Abstract
Fusarium oxysporum includes nonpathogenic strains and pathogenic strains that can induce necrosis or tracheomycosis in plants. The objective of this study was to compare the abilities of a pathogenic strain (Foln3) and a nonpathogenic strain (Fo47) to colonize flax roots and to induce early physiological responses in flax cell culture suspensions. Both strains colonized the outer cortex of the root; however, plant defense reactions, i.e., the presence of wall appositions, osmiophilic material, and collapsed cells, were less frequent and less intense in a root colonized by Foln3 than by Fo47. Early physiological responses were measured in flax cell suspensions confronted with germinated microconidia of both strains. Both pathogenic (Foln3) and nonpathogenic strains (Fo47) triggered transient H2O2 production in the first few minutes of the interaction, but the nonpathogenic strain also induced a second burst 3 h postinoculation. Ca2+ influx was more intense in cells inoculated with Fo47 than in cells inoculated with Foln3. Similarly, alkalinization of the extracellular medium was higher with Fo47 than with Foln3. Inoculation of the fungi into flax cell suspensions induced cell death 10 to 20 h postinoculation, with a higher percentage of dead cells observed with Fo47 than with Foln3 beginning at 14 h. This is the first report showing that early physiological responses of flax cells can be used to distinguish pathogenic and nonpathogenic strains of the soil-borne fungus F. oxysporum.
The fungal species Fusarium oxysporum is ubiquitous in soils worldwide as both plant pathogenic and nonpathogenic strains. Among the pathogenic strains some induce root rots, but the most devastating induce vascular wilts in various crops of economic importance. The wilt-inducing strains are characterized by their ability to enter the plant through the roots and to spread throughout entire vascular system, inducing yellowing, wilting, and finally the death of the plant. Nonpathogenic strains of F. oxysporum are common in soils, where they survive saprophytically, although these strains also can colonize the root surface of plants without inducing disease. Until now, host plants inoculations were required to distinguish pathogenic and nonpathogenic strains of F. oxysporum (4).
Interactions between susceptible or resistant cultivars of host plants and pathogenic strains of F. oxysporum have been studied for many years (15, 18, 19, 65). The mechanisms leading to disease or the absence of disease are still poorly understood (26, 35, 40), and both pathogenic and nonpathogenic strains can penetrate into roots and trigger plant defense reactions (55). Defense reactions appeared earlier and were more intense in response to invasion by nonpathogenic strains than by the pathogens, suggesting that there was specific recognition of each fungus by the plant.
The successful recognition of a pathogen by a plant elicits a battery of defense responses and results in an incompatible interaction, leading to the resistance of the plant. A common expression of host resistance and a frequent expression of resistance by plants that are not hosts is the hypersensitive response (HR), which is associated with the death of plant cells surrounding the site of pathogen infection within a few hours after pathogen contact (39). Thus, the initial phases of plant-pathogen interactions are crucial for the establishment of the fungus within the plant.
During the last decade, cell cultures have been used as a convenient system for identifying early physiological events in plant-pathogen interactions. The earliest detectable events are rapid ion fluxes (especially of Ca2+ and H+) across the plasma membrane, protein phosphorylation, and rapid generation of active oxygen species. The involvement of calcium in the signaling process, leading to plant defense responses, is well established (3, 7, 41, 49, 63, 71, 72), and in plant-microbial interactions calcium influx and cytosolic calcium elevation are critical events after elicitor perception (16, 45, 47). Active oxygen species are assumed to have antimicrobial effects and to be involved in oxidative cross-linking of cell wall proteins, lignification of cell walls, phytoalexin production, and defense gene expression (6, 36, 37, 50, 64, 68).
In the plant-F. oxysporum interaction, later defense responses have been well characterized. These responses include reinforcement of cell walls (8, 13, 59, 60) and accumulation of potential antimicrobial compounds such as pathogenesis-related proteins and phytoalexins (22, 29, 31, 58). One study only reported early events of interactions between crude extracts of fungal walls of F. oxysporum f. sp. melonis and melon cell suspensions (24).
For the present study the nonpathogenic strain Fo47, a well-studied biological control agent (30, 66), a pathogenic strain of F. oxysporum f. sp. lini (Foln3), and flax were selected because they constitute a model used for many years in the laboratory and in greenhouses to study the interactions between the plant and pathogenic and nonpathogenic strains of F. oxysporum. Our objectives here were (i) to describe and compare the infection processes of flax roots inoculated with Fo47 and Foln3 and (ii) to compare the early plant reactions (H2O2 production, Ca2+ influx, alkalization of the extracellular medium, and cell death) in response to the pathogen and the nonpathogen with cell suspensions and microconidia of F. oxysporum. Our working hypothesis was that the early physiological responses of cells would enable us to differentiate the compatible from the incompatible interaction. Indeed, numerous studies of other plant-pathogen interactions have been conducted that led to the differentiation of compatible and incompatible interactions (10, 2). To our knowledge, the present study is the first time this type of approach has been used with F. oxysporum, a soil-borne fungus.
MATERIALS AND METHODS
Fungal strains.
The pathogenic strain F. oxysporum f. sp. lini Foln3, isolated from a diseased flax plant in French Brittany, and the nonpathogenic strain F. oxysporum Fo47, isolated from a suppressive soil from the ChÂteaurenard region of France, were used in the present study. The American Type Culture Collection accession numbers for these strains are MYA-1201 and MYA-1198 for Foln3 and Fo47, respectively. Their pathogenic and nonpathogenic characteristics are regularly checked by inoculation of flax plants in greenhouse experiments. Both fungal strains were cryopreserved by freezing microconidial suspensions at −80°C in 50% glycerol. Before use, the fungi were transferred to potato dextrose agar (Sigma-Aldrich, Saint Quentin Fallavier, France).
Inoculum preparation and inoculation of roots.
The fungi were grown in 150 ml of a 10-g/liter malt broth (Biokar Diagnostics, Beauvais, France) in a 250-ml flask at 25°C on a rotary shaker at 125 rpm. After 5 days of growth, fungal cultures were filtered through a sterile number 2 sintered glass funnel (40- to 100-μm-pore-size mesh). The microconidia left in the filtrate were washed three times by centrifugation (11,000 × g, 20 min) and then resuspended in sterile distilled water. The density of the microconidial suspension was determined with a hemocytometer and adjusted by dilution to the desired concentration for root inoculation (106 microconidia ml−1).
