Abstract
Growth hormone receptor (GHR) has been demonstrated to be nuclear localized both in vivo and in vitro, but the significance of this observation has remained elusive. Here we show that nuclear GHR is strongly correlated with proliferative status in vivo by using a liver regeneration model. In vitro, nuclear translocation of the GH receptor is GH-dependent and appears to be mediated by the Importin system. Constitutive nuclear targeting of GHR in murine pro-B cells is associated with constitutive activation of STAT5, a transforming agent in lymphoma and other cell types. This activation is abrogated by inhibition of JAK2 and appears to be driven by autocrine murine GH action coupled with enhanced nuclear uptake of phospho-STAT5. Nuclear targeting induces dysregulated cell cycle progression in the pro-B cell line, associated with constitutive up-regulation of the proliferation inducers Survivin and Mybbp, the metastasis related Dysadherin, and other tumor markers. GHR nuclear-targeted cells generate aggressive metastatic tumors when injected into nude mice, which display nuclear localized GHR strikingly similar to that seen in human lymphomas. We conclude that aberrant nuclear localization of GHR is a marker of high proliferative status and is sufficient to induce tumorigenesis and tumor progression.
Keywords: nuclear translocation, autocrine GH, importin, constitutive STAT5
Growth hormone (GH) is the major regulator of postnatal somatic growth and an important metabolic regulator. Its actions are mediated via the GH receptor (GHR), which is widely expressed by GH target cells. The GHR is a class 1 cytokine receptor and, as a plasma membrane-bound receptor, is thought to signal exclusively from the cell membrane. However, the majority of GHR are generally located not at the cell surface but within the cell. The most intriguing subcellular location for the GHR has been the nucleus (1). The use of monoclonal antibodies combined with confocal laser-scanning microscopy (CLSM) (2) and immunogold electron microscopy (3) has allowed precise definition of subnuclear localization of the GHR to the inner and outer nuclear membranes, nucleoplasm, and chromatin.
It is now apparent that several growth factors, cytokines, and their receptors become nuclear-localized, with nuclear localization in many cases being necessary for full function, including cell proliferation (4–6). The functional role of the nuclear GHR is currently unknown, although a common feature of tissues and cells demonstrating nuclear GHR appears to be the potential for a high proliferative status; examples include the chondrocytes of the epiphysial growth plate (7), the gastrointestinal tract (8), placenta (9), and preimplantation embryos (10). Nuclear-localized GHR and high GHR expression have also been reported in a number of cancers, including breast cancer (11), colorectal carcinoma (12), hepatocellular carcinoma (13), and melanoma (14). Despite this, rigorous correlation of nuclear GHR with proliferative status has not been reported. Here we show that such localization is not only associated with cell proliferation, but that constitutive nuclear localization can result in a fully transformed phenotype.
Results
Nuclear Localization of the GHR Is Strongly Correlated with Proliferative Status in Regenerating Liver.
Regenerating liver represents a controlled model of proliferation in a normal tissue, whereby after partial hepatectomy, the liver undergoes an almost total regeneration in 9 days, with hepatocytes reaching their maximum mitotic index at 36 h after hepatectomy (15). The liver is a key GH target, with GH known to be required for liver regeneration (16).
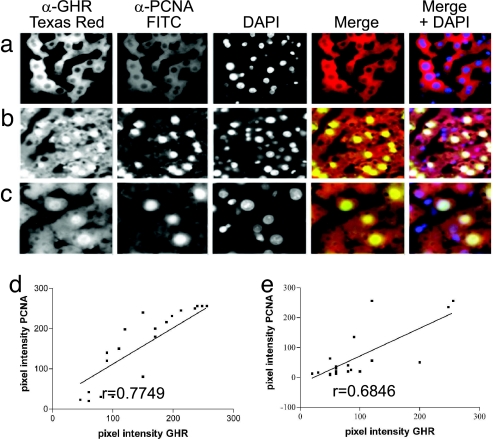
We removed liver sections from sham-operated and partially hepatectomized rats at 24 h after surgery to coincide with the first wave of DNA synthesis before the first cell division. A well characterized antibody to the extracellular domain of the GHR (mAb 263) (17) was used to detect GHR expression and localization in this regenerative phase (Fig. 1 a–c). Proliferative status at the cellular level was ascertained as proliferating cell nuclear antigen (PCNA), by using mAb PC10 conjugated directly to fluorescein. GHR-positive nuclei and PCNA-positive nuclei were counted and expressed as a percentage of total nuclei. PCNA-positive cells comprised 52 ± 9% (mean ± SEM, n = 3 animals) of cells in the regenerating liver compared with 2.4 ± 1.4% in the control liver. Cells that exhibited nuclear immunoreactivity for GHR comprised 67% ± 11% in the regenerating liver compared with 9% ± 2% in the control liver. Furthermore, a strong correlation of r = 0.78 (P < 0.0001) existed at a cellular level between PCNA expression and nuclear GHR (Fig. 1 e and f). This finding provides in vivo evidence relating to a functional role for nuclear GHR.
