Abstract
Steroid hormones play an important role in modulating social behavior in many species. Estrogens are thought to act on an interconnected network of hypothalamic and limbic brain areas to affect aggressive behavior, although the specific nuclei unknown remain unspecified. We show that individual variation in estrogen receptor alpha (ERα) immunoreactivity in the lateral septum (LS), ventral bed nucleus of the stria terminalis (vBNST), and anterior hypothalamus (AHA) of CD-1 mice is positively correlated with aggressive behavior. When males were treated with fadrozole (an aromatase inhibitor), aggressive behavior was reduced, although castration did not reduce aggression. These results suggest that estrogens modulate aggressive behavior by acting on a circuit that includes the LS, vBNST, and AHA and that the source of estrogens is nongonadal. Fadrozole also decreased c-fos expression in the lateral septum following aggressive encounters. Although the effects of estrogen on aggression appear to involve regulation of neuronal activity in the LS, additional processes are likely involved. These results suggest that estrogen acts in a specific subset of a complex network of nuclei to affect aggressive behavior.
Keywords: Estrogen receptor, Aromatase, Lateral septum, Social behavior, Individual differences
Introduction
Investigations into the mechanistic bases of aggressive behavior have identified several neurochemical systems that affect aggression (Nelson and Chiavegatto, 2001; Miczek et al., 2002). Over the past decade, many investigations into the molecular bases of aggressive behavior in mammals have utilized transgenic mice in which a specific gene is deleted (Saudou et al., 1994; Nelson et al., 1995; Wersinger et al., 2002). An advantage of using knock-out mice is that a specific protein can be removed from the system allowing for focused observations on the effects of a single gene. Until recently, tissue-specific gene knock-outs have not been available, so most studies of knock-out mice have been able to provide only limited anatomical detail on where neurochemicals act to affect behavior (but see Chiavegatto et al., 2001; Stowers et al., 2002). Furthermore, knock-out studies typically cannot examine the mechanistic bases of individual differences in behavior. Thus, studies of genetically unmodified populations form a critical component to investigations into the physiological regulation of behavior.
Estrogens are considered important modulators of social behavior, including aggression (Trainor et al., in press). Estrogen increases aggression in quail (Schlinger and Callard, 1990) and song sparrows (Soma et al., 2000); in both species, increased aromatase activity in the brain is associated with increased aggression (Schlinger and Callard, 1989; Soma et al., 2003). Hormone manipulation studies on laboratory mice indicate that estrogen increases the probability that males will attack intruders in resident–intruder aggression tests, although the efficacy of estrogen depends on the strain (Simon, 2002). Estrogen does not universally increase aggression. For example, treatment of California mice (Peromyscus californicus) housed in long days with an aromatase inhibitor increased attack latency (Trainor et al., 2004). Context-dependent effects of estrogen on behavior may be extremely important because most studies of aggression and its components (e.g., hostility) in humans indicate that estrogens tend to decrease components of aggression (reviewed in Trainor et al., in press). One way in which the directional effects of estrogen on behavior can be modulated is through alterations in estrogen receptor subtypes.
To date, the best evidence for estrogen receptor subtype-specific effects on behavior is from gene knock-out studies (Ogawa et al., 2005). There are at least two subtypes of the estrogen receptor: the classical or α estrogen receptor (White et al., 1987) and the β estrogen receptor (Kuiper et al., 1996; Mosselman et al., 1996). Deletion of the ERα gene decreases intermale aggressive behavior (Ogawa et al., 1998; Scordalakes and Rissman, 2003). Studies on estrogen receptor β knock-out (ERβKO) suggest that ERβ may decrease aggressive behavior, although this effect apparently depends on age and sexual experience (Ogawa et al., 1999; Nomura et al., 2002). Thus, expression of ER subtypes could have critical effects on aggressive behavior, although the specific brain nuclei involved remain unclear.
