Abstract
HTLV-I infection is associated with the development of adult T cell leukemia (ATL) and the neuroinflammatory disease HAM/TSP. There are quantitative and qualitative differences in the antiviral cytotoxic T cell (CTL) response in ATL and HAM/TSP although the underlying mechanisms are unclear. Here, we demonstrate that the HTLV-I Tax trans-activating protein is a transcriptional activator of CD40 Ligand (CD40L), a critical regulator of dendritic cell maturation and adaptive immunity. Tax activates CD40L expression via a cyclosporin A insensitive pathway that is also independent of NF-κB. Although Tax upregulates CD40L gene expression, CD40L expression is absent in Tax-expressing HTLV-I transformed cell lines via an epigenetic mechanism involving methylation. T lymphocytes cultured ex vivo from ATL patients, but not HAM/TSP or normal controls, exhibit a potent block in the induction of CD40L, but not CD69. However, the CD40L gene is not silenced by methylation in ATL patients, thus CD40L is downregulated by distinct mechanisms in HTLV-I transformed cell lines and ATL patients.
Keywords: HTLV-I, Tax, ATL, HAM/TSP, CD40L, NFAT, NF-κB, methylation, gene expression
Introduction
Human T cell leukemia virus type I (HTLV-I) infection is associated with adult T cell leukemia (ATL) and a neuroinflammatory disease termed HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP). The HTLV-I Tax protein is a potent oncogene that regulates viral and cellular gene expression and disrupts normal control of the cell cycle (Jeang et al., 2004). Tax exerts its oncogenic activity, in part, by modulating the expression of a large number of host genes, particularly those involved in lymphocyte activation, survival and transcription (Harhaj et al., 1999; Ng et al., 2001). The effect of Tax on cellular gene expression is largely mediated by activation of signaling pathways such as NF-κB, CREB, AP-1 and Nuclear factor of activated T cells (NFAT) (Hall and Fujii, 2005; Harhaj and Harhaj, 2005).
CD40 ligand (CD40L), or CD154, is a member of the tumor necrosis factor (TNF) superfamily and interacts with CD40 to mediate humoral and cellular immune responses against pathogens (Foy et al., 1996). CD40L expression is mostly restricted to activated T lymphocytes and is expressed transiently whereas CD40 is expressed in a wide variety of cells, including B lymphocytes and dendritic cells (DCs) (van Kooten and Banchereau, 2000). Engagement of CD40 is required for B cell maturation, survival and immunoglobulin class switching (van Kooten and Banchereau, 2000). In DCs, triggering of CD40 leads to maturation and efficient priming of CD8+ T lymphocytes (Clarke, 2000). The importance of CD40 and CD40L for immune responses in vivo is underscored by the genetic disease hyper-IgM syndrome caused by mutations in either CD40L or CD40 that result in ineffective T cell responses and impaired immunoglobulin isotype class switching (Aruffo et al., 1993; Bhushan and Covey, 2001).
CD40L is expressed on activated naïve T lymphocytes in two distinct phases, early, which occurs within 24 hours of antigen receptor signaling, and late which occurs after 24 hours (Lee et al., 2002). Cytokines such as IL-4 and IL-12 influence the expression of CD40L during the late but not early phase (Lee et al., 2002). Whereas IL-4 expression is associated with the absence of CD40L during the late phase, IL-12 is associated with prolonged expression of CD40L during the late phase (Lee et al., 2002). Thus, the duration of CD40L expression is tightly regulated and has important biological consequences. Increased and prolonged expression of CD40L on activated T lymphocytes has been associated with autoimmune diseases such as systemic lupus erythematosus (Koshy, Berger, and Crow, 1996). Overexpression of CD40L has also been associated with other autoimmune diseases such as inflammatory bowel disease (IBD) (Liu et al., 1999) and rheumatoid arthritis (MacDonald et al., 1997). Conversely, a lack of CD40L expression in response to anti-CD3 and anti-CD28 stimulation has been associated with immunodeficiency which is observed with HIV-1 infection (Zhang et al., 2004).
The transcription of CD40L is tightly regulated in activated T lymphocytes. CD40L expression requires signals generated from T cell antigen receptor signaling, including NFAT and NF-κB (Cron, 2003). CD40L expression is potently inhibited by cyclosporin A in activated primary T lymphocytes, suggesting a requirement for the calcium responsive phosphatase calcineurin which serves to dephosphorylate NFAT transcription factors, leading to their nuclear localization (Fuleihan et al., 1994). CD40L may also be regulated, in a coordinated manner with CD40, by the AT-hook transcription factor AKNA (Siddiqa et al., 2001). A recent study also supports a critical role for the early growth response 1 (Egr-1) transcription factor in CD40L expression (Cron et al., 2006). The CD40L upstream promoter region, consisting of a 1.3 kb 5'-flanking region, contains cis-elements for several transcription factors, including NFAT (Lobo et al., 2000; Schubert et al., 1995), NF-κB (Srahna et al., 2001), AP-1 (Tsytsykova, Tsitsikov, and Geha, 1996) and Egr family members (Cron et al., 2006). It is likely that optimal CD40L expression requires the cooperation of multiple families of transcription factors that converge on the CD40L promoter. An additional enhancer composed of an NF-κB site has been identified in the 3'-flanking region of the CD40L gene (Schubert et al., 2002). CD40L is also regulated at the post-transcriptional level in activated T lymphocytes where a complex of proteins assembles on the 3'UTR region of CD40L mRNA and regulates stability of the mRNA (Ford et al., 1999).
Whereas ATL patients exhibit immunodeficiency and ineffective anti-HTLV-I cell-mediated immunity (Kozako et al., 2006; Taguchi and Miyoshi, 1989), HAM/TSP patients experience neuroinflammation and possess extremely high frequencies of circulating anti-HTLV-I CD8+ T cells (Kubota et al., 2000). The mechanisms accounting for these highly divergent immune responses in ATL and HAM/TSP are largely unknown. We hypothesize that distinct patterns of gene expression in infected cells from ATL and HAM/TSP patients may play a role in disease progression and pathogenesis. We have previously demonstrated that CD40 is aberrantly expressed in HTLV-I transformed cell lines and is upregulated by Tax (Harhaj et al., 2005). In a gene array analysis in our previous study, we also observed that CD40L may be regulated by Tax (Harhaj et al., 2005). In this report, we demonstrate that Tax is a transcriptional regulator of the CD40L gene. Surprisingly, Tax upregulates CD40L expression in a cyclosporin A insensitive manner. Furthermore, CD40L expression is absent in Tax-expressing HTLV-I transformed cell lines due to an epigenetic mechanism involving methylation. In ATL, but not HAM/TSP patients, there is a profound block in the induction of CD40L, but not CD69, in PBMCs stimulated ex vivo that does not appear to be mediated by epigenetic mechanisms. These results may help explain the severe immunodeficiency and defective antiviral CTL responses commonly observed in ATL patients.
