Abstract
The strong ROS (reactive oxygen species) production, part of an antioxidant response of human fibroblasts triggered by DHA (docosahexaenoic acid; C22:6,n−3), served as a model for deciphering the relative contribution of NOX (NADPH oxidase) to ROS production, as the role of this enzymatic system remains controversial. Using hydroxyethidium fluorescence for fibroblast ROS production, RT (reverse transcriptase)–PCR for NOX 4 mRNA quantification and mRNA silencing, we show that ROS production evolves in parallel with the catalytic activity of NOX and is suppressed by siNOX 4 (small interference oligonucleotide RNA directed against NOX 4) silencing. Apocynin and plumbagin, specific inhibitors of NOX, prevent ROS production in this cellular model and confirm the role of NOX 4 for this production. Furthermore, we show that, in cell lysates, NOX 4 activity can be modulated by PUFAs (polyunsaturated fatty acids) at the micromolar level in the presence of calcium: NOX 4 activity is increased by arachidonic acid (C20:4,n−6) (∼175% of the control), and conjugated linoleic acid (C18:2 [9Z,11E]) is a potent inhibitor (50% of the control). Unexpectedly, intracellular superoxide dismutase does not participate in the modulation of this ROS production and the opposite effects of some PUFAs, described in our experiments, could suggest another way of regulating NOX activity.
Keywords: docosahexaenoic acid, fibroblast, haem oxygenase, NADPH oxidase (NOX), polyunsaturated fatty acid, reactive oxygen species
Abbreviations: AA, arachidonic acid (C20:4,n−6); BCA, bicinchoninic acid; CLA, conjugated linoleic acid (C18:2 [9Z,11E]); DHA, docosahexaenoic acid (C22:6,n−3); DHA-met, docosahexaenoic methyl ester; DMEM, Dulbecco's modified Eagle's medium; DPI, diphenyl iodonium; DUOX, dual oxidase; E+, ethidium; E_OH+, hydroxyethidium; EPA, eicosapentaenoic acid (C20:5,n−3); gp91phox, one of the two integral membrane proteins making up flavocytochrome b558; HE, hydroethidine; HO-1, haem oxygenase 1; IU, international unit; LC/MS, liquid chromatography MS; NOX, NADPH oxidase; p22phox, the other subunit making up flavocytochrome b558; PUFA, polyunsaturated fatty acid; QPCR, quantitative PCR; ROS, reactive oxygen species; RT, retention time; RT–PCR, reverse transcriptase–PCR; siNOX 4, small interference oligonucleotide RNA directed against NOX 4; siRNA, small interfering RNA; SOD, superoxide dismutase; TIC, total ion current; XO, xanthine oxidase
INTRODUCTION
Several novel enzymes, homologues of gp91phox, one of the two integral membrane proteins making up flavocytochrome b558 and the catalytic unit of the protein complex responsible for the oxidative burst in polymorphonuclear cells, have been identified in mammals [1–4]. The identification of new members of this family of enzymes has been an important step in understanding the role of ROS (reactive oxygen species) in the regulation of many biological processes, although their detailed involvement has not yet been fully defined. They are able to produce O2•−, which, more and more, seems implicated in cell signal transduction and communications [5]. For instance, NOXs (NADPH oxidases) may serve as an oxygen sensor [6], may be required for cell proliferation [7,8], may participate in vascular reactivity or smooth-muscle contractility [9,10], regulate the expression of some proteins [11,12] and play a role in signal transduction [13]. NOXs and DUOXs (dual oxidases) generate ROS in a regulated manner in response to different signals such as growth factors, cytokines, calcium etc. Surprisingly, overproduction of ROS due to NOX dysfunction is now suggested to be associated with several diseases, leading to increased bioavailability of ROS and subsequent oxidative damage [14–17].
Recently, we described the induction of glutathione synthesis by human fibroblasts triggered with DHA-met (docosahexaenoic methyl ester) [18]. The antioxidant response enhanced by human fibroblasts triggered with DHA-met was associated with strong production of ROS. While fatty acids are frequently described as potential NOX activators in endothelial cells or neutrophils [19–23], and more and more data report on NOX activity in fibroblasts [24–27], few results confirm the effect of fatty acids on fibroblasts and the type of NOX homologue involved in this ROS production. We were therefore interested in deciphering the relative contribution of NOX to ROS production in our cellular model triggered by PUFAs (polyunsaturated fatty acids).
MATERIALS AND METHODS
Chemicals
Chemicals were obtained as follows: lucigenin, DPI (diphenyl iodonium), SOD (superoxide dismutase), xanthine, plumbagin and apocynin from Sigma–Aldrich (Saint Quentin Fallavier, France); AA (arachidonic acid; C20:4,n−6), DHA (docosahexaenoic acid; C22:6,n−3), DHA-met, CLA (conjugated linoleic acid; C18:2 [9Z,11E]) and EPA (eicosapentaenoic acid; C20:5,n−3) from Cayman Chemical (Interchim, Montluçon, France); XO (xanthine oxidase) and NADPH from Roche (Roche, Meylan, France); and HE (hydroethidine) from Fluoprobe (Interchim). All other reagents were of analytical grade. All concentrations are final concentrations in incubation or culture medium.