Flax seeds (Linum usitatissimum) of cultivar Viking were surface sterilized in 1.25% sodium hypochlorite for 20 min and then rinsed three times in sterilized distilled water. Seeds were germinated on malt (10 g liter−1) agar. Ten seeds were placed on the surface of the nutrient agar in petri dishes that were kept at an inclination of 60° and incubated in the dark for 3 days at 25°C. Seedlings with a 1.5-cm-long radicle were removed from the agar surface and inoculated with the fungus. Inoculation resulted from dipping the radicles in a microconidial suspension (106 microconidia ml−1) for 1 h. Seedlings were aseptically transferred into tubes containing a sterile nutrient solution (Hydrokani nutrient solution; Hydro-Azote, Neuilly, France). The plants were cultivated in a growth chamber at 25°C during the day and 22°C at night with a day-and-night cycle of 16 and 8 h, respectively. Control plants were dipped in sterile distilled water and then cultivated, as were the inoculated plants. Radicles were sampled 4 days after inoculation. For each fungal strain, 10 inoculated plants and 5 control plants were processed for microscopic observations of root sections.
In planta cytological investigations.
Each radicle was examined under a stereomicroscope to determine the amount of root surface colonized by the fungus. The distal part of the root, where secondary roots were present, was sampled, and the secondary roots were removed before processing the taproot. A 5-mm piece of the taproot was fixed with 2% glutaraldehyde in phosphate buffer (0.1 M, pH 7.2) for 4 h at 20 to 22°C. After a wash in phosphate buffer, the samples were postfixed with 1% osmium tetroxide in the same buffer for 1 h at room temperature. The samples were then dehydrated in a graded series of ethanol, followed by propylene oxide, and embedded in a Epon-Araldite mixture (32). Semithin (0.5 μm) and ultrathin (90 nm) sections were cut with a Reichert Ultracut E ultramicrotome (Leica, Rueil-Malmaison, France). Semithin sections were stained with 1% aqueous toluidine blue-methylene blue in 1% sodium tetraborate and then examined under a bright-field light microscope (Leica). Ultrathin sections were contrasted with a methanolic solution of uranyl acetate (2.5%), followed by the addition of Reynold's lead citrate. Microscopic observations were made with a Hitachi 600 electron microscope operating at 75 kV. For each sample, 10 semithin and 5 ultrathin sections were observed. The light and electron micrographs shown in the figures are representative images from these large data collections.
Inoculum preparation for inoculation of cell cultures.
The flax cells were challenged either with microconidia or germinated microconidia of either Foln3 or Fo47. The fungi were grown at 25°C on a rotary shaker (125 rpm) in minimal liquid medium (21) in which sucrose was replaced by 5 g of glucose liter−1. After 5 days of growth, cultures were filtered through a sterile number 2 sintered glass funnel. The microconidia left in the filtrate were washed once by centrifugation (11,000 × g, 20 min), resuspended in standard buffer, and used to inoculate the cell suspensions at the rate of 106 microconidia g (fresh weight) of cells (FWC)−1.
When cell suspensions were inoculated with germinated microconidia, the microconidia were transferred to minimal liquid medium enriched with 1 g of glucose liter−1 and incubated at 25°C on a rotary shaker (125 rpm). After 16 h of incubation, microconidia with germ tubes 90 to 120 μm long were collected on a sterile number 1 sintered glass funnel (100- to 160-μm-pore-size mesh), washed three times with sterilized distilled water, and resuspended in standard buffer before being inoculated into cell suspensions at the rate of 106 microconidia g FWC−1.
Cell cultures.
Cell suspensions of the flax (L. usitatissimum) cultivar Viking were obtained from C. Morvan (University of Rouen, Rouen, France). These cultures were derived from hypocotyl calli and were cultivated in a modified Murashige and Skoog medium (53) supplemented with 1 mg of kinetin and 0.1 mg of dichlorophenoxyacetic acid (61) liter−1. The pH of the medium was adjusted to 6 before autoclaving. Cultures were maintained in 250-ml Erlenmeyer flasks on a rotary shaker (125 rpm) at 25°C in the dark. Cells were transferred to fresh medium every 7 days. One day prior to utilization, log-phase cell cultures were subcultured by transferring 60 ml of cells to 100 ml of fresh medium. For the experiments, cells were harvested by gentle vacuum filtration through a sterile number 2 sintered glass funnel and washed three times by vacuum filtration with a standard buffer. Cells were resuspended in standard buffer to reach a concentration of 0.1 g FWC ml of standard buffer−1, distributed into Erlenmeyer flasks, and incubated for 90 min (unless indicated) on a rotary shaker water bath at 24°C and 150 rpm before the addition of microconidia of F. oxysporum. The standard buffer contained 175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4, and 2 mM morpholinoethanesulfonic acid (MES; pH 6.2) (43).
H2O2 measurement.
H2O2 was quantified by measuring the chemiluminescence of luminol reacting with H2O2 in the presence of peroxidase (69). Portions (30 ml) of the cell suspensions were incubated in 50-ml Erlenmeyer flasks for 15 min and then inoculated with either microconidia or germinated microconidia of the fungi. Chemiluminescence was monitored at 10-min intervals for 5 h. Aliquots (250 μl) of the cell suspensions were withdrawn and then mixed with 5 μl of peroxidase (0.5 μg μl−1; Sigma-Aldrich), 50 μl of 0.5 mM luminol, and 350 μl of 50 mM MES buffer (pH 6.7) containing 175 mM mannitol, 0.5 mM K2SO4, and 5 mM CaCl2. H2O2 production was measured with a luminometer (Lumat LB 9507; EG&G Berthold, Bad Wilbad, Germany) integrating relative luminescent units over a period of 10 s. H2O2 equivalents in nmol H2O2 g FWC−1 were calculated by using calibration curves obtained with known amounts of H2O2 added to control flax cell suspensions.
Diphenylene iodonium (DPI; Sigma-Aldrich) was used to determine whether a NADPH oxidase-like enzyme was responsible for H2O2 production. DPI was dissolved in dimethyl sulfoxide and added 10 min before the addition of germinated microconidia to a final concentration of 15 μM.
Ca2+ influx measurement.
Ca2+ uptake was measured as described by Tavernier et al. (63). 45CaCl2 (0.40 GBq mg−1; Amersham) was added at a rate of 33 mMBq of 45Ca2+ g FWC−1 to 50-ml cell suspensions in 100-ml Erlenmeyer flasks 5 min before the addition of germinated microconidia. Triplicate 2-ml samples of the cell suspensions were collected at 30-min intervals for 150 min and filtered on GF/A glass microfiber filters (Whatman, Kent, United Kingdom) that were washed once for 1 min and twice for 30 s with 10 ml of buffer (175 mM mannitol, 0.5 mM K2SO4, 2 mM MES [pH 6.2]) containing 2 mM LaCl3 to displace any externally bound 45Ca2+. Cells were scraped from the filters, placed in scintillation vials, dried overnight at 60°C, and weighed. A total of 5 ml of Ready-Safe cocktail (Ultima Gold; Perkin-Elmer, Courtaboeuf, France) was added to each vial; the vials were then shaken for 1 min before counting in a scintillation counter (Tri-Carb 2100TR;, Perkin-Elmer). The results were expressed as micromoles of Ca2+ g (dry weight) of cells (DWC)−1.