Functional neuroanatomical studies of aggressive behavior have used indirect markers of neuronal activity to identify a behavioral circuit that includes the lateral septum (LS), bed nucleus of the stria terminalis (BNST), anterior hypothalamus (AHA), and medial amygdala (MEA) (Newman, 1999; Delville et al., 2000; Goodson, 2005). Immunocytochemistry studies indicate that most of these brain areas also express ERα (Wood and Newman, 1995; Shughrue et al., 1997), but no direct link between ERα in these brain areas and aggressive behavior has been established. Although it remains unspecified precisely how estrogens alter this neurocircuitry to affect behavior, some clues have emerged. Both male (Imwalle et al., 2002) and female (Choleris et al., 2003) ERα knock-out mice exhibit deficits in social recognition tasks, which suggests that reduced aggressive behavior in male ERα knock-out mice could be influenced by deficiencies in the processing of social stimuli. To date, no study has examined how indirect markers of neuronal activity following aggressive behavior are affected by manipulations of estrogenic signaling (e.g., Wersinger et al., 2002).
Our study is an attempt to integrate findings on estrogenic control of behavior with variability in estrogen receptor expression within the brain. First, we tested whether photoperiod affected ERα immunoreactivity (-ir) in CD-1 mice (Mus musculus) because observations from another study suggested that photoperiodic ERα-ir regulation may be uncoupled from reproductive suppression (Trainor and Nelson unpublished). Next, we examined correlational relationships between ERα-ir, c-fos, and aggression. We expected that ERα-ir immunoreactivity would be correlated with aggression in regions of the brain in which aggression tests are known to increase markers of neuronal activity such as the LS, BNST, AHA, and MEA (Kollack-Walker and Newman, 1999; Delville et al., 2000). CD-1 mice are ideal for identifying individual differences in brain function and behavior; this strain of mice has been previously used to identify individual differences in learning and memory (Matzel et al., 2003). We tested whether estrogen directly affects aggression by blocking the production of estrogen (via fadrozole). Furthermore, we hypothesized that fadrozole would affect behavior by reducing neuronal activity in brain areas in which ERα-ir was correlated with aggression. We tested this hypothesis by the examining the effects of hormone manipulations on c-fos expression (an indirect marker of neuronal activity).
Methods
Animals
In the correlational experiment, male CD-1 mice (M. musculus) were randomly assigned to be housed in long (16L:8D) or short (8L:16D) day lengths and provided with filtered tap water and Harlan Teklad 8640 food (Madison, WI) ad libitum. In the hormone manipulation experiment, all CD-1 males were housed in long days and provided with water and Harlan Teklad 2016 food (phytoestrogen free) ad libitum. All behavioral observations were conducted with the wire food hopper and filter lid in place as preliminary observations indicated that most mice would not exhibit aggression when the food hopper was removed. Animals were maintained in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experiment 1: correlational study
After 8 weeks of housing in long or short days, all males were tested in resident–intruder aggression tests. Three days before testing in aggression tests, all males were anesthetized with isoflurane and a retroorbital blood sample was collected. For each aggression test, a sexually inexperienced, weight-matched male was introduced into each resident’s home cage for 10 min. Intruders were group housed and kept in 16L:8D. The number of bites, tail rattles, bouts of boxing, and the total amount of time allogrooming and autogrooming were recorded from videotaped observations. Furthermore, the latency to first bite was determined (assigning a value of 600 s if no biting occurred). Forty-five minutes after testing in aggression tests, males were lightly anesthetized with isoflurane and rapidly decapitated. Brains were quickly removed and fixed in 5% acrolein in PBS at 4°C overnight. Each brain was then transferred to 30% sucrose in PBS for 24 h, frozen on dry ice, and stored at −80°C. Trunk blood was collected in heparinized tubes on ice, centrifuged, and plasma removed for testosterone (T) RIA. Total testosterone was measured with a 125I testosterone RIA kit (DSL-4100; Diagnostic Systems Laboratories, Webster, TX) that uses a specific antibody that does not cross-react with DHT. The intraassay CV was 7.57%, and the detection limit was 0.1 ng ml−1.
Experiment 2: hormone manipulation study
To examine the effects of estrogen on the brain and behavior, we conducted a hormone manipulation experiment. To test whether testosterone increased aggression, one group of males (n = 16) was castrated and implanted with an empty Silastic implant (i.d. 1.47 mm, o.d. 1.96 mm). This group was compared with a second group (n = 10) of castrated males that received a Silastic implant containing 5 mm of T and an osmotic minipump (Alzet 2002) filled with saline (T/SAL). To test whether estrogens affected aggressive behavior, males in a third group (n = 12) of castrated males with T implants were given an osmotic minipump filled with fadrozole (0.25 mg/kg), a potent aromatase inhibitor (T/FAD). Finally, a fourth group (n = 11) of males received a sham surgery and remained intact. Thirteen days after castration and implant surgery, all males were tested in 10 min aggression tests as described above. Forty minutes after the end of each test, each male was lightly anesthetized with isoflurane and rapidly decapitated. Brains were collected as described above. Brains from castrated (n = 7), T/SAL (n = 6), and T/FAD (n = 8) males were processed for c-fos ICC. Although we collected brains from intact males, we did not process these brains for c-fos because behavioral results indicated that there were no differences between intact and T/SAL animals.