Cell culture
Human dermal fibroblasts, a gift from Dr Odile Damour (Edouard Herriot Hospital Cornea Bank, Lyon, France), were grown in DMEM (Dulbecco's modified Eagle's medium) containing 10% (v/v) fetal calf serum (Gibco, Pontoise, France), 2.5 μg/ml fungizone, 98 units/ml penicillin and 98 μg/ml streptomycin (Cambrex, Emerainville, France). Cultures were maintained at 37 °C in a humidified atmosphere with 5% CO2. Cells were harvested after trypsinization and three PBS washes. The total cell lysates were obtained by two successive thawing–freezing cycles in 25 mM Tris/HCl buffer (buffer A) (pH 7.4) containing 0.1% Tween 20, with 15 s periods in an ultrasound bath. The total cell lysates were stored at −80 °C until analysis.
Protein measurement
Proteins were quantified by the BCA (bicinchoninic acid) method (BCA from Pierce, Interchim) according to the manufacturer's instructions.
HPLC analysis of E_OH+ (hydroxyethidium)
HE, E+ (ethidium) and E_OH+, the oxidation product of HE by O2•−, were separated by HPLC according to a method modified from Zhao et al. [28]. The HPLC system (Agilent HP 1100; Agilent Technologies, Massy, France) was equipped with a fluorescence (Kontron SFM25, Paris, France), or UV (Thermo Spectra system UV 6000 LP; Thermo Electron, Cergy-Pontoise, France) detector, or a mass spectrometer (Agilent LC/MSD SL) single quadrupole detector. The mobile phase was 15 mM ammonium acetate, adjusted to pH 4 with acetic acid and 35% (v/v) methanol. The stationary phase was a C18 reverse-phase column (Atlantis™; 2.1 mm×150 mm, dC18, 5 μm; Waters SAS, Guyancourt, France).
Samples were positive controls of superoxide anion production obtained by incubation at 37 °C of xanthine (500 μM) and XO [0.2 IU (international units)/ml] or cells and cell culture medium. One sample volume was mixed with 2 vol. of methanol chilled on ice and then centrifuged before injection. HE, E+ and E_OH+ were separated by gradient analysis with a linear increase in methanol concentration from 35 to 90% over 13 min at a flow rate of 0.2 ml/min, with a 5 μl injection volume. In a first step, the elution was monitored by a variable UV detector at 284 and 360 nm and a fluorescence detector with λex and λem of 465 and 575 nm respectively. For the mass spectrometer detector, detection was optimized in an ESI+ (positive electrospray ionization mode) with E_OH+ and set at 350 °C (drying gas), fragmentor at 175 V and capillary voltage at 3 kV. Chromatograms were recorded as TIC (total ion current) and in single ion monitoring mode at m/z+=314 for E+, 330 for E_OH+ and 301 for the main fragment obtained after cleavage of E_OH+ [28] as a control. HPLC peak areas were normalized to protein concentration.
Cell culture and measurements on fibroblasts grown with DHA-met or other PUFAs
Fibroblasts were grown either for 4, 8, 24 or 48 h in DMEM with 15 μM of DHA-met (final concentration) or for 4 h with AA, CLA and EPA at the same concentration.
In order to confirm the implication of NOX in ROS production, fibroblasts were grown in the presence of 2.5 μM apocynin or 1 μM plumbagin or 4 μM DPI, with and without 15 μM DHA-met. Culture controls were carried out with ethanol and DMSO in the culture medium.
For each culture condition, O2•− production was measured either by LC/MS (liquid chromatography MS) or by flow cytometry on intact cells. mRNA quantification by QPCR (quantitative PCR) was carried out on total cell lysates.
SOD activity
SOD activity was measured by a colorimetric assay, the RANSOD kit (RANDOX Laboratories, Montpellier Fréjorgues, France); O2•−, generated by a xanthine/XO reaction, oxidizes INT [2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyltetrazolium chloride] into a red formazan, and this oxidation is inhibited by SOD. Measurements were performed according to the manufacturer's instructions on a Delta Kone automatic analyser.
mRNA quantification by QPCR
Total RNA was isolated from fibroblasts by the total RNA isolation kit (Rneasy® mini kit; Qiagen, Courtaboeuf, France). Reverse transcription was performed with 1 μg of total RNA for each condition with a first-strand cDNA synthesis kit (Amersham, Orsay, France). QPCR was carried out on a Light Cycler® (Roche) by normalizing all cDNA to β-actin.
Sequences and fragment sizes of human-specific primers used for β-actin, NOX 1, 2 and 4, p22phox (the other subunit making up flavocytochrome b558) and SOD 1 are shown in Table 1.