Measurement of extracellular pH.
Extracellular pH was measured directly in 10-ml flax cell suspensions equilibrated in standard buffer (175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4, 2 mM MES [pH 6.2]) in 25-ml Erlenmeyer flasks. pH was monitored every 10 min for 4 h after addition of germinated microconidia.
Detection of cell death.
The vital dye neutral red was used to assess cell death (14). Accumulation of the dye within the vacuole was observed by light microscopy. Cells that lost membrane integrity and did not retain the neutral red staining were considered to be dead (54). Cell suspensions of 30 ml in 25-ml Erlenmeyer flasks were inoculated with germinated conidia, and triplicate samples of 500 μl were withdrawn immediately after inoculation and every 2 h from 10 to 20 h. Aliquots of cell suspensions were washed twice with 1 ml of a buffer containing 175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4, and 2 mM HEPES (pH 7.0), followed by incubation for 5 min in the same buffer supplemented with neutral red (stock solution of 1 mg ml−1 in water) to a final concentration of 0.01%. For each sample, two independent observations of a minimum of 200 cells were made.
All experiments with cell cultures were repeated three to six times, with triplicate samples. The results presented correspond to the mean with the standard deviations (SD) of data obtained in a representative experiment. For each experiment, controls were run with microconidia or cells only.
RESULTS
Patterns of flax root colonization by Fo47 and Foln3.
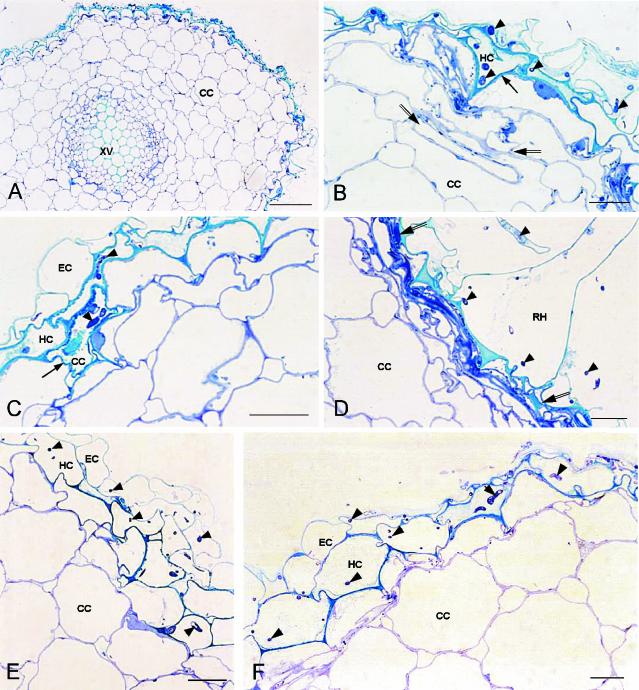
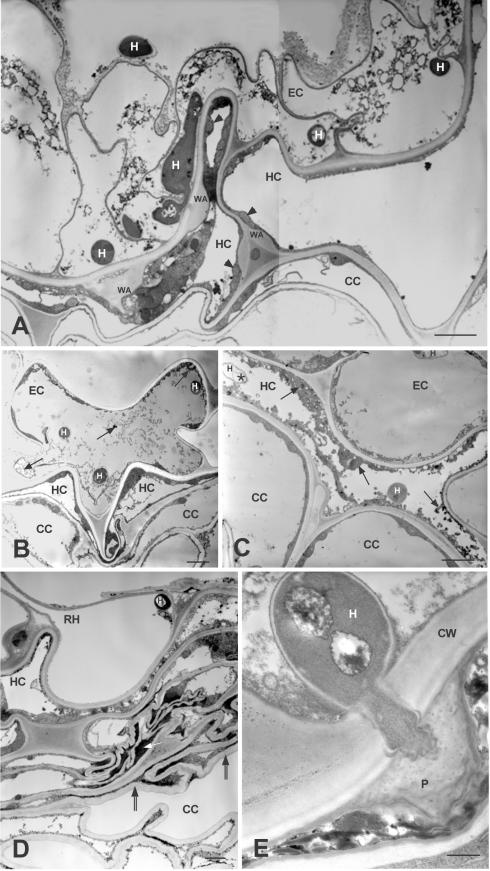
Sections of noninoculated control plants (not shown) had the normal structure of flax roots (67). Four days postinoculation, the nonpathogen Fo47 actively colonized the root surface. Hyphae penetrated into epidermal cells, and the hypodermis was heavily colonized. Few cortical cells were infected by the fungus, and the entire cortex appeared to be healthy (Fig. 1A). Cells infected by Fo47 were irregular in shape, with some appearing as triangles (Fig. 1B) and others as necrotic, with a dense turquoise blue staining when treated with toluidine blue-methylene blue solution (Fig. 1C). Frequently, under the epidermis the outer cortex cells were flattened (Fig. 1B) or collapsed and formed a layer of necrotic tissue at the periphery of the root (Fig. 1D). Based on transmission electron microscopy (TEM) observations, infected cells, regardless of location in the epidermis, hypodermis, or outer cortex, were filled with electron-dense material (Fig. 2B and C), and contained degraded fungal hyphae (Fig. 2C). Collapsed cells that contained an osmiophilic compact material and convoluted cell walls formed necrotic tissues (Fig. 2D). Cells colonized by Fo47 showed clear defense reactions. Wall appositions were observed in reaction to hypha growth; they were surrounded by a layer of cytoplasm with numerous mitochondria (Fig. 2A). Papillae occurred in reaction to fungal penetration pegs between cells (Fig. 2E). Less frequently, epidermal cells invaded by the fungus had a disorganized cytoplasm, without showing wall modifications, although the hypodermal adjacent cells had wall appositions (Fig. 2A).
FIG. 1.
Light micrographs of semithin cross sections showing colonization of flax root tissue at 4 days postinoculation with either the nonpathogenic strain F. oxysporum Fo47 or the pathogenic strain F. oxysporum Foln3 (toluidine blue-methylene blue staining). (A) Colonization of the root surface, the epidermis, and the hypodermis by the nonpathogen Fo47. Scale bar, 50 μm. (B to D) Plant host reactions against colonization by the nonpathogen Fo47. (B) Roughly triangular hypodermal cell (arrow). Some noncolonized cortical cells were flattened (between double arrows). (C) Necrotic cortical cell (arrow) intensively stained in turquoise blue. (D) Necrotic tissues under a colonized root hair. (E and F) Colonization of the epidermis, the hypodermis, and one layer of cortical cells by the pathogen Foln3. The cells appear only slightly disturbed. Hyphae are indicated by arrowheads. Scale bars (B to F), 10 μm. Abbreviations: CC, cortical cell; EC, epidermal cell; HC, hypodermal cell; RH, root hair; XV, xylem vessel.
FIG.2.
