Abstract
We reported previously in HepG2 cells that estradiol induces cell cycle progression throughout the G1–S transition by the parallel stimulation of both PKC-α and ERK signaling molecules. The analysis of the cyclin D1 gene expression showed that only the MAP kinase pathway was involved. Here, the presence of rapid/nongenomic, estradiol-regulated, PI3K/AKT signal transduction pathway, its modulation by the levels of the tumor suppressor PTEN, its cross-talk with the ERK pathway, and its involvement in DNA synthesis and cyclin D1 gene promoter activity have all been studied in HepG2 cells. 17β-Estradiol induced the rapid and biphasic phosphorylation of AKT. These phosphorylations were independent of each other, being the first wave of activation independent of the estrogen receptor (ER), whereas the second was dependent on ER. Both activations were dependent on PI3K activity; furthermore, the ERK pathway modulated AKT phosphorylation by acting on the PTEN levels. The results showed that the PI3K pathway, as well as ER, were strongly involved in both G1–S progression and cyclin D1 promoter activity by acting on its proximal region (-254 base pairs). These data indicate that in HepG2 cells, different rapid/nongenomic estradiol-induced signal transduction pathways modulate the multiple steps of G1–S phase transition.
INTRODUCTION
17β-Estradiol (E2) can trigger DNA synthesis and cell cycle progression in different cell types (Sutherland et al., 1983; Castoria et al., 1999; Marino et al., 2001a) by regulating the expression of the genes involved in the cell cycle machinery (Altucci et al., 1996; Foster et al., 2001). In particular, E2 can induce cyclin D1 gene transcription, even though its gene promoter region does not contain any estrogen-responsive element (ERE) or ERE-like sequence (Herbert et al., 1994; Sabbah et al., 1999), recruiting different transcription factors depending on the cell context. Moreover, the rapid (1–6 h) E2-induced cyclin D1 gene expression reported in several cell lines, even in the presence of an estrogen receptor lacking the DNA binding domain, suggests that E2-induced rapid/nongenomic mechanisms are sufficient to induce cyclin D1 overexpression (Marino et al., 2002). Several cyclin D1 activation mechanisms have been reported. In particular, we identified the E2-induced rapid extranuclear molecular events in HepG2 cells (e.g., mitogen-activated protein kinase/extracellular regulated kinase [MAPK/ERK] and phospholipase C/protein kinase C [PLC/PKC] activation), showing also that only the E2-induced rapid/nongenomic phosphorylation of ERK was necessary for the E2-induced cyclin D1 transcription. Furthermore, the destruction of the TRE motif present in the cyclin D1 promoter, -848 base pairs (bp), caused the complete loss of estrogen responsiveness. In mammary carcinoma cells, it has been reported (Sabbah et al., 1999; Castro-Rivera et al., 2001; Nagata et al., 2001) that the three GC-rich SP1 sites at -143 to -110 bp and the CRE motif at -96 bp regions of the promoter are the major mediators for the induction of the cyclin D1 promoter by E2 via protein kinase A. In addition, recent data in MCF7 cells indicate a role for the phosphatidylinositol 3-kinase (PI3K) but not for ERK in E2-induced cyclin D1 -1800 bp promoter activity (Castoria et al., 2001).
The PI3Ks compose a family of lipid kinases that phosphorylate the 3′ position of the inositol ring of the phosphatidyl inositol(4)phosphate (PI-4-P), the phosphatidyl inositol(4, 5)bisphosphate (PI-4,5-P2) to generate PI-3,4-P2 and PI-3,4,5-P3, respectively (Scheid et al., 2002), which shows an affinity for certain protein modules, such as pleckstrin homology domain, implicated in several cellular processes, including cell survival, DNA synthesis, protein trafficking, and metabolism (for review, see Scheid and Woodgett, 2001). The role of PI3K in growth involves the serine/threonine kinase, AKT/PKB, translocation in proximity to phosphoinositide-dependent kinase 1, PDK1, resulting in AKT/PKB phosphorylation (Scheid and Woodgett, 2001). The PKB/AKT activation drives cells through many biological functions, including gene expression, cell cycle, survival, glucidic metabolism, endocytosis and vesicular trafficking, cell transformation, and oncogenesis (Coffer et al., 1998; Stein and Waterfield, 2000). AKT/PKB phosphorylation is negatively regulated by the PTEN/MMAC1/TEP1 tumor suppressor gene protein product, which is a phosphatase that dephosphorylates the 3′ position to reverse the reactions catalyzed by PI3K (Maehama and Dixon, 1998; Cantley and Neel, 1999). The overexpression of PTEN blocks cell cycle progression and induces apoptosis in cells (Furnari et al., 1998; Ramaswamy et al., 1999). Although the importance of the PI3K/PTEN pathway in cell growth is well established, its cross-talk with ERK pathway and its role in E2-regulated cyclin D1 promoter activity are not understood.
In this study, we show that PI3K is required for E2-stimulated HepG2 cell growth. We provide evidence that the ERK pathway rapidly reduces the levels of PTEN, allowing E2-induced phosphorylation of AKT. Our data indicate that both the PI3K and the ERK pathways coordinately regulate cyclin D1 promoter activity, allowing HepG2 cell cycle progression.
MATERIALS AND METHODS
Reagents
17β-Estradiol, 17α-estradiol, gentamicin, penicillin, RPMI-1640 (with or without phenol red), fetal calf serum, and charcoal-stripped fetal calf serum were purchased from Sigma Chemical (St. Louis, MO). The MAP kinase cascade inhibitors PD 98059 and U 0126 and the PI3 kinase inhibitors wortmannin and Ly 294002 were obtained from Calbiochem (San Diego, CA). The estrogen receptor inhibitor ICI 182,780 was obtained from Tocris (Ballwin, MO). Methyl-1-[3H]thymidine (specific activity, 81 Ci/mmol) was purchased from Amersham-Pharmacia (Little Chalfont, UK). Lipofectamine reagent was obtained from Gibco-BRL Life Technology (Gaithersburg, MD). The luciferase kit was obtained from Promega (Madison, WI). The GenElute Plasmid Maxiprep Kit was obtained from Sigma. The Bradford protein assay was obtained from Bio-Rad Laboratories (Hercules, CA). The monoclonal anti–phospho-AKT antibody was obtained from New England Biolabs (Beverly, MA); the monoclonal and polyclonal anti-AKT, anti-PTEN, and anti–β-actin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). CDP-Star chemiluminescence reagent for Western blot was obtained from NEN (Boston, MA).
