen for exchanging data prior to publication. We thank Drs. Anthony Bretscher, John Cooper, David Drubin, Philip James, Yasushi Matsui, John Pringle, Howard Riezman, Bertrand Seraphin, and Barbara Winsor for yeast strains, plasmids, and libraries. We are grateful to Dr. Masayuki Takahashi for helpful advice on biochemical analysis of Myo5p and for providing rabbit skeletal muscle actin. We also thank Drs. David Amberg and Malcolm Whiteway for valuable discussions, Dr. Masahiko Watanabe for the DNA sequencer, and Tomoe Hirai, Aiko Ishioh, and Eriko Itoh for technical assistance. This work was supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K.F.-K. and K.T.).
Note added in proof: R. P. Jansen's group has also reported that She4p is required for class I and class V myosin function. [Wesche, S., Arnold, M., and Jansen, R.P. (2003). The UCS domain protein She4p binds to myosin motor domains and is essential for class I and class V myosin function. Curr. Biol. 13, 715–724.]
Abbreviations used: GFP, green fluorescent protein; GST, glutathione S-transferase; LY, lucifer yellow-carbohydrazide; TRITC, tetramethylrhodamine isothiocyanate.
References
- Adams, A.E., and Pringle, J.R. (1984). Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98, 934-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatep, R., Kirkpatrick, R.D., Parchaliuk, D.L., Woods, R.A., and Gietz, R.D. (1998). Transformation of Saccharomyces cerevisiae by the lithium acetate/single-stranded carrier DNA/polyethylene glycol (LiAc/ss-DNA/PEG) protocol. Technical Tips Online. Available at: http://research.bmn.com/tto. Search for “lithium acetate” from the opening page. Accessed May 2, 2003.
- Anderson, B.L., Boldogh, I., Evangelista, M., Boone, C., Greene, L.A., and Pon, L.A. (1998). The Src homology domain 3 (SH3) of a yeast type I myosin, Myo5p, binds to verprolin and is required for targeting to sites of actin polarization. J. Cell Biol. 141, 1357-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao, W., and Pilgrim, D. (2000). Caenorhabditis elegans UNC-45 is a component of muscle thick filaments and colocalizes with myosin heavy chain B, but not myosin heavy chain A. J. Cell Biol. 148, 375-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, M.K., McCollum, D., Chang, L., Wong, K.C., Naqvi, N.I., He, X., Sazer, S., and Gould, K.L. (1998). Isolation and characterization of new fission yeast cytokinesis mutants. Genetics 149, 1265-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral, J.M., Bauer, C.C., Ortiz, I., and Epstein, H.F. (1998). Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. J. Cell Biol. 143, 1215-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral, J.M., Hutagalung, A.H., Brinker, A., Hartl, F.U., and Epstein, H.F. (2002). Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 295, 669-671. [DOI] [PubMed] [Google Scholar]
- Bartel, P., Chien, C.T., Sternglanz, R., and Fields, S. (1993). Elimination of false positives that arise in using the two-hybrid system. Biotechniques 14, 920-924. [PubMed] [Google Scholar]
- Berteaux-Lecellier, V., Zickler, D., Debuchy, R., Panvier-Adoutte, A., Thompson-Coffe, C., and Picard, M. (1998). A homologue of the yeast SHE4 gene is essential for the transition between the syncytial and cellular stages during sexual reproduction of the fungus Podospora anserina. EMBO J. 17, 1248-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, E., Maddox, P., Lew, D.J., Salmon, E.D., McMillan, J.N., Yeh, E., and Pringle, J.R. (1998). Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol. 142, 1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobola, N., Jansen, R.P., Shin, T.H., and Nasmyth, K. (1996). Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell 84, 699-709. [DOI] [PubMed] [Google Scholar]
- Bohl, F., Kruse, C., Frank, A., Ferring, D., and Jansen, R.P. (2000). She2p, a novel RNA-binding protein tethers ASH1 mRNA to the Myo4p myosin motor via She3p. EMBO J. 19, 5514-5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockerhoff, S.E., Stevens, R.C., and Davis, T.N. (1994). The unconventional myosin, Myo2p, is a calmodulin target at sites of cell growth in Saccharomyces cerevisiae. J. Cell Biol. 124, 315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, S.S. (1997). Myosins in yeast. Curr. Opin. Cell Biol. 9, 44-48. [DOI] [PubMed] [Google Scholar]
- Cadwell, R.C., and Joyce, G.F. (1992). Randomization of genes by PCR mutagenesis. PCR Methods Appl. 2, 28-33. [DOI] [PubMed] [Google Scholar]
- Cope, M.J., and Hodge, T. (2000). A Multiple Alignment of 139 Myosin Sequences and a Phylogenetic Tree. The Myosin Home Page.Available at: www.mrc-lmb.cam.ac.uk/myosin/myosin.html. Accessed April 23, 2003.