TEM images of ultrathin cross sections showing the defense reactions of flax cells to colonization by the nonpathogenic strain F. oxysporum Fo47 at 4 days postinoculation. (A) Colonized epidermal cells show a disorganization of the cytoplasm. Misshapen hypodermal cells present several wall appositions bordered with a layer of cytoplasm presenting numerous mitochondria (arrowheads). Cortical cells are flattened. (B) Colonized epidermal cell misshapen and filled with an osmiophilic material (arrows). (C) Colonized hypodermal cells, flattened and filled with an abundant osmiophilic material (arrows) and degenerated hyphae (asterisk), can be seen. (D) Detail image of Fig. 1D showing necrotic tissues under a colonized root hair: collapsed cells containing osmiophilic compact material (white arrow) and convoluted cell walls (between double arrows). Scale bars (A to D), 2.5 μm. (E) Penetration of a hypha halted by the formation of a papilla. Scale bar, 0.5 μm. Abbreviations: CC, cortical cell; CW, cell wall; EC, epidermal cell; H, hypha; HC, hypodermal cell; P, papilla; RH, root hair; WA, wall apposition.
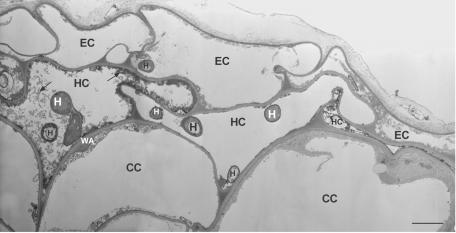
Roots inoculated with Foln3 also were extensively colonized on the surface by the fungus. Hyphae penetrated the epidermal cells and colonized the hypodermis and the first layer of cortical cells, which did not appeared to be heavily disturbed (Fig. 1E). Plant host reactions were observed in some areas and appeared to be similar to but were both less abundant and less intense than those described for Fo47 (Fig. 1F). By TEM observations, cells colonized by Foln3 had little or no reaction to fungal invasion. When reactions were observed, colonized cells appeared collapsed or showed wall appositions and/or osmiophilic material (Fig. 3).
FIG. 3.
TEM images of ultrathin cross sections showing colonization of flax root tissues at 4 days postinoculation with the pathogenic strain F. oxysporum Foln3. Colonization of epidermal and hypodermal cells and the defense reactions (osmiophilic material [arrow], wall appositions, and collapsed cells) are similar but less intense than those observed in root tissues colonized by the nonpathogenic strain Fo47 (see Fig. 2). Scale bar, 2.5 μm. Abbreviations: CC, cortical cell; EC, epidermal cell; H, hypha; HC, hypodermal cell; WA, wall apposition.
Early physiological events in flax cell cultures. (i) Choice of the type of fungal propagules and dose of inoculation.
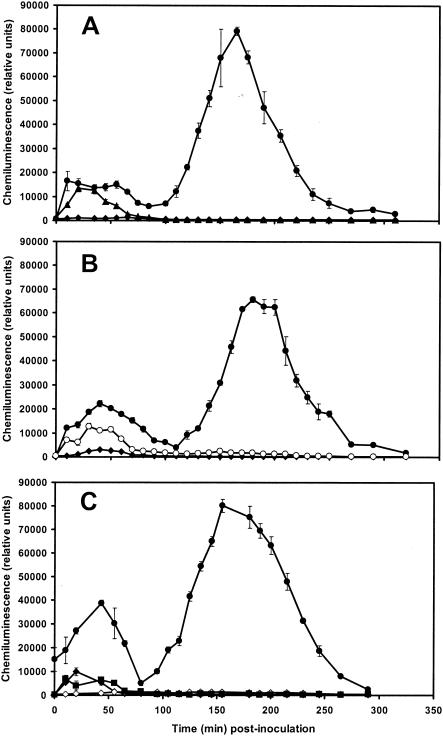
Microconidia and nongerminated microconidia of the nonpathogenic strain were compared in preliminary experiments. Only germinated microconidia induced clear early physiological events (Fig. 4A). Both microconidia and germinated microconidia rapidly induce initial H2O2 production, as seen by chemiluminescence (11 nmol of H2O2 g FWC−1) appearing 35 min postinoculation, but only the germinated microconidia induced a second increase in H2O2 production that peaked at 157 nmol of H2O2 g FWC−1 at 165 min postinoculation (Fig. 4A). Neither the dead (heated for 20 min at 100°C) germinated conidia nor the culture filtrate induced the second burst (data not shown).
FIG. 4.
Kinetics of H2O2 production induced in flax cell suspensions. H2O2 production was measured by using chemiluminescence of luminol and was monitored at 10-min intervals for 5 h. (A) Inoculation with 106 microconidia or 106 nongerminated microconidia g FWC−1 from the nonpathogenic F. oxysporum strain Fo47. Symbols: •, cells inoculated with germinated microconidia; ▴, cells inoculated with microconidia; ♦, control cells. The results are from one repetition in three independent experiments. The data are the means ± the SD of triplicate assays. (B) Inoculation with 106 germinated microconidia g FWC−1 from either the nonpathogenic strain Fo47 or the pathogenic strain Foln3 of F. oxysporum. Symbols: •, cells inoculated with germinated microconidia of Fo47; ○, cells inoculated with germinated microconidia of Foln3; ♦, control cells. The results are from one repetition in six independent experiments. Data are the means ± the SD of triplicate assays. (C) Inoculation with 106 germinated microconidia g FWC−1 from the nonpathogenic strain Fo47 with or without DPI (15 μm) added 10 min before inoculation. Symbols: •, cells inoculated with germinated microconidia; ▪, cells inoculated with microconidia and treated with DPI; ♦, control cells; ⋄, control cells treated with DPI. The results are from one repetition in three independent experiments. The data are the means ± the SD of triplicate assays.
In a second experiment, cells were inoculated with two levels of germinated microconidia: 105 and 106 microconidia g FWC−1. The second burst of H2O2 production was six times larger with 106 germinated microconidia than with 105 germinated microconidia (data not shown).
(ii) Differences between pathogenic and nonpathogenic strains in H2O2 production.
Germinated microconidia of the pathogenic strain Foln3 induced only the first phase of H2O2 production (Fig. 4B), this first burst being slightly lower than that induced by Fo47. The biphasic production of H2O2 was observed with germinated microconidia of Fo47. Neither the fungal propagules alone nor the flax cells alone produced a significant increase in H2O2 production (data not shown). When DPI (15 μM) was added to flax cells 10 min before inoculation of the germinated microconidia, the intensity of the first burst induced by both Foln3 (data not shown) and Fo47 was reduced by 80%, and the second burst induced by Fo47 was suppressed (Fig. 4C). Controls stained with neutral red showed that DPI did not affect the viability of either flax cells or the fungi.
(iii) Differences between pathogenic and nonpathogenic strains in Ca2+ influx.