Fig. 1.
Nuclear localization of GHR in regenerating rat liver. (a–c) Liver from a control animal (a) and 24 h after hepatectomy (b and c) is shown. GHR localization was detected with MAb 263 (left image) and proliferation as PCNA with mAb PC10-FITC (second image). Nuclei were stained with DAPI (third image). The merged image in the fourth image is for GHR and PCNA immunoreactivity. Blue nuclei in Right represent DAPI staining, whereas white nuclei colocalize all three. Higher magnification (×60) shows more detail in regenerating liver 24 h after hepatectomy (c). Negative controls for staining specificity where primary antibody (mAb 263) was omitted showed no staining (not shown). (d and e) Correlation plots show the correlation between fluorescence intensity for mAb263 (GHR) and mAb PC10 (PCNA) in the nuclei of randomly chosen hepatocytes at 24 h after confocal scanning light microscopy in Hx (d) and sham Hx (e) livers.
GHR Undergoes Regulated Nuclear Translocation in Cell Models.
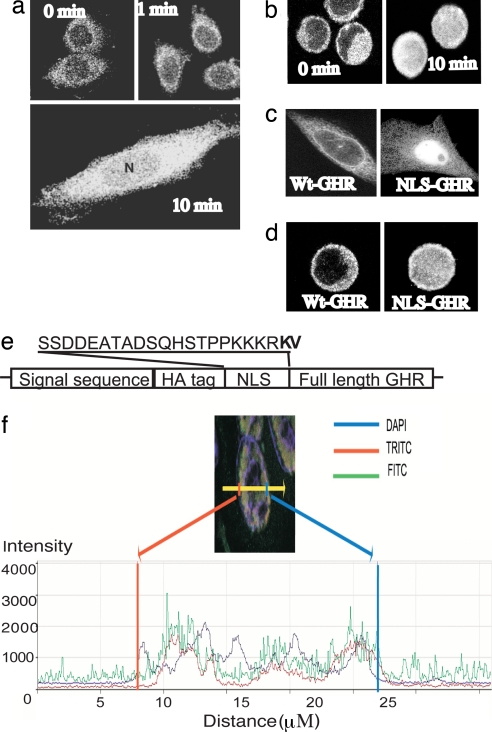
We have utilized a cell model of GH-driven proliferation, the progenitor B cell line BaF/3 stably expressing GHR, to investigate the role of nuclear GHR in proliferation. In this model, and in CHO-K1 lines, we find that nuclear localization of the GHR WT occurs as a regulated cell cycle-dependent GH-mediated event, recapitulating previous findings (3) (Fig. 2). mAb 263-reactive nuclear GHR is essentially absent in cells arrested in G1 phase by serum starvation for 10 h but appears rapidly after addition of a physiological dose of GH. To confirm this, an HA tag was placed at the N terminus of the mature GHR WT to verify the mAb 263 staining pattern with the high-specificity high-affinity mAb HA11. This showed an identical nuclear staining pattern (Fig. 2b).
Fig. 2.
Nuclear translocation of GHR occurs in response to GH. Immunofluorescence/CLSM imaging shows rapid GH-dependent nuclear translocation of GHR in CHO-K1 and BaF stable lines. GHR was detected by mAb 263 and FITC-coupled secondary antibody. (a) After 10-h serum starve, nuclear immunofluorescence was absent in CHO-GHR cells. (b) Addition of GH (100 ng/ml, 5 nM) resulted in rapid accumulation of GHR around the nuclear membrane at 1 min, followed by nuclear localization, which reaches a maximum at 10-min CLSM with anti-HA for HA-tagged GHR also shows rapid GH-dependent nuclear translocation of GHR in BaF-GHR WT stable lines. (c and d) Addition of the T-Ag NLS to the N terminus of mature GHR resulted in constitutive nuclear localization of GHR in the absence of GH, in both CHO-K1 (c) and BaF (d) cells. (e) Location and sequence of NLS are shown in e. (f) Quantification of 4.1 μm confocal scanning light microscopy z-scan of NLS-GHR transiently transfected COS cell, scan across one cell. Nucleus is DAPI stain (blue line), HA tag (N terminus, FITC, green) colocalizes with FLAG tag (C terminus, TRITC, red), quantified by scan across nucleus.