All the other products were from Sigma. Analytical or reagent grade products, without further purification, were used.
Cell Culture
HepG2 cells were grown routinely in 5% CO2 in air in modified, phenol red–free, RPMI-1640 medium containing 10% (vol/vol) charcoal-stripped fetal calf serum, l-glutamine (2 mM), gentamicin (10 μg/ml), and penicillin (100 U/ml). Cells were passaged every 4 d, and media were changed every 2 d.
DNA Synthesis
DNA synthesis was assayed by incubating subconfluent cells (70–80%) with methyl-1-[3H]thymidine (final concentration, 1 μCi/ml). Cells were treated simultaneously with E2 (final concentration, 10 nM) or vehicle (ethanol/PBS 1:10 vol/vol). Ly 294002 (final concentration, 10 μM) or ICI 182,780 (final concentration, 1 μM) was added 15 min before E2 and methyl-1-[3H]thymidine. Wortmannin (final concentration, 10 μM) was added simultaneously to E2 and labeled thymidine. Thymidine incorporation was assayed 1 h after E2 administration as previously reported (Marino et al., 2001a).
Plasmids
The gene reporter plasmids pXP2-D1-2966-luciferase, pXP2-D1Δ-254-luciferase (-254), and pXP2-D1Δ-20-luciferase (-20) and the plasmids containing the vector expression for pCR3.1-β-galactosidase have been described previously (Herbert et al., 1994; Marino et al., 2002). Plasmids were purified for transfection with a plasmid preparation kit according to the manufacturer's instructions. A luciferase dose–response curve showed that the maximum effect was present when 1 μg of DNA was transfected with 1 μg of pCR3.1-β-galactosidase to normalize transfection efficiency (∼55–65%).
Transfection and Luciferase Assay
Cells were grown to ∼70% confluence, then transfected using Lipofectamine reagent according to the manufacturer's instructions. Six hours after transfection, the medium was changed, and 24 h thereafter, cells were stimulated with 10 nM E2 for 6 h. In some experiments, the HepG2 cells were treated with E2–BSA conjugate (β-estradiol 6-(0-carboxy-methyl)oxime:BSA, 32 mol E2/mol BSA). This form of macromolecular bound estrogen does not pass through the plasma membrane and is much more water soluble than free E2 (Zheng et al., 1996). Before each experiment, E2–BSA was dissolved in phenol-free growth medium (0.2 mg/ml), mixed with dextran and charcoal, centrifuged, and passed through a 0.22-mm filter (Russell et al., 2000; Marino et al., 2002; Seo and Leclercq, 2002). In these conditions, free E2 was retained in the resin (>3‰) (Stevis et al., 1999), and the filtrate contained E2–BSA. When indicated, Ly 294002 or wortmannin (PI3K inhibitors) was added 15 min before E2, and reporter plasmid expression was evaluated 6 h thereafter. The cell lysis procedure and the subsequent measurement of luciferase gene expression were performed by use of the luciferase kit according to the manufacturer's instructions with a EC & G Berthold luminometer.
Electrophoresis and Immunoblotting
After treatment with inhibitors (10 μM PD 98059 or 10 μM U 0126 [MAP kinase cascade inhibitors] or 10 μM Ly 294002 or 10 μM wortmannin [PI3K inhibitors] or 1 μM ICI 182,780) or hormone, cells were lysed as described previously (Marino et al., 2001b) and solubilized in 0.125 M Tris HCl (pH 6.8) containing 10% SDS (wt/vol), 1 mM phenylmethylsulfonyl fluoride, and 5 μg/ml leupeptin and boiled for 2 min. Proteins were quantified using the Bradford protein assay (Bradford, 1976). Solubilized proteins (20 μg) were resolved using 7.5% SDS-PAGE at 100 V for 1 h. The proteins were then electrophoretically transferred to nitrocellulose for 45 min at 150 V at 4°C. The nitrocellulose was treated with 3% bovine serum albumin in 138 mM NaCl, 26.8 mM KCl, 25 mM Tris HCl (pH 8.0), 0.05% Tween-20, and 0.1% BSA and then probed at room temperature for 1 h with anti–phospho-AKT, anti–phospho-ERK, or anti-PTEN antibodies. The nitrocellulose was stripped with Restore Western blot stripping buffer (Pierce Chemical, Rockford, IL) for 10 min at room temperature and then probed with anti-AKT (1 μg/ml). Anti–β-actin antibody (1 μg/ml) was used to normalize the sample loading. Antibody reaction was visualized with chemiluminescence reagent for Western blot.
RESULTS
PI3K Is Required for E2-induced DNA Synthesis
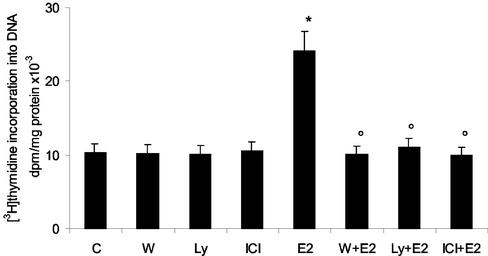
To determine whether PI3K is essential for E2-stimulated growth in HepG2 cells, we tested the effect of the selective PI3K inhibitors Ly 294002 and wortmannin on E2-induced DNA synthesis (Figure 1). The pretreatment of cells with Ly 294002 or wortmannin inhibits E2-induced DNA synthesis as measured by [3H]thymidine incorporation. The pretreatment of the cells with the pure anti–estrogen receptor (ER) inhibitor ICI confirmed the ER dependence on E2-induced effects (Figure 1).