Immunocytochemistry
Brains were sectioned at 40 μm on a cryostat and alternate free-floating sections were processed for ERα or c-fos immunocytochemistry. Sections were washed 3 times in PBS and then incubated in 1% sodium borohydride in PBS for 10 min. Sections were then rinsed in 20% normal goat serum and 0.3% hydrogen peroxide in PBS for 20 min. Sections were incubated in either primary ERα antibody (C1355, Upstate Biotechnology, 1:50K) or c-fos (rabbit anti c-fos, Chemicon 1:10K) in 1% normal goat serum in 0.5% Triton-X PBS (PBS + TX) for 48 h. Next, the sections were rinsed 3 times in PBS and incubated for 90 min with biotinylated goat–antirabbit antibody (Vector Laboratories) in PBS + TX. The sections were then rinsed 3 times in PBS and then incubated for either 30 min (ERα) or 60 min (c-fos) in avidin–biotin complex (ABC Elite kit, Vector Laboratories). After 3 rinses in PBS, the sections were developed in diaminobenzidine for 2 min. Sections were mounted, dehydrated and coverslipped with Permount. Sections containing the LS, anterodorsal BNST (dBNST), anteroventral BNST (vBNST), MEA, MPOA, AHA, and lateral hypothalamus (LH) were identified using the mouse atlas by Paxinos and Franklin (2002). The dBNST was defined as immediately dorsal to the anterior commissure and lateral to the lateral ventricle (Bregma 0.14). The vBNST was defined as immediately ventral to the anterior commissure (Bregma 0.14). For measurements of c-fos immunoreactivity, we also counted cells in the paraventricular nucleus (PVN). Images were captured at the same time using a Nikon E800 microscope. Immunopositive cells were counted with the aid of Neurolucida software (Microbrightfield, Burlington, VT). Control sections in which primary antibodies were not added showed no specific staining.
Statistical analyses
The effect of photoperiod on ERα cell counts was tested with unpaired t tests, and Pearson correlations were used to examine the relationships between ERα cell counts and aggressive behavior. In the hormone manipulation experiment, variability in aggressive behavior within the castrated group caused heterogeneous between group variance, so we used non-parametric Kruskal–Wallis tests followed by pair-wise Mann–Whitney U tests. A chi-square test was used to test whether hormone manipulations influenced the probability that males attacked intruders. Group differences in c-fos immunoreactivity were tested with one-way ANOVAs, and relationships between c-fos cell counts and aggressive behavior were examined with Pearson correlations. Mean differences were considered statistically significant when P < 0.05 (Fig. 1).
Fig. 1.

Estrogen receptor alpha immunoreactivity in the (a) lateral septum, (b) ventral bed nucleus of the stria terminalis, (c) lateral hypothalamus, (d) anterior hypothalamus, medial preoptic area (e), and medial amygdala (f). Landmarks are abbreviated as: anterior commissure (ac), lateral ventricle (lv), third ventricle (3v), fornix (fo), optic tract (opt). Scale bar = 1 mm.
Results
Effects of photoperiod on brain and behavior
There were no significant effects of photoperiod on ERα immunoreactivity in any brain area examined, and there were no significant differences in aggressive behavior or pre-encounter or post-encounter T.
Relationships between ERα immunoreactivity and behavior
Across both photoperiods, aggressive behavior was consistently correlated with ERα immunoreactive (-ir) cells in several hypothalamic and limbic brain areas. The number of bites during 10 min tests was positively correlated with ERα-ir in the vBNST (Fig. 2a, r = 0.67, P = 0.02), LS (Fig. 2b, r = 0.80, P = 0.01), and AHA (Fig. 2c, r = 0.59, P = 0.04). There was a non-significant correlation between biting and ERα-ir in the dBNST (r = 0.54, P = 0.08). Attack latency was negatively correlated with ERα-ir cells in the vBNST (r = −0.76, P < 0.01) and LS (r = −0.60, P < 0.05), but not the dBNST. The number of ERα-ir cells in the MEA, MPOA, and LH was not significantly correlated with any aggressive behaviors (all P’s > 0.05).