Both the BaF/3 and CHO-K1 cell lines were then used to determine functional effects arising from the addition of the canonical tumor antigen (T-Ag) nuclear localization sequence (NLS) at the N terminus of the mature GHR extracellular domain (GHR NLS). Inclusion of an intact leader sequence before the HA tag, NLS, and mature GHR ensured that the GHR NLS was processed and trafficked to the surface in a similar manner to GHR WT. Comparable levels of GHR were demonstrated on the surface of both cell lines by using 125I-GH-binding studies with intact cells (see Fig. 4d) and by FACS analysis after immunofluorescent staining with both mAb 263 and anti-HA (not shown). Despite WT surface expression, the NLS was able to constitutively target the GHR NLS to the nucleus. As expected, the level of nuclear GHR was much higher in the GHR NLS-expressing cells, as detected by CLSM (Fig. 2 c and d) and 125I-GH-binding studies with purified nuclear fractions (not shown). Therefore, the nuclear localization of the GHR had become independent of the stringent regulation apparent for the GHR WT and was instead constitutive. Fig. 2 also shows that the full length NLS receptor is present throughout the nucleus, by confocal scanning light microscopy z-scan [and supporting information (SI) Fig. 7].
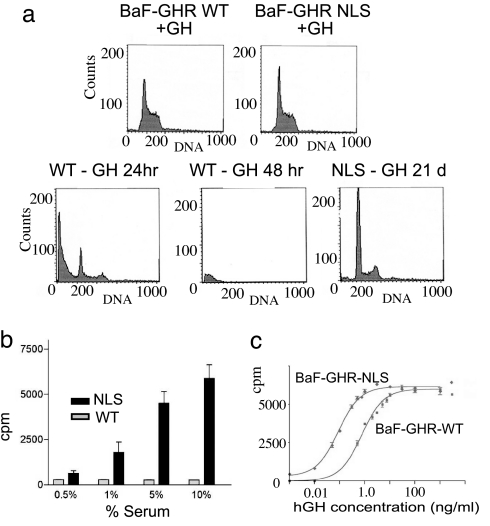
Fig. 4.
Effect of increased GHR nuclear targeting in a cell model. In a subconfluent culture with GH support, both BaF-GHR WT and NLS lines undergo mitosis, with 60% in G1 phase and 40% in S/M phase (a Upper). However, upon the withdrawal of cytokine support (in the form of GH or IL-3), only the BaF-GHR NLS lines proliferate (a Lower; only 21-day sort shown for NLS line, exemplifying earlier timepoints). (b) BaF-GHR NLS lines continue to proliferate in the absence of GH and IL-3, with the support of fetal bovine serum. (c) NLS-GHR lines show a dramatic increase in the sensitivity to GH as measured by [3H]thymidine incorporation assay (20-fold decrease in ED50, i.e., 10 pM for NLS, compared with 200 pM for WT in 1.0% serum). Results were confirmed in three separate population studies and clonal lines from three separate transfections.
Nuclear Import of GHR Extracellular Domain Depends on Importin (Imp) α/β.
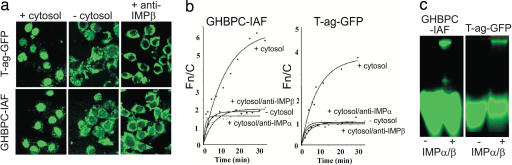
Nuclear import requires recognition by the cellular nuclear import machinery and, in particular, by members of the NLS-recognizing IMP superfamily. The best-understood NLS-dependent nuclear import pathway is that mediated by the IMPα/β heterodimer (18). It is known that the extracellular domain of the GHR (GHBP) can translocate to the nucleus, because antibodies to the alternatively spliced C terminus of the GHBP reveal nuclear localization in vivo (19). To ascertain the role of IMPs in GHR nuclear localization, a GHR extracellular domain construct (GHBPC) was created that possessed a Cys residue substituted at position 237 to enable fluorescent labeling by using the Cys-specific fluorescein derivative iodoacetamido-fluorescein (IAF) (SI Text). GHBPC-IAF accumulated in the nucleus of mechanically perforated cells in the presence of exogenous cytosol (Fig. 3a) to maximal levels (Fig. 3b) over five times those in the cytoplasm, comparing well to the levels for the control protein T-Ag-GFP containing the T-Ag NLS (nuclear to cytoplasmic ratio (Fn/c) of nearly 4). Half-maximal accumulation was achieved somewhat more slowly (≈7 min) than for T-Ag-GFP (≈5 min). Nuclear accumulation in the absence of exogenous cytosol was severely reduced as for T-Ag-GFP, implying that cytosolic factors were required for GHBP nuclear import. Accumulation in the presence of cytosol depended on both IMPα and IMPβ, because antibodies to either reduced nuclear accumulation of GHBPC-IAF to levels comparable to those in the absence of cytosol.
Fig. 3.
Nuclear accumulation of GHR in vitro is mediated by a classical nuclear import pathway involving Impα and -β. (a) CLSM visualization showing localization of T-Ag-GFP (Upper) and GHBPC (GHBPC-IAF) (Lower) in mechanically perforated HTC rat hepatoma cells in the absence or presence of cytosol or cytosol with anti-IMPβ antibody. (b) Quantitative measurements of the kinetics of nuclear accumulation of GHBPC (Left) and T-Ag-GFP (Right), +/− exogenous cytosol in the absence or presence of specific antibodies to IMPα or IMPβ. [Data were fitted for the function Fn/c(t) = Fn/cmax × (1− e-kt], where t is time, Fn/cmax is the maximal level of nuclear accumulation, and k is the first-order rate constant. (c) Recognition of GHBPC by the IMP α/β heterodimer as indicated by native gel electrophoresis imaging; 25 pmol GHBPC-IAF or T-Ag-GFP was incubated in the absence or presence of 10 μM IMPα/β heterodimer for 15 min at 20°C, before electrophoresis.