Figure 1.
E2-induced DNA synthesis requires PI3K in HepG2 cells. [3H]thymidine incorporation into DNA has been evaluated as described in MATERIALS AND METHODS in HepG2 cells treated with the PI3K inhibitors Ly 294002 (Ly) and wortmannin (W) (10 μM each) or with the pure antiestrogen ICI 182,780 (ICI) (1 μM) and then stimulated for 1 h with vehicle (C) or 17β-estradiol (E2) (10 nM). Data are expressed as disintegrations per minute (dpm) per total protein extracted ± SD of four independent experiments. *p < 0.001 compared with respective control values (C), determined using Student's t test. °p < 0.001 compared with respective E2 values (E2), determined using Student's t test.
E2 Rapidly Activates PI3K-dependent AKT Phosphorylation
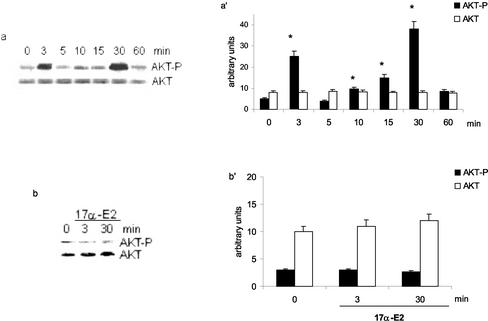
The serine/threonine kinase AKT/PKB is a major PI3K target, and its activation through the phosphorylation of Thr308/Ser473 mediates many of the downstream cellular effects of PI3K. To investigate the role of the PI3K/AKT pathway in the E2-induced HepG2 cell cycle progression, we analyzed the hormone's effects on AKT activity. Figure 2a shows that E2 stimulated the biphasic phosphorylation of AKT. A transient stimulation was initially detectable after 3 min of hormonal treatment and decreased toward basal levels after 5 min. A second wave of AKT phosphorylation started 10 min after hormone administration, with a peak 30 min later. The total amount of AKT protein did not change, as detected when the same filter was reprobed with anti-AKT antibody.
Figure 2.
AKT phosphorylation is specifically induced by E2 in Hep G2 cells. Time course of AKT phosphorylation (a) and effect of the stereoisomer 17α-estradiol on AKT phosphorylation (b) in HepG2 cells. Western blot analyses were performed as described in MATERIALS AND METHODS on control (0) and on E2-treated cells (E2) (10 nM) at different times. The same filters were reprobed with anti-AKT antibody. a′ and b′, densitometric analysis (means ± SD) of three independent experiments. *p < 0.001 compared with respective control values (0), determined using Student's t test.
The specificity of the biphasic E2-induced AKT phosphorylation was confirmed by the powerlessness of the stereoisomer 17-α-estradiol to induce a similar activation (Figure 2b).
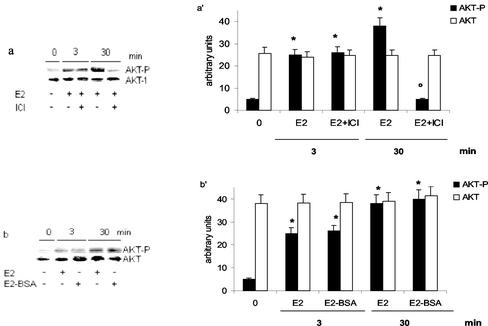
The role of ER was assessed by using the pure antiestrogen ICI (Figure 3a). Surprisingly, the ER inhibitor prevented only 30 min E2-induced AKT phosphorylation. To test whether the E2-induced AKT phosphorylation could be mediated by the binding of E2 to the cell surface receptors, HepG2 cells were treated with the E2–BSA conjugate, which does not pass through the plasma membrane (Zheng et al.,1996). As shown in Figure 3b, after E2–BSA stimulation, the increase in the AKT phosphorylation was similar to that induced by the free E2, thus suggesting the probable involvement of a membrane ER in the E2 effects.
Figure 3.
Involvement of estrogen receptor in E2-induced AKT phosphorylation in HepG2 cells. Western blot analyses of AKT phosphorylation were performed as described in MATERIALS AND METHODS in HepG2 cells stimulated with vehicle (-) or with 17β-estradiol (E2) (10 nM) for 3 and 30 min with or without 15 min of pretreatment with the pure antiestrogen ICI 182,780 (ICI) (1 μM) (a) or on E2–BSA-stimulated cells (10 nM) for 3 and 30 min (b). The same filters were reprobed with anti-AKT antibody. a′ and b′, densitometric analysis (means ± SD) of three independent experiments. *p < 0.001 compared with respective control values (0), determined using Student's t test. °p < 0.001 compared with respective estradiol values (E2), determined using Student's t test.
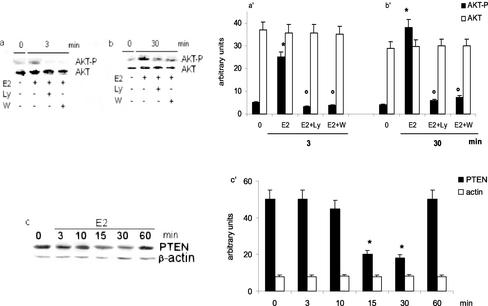
Consistent with the view that AKT is a target for PI3K, the Ly 294002 and wortmannin pretreatments of HepG2 cells blocked the E2-induced AKT phosphorylation both after 3 min and after 30 min of hormone stimulation (Figure 4, a and b). The levels of PTEN were analyzed to assess a role for the tumor suppressor PTEN, which recognizes PI-3,4,5-P3 as the principal substrate in the E2-induced AKT phosphorylation. E2 decreased the PTEN levels at 15 and 30 min after the stimulation, as shown by the time course in Figure 4c.
Figure 4.