Fig. 2.

Correlations between ERα immunoreactivity and number of bites in the (a) vBNST, (b) LS, (c) AHA, (d) MPOA, (e) LH, and (f) MEA.
In the medial amygdala, there were significant positive correlations between c-fos and ERα-ir in the dBNST (r = 0.79, P < 0.01), LS (r = 0.70, P = 0.02), and AHA (r = 0.61, P = 0.04) and a non-significant correlation in the vBNST (r = 0.55, P = 0.06). There was no correlation between c-fos in the MEA and ERα-ir in the other areas measured (all P’s > 0.05). There were no significant correlations between c-fos and ERα-ir in any other brain areas. There were no significant correlations between aggressive behavior and c-fos (all P’s > 0.05).
Effects of hormone manipulation on behavior and c-fos immunoreactivity
Omnibus Kruskal–Wallis tests detected significant effects of hormone manipulations on tail rattling (Fig. 3, χ3 = 8.65, P = 0.034) and biting (Fig. 3, χ3 = 9.37, P = 0.025), but not boxing (Fig. 3, χ3 = 5.89, P = 0.117) or attack latency (χ3 = 1.66, P = 0.646). Castrated males treated with T/FAD exhibited significantly fewer bites during aggression tests than intact males (U = 34, P < 0.05), T/SAL males (U = 31, P < 0.05), or castrated males with empty implants (U = 41, P < 0.01). Tail rattling in the T/FAD group was significantly reduced compared to castrated males with empty implants (U = 42, P = 0.008), and marginally lower than T/SAL males (U = 37, P = 0.06). Castrated males with empty implants did not differ from either intact or castrated males treated with T/SAL for biting, boxing, or tail rattling (all P’s > 0.05). Hormone manipulations did not significantly affect the probability that males attacked intruders (χ3 = 5.25, P > 0.05).
Fig. 3.

Effects of hormone manipulations on aggressive behavior in CD-1 mice. Bars represent mean ± SEM for intact males (open bars, n = 11) castrated males with empty implants (striped bars, n = 16), castrated males with T implants (black bars, n = 10), and castrated males with T implants and fadrozole (gray bars, n = 12). *Mann–Whitney U test P < 0.05 compared to castrated males treated with T and fadrozole.
Hormone manipulations significantly affected c-fos immunoreactivity following aggression tests in the LS (F2,18 = 7.52, P < 0.01). Castrated males treated with T/SAL had significantly more c-fos immunoreactive cells in the LS following an aggression test compared to castrated males with empty implants (Fig. 4, P < 0.05) and castrated males treated with T/FAD (Fig. 4, P < 0.01). Similar non-significant patterns were observed in the vBNST (F2,18 = 1.91, P = 0.17), AHA (F2,18 = 1.85, P = 0.18), and PVN (F2,18 = 2.84, P = 0.08). There were no significant effects of hormone manipulations on c-fos in the dBNST, MPOA, LH, or MEA (all P’s > 0.05). Although hormone manipulations did not affect the total number of c-fos immunopositive cells in brain areas outside the LS, hormone manipulations apparently affected the relationships between c-fos expression and aggressive behavior. Biting behavior was significantly correlated with c-fos immunoreactive cells in the vBNST and dBNST of T/SAL males, but not T/FAD males or castrated males with empty implants (Table 1). Likewise, attack latency was negatively correlated with c-fos-ir in the vBNST, LS, PVN, AHA, and MEA in T/SAL males, but not T/FAD or castrated males with empty implants (Table 1; Fig. 5).
Fig. 4.

Effects of hormone manipulations on c-fos immunoreactivity in CD-1 mice. Bars represent mean ± SE for castrated males with empty implants (white bars, n = 7), castrated males with T implants (black bars, n = 6), and castrated males with T implants and fadrozole (gray bars, n = 8). *P < 0.05 from castrated + T.
Table 1.
