Abstract
Recent evidence has demonstrated the importance of bone marrow-derived mesenchymal stem cells (BM-MSCs) in the repair of damaged myocardium. The molecular mechanisms of engraftment and migration of BM-MSCs in the ischemic myocardium are unknown. In this study, we developed a functional genomics approach toward the identification of mediators of engraftment and migration of BM-MSCs within the ischemic myocardium. Our strategy involves microarray profiling (>22,000 probes) of ischemic hearts, complemented by reverse transcription-polymerase chain reaction and fluorescence-activated cell sorting of corresponding adhesion molecule and cytokine receptors in BM-MSCs to focus on the coexpressed pairs only. Our data revealed nine complementary adhesion molecules and cytokine receptors, including integrin β1, integrin α4, and CXC chemokine receptor 4 (CXCR4). To examine their functional contributions, we first blocked selectively these receptors by preincubation of BM-MSCs with specific neutralizing antibodies, and then we administered these cells intramyocardially. A significant reduction in the total number of BM-MSC in the infarcted myocardium was observed after integrin β1 blockade but not integrin α4 or CXCR4 blockade. The latter observation is distinctively different from that reported for hematopoietic stem cells (HSCs). Thus, our data show that BM-MSCs use a different pathway from HSCs for intramyocardial trafficking and engraftment.
INTRODUCTION
Cardiac repair and remodeling after ischemic injury involves myocyte hypertrophy, collagen deposition, and possibly ventricular dilatation (Sutton and Sharpe, 2000). Recent provocative data suggest that stem cells, either resident in the heart or originating from the bone marrow, may play an important role in the repair and regeneration of the injured myocardium (Anversa and Nadal-Ginard, 2002). We and others have shown that intramyocardial transplantation of bone marrow-derived stem cells (BMSCs) can promote cardiac repair with resulting functional improvement and reduced infarct size (Kocher et al., 2001; Mangi et al., 2003; Amado et al., 2005). In addition to direct transplantation, mobilization of BMSCs with cytokines such as granulocyte-colony stimulating factor (G-CSF) and stem cell factor has been reported to enhance myocardial repair and improve cardiac function (Anversa and Nadal-Ginard, 2002; Askari et al., 2003). However, in a recent trial, the subcutaneous administration of G-CSF after acute myocardial infarction (MI) did not lead to further improvement in ventricular function compared with conventional treatment (Ripa et al., 2006). These controversial findings suggest the need to understand the molecular mechanisms involved with stem cell migration and engraftment into the infarcted myocardium.
It has been reported that hematopoietic stem cells (HSCs) migrate in response to stromal-derived factor (SDF)-1α, the ligand for the CXC chemokine receptor 4 (CXCR4) (Wright et al., 2002), and the up-regulation of SDF-1 in the ischemic myocardium mediates homing of HSCs via its direct interaction of CXCR4 on the stem cells (Askari et al., 2003; Abbott et al., 2004). However, much controversy exists over the ability of HSCs to transdifferentiate into cardiac myocytes (Balsam et al., 2004; Nygren et al., 2004). Recent data suggest that that mesenchymal stem cells (MSCs) may be mobilized from BM, migrate into the infarcted myocardium and differentiate into cardiac myocytes (Mangi et al., 2003; Kawada et al., 2004). The molecular mediators involved with MSC migration and engraftment are unknown. In this study, we developed a functional genomics strategy to identify the mediators of bone marrow-derived mesenchymal stem cells' (BM-MSCs) intramyocardial migration and engraftment in the infarcted tissue. We focus our investigation on the events that occur within the heart that mediate the movement and engraftment of MSCs from the nonischemic to the ischemic regions. Our approach is based on the hypothesis that specific chemoattractant molecules and adhesion molecules in the ischemic myocardium are up-regulated and interact specifically with corresponding receptors on BM-MSCs to induce migration and engraftment. Accordingly, we generated expression profiles of myocardial infarction (MI) heart to identify the chemokines, cytokines, and adhesion molecules that are up-regulated in myocardial ischemic injury, and we narrow our study to those whose corresponding receptors and ligands that are expressed in BM-MSCs (Figure 1A). We then used a functional approach to define the contribution of selected candidate molecules by evaluating the blocking effect of specific monoclonal antibodies on allogenic BM-MSC transplantation into mouse heart in vivo. Our data showed that distinctly different from that reported for HSCs, integrin β1, but not integrin α4 or CXCR4, is important for MSC migration and engraftment in the infarcted myocardium.
Figure 1.
(A) Strategy using genomics to identify potential receptor/ligand pairs involved in stem cell homing and trafficking. (B) Real-time PCR showing increased expression of numerous cytokines and adhesion molecules in MI versus sham hearts after 24 h (p < 0.05 except VEGF-α). Sele, endothelial selectin; TNFRII, tumor necrosis factor receptor II; CC, chemokine (C-C motif); CXC, chemokine (C-X-C motif); FN, fibronectin; Lam, laminin.
