In 2006, the first vaccine against human papillomavirus (HPV) infection, Gardasil (Merck Frosst), was approved for use in Canada. A second vaccine, Cervarix (GlaxoSmith Kline), is currently undergoing regulatory review by Health Canada. Both vaccines are designed with the goal of preventing cervical cancer. This article provides an overview of HPV infection and the HPV vaccines, Gardasil and Cervarix.
HPV epidemiology and disease outcomes
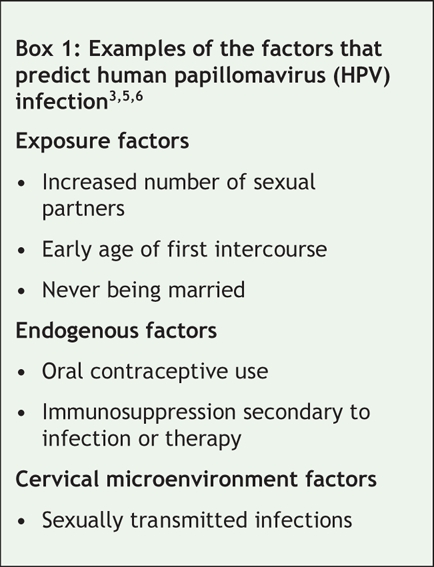
HPV is one of the most common sexually transmitted infections.1 The global age-standardized prevalence of HPV among women varies by location, ranging from 1.4% in Spain to 25.6% in Nigeria.2 In Canada, although the prevalence varies by location, age, ethnic background and risk,3 the most comprehensive population-based data indicate that the age-adjusted prevalence of HPV (all types) is 16.8% and that the prevalence of HPV types 16 and 18 is 10.6% and 3.5% respectively.4 The highest prevalence rates are among women under 20 years of age (all HPV types 26.9%, all high-risk types 20.6%, high-risk HPV types 16 and/or 18 16.7%).4 Factors that are predictors of HPV infection are presented in Box 1.
Box 1.
The HPV family consists of over 100 DNA viruses. There are about 40 types of HPV that can infect the genital tract.7 These viruses are divided into 2 groups (high or low risk) based on their oncogenic potential. The epidemiologic evidence that links HPV infection with cervical cancer includes data from case series, case–control and cohort studies.8 In a large case series of 1000 cervical cancer cases in 22 countries, HPV DNA was detected in 99.7% of specimens.8 Case–control studies in 22 countries (sponsored by the International Agency for Research on Cancer) demonstrated consistently high odds ratios (ORs) for the risk of cervical cancer associated with persistent HPV infection (squamous cell carcinoma OR 90.0, adenocarcinoma OR 81.3).8 In a 10-year cohort study of 20 810 women, the cumulative incidence of cervical intraepithelial neoplasia (grade 3) among women positive for HPV type 16 was 17.2% (95% confidence interval [CI] 11.5%–22.9%), and the incidence among women positive for HPV type 18 was 13.6% (95% CI 3.6%–23.7%). However, the risk among women who tested positive for high-risk HPV types other than types 16 and 18 was 3.0% (95% CI 1.9%–4.2%). The 10-year incidence of cervical intraepithelial neoplasia (grade 3) among women negative for oncogenic high-risk HPV infections was 0.8% (95% CI, 0.6%–1.1%).9 This study provides a conservative estimate of risk, given the aggressive management of grade 1 and 2 cervical intraepithelial neoplasia lesions among the trial cohort. In addition to the epidemiologic evidence, the biologic mechanism of HPV carcinogenesis has been clearly delineated.8
Globally, HPV types 16 and 18 account for 65%–77% of cervical cancers. HPV types 16 and 18 account for 41%–57% of high-grade cervical squamous intraepithelial lesions, 15%–32% of low-grade squamous intraepithelial lesions and 8%–19% of atypical squamous cells of undetermined significance.10 Six HPV genotypes (types 31, 33, 35, 45, 52 and 58) account for an additional 20% of cervical cancers worldwide.10