Correlations between c–fos expression and aggression from hormone manipulation experiment
| Castrated + empty
|
Castrated + T + SAL
|
Castrated + T + FAD
|
||||
|---|---|---|---|---|---|---|
| Biting | Attack latency | Biting | Attack latency | Biting | Attack latency | |
| vBNST | r = 0.38 | r = −0.32 | r = 0.90 *** | r = −0.96 *** | r = 0.07 | r = −0.16 |
| dBNST | r = −0.53 | r = 0.66 | r = 0.81 * | r = −0.59 | r = −0.78 a | r = 0.49 |
| LS | r = −0.13 | r = 0.17 | r = 0.68 | r = −0.81 * | r = 0.34 | r = −0.68 |
| MPOA | r = −0.22 | r = 0.16 | r = 0.11 | r = −0.35 | r = 0.36 | r = −0.18 |
| AHA | r = −0.03 | r = −0.18 | r = 0.68 | r = −0.86 ** | r = −0.44 | r = −0.17 |
| PVN | r = 0.16 | r = −0.4 | r = 0.62 | r = −0.86 ** | r = 0.27 | r = 0.18 |
| MA | r = −0.17 | r = 0.38 | r = 0.75a | r = −0.87 ** | r = −0.26 | r = −0.38 |
P = 0.05.
P < 0.05.
P < 0.01.
P < 0.001.
Fig. 5.
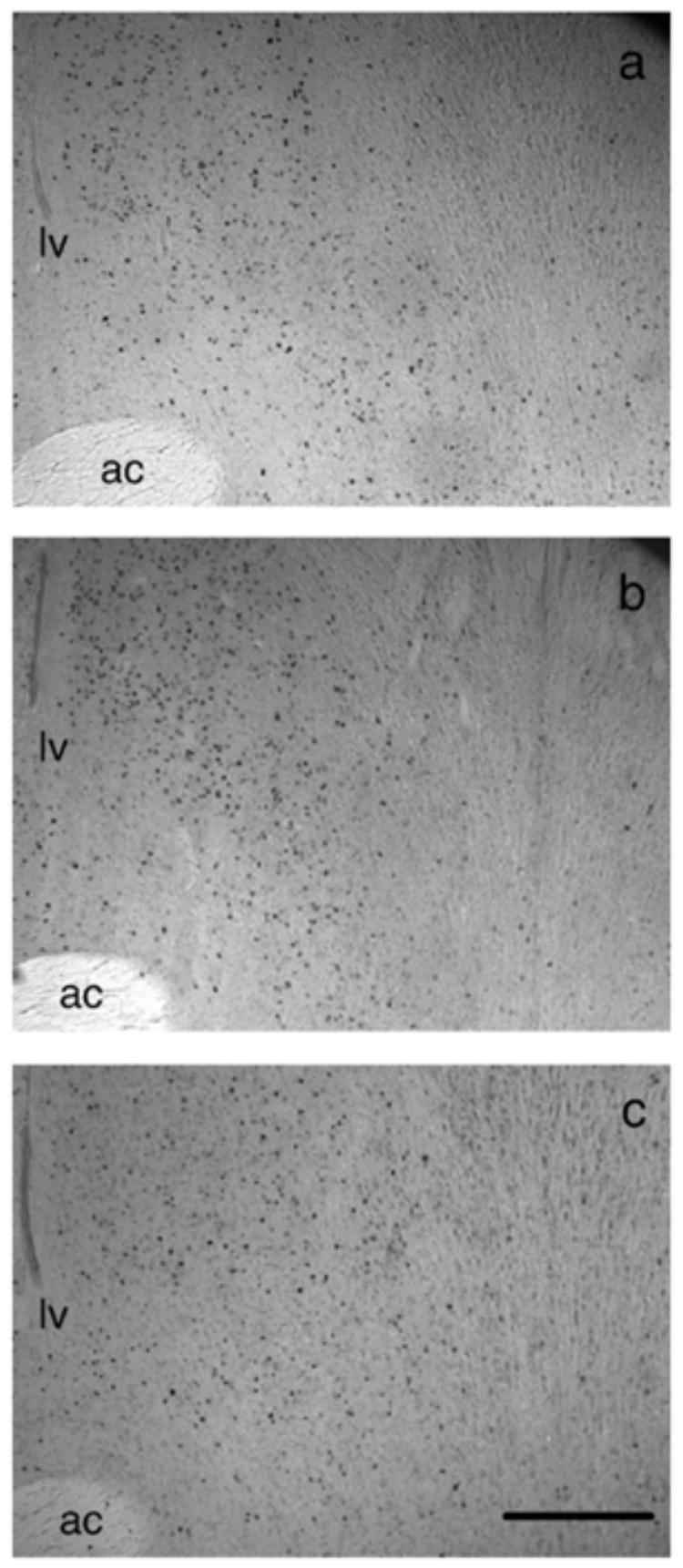
Photomicrographs of the lateral septum from (a) castrated, (b) castrated + T, (c) castrated + T + fadrozole males. Landmarks are abbreviated as: anterior commissure (ac), lateral ventricle (lv). Scale bar = 1 mm.