MATERIALS AND METHODS
Expression Profiling of Acute Ischemic Injury
BALB/c mice (female, 8–10 wk old; Harlan, Indianapolis, IN) were used with approval of the Harvard Medical Area Standing Committee on Animals. Myocardial infarctions were created by permanent ligation of left anterior descending (LAD) coronary artery as described previously (Min et al., 2002). Hearts were removed after 1, 8, and 24 h, and they were examined (n = 3 at each time point). The infarcted zone and bordering regions were carefully dissected away from the normal myocardium, and they were used for RNA extraction with TRIzol Reagent (Invitrogen). Corresponding regions from sham-operated littermates were used as controls (n = 3/time point). Total RNA was used for hybridization to Affymetrix Expression Set MOE430 oligonucleotide arrays (Affymetrix, Santa Clara, CA) according to the manufacturer's protocol. Affymetrix Microarray Suite version 5.0 was used to determine genes that were differentially expressed according to detection, change, and signal log ratio (>0.6 SLR) parameters. Genes that met the criteria for such data analysis metrics were further studied using real-time polymerase chain reaction (PCR) to verify differential expression. Primer sets for intercellular adhesion molecule-1 (ICAM-1), interleukin (IL)-1B, IL-6, endothelial selectin (Sele), tissue inhibitor of metalloproteinases-1 (TIMP-1), tumor necrosis factor receptor II (TNFRII), and vascular cell adhesion molecule (VCAM-1) were obtained from R&D Systems (Minneapolis, MN). The other primer sequences are as follows: SDF-1 forward GTCCTCTTGCTGTCCAGCTC, reverse AGATGCTTGACGTTGGCTCT; CCL6 forward GGCTGGCCTCATACAAGAAA, reverse TCCCCTCCTGCTGATAAAGA; CCL7 forward GTGTCCCTGGGAAGCTGTTA, reverse AGAAAGAACAGCGGTGAGGA; CXCL2 forward AGTGAACTGCGCTGTCAATG, reverse TCCAGGTCAGTTAGCCTTGC; fibronectin-1 forward AATCCAGTCCACAGCCATTC, reverse TAGTGGCCACCATGAGTCCT; laminin-1 forward AGTGGAAGGAATGGTTCACG, reverse TGCCAGTAGCCAGGAAGACT; vascular endothelial growth factor (VEGF)-1α forward AGAGCAACATCACCATGCAG, reverse CAGTGAACGCTCCAGGATTT; CXCR4 forward TGGAACCGATCAGTGTGAGT, reverse GACCAGGATCACCAATCCAT; IL-6 receptor-α forward ATGCTCCCTGAATGATCACC, reverse TTGTCACCCTCCAGGATCTC; IL-6 signal transducer forward CATGCTTTCAGGCTTTCCTC; reverse CCATACATGAAGTGCCATGC; CCR2 forward TGGCTGTGTTTGCCTCTCTA, reverse CGAAACAGGGTGTGGAGAAT; CXCR2 forward TGCCTCCTACCCATCAGAAC, reverse GACCTTTGGAAGAGCAGTCG; E-selectin ligand-1 forward AGGCGCTTCAGACACTGATT, reverse CAACTTCCAATCCCGAGAGA; integrin β1 forward CTGATTGGCTGGAGGAATGT, reverse TGAGCAATTGAAGGATAATCATAG; integrin β2 forward AGTTCGACTACCCATCCGTG; reverse GTTGCTGGAGTCGTCAGACA; integrin α1 forward TTGAGGGCACAAACAGACAG, reverse TCATCCAGGCCACAGTGTAA; integrin αL forward TTGAGGGCACAAACAGACAG, reverse TCATCCAGGCCACAGTGTAA; integrin αM forward CTTCTGGTCACAGCCCTAGC, reverse ACACTGGTAGAGGGCACCTG; integrin α4 forward TCTATCGTGACTTGTGGGCA, reverse AGTCCAGTACGATGATCCCG; integrin α5 forward AGCTGGATGTGTATGGGGAG, reverse CAGCTCAGGCTGGAGAAGTT; integrin α6 forward ATCACGGCTTCTGTGGAGAT, reverse GGATGCCTTTTTGAATTGGA; integrin α8 forward CTCACCTTGTCGAAACAGCA, reverse CATCATAGGAAGCTGGAGCC; and integrin α9 forward AGAGGAACTGGTGGTCATGG, reverse GGATGGATGAGAGAAGTGGC.
Bone Marrow Mesenchymal Stem Cells
BM-MSCs were isolated from the bone marrow of BALB/c mice as described previously (Peister et al., 2004). Briefly, nucleated cells were isolated from the bone marrow with a density gradient (Ficoll-Paque; Pharmacia, Sweden) and cultured in a growth medium consisting of α-minimal essential medium (α-MEM; Invitrogen, Carlsbad, CA) supplemented with 17% fetal bovine serum (FBS) on uncoated polystyrene dishes at 37°C with 5% CO2 for 24 h. Then, the culture was washed with PBS to remove the unattached cells. The attached cells were maintained in the growth medium to reach 80% confluence. The cells that were lifted by incubating with trypsin/EDTA for 2 min at 37°C were collected and the cells that did not detach in 2 min were discarded. The collected cells were expanded by seeding into new plates at a density of 50 cells/cm2. When reaching 80% confluence, only the cells that were lifted by incubating with trypsin/EDTA for 2 min at 37° were collected. Cells in passage 4 to 5 were used for the study. FACS analysis of the cells indicated that they were negative for hematopoietic linage markers CD45, CD19 (Figure 3A), CD3, CD14, and Flk-1 (data not shown) and positive for Sca-1, CD105 and CD29 (Figure 3A). When cultured in induction media (Peister et al., 2004), the cells differentiated into adipocytes, osteoblasts, and chondrocytes (data not shown).
Figure 3.
Protein expression of receptor/ligand pairs. (A) Flow cytometric analysis of BM-MSC surface receptors. Aliquots of cultured BM-MSCs were incubated with FITC- or PE-conjugated monoclonal antibodies against CD45, CD14, CD29, CD49d, CD105, CXCR4, IL-6 receptor α chain, or Sca-1. Cells stained with isotype control IgG conjugated to FITC served as a negative controls (gray peak). Representative results from one of three individual experiments were shown. Values represent percentages of positive cells. (B–D) Immunohistochemical staining for ICAM-1 (B), VCAM-1 (C), and tenascin-C (D). Murine heart sections, 48 h (B and C) and 72 h (D) after MI, were stained with anti-ICAM-1, VCAM-1, or tenascin-C (green) mAb. Myocytes were stained red except in D, and nuclei were stained with blue.
Determination of Corresponding Ligands/Receptors on BM-MSCs
Total RNA from cultured murine BM-MSCs was isolated, and reverse transcription-polymerase chain reaction (RT-PCR) was used to determine the expression of receptors corresponding to several adhesion molecules/extracellular matrix (ECM) proteins and chemokines/cytokines identified through profiling. RT-PCR was used to determine the expression of receptors corresponding to several adhesion molecules/ECM proteins, and chemokines/cytokines identified through profiling. RNA samples from murine peripheral blood mononuclear cells (PBMCs), juxtaglomerular cells (JGCs, from JG cell line As4.1; American Type Culture Collection, Manassas, VA) (Klar et al., 2002), vascular smooth muscle cells (VSMCs, isolated from murine thoracic aortae), and skin keratinocytes were used for comparison.
Flow Cytometric Analysis of Murine BM-MSCs
Cultured BM-MSCs were harvested by trypsinization. Cell aliquots were incubated with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibody (mAb) (BD Biosciences PharMingen, San Diego, CA) against CD45, CD14, CD29 (integrin β1), CD49d, CD105 (SH2), CXCR4, Sca-1, or CD126 (IL-6 receptor α chain), and they were analyzed (FACScan; BD Biosciences, San Jose, CA). For each analysis, an aliquot of cells was also stained with isotype control IgG-conjugated to FITC or PE as a negative control.