HPV infections are also associated with other genitourinary cancers (e.g., anal, penile, vaginal, vulvar), head and neck cancers (e.g., conjunctivae, mouth, oropharynx, larynx) and nonmalignant diseases (e.g., genital warts, recurrent respiratory papillomatosis).8,10–13 Of genital warts, 90%–100% of cases are caused by HPV types 6 and 11, although 20%–50% of lesions may be coinfected with high-risk types of HPV.12 The International Agency for Research on Cancer has declared certain high-risk HPV genotypes, including HPV types 16 and 18, as group 1 carcinogens and has declared low-risk HPV types 6 and 11 as possible carcinogens (group 2b).11
Infection with a high-risk type of HPV is a necessary, but not sufficient, cause of cervical cancer. The development of a precancerous lesion (squamous intraepithelial lesion) from a persistent HPV infection can take from 1 to 10 years, and the development of invasive cervical cancer can take an additional 10 years.14 Thus, there is a long lag period from infection to invasive disease. Cofactors that contribute to disease progression include a history of smoking, long-term use of oral contraceptives, high parity and sexually transmitted infections.8
The global annual incidence of cervical cancer ranges from 56.9/100 000 in Zimbabwe to less than 10/100 000 in developed countries including Canada, Switzerland and the Netherlands (Figure 1).13 Each year in Canada there are about 1300 cases of cervical cancer and about 400 deaths attributed to this disease.15 The lifetime risk of cervical cancer among Canadian women is 0.7%, or 1 in 138 women.15 Although the age-standardized incidence rates of cervical cancer in Canada have been reduced by half since the 1970s (primarily due to cervical cytology [i.e., Papanicolaou smear] screening programs), the decline in cervical cancer incidence rates has plateaued in the last decade.15 Sensitivity of Pap smear screening programs for the detection of precancerous lesions varies widely;16 thus, about 40% of cervical cancers in British Columbia (personal communication, Dr. Andy Coldman, BC Cancer Agency, 2007) and 50% of cervical cancers in the United States are detected in women who are routinely screened.17
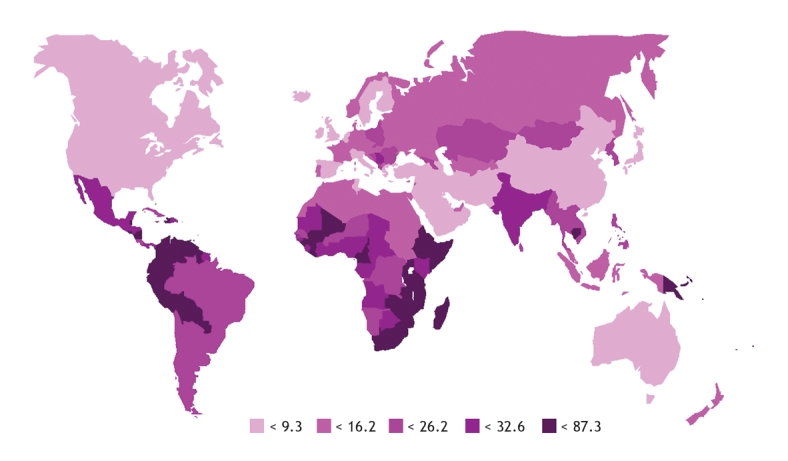
Figure 1: Worldwide age-standardized annual incidence (per 100 000) of cervical cancer (all ages). Reproduced with permission from Elsevier (Vaccine 2006;24[Suppl 3]:11–25).13
HPV vaccine development and clinical trial issues
Identification of HPV as an etiologic link to cervical cancer in the early 1990s stimulated the race for creation of an HPV vaccine. Vaccine development has involved attention to the design of vaccine components (e.g., antigen expression, choice of adjuvants, formulation and patent rights) and the development of serologic tests to measure immunity and tests to detect the specific type of HPV infection.18
Interpretation of the immune response induced by the HPV vaccines is complicated by 2 factors. First, serologic correlates of immunity to HPV infection are unknown. For example, the minimum antibody threshold at which an individual is protected from natural HPV infections is unknown, as is how this level compares with vaccine-induced antibody titres. In addition, antibody titres from GlaxoSmithKline and Merck cannot be directly compared because the companies use different serologic assays. These assays have not been validated against each other or against an international standard, as the latter has not been developed.19
