Abstract
Background
In order to estimate whether multi-spacer typing (MST), based on the sequencing of variable intergenic spacers, could serve for the identification of Rickettsia at the species level, we applied it to 108 rickettsial isolates or arthropod amplicons that include representatives of 23 valid Rickettsia species.
Results
MST combining the dksA-xerC, mppA-purC, and rpmE-tRNAfMet spacer sequences identified 61 genotypes, allowing the differentiation of each species by at least one distinct genotype. In addition, MST was discriminatory at the strain level in six species for which several isolates or arthropod amplicons were available.
Conclusion
MST proved to be a reproducible and high-resolution genotyping method allowing clear identification of rickettsial isolates at the species level and further additional differentiation of strains within some species.
Background
Rickettsiae are obligate intracellular, Gram-negative bacteria, the vectors and reservoirs of which are mainly arthropods, although they may also infect vertebrate hosts [1]. Currently, the genus Rickettsia contains 24 validated species, 22 of which are classified into 2 groups: i) the typhus group including R. prowazekii [2] and R. typhi [3]; and ii)the spotted fever group made of R. rickettsii [4], R. conorii [5], R. africae [6], R. sibirica [7], R. slovaca [8], R. honei [9], R. japonica [10], R. australis [11], R. akari [12], R. felis [13], R. aeschlimannii [14], R. helvetica [15], R. massiliae [16], R. rhipicephali [17], R. montanensis [18], R. parkeri [19], R. heilongjiangensis [20], R. tamurae [21], R. asiatica [22], and R. peacockii [23]. Among spotted fever rickettsiae, R. peacockii is the only validated species for which no established strain is available. Two additional species, i.e., R. bellii [24] and R. canadensis [25], initially classified within a third group named "ancestral group" [26], may not belong to a single group [20] but lie outside from the former two groups. In addition to the 24 recognized species, more than 20 rickettsial isolates which have not been fully characterized or have not received a species designation, have been described and their current classification is uncertain [27].
Various DNA-based techniques have been developed for the species identification of rickettsiae. Phylogenetic analysis based on the 16S rRNA gene sequences have frequently been used, but since the sequences are highly conserved, significant inferences about intragenus phylogeny are not possible [1,28]. In recent years, several genes have been sequenced for most of the known rickettsial isolates, including the citrate synthase – encoding gene (gltA) [29], and the Rickettsia-specific ompA [30], ompB [31], sca1 [32], sca2 [33], and sca4 [34] genes, encoding autotransporter proteins. The usefulness of DNA taxonomy has been recognized for living organisms [35] and Maiden et al. [36] have demonstrated the usefulness of multiple gene sequencing for prokaryotic taxonomy. Using multi-gene sequencing, we proposed gene sequence-based criteria for the identification of rickettsial isolates at the genus, group and species levels [20]. However, we observed that such a method lacks intra species discriminatory power [37]. As a consequence, there is no current method that both identifies rickettsiae at the species and strain levels, and produces results that are reproducible and comparable among laboratories.
Recently, we developed a new genotyping tool named Multi-Spacer Typing (MST), based on the assumption that intergenic spacers, which are less subject to evolutionary pressure than coding sequences, would be more appropriate for typing bacteria at the strain level than genes [37]. We demonstrated that MST using three intergenic spacers was suitable for typing R. conorii (31 genotypes among 42 tested strains), R. sibirica (3 genotypes among 14 tested strains), and R. prowazekii (4 genotypes among 15 studied strains) at the strain level, and produced species-specific signatures [37-39]. Herein, we hypothesized that the three intergenic spacers successfully used for R. conorii, being also present in R. sibirica and R. prowazekii, could also exhibit species-specific signatures in other Rickettsia species. Thus, in order to estimate the suitability of MST for identifying rickettsiae at the species level, we applied it to 20 additional species for which isolates are available [see Additional file 1].
Results
PCR amplification and sequencing
The dksA-xerC, mppA-purC, and rpmE-tRNAfMet intergenic spacers were amplified from all the strains of Rickettsia studied except R. canadensis and R. bellii, for which the mppA-purC spacer PCR could not amplify any fragment.