Native gel electrophoresis (Fig. 3c) was used to test whether IMPα/β was able to recognize GHBPC directly. This showed that IMPα/β binds GHBPC, resulting in altered mobility in the native gel (Fig. 3c Left), in comparable fashion to the control molecule T-ag-GFP (Fig. 3c Right). These results are consistent with GHR nuclear import depending on IMPα/β, as it is for ErbB2 (6). However, they do not address the issue of how the membrane-bound receptor enters the cytoplasm. This translocation may use the Sec61 ER translocon, because proteomics analysis shows that the GHR associates with BiP (H.S.C. and M.J.W., unpublished work), which binds to the luminal side of this translocon.
Effects of Increased GHR Nuclear Targeting on Proliferative Status in Vitro.
We asked whether GHR NLS results in a change in the proliferative status of BaF/3 cells. BaF/3 proliferation normally depends on the presence of IL3, but stable transfection with other cytokine receptors (e.g., GHR) enables these cells to grow with the relevant cytokine in the absence of IL3. Unlike mature B cells, BaF/3 cells have no endogenous GHRs. Although no proliferative difference was observed between GHR WT and GHR NLS lines on the maximal dose of GH (Fig. 4a Top), there was a striking difference when GH was withdrawn from the media. BaF-WT GHR lines rapidly exited the cell cycle (Fig. 4a Lower Left and Center). In contrast, BaF-GHR NLS lines proliferated in serum alone, even in the absence of either IL3 or GH (Fig. 4a Lower Right). This effect was quantified by using3H-thymidine incorporation. No 3H-thymidine incorporation was evident in BaF-GHR WT lines in response to serum alone, whereas robust proliferation was evident in BaF-GHR NLS lines (Fig. 4b). GH dose–response proliferation assays also showed that the NLS lines were exquisitely sensitive to low levels of GH (20-fold decrease in ED50; Fig. 4c and SI Fig. 8). This difference was not a result of increased surface receptor expression, as determined by Scatchard analysis (SI Fig. 8), and verified by FACS analysis (not shown). Increased sensitivity was also not the result of altered affinity for GH, as this was not different between WT and NLS-GHR cells (SI Fig. 8).
Microarray Analysis of BaF/3 Lines.
Microarray analysis was used to identify genes expressed differentially between BaF-WT GHR and BaF-GHR NLS lines (SI Fig. 9). The constitutive transcripts up-regulated in BaF-GHR NLS were increased 2.5–4 fold in GHR-WT cells 1 h after GH addition. These transcripts included some that increase in highly proliferating tissues (regenerating liver or neuronal tissue recovering from trauma) (Phgdh, F2r, and Ctsc), and in cancers, (Phgdh, F2r, Ctsc, Mybbp1a, Eif5a, and dysadherin). Northern blot analysis was performed for some of these transcripts (Phgdh, Ctsc, Mybbp1a, and Survivin) and showed they remain up-regulated during the entire 6-h experiment in BaF-GHR NLS lines (SI Fig. 9). Among the transcripts down-regulated in BaF-GHR NLS relative to BaF-WT GHR was Gata-1, which is normally associated with maturation/differentiation of erythroid, megakaryocytic, and hematopoietic lineages. Other transcripts were present at low levels in BaF-GHR NLS [Stim1, Kai (tumor suppressors), Emb, Rfc2, and Mki67], and their expression was repressed in BaF WT-GHR cells by GH treatment for 1 h (data not shown).
The majority of GH-regulated genes, including metabolic enzymes, did not show differential regulation in the two cell lines, suggesting that basic metabolic functions of GH are not influenced by nuclear targeting of the GHR and are not regulated by nuclear translocation of GHR (SI Table 1).
Oncogenic Transformation by Nuclear GHR.