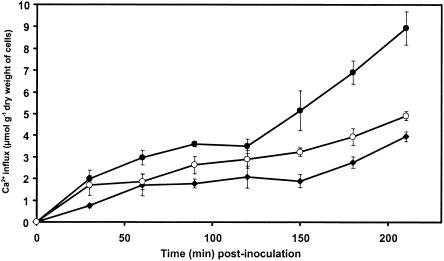
Ca2+ influx induced by germinated microconidia was more intense in cells inoculated with the nonpathogenic strain Fo47 than in cells inoculated with the pathogenic strain Foln3 (Fig. 5). Ca2+ influx induced by Fo47 increased progressively until 90 min postinoculation and then stabilized before increasing again sharply to reach 8.9 μmol g DWC−1 at the end of the experiment, i.e., at 210 min postinoculation. The Ca2+ influx induced by Foln3 was always higher than that observed in the control cells but was lower than that induced by Fo47. The difference between the two treatments increased with time and was highest at the end of the experiment (4.9 versus 8.9 μmol g DWC−1). Ca2+ influx in germinated microconidia incubated without flax cells was not significant during the time course of the experiment.
FIG. 5.
Time course of Ca2+ influx in flax cells inoculated with 106 germinated microconidia g FWC−1 from either the nonpathogenic strain Fo47 or the pathogenic strain Foln3 of F. oxysporum. At 5 min before inoculation, cell suspensions were incubated with 45Ca2+ (33 mMBq g FWC−1). Aliquots were collected at 30-min intervals for 150 min and treated as described in Materials and Methods. 45Ca2+ influx was determined by liquid scintillation counting. Symbols: •, cells inoculated with germinated microconidia of Fo47; ○, cells inoculated with germinated microconidia of Foln3; ⧫, control cells. The results are from one repetition in three independent experiments. The data are means ± the SD of triplicate assays.
(iv) Differences between pathogenic and nonpathogenic strains in alkalinization of the extracellular medium.
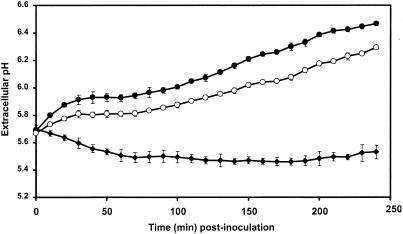
Flax cells responded to inoculation by germinated microconidia of both strains of F. oxysporum by alkalization of the extracellular medium (Fig. 6). In comparison to the control cells, Fo47 and Foln3 induced an alkalization of 0.94 and 0.76 pH units, respectively, by 4 h after inoculation. The germinated microconidia incubated without cells did not alter the extracellular pH.
FIG. 6.
Time course of extracellular alkalinization in flax cell suspensions inoculated with 106 germinated microconidia g FWC−1 from either the nonpathogenic F. oxysporum strain Fo47 or the pathogenic F. oxysporum strain Foln3. Extracellular pH of flax cell suspensions was measured at 10-min intervals for 4 h. Symbols: •, cells inoculated with germinated microconidia of Fo47; ○, cells inoculated with germinated microconidia of Foln3; ⧫, control cells. The results are from one repetition of six independent experiments. The data are the means ± the SD of triplicate assays.
(v) Differences between pathogenic and nonpathogenic strains in cell death.
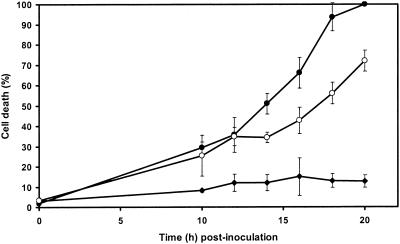
In control cell suspensions, the percentage of dead cells increased from 3% at the beginning of the assay to 15% after 20 h of incubation (Fig. 7). Inoculation of flax cell suspensions with germinated microconidia of the pathogenic strain Foln3 and the nonpathogenic strain Fo47 induced cell death at the same rate until 12 h postinoculation. A higher percentage of dead cells was observed in cultures treated with Fo47 than in cultures treated with Foln3 from 14 h postinoculation to the end of the experiment (20 h). By 18 h postinoculation, 93% of the cells were dead in cultures inoculated with Fo47 compared to 56% mortality in cultures inoculated with Foln3.
FIG. 7.
Time course of cell death in cultured flax cells inoculated with 106 germinated microconidia g FWC−1 from either the nonpathogenic F. oxysporum strain Fo47 or the pathogenic F. oxysporum strain Foln3. The percentage of dead cells was estimated at the time of inoculation and then every 2 h from 10 to 20 h by staining with neutral red. Symbols: •, cells inoculated with germinated microconidia of Fo47; ○, cells inoculated with germinated microconidia of Foln3; ⧫, control cells. The results are from one repetition in three independent experiments. The data shown are the means ± the SD of triplicate assays. For each assay, two independent observations of a minimum of 200 cells were made.
DISCUSSION
Microscope observations revealed differences in plant reactions in response to invasion by pathogenic and nonpathogenic strains of F. oxysporum, but the intensities of the responses were difficult to quantify. In contrast, the physiological studies showed significant differences in the early events induced by both fungi in flax cell suspensions. H2O2 production and Ca2+ influx showed the greatest differences.
Inoculation of flax cell suspensions with germinated microconidia of the nonpathogenic strain Fo47 induced a biphasic production of H2O2, whereas interaction with the pathogenic strain Foln3 only induced the first oxidative burst. Therefore, the second phase of H2O2 production is specific for the interaction with the nonpathogenic strain Fo47. This second phase of H2O2 production is induced only by nongerminated microconidia of Fo47 and not by either the microconidia or the culture filtrate. This result suggests that the discriminating molecules that trigger the early plant defense responses are present at the surface of the germ tubes emerging from the microconidia. This observation is consistent with the fact that the germ tube is the infection peg that penetrates into the epidermal root cells. Biphasic production of H2O2 has rarely been reported in cell suspensions treated with elicitors (28, 42). However, two-phase kinetics is common in cell suspensions challenged with bacteria (9, 12, 33, 34, 43, 44, 46, 48, 56) or fungal propagules (2). In these studies, the second burst was found to be associated with an incompatible reaction between either a virulent strain interacting with a resistant cultivar or a non-host plant species (44, 46) or an avirulent strain interacting with a susceptible cultivar (48, 56). This second burst of H2O2 production may be involved in pathways leading to restriction of fungal growth in roots (50). Since DPI can block the oxidative burst attributed to an NADPH oxidase (17, 25, 47, 57, 62) in different systems, the effect of DPI on H2O2 production suggests that an NADPH oxidase is involved in the production of H2O2 in the F. oxysporum-flax model.