Effect of E2 on AKT upstream signaling molecules in HepG2 cells. Involvement of PI3K in E2-induced AKT phosphorylation was studied by Western blot analysis as described in MATERIALS AND METHODS on HepG2 cells treated with the PI3K inhibitors Ly 294002 (Ly) and wortmannin (W) (10 μM each) and stimulated for 3 or 30 min with vehicle (0) or estradiol (E2) (10 nM) (a and b). Time course of PTEN levels (c) was analyzed by Western blot on control (0) and on E2-treated (10 nM) HepG2 cells at different times. The same filters were reprobed with anti-AKT or anti–β-actin antibodies. a′, b′ and c′, densitometric analysis (means ± SD) of three independent experiments. *p < 0.001 compared with respective control values (0), determined using Student's t test. °p < 0.001 compared with respective E2 values (E2), determined using Student's t test.
Cross-Talk Between PI3K and ERK Signal Transduction Pathways and the Effect on the Cyclin D1 Promoter Activity
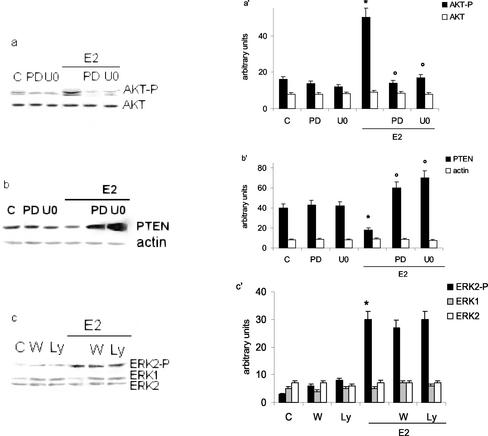
The accumulation of cyclin D1 protein after E2 stimulation is a critical event in G1 progression. We previously reported the accumulation of E2-induced cyclin D1 mRNA and protein 1–6 h after hormone stimulation. Furthermore, our data indicated that E2-induced DNA synthesis and cyclin D1 transcription were strictly dependent on rapid (10 min) and ER-dependent ERK phosphorylation; moreover, we demonstrated that the TRE motif located at -848 bp of cyclin D1 promoter was the target for E2-induced ERK activation (Marino et al., 2002). To assess whether this E2-induced pathway is synergic or parallel to the PI3K pathway in modulating cyclin D1 transcription, we first examined the effect of the MAP kinase pathway inhibitors on the ER-dependent AKT phosphorylation and PTEN levels 30 min after E2 stimulation. The MAP kinase cascade inhibitor (PD 98059 or U 0126) pretreatment completely blocked the E2-induced AKT phosphorylation (Figure 5a) and caused the parallel increase of PTEN levels (Figure 5b). The cell pretreatment with the inhibitors alone did not change AKT phosphorylation or PTEN levels.
Figure 5.
Cross-talk between PI3K/AKT and MAP kinase/ERK pathways via modulation of PTEN levels in Hep G2. Western blot analyses of both AKT and ERK phosphorylation (a and c, respectively) and PTEN levels (b) in Hep G2 cells were performed as described in MATERIALS AND METHODS on control (C) and on stimulated cells for 10 (c) or 30 (a and b) min with E2 (10 nM) after 15 min of pretreatment with the MAP kinase cascade inhibitors PD 98059 (PD) and U 0126 (U0) (10 μM either) or with PI3K inhibitors Ly 294002 (Ly) and wortmannin (W) (10 μM each). The same filters were reprobed with anti-AKT or anti–β-actin or anti-ERK antibodies. a′, b′ and c′, densitometric analysis (means ± SD) of three independent experiments. *p < 0.001 compared with respective control values (C), determined using Student's t test. °p < 0.001 compared with respective estradiol values (E2), determined using Student's t test.
The pretreatment of HepG2 cells with the PI3K inhibitors Ly 294002 or wortmannin did not affect the E2-induced ERK phosphorylation after 10 min of hormone treatment (Figure 5c), even though these concentrations completely blocked AKT phosphorylation.
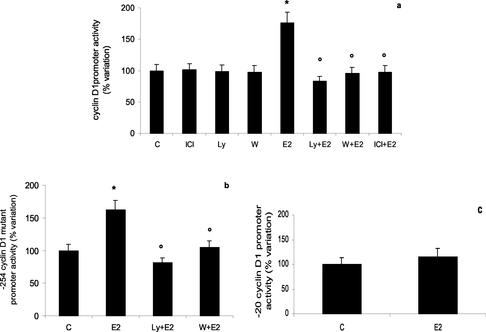
To verify whether the PI3K signal transduction pathway had a role in regulating cyclin D1 promoter activity and whether this signaling pathway worked on a different regulatory element(s) present in the cyclin D1 promoter, we transiently transfected HepG2 cells with either the complete cyclin D1 promoter construct (-2966 bp) or the deletion mutants of cyclin D1 promoter construct (-254 bp and -20 bp) linked to luciferase gene. E2 induced the transcription of -2966 and -254 cyclin D1 constructs (Figure 6, a and b). PI3K inhibitor pretreatment completely prevented E2 effects on both constructs. Conversely, E2 was ineffective in stimulating the activity of the minimal cyclin D1 promoter construct (-20, Figure 6c).
Figure 6.
Cyclin D1 gene promoter regulation by estradiol-induced PI3K pathway in Hep G2 cells. Luciferase assay detection on HepG2 cells transfected with pXP2-D1–2966-luciferase (a), pXP2-D1Δ-254-luciferase (-254) (b), and pXP2-D1Δ-20-luciferase (-20) (c). After transfection, cells were treated 6 h with vehicle (C) or E2 (10 nM) in the presence or absence of PI3K inhibitors Ly 294002 (Ly) or wortmannin (W) (10 μM each) or pure antiestrogen ICI 182,780 (ICI) (1 μM) (a and b). Data are expressed as percentage variation with respect to the controls and are the means ± SD of four independent experiments. *p < 0.001 compared with respective control values (C), determined using Student's t test. °p < 0.001 compared with respective estradiol values (E2), determined using Student's t test.