Vaccine trials have examined a number of end points, including transient and persistent (lasting 4 months or longer) HPV infection, abnormal Pap smear results, histology (e.g., grade 1–3 cervical intraepithelial neoplasia lesions, adenocarcinoma in situ, cancer of the cervix and other genital sites) and other end points related to HPV types covered and not covered by the vaccine. Trials could not assess cervical cancer alone as an outcome, given the lengthy delay between HPV infection and onset of cervical cancer and the fact that the standard of care for precancerous cervical lesions (cervical intraepithelial neoplasia, grade 2–3) precludes watchful waiting. Thus, the World Health Organization advocates assessment of vaccine efficacy against the following disease outcomes: persistent infection (6 months or longer) and cervical intraepithelial neoplasia (grade 2 or higher).20
HPV vaccines
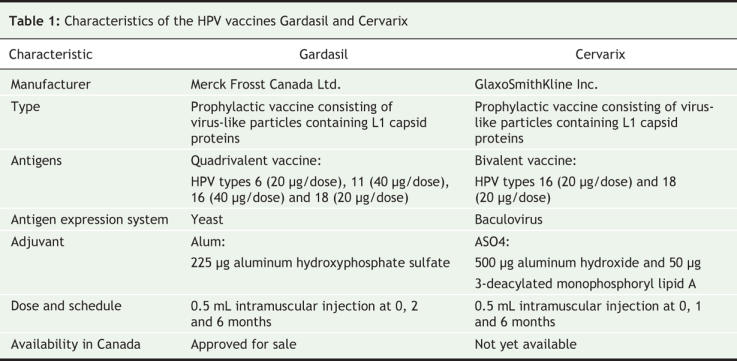
Gardasil is a quadrivalent vaccine that offers protection against HPV types 6 and 11, which are responsible for 90% of genital warts, and HPV types 16 and 18, which are associated with 70% of cervical cancers. This vaccine is formulated with a classic alum adjuvant. Data on this vaccine's safety, immunogenicity, efficacy and effectiveness are now available from 5-year phase 221–23 and 3-year phase 324–27 trials that included 20 000 participants.
Cervarix is a bivalent vaccine that protects against HPV types 16 and 18. The vaccine is formulated with a new ASO4 adjuvant that contains monophosphoryl lipid A, a derivative of bacterial cell walls. ASO4 is also incorporated in Fendrix (hepatitis B vaccine) and a candidate vaccine against herpes simplex virus, neither of which are approved for use in Canada. Data are available from 5-year phase 2 and 15-month phase 3 trials that included 18 000 participants.28–31 A comparison of the characteristics of Gardasil and Cervarix is provided in Table 1.
Table 1
Clinical trial outcomes
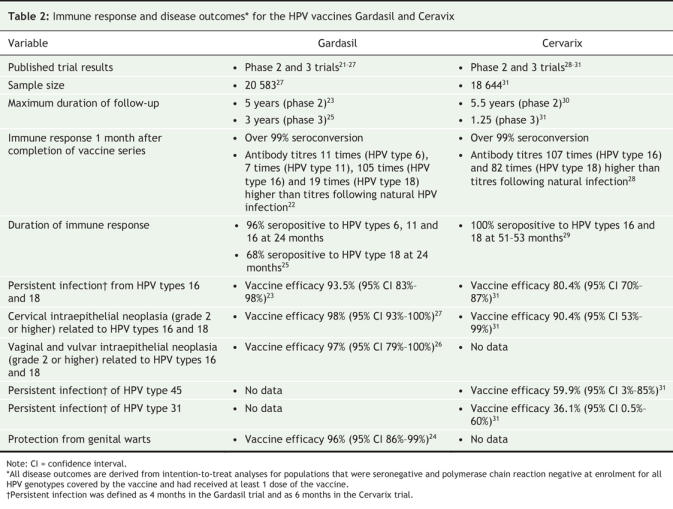
Table 2 provides data on the immune response to and efficacy of Gardasil and Cervarix from published phase 2 and 3 trials. Immune response data are derived from trial participants who had not been exposed to the HPV genotypes covered by the vaccines at trial enrolment through to completion of the 3-dose immunization series (per-protocol efficacy population). Efficacy data are derived from trial participants who are naive to the HPV genotypes covered by the vaccine at enrolment and who received 1 dose or more of the vaccine (intention-to-treat population). Per-protocol efficacy analysis represents “a perfect world,” where all vaccine doses are given on time and before the individual has been exposed to HPV. In contrast, intention-to-treat analysis represents a “real world” scenario, where the vaccine schedule has not necessarily been adhered to and the individual may be exposed to HPV before being fully vaccinated.