Characterization of Blocking Antibodies
The azide-free anti mouse CD49d mAb (IgG, clone PS/2; Accurate Chemical & Scientific, Westbury, NY) was used previously to block neutrophil migration in vivo (Petit et al., 2002; Bowden et al., 2002). The azide-free anti-CXCR4 IgG (Torrey Pine Biolabs, Houston, TX) was shown to neutralize CXCR4 and block SDF1-mediated leukocyte mobilization in mice (Petit et al., 2002; Bowden et al., 2002). The azide-free CD29 blocking mAb (IgM, clone Ha2/5) was purchased from BD Biosciences PharMingen. The saturating concentrations of the blocking antibodies were determined by flow cytometry as described previously (Ridger et al., 2001b). The antibodies were further verified for their capacity in blocking the receptor bindings to their ligands. SDF-1 (United States Biological, Swampscott, MA) and VCAM-1 (R&D Systems) were FITC conjugated using ProtOn Fluorescein Labeling kit (Vector Laboratories, Burlingame, CA) following the manufacturer's instruction. The saturating concentrations of the FITC-conjugated SDF-1 and VCAM-1 to the cells were titrated. Mouse T lymphocytes from EL4 cell line (American Type Culture Collection) grown in RPMI 1640 medium (Invitrogen) containing 10% FBS and l-glutamine were washed, preincubated with anti-CXCR4 or control IgG in RPMI 1640 medium containing 0.5% bovine serum albumin (BSA) at a concentration of 10 μg/ml for 20 min at room temperature, and then incubated with FITC-labeled SDF-1 for 30 min at 37°C. Mouse bone marrow-nucleated cells isolated with a Ficoll density gradient were cultured in α-MEM containing 17% FBS on plastic tissue culture dishes for 10 d. After washes, the adherent cells were detached and used for in vitro tests of anti-CD49d. The cells were resuspended in α-MEM containing 0.5% BSA, stimulated with 100 ng/ml SDF-1 (Ganju et al., 1998) to induce CD49d binding (Glodek et al., 2003), and incubated with IgG or anti-CD49d at a concentration of 10 μg/ml for 20 min at room temperature. The cells were then incubated with FITC-labeled VCAM-1 for 30 min at 37°C. After washes, the cells were fixed with 1% paraformaldehyde in PBS and analyzed on FACS to determine the cells with ligand binding. Cells stained with FITC-conjugated nonimmune IgG were used as a negative control.
Chemotaxis Assay
EL4 cell chemotaxis assay was performed in 24-well plates containing 5-μm porosity inserts (Costar, Kennebunk, ME) (Ganju et al., 1998). The expression of CXCR4 in these cells was verified by FACS analysis by using an FITC-conjugated anti-mouse CXCR4 mAb (BD Biosciences PharMingen). The cells were washed twice with serum-free RPMI 1640 medium and suspended as 1 × 106/ml in RPMI 1640 medium and H199 medium (1:1) containing 0.5% bovine serum albumin. Cells (105) in 100 μl were loaded onto the top wells. Then, 100 ng/ml SDF-1 was added to the bottom chamber with a total volume of 0.6 ml. Cells migrating to the bottom well were collected after 3 h and counted. Chemotaxis of passage 0 adherent mouse bone marrow nucleated cells was performed a in 48-well microchemotaxis chamber (NeuroProbe, Gaithersburg, MD) with 8-μm pore fibronectin-coated filter (Ceradini et al., 2004). The cells were suspended in serum-free α-MEM containing 0.5% BSA at a concentration of 0.5 × 106/ml. Then, 25,000 cells in 50 μl/well were loaded onto the upper chambers. The lower chambers were filled with serum-free α-MEM containing 100 or 500 ng/ml SDF-1. After 4-h incubation, the nonmigrating cells were completely wiped from the top surface of the filters, and the migrating cells adhering to the undersurface of the filters were stained with Hoeschst and quantified with an imaging software (IPlab; Scanalytics, Fairfax, VA). To assess the effect of anit-CXCR4, the cells were preincubated with anti-CXCR4 or control IgG at a concentration of 10 μg/ml for 20 min at room temperature before chemotaxis test. Each experiment was performed twice in six replicate wells.
Cell Adhesion Assay
Cell adhesion assays were performed in 48-well plates that were coated with 20 ng/ml fibronectin or recombinant human VCAM-1 (150 ng/well; R&D Systems) (Glodek et al., 2003). Wells were then washed three times with Hanks' balanced salt solution (HBSS) containing HEPES and blocked with 2% BSA in PBS for 1 h at 37°C. Then, 2% BSA in PBS alone-coated wells was used as negative control. For CD29 blockade, 104 MSCs per well were seeded on fibronectin-coated plates in the presence of isotype control IgM or anti-CD29 blocking mAb at a concentration of 40 μg/ml, and cells were incubated in α-MEM for 3 h at 37°C. Cells were photographed for assessment of adhesion and spreading. For CD49d blockade, 105 per well of passage 0 adherent mouse bone marrow-nucleated cells were stimulated with 100 ng/ml SDF-1 (Ganju et al., 1998) to induce CD49d-mediated cell adhesion (Glodek et al., 2003), incubated with 2.5 or 10 μg/ml CD49d mAb or 10 μg/ml isotype IgG for 30 min at 37°C, and then placed into VCAM-1–coated wells for 30 min at 37°C. The nonadherent cells were removed by three washes with HBSS, and the cells adhered were detached and counted. The same experiment was performed twice in quadruplet wells for each variable.
Intramyocardial Delivery of BM-MSCs
Female BALB/c mice (8–10 wk old; body weight 22–26 g) underwent permanent occlusion of LAD coronary artery. BM-MSCs isolated from male BALB/c mice (5–7 wk old) were transduced with retroviral green fluorescent protein (GFP) as described previously (Mangi et al., 2003). After sorting, >98% of BM-MSCs were GFP positive. One hour after ligation, 3 × 105 GFP-positive BM-MSCs were intramyocardially injected at a site slightly above the ligature in 20 μl of PBS after incubation with blocking antibody or isotype control as described in the Results. Seventy-two hours later, the hearts were arrested in diastole with KCl and harvested after PBS perfusion. The hearts were transversely dissected at the ligation level. The BM-MSCs in the myocardium below the ligature were assessed by real-time PCR and histology.
Immunohistochemical Staining
Frozen tissue sections from the heart 48 h after infarct were incubated with rat mAb against mouse ICAM-1 (eBioscience, San Diego, CA), VCAM-1 (Cymbus Biotechnology, Eastleigh, Southampton, United Kingdom), or tenascin-C (Chemicon, International, Temecula, CA) followed by sequential incubations with anti-rat biotin and FITC-conjugated anti-biotin antibody (Sigma-Aldrich, St. Louis, MO). Myocytes were stained with a mouse mAb against sarcomeric α-actin (Sigma-Aldrich) and Cy3-conjugated secondary antibody (Sigma-Aldrich). Nuclei were stained with Hoechst. The samples were visualized under a fluorescence microscope (Ecliose 80i; Nikon, Tokyo, Japan).
Histological Assessment of BM-MSCs in the Myocardium
The apical myocardium below the ligation of the heart was sectioned. Ten sections (20 μm in thickness) at 100-μm intervals down to the apex from the ligation were immunostained for GPF-positive cells. GFP was detected with an anti-GFP antibody (United States Biological) and an FITC-conjugated secondary antibody (Sigma-Aldrich). The area of GFP-positive BM-MSCs in each tissue section was measured using IPLab software (Scanalytics). The volume of BM-MSCs in the myocardium was determined by totaling the GFP-positive cell volumes between each two adjacent sections (average GFP-positive area of two adjacent sections times the interval [100 μm]).