Inoculation of flax cell suspensions with germinated microconidia of the nonpathogenic strain Fo47 induced a Ca2+ influx much more intense than that induced with the pathogenic strain. Moreover, the two phases of Ca2+ influx are superimposed on the two successive oxidative bursts. In particular, the Ca2+ influx increased sharply 2 h postinoculation, as did the H2O2 production, and this suggests that H2O2 levels are regulated through calcium signaling. Since the intensity of the spatiotemporal changes in intracellular calcium concentrations is responsible for the specificity of the physiological responses (5, 27, 51), the difference in Ca2+ influx in cells confronted by pathogenic and nonpathogenic strains could activate different signaling cascades and lead to the incompatible reactions associated with the nonpathogenic strains.
Both the nonpathogenic strain, Fo47, and the pathogenic strain, Foln3, triggered an increase in extracellular pH. This increase was higher for Fo47 than for Foln3. We found these results to be similar to those of Baker et al. (11) and Orlandi et al. (56) when they compared the interactions between soybean cell suspensions exposed to compatible and incompatible races of Pseudomonas syringae pv. glycenea and pathogens of other host species.
Both strains of F. oxysporum induced cell death, but the kinetics were different. The cells survived for a longer period in the presence of the pathogenic strain Foln3 than in the presence of the nonpathogenic strain Fo47. These results are in agreement with those of Able et al. (1) that showed that the kinetics of cell death were different in tobacco cell suspensions of a susceptible versus a resistant cultivar inoculated with different races of Phytophthora parasitica var. nicotianae. Similarly, Che et al. (20) showed that the number of dead cells was always significantly higher in the incompatible than in the compatible reactions in cultured rice cells challenged with strains of Pseudomonas avenae.
Hyphae of the nonpathogenic strain Fo47 are present in senescent cells that either did not show any defense reaction or most frequently appeared misshapen and probably filled with phenolic compounds, as indicated by the intensive turquoise blue staining in light microscopy and high density observed by TEM. The growth of the nonpathogenic strain was restricted by papillae and necrotic lesions made of collapsed cells around the penetration points. This phenomenon could be compared to the HR, which is characterized by a rapid induction of a localized necrosis at the penetration site of the pathogen (38, 39). Colonization of the root by the pathogen Foln3 induced less intense, but similar types of defense reactions than those observed in response to colonization by Fo47. The fact that the flax cultivar Viking has been selected for its limited susceptibility to Fusarium wilt could explain the observed defense reactions, which could have been less intense with a highly susceptible cultivar.
Taken together, these results allow us to compare the interactions between a susceptible cultivar of flax and the nonpathogenic strain Fo47 with incompatible reactions described for other plant pathogen models and the interactions with the pathogenic strain Foln3, with compatible reactions leading to disease. In most cases, the models chosen to study these early stages of the interactions involved either an aerial pathogen and a resistant cultivar of the plant or an avirulent strain and a susceptible cultivar, with both types of interactions leading to an HR. This HR has been well characterized; it corresponds to an incompatible reaction in which plant cells in contact with the pathogen die rapidly, the surrounding cells creating physical and biochemical barriers preventing the growth of the pathogen (23, 38, 52). To our knowledge, the HR has never been reported in an interaction between a root and a fungal pathogen. The only example related to root attacks concerns the HR described in the case of plant resistant to nematode (70). The observations reported here were made 4 days after inoculation, and it would be necessary to observe the root-Fo47 interaction a few hours after the fungus enters into contact with the root to investigate an HR process.
The present study is the first to address the cascade of events taking place in cells inoculated with germinated microconidia of a pathogenic or a nonpathogenic strain of F. oxysporum. Our results, although based on only two strains, show clear differences between these two types of interactions and are analogous to the compatible and incompatible reactions described for other plant-pathogen interactions. A critical question is whether these results can be generalized to other pathogenic wilt-inducing and nonpathogenic strains. We will test this hypothesis by inoculating flax cells with various strains of F. oxysporum that are either nonpathogenic or pathogenic on other plant species.
Acknowledgments
The electron microscope observations were made at the Center for Microscopy Applied to Biology, University of Burgundy, Burgundy, France. We thank Claudine Morvan for the flax cell cultures, Krystyna Stawiecki for technical assistance, Josette Relot and Claude Humbert for photography, Martine Janisz for bibliography assistance, and L. C. Van Loon and Michel Nicole for stimulating discussions.
REFERENCES
- 1.Able, A. J., D. I. Guest, and M. W. Sutherland. 1998. Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var. nicotianae. Plant Physiol. 117:491-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Able, A. J., D. I. Guest, and M. L. Sutherland. 2000. Hydrogen peroxide yields during the incompatible interaction of tobacco suspension cells inoculated with Phytophthora nicotianae. Plant Physiol. 124:899-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Able, A. J., D. I. Guest, and M. W. Sutherland. 2001. Relationship between transmembrane ion movements, production of reactive oxygen species and the hypersensitive response during the challenge of tobacco suspension cells by zoospores of Phytophthora nicotianae. Physiol. Mol. Plant Pathol. 58:189-198. [Google Scholar]
- 4.Alabouvette, C., V. Edel, P. Lemanceau, C. Olivain, G. Recorbet, and C. Steinberg. 2001. Diversity and interactions among strains of Fusarium oxysporum: application to biological control, p. 131-157. In M. J. Jegger and N. J. Spence (ed.), Biotic interactions in plant-pathogen associations. CAB International, London, England.