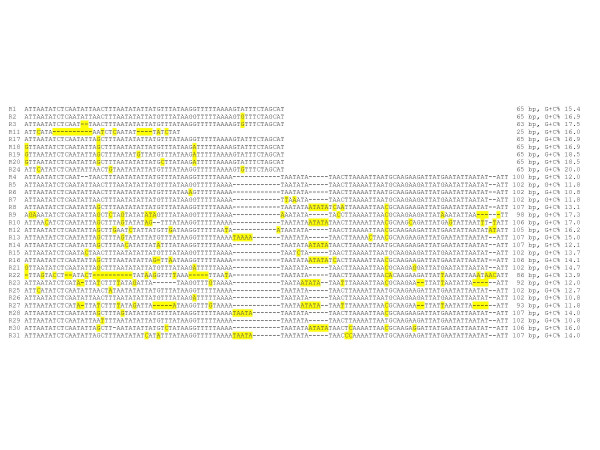
The size of the dksA-xerC spacer ranged from 92 for R. prowazekii to 687-bp for R. montanensis. Depending on species and strains, the dksA-xerC spacer was made of 31 different repeats named R1 to R31 that varied in size, from 25- to 107-bp, in number and in arrangement. The repeats were classified into two groups depending on their sizes, from 25- to 65-bp for the first group which includes 9 different repeats, and 86- to 107-bp for the second group. Sequences of both groups share a highly conserved 5'-part, and differ in their 3'-end. The sequences of these repeats are shown in Figure 1; and their repartition within the different Rickettsia strains reported in Additional file 2. Overall, the dksA-xerC spacer allowed the identification of 42 different genotypes. These included 19 genotypes within the R. conorii species, two within the R. felis species, three within the R. sibirica species, and two within the R. slovaca species. Other species each exhibited a unique dksA-xerC spacer sequence, except R. africae which had the same sequence as R. conorii subsp. Caspia isolate Chad, R. parkeri which had the same sequence as R. conorii subsp. Caspia isolate A-167, and R. massiliae which had the same sequence as R. rhipicephali.
Figure 1.
Repeated sequences making up the dksA-xerC intergenic spacers of Rickettsia species.
MppA-purC amplicons ranged from 80-bp for R. akari to 180-bp for R. felis. The degree of pairwise nucleotide sequence similarity ranged from 50.5% between R. conorii and R. felis to 100% between R. honei and R. africae or R. helvetica and R. tamurae. Using the BLAST software, we identified, within mppA-purC spacer sequences, the presence of a 25- to 102-bp Rickettsia palindromic element, depending on species, that has a high degree of similarity with the Rickettsia palindromic element 1 (RPE-1) family [40,41]. Thirty-one distinct genotypes were identified among mppA-purC spacer sequences [see Additional file 2]. R. conorii strains were classified into eight genotypes, R. felis strains and amplicons into three genotypes, R. massiliae strains and amplicons into two genotypes, R. sibrica strains into three genotypes, and R. slovaca strain and amplicons into two genotypes. Each of the other species had a unique mppA-purC genotype, with the exception of R. africae, which exhibited the same genotype as R. honei, and R. asiatica and R. tamurae which exhibited the same genotype as R. helvetica.
The size of the rpmE-tRNAfMet spacer sequence ranged from 175 for R. typhi to 402-bp for R. asiatica. The degree of pairwise nucleotide sequence similarity ranged from 69.6% between R. typhi and R. aeschlimannii to 100% between R. honei and R. africae. Using the BLAST software, we identified, within rpmE-tRNAfMet spacer sequences, the presence of a Rickettsia palindromic element that has a high degree of similarity with the Rickettsia palindromic element 2 (RPE-2) family [40,41]. Twenty-nine distinct genotypes were identified among rpmE-tRNAfMet spacer sequences [see Additional file 2]. R. conorii strains were classified into four genotypes, R. felis strain and amplicons into two genotypes, R. prowazekii strains and amplicons into three genotypes, and R. sibirica strains into two genotypes. Each of the other species exhibited a unique rpmE-tRNAfMet spacer sequence, except R. africae and R. honei, which had the same sequence.