Quantification of BM-MSCs in the Myocardium by Real-Time PCR
Real-time PCR was used to quantify BM-MSCs in the myocardium by measuring the amount of Y-chromosome–specific sequence derived from the male BM-MSCs. Genomic DNA was extracted from the myocardium below the ligation, by using a QIAamp DNA Blood Mini kit (QIAGEN, Valencia, CA). Real-time PCR was carried out using a 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). Primers and probes for murine Y-chromosome–specific TSPY gene and β-actin (Wang et al., 2002) were synthesized by Applied Biosystems. Standard curves were generated by serially diluting genomic DNA prepared from mouse BM-MSCs into samples containing 200 ng of genomic DNA from a mouse infarcted heart. PCR was performed for 50 cycles with denaturation at 95°C for 15 s and annealing at 59°C for 1 min, by using Master Mix (Applied Biosystems). β-Actin gene was used as an internal control to normalized equal loading of DNA per reaction. Assuming each MSC contains one copy of Y chromosome and 5 pg of DNA per diploid nucleus, the numbers of BM-MSCs in the myocardium below the ligation were determined (Lee et al., 2006).
Statistical Analysis
All values are expressed as mean ± SD. Student's paired t test was performed for comparison of data between the control and treated samples.
RESULTS
Expression Profile of Animal Model of Myocardial Infarction
To identify the chemokines, cytokines, and adhesion molecules that are up-regulated in myocardial ischemic injury, we generated expression profiles of MI heart. Samples from murine myocardial infarcts created by LAD coronary artery was analyzed on Affymetrix Expression Set MOE430 oligonucleotide arrays. Because our goal was to identify cytokines and adhesion receptors involved in trafficking, homing, and engraftment of BM-MSCs into ischemic myocardium, we focused on a subset of 461 probes (of >22,000 probes on this array) related to cell adhesion, chemokines, cytokines, and chemotaxis (determined by using the Gene Ontology classification system as well as a thorough evaluation of the current literature). Using Affymetrix MAS software, 175 probes met criteria for “presence” in at least four of six independent hybridizations, and these probes were further analyzed for either a mean SLR >0.6 from all nine comparisons at each time point (3 MI × 3 sham) or a change metrics of increase/marginal increase or decrease/marginal decrease in the majority of the comparisons (>4/9). The results indicated that at 1 h after LAD occlusion, the number of genes differentially expressed between hearts of MI and sham animals was modest but increased progressively at 24 h. A composite list of 46 genes is shown in Table 1. Twenty genes were differentially expressed at 8 h, 32 genes were found at 24 h, and 14 genes were shared at both time points (data not shown). Real-time PCR was performed for 35 of these apparently up-regulated genes. Thirty-four were confirmed to exhibit significant increases in expression. A subset of them that were up-regulated at 24 h post-MI are shown in Figure 1B. This included several cytokines such as IL-1β, IL-6, SDF-1, TIMP-1, and cell adhesion molecules (such as fibronectin-1 [FN-1]), ICAM-1, E-selectin, and VCAM-1).
Table 1.
Selected differentially expressed transcripts in MI vs. sham
| Transcript | Abbreviation | Transcript | Abbreviation |
|---|---|---|---|
| Up-regulated significantly | |||
| Actin, β, cytoplasmic | Actb | Integrin α 6 | Itga6 |
| A disintegrin-like and metalloprotease | Adamts1 | Macrophage migration inhibitory factor | Mif |
| Chemokine (C-C motif) ligand 2 | Ccl2 | Matrix metalloproteinase 14 | Mmp14 |
| Chemokine (C-C motif) ligand 6 | Ccl6 | Matrix metalloproteinase 8 | Mmp8 |
| Chemokine (C-C motif) ligand 7 | Ccl7 | Nuclear factor-κB light chain gene enhancer in B-cells inhibitor | Nfkbia |
| Chemokine (C-C motif) ligand 9 | Ccl9 | Platelet factor 4 | Pf4 |
| Chemokine (C-C motif) receptor 1 | Ccr1 | Plasminogen activator, tissue | Plat |
| Chemokine (C-C) receptor 2 | Ccr2 | Urokinase plasminogen activator receptor | Plaur |
| Procollagen, type I, alpha 1 | Col1a1 | Proplatelet basic protein | Ppbp |
| Chemokine (C-X-C motif) ligand 1 | Cxcl1 | Ribosomal protein L13a | Rpl13a |
| Chemokine (C-X-C motif) ligand 2 | Cxcl2 | Selectin, endothelial cell | Sele |
| Chemokine (C-X-C motif) receptor 6 | Cxcr6 | Secreted acidic cysteine-rich glycoprotein | Sparc |
| Fibronectin 1 | Fn1 | Transforming growth factor, β1 | Tgfb1 |
| Intercellular adhesion molecule | Icam1 | Transforming growth factor, β2 | Tgfb2 |
| Interferon-related developmental regulator 1 | Ifrd1 | Thrombospondin 1 | Thbs1 |
| Interleukin 1 receptor, type II | Il1r2 | Tissue inhibitor of metalloproteinase 1 | Timp1 |
| Interleukin 1 receptor antagonist | Il1rn | Tenascin-C | Tnc |
| Interleukin 6 | Il6 | Vascular cell adhesion molecule 1 | Vcam1 |
| Integrin α 5 | Itga5 | Vascular endothelial growth factor A | Vegfa |
| Down-regulated significantly | |||
| Catenin α-like 1 | Catnal1 | Matrix metalloproteinase 2 | Mmp2 |
| Cystatin C | Cst3 | Tissue inhibitor of metalloproteinase 2 | Timp2 |
| Interleukin 10 receptor, β | Il10rb | Transcription factor 4 | Tcf4 |
| Kit ligand | Kitl | Vitronectin | Vtn |
Expression Profile of BM-MSC Receptors
Although some of the adhesion molecules and cytokines identified by the expression profiling are known to be involved in the acute inflammatory response to myocardial ischemia, we postulated that some of these genes might be important for stem cell trafficking and engraftment through interactions with their receptors on BM-MSCs. To investigate this, we first determined whether their corresponding receptors or ligands are expressed in BM-MSCs. Indeed, our BM-MSCs expressed nine counter-receptors to eight cytokines that are up-regulated in the ischemic myocardium (Figure 2A). To examine the selectivity of gene expression, we studied several different cell types as controls, including PBMC-cultured JGCs and VSMCs. The receptors CXCR4 (for SDF-1), IL6RA and IL6ST (for IL-6), and CC chemokine receptor-2 (CCR2) (for CC chemokine receptor ligand-7 [CCL7]) were expressed by BM-MSCs as well as PBMCs but not by JGCs or VSMCs. CXCR2 for CXCL2 was expressed by PBMCs but not by BM-MSCs. The data support the notion that BM-MSCs express a selective set of membrane proteins that are distinct from hematopoietic, vascular, and other cells. We also examined the status of cell adhesion molecules in these cells. E-selectin ligand was universally expressed in all four cell types studied, including BM-MSCs. Several members of the integrin family were also expressed. Very late antigen 4 (VLA-4, integrin α4/β1) and integrin α6/β1 were expressed by both BM-MSCs and PBMCs, whereas integrin α8/β1 and α9/β1 was expressed in BM-MSCs, VSMCs, and JGCs but not in PBMCs (Figure 2A). All four isoforms (A, B, C, and D) of integrin β1 were expressed by BM-MSCs at varying levels with β1A the highest, but β1D was not detected in dermal keratinocytes (Figure 2B).