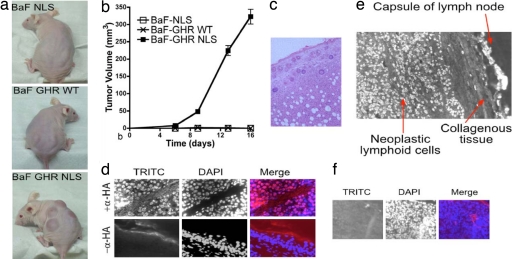
Previous studies have used BaF/3 cells as a model of oncogenic transformation into pre-B cell leukemias in vivo, induced by oncogenes such as v-Abl, TEL-JAK, or NOK (20). We therefore examined the ability of BaF-WT GHR and BaF-GHR NLS to form tumors in an athymic nude mouse model. s.c. inoculation of BaF-GHR NLS cells led to rapid tumor formation in vivo, whereas BaF-WT GHR did not form tumors (Fig. 5 a and b). Importantly, no tumors were observed in the BaF-WT GHR group over 50 days. s.c. tumor samples and lymph nodes were taken from all BaF-GHR NLS mice, as well as a liver sample from one of the group, and stained by H&E (Fig. 5c). Immunofluorescence to the HA tag of the introduced GHR was used to verify that the tumors were composed of the BaF-GHR NLS cells (Fig. 5d). The BaF-GHR NLS cells were noted to have invaded the stromal compartment in each case. Moreover, the tumor cells were also observed in all lymph nodes collected and in the liver sections.
Fig. 5.
s.c. inoculation of BaF-GHR NLS cells results in rapid tumor formation in vivo. (a) BaF-GHR NLS cells give rise to aggressive metastatic tumors when injected s.c. into nude mice. (b) Quantitative data over a 16-day period are shown graphically. (d Upper) No tumors were observed in the BaF-GHR WT or the BaF-NLS (as a negative control for the NLS sequence) group. HA-GHR immunofluorescence by using TRITC second antibody shows the tumors are derived from BaF HA-GHR NLS cells. (d Lower) A negative control omitting the primary anti-HA antibody shows staining specificity. (c) s.c. tumor samples and lymph nodes were taken from all BaF GHR NLS mice, as well as a liver sample from one of the group, and stained by H&E. (e) Nuclear localization of GHR in human lymphoma. (f) Negative control for staining in human lymphoma where primary antibody (mAb 263) is omitted.
To establish the clinical relevance of these observations, sections of highly proliferative lymphomas from four patients were immunostained for GHR (see SI Text). Intense nuclear localization of the receptor was evident in most neoplastic lymphoid cells within the encapsulated compartment of the lymph node, whereas few nuclei were stained in normal lymph nodes (Fig. 5e and SI Fig. 10). These results suggest that nuclear GHR is associated with tumorigenesis and tumor metastasis.
Mechanism of Transformation.
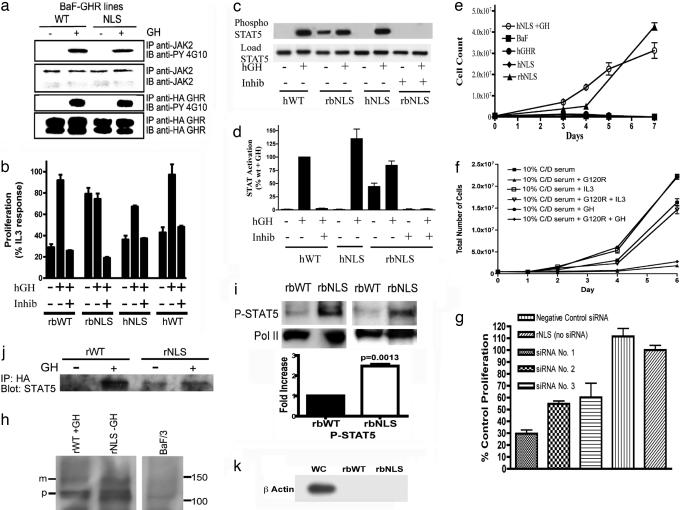
To ascertain the basis for transformation by nuclear localization, we examined signaling intermediates in BaF-GHR lines. As shown in Fig. 6a, tyrosine phosphorylation of both JAK2 and the GHR in the BaF-NLS was comparable to that in BaF-WT cells in response to GH addition, with no convincing evidence of constitutive phosphorylation. However, constitutive phosphorylation of STAT5 was evident in the NLS cells (Fig. 6 c, d, and i), and this could be abrogated by addition of a potent JAK2 inhibitor, JAK inhibitor 1. Because STAT5 is a growth and transforming agent (21, 22) we measured constitutive proliferation in the presence of the JAK2 inhibitor and found it to be blocked in the BaF-NLS cells (Fig. 6b). To determine whether the constitutive STAT5 activation was a result of activation of the rabbit GHR by autocrine mouse GH, we made BaF lines expressing human GHR-NLS, because the human GHR cannot be activated by mouse GH. As shown in Fig. 6e, these cells did not proliferate independently of added human GH and did not display constitutive STAT5 phosphorylation, although the human GHR was indeed nuclear targeted in these cells (SI Fig. 11b). Accordingly, our BaF cells express the transcript for mouse GH (SI Fig. 11a), verifying a previous study reporting expression of mouse GH protein in BaF cells (23). Moreover, siRNA knockdown of endogenous murine GH blocked constitutive proliferation (Fig. 6g), as did addition of exogenous G120R human GH antagonist (Fig. 6f). The presence of glycosylated mature GHR in highly purified nuclei (Fig. 6h) is consistent with nuclear translocation of full length receptor from the cell surface (although immature form is also present). Importantly, nuclear uptake of phospho-STAT5 in response to exogenous GH was enhanced in NLS-GHR BaF cells, and STAT5 was coimmunoprecipitated with HA-GHR in the nucleus, even in the absence of GH with NLS-GHR BaF cells (Fig. 6j). More phospho-STAT5 is present in purified nuclei of NLS-GHR cells both in the basal state (2.0-fold) and after GH (Fig. 6i). confocal scanning light microscopy immunofluorescence also showed increased nuclear STAT5 in NLS-GHR cells, which colocalizes with the HA-GHR epitope (SI Fig. 12).