MST genotyping
By combining the genotypes obtained from the three intergenic spacers we studied, we identified 61 MST genotypes among the 108 strains and arthropod amplicons. Thirty-one MST genotypes were identified within the R. conorii species, three within R. felis, two within R. massiliae, three within R. prowazekii, three within R. sibirica, and three within R. slovaca. Each other studied species had a unique MST genotype. Each Rickettsia species was identified on the basis of a specific dksA-xerC sequence except for R. africae, R. conorii subsp. Caspia, R. massiliae, R. parkeri and R. rhipicephali for which the three spacer sequences were necessary.
Discussion
The ability to differentiate bacteria beyond the species level is essential for identifying and tracking infectious disease outbreaks and to improve our knowledge of the population genetics, epidemiology, and ecology of bacterial pathogens. In the past decades, a variety of phenotypic and genotypic methods have been used for microbial typing, identification, and classification. Commonly used subtyping methods, such as serotyping, phage typing, ribotyping, and pulsed-field gel electrophoresis, are time-consuming. PCR and DNA sequence-based typing methods have emerged as the most rapid, reliable, and simple ways to characterize and type microorganisms and can reduce interpretation ambiguity. To be reliable, a typing method should not be influenced by sequence variations that would occur during culture passage.
Rickettsia species, as intracellular bacteria, undergo lower rates of genomic changes than extracellular bacteria, and their genomes, with the exception of R. bellii [42], are highly collinear [42-46]. As a consequence, intergenic spacers, previously demonstrated to be more variable than coding sequences [37], are a valuable target for genotyping these bacteria. The most widely used intergenic spacer in bacteria, i. e., 16S–23S rRNA, is not useable in Rickettsia species as both genes are not contiguous [44]. We have previously demonstrated the versatility of MST by applying it to several bacterial species in addition to rickettsiae, including Yersinia pestis [47], Coxiella burnetii [48], Bartonella quintana [49] and Bartonella henselae [50]. For rickettsiae, we have demonstrated that it is insensitive to culture passage in both R. conorii and R. prowazekii [37,38]. This makes MST a reliable typing method for strain tracing. The three intergenic spacers used in our study were previously selected among the most variable intergenic spacers conserved in both the R. conorii and R. prowazekii genomes [37]. We could detect the three spacers in all studied rickettsial strains and arthropod amplicons except for the mppA-purC spacer that could not be found in R. canadensis and R. bellii. This was explained in the latter species by the fact that the mppA and purC genes are not contiguous in the genome [42]. To date, only one gene, i. e., sca1, a member of the sca-family of auto-transporter genes, has been found in all Rickettsia species [32]. However, this gene was shown to be discriminatory at the species level but not at the intra-species level. In contrast, MST identified 61 distinct genotypes that include genotypes specific of each of the 23 studied species. To the best of our knowledge, this is the first reproducible method that allows identification of rickettsiae at the species and strain levels and can be applied to both isolates and clinical or arthropod specimens. In addition to the previously studied R. conorii, R. prowazekii and R. sibirica species for which MST has demonstrated intra-species variability, we showed that this method was also discriminatory for R. felis, R. massiliae, and R. slovaca strains and amplicons. Therefore, the discriminatory power of MST was highly correlated to the analyzed species. For R. africae, for which a large number of strains and tick amplicons from various geographic origins were tested, this lower genetic diversity may reflect a more recent emergence of this species than R. conorii. However, we cannot exclude that the observed differences could be due to a bias in specimen selection.
We identified repeated elements within the three studied spacers. The high density of repeated sequences appears to be a characteristic of Rickettsia genomes [40,41,45]. These repeated sequences are classified into several categories. The most remarkable of these elements are the RPEs, which represent 3.2% of the R. conorii genome [41]. The RPEs identified within the mppA-purC and rpmE-tRNAfMet spacers belong to the RPE-1 and RPE-2 families, respectively. Although usually confined to intergenic regions in prokaryotes [51], some RPE families, including the RPE-1 and RPE-2 families, are also found inserted in-frame within genes in Rickettsia genomes [41,45]. Their exact function at that location is unknown but it has been suggested that they may play a role in the creation and the modification of proteins [41]. The presence of RPEs in the studied intergenic spacers may in part explain their high rate of interspecies nucleotide variability.