Figure 2.
(A) RT-PCR showing expression of receptors/ligands in BM-MSCs, PBMCs, JGCs, and VSMCs. Items displayed in red were shown to be up-regulated in ischemic myocardium compared with sham at 24 h by RT-PCR. Items displayed in blue were shown to be counterreceptors/ligands expressed by MSCs. IL6RA, IL-6 receptor, α; IL6ST, IL-6 signal transducer; CCR, CC receptor; Selel, E-selectin-1 ligand; VN, vitronectin; Tnc, tenascin-C; Itg, integrin. The other abbreviations were donated in the legend of Figure 1. (B) RT-PCR (30 cycles) showing expression of integrin β1 isoforms (A, B, C, and D) in BM-MSCs and dermal keratinocytes.
Protein Expression of Receptor/Ligand Pairs
The receptors on BM-MSCs and corresponding ligands in ischemic myocardium were further examined by FACS and immunohistochemistry. Our cultured BM-MSC exhibited differential expression patterns of various receptors as determined by FACS (Figure 3A). Although some of the α integrins demonstrated an attenuation of surface expression with successive passages, the integrin β1 (CD29) expression remained unchanged, ∼99% through the fifth passage (data not shown). Immunohistochemistry performed on ischemic myocardium validated the up-regulation of ECM proteins, including ICAM-1 (Figure 3B) and VCAM-1 (Figure 3C) at 48 h and tenascin-C at 72 h (Figure 3D) after MI.
Functional Validation of Receptor/Ligand Pairs with Antibody Blockade
To prove the functional role of these molecules for BM-MSC attachment to ischemic myocardium and migration within the infarct area, we studied the effect of ex vivo incubation of the cell with blocking monoclonal antibodies directed against potentially important ligands. FACS analysis indicated that incubation with antibody against CD29 blocked 85% of the cell surface receptor in BM-MSCs. Moreover, adhesion assay demonstrated that immunoblockade of CD29 dramatically reduced BM-MSC attachment to fibronectin-coated plates (Figure 4, A and B). To examine whether blockade of CD29 in BM-MSCs caused increased apoptosis, we performed trypan blue exclusion assay and annexin V and caspase 3 analyses. The results showed that blockade of CD29 did not increase cell death and apoptosis (Supplemental Figure 1). To test the in vivo relevance of the interaction between CD29 in the BM-MSCs and its ligands in the ischemic myocardium, female mice underwent permanent occlusion of left anterior descending coronary artery, and 3 × 105 BM-MSCs, derived from male mice and transduced with GFP gene, were injected into the left ventricular myocardium at a site above the ligature. To assess the quantity of BM-MSCs that had migrated into the infarcted myocardium, we performed real-time PCR assay of the Y-chromosome–specific TSPY genomic sequence that was only present in the male-derived BM-MSCs. In addition, we conducted histological assessment of GFP-positive BM-MSCs. Real-time PCR analysis indicated that the blockade reduced the amount of BM-MSCs in the ischemic myocardium by 45% compared with control group (i.e., mouse hearts injected with BM-MSCs treated with equal amount of nonimmune IgM; Figure 4C; n = 5; p = 0.012).
Figure 4.
Effect of CD29 blockade on BM-MSC adhesion, migration, and engraftment. (A and B) Blocking mAb against CD29 (B) reduced BM-MSCs attachment and spreading onto the fibronectin-coated plates compared with control IgM (A). (C) Real-time PCR assessment of BM-MSC migration and engraftment into the infarcted myocardium. BM-MSCs derived from male mice were incubated with anti-CD29 mAb or control IgM, and then they were injected into the myocardium of female mice after MI above the ligation. Seventy-two hours later, the BM-MSCs in the apical region of the heart below the ligation was assessed by real-time PCR assay of the Y-chromosome–specific DNA sequence. BM-MSCs incubated with antibody against CD29 had reduced accumulation in the apical region compared with the cells treated with control IgM (n = 5; **p = 0.012).
The amount of BM-MSCs in the infarcted myocardium below the ligation was further assessed by immunohistochemistry analysis of GFP-positive BM-MSCs. Injected in a site above the ligation, control BM-MSCs (incubated with nonimmune IgM) migrated from the injected site and “homed” to the left ventricular wall infarct (Figure 5, A, C, and E), whereas a dramatically reduced BM-MSC presence was seen in the infarcted myocardium that was injected with BM-MSCs pretreated with CD29 blocking antibody (Figure 5, B, D, and F). The total volume of BM-MSCs in the infarcted myocardium (below the ligation) showed a 39% reduction in these cells pretreated with anti-CD29 antibody compared with cells pretreated with nonimmune IgM (Figure 5G; n = 6; p = 0.004). To examine the possibility that inflammatory cells in the infarcted myocardium contribute to the GFP evaluation by uptake or cell fusion, we performed costaining studies of the myocardium 3 d post-MI for GFP and for neutrophils (anti-mouse neutrophil mAb MCA771GA; Serotec, Oxford, United Kingdom), macrophage (anti-mouse Mac-3 mAb), and toxic T lymphocytes (anti-mouse CD8 mAb). We found abundant neutrophils and macrophages (but a much lower amount of toxic T cells) in the infarct (Dewald et al., 2004; Vandervelde et al., 2006). None of the inflammatory cells were found GFP positive (data not shown), indicating that cell fusion or uptake of GFP by inflammatory cells, if existed, was extremely rare.
Figure 5.
CD29 blockade reduced the accumulation of BM-MSCs in the infarcted myocardium. BM-MSCs incubated with control IgM (A, C, and E) or anti-CD29 mAb (B, D, and F) were injected into the myocardium at one site above the ligation. Seventy-two hours later, sections of the heart below the ligation were immunostained for GPF-positive BM-MSCs (green). BM-MSCs treated with anti-CD29 blocking mAb (B and D) had reduced accumulation in the heart than BM-MSCs incubated with control IgM (A and C). BM-MSCs incubated with control IgM (E) were found to have migrated from the injection site, and they homed to the entire left ventricular wall infarct, whereas reduced BM-MSC migration and accumulation were seen in the BM-MSCs incubated with anti-CD29 (F). Myocytes (red) were detected by anti-sarcomeric α-actin, and nuclei (blue) were stained with Hoechst. (G) The area of GFP-positive BM-MSCs in each section was quantified. Treatment of BM-MSCs with CD29 blocking mAb reduced BM-MSC volume in the apical region of the hearts compared with incubation of the cells with control IgM (n = 6; **p = 0.004).