DISCUSSION
The ER is a ligand-inducible transcriptional enhancer that modulates the transcription of E2-induced target genes. Controversy exists, however, concerning whether E2-activated ER has a role outside the nucleus, particularly in mediating the E2 mitogenic effects (Lee and Yee, 1995; Lupu et al., 1996; Castoria et al., 1999; Marino et al., 2001b; Nadal et al., 2001). In addition to the accepted model for the E2 action mechanism, there is emerging evidence that E2-regulated mitogenesis starts with the activation of rapid/nongenomic signaling molecules often originating from the cell membrane (Castoria et al., 1999; Marino et al., 2001a, 2001b, 2002). This activation has been investigated with the primary focus on the MAP kinase pathway in mammary gland cells, MCF7, but with contradictory results (Migliaccio et al., 1996; Improta-Brears et al., 1999; Lobenhofer et al., 2000; Caristi et al., 2001; Castoria et al., 2001). Similar contradictions, although less well documented, are reported on E2-induced PI3K/AKT activation and on its role in regulating DNA synthesis and the cyclin D1 promoter activity (Lobenhofer et al., 2000; Castoria et al., 2001; Sun et al., 2001); furthermore, an acceptable definition of the cross-talk with other signaling pathways (i.e., MAP kinase) is lacking. The failure in summarizing a general model of E2 action working in MCF7 cells is probably attributable to the changes in the cell context occurring in these cells. Either synchronization procedures or the different ERα-to-ERβ ratio present in MCF7 cells, depending on their malignancy (Roger et al., 2001; Liu et al., 2002), could turn signal transduction pathways on or off differently.
Conversely, the HepG2 cell line represents a valuable experimental model for studying the role played by E2-induced nongenomic actions on hepatic cell proliferation. In fact, these cells retain many of the differentiated characteristics of quiescent hepatocytes, including E2 responsiveness (Archer et al., 1985, 1986; Chen et al., 1999; Graf et al., 2001). Like other liver-derived cells, HepG2 cells cultured in a medium not supplemented with estrogen maintain only the ERα expression (Tam et al., 1986; Couse et al., 1997; Farsetti et al., 1998; Taylor and Al-Azzawi, 2000), although at levels insufficient to induce a ligand-dependent trans-activation of synthetic ERE-containing target genes (Marino et al., 2001b). In this experimental model, we have recently identified two nongenomic actions of E2 (PKC-α and ERK-2 activation) that work in parallel to modulate distinct steps of E2-induced cell cycle progression (Marino et al., 2002).
Here, using a pharmacological approach, we found that the disruption of the PI3K cascade by specific inhibitors prevented the ability of HepG2 cells to enter the S phase in response to E2. We also demonstrated that E2 activates the AKT phosphorylation via PI3K and ERK. In fact, only after ERK activation (15 min after E2 administration) was a sustained, ER-dependent AKT phosphorylation detected (15–30 min). The ERK modulation of AKT phosphorylation is connected to the tumor suppressor PTEN levels, as demonstrated by the ability of MAP kinase cascade inhibitors to prevent E2-induced reduction of the PTEN levels. Both the PI3K inhibitors used prevented the cyclin D1 promoter activity. Noteworthy was the effect also present in the deletion mutant of cyclin D1 promoter construct (i.e., -254 bp), which contains the GC-rich region of SP1 and CRE motif.
These results showed a peculiar biphasic PI3K/AKT activation, partially related to PTEN levels, and identified the region of cyclin D1 promoter affected by this activation cascade. The timing of E2-induced AKT phosphorylation agrees with those reported in MCF7 (i.e., 2–10 min) (Improta-Brears et al., 1999; Castoria et al., 2001), neuronal, and astroglial cells (from 15 min with the highest level at 30 min) (Ivanova et al., 2002). Furthermore, we have shown the biphasic AKT phosphorylation with the earliest activation (3 min) to be unresponsive to the pure anti-ER, ICI, and the second (30 min) to be prevented by ICI. No activation was induced after the administration of the stereoisomer 17α-E2, indicating the specificity of E2-induced AKT phosphorylation. Moreover, both waves of AKT activation were mimicked by the plasma membrane–nonpermeating E2–BSA and were strictly linked to PI3K. These data, together with the rapidity of AKT phosphorylation (3 and 30 min), imply that the activation of PI3K starts from the plasma membrane. It has been proposed that a putative plasma membrane ER might be involved in rapid/nongenomic effects of E2 in several cell types (Levin, 2002), although its characterization remains unsolved. Recently, a γ-adrenergic membrane ER, which could be responsible for E2 initiating rapid signal in pancreatic β cells (i.e., increase of Ca2+ entry), has been described (Ropero et al., 2002). This receptor seems to be structurally unrelated to ERα, being activated even in the presence of ICI. It is possible that the first activation of AKT, unresponsive to ICI, is dependent on this kind of receptor. By contrast, a fraction of ERα appears to be able to translocate from the nucleus (Song et al., 2002) and localize close to the plasma membrane (Norfleet et al., 1999; Marino et al., 2002; Razandi et al., 2002). This kind of ER could be accounted for in the second wave of AKT phosphorylation.
Conversely, it is known that PI3K catalyzes the phosphorylation in the 3′ position of the inositol ring of phosphoinositides (mainly PI-4-P and PI-4,5-P2), producing an accumulation of second messengers (i.e., PI-3,4-P2, PI-3,4,5-P3) that are poor substrates for PLC but can recruit a variety of cytosolic signaling proteins on plasma membrane. AKT is one of these proteins: the link with the phosphoinositides enables AKT to localize on the plasma membrane and to open up the catalytic site. A second serine/threonine kinase, PDK1, also binds the phosphoinositides and colocalizes with AKT, phosphorylating the activation loop of the exposed catalytic domain (Scheid et al., 2002). The prevalent paradigm is that AKT phosphorylation is regulated by PTEN levels. Characterization of the lipid phosphatase activity of PTEN demonstrates that it shows specificity for phosphatidyl inositols phosphorylated at the 3′ position, and indeed, overexpression of PTEN in mammalian cells disrupted the PI3K-dependent production of PI-3,4,5-P3. Furthermore, the expression of the PTEN catalytically inactive mutant PTEN-C124S, which may function as a substrate trap, resulted in the accumulation of PI-3,4,5-P3, indicating that PTEN may function in vivo to antagonize the PI3K-dependent signaling (Furnari et al., 1997; Weng et al., 2001). These results could explain the presence of the growth factor–induced PI3K/AKT activations, probably mediated by the other PI3K product, PI-3,4-P2, which was still observed in the presence of high levels of PTEN (Cantley and Neel, 1999). In our system, the high levels of PTEN, parallel to the E2-induced earlier AKT phosphorylation, suggest that such activation may be dependent on PI-3,4-P2; furthermore, it is transient and redundant with respect to E2-induced proliferation, because no ER-independent effects on E2-induced DNA synthesis have been detected in HepG2 cells.