Within the third intergenic spacer, i.e., dksA-xerC, we have previously identified a second category of repeated elements named variable number of tandem repeats (VNTRs). These repeats are present at a single genomic locus and show interindividual length variability [51]. Such VNTRs are recognized in eukaryotes as source of DNA variability [52], and are also described in bacteria, where they are found within genes and mainly play a role in implementing size variation of membrane-associated proteins. Such a location is found within the ompA gene in rickettsiae. The main characteristic of the VNTRs herein described is their unusual presence within a non-coding sequence. We identified 31 repeat types, classified into two groups depending on their sizes. Another feature of these VNTRs is that their number and arrangement vary depending on strains and species, from a single repeat to 10 repeats. Such a variability makes the dksA-xerC the most discriminatory of the three spacers, with 42 genotypes identified. In a recent work, Vitorino et al. proposed that PCR amplification of VNTRs within the dksA-xerC might serve to identify rickettsial isolates [53]. However, these authors only took into account the size of the VNTRs, not their sequence variability. Using optimal conditions of electrophoresis resolution that would discriminate size differences of 1-bp, which may be difficult to achieve in routine laboratories, PCR amplification of VNTRs would discriminate only 22 different types among our 42 dksA-xerC genotypes. As an example, this would not differentiate R. felis from R. massiliae and R. helvetica, all three being recognized or suspected human pathogens. This supports the MST method that relies on DNA sequences.
Conclusion
MST using the dksA-xerC, mppA-purC, and rpmE-tRNAfMet intergenic spacers is an efficient and reproducible typing method able to identify 23 validated Rickettsia species. It also allows the discrimination at the strain level in six species. Thus, it constitutes a powerful tool for identification of these bacteria, in particular within clinical specimens.
Methods
Rickettsial strains or PCR amplicons
The strains or PCR amplicons used in this study are listed in Additional file 1. Rickettsial strains were propagated at 32°C on Vero cell (ATCC CRL-1587) monolayers in Eagle's minimal essential medium (MEM, Seromed, Berlin, Germany) supplemented with 4% fetal bovine serum (Seromed) and 2 mM glutamine. When cells stained with Gimenez were heavily infected (3 to 5 days), the cultures were harvested, centrifuged (12,000 × g for 10 min), resuspended in MEM and stored at -70°C until processed further.
For the three Rickettsia species previously studied by MST, we included a representative strain of each distinct genotype, i.e., 27 strains for R. conorii subsp. conorii [37], two for R. conorii subsp. caspia, and one each for R. conorii subsp. indica and R. conorii subsp. israelensis [54]; one strain for R. sibirica subsp. sibirica, and two for R. sibirica subsp. mongolitimonae [39]; and two strains and a louse amplicon for R. prowazekii [38].
For the other 20 valid Rickettsia species studied, we included a minimum of one strain and, when available, several strains and/or arthropod amplicons [see Additional file 1]. These extra-strains or arthropod amplicons had been identified using gltA and ompB gene amplification and sequencing as previously described [28,29]. All studied strains are detailed in Additional file 1.
PCR amplification and sequencing
Genomic DNA was extracted from rickettsial cultures using the QIAamp Tissue kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. We used the primers dksAF: 5'-TCCCATAGGTAATTTAGGTGTTTC-3' and dksAR: 5'-TACTACCGCATATCCAATTAAAAA-3', mppAF: 5'-GCAATTATCGGTCCGAATG-3' and mppAR: 5'-TTTCATTTATTTGTCTCAAAATTCA-3', and rpmEF: 5'-TTCCGGAAATGTAGTAAATCAATC-3' and rpmER: 5'-TCAGGTTATGAGCCTGACGA-3', to amplify the intergenic spacers dksA-xerC, mppA-purC, and rpmE-tRNAfMet, respectively. The primers were obtained from Eurogentec (Seraing, Belgium). PCR reactions were carried out in a PTC-200 automated thermal-cycler (MJ Research, Waltham, Mass.) using the previously described conditions [37]. PCR products were purified using a QIAquick Spin PCR purification kit (QIAGEN) as described by the manufacturer. Sequencing reactions were carried out using the d-Rhodamine Terminator cycle sequencing ready reaction kit with Amplitaq Polymerase FS (Applied Biosystems, Coignieres, France) as described by the manufacturer. For all PCR products, sequences from both DNA strands were determined twice. Sequencing products were resolved using an ABI 3100 automated sequencer (Applied Biosystems). Sequence analysis was performed using the software package ABI Prism DNA Sequencing Analysis Software version 3.0 (Applied Biosystems). Sterile water was used as a negative control in each assay.