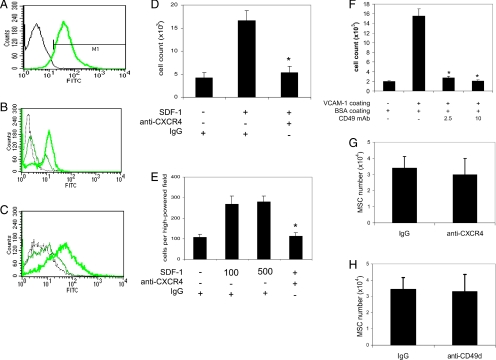
We applied the same blocking antibodies against CD49d (integrin α4) and CXCR4 as were used in previous studies (Bowden et al., 2002; Petit et al., 2002). We examined the blocking ability of the antibodies. We found that anti-CXCR4 reduced FITC-labeled SDF-1 binding to EL4 T lymphocytes, 90% of them expressed CXCR4 (Figure 6, A and B). We tested anti-CD49d on passage 0 adherent cells from culture of mouse bone marrow-nucleated cells, and we found that anti-CD49d inhibited FITC-labeled VCAM-1 binding to the cells (Figure 6C). Furthermore, anti-CXCR4 reduced SDF-1–induced migration of EL4 T lymphocytes (Figure 6D; p < 0.00001) and passage 0 adherent cells from culture of mouse bone marrow-nucleated cells (Figure 6E; p < 0.00001), and anti-CD49d inhibited attachment of the passage 0 adherent cells to VCAM-1–coated plates (Figure 6F; p < 0.0001). However, when BM-MSCs pretreated with blocking antibodies specifically against CXCR4 (Figure 6G; n = 5) or CD49d (Figure 6H; n = 6) were injected into the myocardium, in contrast to our result with CD29 antibody, we observed no statistically significant differences in the quantity of BM-MSCs in the infarcted myocardium (below the ligation) compared with injection of BM-MSCs pretreated with control IgG.
Figure 6.
Effect of CXCR4 or CD49d blockade on BM-MSC intramyocardial homing and engraftment to the infarcted myocardium. (A) FACS analysis indicated that >90% of EL4 cells expressed CXCR4. (B) EL4 cells were preincubated with anti-CXCR4 (peak in middle) or control IgG (peak on right) at a concentration of 10 μg/ml, and then they were incubated with FITC-labeled SDF-1. EL4 cells with SDF-1 binding were determined by FACS. Cell incubated with FITC-labeled nonimmune IgG were used as a negative control (gray peak). (C) Passage 0 adherent cells from culture of mouse bone marrow-nucleated cells were first incubated with anti-CD49d (peak in middle) or control IgG (peak on right) at a concentration of 10 μg/ml, and then they were incubated with FITC-labeled VCAM-1. Cells with VCAM-1 binding were determined by FACS. Cell incubated with FITC-labeled nonimmune IgG were used as a negative control (gray peak). (D and E) Anti-CXCR4 (10 μg/ml) reduced SDF-1–mediated migration of EL4 cell (D) and passage 0 adherent mouse bone marrow-nucleated cells (E). Each experiment was performed twice in six replicate wells, p < 0.00001 in D and E. (F) Anti-CD49d (2.5 and 10 μg/ml) inhibited attachment of passage 0 adherent mouse bone marrow-nucleated cells. The experiment was performed twice in quadruplet wells for each variable (p < 0.0001 for both antibody doses). (G and H) A similar procedure as described in Figure 5 was used for BM-MSC injection and assessment by real-time PCR. Treatment of BM-MSCs with anti-CXCR4 (G; n = 6, p = 0.83) or anti-CD49d (H; n = 5, p = 0.31) had no significant effect on the amount of BM-MSCs accumulated in the infarcted myocardium compared with treatment with control IgG.
We performed additional experiments with injections of 10-μm microspheres (Vector Laboratories) into the myocardium of infarcted or sham-operated animals, and we found that very few particles remained in the myocardium after 72 h in either sham or MI hearts (data not shown). These data demonstrate that the retention of BM-MSCs in the ischemic myocardium involves specific mediators and cell adhesion.
DISCUSSION
Myocardial infarction is a leading cause of heart failure and death in developed countries. The application of cell-based therapy for the treatment of heart disease remains in its preliminary phase, but it has shown some promise as seen in several early trials (Strauer et al., 2001; Assmus et al., 2002; Perin et al., 2003; Stamm et al., 2003; Britten et al., 2003; Tse et al., 2003). However, cell therapy encounters significant challenges in isolation techniques, scalability, reproducibility, and ease of clinical application. An alternative to cell therapy is to identify the molecules that mediate homing and engraftment of stem cells to the ischemic myocardium and to develop molecular therapies based on these discoveries.
SDF-1 has been shown to be important for the trafficking of BM-HSCs, and its intramyocardial administration seems to enhance BM-HSC homing to the ischemic myocardium (Askari et al., 2003; Abbott et al., 2004). Recent study indicated that up-regulation of SDF-1 by hypoxic endothelial cells was required for the attachment and transendothelial migration of the circulating CXCR4-positive endothelial progenitor cells (Ceradini et al., 2004). However, it has not been shown that this pathway is involved with BM-MSCs homing to the ischemic myocardium. Because recent data have demonstrated that MSCs mobilized from the bone marrow, rather than HSCs, are involved in myocyte regeneration (Mangi et al., 2003; Balsam et al., 2004; Kawada et al., 2004), the elucidation of the pathway mediating MSC homing and trafficking is obviously important.
In this study, we report a functional genomics strategy to determine the signals that mediate intramyocardial migration and engraftment of BM-MSCs to ischemic tissue, and we provide “proof of concept” for this approach. We injected BM-MSCs to study the migration within the heart from the border zone to the infarcted myocardium, and subsequently the engraftment of the cells in the ischemic myocardium. We identified integrin β1 but not integrin α4 or CXCR4 as a distinctive pathway for BM-MSC intramyocardial migration and engraftment. Our strategy involves 1) generating gene expression profiles of murine acute MI hearts to determine the early events involved in stem cell homing and myocardial repair, 2) narrowing the number of candidates to only these whose counterreceptors are expressed in BM-MSCs, and 3) proving the functional role of the verified ligands in vivo by examining the effect of blocking antibodies on allogenic BM-MSC transplantation in murine acute MI hearts. Using Affymetrix microarrays and real-time PCR, we first found that, compared with hearts from sham-operated animals, MI hearts showed significantly increased expression of selective chemokines, cytokines, and cell adhesion molecules, including ICAM-1, IL-6, SDF-1, Sele, VCAM-1, FN-1, and Lam-1. To narrow our focus to those that are involved with important cell–cell/cell–matrix interactions between ischemic myocardium and BM-MSCs, we verified the expression of corresponding receptor/ligand pairs on BM-MSCs, and we identified nine potential targets, including CXCR4, integrin α4/β1, integrin α5/β1(Figures 1A and 2A). These ligand–receptor interactions, which were shown previously to be relevant to stem cell and cardiac biology, may play an important role in cardiac repair by influencing homing, migration, and engraftment of BM-MSCs.