Discussion
Our data from correlational and hormone manipulation experiments suggest that estrogen modulates aggressive behavior in CD-1 mice by acting in discrete subsections of the hypothalamus and limbic system, specifically a subnetwork that includes that LS, vBNST, and AHA. Fadrozole decreased aggressive behavior, and aggression was positively associated with estrogen receptor immunoreactivity in the LS, vBNST, and AHA, but not the MPOA, LH, or MEA. These correlations are consistent with previous c-fos (Kollack-Walker and Newman, 1995; Delville et al., 2000) and lesion (Albert et al., 1992) studies that suggest that the AHA, but not the MPOA, modulates aggressive behavior. Based on these results, we expected that fadrozole treatment would reduce c-fos in the LS, vBNST, and AHA in response to intruders. Hormone manipulations significantly affected c-fos expression in LS, although weaker changes in c-fos may be present in the vBNST, AHA, and PVN. Hormone manipulations appeared to have a much stronger effect on the relationships between c-fos and aggressive behavior. These results suggest that estrogen acts in specific nuclei to modulate aggressive behavior.
Prior work on the neuroendocrine mechanisms of aggression has identified a collection of hypothalamic and limbic brain areas which are activated during aggressive behavior (Kollack-Walker and Newman, 1995; Goodson, 2005). Many of these brain areas contain estrogen receptors (Simerly et al., 1990; Wood and Newman, 1995), and several knock-out studies indicate that estrogen receptors modulate aggressive behavior. We show that ERα-ir in the CD-1 mouse brain is positively associated with aggression and that this relationship is confined to particular nuclei, specifically the LS, vBNST, and AHA. These results are consistent with ERα knock-out studies which show that deletion of ERα is associated with decreased aggression (Ogawa et al., 1998; Scordalakes and Rissman, 2003) and suggest that the effect of ERα deletion is not solely an organizational effect. Although most nuclei examined in this study are known to be activated in both sexual and aggressive contexts, the majority of studies suggest that the AHA is minimally activated in sexual contexts and the MPOA is minimally activated during aggression (Joppa et al., 1995; Kollack-Walker and Newman, 1995; Kelliher et al., 1998). Our results largely agree with these observations. The LS and vBNST are activated by both aggressive and sexual contexts, and it would be interesting to test whether variability in ERα expression in these nuclei has consequences for sexual behavior. There were no significant correlations between ERα-ir and aggressive behavior in the LH or MEA. It is possible that estrogen action may act in these brain areas to affect aggression, or the afferents from estrogen sensitive areas such as the BNST (Dong and Swanson, 2006) or AHA (Delville et al., 2000) might regulate neuronal activity in the MEA. Post-encounter c-fos in the MEA was positively correlated with ERα-ir in the dBNST, LS, and AHA. However, these data do not support the hypotheses that individual variation in ERα expression in the LH and MEA mediates individual variation in behavior.