Sequences were deposited in GenBank under the accession numbers detailed in Additional file 1.
Authors' contributions
The individual parts of the work presented in the paper were conducted as follows: PEF participated in the design of the study, carried out the molecular genetic studies, analyzed the sequences and drafted the manuscript. DR conceived the study and helped to draft the manuscript. Both authors read and approved the final manuscript.
Supplementary Material
Rickettsia strains studied. The Table details the strains studied and their GenBank accession numbers.
Genotypes obtained from each Rickettsia strain studied. The Table contains all genotypes obtained from the Rickettsia strains studied.
Acknowledgments
Acknowledgements
The authors thank Christopher D. Paddock for providing R. parkeri strain Portsmouth, Annick Bernard, Laurence Delaunay and Jean-Luc Llabres for their technical help.
Contributor Information
Pierre-Edouard Fournier, Email: Pierre-Edouard.Fournier@medecine.univ-mrs.fr.
Didier Raoult, Email: didier.raoult@gmail.com.
References
- Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719. doi: 10.1128/cmr.10.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha-Lima H. Zur aetiologie des fleckenfiebers. Berliner Klinishe Wochenschrift. 1916;53:567–569. [Google Scholar]
- Philip CB. Nomenclature of the pathogenic rickettsiae. Am J Hyg. 1943;37:301–309. [Google Scholar]
- Brumpt E. Les spirochétoses. In: Roger , Vidal and Teissier , editor. Nouveau traité de Médecine. Paris, Masson; 1922. pp. 491–531. [Google Scholar]
- Brumpt E. Longévité du virus de la fièvre boutonneuse (Rickettsia conorii n.sp.) chez la tique Rhipicephalus sanguineus. C R Séances Soc Biol Filiales. 1932;110:1199–1209. [Google Scholar]
- Kelly PJ, Beati L, Mason PR, Matthewman LA, Roux V, Raoult D. Rickettsia africae sp nov, the etiological agent of African tick bite fever. Int J Syst Bact. 1996;46:611–614. doi: 10.1099/00207713-46-2-611. [DOI] [PubMed] [Google Scholar]
- Zdrodovskii PF. Systematics and comparative characterization of endemic rickettsioses. Zhur Mikrobiol Epidemiol. 1949;10:19–28. [Google Scholar]
- Sekeyova Z, Roux V, Xu WB, Rehacek J, Raoult D. Rickettsia slovaca sp.nov., a member of the spotted fever group rickettsiae. Int J Syst Bacteriol. 1998;48:1455–1462. doi: 10.1099/00207713-48-4-1455. [DOI] [PubMed] [Google Scholar]
- Stenos J, Roux V, Walker D, Raoult D. Rickettsia honei sp.nov., the aetiological agent of Flinders Island spotted fever in Australia. Int J Syst Bacteriol. 1998;48:1399–1404. doi: 10.1099/00207713-48-4-1399. [DOI] [PubMed] [Google Scholar]
- Uchida T, Uchiyama T, Kumano K, Walker DH. Rickettsia japonica sp. nov., the etiological agent of spotted fever group rickettsiosis in Japan. Int J Syst Bacteriol. 1992;42:303–305. doi: 10.1099/00207713-42-2-303. [DOI] [PubMed] [Google Scholar]
- Philip CB. Miscellaneous human rickettsioses. In: Pullen RL, editor. Communicable diseases. Philadelphia, Lea and Feibiger Co.; 1950. pp. 781–788. [Google Scholar]
- Huebner RJ, Jellison WL, Pomerantz C. Rickettsialpox- a newly recognized rickettsial disease. IV Isolation of a rickettsia apparently identical with the causative agent of rickettsialpox from Allodermanyssus sanguineus a rodent mite. Public Health Rep. 1946;61:1677–1682. [PubMed] [Google Scholar]