- 5.Allen, G. J., S. P. Chu, K. Schumacher, C. T. Shimazaki, D. Vafeados, A. Kemper, S. D. Hawke, G. Tallman, R. Y. Tsien, J. F. Harper, J. Chory, and J. I. Schroeder. 2000. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289:2338-2342. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez, M. E., R. I. Pennell, P. J. Meijer, A. Ishikawa, R. A. Dixon, and C. Lamb. 1998. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773-784. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson, M. M., S. L. Midland, J. J. Sims, and N. T. Keen. 1996. Syringolide 1 triggers Ca2+ influx, K+ efflux, and extracellular alkalization in soybean cells carrying the disease-resistance gene RPg4. Plant Physiol. 112:297-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baayen, R. P., C. Van Eijk, and D. M. Elgersma. 1989. Histology of roots of resistant and susceptible carnation cultivars from soil infested with Fusarium oxysporum f. sp. dianthi. Neth. J. Plant Pathol. 95:3-13. [Google Scholar]
- 9.Baker, C. J., and E. W. Orlandi. 1995. Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33:299-321. [DOI] [PubMed] [Google Scholar]
- 10.Baker, C. J., N. R. O'Neill, L. D. Keppler, and E. W. Orlandi. 1991. Early responses during plant-bacteria interactions in tobacco cell suspensions. Phytopathology 81:1504-1507. [Google Scholar]
- 11.Baker, C. J., C. J. Mock, J. A. Glazener, and E. W. Orlandi. 1993. Recognition responses in pathogen/non-host and race/cultivar interactions involving soybean (Glycine max) and Pseudomonas syringae pathovars. Physiol. Mol. Plant Pathol. 43:81-94. [Google Scholar]
- 12.Baker, C. J., G. L. Harmon, J. A. Glazener, and E. W. Orlandi. 1995. A noninvasive technique for monitoring peroxidative and H2O2-scavenging activities during interactions between bacterial plant pathogens and suspension cells. Plant Physiol. 108:353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benhamou, N., and C. Garand. 2001. Cytological analysis of defense-related mechanisms induced in pea root tissues in response to colonization by nonpathogenic Fusarium oxysporum Fo47. Phytopathology 91:730-740. [DOI] [PubMed] [Google Scholar]
- 14.Binet, M.-N., C. Humbert, D. Lecourieux, M. Vantard, and A. Pugin. 2001. Disruption of microtubular cytoskeleton induced by cryptogein, an elicitor of hypersensitive response in tobacco cells. Plant Physiol. 125:564-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop, C. D., and R. M. Cooper. 1983. An ultrastructural study of root invasion in three vascular wilt diseases. Physiol. Plant Pathol. 22:15-27. [Google Scholar]
- 16.Blume, D., T. Nürnberger, N. Nass, and D. Scheel. 2000. Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12:1425-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borden, S., and V. J. Higgins. 2002. Hydrogen peroxide plays a critical role in the defence response of tomato to Cladosporium fulvum. Physiol. Mol. Plant Pathol. 61:227-236. [Google Scholar]
- 18.Brammall, R. A., and V. J. Higgins. 1988. A histological comparison of fungal colonization in tomato seedlings susceptible of resistant to Fusarium crown and root rot disease. Can. J. Bot. 66:915-925. [Google Scholar]
- 19.Charest, P. M., G. B. Ouellette, and F. J. Pauzé. 1984. Cytological observations of early infection process of Fusarium oxysporum f. sp. radicis lycopersici. Can. J. Bot. 62:1232-1244. [Google Scholar]
- 20.Che, F. S., M. Iwano, N. Tanaka, S. Takayama, E. Minami, N. Shibuya, I. Kadota, and A. Isogai. 1999. Biochemical and morphological features of rice cell death induced by Pseudomonas avenae. Plant Cell Physiol. 40:1036-1046. [Google Scholar]
- 21.Correll, J. C., C. J. R. Klittich, and J. F. Leslie. 1987. Nitrate nonutilizing mutants of Fusarium oxysporum and their use in vegetative compatibility tests. Phytopathology 77:1640-1646. [Google Scholar]
- 22.Daayf, F., M. El-Bellaj, M. El-Hassni, F. J'Aiti, and I. El-Hadrami. 2003. Elicitation of soluble phenolics in date palm (Phoenix dactylifera) callus by Fusarium oxysporum f. sp. albedinis culture medium. Environ. Exp. Bot. 49:41-47. [Google Scholar]
- 23.Dangl, J. L., R. A. Dietrich, and M. H. Richberg. 1996. Death don't have no mercy: cell death programs in plant-microbe interactions. Plant Cell 8:1793-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Donato, M., C. Mozzetti, P. Chiavazza, R. Rubino, and A. Matta. 1997. Active oxygen production in cell-free melon homogenates elicited with hyphal wall components of Fusarium oxysporum f. sp. melonis. I. A response correlated with race-specific resistance. J. Plant Pathol. 78:35-43. [Google Scholar]
- 25.Desikan, R., J. T. Hancock, M. J. Coffey, and S. J. Neill. 1996. Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett. 382:213-217. [DOI] [PubMed] [Google Scholar]
- 26.Di Pietro, A., F. I. Garcia-Maceira, E. Mégkecz, and M. I. G. Roncero. 2001. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 39:1140-1152. [PubMed] [Google Scholar]
- 27.Dolmetsch, R. E., R. S. Lewis, C. C. Goodnow, and J. I. Healy. 1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386:855-858. [DOI] [PubMed] [Google Scholar]
- 28.Dorey, S., M. Kopp, P. Geoffroy, B. Fritig, and S. Kauffmann. 1999. Hydrogen peroxide from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiol. 121:163-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duijff, B. J., D. Pouhair, C. Olivain, C. Alabouvette, and P. Lemanceau. 1998. Implication of systemic induced resistance in the suppression of fusarium wilt of tomato by Pseudomonas fluorescens WCS417r and nonpathogenic Fusarium oxysporum Fo47. Eur. J. Plant Pathol. 104:903-910. [Google Scholar]
- 30.Fravel, D., C. Olivain, and C. Alabouvette. 2003. Fusarium oxysporum and its biocontrol. New Phytol. 157:271-279. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs, J. G., Y. Moënne-Loccoz, and G. Défago. 1997. Nonpathogenic Fusarium oxysporum strain Fo47 induces resistance to Fusarium wilt in tomato. Plant Dis. 81:492-496. [DOI] [PubMed] [Google Scholar]
- 32.Glauert, A. M., and C. Hall. 1991. Epoxy resins: an update on their selection and use. Eur. Microsc. Anal. 13:13-18. [Google Scholar]
- 33.Glazener, J. A., E. W. Orlandi, and C. J. Baker. 1996. The active oxygen response of cell suspensions to incompatible bacteria is not sufficient to cause hypersensitive cell death. Plant Physiol. 110:759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glazener, J. A., E. W. Orlandi, G. L. Harmon, and C. J. Baker. 1991. An improved method for monitoring active oxygen in bacteria-treated suspension cells using luminol-dependent chemiluminescence. Physiol. Mol. Plant Pathol. 39:123-133. [Google Scholar]
- 35.Gomez-Gomez, E., M. C. Ruiz-Roldan, A. Di Pietro, M. I. Roncero, and C. Hera. 2002. Role in pathogenesis of two endo-β-1,4-xylanase genes from the vascular wilt fungus Fusarium oxysporum. Fungal Genet. Biol. 35:213-222. [DOI] [PubMed] [Google Scholar]
- 36.Grant, M., and J. Mansfield. 1999. Early events in host-pathogen interactions. Curr. Opin. Plant Biol. 2:312-319. [DOI] [PubMed] [Google Scholar]