Fig. 6.
Mechanistic studies. (a) Activation of WT and NLS GHR in BaF lines as determined by tyrosine phosphorylation of JAK2 and GHR in phosphotyrosine immunoblots. BaF-GHR WT or NLS lines were serum-starved for 6 h and then treated with human GH (100 ng/ml) (+) or saline (−) for 10 min. (b) Proliferative response of BaF lines, and abrogation of this with JAK2 inhibitor 1 (Calbiochem, San Diego, CA) at 0.4 μM, using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay (MTT) (SI Text). (c) STAT5 activation in BaF lines showing constitutive activation in BaF-NLS and abrogation by JAK inhibitor, with quantification for three independent experiments (d). (e) Proliferation of BaF lines from 0.5 × 106 cells showing lack of constitutive proliferation with NLS-human GHR cells. (f) G120R (0.6 μM) blocks constitutive proliferation of NLS-rbGHR expressing cells growing in charcoal stripped serum (g) siRNA to murine GH inhibits constitutive proliferation in charcoal stripped serum (h) mature and precursor full-length GHR in highly purified BaF cell nuclei prepared according to (28). (i) Enhanced basal (2.5-fold by densitometry) phospho-STAT5 (Cell Signaling clone 14H2, Y694) in purified nuclei of NLS-rbGHR BaFcells. Histogram shows quantification of three separate preparations, mean ± SEM (j) STAT5 coimmunoprecipitation with nuclear GHR, even in the basal state with NLS-GHR BaF cells. (k) β-Actin immunoblot comparing whole-cell and nuclear β-actin with 10 μg of protein, demonstrating purity of nuclei in i.
Discussion
We report here that nuclear GHR correlates with high proliferative status in vivo (in the regenerating rat liver model) and in vitro (BaF lines), and that constitutive nuclear targeting of GHR causes a dysregulation of proliferative arrest and induction of cell cycle progression, resulting in oncogenesis. This appears to be a result of enhanced nuclear translocation of phospho-STAT5 generated at the cell surface by autocrine GH, most probably in association with nuclear-targeted GHR. Other proliferative signals such as phosphatidyl inositol 3-kinase/Akt presumably contribute to the constitutive proliferation (23). Johnson et al. (5) have shown that the liganded IFN-γ receptor can translocate phospho-STAT1 to the nucleus, and our data are consistent with this mechanism. However, we cannot exclude direct transcriptional actions by the nuclear receptor as reported for the epidermal growth factor receptor (4).
The normally limited period of nuclear localization of GHR after GH addition (1 h; ref. 3), which coincides with cell cycle initiation, may prevent cell transformation. However, we suggest that excessive nuclear uptake or extended residence time of GHR in the nucleus could result in cell transformation. We note that, although the GHR lacks a consensus NLS, it does associate with Impαβ (Fig. 3), and this interaction could be enhanced through association with NLS-containing proteins such as CoAA, which bind to the GHR in a GH-dependent manner (B.L.C.-C, J.W.W., and M.J.W., unpublished work). We also note that it is likely that the GHR translocates from the cell surface, because a portion of nuclear GHR possesses mature glycosylation and has similar kinetics of nuclear uptake as exogenous GH (Fig. 6 and ref. 2).
The recent finding that autocrine production of GH in human breast epithelial cells is able to induce cellular transformation through a GHR-dependent mechanism (24, 25), whereas exogenous human GH is nontransforming, is relevant to our findings. There is a positive correlation between human mammary proliferative disorders (particularly metastatic carcinoma) and autocrine human GH (26), and the enhanced sensitivity of nuclear GHR to the proliferative action of GH would mean that such cells would be driven to proliferation and potentially transformation. The increased dysadherin transcript expression we observed is consistent with the metastatic ability of cells with nuclear-localized GHR, because dysadherin is thought to destabilize cadherin-based cell-cell contacts (27). Similarly, constitutive expression of transcripts for the proliferation-related proteins Survivin, MybBP1a,and PhgDH in cells with nuclear-targeted GHR supports its role in proliferation.
The ability of nuclear-localized GHR to induce tumor formation could have important clinical implications. Our findings indicate nuclear GHR as a novel target in the treatment of proliferative disorders, and strategies aimed at blocking nuclear translocation of the GHR could result in useful cancer therapeutics.