- Bouyer DH, Stenos J, Crocquet-Valdes P, Moron C, Vsevolod P, Zavala-Velasquez JE, Foil L, Stothard D, Azad A, Walker D. Rickettsia felis : molecular characterization of a new member of the spotted fever group. Int J Syst Evol Microbiol. 2001;51:339–347. doi: 10.1099/00207713-51-2-339. [DOI] [PubMed] [Google Scholar]
- Beati L, Meskini M, Thiers B, Raoult D. Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int J Syst Bacteriol. 1997:548–554. doi: 10.1099/00207713-47-2-548. [DOI] [PubMed] [Google Scholar]
- Beati L, Peter O, Burgdorfer W, Aeschlimann A, Raoult D. Confirmation that Rickettsia helvetica sp. nov. is a distinct species of the spotted fever group of rickettsiae. Int J Syst Bacteriol. 1993;43:521–526. doi: 10.1099/00207713-43-3-521. [DOI] [PubMed] [Google Scholar]
- Beati L, Raoult L. Rickettsia massiliae sp.nov., a new spotted fever group rickettsia. Int J Syst Bacteriol. 1993;43:839–840. doi: 10.1099/00207713-43-4-839. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Brinton LP, Krinsky WL, Philip RN. Rickettsia rhipicephali : a new spotted fever group rickettsia from the brown dog tick Rhipicephalus sanguineus. In: Kazar J, Ormsbee RA and Tarasevich IV, editor. Rickettsiae and rickettsial diseases. Bratislava, House of the Slovak Academy of Sciences; 1978. pp. 307–316. [Google Scholar]
- Weiss E, Moulder JW. Order I Rickettsiales, Gieszczkiewicz 1939. In: Krieg NR and Holt JG, editor. Bergey's manual of systematic bacteriology. 1. Baltimore, Williams & Wilkins; 1984. pp. 687–703. [Google Scholar]
- Lackman DB, Bell EJ, Stoenner HG, Pickens EG. The Rocky mountain spotted fever group of rickettsiae. Health Lab Sci. 1965;2:135. [PubMed] [Google Scholar]
- Fournier PE, Dumler JS, Greub G, Zhang J, Yimin W, Raoult D. Gene sequence-based criteria for the identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier PE, Takada N, Fujita H, Raoult D. Rickettsia tamurae sp. nov., isolated from Amblyomma testudinarium ticks. Int J Syst Evol Microbiol. 2006;56:1673–1675. doi: 10.1099/ijs.0.64134-0. [DOI] [PubMed] [Google Scholar]
- Fujita H, Fournier PE, Takada N, Saito T, Raoult D. Rickettsia asiatica sp. nov., isolated in Japan. Int J Syst Evol Microbiol. 2006;56:2365–2368. doi: 10.1099/ijs.0.64177-0. [DOI] [PubMed] [Google Scholar]
- Niebylski ML, Schrumpf ME, Burgdorfer W, Fischer ER, Gage KL, Schwan TG. Rickettsia peacockii sp.nov., a new species infecting wood ticks, Dermacentor andersoni, in Western Montana. Int J Syst Bacteriol. 1997:446–452. doi: 10.1099/00207713-47-2-446. [DOI] [PubMed] [Google Scholar]
- Philip RN, Casper EA, Anacker RL, Cory J, Hayes SF, Burgdorfer W, Yunker E. Rickettsia bellii sp. nov. : a tick-borne rickettsia, widely distributed in the United States, that is distinct from the spotted fever and typhus biogroups. Int J Syst Bacteriol. 1983;33:94–106. [Google Scholar]
- McKiel YA, Bell EJ, Lackman DB. Rickettsia canada : a new member of the typhus group of rickettsiae isolated from Haemaphylasis leporispalustris ticks in Canada. Can J Microbiol. 1967;13:503–510. doi: 10.1139/m67-065. [DOI] [PubMed] [Google Scholar]
- Stothard DR, Fuerst PA. Evolutionary analysis of the spotted fever and typhus groups of Rickettsia using 16S rRNA gene sequences. Syst Appl Microbiol. 1995;18:52–61. [Google Scholar]
- Roux V. Phylogenetic analysis and taxonomic relationships among the genus Rickettsia. In: Raoult D and Brouqui P, editor. Rickettsiae and Rickettsial diseases at the turn of the third millinium. Marseille, Elsevier; 1999. pp. 52–66. [Google Scholar]