Table 1. Primer sequences used for QPCR.
| Name | Accession no. | Direction | Primer sequence | Fragment length (bp) |
|---|---|---|---|---|
| β-Actin | NM_001101 | Forward | 5′-TCGTGCGTGACATTAAGGAG-3′ | 262 |
| Reverse | 5′-AGCACTGTGTTGGCGTACAG-3′ | |||
| NOX 1 | AJ 438989 | Forward | 5′-TCGAACAACAATATTCACCA-3′ | 225 |
| Reverse | 5′-TGGCCTTGTCAAAGTTTAAT-3′ | |||
| NOX 2 | NM_000397 | Forward | 5′-AGAGTTCGAAGACAACTGGA-3′ | 233 |
| Reverse | 5′-CCTCCTTCAGGGTTCTTTAT-3′ | |||
| NOX 4 | NM_016931 | Forward | 5′-CTTTTGGAAGTCCATTTGAG-3′ | 231 |
| Reverse | 5′-GTCTGTTCTCTTGCCAAAAC-3′ | |||
| p22phox | NM_000101 | Forward | 5′-CTTCACCCAGTGGTACTTT-3′ | 174 |
| Reverse | 5′-CGAACATAGTAATTCCTGGTA-3′ | |||
| SOD 1 | NM_000454 | Forward | 5′-AAGTACAAAGACAGGAAACG-3′ | 193 |
| Reverse | 5′-AGCAACTCTGAAAAAGTCAC-3′ |
Silencing of NOX 4
To confirm the involvement of NOX expression in DHA treatment, we inhibited NOX 4 expression with siRNA (small interfering RNA). Twenty-one-nucleotide double-stranded siRNAs (from Qiagen HP genome-wide siRNA databank, catalogue number: SI00117663; http://www.qiagen.com; 5 nM), targeting the NOX 4 sequence, were used. Non-silencing fluorescein-labelled control (5 nM) was used as the negative siRNA control (scrambled siRNA).
siRNA transfection to fibroblasts was performed according to the manufacturer's instructions and was monitored with the control siRNA labelled with fluorescein (scrambled siRNA). Quantitative RT–PCR (reverse transcriptase PCR) was performed to compare NOX 4 mRNA degradation in the presence and absence of siNOX 4 (small interference oligonucleotide RNA directed against NOX 4).
After 36 h silencing, fibroblasts were triggered by DHA for 4 h and cellular ROS production on whole cells was performed by flow cytometry.
Evaluation of cellular ROS production by flow cytometry
In order to assess the production of ROS and superoxide anion, fibroblasts were loaded with HE. After 0, 4, 8, 24 and 48 h of culture, cells were harvested and washed twice with PBS, and incubated with 2 μM of HE in PBS at 37 °C for 30 min. Then the cell suspension was submitted to fluorescent flow cytometry analysis (FACScan flow cytometer; BD Biosciences) on a logarithmic scale for 10000 events (cell counts). The oxidation of HE was measured, with the excitation set at 488 nm, as an increase in fluorescence at 585 nm.
Statistical analysis
Statistical analysis was carried out by GraphPad analysis (version 2.1). All results are expressed as means±S.E.M. Comparisons among groups were performed by the Student's t test or one-way ANOVA, with Dunnett's multiple range tests. The significance level was 0.05.
RESULTS
Effects of inhibitors and fatty acids on whole cells at 4 h
We investigated the fluorescence of ROS production of fibroblasts triggered for 4 h with DPI (results not shown), apocynine, plumbagin and/or with fatty acids. The vehicle controls – DMSO for DPI, ethanol for apocynin, plumbagin and DHA-met – had no significant effect on ROS production. DPI increased the production of ROS at 4 h to 143±4% of the control, and this increase was cumulative with that due to DHA, reaching 185±1%.
On the other hand, E_OH+ fluorescence induced by DHA alone was 165±18% of the control and that of the inhibitors, alone or with DHA, was between 104 and 112% of the control (Figure 1A).
Figure 1. Effects of specific inhibitors (A) and fatty acids (B) on whole cells (fibroblasts grown for 4 h).
Each bar corresponds to the mean for at least three assays (*P<0.05, **P<0.01, ***P<0.001). (A) Apocynin or plumbagin inhibition of the fluorescence emitted by fibroblasts triggered by DHA. Controls correspond to fibroblasts grown for 4 h with ethanol (negative), DHA (positive) or inhibitor alone. (B) Enhancement of fluorescence of fibroblasts triggered with 15 μM of AA, DHA or EPA for 4 h. Unlike other PUFAs, CLA decreases the fluorescence. Control corresponds to fibroblasts triggered with ethanol for 4 h.
The effects of fatty acids on fluorescence produced by whole cells (through NOX activity) are shown in Figure 1(B): both DHA and EPA significantly increased cell fluorescence (148±23% and 143±16%) and only AA increased the signal up to 202±10%. Fluorescence of fibroblasts triggered by CLA for 4 h was lower than that of the control, but not significantly.
HPLC analysis of E_OH+
As described by Zhao et al. [28], HE is known to produce a specific oxidation product, named E_OH+, in the presence of superoxide anion. In order to demonstrate the production of superoxide by fibroblasts triggered by fatty acids, we performed LC/MS analysis of E_OH+ on different samples including culture media and cells.