The second, prolonged and ER-dependent, AKT phosphorylation may be linked primarily to the production of PI-3,4,5-P3; in fact, it is paralleled by the decrease of PTEN levels. Remarkably, the disruption of the MAP kinase cascade by specific inhibitors prevented the E2-induced PTEN level reduction, suggesting that ERK may modulate the PTEN levels. PTEN was discovered only in 1997, and it has been the focus of particularly intense interest because of its central role in suppressing malignancy, functioning primarily as a PI-3,4,5-P3 phosphatase to regulate the crucial signal transduction pathway of PI3K (Myers et al., 1998; Yamada and Araki, 2001). Here, for the first time, a PTEN role in E2-induced cell proliferation has been reported.
Our results show that the disruption of the PI3K cascade by specific inhibitors prevents the E2-induced thymidine incorporation in DNA and confirm the role of cyclin D1 as a key intermediate in the E2-induced progression of cells through the G1 phase of the cell cycle, even though no ERE-like sequence in the promoter of cyclin D1 gene has been detected (Herbert et al., 1994). The E2 activation of cyclin D1 promoter is ER dependent, despite the HepG2 cells' containing low levels of ER unable to trans-activate ERE-containing reporter genes. Altogether, these data indicate that E2-induced cyclin D1 transcription is independent of the DBD domain of ER (Marino et al., 2002), and we have further demonstrated here that it depends on the rapid/nongenomic effects. The effect of E2 on cyclin D1 promoter is present with the mutant -254, which still contains the Oct/Sp1 and the CRE motifs but lacks the TRE, the E2F, and the E-box (Herbert et al., 1994). All these data sustain the notion that the effect of PI3K pathway on the cyclin D1 promoter is addressed to the regulatory elements present in this proximal promoter region. Thus, the E2-dependent activation of cyclin D1 must be considered a multifactorial process involving distinct regulatory elements localized in the cyclin D1 promoter (Sabbah et al., 1999; Castro-Rivera et al., 2001; Liu et al., 2002), each of which can be affected by rapid/nongenomic mechanisms.
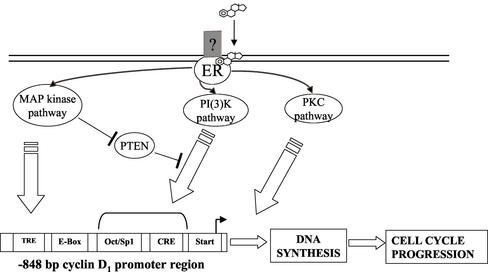
In conclusion, E2 induces parallel signaling pathways, which, in turn, play different roles in modulating HepG2 cell proliferation. In particular, the E2-induced PKC-α is strongly related to DNA synthesis but is not involved in cyclin D1 promoter activity (Marino et al., 2002), which suggests that its role is focused on the steps after cyclin D1 induction. Conversely, E2-induced MAP kinase and PI3K pathways are strongly involved in both DNA synthesis and cyclin D1 promoter activity (Marino et al., 2002 and present results). Altogether, these findings, summarized in Figure 7, represent a new model of E2-induced cell proliferation.
Figure 7.
Model of cell cycle progression triggered by rapid E2-induced signaling pathways in HepG2 cells. E2, via a putative membrane ER, mediates the rapid activation of MAP kinase, PKC, and PI(3)K signaling pathways that cross-talk and drive cells through G1–S phase transition inducing DNA synthesis: MAP kinase and PI3K signals regulate cyclin D1 transcription, whereas the role of PKC is focused on the following steps of the cell cycle regulation.
Acknowledgments
The generous gift of human cyclin D1-luciferase reporter genes from Prof. A. Weisz and F. Bresciani (Dipartimento di Patologia Generale, Seconda Università degli Studi di Napoli) is gratefully acknowledged. The assistance of Irene Cardillo and the editorial assistance of Peter DeMuro are also acknowledged. This work was supported by grants from Ministero Istruzione, Università, Ricerca (PRIN 2001–02) and Fondi Italiani Ricerca di Base 2003 to M.M.