Table 2
Immune response among young women (16–26 years)
Both Gardasil and Cervarix are highly immunogenic, with vaccine-induced antibody titres that are many times higher than those induced by natural HPV infections. Gardasil-induced antibody titres peak 7 months following initiation of the vaccine series. The titres then decline, reaching a plateau 18–24 months later. This plateau is maintained for at least 5 years, with 5-year levels that are similar to the titres naturally induced by HPV types 6 and 18 and that are higher than the titres naturally induced by HPV types 11 and 16.23 At 24-months follow-up, over 96% of participants in the Gardasil trial were seropositive for HPV types 6, 11 and 16; however, only 68% of participants were seropositive for HPV type 18.25 The significance of this reduction remains unclear given that immune memory is induced by the vaccine.32
Cervarix-induced antibody titres follow the same profile as Gardasil, with 2 differences. The 18-month plateau level is many-fold higher than the levels induced by natural infection and, after 51–53 months, 100% of women were seropositive for both HPV types 16 and 18.29
Immune response among young adolescent females (9–15 years)
Both vaccines are highly immunogenic in young adolescents, with titres that are 1.7 to 2.4 times higher than those among women aged 16–26 years.33–35 This response is more pronounced for younger adolescents (aged 9–13 years).34 Two doses of Gardasil (0 and 2 months) in adolescents aged 10–15 years produced antibody titres that were equivalent to those produced by 3 doses (0, 2 and 6 months) in women aged 16–26 years for 3 of the 4 vaccine genotypes;33 however, the sustainability of this response over time has not been evaluated.
Vaccine efficacy
Among the per-protocol efficacy population, a full series of both vaccines is highly efficacious (96%) in preventing persistent infection with the HPV genotypes23,29 covered by the vaccine. Efficacy against persistent infection in an intention-to-treat population was 94% and 80% for Gardasil and Cervarix respectively.25,31 Vaccine efficacy for precancerous lesions (cervical intraepithelial neoplasia, grade 2 or higher) caused by HPV types 16 and 18 is 98% for Gardasil and 90% for Cervarix.27,31 In addition, Gardasil offers 100% protection against vulvar intraepithelial neoplasia (grade 2–3) and vaginal intraepithelial neoplasia (grade 2–3) caused by HPV types 16 and 18.26 Gardasil is also 96% efficacious in preventing genital warts.36
In the Cervarix trials, modest cross protection was documented against infection of HPV type 45 (vaccine efficacy 60%) and, to a lesser extent, against HPV types 31 and 52 (vaccine efficacy 36% and 32% respectively).31 The cross-protective effect of Gardasil has not yet been reported. HPV types 45, 31 and 52 are estimated to cause 12% of cervical cancers.10
Phase 3 data on the effectiveness of Gardasil among women aged 15–26 years are available.24,25,27 The general trial population included all women who met the inclusion criteria (less than 5 life-time sexual partners) who potentially had been infected with HPV types covered by the vaccine at or before trial enrolment and who may have received fewer than 3 vaccine doses. In this population, vaccine efficacy against cervical disease was very low (vaccine-specific types 44%–55%, all types 17%–20%), thus demonstrating that the vaccine is not effective if adminstered to women who are already infected with vaccine-specific HPV types. These results highlight 2 issues. First, Gardasil should be used as a prophylactic vaccine and therefore should be offered to females before they are at risk of HPV infection. Second, all vaccinated females should continue to participate in Pap smear screening programs because they remain at risk of adverse gynecological outcomes from other high-risk HPV genotypes.