- Roux V, Raoult D. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res Microbiol. 1995;146:385–396. doi: 10.1016/0923-2508(96)80284-1. [DOI] [PubMed] [Google Scholar]
- Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol. 1997;47:252–261. doi: 10.1099/00207713-47-2-252. [DOI] [PubMed] [Google Scholar]
- Fournier PE, Roux V, Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int J Syst Bacteriol. 1998;48:839–849. doi: 10.1099/00207713-48-3-839. [DOI] [PubMed] [Google Scholar]
- Roux V, Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB) Int J Syst Evol Microbiol. 2000;50:1449–1455. doi: 10.1099/00207713-50-4-1449. [DOI] [PubMed] [Google Scholar]
- Ngwamidiba M, Blanc G, Raoult D, Fournier PE. Sca1, a previously undescribed paralog from autotransporter protein-encoding genes in Rickettsiaspecies. BMC Microbiol. 2006;6:12. doi: 10.1186/1471-2180-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwamidiba M, Blanc G, Ogata H, Raoult D, Fournier PE. Phylogenetic Study of Rickettsia Species Using Sequences of the Autotransporter Protein-Encoding Gene sca2. Ann N Y Acad Sci. 2005;1063:94–99. doi: 10.1196/annals.1355.015. [DOI] [PubMed] [Google Scholar]
- Sekeyova Z, Roux V, Raoult D. Phylogeny of Rickettsia spp. inferred by comparing sequences of 'gene D', which encodes an intracytoplasmic protein. Int J Syst Evol Microbiol. 2001;51:1353–1360. doi: 10.1099/00207713-51-4-1353. [DOI] [PubMed] [Google Scholar]
- Tautz D, Arctander P, Minelli A, Thomas RH, Vogler AP. A plea for DNA taxonomy. Trends Ecol Evol. 2003;18:70–74. doi: 10.1016/S0169-5347(02)00041-1. [DOI] [Google Scholar]
- Maiden MCJ, Bygraves JA, Feil E, Morelli G, Russel JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier PE, Zhu Y, Ogata H, Raoult D. Use of highly variable intergenic spacer sequences for multispacer typing of Rickettsia conorii strains. J Clin Microbiol. 2004;42:5757–5766. doi: 10.1128/JCM.42.12.5757-5766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Fournier PE, Ogata H, Raoult D. Multispacer Typing of Rickettsia prowazekii Enabling Epidemiological Studies of Epidemic Typhus. J Clin Microbiol. 2005;43:4708–4712. doi: 10.1128/JCM.43.9.4708-4712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier PE, Zhu Y, Yu X, Raoult D. Proposal to create subspecies of Rickettsia sibirica and an emended description of Rickettsia sibirica. Ann N Y Acad Sci. 2006;1078:597–606. doi: 10.1196/annals.1374.120. [DOI] [PubMed] [Google Scholar]
- Ogata H, Audic S, Barbe V, Artiguenave F, Fournier PE, Raoult D, Claverie JM. Selfish DNA in protein-coding genes of Rickettsia. Science. 2000;290:347–350. doi: 10.1126/science.290.5490.347. [DOI] [PubMed] [Google Scholar]
- Ogata H, Audic S, Abergel C, Fournier PE, Claverie JM. Protein coding palindromes are a unique but recurrent feature in Rickettsia. Genome Res. 2002;12:808–816. doi: 10.1101/gr.227602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H, La Scola B, Audic S, Renesto P, Blanc G, Robert C, Fournier PE, Claverie JM, Raoult D. Genome Sequence of Rickettsia bellii Illuminates the Role of Amoebae in Gene Exchanges between Intracellular Pathogens. PLoS Genet. 2006;2:e76. doi: 10.1371/journal.pgen.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson SGE, Zomorodipour A, Andersson JO, Sicheritz-Pontén T, Alsmark UCM, Podowski RM, Näslund AK, Eriksson AS, Winkler HH, Kurland CG. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- Ogata H, Audic S, Renesto-Audiffren P, Fournier PE, Barbe V, Samson D, Roux V, Cossart P, Weissenbach J, Claverie JM, Raoult D. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science. 2001;293:2093–2098. doi: 10.1126/science.1061471. [DOI] [PubMed] [Google Scholar]