- Dulic, V., Egerton, M., Elguindi, I., Raths, S., Singer, B., and Riezman, H. (1991). Yeast endocytosis assays. Methods Enzymol. 194, 697-710. [DOI] [PubMed] [Google Scholar]
- Elble, R. (1992). A simple and efficient procedure for transformation of yeasts. Biotechniques 13, 18-20. [PubMed] [Google Scholar]
- Evangelista, M., Klebl, B.M., Tong, A.H., Webb, B.A., Leeuw, T., Leberer, E., Whiteway, M., Thomas, D.Y., and Boone, C. (2000). A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 148, 353-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli, M.I., and Riezman, H. (1996). Role of type I myosins in receptor-mediated endocytosis in yeast. Science 272, 533-535. [DOI] [PubMed] [Google Scholar]
- Geli, M.I., and Riezman, H. (1998). Endocytic internalization in yeast and animal cells: similar and different. J. Cell Sci. 111, 1031-1037. [DOI] [PubMed] [Google Scholar]
- Geli, M.I., Lombardi, R., Schmelzl, B., and Riezman, H. (2000). An intact SH3 domain is required for myosin I-induced actin polymerization. EMBO J. 19, 4281-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, R.D., and Sugino, A. (1988). New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74, 527-534. [DOI] [PubMed] [Google Scholar]
- Goodson, H.V., Anderson, B.L., Warrick, H.M., Pon, L.A., and Spudich, J.A. (1996). Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): myosin I proteins are required for polarization of the actin cytoskeleton. J. Cell Biol. 133, 1277-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente, L. (1983). Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 101, 181-191. [DOI] [PubMed] [Google Scholar]
- Hollenberg, S.M., Sternglanz, R., Cheng, P.F., and Weintraub, H. (1995). Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol. Cell. Biol. 15, 3813-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura, H., Tanaka, K., Hihara, T., Umikawa, M., Kamei, T., Takahashi, K., Sasaki, T., and Takai, Y. (1997). Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16, 2745-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P., Halladay, J., and Craig, E.A. (1996). Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, R.P., Dowzer, C., Michaelis, C., Galova, M., and Nasmyth, K. (1996). Mother cell-specific HO expression in budding yeast depends on the unconventional myosin Myo4p and other cytoplasmic proteins. Cell 84, 687-697. [DOI] [PubMed] [Google Scholar]
- Johnson, J.L., and Craig, E.A. (1997). Protein folding in vivo: unraveling complex pathways. Cell 90, 201-204. [DOI] [PubMed] [Google Scholar]
- Johnston, G.C., Prendergast, J.A., and Singer, R.A. (1991). The Saccharomyces cerevisiae MYO2gene encodes an essential myosin for vectorial transport of vesicles. J. Cell Biol. 113, 539-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova, T.S., Reck-Peterson, S.L., Elkind, N.B., Mooseker, M.S., Novick, P.J., and Cooper, J.A. (2000). Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell 11, 1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin, J.V., and Adams, A.E. (1984). Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J. Cell Biol. 98, 922-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmar, M., Durrwang, U., Kliche, W., Manstein, D.J., and Kull, F.J. (2002). Crystal structure of the motor domain of a class-I myosin. EMBO J. 21, 2517-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler, T., Shevchenko, A., and Li, R. (2000). Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol. 148, 363-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie, S.H., and Brown, S.S. (1994). Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J. Cell Biol. 125, 825-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott, J., and Li, R. (1998). Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J. Cell Biol., 140, 355-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, R.M., Singer, R.H., Meng, X., Gonzalez, I., Nasmyth, K., and Jansen, R.P. (1997). Mating type switching in yeast controlled by asymmetric localization of ASH1mRNA. Science 277, 383-387. [DOI] [PubMed] [Google Scholar]
- Long, R.M., Gu, W., Lorimer, E., Singer, R.H., and Chartrand, P. (2000). She2p is a novel RNA-binding protein that recruits the Myo4p-She3p complex to ASH1mRNA. EMBO J. 19, 6592-6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M.S., McKenzie, A.III, Demarini, D.J., Shah, N.G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J.R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953-961. [DOI] [PubMed] [Google Scholar]