Results
Tax upregulates the expression of CD40L mRNA and protein
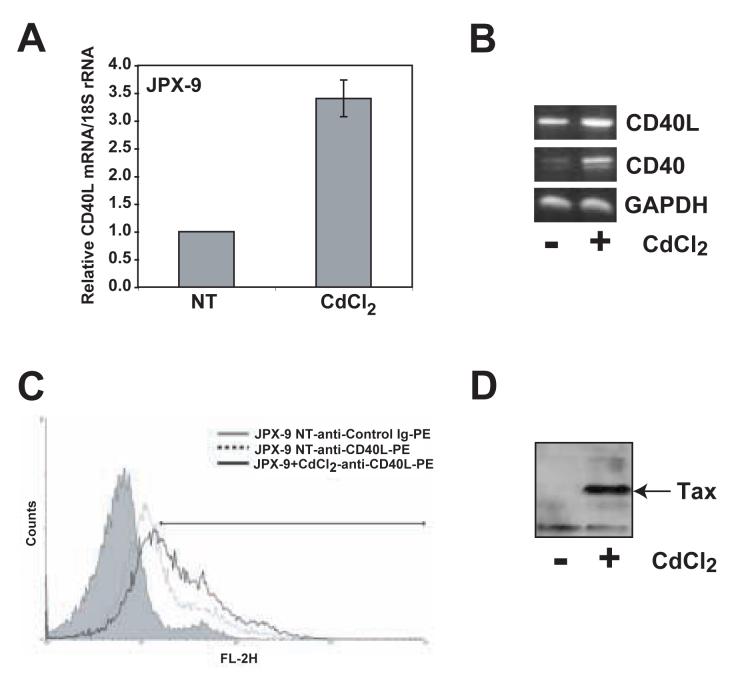
Tax promotes the uncontrolled proliferation and immortalization of T lymphocytes by deregulating cellular signaling pathways and gene expression. In a previous study from our laboratory, we used gene array analysis to identify several genes from the TNF/TNFR superfamilies that may be regulated by Tax (Harhaj et al., 2005). One of the putative Tax regulated genes was CD40L, an important costimulatory molecule expressed on activated T lymphocytes that galvanizes adaptive immune responses. To confirm that Tax was indeed upregulating the expression of CD40L, we used the Tax-inducible Jurkat subline JPX-9 which stably expresses Tax under the control of a metallothionein promoter (Fujii et al., 1991). JPX-9 cells were treated with CdCl2 for 24 hr to induce Tax expression and total RNA was isolated from the cells for Real-Time RT-PCR analysis. CD40L mRNA was upregulated by Tax as demonstrated by Real-Time RT-PCR (Fig. 1A) and RT-PCR (Fig. 1B). Furthermore, we used flow cytometry to demonstrate that CD40L protein was upregulated on the cell surface of CdCl2-treated JPX-9 cells (Fig. 1C). As expected, Tax expression was induced by CdCl2 treatment (Fig. 1D).
Figure 1. HTLV-I Tax induces the expression of CD40L in JPX-9 cells.
Tax expression in JPX-9 cells was induced by incubating the cells with 15 μM CdCl2 for 24 hours. Tax-induced CD40L expression was analyzed at mRNA and protein levels. (A) Real time PCR analysis of CD40L mRNA expression in JPX-9 cells with or without CdCl2. CD40L mRNA expression was normalized to expression of 18S rRNA in the same real time PCR reaction. The graph depicts the results of 3 experiments. (B) RT-PCR analysis of CD40L (top panel), CD40 (middle panel) and GAPDH (lower panel) mRNA expression in JPX-9 cells. (C) Flow cytometry analysis of cell surface CD40L protein expression in JPX-9 cells with or without CdCl2. Cells were stained with PE-conjugated anti-CD40L antibody. Control cells were stained with irrelevant antibody of the same isotype as the anti-CD40L antibody. At least 10,000 gated cells were analyzed for PE expression. (D) CdCl2 induced expression of HTLV-I Tax was confirmed by Western blot analysis of JPX-9 whole cell lysates using anti-Tax hybridoma supernatant.
Tax-mediated upregulation of CD40L does not require NF-κB and is insensitive to cyclosporin A
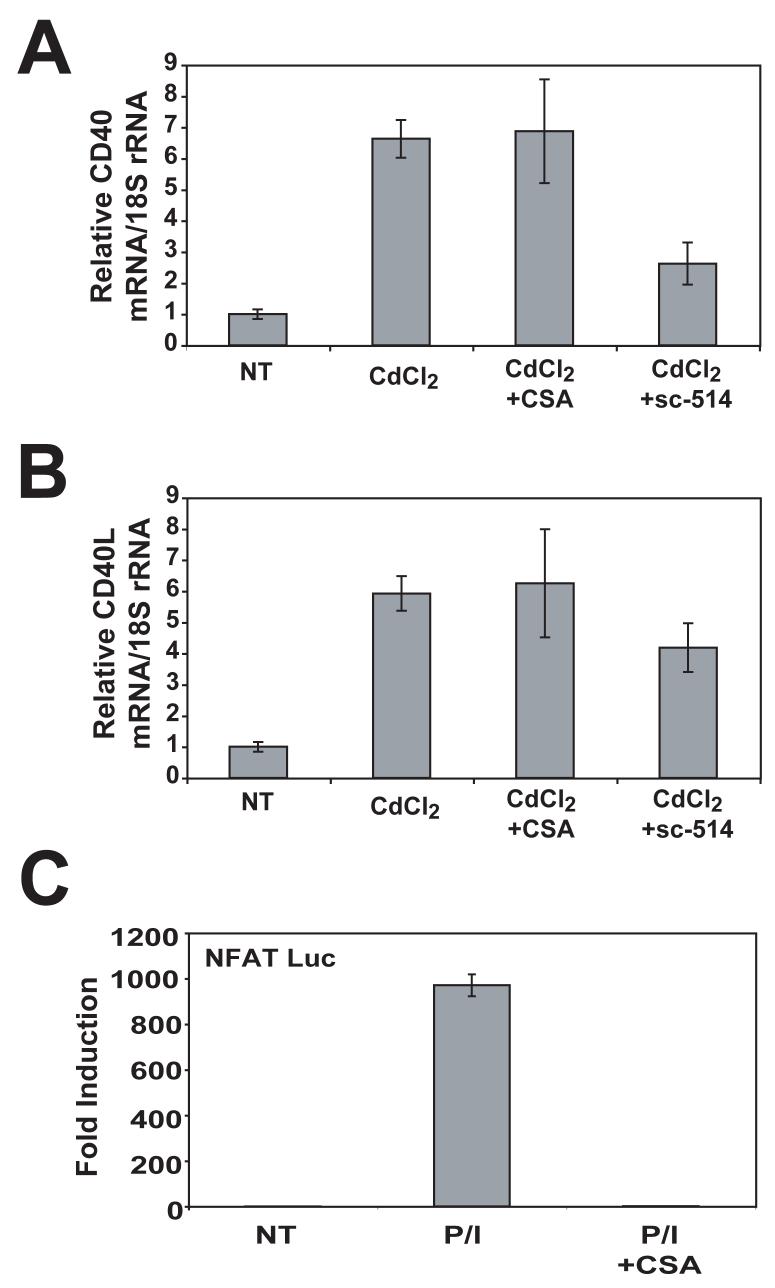
To determine the signaling pathways that governed Tax-induced CD40L induction, we used pharmacological inhibitors of NF-κB and calcineurin, sc-514 and cyclosporin A (CsA) respectively. CD40L mRNA is strongly induced in activated T lymphocytes and requires multiple signals including calcium signaling that promotes calcineurin-dependent NFAT activation (Cron, 2003). Treatment of activated T lymphocytes with CsA leads to potent inhibition of CD40L transcription (Fuleihan et al., 1994). In addition, NF-κB has been reported to play a role in CD40L expression induced by antigen receptor signaling (Srahna et al., 2001). To determine if Tax required calcineurin for the induction of CD40L, we treated JPX-9 cells with CdCl2 to induce Tax expression in the absence or presence of CsA followed by Real-Time RT-PCR analysis. As seen in Fig. 2B, Tax expression resulted in about a six-fold increase in CD40L mRNA, and surprisingly CsA had no effect on Tax-mediated induction of CD40L. However, CsA completely inhibited an NFAT-Luc reporter activated by PMA and ionomycin (Fig. 2C). To examine the role of NF-κB in Tax-mediated CD40L upregulation, we treated JPX-9 cells with the IKK inhibitor sc-514 (Jeong et al., 2005). Although sc-514 inhibited Tax induction of CD40 (Fig. 2A), a known NF-κB-dependent Tax target, it had little, if any, effect on Tax upregulation of CD40L (Fig. 2B). These results indicate that Tax requires neither calcineurin nor NF-κB to activate CD40L expression. We also found that Tax activated the CD40L promoter (a 5kb fragment upstream of the transcriptional start site) by 2-3 fold in an NFAT and NF-κB independent manner (data not shown).