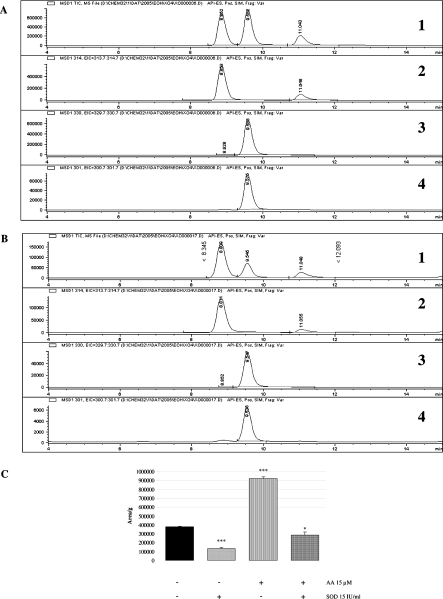
As a positive control for superoxide anion production, we used a xanthine–XO assay for which typical chromatograms are shown in Figure 2(A). The TIC chromatogram is shown on the upper graph, and specific ion current chromatograms are displayed for m/z+=314 for E+ [Figure 2A(2)], 330 for E_OH+ [Figure 2A(3)] and 301 for the main fragment of 330 [Figure 2A(4)]. Obviously m/z+=330 and 301 have the same RT (retention time), which is different from that of both HE (RT=11.04) and E+ (RT=8.85). Figure 2(B) shows similar chromatograms obtained for fibroblasts triggered for 2 h with 15 μM AA and 5 μM HE. Figure 2(C) displays the results (mean of triplicate) for culture media, under the same conditions, with a 2.4-fold increase in E_OH+ production and a production similar to ethanol control if SOD (15 IU/ml) is added. These experiments are a further confirmation of superoxide anion production by NOX of fibroblasts triggered by different PUFAs.
Figure 2. LC/MS analysis of E_OH+ produced from O2•− with HE.
(A, B) Chromatograms of a xanthine–XO assay and of a total cell lysate respectively. TIC chromatograms are shown in (A1, B1). Specific chromatograms are displayed at m/z+=314 for E+ (at RT=8.8) in (A2, B2), at m/z+=330 for E_OH+ (at RT=9.6) in (A3, B3) and at m/z+=301 for the main fragment of E_OH+ (at RT=9.6) in (A4, B4). (C) E_OH+ production in cell culture medium by fibroblasts triggered for 2 h by AA at 15 μM, with or without SOD at 15 IU/ml. Each bar is the mean for triplicates (*P<0.05, ***P<0.001).
Time course analysis of cell culture with DHA-met
We grew human fibroblasts with 15 μM of DHA-met for up to 48 h. Fluorescent measurements of O2•− production were performed at 0, 4, 8, 24 and 48 h. At the same time points, different mRNAs were quantified by quantitative RT–PCR.
During this time period, neither SOD 1 mRNA expression nor total SOD catalytic activity were significantly different from the control (results not shown).
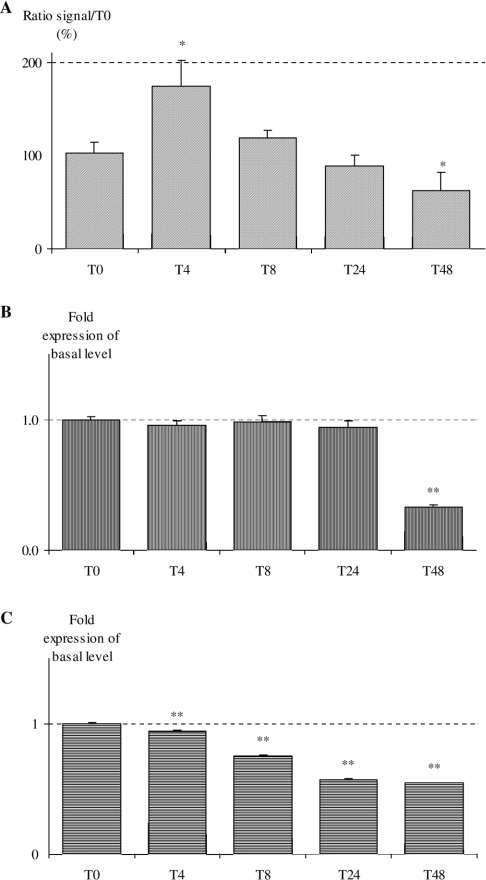
O2•− production measured by fluorescence of E_OH+ (Figure 3A) reached a peak at 4 h (174±28%) and then decreased until 48 h. Cell culture controls (fibroblasts at zero time and fibroblasts grown with ethanol for 48 h) were not significantly different from each other.
Figure 3. Time course of ROS production (A) and mRNA expression (B, C) in fibroblasts grown with 15 μM DHA-met for 48 h.
Ethanol is the vehicle control at 48 h. All results are expressed as a ratio with the reference at zero time (*P<0.05, **P<0.01). (A) Evaluation of total ROS production by E_OH+ fluorescence. (B) NOX 4 mRNA expression. (C) p22phox mRNA expression.