- Ogata H, Renesto P, Audic S, Robert C, Blanc G, Fournier PE, Parinello H, Claverie JM, Raoult D. The Genome Sequence of Rickettsia felis Identifies the First Putative Conjugative Plasmid in an Obligate Intracellular Parasite. PLoS Biol. 2005;3:e248. doi: 10.1371/journal.pbio.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod MP, Qin X, Karpathy SE, Gioia J, Highlander SK, Fox GE, McNeill TZ, Jiang H, Muzny D, Jacob LS, Hawes AC, Sodergren E, Gill R, Hume J, Morgan M, Fan G, Amin AG, Gibbs RA, Hong C, Yu XJ, Walker DH, Weinstock GM. Complete genome sequence of Rickettsia typhi and comparison with sequences of other rickettsiae. J Bacteriol. 2004;186:5842–5855. doi: 10.1128/JB.186.17.5842-5855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drancourt M, Roux V, Dang LV, Lam TH, Castex,D., Chenal-Francisque V, Ogata H, Fournier PE, Crubezy E, Raoult D. Genotyping, Orientalis-like Yersinia pestis, and plague pandemics. Emerg Infect Dis. 2004;10:1585–1592. doi: 10.3201/eid1009.030933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazunova O, Roux V, Freylikman O, Sekeyova Z, Fournous G, Tyczka J, Tokarevich N, Kovacava E, Marrie TJ, Raoult D. Coxiella burnetii genotyping. Emerg Infect Dis. 2005;11:1211–1217. doi: 10.3201/eid1108.041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucault C, La Scola B, Lindroos H, Andersson SG, Raoult D. Multispacer typing technique for sequence-based typing of Bartonella quintana. J Clin Microbiol. 2005;43:41–48. doi: 10.1128/JCM.43.1.41-48.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Chomel BB, Maruyama S, Guptil L, Sander A, Raoult D, Fournier PE. Multispacer typing to study the genotypic distribution of Bartonella henselae populations. J Clin Microbiol. 2006;44:2499–2506. doi: 10.1128/JCM.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van belkum A, Scherer S, van Alphen L, Verbrugh H. Short-sequence DNA repeats in prokaryotic genomes. Microbiol Mol Biol Rev. 1998;62:275–293. doi: 10.1128/mmbr.62.2.275-293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Elder JF, Jr., Laughlin TF, Davis WP. Genetic variation in clonal vertebrates detected by simple-sequence DNA fingerprinting. Proc Natl Acad Sci U S A. 1990;87:5653–5657. doi: 10.1073/pnas.87.15.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorino L, de Sousa R, Bacellar F, Ze-Ze L. Characterization of a tandem repeat polymorphism in Rickettsia strains. J Med Microbiol. 2005;54:833–841. doi: 10.1099/jmm.0.45956-0. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Fournier PE, Eremeeva M, Raoult D. Proposal to create subspecies of Rickettsia conorii based on multi-locus sequence typing and an emended description of Rickettsia conorii. BMC Microbiol. 2005;5:11. doi: 10.1186/1471-2180-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rickettsia strains studied. The Table details the strains studied and their GenBank accession numbers.
Genotypes obtained from each Rickettsia strain studied. The Table contains all genotypes obtained from the Rickettsia strains studied.