References
- Altucci, L., Addeo, R., Citatiello, L., Davois, S., Parker, M.G., Truss, M., Beato, M., Sica, V., Bresciani, F., and Weisz, A. (1996). 17β-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G1-arrested human breast cancer cell. Oncogene 12, 2315-2324. [PubMed] [Google Scholar]
- Archer, T.K., Tam, S.P., and Deeley, R.G. (1986). Kinetics of estrogen-dependent modulation of apolipoprotein A-I synthesis in human hepatoma cells. J. Biol. Chem. 261, 5067-5074. [PubMed] [Google Scholar]
- Archer, T.K., Tam, S.P., Deugau, K.V., and Deeley, R.G. (1985). Apolipoprotein C-II mRNA levels in primate liver: induction by estrogen in the human hepatocarcinoma cell line, HepG2. J. Biol. Chem. 260, 1676-1681. [PubMed] [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 772, 248-254. [DOI] [PubMed] [Google Scholar]
- Cantley, L.C., and Neel, B.G. (1999). New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 96, 4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caristi, S., Galera, J.L., Matarese, F., Imai, M., Caporali, S., Cancemi, M., Altucci, L., Cicatiello, L., Teti, D., Bresciani, F., and Weisz, A. (2001). Estrogens do not modify MAP kinase-dependent nuclear signaling during stimulation of early G(1) progression in human breast cancer cells. Cancer Res. 61, 6360-6366. [PubMed] [Google Scholar]
- Castoria, G., Barone, M.V., Di Domenico, M., Bilancio, A., Ametrano, D., Migliaccio, A., and Auricchio, F. (1999). Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J. 18, 2500-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoria, G., Migliaccio, A., Bilancio, A., Di Domenico, M., de Falco, A., Lombardi, M., Fiorentino, R., Varricchio, L., Barone, M.V., and Auricchio, F. (2001). PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated M.C.F-7 cells. EMBO J. 20, 6050-6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Rivera, E., Samudio, I., and Safe, S. (2001). Estrogen regulation of cyclin D1 gene expression in Z.R-75 breast cancer cells involves multiple enhancer elements. J. Biol. Chem. 276, 30853-30861. [DOI] [PubMed] [Google Scholar]
- Chen, J., Lavigne, J.A., Trush, M.A., and Yager, J.D. (1999). Increased mitochondrial superoxide production in rat liver mitochondria, rat hepatocytes, and HepG2 cells following ethinyl-estradiol treatment. Toxicol. Sci. 51, 224-235. [DOI] [PubMed] [Google Scholar]
- Coffer, P.J., Jin, J., and Woodgett, J.R. (1998). Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335, 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse, J.F., Lindzey, J., Grandien, K., Gustafsson, J.A., and Korach, K.S. (1997). Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERα) and estrogen receptor-beta (ER) messenger ribonucleic acid in the wild-type and ER-knockout mouse. Endocrinology 138, 4613-4621. [DOI] [PubMed] [Google Scholar]
- Farsetti, A., Moretti, F., Narducci, M., Misiti, S., Nanni, S., Andreoli, M., Sacchi, A., and Pontecorvi, A. (1998). Orphan receptor hepatocyte nuclear factor-4 antagonises estrogen receptor-mediated induction of human coagulation factor XII gene. Endocrinology 139, 4581-4589. [DOI] [PubMed] [Google Scholar]
- Foster, J.S., Henley, D.C., and Ahamed, S. (2001). Estrogens and cell-cycle regulation in breast cancer. Trends Endocrinol. Metab. 12, 320-327. [DOI] [PubMed] [Google Scholar]
- Furnari, F.B., Huang, H.J., and Cavenee, W.K. (1998). The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 58, 5002-5008. [PubMed] [Google Scholar]
- Furnari, F.B., Lin, H., Huang, H.S., and Cavenee, W.K. (1997). Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc. Natl. Acad. Sci. USA 94, 12479-12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf, G.A., Roswell, K.L., and Smart, E.J. (2001). 17β-Estradiol promotes the up-regulation of SR-BII in HepG2 cells and in rat liver. J. Lipid Res. 42, 1444-1449. [PubMed] [Google Scholar]
- Herbert, B., Truss, M., Beato, M., and Müller, R. (1994). Inducible regulatory elements in the human cyclin D1 promoter. Oncogene 9, 1295-1304. [PubMed] [Google Scholar]
- Improta-Brears, T., Whorton, A.R., Codazzi, F., York, J.D., Meyer, T., and McDonnell, D.P. (1999). Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc. Natl. Acad. Sci. USA 96, 4686-4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova, T., Mendez, P., Garcia-Segura, L.M., and Beyer, C. (2002). Rapid stimulation of the PI3-kinase/Akt signalling pathway in developing midbrain neurones by oestrogen. J. Neuroendocrinol. 14, 73-79. [DOI] [PubMed] [Google Scholar]
- Lee, A.V., and Yee, D. (1995). Insulin-like growth factors and breast cancer. Biomed. Pharmacother. 49, 415-421. [DOI] [PubMed] [Google Scholar]
- Levin, E.R. (2002). Cellular functions of plasma membrane estrogen receptors. Steroids 67, 471-475. [DOI] [PubMed] [Google Scholar]
- Liu, M.M., Albanese, C., Anderson, C.M., Hilty, K., Webb, P., Uht, R.M., Price, R.H., Jr., Pestell, R.G., and Kushner, P.J. (2002). Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J. Biol. Chem. 277, 24353-24360. [DOI] [PubMed] [Google Scholar]
- Lobenhofer, E.K., Huper, G., Iglehart, J.D., and Marks, J.R. (2000). Inhibition of mitogen-activated protein kinase and phosphatidylinositol 3-kinase activity in MCF-7 cells prevents estrogen-induced mitogenesis. Cell Growth Differ. 11, 99-110. [PubMed] [Google Scholar]
- Lupu, R., Cardillo, M., Cho, C., Harris, L., Hijazi, M., Perez, C., Rosenberg, K., Yang, D., and Tang, C. (1996). The significance of heregulin in breast cancer tumor progression and drug resistance. Breast Cancer Res. Treat. 38, 57-66. [DOI] [PubMed] [Google Scholar]
- Maehama, T., and Dixon, J.E. (1998). The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375-13378. [DOI] [PubMed] [Google Scholar]