Integrins have been known to play a key role in cell adhesion, migration, and chemotaxis (Gao and Issekutz, 1997; Werr et al., 1998; Ridger et al., 2001a; Lindbom and Werr, 2002; Imhof and Aurrand-Lions, 2004). Localization of leukocytes to extravascular sites of inflammation is a function of repeated adhesive and de-adhesive events. After extravasation, leukocytes migrate toward a source of inflammation in response to locally elaborated chemotaxins and cytokines. Stimulated by a chemotactic gradient, leukocytes traverse the ECM by way of transient interactions between integrin receptors and components of the ECM and that serve as adhesive ligands (Lauffenburger and Horwitz, 1996; Palecek et al., 1997). Integrins have been known to contribute to the process of neutrophil locomotion include members of CD29 and CD18 (Gao and Issekutz, 1997; Werr et al., 1998; Imhof and Aurrand-Lions, 2004). CD29 also involves cell-to-cell adhesion (Behzad et al., 1996; Werr et al., 1998), which may be important for the anchorage of the engrafted cells. We hypothesized that a similar mechanism was used for the engrafted BM-MSCs homing to the infarct. In this study, we demonstrated that BM-MSCs expressed many integrins on their surface, including CD29 and CD49d, and their binding partners were up-regulated in the ischemic myocardium. In agreement with previous findings (Pittenger and Martin, 2004), our BM-MSCs expressed high level of CD29. Theoretically, CD29 has four isoforms that are formed by alternative mRNA splicing and differentially expressed in different cell types (Balzac et al., 1993). In this study, we show that BM-MSCs, as multipotent stem cells, express mRNA of all four isoforms, with CD29A the highest level which is the major isoform involved in cell adhesion and migration (Balzac et al., 1993). Correspondingly, the expression CD29 ligands tenascin-C, fibronectin, VCAM-1, and laminin (Lam) are found increased in the ischemic myocardium in this study. Tenascin-C is highly expressed during embryogenesis (Crossin et al., 1986), whereas its expression is very low after birth. In this study, we show that tenascin-C is expressed in the ischemic border zone of the infarcted myocardium 3 d post-MI. This is consistent with a previous study where tenascin-C was found to reappear in interstitial fibroblasts in the border zone within 24 h of MI in rats, decrease at day 7 (Imanaka-Yoshida et al., 2001). Tenascin-C possesses adhesive as well as “de-adhesion” activities, which depend on ECM and cell surface receptor binding. These special features facilitates cell migration during wound healing (Murphy-Ullrich, 2001; Tamaoki et al., 2005). Fibronectin has long been known to play an important role in mediating cell adhesion and migration (Larsen et al., 2006). Rapid up-regulation of fibronectin in the infarcted myocardium has been reported previously after MI (Knowlton et al., 1992; Kossmehl et al., 2005). The up-regulation of fibronectin is ahead of collagens, suggesting its involvement in the acute phase of MI (Knowlton et al., 1992). In 5 h after acute MI in pigs, increased expression of fibronectin was found in fibroblast-like cells in the infarct (Kossmehl et al., 2005). Based on these findings, we decided to study whether this particular class of integrins may be responsible for stem cell homing and engraftment. Indeed, we found that there were significantly lower numbers of BM-MSCs engrafted and migrated into ischemic myocardium if pretreated with antibody against CD29, suggesting a crucial role of CD29 in stem cell cardiac engraftment. Similarly, a previous study shows that blockade of CD29 diminished neutrophil migration to the lung inflammation (Ridger et al., 2001a).
CD49d has been known to be involved in leukocyte transendothelial migration (Ridger et al., 2001a). Of note, our results did not show a statistically significant difference after CD49d was blocked with antibodies before injection. Consistent with our finding, a recent study shows that blockade of CD49d in endothelial progenitor cells does not affect their homing and engraftment into ischemic sites in MI hearts or ischemic limbs (Qin et al., 2006).
In this study, different from that observed in CD34+ hematopoietic cells (Askari et al., 2003; Abbott et al., 2004), blockade of CXCR4 in BM-MSCs with a neutralizing antibody did not reduce their intramyocardial migration and engraftment into the ischemic myocardium. This may be due to the fact the level of CXCR4 expression in BM-MSCs is much lower compared with CD34+ hematopoietic cells (Askari et al., 2003; Abbott et al., 2004; Ceradini et al., 2004). A limitation in our study is that our cells are cultivated and their behavior may differ from endogenous BM-MSCs. Notably, a recent study shows that a small subpopulation of bone marrow-adherent cells that are small in size, seen in the colonies of the earliest passages, express high levels of CXCR4, exhibit greater engraftment after systemic infusion (Lee et al., 2006). However, these small cells depend on the larger cells for survival and diminish quickly with successive passages, and they almost disappear in passage 3 (Colter et al., 2001). Nevertheless, it is the larger and uniformly sized MSCs that are being used for transplantation for cardiac repair (Mangi et al., 2003; Amado et al., 2005). Our data, together with those in published literature (Askari et al., 2003; Abbott et al., 2004) would suggest that MSCs and HSCs may use distinctive classes of surface adhesion receptors to establish functional interactions with resident cells or the ECM in the ischemic myocardium, thereby differentially influencing intramyocardial homing and trafficking.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants HL-35610, HL-058516, HL-072010, and HL-073219 (all to V.J.D.) from the National Heart, Lung, and Blood Institute, National Institutes of Health. Part of this work was done while J.I. was a Doris Duke Charitable Foundation clinical research fellow at Harvard Medical School. Y.W. is a recipient of a Canadian Institutes of Health Research Fellowship Award.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-02-0166) on May 16, 2007.