Vaccine safety
Both vaccines have a good safety profile. Cervarix and Gardasil both produced local reactions that were 6%–8% more frequent than reactions produced by an alum placebo.28,36 Cervarix-induced local reactions were 12%–22% more frequent than reactions produced by an investigational hepatitis A vaccine.31 There was no difference noted in the frequency of systemic adverse events among those who received the vaccine or the placebo.28,31,36
Gardasil is not approved for pregnant women, although data on 1900 women who became pregnant during the vaccine trials indicate that adverse events (including congenital anomalies) were similar among recipients of the vaccine and the placebo.36,37
Data are available on 1350 pregnancies that occurred during the Cervarix phase 3 trial.31 No differences in pregnancy outcomes were reported among those who received the vaccine or the placebo.
Recommendations
The National Advisory Committee on Immunization recently released its recommendations for the use of Gardasil in females aged 9–26 years.3 Because of limited data, Gardasil is not recommended for females less than 9 years of age, males (all ages) or pregnant women. Because of a lack of efficacy data, Gardasil is not recommended for women over 26 years of age; however, women in this group may receive the vaccine after an individual consultation. Full recommendations can be viewed at www.naci.gc.ca.
Recommendations for Cervarix use will be made once this vaccine has been reviewed by the regulators and is approved for use in Canada.
Discussion
Cervical cancer ranks as the 12th most common type of incidental cancer among Canadian women and ranks second globally.13 In 2002, worldwide there were an estimated 493 000 cases and 274 000 deaths due to cervical cancer.13 Although substantial reductions in cervical cancer incidence and mortality have been made in developed countries (primarily because of the implementation of cervical cytology screening programs), the absence of such programs in the developing world, barriers to access and acceptability of cytology screening in population subgroups in the developed world and poor test performance (high false-negative rate) contribute to this high mortality rate.
Gardasil and Cervarix are both empty virus-like particle vaccines that were created using recombinant technology. Both vaccines are safe. Both vaccines provide protection against infection by HPV types 16 and 18, 2 oncogenic HPV genotypes that cause about 70% of cervical cancers. Both vaccines protect against high-grade cervical disease (cervical intraepithelial neoplasia, grade 2 and higher). In addition, Gardasil trials have demonstrated that this vaccine is efficacious against high-grade vaginal and vulvar lesions. Gardasil also offers protection against the 2 HPV genotypes that are responsible for about 90% of genital warts. The preliminary data from the phase 3 trials of Cervarix have demonstrated 2 differences from Gardasil: a slightly lower efficacy against cervical disease and modest cross protection against 3 additional oncogenic genotypes that are responsible for 12% of cervical cancers. Given that these 2 vaccines contain identical virus-like particle antigens against oncogenic types of HPV, long-term follow-up studies will help to discern the significance of these differences.
Information available on HPV vaccines is evolving rapidly, with new peer-reviewed publications becoming available each month. In addition, there are a number of trials planned or underway that will contribute to our understanding of these vaccines. Trials of Gardasil among men are under way, and there are plans to test a 3-dose regimen of Gardasil among people who are immunocompromised and to test a 2-dose regimen among adolescents. GlaxoSmithKline has announced plans to conduct a head-to-head comparison of Cervarix and Gardasil, and both GlaxoSmithKline and Merck are conducting trials involving older women and are planning trials of second-generation vaccines that will offer protection against additional high-risk HPV genotypes.
Although Gardasil and Cervarix have demonstrated favourable beginnings, there are still a number of knowledge gaps (Box 2).38 Planned long-term follow-up of phase 3 trials and population-based studies39,40 are required to fill in these knowledge gaps.
Box 2.

Conclusion
Both Cervarix and Gardasil represent superb technological achievements. We strongly recommend a universal publicly funded vaccination program aimed at immunizing adolescent females before they are at risk of HPV infection. Although there are knowledge gaps, especially about long-term efficacy, this is not unusual at the outset of any new vaccine program. Careful ongoing evaluation of the performance of the HPV-vaccine program and of HPV-infection epidemiology and surveillance of HPV-induced cancers will be essential. We look forward to trial data supporting efficacy in males so that young males can also benefit from the potential protection afforded by this vaccine.