Figure 2. HTLV-I Tax induction of CD40L mRNA is independent of calcineurin and NF-κB.
(A) JPX-9 cells were untreated or stimulated with CdCl2 (15 μM) and cyclosporin A (1 μg/ml) or sc-514 (25 μm) for 24 hours. Real time PCR for CD40 was performed, and CD40 mRNA expression was normalized to 18S rRNA in the same real time PCR reaction. Error bars represent SEM, with n=3 for each condition. This experiment was repeated one additional time with similar results. (B) JPX-9 cells were treated as in Panel A, and real time PCR was performed for CD40L. CD40L mRNA expression was normalized to 18S rRNA in the same real time PCR reaction. (C) Jurkat-E6 cells were transfected with 0.25 μg NFAT luciferase reporter and 0.01 μg pRL-TK luciferase. Transfected cells were treated with or without PMA/ionomycin and cyclosporin A for 7 hours, harvested, and a dual luciferase assay was performed. NFAT luciferase values were normalized to renilla luciferase. Error bars represent SEM, with n=3 for each condition.
CD40L expression is silenced by methylation in Tax-expressing HTLV-I transformed cell lines
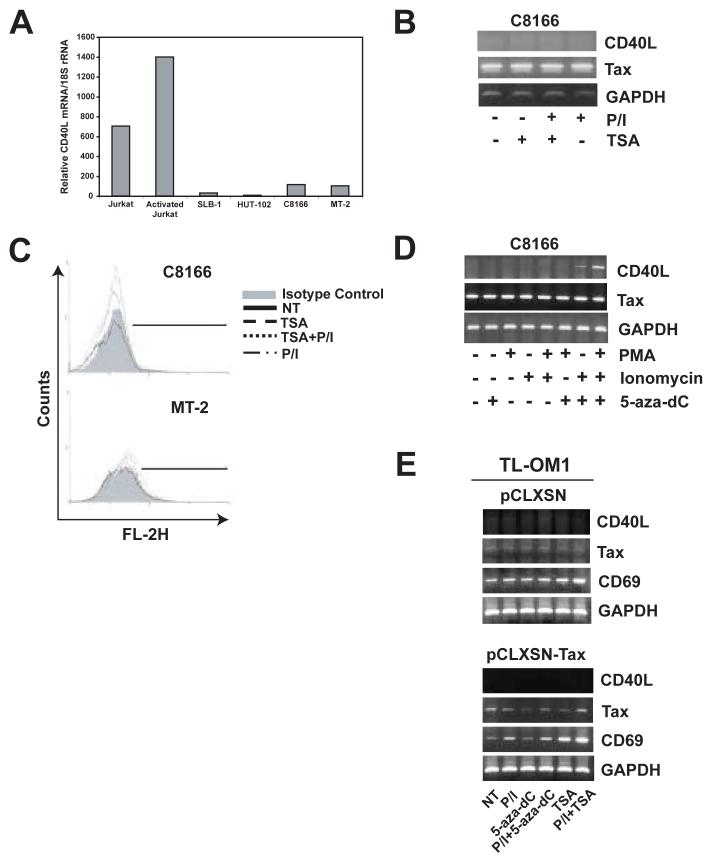
We then analyzed CD40L expression in HTLV-I transformed cell lines by Real-Time RT-PCR. There was no CD40L mRNA detected in all of the HTLV-I transformed cell lines, including SLB-1, HUT-102, C8166 and MT-2 (Fig. 3A). CD40L protein was also absent from the cell surface of these cell lines (data not shown). These results are in agreement with our previous study demonstrating that CD40L is downregulated in immortalized T lymphocytes using an in vitro coculture model of HTLV-I infection (Harhaj et al., 1999). As expected, we readily detected CD40L mRNA in Jurkat cells that were either untreated or treated with PMA and ionomycin (Fig. 3A). CD40L mRNA was also not expressed when HTLV-I transformed cell lines were treated with PMA and ionomycin (Fig. 3B). We hypothesized that the CD40L gene may be silenced by an epigenetic mechanism in these cell lines. Histone deacetylation and methylation are two common mechanisms of epigenetic silencing of genes (Baylin and Ohm, 2006). To determine if CD40L was silenced by histone deacetylation, we treated cells with the HDAC inhibitor trichostatin A (TSA). Treatment of C8166 and MT-2 cells with either TSA alone or in combination with PMA and ionomycin did not restore CD40L expression (Fig. 3B and C). However, when C8166 cells were treated with the demethylating agent 5-aza-dc together with ionomycin but not PMA, CD40L expression was restored (Fig. 3D). When cells were treated with 5-aza-dc together with both PMA and ionomycin, the restoration of CD40L was even more dramatic (Fig. 3D). In MT-2 cells, 5-aza-dc together with PMA and ionomycin also induced CD40L, although not to the same degree as C8166 cells (data not shown). These results suggest that CD40L is silenced by methylation, but not deacetylation, in Tax-expressing HTLV-I transformed cell lines. Further, it is apparent that Tax expression is no longer sufficient for CD40L upregulation in these cells in the presence of 5-aza-dc since PMA and ionomycin treatment is also required.
Figure 3. CD40L is silenced by methylation, but not deacetylation, in Tax-expressing HTLV-I-infected T cell lines.
(A) Real time PCR analysis of CD40L mRNA expression in C8166, MT-2, SLB-1, HUT-102 HTLV-I-infected T cell lines and Jurkat cells non-treated and activated by PMA (10ng/ml)/ionomycin (2μM) treatment. Bars represent relative CD40L mRNA levels normalized to 18S rRNA in the same real time PCR reaction. (B) RT-PCR analysis of CD40L, Tax, and GAPDH expression in C8166 cells treated with or without Trichostatin A (100 nM) for 72 hours and PMA and/or ionomycin for the last 6 hours. (C) Cell surface expression of CD40L protein in C8166 and MT-2 HTLV-I-infected T cell lines with or without treatment with PMA and ionomycin for 6 hours and/or Trichostatin A (500 nM) for 24 hours. Cells were stained with PE conjugated anti-CD40L antibody. Control cells were stained with irrelevant antibody of the same isotype as the anti-CD40L antibody. PE expression was analyzed on 10,000 gated cells using FACScan flow cytometer. (D) RT-PCR analysis of CD40L, Tax, and GAPDH expression in C8166 cells treated with or without 5-aza-2'-deoxycytidine (1 μM) for 72 hours and PMA and/or ionomycin for the last 6 hours. (E) RT-PCR analysis of CD40L, Tax, CD69 and GAPDH in TL-OM1 cells infected with either pCLXSN or pCLXSN-Tax. Cells were treated overnight with either PMA/ionomycin alone, TSA (500 nM) alone or in combination with PMA/ionomycin, 5-aza-dc (1 μm) for 3 days either alone or together with PMA/ionomycin overnight.
To determine the exact role of Tax in CD40L induction by 5-aza-dc and PMA/ionomycin, we next examined TL-OM1 cells which are leukemic cells derived from an ATL patient. This cell line may be more representative of ATL tumors since it lacks Tax expression but retains constitutive NF-κB activation (Hironaka et al., 2004). TL-OM1 cells were infected with a Tax-expressing retroviral vector (pCLXSN-Tax) or empty vector (pCLXSN). After infection, cells were treated with either PMA/ionomycin, 5-aza-dc or TSA with and without PMA/ionomycin. Surprisingly, CD40L expression was unable to be restored with any of the conditions, either in empty vector or Tax-infected cells (Fig. 3E). The housekeeping gene GAPDH was similarly expressed in all of the samples. CD69 was significantly upregulated after TSA and PMA/ionomycin treatment, especially in the presence of Tax (Fig. 3E). Thus, at least in TL-OM1 cells, CD40L expression is unable to be restored by 5-aza-dc, TSA or Tax expression.