- Madania, A., Dumoulin, P., Grava, S., Kitamoto, H., Scharer-Brodbeck, C., Soulard, A., Moreau, V., and Winsor, B. (1999). The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol. Biol. Cell 10, 3521-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J.H. (1972). Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Mochida, J., Yamamoto, T., Fujimura-Kamada, K., and Tanaka, K. (2002). The novel adaptor protein, Mti1p, and Vrp1p, a homolog of Wiskott-Aldrich syndrome protein-interacting protein (WIP), may antagonistically regulate type I myosins in Saccharomyces cerevisiae. Genetics 160, 923-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker, M.S., and Cheney, R.E. (1995). Unconventional myosins. Annu. Rev. Cell Dev. Biol. 11, 633-675. [DOI] [PubMed] [Google Scholar]
- Munchow, S., Sauter, C., and Jansen, R.P. (1999). Association of the class V myosin Myo4p with a localised messenger RNA in budding yeast depends on She proteins. J. Cell Sci. 112, 1511-1518. [DOI] [PubMed] [Google Scholar]
- Oberholzer, U., Marcil, A., Leberer, E., Thomas, D.Y., and Whiteway, M. (2002). Myosin I is required for hypha formation in Candida albicans. Eukaryot. Cell 1, 213-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki, K., Tanaka, K., Imamura, H., Hihara, T., Kameyama, T., Nonaka, H., Hirano, H., Matsuura, Y., and Takai, Y. (1996). Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15, 2196-2207. [PMC free article] [PubMed] [Google Scholar]
- Pruyne, D., and Bretscher, A. (2000). Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113, 365-375. [DOI] [PubMed] [Google Scholar]
- Rayment, I., Rypniewski, W.R., Schmidt-Base, K., Smith, R., Tomchick, D.R., Benning, M.M., Winkelmann, D.A., Wesenberg, G., and Holden, H.M. (1993a). Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261, 50-58. [DOI] [PubMed] [Google Scholar]
- Rayment, I., Holden, H.M., Whittaker, M., Yohn, C.B., Lorenz, M., Holmes, K.C., and Milligan, R.A. (1993b). Structure of the actin-myosin complex and its implications for muscle contraction. Science 261, 58-65. [DOI] [PubMed] [Google Scholar]
- Reck-Peterson, S.L., Tyska, M.J., Novick, P.J., and Mooseker, M.S. (2001). The yeast class V myosins, Myo2p and Myo4p, are nonprocessive actin-based motors. J. Cell Biol. 153, 1121-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaut, G., Shevchenko, A., Rutz, B., Wilm, M., Mann, M., and Seraphin, B. (1999). A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17, 1030-1032. [DOI] [PubMed] [Google Scholar]
- Rodriguez, J.R., and Paterson, B.M. (1990). Yeast myosin heavy chain mutant: maintenance of the cell type specific budding pattern and the normal deposition of chitin and cell wall components requires an intact myosin heavy chain gene. Cell Motil. Cytoskeleton 17, 301-308. [DOI] [PubMed] [Google Scholar]
- Sayle, R.A., and Milner-White, E.J. (1995). RASMOL: biomolecular graphics for all. Trends Biochem. Sci. 20, 374-376. [DOI] [PubMed] [Google Scholar]
- Scherrer, L.C., Dalman, F.C., Massa, E., Meshinchi, S., and Pratt, W.B. (1990). Structural and functional reconstitution of the glucocorticoid receptor-hsp90 complex. J. Biol. Chem. 265, 21397-21400. [PubMed] [Google Scholar]
- Schott, D.H., Collins, R.N., and Bretscher, A. (2002). Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J. Cell Biol. 156, 35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers, J.R. (1999). Myosins, 2nd ed., Oxford, UK: Oxford University Press.
- Sherman, F. (1991). Getting started with yeast. Methods Enzymol. 194, 3-21. [DOI] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil, A., and Herskowitz, I. (1996). Identification of asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell 84, 711-722. [DOI] [PubMed] [Google Scholar]
- Takizawa, P.A., Sil, A., Swedlow, J.R., Herskowitz, I., and Vale, R.D. (1997). Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature 389, 90-93. [DOI] [PubMed] [Google Scholar]
- Takizawa, P.A., and Vale, R.D. (2000). The myosin motor, Myo4p, binds Ash1 mRNA via the adapter protein, She3p. Proc. Natl. Acad. Sci. USA 97, 5273-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, A.H., et al. (2001). Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294, 2364-2368. [DOI] [PubMed] [Google Scholar]
- Venolia, L., and Waterston, R.H. (1990). The unc-45gene of Caenorhabditis elegans is an essential muscle-affecting gene with maternal expression. Genetics 126, 345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, F.Z., Shiels, G., and Orr, E. (1987). The yeast MYO1gene encoding a myosin-like protein required for cell division. EMBO J. 6, 3499-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland, B., McCaffery, J.M., Xiao, Q., and Emr, S.D. (1996). A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J. Cell Biol. 135, 1485-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland, B., Emr, S.D., and Riezman, H. (1998). Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr. Opin. Cell Biol. 10, 513-522. [DOI] [PubMed] [Google Scholar]
- Wong, K.C., Naqvi, N.I., Iino, Y., Yamamoto, M., and Balasubramanian, M.K. (2000). Fission yeast Rng3p: an UCS-domain protein that mediates myosin II assembly during cytokinesis. J. Cell Sci. 113, 2421-2432. [DOI] [PubMed] [Google Scholar]