Quantification of mRNA for NOX 4 and p22phox
First, we investigated the mRNA expression of the different NOX homologues (NOX 1, 2 and 4) in human fibroblasts by agarose gel electrophoresis and RT–PCR. Amplification was detected only with NOX 4 and p22phox primers on fibroblasts, whereas NOX 2 primers were able to detect mRNA expression in leucocytes as a control.
Secondly, the time course of mRNA expression for NOX 4 showed a significant decrease at 48 h (Figure 3B). During the same time period, p22phox mRNA expression decreased gradually (Figure 3C), and the absence of mRNA expression for NOX 1 and 2 was confirmed.
Silencing of NOX 4
To confirm the involvement of NOX 4 expression in response to DHA treatment, we inhibited NOX 4 expression with siRNA.
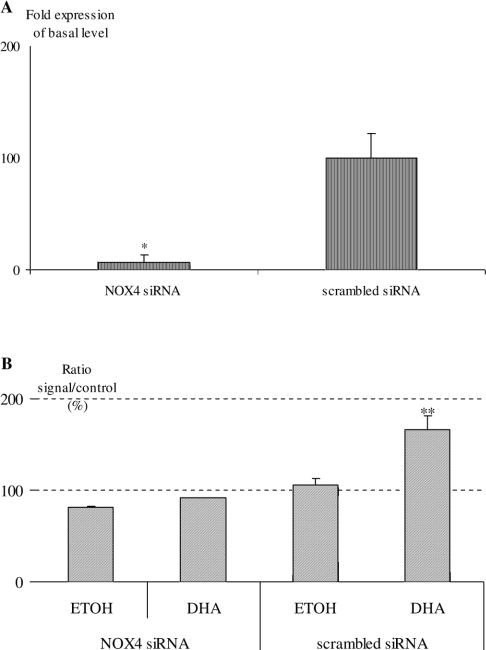
After 36 h silencing, quantitative RT–PCRs were performed to compare NOX 4 mRNA degradation in the presence and absence of siNOX 4 (Figure 4A). Expression remained at only 6% of basal level.
Figure 4. Silencing of NOX 4 in human fibroblasts.
(A) Quantitative RT–PCR of NOX 4 mRNA after 36 h silencing in the presence and absence of siNOX 4 on fibroblasts. (B) ROS production measured by E_OH+ on whole cells triggered by DHA for 4 h, after 36 h silencing in the presence and absence of siNOX 4. Non-silencing control (5 nM) was used as the negative siRNA control (scrambled siRNA) (*P<0.05, **P<0.01). ETOH, ethanol.
Fibroblasts treated with siNOX 4 were triggered by DHA for 4 h. Neither NOX catalytic activity on total cell lysates (results not shown) nor fluorescent ROS production performed on whole cells (Figure 4B) showed any activation under DHA and siNOX 4.
DISCUSSION
NOXs, cellular sources of ROS, use O2 and NADPH as substrates and FAD as a coenzyme. As sources of ROS, they are detectable by lucigenin luminescence, a fast tool for screening of NOX catalytic activity. However, lucigenin specificity is often questioned and we were strongly advised to use E_OH+ analysis either by fluorescence or by LC/MS for O2•− production. NOXs, particularly NOX 1 and 2, are claimed to be activated by some fatty acids [19–23]. In human fibroblasts, Dhaunsi et al. [24] reported the effect of only very-long-chain fatty acids on NOX 2 activity. In our model, with fibroblasts grown with DHA-met (C22:6,n−3), an increase in ROS production at 4 h was observed [18]. This model therefore seemed appropriate for deciphering the relative contribution of NOX isoenzymes to this ROS production and for investigating the potential modulation of this catalytic activity by fatty acids.
Preliminary results showed that ROS production obtained with cell lysates incubated with NADPH was totally inhibited by DPI (a well-known NOX inhibitor acting on FAD coenzyme) and with added SOD, the enzyme responsible for physiological O2•− degradation. As demonstrated by nitrogen bubbling, this signal was also dependent on O2. The latter inhibition was within a very close range of those obtained with DPI or SOD, suggesting that a unique catalytic activity, dependent on a FAD coenzyme, required NADPH and O2 for O2•− production, as these ROS were SOD-sensitive. Moreover, inhibition was in the same range for specific NOX inhibitors (plumbagin [29] and apocynin) as well as for non-specific inhibitors (DPI or SOD, specific for O2•− but not for NOX). These results strongly suggested that this ROS production was due essentially to NOX activity.
To confirm this hypothesis, we investigated the action of NOX inhibitors such as apocynin, plumbagin or DPI on whole cells triggered by DHA. As shown in Figure 1(A), apocynin, a specific NOX inhibitor that prevents NOX subunit assembly, totally inhibited ROS production under DHA, thus confirming the participation of NOX in the production of ROS at 4 h. Moreover, plumbagin, described as a NOX 4 inhibitor [29], also totally inhibited ROS production under DHA, thus defining NOX 4 as the NOX homologue of our fibroblasts.