T lymphocytes stimulated ex vivo from ATL, but not HAM/TSP patients, exhibit a potent defect in CD40L induction
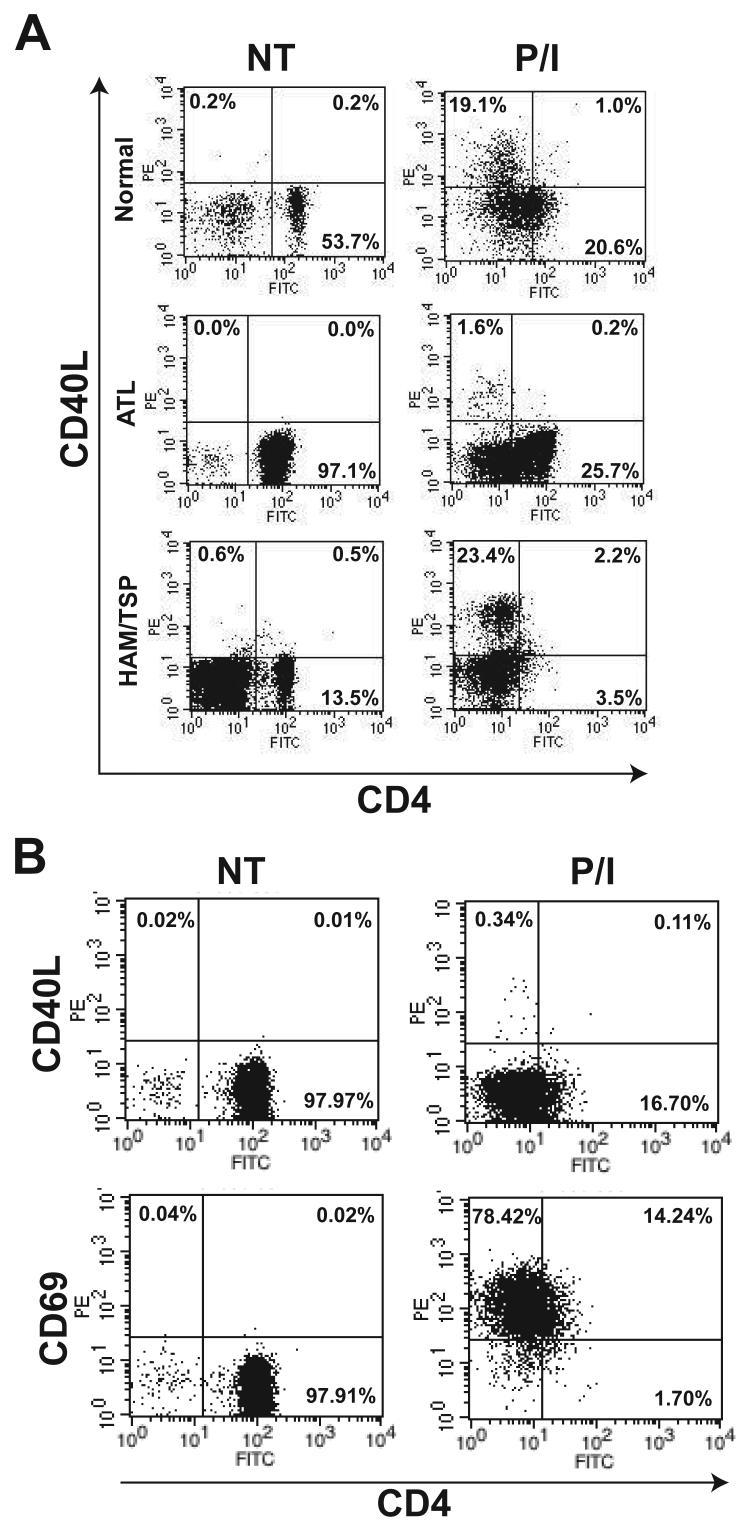
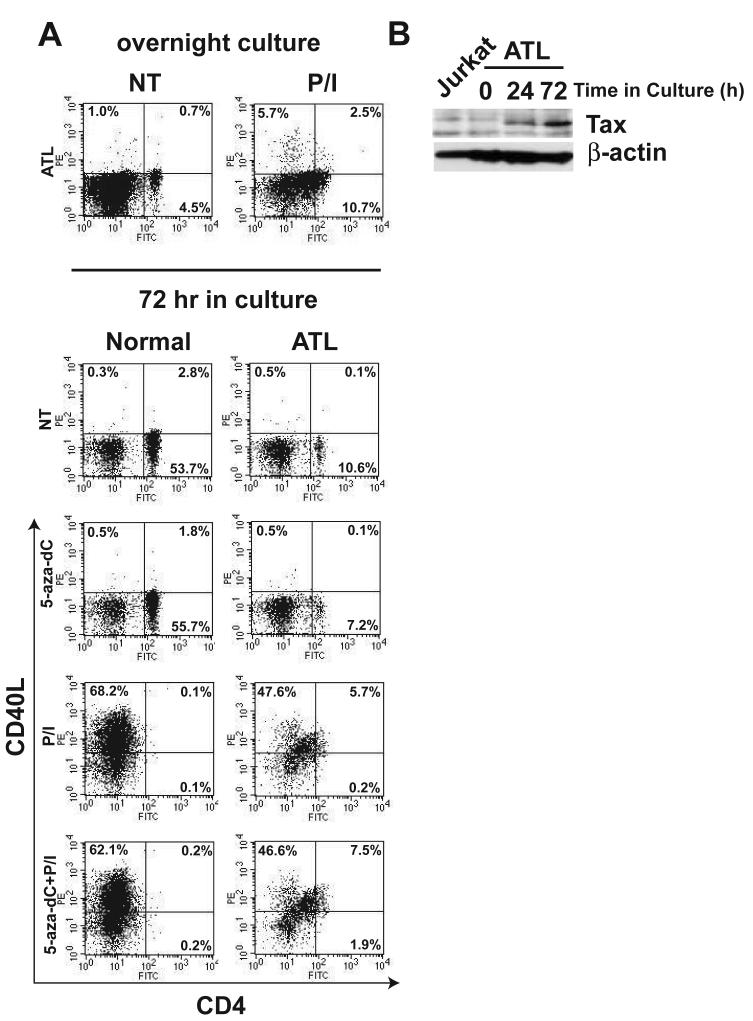
We next examined CD40L expression in HTLV-I-infected patients. PBMCs were isolated from ATL, HAM/TSP or uninfected normal donors and stained with antibodies for flow cytometry experiments. In normal donors, CD40L was not expressed on resting cells, but was induced after treatment with PMA and ionomycin (Fig. 4A). In HAM/TSP patients (a total of two were examined), there was a normal induction of CD40L by PMA and ionomycin within CD4+CD25+ lymphocytes (Fig. 4A). However, there was a profound block of CD40L expression in response to PMA and ionomycin treatment in an ATL patient (acute ATL; white blood cell count (WBC) 148,000 cells/mm3) (Fig. 4A). A defect in mitogen-mediated induction of CD40L was also observed in three other ATL patients we examined, two of which were diagnosed with acute ATL (WBC 42,000 and 68,000 cells/mm3) and the other with unfavorable chronic ATL (WBC 45,000 cells/mm3). Importantly, defective CD40L induction was not observed in any normal controls or HAM/TSP patients. Interestingly, mitogen-induced activation of CD69, which only requires PMA stimulation (Taylor-Fishwick and Siegel, 1995), was unaffected in ATL patients (Fig. 4B). Downregulation of CD4, which is dependent on PMA stimulation (Bigby et al., 1990), was similarly unaffected in ATL (Fig. 4A). These results raise the intriguing possibility that calcium signaling may be defective in ATL patients. Furthermore, the block in CD40L induction is specific for ATL patients in the context of HTLV-I infection.