Experimental Procedures
Animal Experiments.
Partial hepatectomy was performed on male 12-week-old Wistar rats according to ref. 29, with authorization from the University of Queensland Animal Ethics Committee. At 24 h after surgery, the animals were killed, and the remnant liver removed and transferred into 4% paraformaldehyde fixative.
Immunofluorescence Staining, Microscopy, and Quantification.
Paraformaldehyde-fixed OCT section of livers were examined for GHR immunoreactivity by using mAb 263 (17), revealed by biotinylated anti-mouse IgG and Streptavidin-Texas red, as detailed in SI Text. Proliferating cells were identified with FITC-conjugated PCNA antibody (clone PC10).
GHR in CHO-K1 and BaF/3 cells was visualized by using mAb 263 or HA (clone 16B12) or FLAG antibody followed by anti-mouse TRITC, as in SI Text. DAPI was used to visualize nuclei.
Plasmid Construction.
An HA-tagged rabbit GHR (rbGHR) construct (GHR WT) was created as described (30), together with an NLS sequence-containing plasmid (GHR NLS). The NLS rbGHR was created by ligating annealed double-stranded oligonucleotides coding for the NLS of the simian virus 40 large T-ag between the HA tag and the full-length GHR.
Nuclear Import Assay.
Nuclear import kinetics was analyzed at the single-cell level in vitro by using mechanically perforated HTC cells with CLSM, as described in ref. 31 and in SI Text.
Cell Culture and Treatments.
BaF/3 cells were grown in RPMI medium 1640/10% Serum Supreme/100 units/ml IL-3. BaF/3 cells expressing WT and mutant GHR were supplemented with 5 nM human GH instead of IL-3 to maintain receptor expression. Cells for the GH-stimulation experiments were incubated in 0.5% serum for 10 h and then treated with 100 ng/ml human GH for times as indicated. Proliferation assays using [3H]thymidine incorporation were as described (30).
Microarray Procedures.
Fluorescently labeled cDNA probes were prepared from 30 μg of total RNA and labeled with Alexa 555 for the BaF probes or Alexa 648 for the reference probes. The probes were hybridized to Compugen Oligo arrays, then scanned on a G418 scanner (Genetic Microsystems, Woburn, MA).
Tumorigenicity in Nude Mice.
Two million cells in 50 μl of RPMI medium 1640/10% FCS were injected s.c. into each of four sites on three 6- to 8-week-old male athymic nu/nu mice. 3D measurement was performed twice weekly and the tumor volume expressed as mm3. Statistical analysis of tumor growth curves was performed by using a permutation test with 10,000 iterations.
Supplementary Material
Acknowledgments
We thank Dr. Vicki Whitehall for the data presented in SI Fig. 10 and both Kathryn Tunny and Johanna Barclay for invaluable technical assistance. This work was supported by grants from the National Health and Medical Research Council (Australia) (to M.J.W. and D.A.J.).
Abbreviations
- GH
growth hormone
- GHR
GH receptor
- PCNA
proliferating cell nuclear antigen
- GHR NLS
canonical T-Ag NLS at the N terminus of the mature GHR extracellular domain
- IMP
Importin
- T-ag
tumor antigen
- IAF
iodoacetamido-fluorescein
- NLS
nuclear localization sequence.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GEO Database, www.ncbi.nlm.nih.gov/geo (accession no. GSE2898).
This article contains supporting information online at www.pnas.org/cgi/content/full/0600181104/DC1.