Unlike apocynin, DPI acts by covalent binding to FAD; in this manner, it is a general inhibitor of all enzymes requiring FAD as a coenzyme. As described in the literature [30,31], DPI induces oxidative stress and apoptosis via mitochondrial O2•− production. Our results confirmed these observations, and also that DPI, active on many enzymes ex vivo, is not relevant for the investigation of NOX activity in intact cells.
Quantification by RT–PCR of mRNA for NOXs confirmed that mRNA for NOX 4 was the only NOX homologue mRNA present to explain ROS production. After 36 h silencing with siRNA for NOX 4, quantitative RT–PCR showed NOX 4 mRNA degradation (Figure 4A). Furthermore fibroblasts silenced by siNOX 4 (for 36 h) and triggered by DHA for 4 h displayed no cellular ROS production (Figure 4B) unlike fibroblasts silenced by the scrambled siRNA. Associated with the fact that NOX catalytic activity measurement [32] and E_OH+ fluorescence on whole cells show a similar pattern, these results strongly confirm the role of NOX 4 as the only NOX isoenzyme for ROS production in human fibroblasts and are in agreement with very recent publications on fibroblasts [25,27,33].
Lipids in general, and AA in particular, are known to interact with NOX in phagocytic and non-phagocytic cells [19–24], and a recent publication has implicated very-long-chain fatty acids in the activation of NOX in some peroxisomal disorders of human dermal fibroblasts [24]. In a previous publication [32], we investigated the effects of DHA, free or as a methyl ester, and of AA on fibroblast extracts: in our experiments, neither calcium, described as a NOX 5 activator [34], nor AA alone, known as a gp91phox activator [21,22], had any effect on NOX catalytic activity of fibroblast lysates. However, AA associated with calcium strongly increased NOX activity (175% of the control); this result recalls the work of Cui and Douglas [19]. According to these authors, AA activates the c-Jun N-terminal kinase through NOX in rabbit proximal tubular epithelial cells. In our model, NOX catalytic activity was due to NOX 4, as demonstrated by RT–PCR. In agreement with previous results on Renox, the first name of NOX 4 [19,21], NOX 4 was responsive to AA and required Ca2+ mobilization.
Unexpectedly, neither DHA (free or as a methyl ester) nor EPA, both with calcium, activated NOX on cell lysates [32], whereas they strongly induced NOX activity on whole fibroblasts triggered for 4 h (Figure 1B). Very recently, we showed that, in fibroblasts triggered by DHA-met, DHA increased 3-fold and induced a profound change in total cell lipid composition [18] with a low cellular AA increase. These results strongly suggest that, when PUFAs induce a huge O2•− production, it could be due to release of AA by membranes, with subsequent NOX activation. Thus this increase in O2•− production in the first four hours could play the role of an intracellular messenger, a role already suggested in the model by Cui and Douglas [19] in 1997 after AA activation and more recently by Colston et al. [25] in 2005. A general mechanism involving AA and calcium should be in agreement with the results of Bouzidi et al. [35], who reported this association as a NOX activator with an effect mediated by the myeloid-related proteins (S100A8/A9), which bind both calcium and AA. Furthermore, the hypothesis that AA is released from membranes could explain the similar activation of NOX obtained with many lipids: according to Rouhanizadeh et al. [23], 'the specific mechanism(s) by which ox-PAPC (oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine) induces NADPH oxidase activity and O2•− production remain to be defined and the effect may be similar to that reported for lysophosphatidylcholine (LPS) which stimulates monocyte chemoattractant protein-1 (MCP-1) expression and O2•− production by NADPH oxidase. Similar activation is also reported for other lysolipids such as phosphatidic acid and platelet-activating factors, which induce mitogenic signaling pathways.'
We previously showed that fibroblasts triggered by DHA [18] and other PUFAs, except CLA [36], produce superoxide anion and activate an antioxidant response. Now we demonstrate that this ROS production is due to NOX 4 activation. During this antioxidant response, we were unable to detect any change either in mRNA SOD 1 expression or total cellular catalytic SOD activity. For this reason, regulation of ROS production by cellular SOD seems to be excluded, at least during the relatively short time periods of our experiments. However, during the antioxidant response triggered by different PUFAs [18,36], we demonstrate the induction of HO-1 (haem oxygenase 1), a typical enzyme of the antioxidant response, as it contains some antioxidant responsive elements in its gene-promoter region. HO-1 produces CO through haem degradation and its induction strongly suggests that, apart from regulation at the transcriptional level [37], confirmed by a decrease in NOX 4 and p22phox mRNA expression, a further factor of regulation for NOX activity, as suggested recently [32], could be through CO inhibition [38] or haem degradation [39].
As CLA is the only PUFA able to induce an antioxidant response (testified by glutathione synthesis up-regulation) without NOX 4 activation, our cellular model may also be useful to explore the different facets and steps leading to a ‘physiological’ defence mechanism, the antioxidant response, and question the exact role of ROS for its signalling.
Acknowledgments
We thank Ms Sarah Somerville [International Agency for Research on Cancer (IARC), Lyon, France] for careful English editing.