Figure 4. CD40L, but not CD69, induction is defective in peripheral blood mononuclear cells of ATL patients.
(A) Flow cytometry analysis of CD40L and CD4 cell surface expression in PBMCs obtained from normal and ATL or HAM/TSP patients. Cells were either untreated or treated overnight with PMA (10 ng/ml) and ionomycin (2μM). Cells were stained with PE-conjugated anti-CD40L antibody, PE-Cy5-conjugated anti-CD25 and FITC-conjugated anti-CD4 antibody. The data are presented as a two-dimensional dot plot depicting cells that were within the gated region of the light scattering plot. Gating was done using FSC and SSC properties and for each test, at least 10,000 gated cells were analyzed. The numbers in each panel denote the percentage of cells falling within the indicated regions. (B) Flow cytometry analysis of PBMCs from an acute ATL patient. Cells were treated as in panel A and stained with either PE-conjugated anti-CD40L or anti-CD69 together with PE-Cy5-conjugated anti-CD25 and FITC-conjugated anti-CD4 antibody. Analysis was performed as described in panel A.
To determine if the expression of CD40L was downregulated by methylation in ATL patients, we obtained PBMCs from the same ATL patient shown in Fig. 4A at a later time and treated with 5-aza-dc for 72 hr together with PMA and ionomycin treatment for the final 12 hr of culture. First, we confirmed that ex vivo stimulation of these cells with PMA and ionomycin for 12 hr did not result in efficient induction of CD40L (Fig. 5A top panel). Treatment of cells with 5-aza-dc alone for 72 hr was unable to induce CD40L expression in ATL cells (Fig. 5A). However, PMA and ionomycin treatment resulted in a robust induction of CD40L, but only after cells were cultured for 72 hr (Fig. 5A). It has been reported that the tax gene is silenced, mutated or absent in vivo in ATL patients (Takeda et al., 2004). We performed immunoblotting for Tax from the same ATL patient at different time points in cell culture, and Tax was clearly absent in PBMCs ex vivo but was expressed after culturing the cells for 24 and 72 hr. (Fig. 5B). Thus, Tax expression, or possibly another factor induced by culturing the cells in vitro, may be essential to restore the normal induction of CD40L expression in ATL patients.
Fig. 5. CD40L is not silenced by methylation in ATL patients.
(A) PBMCs from an ATL patient were either untreated or treated overnight with PMA and ionomycin and subjected to flow cytometry (top panel). PBMCs from the same ATL patient or normal control were either untreated or incubated with 5-aza-2'-deoxycytidine (1 μM) for 72 hours and/or PMA and ionomycin for the final 12 hours (bottom panel). Cells were stained and analyzed as in panel A. (B) PBMCs obtained from the same ATL patient used in panels A and B were incubated at 37°C and 5% CO2 for 0, 24 and 72 hours. Whole cell lysates were subjected to SDS-PAGE and Western blot analysis was performed with anti-Tax (top panel) or anti-β–actin antibodies (lower panel).
Discussion
In this study, we have demonstrated that the HTLV-I Tax oncoprotein is a transcriptional regulator of CD40L. Tax expression correlates with the upregulation of CD40L mRNA and protein in T lymphocytes. However, Tax appears to use mechanisms distinct from T cell antigen receptor signaling to induce CD40L since it requires neither calcineurin nor NF-κB for upregulation of CD40L mRNA. In certain Tax-expressing HTLV-I transformed cell lines such as C8166, CD40L is absent due to epigenetic silencing by methylation. In other HTLV-I transformed cell lines that lack Tax expression such as TL-OM1, CD40L is likely downregulated by distinct mechanisms. We also examined the expression of CD40L in patients infected with HTLV-I and observed defective CD40L induction in response to mitogens in ATL but not HAM/TSP patients. However, CD40L is not silenced by methylation in ATL patients, and can be restored by mitogen treatment after culturing the cells for 72 hr, coincident with induction of Tax. Thus, CD40L is dysregulated by distinct mechanisms in HTLV-I transformed cell lines and ATL patients.
The transcriptional regulation of the CD40L gene in response to antigen receptor signaling in T lymphocytes has been extensively studied (Cron, 2003). It is well established that CD40L requires multiple signals for its activation, with one of the most critical ones being calcium signaling that mediates NFAT activation (Fuleihan et al., 1994). In addition to NFAT, there have been reports that NF-κB (Srahna et al., 2001), AP-1 (Tsytsykova, Tsitsikov, and Geha, 1996) and Egr-1 (Cron et al., 2006) also play important roles in CD40L regulation. We are currently directing our efforts to identify factors essential for Tax activation of CD40L. Clearly, Tax upregulation of CD40L mRNA occurs in the absence of NFAT and NF-κB activation. Therefore, Tax induction of CD40L is the first known example, to our knowledge, of transcriptional upregulation of the CD40L gene in a cyclosporin A insensitive manner. It is unknown why Tax would acquire a mechanism for CD40L induction that is distinct from antigen receptor signaling and independent of NFAT or NF-κB. Tax is a potent activator of NF-κB, and activation of NF-κB is a hallmark of HTLV-I transformed cell lines and ATL patients (Sun and Yamaoka, 2005). Furthermore, Tax activates NFAT by promoting the dephosphorylation and nuclear translocation of NFAT1 in a cyclosporin A sensitive manner, and NFAT1 is constitutively activated in HTLV-I transformed cell lines (Good et al., 1997). It is possible that HTLV-I may require CD40L in a transient manner for a function related to the viral life cycle, and therefore may not need constitutive expression of CD40L, which would occur in cells with constitutive NF-κB and NFAT activation. Furthermore, constitutive expression of CD40L may lead to hyperstimulation of anti-viral immune responses and autoimmunity, similar to what is observed in HAM/TSP. However, we did not observe constitutive expression of CD40L in the small number of HAM/TSP patients we examined, although the basal levels of CD40L were slightly higher compared to uninfected control PBMCs (Fig. 4A).
We have found that CD40L expression is downregulated by an epigenetic mechanism involving methylation in certain Tax-expressing HTLV-I transformed cell lines. CD40L expression is restored upon treating HTLV-I transformed C8166 cells with 5-aza-dc in combination with PMA and ionomycin. It is unclear why treatment with 5-aza-dc alone is not sufficient to rescue CD40L expression in these cells, since Tax is highly expressed in these cell lines. These results would imply that Tax regulation of CD40L expression is uncoupled in HTLV-I transformed cell lines. This notion is further supported by our results with the TL-OM1 cell line that lacks Tax expression. Retroviral-vector-mediated introduction of Tax in these cells was unable to induce CD40L expression, even in the presence of 5-aza-dc and PMA/ionomycin treatment (Fig. 3E). Thus, it appears that there are distinct mechanisms of CD40L downregulation in different HTLV-I-transformed cell lines, although the exact mechanism of CD40L downregulation in TL-OM1 cells or ATL patients is uncertain. Furthermore, it is unclear if the cd40l gene is directly silenced by methylation or indirectly by silencing of a regulator of cd40l gene expression. Aberrant methylation of CpG islands in gene promoters is a common mechanism of gene inactivation in the progression of many cancers (Baylin and Ohm, 2006). The cd40l gene, to our knowledge, has not been identified previously as a direct target of methylation. The cd40l genomic region, including the promoter (up to 5kb upstream of the start site) and exon/intron sequences, are devoid of CpG islands, thus CD40L may not be a direct target for methylation. One candidate for an upstream regulator of CD40L that may be silenced is egr3, which has been shown to be silenced directly by methylation in ATL patients (Yasunaga et al., 2004). However, it is unlikely that downregulation of egr family members is linked to CD40L silencing in HTLV-I transformed cell lines since we found that egr1-3 are indeed expressed in C8166 cells (data not shown).