- Marino, M., Acconcia, F., Bresciani, F., Weisz, A., and Trentalance, A. (2002). Distinct nongenomic signal transduction pathways controlled by 17β-estradiol regulate DNA synthesis and cyclin D1 gene transcription in HepG2 cells. Mol. Biol. Cell 13, 3720-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, M., Distefano, E., Pallottini, V., Caporali, S., Ceracchi, G., and Trentalance, A. (2001a). β-Estradiol stimulation of DNA synthesis requires different PKC isoforms in HepG2 and MCF7 cells. J. Cell. Physiol. 188, 170-177. [DOI] [PubMed] [Google Scholar]
- Marino, M., Distefano, E., Trentalance, A., and Smith, C.L. (2001b). Estradiol-induced IP3 mediate the estrogen receptor activity expressed in human cells. Mol. Cell. Endocrinol. 182, 19-26. [DOI] [PubMed] [Google Scholar]
- Migliaccio, A., Di Domenico, M., Castoria, G., de Falco, A., Contempo, P., Nola, E., and Auricchio, F. (1996). Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 15, 1292-1300. [PMC free article] [PubMed] [Google Scholar]
- Myers, M.P., Pass, I., Batty, I.H., Van der Kaay, J., Stolarov, J.P., Hemmings, B.A., Wigler, M.H., Downes, C.P., and Tonks, N.K. (1998). The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc. Natl. Acad. Sci. USA 95, 13513-13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal, A., Ropero, A.B., Fuentes, E., and Soria, B. (2001). The plasma membrane estrogen receptor: nuclear or unclear? Trends Pharmacol. Sci. 22, 597-599. [DOI] [PubMed] [Google Scholar]
- Nagata, D., Suzuki, E., Nishimatsu, H., Satonaka, H., Goto, A., Omata, M., and Hirata, Y. (2001). Transcriptional activation of the cyclin D1 gene is mediated by multiple cis-elements, including SP1 sites and a cAMP-responsive element in vascular endothelial cells. J. Biol. Chem. 276, 662-669. [DOI] [PubMed] [Google Scholar]
- Norfleet, A.M., Thomas, M.L., Gametchu, B., and Watson, C.S. (1999). Estrogen receptor-α detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology 140, 3805-3814. [DOI] [PubMed] [Google Scholar]
- Ramaswamy, S., Nakamura, N., Vazquez, F., Batt, D.B., Perera, S., Roberts, T.M., and Sellers, W.R. (1999). Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 96, 2110-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi, M., Oh, P., Pedram, A., Schnitzer, J., and Levin, E.R. (2002). ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol. Endocrinol. 16, 100-115. [DOI] [PubMed] [Google Scholar]
- Roger, P., Sahla, M.E., Makela, S., Gustafsson, J.A., Baldet, P., and Rochefort, H. (2001). Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 61, 2537-2541. [PubMed] [Google Scholar]
- Ropero, A.B., Soria, B., and Nadal, A. (2002). A nonclassical estrogen membrane receptor triggers rapid differential actions in the endocrine pancreas. Mol. Endocrinol. 16, 497-505. [DOI] [PubMed] [Google Scholar]
- Russell, K.S., Haynes, M.P., Sinha, D., Clerisme, E., and Bender, J.R. (2000). Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc. Natl. Acad. Sci. USA 97, 5930-5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah, M., Courilleau, D., Mester, J., and Redeuilh, G. (1999). Estrogen induction of the cyclin D1 promoter: involvement of a camp response-like element. Proc. Natl. Acad. Sci. USA 96, 11217-11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid, M.P., Marignani, P.A., and Woodgett, J.R. (2002) Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol. Cell. Biol. 22, 6247-6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid, M.P., and Woodgett, J.R. (2001). PKB/AKT: functional insights from genetic models. Nat. Rev. Mol. Cell. Biol. 2: 760-768. [DOI] [PubMed] [Google Scholar]
- Seo, H.S., and Leclercq, G. (2002). Evaluation of potential implication of membrane estrogen binding sites on ERE-dependent transcriptional activity and intracellular estrogen receptor-α regulation in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 80, 109-123. [DOI] [PubMed] [Google Scholar]
- Song, R.X.-D., McPherson, R.A., Adam, L., Bao, Y., Shupnik, M., Kumar, R., and Santen, R.J. (2002). Linkage of rapid estrogen action to MAPK activation by ERα-Shc association and Shc pathway activation. Mol. Endocrinol. 16, 116-127. [DOI] [PubMed] [Google Scholar]
- Stein, R.C., and Waterfield, M.D. (2000). PI3-kinase inhibition: a target for drug development? Mol. Med. Today 6, 347-357. [DOI] [PubMed] [Google Scholar]
- Stevis, P.E., Deecher, D.C., Suhadolnik, L., Mallis, L.M., and Frail, D.E. (1999). Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology 140, 5455-5458. [DOI] [PubMed] [Google Scholar]
- Sun, M., Paciga, J.E., Feldman, R.I., Yuan, Z., Coppola, D., Lu, Y.Y., Shelley, S.A., Nicosia, S.V., and Cheng, J.O. (2001). Phosphatidylinositol-3-OH kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ER) via interaction between ER and PI3K. Cancer Res. 61, 5985-5991. [PubMed] [Google Scholar]
- Sutherland, R.L., Reddel, R.R., and Green, M.D. (1983). Effects of estrogens on cell proliferation and cell cycle kinetics: a hypothesis on the cell cycle effects of antiestrogens. Eur. J. Cancer Clin. Oncol. 19, 307-318. [DOI] [PubMed] [Google Scholar]
- Tam, S.P., Hachè, R.J.G., and Deeley, R.G. (1986). Estrogen memory effect in human hepatocytes during repeated cell division without hormone. Science 234, 1234-1237. [DOI] [PubMed] [Google Scholar]
- Taylor, A.H., and Al-Azzawi, F. (2000). Immunolocalisation of oestrogen receptor beta in human tissues. J. Mol. Endocrinol. 24, 145-155. [DOI] [PubMed] [Google Scholar]
- Weng, L.P., Brown, J.L., and Eng, C. (2001). PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum. Mol. Genet. 10, 599-604. [DOI] [PubMed] [Google Scholar]
- Yamada, K.M., and Araki, M. (2001). Tumor suppressor PTEN: modulator of cell signalling, growth, migration and apoptosis. J. Cell Sci. 114, 2375-2382. [DOI] [PubMed] [Google Scholar]
- Zheng, J., Ali, A., and Ramirez, V.D. (1996). Steroids conjugated to bovine serum albumin as tools to demonstrate specific steroid neuronal membrane binding sites. J. Psychiatry Neurosci. 21, 187-197. [PMC free article] [PubMed] [Google Scholar]