References
- 1.Meier B., Jesaitis A. J., Emmendorffer A., Roesler J., Quinn M. T. The cytochrome b-558 molecules involved in the fibroblast and polymorphonuclear leucocyte superoxide-generating NADPH oxidase systems are structurally and genetically distinct. Biochem. J. 1993;289:481–486. doi: 10.1042/bj2890481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng G., Cao Z., Xu X., Van Meir E. G., Lambeth J. D. Homologs of gp91phox: cloning and tissue expression of NOX 3, NOX 4, and NOX 5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 3.Rao P. V., Maddala R., John F., Zigler J. S., Jr Expression of nonphagocytic NADPH oxidase system in the ocular lens. Mol. Vis. 2004;10:112–121. [PubMed] [Google Scholar]
- 4.Shiose A., Kuroda J., Tsuruya K., Hirai M., Hirakata H., Naito S., Hattori M., Sakaki Y., Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 5.Lambeth J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 6.Jones R. D., Hancock J. T., Morice A. H. NADPH oxidase: a universal oxygen sensor? Free Radical Biol. Med. 2000;29:416–424. doi: 10.1016/s0891-5849(00)00320-8. [DOI] [PubMed] [Google Scholar]
- 7.Arnold R. S., Shi J., Murad E., Whalen A. M., Sun C. Q., Polavarapu R., Parthasarathy S., Petros J. A., Lambeth J. D. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase NOX 1. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abid M. R., Kachra Z., Spokes K. C., Aird W. C. NADPH oxidase activity is requierd for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–256. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- 9.Souza H. P., Laurindo F. R. M., Zeigelstein R. C., Berlowitz C. O., Zweier J. L. Vascular NAD(P)H oxidase is distinct from the phagocytic enzyme and modulates vascular reactivity control. Am. J. Physiol. Heart. Circ. Physiol. 2001;280:H658–H667. doi: 10.1152/ajpheart.2001.280.2.H658. [DOI] [PubMed] [Google Scholar]
- 10.Thabut G., El-Benna J., Samb A., Corda S., Megret J., Leseche G., Vicaut E., Aubier M., Boczkowski J. Tumor necrosis factor-α increases airway smooth muscle oxidants production through a NADPH oxidase-like system to enhance myosin light chain phosphorylation and contractility. J. Biol. Chem. 2002;277:22814–22821. doi: 10.1074/jbc.M200315200. [DOI] [PubMed] [Google Scholar]
- 11.Rupin A., Paysant J., Sansilvestri-Morel P., Lembrez N., Lacoste J.-M., Cordi A., Verbeuren T. J. Role of NADPH oxidase-mediated superoxide production in the regulation of E-selectin expression by endothelial cells subjected to anoxia/reoxygenation. Cardiovasc. Res. 2004;63:323–330. doi: 10.1016/j.cardiores.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Fan J., Fey R. S., Rahman A., Malik A. B. Role of neutrophil NADPH oxidase in the mechanisim of tumor necrosis factor-α-induced NF-κB activation and intracellular adhesion molecule-1 expression in endothelial cells. J. Biol. Chem. 2002;277:3404–3411. doi: 10.1074/jbc.M110054200. [DOI] [PubMed] [Google Scholar]
- 13.Mahadev K., Motoshima H., Wu X., Ruddy J. M., Arnold R. S., Cheng G., Lambeth J. D., Goldstein B. J. The NAD(P)H oxidase homolog NOX 4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer J. W., Schmitt M. E. A central role for the endothelial NADPH oxidase in atherosclerosis. FEBS Lett. 2000;472:1–4. doi: 10.1016/s0014-5793(00)01397-1. [DOI] [PubMed] [Google Scholar]
- 15.Kaminski K. A., Bonda T. A., Korecki J., Musial W. J. Oxidative stress and neutrophil activation – the two keystones of ischemia/reperfusion injury. Int. J. Cardiol. 2002;86:41–59. doi: 10.1016/s0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 16.Park Y. M., Park M. Y., Suh Y.-L., Park J. B. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem. Biophys. Res. Commun. 2004;313:812–817. doi: 10.1016/j.bbrc.2003.11.173. [DOI] [PubMed] [Google Scholar]
- 17.Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J. C., Pouzet C., Samadi M., Elbim C., et al. NAD(P)H oxidase NOX 4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arab K., Rossary A., Flourié F., Tourneur Y., Steghens J.-P. Docosahexaenoic acid enhances the antioxidant response of human fibroblasts by upregulating γ-glutamyl-cysteinyl ligase and glutathione reductase. Br. J. Nutr. 2006;95:18–26. doi: 10.1079/bjn20051626. [DOI] [PubMed] [Google Scholar]