A previous study examined CD40L expression in T lymphocytes derived from HAM/TSP and ATL patients that were cultured in vitro in the presence of PHA and IL-2 (Makino et al., 2001). Makino et al. demonstrated that CD40L expression is significantly lower on CD4+ T lymphocytes from ATL versus HAM/TSP patients after culturing for 1 and 3 weeks, although they did not determine if Tax was expressed in their cultured cells (Makino et al., 2001). Our current study indicates that there is a potent block in the induction of CD40L ex vivo, selectively in ATL but not HAM/TSP patient PBMCs. Culturing ATL PBMCs in vitro leads to a progressive increase in Tax expression which may endow cells with the capacity to upregulate CD40L in response to mitogen stimulation. A recent report has indicated that ATL patients exhibit reduced frequency, diversity and function of HTLV-I-specific CD8+ T cells (Kozako et al., 2006). It is tempting to speculate that the lack of CD40L induction in ATL patients may be linked to the reduced and defective anti-HTLV-I CTLs in these patients. Future studies will be necessary to confirm this hypothesis. In addition, it is necessary to identify the mechanism underlying the defect in CD40L expression in ATL patients. This observation is reminiscent of the defect in CD40L induction observed in HIV-1-infected patients (Chougnet, 2003). However, the defect is likely distinct in HIV-1 infection, since PMA/ionomycin, but not anti-CD3/CD28, induces CD40L expression in CD4+ T cells from HIV-1-infected patients. Anergic T lymphocytes also exhibit a defect in CD40L expression (Bowen, Haluskey, and Quill, 1995), thus an intriguing possibility is that T lymphocytes from ATL patients may be functionally anergic. This may explain why ATL patients are prone to opportunistic infections.
CD40L expression does not appear to be downregulated by methylation in ATL patients, indicating that HTLV-I transformed cell lines such as C8166 or MT-2 may not be very representative of ATL tumors in vivo. TL-OM1 cells, which lack Tax expression, may be more representative of ATL with regard to CD40L downregulation. Introduction of Tax into these cells did not restore CD40L expression, even combined with PMA/ionomycin and 5-aza-dc stimulation. Thus, Tax likely does not play a role in the restoration of silenced CD40L in HTLV-I transformed cell lines. However, one caveat is that the levels of Tax expression observed in the TL-OM1 cells after retroviral infection may not have been high enough to restore CD40L expression. It is known that ATL tumors do not express Tax in vivo, but in vitro culturing of ATL PBMCs leads to a progressive increase in Tax expression over time. Thus, it is critical to perform experiments with unmanipulated ATL tumors to circumvent potential artifacts associated with Tax expression. Besides CD40L, we have not observed a defect in mitogen-induced activation of other genes in ATL patients, suggesting a strict selectivity, although future studies with gene arrays are necessary to confirm this hypothesis. CD69 induction was normal in ATL PBMCs treated with PMA and ionomycin (Fig. 4B). Interestingly, CD69 activation does not require calcium signaling but only a PKC-mediated signal generated by PMA (Taylor-Fishwick and Siegel, 1995). Also, PMA-mediated downregulation of CD4 was normal in the ATL patients. These results indicate that the defect in ATL patients may be linked to a defective calcium signaling pathway or impaired induction of calcium-regulated genes. Future studies will examine the efficiency of calcium mobilization and the induction of calcium regulated genes in T lymphocytes from ATL patients.
Materials and methods
Biological reagents and cell culture
The human T cell lymphocytic cell lines Jurkat and JPX-9 (kindly provided by Drs. Naoki Mori and Masataka Nakamura), the Tax-expressing HTLV-I-transformed T cell lines C8166, MT-2, SLB-1, and HUT-102, and the ATL cell line lacking Tax expression, TL-OM1 (kindly provided by Dr. Michiyuki Maeda) were cultured and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT). JPX-9 cells are subclones of Jurkat cells that stably express the tax gene under the control of a metallothionein promoter (Fujii et al., 1991). Peripheral blood was obtained from normal donors, ATL, and HAM/TSP patients after informed consent, approved by the University of Miami Institutional Review Board. Clinical diagnosis of ATL was made by serologic evidence of HTLV-I by ELISA, confirmed by Western blot, and the identification of circulating leukemic cells of T-cell origin by morphology, flow-cytometry, and gene rearrangement, performed at Jackson Memorial Hospital (JMH)/University of Miami. ATL patients were subclassifed as acute-type leukemia as per Shimoyama et al. (Shimoyama, 1991). Clinical diagnosis of HAM/TSP was made by neurologic symptoms, imaging studies and HTLV-I serology. HAM/TSP patients were diagnosed outside of JMH and referred to our center. Peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare Biosciences Corp., Piscataway, NJ). The anti-CD40L-phycoerythrin (PE) (TRAP1 clone), anti-CD69-PE (clone FN50), anti CD4-FITC (clone RPA-T4), anti-CD25-PE-Cy5 (clone M-A251) and anti-mouse IgG PE, FITC and PE-Cy5 isotype control antibodies were purchased from BD Pharmingen (San Diego, CA). The Tax monoclonal antibody was prepared from a Tax hybridoma (NIH AIDS Research and Reagents Program). The monoclonal β-actin antibody was purchased from AbCam (Cambridge, MA). Trichostatin A (TSA), 5-aza-dc, CdCl2 and cyclosporin A (CsA) were purchased from Sigma (Saint Louis, MO). The IKK inhibitor sc-514 was purchased from EMD Biosciences (San Diego, CA).
Luciferase assays and transient expression analysis
Transient transfections in Jurkat cells were performed with the FuGENE 6 transfection reagent (Roche, Indianapolis, IN) as described previously (Harhaj et al., 2005). Transfections with NFAT-Luc. (BD Biosciences) also included pRL-tK (25 ng) to normalize for transfection efficiency. Dual luciferase assays were performed according to the manufacturer's instructions (Promega, Madison, WI). Data are represented as fold induction of PMA and ionomycin treatment versus no treatment after normalization with pRL-tK values. Error bars indicate the standard error of the mean (SEM) of triplicate samples.
Flow cytometry
Cells (5×105) were incubated with 10 μL of individual PE-conjugated or isotype control antibodies for 30 min in 100 μl of FACS buffer (PBS, 0.05% NaN3, 3% FBS) on ice. Cells were washed once with ice-cold FACS buffer, washed again with ice-cold PBS, and resuspended in 300 μl of 2% paraformaldehyde. Fluorescence-activated flow cytometry was performed with a FACScan 2000 (Becton Dickinson, Franklin Lakes, NJ) flow cytometer. A total of 10,000 events were analyzed for each sample. Live cells were gated for analysis based on forward angle light scatter (FSC) and side angle light scatter (SSC). Single-color and three-color analyses were performed with CellQuest software (Becton Dickinson) and Windows Multiple Document Interface flow cytometry application (WinMDI). Cells from normal donors, ATL and HAM/TSP patients were gated on CD4+CD25+ cells.