- 37.Hammond-Kosack, K. E., and J. D. G. Jones. 1996. Resistance gene-dependent plant defense responses. Plant Cell 8:1773-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heath, M. C. 2000. Hypersensitive response-related death. Plant Mol. Biol. 44:321-334. [DOI] [PubMed] [Google Scholar]
- 39.Heath, M. C. 2000. Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant Biol. 3:315-319. [DOI] [PubMed] [Google Scholar]
- 40.Inoue, I., F. Namiki, and T. Tsuge. 2002. Plant colonization by the vascular wilt fungus Fusarium oxysporum requires FOW1, a gene encoding a mitochondrial protein. Plant Cell 14:1869-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishihara, A., H. Miyagawa, Y. Kuwahara, T. Ueno, and S. Mayama. 1996. Involvement of Ca2+ ion in phytoalexin induction in oats. Plant Sci. 115:9-16. [Google Scholar]
- 42.Jabs, T., M. Tschöpe, C. Colling, K. Hahlbrock, and D. Scheel. 1997. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc. Natl. Acad. Sci. USA 94:4800-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keppler, L. D., and C. J. Baker. 1989. O2− initiated lipid peroxidation in a bacteria-induced hypersensitive reaction in tobacco cell suspensions. Phytopathology 79:555-562. [Google Scholar]
- 44.Keppler, L. D., C. J. Baker, and M. N. Atkinson. 1989. Activated oxygen production during a bacteria-induced hypersensitive reaction in tobacco suspension cells. Phytopathology 79:974-978. [Google Scholar]
- 45.Knight, M. R., A. K. Campbell, S. M. Smith, and A. J. Trewavas. 1991. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352:524-526. [DOI] [PubMed] [Google Scholar]
- 46.Lamb, C., and R. A. Dixon. 1997. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48:251-275. [DOI] [PubMed] [Google Scholar]
- 47.Lecourieux, D., C. Mazars, N. Pauly, R. Ranjeva, and A. Pugin. 2002. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14:2627-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine, A., R. Tenhaken, R. Dixon, and C. Lamb. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583-593. [DOI] [PubMed] [Google Scholar]
- 49.Levine, A., R. I. Pennell, L. E. Alvarez, R. Palmer, and C. Lamb. 1996. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 6:427-437. [DOI] [PubMed] [Google Scholar]
- 50.Mellersh, D. G., I. V. Foulds, V. J. Higgins, and M. C. Heath. 2002. H2O2 plays different roles in determining penetration failure in three diverse plant-fungal interactions. Plant J. 29:257-268. [DOI] [PubMed] [Google Scholar]
- 51.McAinsh, M. R., and A. M. Hetherington. 1998. Encoding specificity in Ca2+ signalling systems. Trends Plant Sci. 3:32-36. [Google Scholar]
- 52.Morel, J. B., and J. L. Dangl. 1997. The hypersensitive response and the induction of cell death in plants. Cell Death Differ. 4:671-683. [DOI] [PubMed] [Google Scholar]
- 53.Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 45:473-497. [Google Scholar]
- 54.Naton, B., K. Hahlbrock, and E. Schmelzer. 1996. Correlation of rapid cell death with metabolic changes in fungus-infected, cultured parsley cells. Plant Physiol. 112:433-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olivain, C., and C. Alabouvette. 1999. Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici discussed in comparaison to a non-pathogenic strain. New Phytol. 141:497-510. [DOI] [PubMed] [Google Scholar]
- 56.Orlandi, E. W., S. W. Hutcheson, and C. J. Baker. 1992. Early physiological responses associated with race-specific recognition in soybean leaf tissue and cell suspensions treated with Pseudomonas syringae pv. glycinea. Physiol. Mol. Plant Pathol. 40:173-180. [Google Scholar]
- 57.Pugin, A., J. M. Frachisse, E. Tavernier, R. Bligny, E. Gout, R. Douce, and J. Guern. 1997. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell 9:2077-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Recorbet, G., G. Bestel-Corre, E. Dumas-Gaudot, S. Gianinazzi, and C. Alabouvette. 1998. Differential accumulation of β-1,3-glucanase and chitinase isoforms in tomato roots in response to colonization by either pathogenic or non-pathogenic strains of Fusarium oxysporum. Microbiol. Res. 153:257-263. [Google Scholar]
- 59.Rodriguez-Galvez, E., and K. Mendgen. 1995. Cell wall synthesis in cotton roots after infection with Fusarium oxysporum. Planta 197:535-545. [DOI] [PubMed] [Google Scholar]
- 60.Salerno, M. I., S. Gianinazzi, and V. Gianinazzi-Pearson. 2000. Effects on growth and comparison of root tissue colonization patterns of Eucalyptus viminalis by pathogenic and nonpathogenic strains of Fusarium oxysporum. New Phytol. 146:317-324. [DOI] [PubMed] [Google Scholar]
- 61.Schaumann, A., M. P. Bruyant-Vannier, F. Goubet, and C. Morvan. 1993. Pectic metabolism in suspension-cultured cells of flax, Linum usitatissimum. Plant Cell Physiol. 34:891-897. [Google Scholar]
- 62.Simon-Plas, F., C. Rustérucci, M. L. Milat, C. Humbert, J. L. Montillet, and J. P. Blein. 1997. Active oxygen species production in tobacco cells elicited by cryptogein. Plant Cell Environ. 20:1573-1579. [Google Scholar]
- 63.Tavernier, E., D. Wendehenne, J. P. Blein, and A. Pugin. 1995. Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction in tobacco cells. Plant Physiol. 109:1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tenhaken, R., A. Levine, L. F. Brisson, R. A. Dixon, and C. Lamb. 1995. Function of the oxidative burst in hypersensitive disease resistance. Proc. Natl. Acad. Sci. USA 92:4158-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tessier, B. J., W. C. Mueller, and A. T. Morgham. 1990. Histopathology and ultrastructure of vascular responses in peas resistant or susceptible to Fusarium oxysporum f. sp. pisi. Phytopathology 80:756-764. [Google Scholar]
- 66.Trouvelot, S., C. Olivain, G. Recorbet, Q. Migheli, and C. Alabouvette. 2002. Recovery of Fusarium oxysporum Fo47 mutants affected in their biocontrol activity after transposition of the Fot1 element. Phytopathology 92:936-945. [DOI] [PubMed] [Google Scholar]
- 67.Turlier, M. F., A. Eparvier, and C. Alabouvette. 1994. Early dynamic interactions between Fusarium oxysporum f. sp. lini and the roots of Linum usitatissimum as revealed by a transgenic GUS-marked hyphae. Can. J. Bot. 72:1605-1612. [Google Scholar]
- 68.Van Breusegem, F., E. Vranova, J. F. Dat, and D. Inzé. 2001. The role of active oxygen species in plant signal transduction. Plant Sci. 161:405-414. [Google Scholar]
- 69.Viard, M. P., P. Martin, A. Pugin, P. Ricci, and J. P. Blein. 1994. Protein phosphorylation is induced in tobacco cells by the elicitor cryptogein. Plant Physiol. 104:1245-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williamson, V. M., and R. S. Hussey. 1996. Nematode pathogenesis and resistance in plants. Plant Cell 8:1735-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu, H., and M. C. Heath. 1998. Role of calcium in signal transduction during the hypersensitive response caused by basidiospore-derived infection of the cowpea rust fungus. Plant Cell 10:585-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimmermann, S., T. Nürnberger, J. M. Frachisse, W. Wirtz, J. Guern, R. Hedrich, and D. Scheel. 1997. Receptor-mediated activation of a plant Ca2+-permeable ion channel involved in pathogen defense. Proc. Natl. Acad. Sci. USA 94:2751-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]