References
- 1.Lobie PE, Barnard R, Waters MJ. J Biol Chem. 1991;266:22645–22652. [PubMed] [Google Scholar]
- 2.Mertani HC, Raccurt M, Abbate A, Kindblom J, Tornell J, Billestrup N, Usson Y, Morel G, Lobie PE. Endocrinology. 2003;144:3182–3195. doi: 10.1210/en.2002-221121. [DOI] [PubMed] [Google Scholar]
- 3.Lobie PE, Wood TJ, Chen CM, Waters MJ, Norstedt G. J Biol Chem. 1994;269:31735–31746. [PubMed] [Google Scholar]
- 4.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 5.Johnson HM, Subramaniam PS, Olsnes S, Jans DA. BioEssays. 2004;26:993–1004. doi: 10.1002/bies.20086. [DOI] [PubMed] [Google Scholar]
- 6.Giri DK, Ali-Seyed M, Li L-Y, Lee D-F, Ling P, Bartholomeusz G, Wang S-C, Hung M-C. Mol Cell Biol. 2005;25:11005–11018. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gevers EF, van der Eerden BC, Karperien M, Raap AK, Robinson IC, Wit JM. J Bone Miner Res. 2002;17:1408–1419. doi: 10.1359/jbmr.2002.17.8.1408. [DOI] [PubMed] [Google Scholar]
- 8.Lobie PE, Breipohl W, Waters MJ. Endocrinology. 1990;126:299–306. doi: 10.1210/endo-126-1-299. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Aragon J, Lobie PE, Muscat GE, Gobius KS, Norstedt G, Waters MJ. Development (Cambridge, UK) 1992;114:869–876. doi: 10.1242/dev.114.4.869. [DOI] [PubMed] [Google Scholar]
- 10.Pantaleon M, Whiteside EJ, Harvey MB, Barnard RT, Waters MJ, Kaye PL. Proc Natl Acad Sci USA. 1997;94:5125–5130. doi: 10.1073/pnas.94.10.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mertani HC, Garcia-Caballero T, Lambert A, Gerard F, Palayer C, Boutin JM, Vonderhaar BK, Waters MJ, Lobie PE, Morel G. Int J Cancer. 1998;79:202–211. doi: 10.1002/(sici)1097-0215(19980417)79:2<202::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Lincoln DT, Kaiser HE, Raju GP, Waters MJ. In Vivo. 2000;14:41–49. [PubMed] [Google Scholar]
- 13.Garcia-Caballero T, Mertani HM, Lambert A, Gallego R, Fraga M, Pintos E, Forteza J, Chevallier M, Lobie PE, Vonderhaar BK, et al. Endocrine. 2000;12:265–271. doi: 10.1385/ENDO:12:3:265. [DOI] [PubMed] [Google Scholar]
- 14.Lincoln DT, Sinowatz F, Kolle S, Takahashi H, Parsons P, Waters M. Anticancer Res. 1999;19:1919–1931. [PubMed] [Google Scholar]
- 15.Haber BA, Mohn KL, Diamond RH, Taub R. J Clin Invest. 1993;91:1319–1326. doi: 10.1172/JCI116332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pennisi PA, Kopchick JJ, Thorgeirsson S, LeRoith D, Yakar S. Endocrinology. 2004;145:4748–4755. doi: 10.1210/en.2004-0655. [DOI] [PubMed] [Google Scholar]
- 17.Wan Y, Zheng YZ, Harris JM, Brown R, Waters MJ. Mol Endocrinol. 2003;17:2240–2250. doi: 10.1210/me.2003-0162. [DOI] [PubMed] [Google Scholar]
- 18.Jans DA, Xiao CY, Lam MH. BioEssays. 2000;22:532–544. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 19.Lobie PE, Garcia-Aragon J, Wang BS, Baumbach WR, Waters MJ. Endocrinology. 1992;130:3057–3065. doi: 10.1210/endo.130.5.1374020. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Yu XZ, Li TS, Song LX, Chen PL, Suo TL, Li YH, Wang SD, et al. Cancer Res. 2004;64:3491–3499. doi: 10.1158/0008-5472.CAN-03-2106. [DOI] [PubMed] [Google Scholar]
- 21.Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A. J Cell Physiol. 2003;197:157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 22.Nieborowska-Skorska M, Wasik MA, Slupianek A, Salomoni P, Kitamura T, Calabretta B, Skorski T. J Exp Med. 1999;189:1229–1242. doi: 10.1084/jem.189.8.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeay S, Sonenshein GE, Kelly PA, Postel-Vinay MC, Baixeras E. Endocrinology. 2001;142:147–156. doi: 10.1210/endo.142.1.7892. [DOI] [PubMed] [Google Scholar]
- 24.Mukhina S, Mertani HC, Guo K, Lee KO, Gluckman PD, Lobie PE. Proc Natl Acad Sci USA. 2004;101:15166–15171. doi: 10.1073/pnas.0405881101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waters MJ, Conway-Campbell BL. Proc Natl Acad Sci USA. 2004;101:14992–14993. doi: 10.1073/pnas.0406396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raccurt M, Lobie PE, Moudilou E, Garcia-Caballero T, Frappart L, Morel G, Mertani HC. J Endocrinol. 2002;175:307–318. doi: 10.1677/joe.0.1750307. [DOI] [PubMed] [Google Scholar]
- 27.Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S. Proc Natl Acad Sci USA. 2002;99:365–370. doi: 10.1073/pnas.012425299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerner C, Holzmann K, Grimm R, Sauermann G. J Cell Biochem. 1998;71:363–7429. doi: 10.1002/(sici)1097-4644(19981201)71:3<363::aid-jcb5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Waynforth HB, Flecknell PA. Experimental and Surgical Technique in the Rat. London: Academic; 1992. [Google Scholar]
- 30.Behncken SN, Billestrup N, Brown R, Amstrup J, Conway-Campbell B, Waters MJ. J Biol Chem. 2000;275:17000–17007. doi: 10.1074/jbc.275.22.17000. [DOI] [PubMed] [Google Scholar]
- 31.Hubner S, Smith HM, Hu W, Chan CK, Rihs HP, Paschal BM, Raikhel NV, Jans DA. J Biol Chem. 1999;274:22610–22617. doi: 10.1074/jbc.274.32.22610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.