RT-PCR
Total RNA was isolated from cells using an RNeasy kit (Qiagen, Valencia, CA) as recommended by the manufacturer. A total of 2 μg of RNA was converted to cDNA in a reverse transcriptase (RT) reaction using the first strand cDNA synthesis kit for RT-PCR (AMV; Roche; Indianapolis, IN). Reactions were primed with random primers (Roche). RT-PCR was performed to amplify the indicated genes in a 50 μl reaction containing 2 μl of cDNA, 1X PCR buffer (Invitrogen), 0.25 μM of forward and reverse primers, 0.2 mM dNTP mix, 1.5 mM MgCl2, and 3 units of Platinum Taq polymerase (Invitrogen). PCR products were resolved by agarose gel electrophoresis. The following sets of primers were used to amplify gene products for RT-PCR reactions: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (263 bp) forward-5'-CCACAGTCCATGCCATCAC, reverse 5'-GCTTCACCACCTTCTTGATG; Tax (429 bp) forward-5'-CGGATACCCAGTCTACGTC, reverse-5'-GAGGTACATGCAGACAACGG; CD40 (833 bp) forward-5'-CATGGTTCGTCTGCCTCTGC, reverse-5'-CACTGTCTCTCCTGCACTGAG; CD40L (785 bp) forward-5' CAT GAT CGA AAC ATA CAA CCA AAC, reverse-5'-CAG AGT TTG AGT AAG CCA AAG G; CD69 (492 bp) forward-5'-CGTAGCAGAGAACAGCTCTTTG, reverse-5'-GTCAGACCCTGTAACGTTGAAC.
Quantitative Real Time RT-PCR
Total RNA was converted to cDNA as described above, and 1μl of cDNA was used as a template in subsequent PCR reaction that was performed on LightCycler Instrument (Roche Diagnostics) using LightCycler® TaqMan Master kit (Roche) and gene specific Hydrolysis (TaqMan) Probes, according to the manufacturer's instruction. Taqman probes for CD40 and CD40L were purchased from Applied Biosystems (Foster City, CA). We used 18S rRNA as an internal control for relative gene expression quantification and for each gene amplification, Cp values (reading obtained at 530 nm) were normalized to the Cp of the 18S rRNA (reading obtained at 560 nm) using calibrator normalized relative quantification in the LightCycler analysis software.
Retroviral vector infections
Retroviral infections were performed as described (Harhaj et al., 2005; Naviaux et al., 1996; Rivera-Walsh et al., 2001). Briefly, 293T cells were transfected with 1 μg of either pCLXSN or pCLXSN-Tax together with pCL-Ampho (1 μg) and VSV-g (0.15 μg) using FuGENE 6 (Roche). Supernatant containing recombinant retroviruses was filtered 36 hr. post-transfection using a 0.45 μM polysulfone filter (Pall-Gelman Acrodisc) and used to resuspend TL-OM1 cells in 6-well plates. Polybrene (8 μg/ml; Sigma) was added to each well, and cells were centrifuged at 1800 rpm for 45 min. The cells were washed with RPMI media the following day, and the 5-aza-dc treatments were initiated the next day.
Western blotting
Western blotting was performed as described previously (Harhaj and Sun, 1999). Cells were lysed in RIPA buffer and proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. Membranes were blocked in 5% milk, incubated with primary antibodies (anti-Tax 1:1000 dilution), anti-β-actin (1:5000 dilution), washed three times with PBST, incubated with mouse Ig-horse radish peroxidase (HRP)-conjugated antibody (GE Healthcare, Piscataway, NJ), washed three times with PBST, and antigen-antibody complexes were detected with Western Lightning Enhanced Chemiluminescence (ECL) reagent (Perkin Elmer, Boston, MA).
Acknowledgements
We thank Drs. Shao-Cong Sun (Penn State College of Medicine), Brian Wigdahl (Drexel University), Michiyuki Maeda (Kyoto University), Gutian Xiao (Rutgers University), Naoki Mori (Ryukyu University), and Masataka Nakamura (Tokyo Medical and Dental University) for reagents. We thank the NIH AIDS Research and Reference Program for cell lines. We thank V. Jurecic of the Molecular Analysis Core Facility at the University of Miami Miller School of Medicine for assistance with Real-Time PCR and J. Philips of the Flow Cytometry Core for assistance with flow cytometry. These studies were supported by a grant from the United States Public Health Service/National Institutes of Health (CA99926 to EWH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, Bajorath J, Grosmaire LS, Stenkamp R, Neubauer M, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72(2):291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6(2):107–16. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- Bhushan A, Covey LR. CD40:CD40L interactions in X-linked and non-X-linked hyper-IgM syndromes. Immunol Res. 2001;24(3):311–24. doi: 10.1385/IR:24:3:311. [DOI] [PubMed] [Google Scholar]
- Bigby M, Wang P, Fierro JF, Sy MS. Phorbol myristate acetate-induced down-modulation of CD4 is dependent on calmodulin and intracellular calcium. J Immunol. 1990;144(8):3111–6. [PubMed] [Google Scholar]
- Bowen F, Haluskey J, Quill H. Altered CD40 ligand induction in tolerant T lymphocytes. Eur J Immunol. 1995;25(10):2830–4. doi: 10.1002/eji.1830251018. [DOI] [PubMed] [Google Scholar]
- Chougnet C. Role of CD40 ligand dysregulation in HIV-associated dysfunction of antigen-presenting cells. J Leukoc Biol. 2003;74(5):702–9. doi: 10.1189/jlb.0403171. [DOI] [PubMed] [Google Scholar]
- Clarke SR. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J Leukoc Biol. 2000;67(5):607–14. doi: 10.1002/jlb.67.5.607. [DOI] [PubMed] [Google Scholar]
- Cron RQ. CD154 transcriptional regulation in primary human CD4 T cells. Immunol Res. 2003;27(23):185–202. doi: 10.1385/IR:27:2-3:185. [DOI] [PubMed] [Google Scholar]
- Cron RQ, Bandyopadhyay R, Genin A, Brunner M, Kersh GJ, Yin J, Finkel TH, Crow MK. Early growth response-1 is required for CD154 transcription. J Immunol. 2006;176(2):811–8. doi: 10.4049/jimmunol.176.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford GS, Barnhart B, Shone S, Covey LR. Regulation of CD154 (CD40 ligand) mRNA stability during T cell activation. J Immunol. 1999;162(7):4037–44. [PubMed] [Google Scholar]
- Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- Fujii M, Niki T, Mori T, Matsuda T, Matsui M, Nomura N, Seiki M. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene. 1991;6(6):1023–9. [PubMed] [Google Scholar]
- Fuleihan R, Ramesh N, Horner A, Ahern D, Belshaw PJ, Alberg DG, Stamenkovic I, Harmon W, Geha RS. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J Clin Invest. 1994;93(3):1315–20. doi: 10.1172/JCI117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good L, Maggirwar SB, Harhaj EW, Sun SC. Constitutive dephosphorylation and activation of a member of the nuclear factor of activated T cells, NF-AT1, in Tax-expressing and type I human T-cell leukemia virus-infected human T cells. J Biol Chem. 1997;272(3):1425–8. doi: 10.1074/jbc.272.3.1425. [DOI] [PubMed] [Google Scholar]