Meenakshi Dawar MD MHSc Canadian Field Epidemiology Program Public Health Agency of Canada Vancouver, BC Shelley Deeks MD MHSc Immunization and Respiratory Infections Division Centre for Infectious Disease Prevention and Control Public Health Agency of Canada Ottawa, Ont. Simon Dobson MD Vaccine Evaluation Centre University of British Columbia Vancouver, BC
@ See related articles pages 462, 464, 469, 480, 484
Key points
• Each year in Canada there are about 1300 new cases and about 400 deaths caused by cervical cancer
• About 40%–50% of cervical cancers occur in women who undergo routine cervical cytology screening
• HPV is the most common sexually transmitted infection
• HPV infection can cause cervical cancer and contributes to other cancers of the genitourinary tract, head and neck
• Two prophylactic vaccines, Gardasil and Cervarix, offer protection against HPV types 16 and 18. These 2 genotypes are responsible for about 70% of cervical cancers
• These vaccines protect against the precursors of cervical cancer (cervical intraepithelial neoplasia, grade 2 and higher). Proof of whether they protect against cervical cancer will take many years of follow-up
• Gardasil protects against genital warts caused by HPV types 6 and 11
• Vaccine-induced antibody levels are much higher than those produced by natural infections. Immunity lasts for at least 5.5 years
• Routine cytology screening is still required, because the vaccines do not protect against all oncogenic types of HPV
• Gardasil is recommended for females aged 9–26 years. Cervarix is not yet available in Canada
Footnotes
This article has been peer reviewed.
Competing interests: None declared for Meenakshi Dawar or Shelley Deeks. Simon Dobson has given several HPV-related talks and has co-chaired a series of classes and research-planning workshops on HPV that were sponsored through educational grants from HPV vaccine manufacturers (Merck, GlaxoSmithKline).
REFERENCES
- 1.Burchell AN, Winer RL, de Sanjosé S, et al. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine 2006;24(Suppl 3):52-61. [DOI] [PubMed]
- 2.Clifford GM, Herrero R, Munoz N, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005;366:991-8. [DOI] [PubMed]
- 3.Dobson S, Deeks S, Money D, and the NACI working group. Statement on human papillomavirus vaccine. Can Commun Dis Rep 2007;33(ACS-2):1-31. [PubMed]
- 4.Moore RA, Fornika DJ, Moravan V, et al. HPV type distribution in North America: a population based study of 5000 British Columbia women [poster]. Twenty-third International Papillomavirus Conference and Clinical Workshop: 2006 Sept. 1–7; Prague.
- 5.Burk RD, Ho GYF, Beardsley L, et al. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J Infect Dis 1996;174:679-89. [DOI] [PubMed]
- 6.Kahn JA, Lan D, Kahn RS. Sociodemographic factors associated with high-risk human papillomavirus infection. Obstet Gynecol 2007;110:87-95. [DOI] [PubMed]
- 7.Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: Underlying mechanisms. Vaccine. 2006; 24(Suppl 3): 106-113. [DOI] [PubMed]
- 8.Muñoz N, Castellsagué X, Berrington de González A, et al. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006;24(Suppl 3):1-10. [DOI] [PubMed]
- 9.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072-9. [DOI] [PubMed]
- 10.Clifford G, Franceschi S, Diaz M, et al. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006;24 (Suppl 3): 26-34. [DOI] [PubMed]
- 11.International Agency for Research on Cancer. Vol 90: Human papillomaviruses. Lyon, France: The Agency; 2005. Available: http://monographs.iarc.fr/ENG/Meetings/vol90.php (accessed 2007 July 20).
- 12.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24 (Suppl 3):35-41. [DOI] [PubMed]
- 13.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006;24(Suppl 3):11-25. [DOI] [PubMed]
- 14.Moscicki AB, Schiffman M, Kjaer S, et al. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine 2006;24(Suppl 3):42-51. [DOI] [PubMed]
- 15.Canadian cancer statistics. Toronto: Canadian Cancer Society/National Cancer Institute of Canada; 2005. p. 57. Available: www.cancer.ca/vgn/images/portal/cit_86751114/48/28/401594768cw_2005stats_en.pdf (accessed 2007 July 20).