- 19.Cui X. L., Douglas J. G. Arachidonic acid activates c-jun N-terminal kinase through NADPH oxidase in rabbit proximal tubular epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3771–3776. doi: 10.1073/pnas.94.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zafari A. M., Ushio-Fukai M., Minieri C. A., Akers M., Lassegue B., Greindling K. K. Arachidonic acid metabolites mediate angiotensin II-induced NADH/NADPH oxidase activity and hypertrophy in vascular smooth muscle cells. Antioxid. Redox. Signal. 1999;1:167–179. doi: 10.1089/ars.1999.1.2-167. [DOI] [PubMed] [Google Scholar]
- 21.Cherny V. V., Henderson L. M., Xu W., Thomas L. L., DeCoursey T. E. Activation of NADPH oxidase-related proton and electron currents in human eosinophils by arachidonic acid. J. Physiol. 2001;535:783–794. doi: 10.1111/j.1469-7793.2001.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao D., Segal A. W., Dekker L. V. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Lett. 2003;550:101–106. doi: 10.1016/s0014-5793(03)00845-7. [DOI] [PubMed] [Google Scholar]
- 23.Rouhanizadeh M., Hwang J., Clempus R. E., Marcu L., Lassegue B., Sevanian A., Hsiai T. K. Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: implication of NADPH oxidase. Free Radical Biol. Med. 2005;39:1512–1522. doi: 10.1016/j.freeradbiomed.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhaunsi G. S., Kaur J., Alsaeid K., Turner R. B., Bitar M. S. Very long chain fatty acids activate NADPH oxidase in human dermal fibroblasts. Cell. Biochem. Funct. 2005;23:65–68. doi: 10.1002/cbf.1173. [DOI] [PubMed] [Google Scholar]
- 25.Colston J. T., de la Rosa S. D., Strader J. R., Anderson M. A., Freeman G. L. H2O2 activates NOX 4 through PLA2-dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett. 2005;579:2533–2540. doi: 10.1016/j.febslet.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 26.Dhaunsi G. S., Paintlia M. K., Kaur J., Turner R. B. NADPH oxidase in human lung fibroblasts. J. Biomed. Sci. 2004;11:617–622. doi: 10.1007/BF02256127. [DOI] [PubMed] [Google Scholar]
- 27.Cucoranu I., Clempus R., Dikalova A., Phelan P. J., Ariyan S., Dikalov S., Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H., Joseph J., Fales H. M., Sokoloski E. A., Levine R. L., Vasquez-Vivar J., Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding Y., Chen Z. J., Liu S., Che D., Vetter M., Chang C. H. Inhibition of NOX 4 activity by plumbagin, a plant-derived bioactive naphtoquinone. J. Pharm. Pharmacol. 2005;57:111–116. doi: 10.1211/0022357055119. [DOI] [PubMed] [Google Scholar]
- 30.Riganti C., Gazzano E., Polimeni M., Costamagna C., Bosia A., Ghigo D. Diphenyleneiodonium inhibits the cell redox metabolism and induces oxidatives stress. J. Biol. Chem. 2004;279:47726–47731. doi: 10.1074/jbc.M406314200. [DOI] [PubMed] [Google Scholar]
- 31.Li N., Ragheb K., Lawler G., Sturgis J., Rajwa B., Melendez J. A., Robinson J. P. DPI induces mitochondrial superoxide-mediated apoptosis. Free Radical Biol. Med. 2003;34:465–477. doi: 10.1016/s0891-5849(02)01325-4. [DOI] [PubMed] [Google Scholar]
- 32.Rossary A., Arab K., Goudable J., Steghens J.-P. Régulation de l'activité des NADPH oxydases en présence d'acides gras. Ann. Biol. Clin. (Paris) 2007;65:33–40. [PubMed] [Google Scholar]
- 33.Li J., Stouffs M., Serrander L., Banfi B., Bettiol E., Charnay Y., Steger K., Krause K.-H., Jaconi M. E. The NADPH oxidase NOX 4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol. Biol. Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banfi B., Molnar G., Maturana A., Steger K., Hegedus B., Demaurex N., Krause K. H. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 35.Bouzidi F., Doussiere J. Binding of arachidonic acid to myeloid-related proteins (S100A8/A9) enhances phagocytic NADPH oxidase activation. Biochem. Biophys. Res. Commun. 2004;325:1060–1065. doi: 10.1016/j.bbrc.2004.10.134. [DOI] [PubMed] [Google Scholar]
- 36.Arab K., Rossary A., Soulère L., Steghens J.-P. Conjugated linoleic acid, unlike other unsaturated fatty acids, strongly induces glutathione synthesis without any lipoperoxidation. Br. J. Nutr. 2006;96:811–819. doi: 10.1017/bjn20061910. [DOI] [PubMed] [Google Scholar]
- 37.Martyn K. D., Frederick L. M., von Loehneysen K., Dinauer M. C., Knaus U. G. Functional analysis of NOX 4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signalling. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 38.Kim H. P., Ryter S. W., Choi A. M. CO as a cellular signaling molecule. Annu. Rev. Pharmacol. Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 39.Taillé C., El-Benna J., Lanone S., Dang M. C., Ogier-Denis E., Aubier M., Boczkowski J. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J. Biol. Chem. 2004;279:28681–28688. doi: 10.1074/jbc.M310661200. [DOI] [PubMed] [Google Scholar]