- Hall WW, Fujii M. Deregulation of cell-signaling pathways in HTLV-1 infection. Oncogene. 2005;24(39):5965–75. doi: 10.1038/sj.onc.1208975. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Good L, Xiao G, Sun SC. Gene expression profiles in HTLV-I-immortalized T cells: deregulated expression of genes involved in apoptosis regulation. Oncogene. 1999;18(6):1341–9. doi: 10.1038/sj.onc.1202405. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Harhaj NS. Mechanisms of persistent NF-kappaB activation by HTLV-I tax. IUBMB Life. 2005;57(2):83–91. doi: 10.1080/15216540500078715. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Harhaj NS, Grant C, Mostoller K, Alefantis T, Sun SC, Wigdahl B. Human T cell leukemia virus type I Tax activates CD40 gene expression via the NF-kappa B pathway. Virology. 2005;333(1):145–58. doi: 10.1016/j.virol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Sun SC. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem. 1999;274(33):22911–4. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- Hironaka N, Mochida K, Mori N, Maeda M, Yamamoto N, Yamaoka S. Tax-independent constitutive IkappaB kinase activation in adult T-cell leukemia cells. Neoplasia. 2004;6(3):266–78. doi: 10.1593/neo.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang KT, Giam CZ, Majone F, Aboud M. Life, death, and tax: role of HTLV-I oncoprotein in genetic instability and cellular transformation. J Biol Chem. 2004;279(31):31991–4. doi: 10.1074/jbc.R400009200. [DOI] [PubMed] [Google Scholar]
- Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. A novel NF-kappaB pathway involving IKKbeta and p65/RelA Ser-536 phosphorylation results in p53 Inhibition in the absence of NF-kappaB transcriptional activity. J Biol Chem. 2005;280(11):10326–32. doi: 10.1074/jbc.M412643200. [DOI] [PubMed] [Google Scholar]
- Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest. 1996;98(3):826–37. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozako T, Arima N, Toji S, Masamoto I, Akimoto M, Hamada H, Che XF, Fujiwara H, Matsushita K, Tokunaga M, Haraguchi K, Uozumi K, Suzuki S, Takezaki T, Sonoda S. Reduced frequency, diversity, and function of human T cell leukemia virus type 1-specific CD8+ T cell in adult T cell leukemia patients. J Immunol. 2006;177(8):5718–26. doi: 10.4049/jimmunol.177.8.5718. [DOI] [PubMed] [Google Scholar]
- Kubota R, Kawanishi T, Matsubara H, Manns A, Jacobson S. HTLV-I specific IFN-gamma+ CD8+ lymphocytes correlate with the proviral load in peripheral blood of infected individuals. J Neuroimmunol. 2000;102(2):208–15. doi: 10.1016/s0165-5728(99)00175-7. [DOI] [PubMed] [Google Scholar]
- Lee BO, Haynes L, Eaton SM, Swain SL, Randall TD. The biological outcome of CD40 signaling is dependent on the duration of CD40 ligand expression: reciprocal regulation by interleukin (IL)-4 and IL-12. J Exp Med. 2002;196(5):693–704. doi: 10.1084/jem.20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Colpaert S, D'Haens GR, Kasran A, de Boer M, Rutgeerts P, Geboes K, Ceuppens JL. Hyperexpression of CD40 ligand (CD154) in inflammatory bowel disease and its contribution to pathogenic cytokine production. J Immunol. 1999;163(7):4049–57. [PubMed] [Google Scholar]
- Lobo FM, Xu S, Lee C, Fuleihan RL. Transcriptional activity of the distal CD40 ligand promoter. Biochem Biophys Res Commun. 2000;279(1):245–50. doi: 10.1006/bbrc.2000.3914. [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Nishioka Y, Lipsky PE, Thomas R. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest. 1997;100(9):2404–14. doi: 10.1172/JCI119781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino M, Utsunomiya A, Maeda Y, Shimokubo S, Izumo S, Baba M. Association of CD40 ligand expression on HTLV-I-infected T cells and maturation of dendritic cells. Scand J Immunol. 2001;54:574–581. doi: 10.1046/j.1365-3083.2001.01003.x. [DOI] [PubMed] [Google Scholar]
- Naviaux RK, Costanzi E, Haas M, Verma IM. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70(8):5701–5. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PW, Iha H, Iwanaga Y, Bittner M, Chen Y, Jiang Y, Gooden G, Trent JM, Meltzer P, Jeang KT, Zeichner SL. Genome-wide expression changes induced by HTLV-1 Tax: evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-kappaB activation. Oncogene. 2001;20(33):4484–96. doi: 10.1038/sj.onc.1204513. [DOI] [PubMed] [Google Scholar]
- Rivera-Walsh I, Waterfield M, Xiao G, Fong A, Sun SC. NF-kappaB signaling pathway governs TRAIL gene expression and human T-cell leukemia virus-I Tax-induced T-cell death. J Biol Chem. 2001;276(44):40385–8. doi: 10.1074/jbc.C100501200. [DOI] [PubMed] [Google Scholar]
- Schubert LA, Cron RQ, Cleary AM, Brunner M, Song A, Lu LS, Jullien P, Krensky AM, Lewis DB. A T cell-specific enhancer of the human CD40 ligand gene. J Biol Chem. 2002;277(9):7386–95. doi: 10.1074/jbc.M110350200. [DOI] [PubMed] [Google Scholar]
- Schubert LA, King G, Cron RQ, Lewis DB, Aruffo A, Hollenbaugh D. The human gp39 promoter. Two distinct nuclear factors of activated T cell protein-binding elements contribute independently to transcriptional activation. J Biol Chem. 1995;270(50):29624–7. doi: 10.1074/jbc.270.50.29624. [DOI] [PubMed] [Google Scholar]
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87) Br J Haematol. 1991;79(3):428–37. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- Siddiqa A, Sims-Mourtada JC, Guzman-Rojas L, Rangel R, Guret C, Madrid-Marina V, Sun Y, Martinez-Valdez H. Regulation of CD40 and CD40 ligand by the AT-hook transcription factor AKNA. Nature. 2001;410(6826):383–7. doi: 10.1038/35066602. [DOI] [PubMed] [Google Scholar]
- Srahna M, Remacle JE, Annamalai K, Pype S, Huylebroeck D, Boogaerts MA, Vandenberghe P. NF-kappaB is involved in the regulation of CD154 (CD40 ligand) expression in primary human T cells. Clin Exp Immunol. 2001;125(2):229–36. doi: 10.1046/j.1365-2249.2001.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Yamaoka S. Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene. 2005;24(39):5952–64. doi: 10.1038/sj.onc.1208969. [DOI] [PubMed] [Google Scholar]
- Taguchi H, Miyoshi I. Immune suppression in HTLV-I carriers: a predictive sign of adult T-cell leukemia. Acta Med Okayama. 1989;43(6):317–21. doi: 10.18926/AMO/30865. [DOI] [PubMed] [Google Scholar]
- Takeda S, Maeda M, Morikawa S, Taniguchi Y, Yasunaga J, Nosaka K, Tanaka Y, Matsuoka M. Genetic and epigenetic inactivation of tax gene in adult T-cell leukemia cells. Int J Cancer. 2004;109(4):559–67. doi: 10.1002/ijc.20007. [DOI] [PubMed] [Google Scholar]
- Taylor-Fishwick DA, Siegel JN. Raf-1 provides a dominant but not exclusive signal for the induction of CD69 expression on T cells. Eur J Immunol. 1995;25(12):3215–21. doi: 10.1002/eji.1830251203. [DOI] [PubMed] [Google Scholar]
- Tsytsykova AV, Tsitsikov EN, Geha RS. The CD40L promoter contains nuclear factor of activated T cells-binding motifs which require AP-1 binding for activation of transcription. J Biol Chem. 1996;271(7):3763–70. doi: 10.1074/jbc.271.7.3763. [DOI] [PubMed] [Google Scholar]
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Yasunaga J, Taniguchi Y, Nosaka K, Yoshida M, Satou Y, Sakai T, Mitsuya H, Matsuoka M. Identification of aberrantly methylated genes in association with adult T-cell leukemia. Cancer Res. 2004;64(17):6002–9. doi: 10.1158/0008-5472.CAN-04-1422. [DOI] [PubMed] [Google Scholar]
- Zhang R, Fichtenbaum CJ, Hildeman DA, Lifson JD, Chougnet C. CD40 ligand dysregulation in HIV infection: HIV glycoprotein 120 inhibits signaling cascades upstream of CD40 ligand transcription. J Immunol. 2004;172(4):2678–86. doi: 10.4049/jimmunol.172.4.2678. [DOI] [PubMed] [Google Scholar]