REFERENCES
- Abbott J. D., Huang Y., Liu D., Hickey R., Krause D. S., Giordano F. J. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- Amado L. C., et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anversa P., Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- Askari A. T., et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- Assmus B., et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- Balsam L. B., Wagers A. J., Christensen J. L., Kofidis T., Weissman I. L., Robbins R. C. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Balzac F., Belkin A. M., Koteliansky V. E., Balabanov Y. V., Altruda F., Silengo L., Tarone G. Expression and functional analysis of a cytoplasmic domain variant of the beta 1 integrin subunit. J. Cell Biol. 1993;121:171–178. doi: 10.1083/jcb.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzad A. R., Chu F., Walker D. C. Fibroblasts are in a position to provide directional information to migrating neutrophils during pneumonia in rabbit lungs. Microvasc. Res. 1996;51:303–316. doi: 10.1006/mvre.1996.0029. [DOI] [PubMed] [Google Scholar]
- Bowden R. A., Ding Z. M., Donnachie E. M., Petersen T. K., Michael L. H., Ballantyne C. M., Burns A. R. Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ. Res. 2002;90:562–569. doi: 10.1161/01.res.0000013835.53611.97. [DOI] [PubMed] [Google Scholar]
- Britten M. B., et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- Ceradini D. J., Kulkarni A. R., Callaghan M. J., Tepper O. M., Bastidas N., Kleinman M. E., Capla J. M., Galiano R. D., Levine J. P., Gurtner G. C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Colter D. C., Sekiya I., Prockop D. J. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc. Natl. Acad. Sci. USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Hoffman S., Grumet M., Thiery J. P., Edelman G. M. Site-restricted expression of cytotactin during development of the chicken embryo. J. Cell Biol. 1986;102:1917–1930. doi: 10.1083/jcb.102.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald O., Ren G., Duerr G. D., Zoerlein M., Klemm C., Gersch C., Tincey S., Michael L. H., Entman M. L., Frangogiannis N. G. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am. J. Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganju R. K., Brubaker S. A., Meyer J., Dutt P., Yang Y., Qin S., Newman W., Groopman J. E. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J. Biol. Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- Gao J. X., Issekutz A. C. The beta 1 integrin, very late activation antigen-4 on human neutrophils can contribute to neutrophil migration through connective tissue fibroblast barriers. Immunology. 1997;90:448–454. doi: 10.1111/j.1365-2567.1997.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodek A. M., Honczarenko M., Le Y., Campbell J. J., Silberstein L. E. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. J. Exp. Med. 2003;197:461–473. doi: 10.1084/jem.20021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka-Yoshida K., Hiroe M., Nishikawa T., Ishiyama S., Shimojo T., Ohta Y., Sakakura T., Yoshida T. Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Lab. Invest. 2001;81:1015–1024. doi: 10.1038/labinvest.3780313. [DOI] [PubMed] [Google Scholar]
- Imhof B. A., Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- Kawada H., et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- Klar J., Sandner P., Muller M. W., Kurtz A. Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflugers Arch. 2002;444:335–344. doi: 10.1007/s00424-002-0818-9. [DOI] [PubMed] [Google Scholar]
- Knowlton A. A., Connelly C. M., Romo G. M., Mamuya W., Apstein C. S., Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin. Invest. 1992;89:1060–1068. doi: 10.1172/JCI115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher A. A., Schuster M. D., Szabolcs M. J., Takuma S., Burkhoff D., Wang J., Homma S., Edwards N. M., Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat. Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Kossmehl P., et al. Increase of fibronectin and osteopontin in porcine hearts following ischemia and reperfusion. J Mol. Med. 2005;83:626–637. doi: 10.1007/s00109-005-0642-8. [DOI] [PubMed] [Google Scholar]
- Larsen M., Wei C., Yamada K. M. Cell and fibronectin dynamics during branching morphogenesis. J. Cell Sci. 2006;119:3376–3384. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D. A., Horwitz A. F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lee R. H., Hsu S. C., Munoz J., Jung J. S., Lee N. R., Pochampally R., Prockop D. J. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107:2153–2161. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- Lindbom L., Werr J. Integrin-dependent neutrophil migration in extravascular tissue. Semin. Immunol. 2002;14:115–121. doi: 10.1006/smim.2001.0348. [DOI] [PubMed] [Google Scholar]
- Mangi A. A., Noiseux N., Kong D., He H., Rezvani M., Ingwall J. S., Dzau V. J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- Min J. Y., Yang Y., Converso K. L., Liu L., Huang Q., Morgan J. P., Xiao Y. F. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J. Appl. Physiol. 2002;92:288–296. doi: 10.1152/jappl.2002.92.1.288. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state. Clin. Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren J. M., Jovinge S., Breitbach M., Sawen P., Roll W., Hescheler J., Taneera J., Fleischmann B. K., Jacobsen S. E. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- Palecek S. P., Loftus J. C., Ginsberg M. H., Lauffenburger D. A., Horwitz A. F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Peister A., Mellad J. A., Larson B. L., Hall B. M., Gibson L. F., Prockop D. J. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Perin E. C., et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- Petit I., et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Pittenger M. F., Martin B. J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- Qin G., et al. Functional disruption of {alpha}4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J. Exp. Med. 2006;203:153–163. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridger V. C., Wagner B. E., Wallace W. A., Hellewell P. G. Differential effects of CD18, CD29, and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J. Immunol. 2001;166:3484–3490. doi: 10.4049/jimmunol.166.5.3484. [DOI] [PubMed] [Google Scholar]
- Ripa R. S., Jorgensen E., Wang Y., Thune J. J., Nilsson J. C., Sondergaard L., Johnsen H. E., Kober L., Grande P., Kastrup J. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113:1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- Stamm C., Westphal B., Kleine H. D., Petzsch M., Kittner C., Klinge H., Schumichen C., Nienaber C. A., Freund M., Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- Strauer B. E., Brehm M., Zeus T., Gattermann N., Hernandez A., Sorg R. V., Kogler G., Wernet P. Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction. Dtsch. Med. Wochenschr. 2001;126:932–938. doi: 10.1055/s-2001-16579-2. [DOI] [PubMed] [Google Scholar]
- Sutton M. G., Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- Tamaoki M., Imanaka-Yoshida K., Yokoyama K., Nishioka T., Inada H., Hiroe M., Sakakura T., Yoshida T. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am. J. Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse H. F., Kwong Y. L., Chan J. K., Lo G., Ho C. L., Lau C. P. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361:47–49. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- Vandervelde S., van Amerongen M. J., Tio R. A., Petersen A. H., van Luyn M.J.A., Harmsen M. C. Increased inflammatory response and neovascularization in reperfused vs. nonreperfused murine myocardial infarction. Cardiovasc. Pathol. 2006;15:83–90. doi: 10.1016/j.carpath.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Wang L. J., Chen Y. M., George D., Smets F., Sokal E. M., Bremer E. G., Soriano H. E. Engraftment assessment in human and mouse liver tissue after sex-mismatched liver cell transplantation by real-time quantitative PCR for Y chromosome sequences. Liver Transpl. 2002;8:822–828. doi: 10.1053/jlts.2002.34891. [DOI] [PubMed] [Google Scholar]
- Werr J., Xie X., Hedqvist P., Ruoslahti E., Lindbom L. β1 Integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J. Exp. Med. 1998;187:2091–2096. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. E., Bowman E. P., Wagers A. J., Butcher E. C., Weissman I. L. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J. Exp. Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.