- 16.Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol 1995;141:680-9. [DOI] [PubMed]
- 17.Crum CP. The beginning of the end for cervical cancer? N Engl J Med 2002;347:1703-5. [DOI] [PubMed]
- 18.Inglis S, Shaw A, Koenig S. Chapter 11: HPV vaccines: Commercial research & development. Vaccine. 2006; 24(Suppl 3): 99-105. [DOI] [PubMed]
- 19.Pagliusi SR, Dillner J, Pawlita M, et al. Chapter 23: International standard reagents for harmonization of HPV serology and DNA assays—an update. Vaccine 2006;24(Suppl 3):193-200. [DOI] [PubMed]
- 20.Pagliusi SR, Aguado MT. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine 2004;23:569-78. [DOI] [PubMed]
- 21.Villa LL, Costa RLR, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomized double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6: 271-8. [DOI] [PubMed]
- 22.Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 2006;24:5571-83. [DOI] [PubMed]
- 23.Villa LL, Costa RLR, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459-66. [DOI] [PMC free article] [PubMed]
- 24.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007;356:1928-43. [DOI] [PubMed]
- 25.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356: 1915-27. [DOI] [PubMed]
- 26.Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomized clinical trials. Lancet 2007;369:1693-702. [DOI] [PubMed]
- 27.Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomized clinical trials. Lancet 2007;369:1861-8. [DOI] [PubMed]
- 28.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet 2004;364:1757-65. [DOI] [PubMed]
- 29.Harper DM, Franco EL, Wheeler C, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006;367:1247-55. [DOI] [PubMed]
- 30.Gall SA, Teixeira J, Wheeler CM, et al. Substantial impact on precancerous lesions and HPV infections through 5.5 years in women vaccinated with the HPV-16/18 L1 VLP ASO4 candidate vaccine. American Assocation for Cancer Research Annual Meeting; 2007 Apr 17; Los Angeles.
- 31.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007;369:2161-70. [DOI] [PubMed]
- 32.Olsson S-E, Villa LL, Costa RLR, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007;26:4931-39. [DOI] [PubMed]
- 33.Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (Types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics 2006;118:2135-45. [DOI] [PubMed]
- 34.Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents. A randomized controlled trial. Pediatr Infect Dis J 2007;26:201-9. [DOI] [PubMed]
- 35.Dubin G for the HPV Vaccine Adolescent Study Investigators. Enhanced immunogenicity of a candidate human papillomavirus (HPV) 16/18 L1 virus-like particle vaccine (VLP) with novel ASO4 adjuvant in pre-teens/adolescents [poster]. Interscience Conference on Antimicrobial Agents and Chemotherapy; 2005 Dec. 16–19; Washington.
- 36.Gardasil (quadrivalent human papillomavirus [types 6, 11, 16, 18] recombinant vaccine) [product monograph]. Kirkland (QC):Merck Frosst Canada Ltd.; 2006.
- 37.Advisory Committee on Immunization Practices. June 29–30, 2006. Record of the Proceedings. Human Papillomavirus Vaccine. p5-6. http://0-www.cdc.gov.mill1.sjlibrary.org/vaccines/recs/acip/downloads/min-jun06.pdf (accessed 2007 July 31).
- 38.Public Health Agency of Canada. Canadian human papillomavirus vaccine research priorities workshop — final report. Can Commun Dis Rep 2006; 32(Suppl 1):1-66.
- 39.Lehtinen M, Idanpaan-Heikkila I, Lunnas T, et al. Population-based enrolment of adolescents in a long-term follow-up trial of human papillomavirus vaccine efficacy. Int J STD AIDS 2006;17:237-46. [DOI] [PubMed]
- 40.Lehtinen M, Apter D, Dubin G, et al. Enrolment of 22 000 adolescent women to cancer registry follow-up for long-term human papillomavirus vaccine efficacy: marching towards outcomes and guarding against guessing. Int J STD AIDS 2006;17:517-21. [DOI] [PubMed]