In the hormone manipulation experiment, we observed that estrogens promote aggression in CD-1 mice. Fadrozole reduced the number of bites compared to saline-treated males. Individual variability in aggressive behavior was quite large, forcing the use of non-parametric analyses. This variability suggests that other factors besides estrogen are important in the regulation of aggression in CD-1 mice. Testicular androgens do not appear to be one of these factors because castration did not reduce aggression. Several recent studies have reported that castration does not affect baseline aggression in rodents (Caldwell et al., 1984; Demas et al., 1999; Hilakivi-Clarke, 1999; Jasnow et al., 2000; Trainor and Marler, 2001; Pinxten et al., 2003). It is possible that an effect of castration on aggression could take longer than the 2-week recovery period we used in experiment 2. However, we consider this possibility unlikely because in preliminary studies castrated CD-1 mice did not reduce aggression after 4 weeks (Trainor and Nelson unpublished). Androgen-dependent vasopressin expression is significantly decreased 3 weeks after castration in rats (Miller et al., 1992), which suggests that the time course of our hormone manipulations is appropriate. It seems more likely that castrated CD-1 mice are affected by non-androgen-based mechanisms of aggression. In the California mouse (P. californicus), castration does not reduce aggression (Trainor and Marler, 2001) yet estrogen manipulations affect aggression (Trainor et al., 2004). Recent studies have suggested that adrenal hormones (Demas et al., 2004), specifically DHEA (Soma et al., 2004), may modulate aggression.
Based on the correlations between aggressive behavior and ERα expression in the LS, vBNST, and AHA, we expected that fadrozole would reduce c-fos (an indirect marker of neuronal activity) expression in these brain areas following aggression tests. Both castration alone and T/FAD reduced c-fos-ir in the LS, but no significant differences in the vBNST or AHA were detected. It is possible that this study lacked sufficient statistical power to detect changes in c-fos in the vBNST or AHA. However, it seems apparent that c-fos activity in the LS is more sensitive to estrogen than other brain regions examined. These data in the LS are consistent with previous studies showing that estradiol increases c-fos expression (Balog et al., 2001; Wang et al., 2004). Castration and fadrozole treatment also disrupted relationships between aggression and c-fos. Attack latency was negatively correlated with c-fos in several brain areas only in T/SAL males, but not castrated or T/FAD-treated males. Correlations were significant in the LS, AHA, MEA, dBNST, and vBNST consistent with previous studies showing that aggression tests increase c-fos expression in these areas (Kollack-Walker and Newman, 1995; Delville et al., 2000; Davis and Marler, 2004; Veening et al., 2005). One possible explanation for these results is that estrogen may affect how males perceived aggressive encounters. Overall, these data do not support the hypothesis that ERα acting in the AHA and vBNST promote aggression via increasing neuronal activity in these nuclei, although it is possible that other markers of neural activation might reveal differences not detected with c-fos. The effects of estrogen on behavior may be mediated via regulation of other neuromodulators. Finally, because all males were tested in aggression tests, our study cannot separate out c-fos due to intruder exposure and c-fos stimulated by fighting. In future experiments, it would be interesting to determine whether estrogen alters the perception of intruders (e.g., Stowers et al., 2002) or alters the behavioral responses to intruders.
In the anterior hypothalamus, several studies have examined the relationship between steroids and vasopressin (AVP). Castration reduces AVP in male rats (De Vries et al., 1985), hamsters (Delville et al., 1996), and prairie voles (Wang and De Vries, 1993), apparently via an estrogen-dependent mechanism (De Vries et al., 1985; Lonstein and De Vries, 1999). Estrogenic upregulation of AVP could increase aggression as AVP acting in the AHA can promote aggression (Ferris and Potegal, 1988). In the lateral septum, studies from many species report that lesions to this area increase aggressive behavior (Albert and Walsh, 1984). One possible mechanism for this effect on aggression is the destruction of inhibitory GABA neurons that project (among other areas) to the anterior hypothalamus (Ferris et al., 1990). Estrogen decreases GABAergic activity in the hippocampus (Rudick et al., 2003) and arcuate nucleus (Parducz et al., 1993), which raises the possibility that increased ERα activity in the lateral septum decreases GABAergic activity in afferents to the anterior hypothalamus. Finally, an interesting possibility for ERα action in the vBNST involves modulation of interleukin signaling. Recent studies have demonstrated that lesions to the vBNST can block interleukin-1β-induced c-fos in corticotrophin releasing hormone neurons of the PVN (Crane et al., 2003). Although not statistically significant, we observed that castration and T/FAD treatment reduced c-fos in the PVN. Further characterization of the activity of ERα cells during aggressive encounters is needed to test these hypotheses.
In summary, although previous studies suggest that ERα can affect aggression, the details of where in the brain this occurs have been unclear. We demonstrate that individual differences in ERα-ir in the LS, vBNST, and AHA of CD-1 mice were positively correlated with aggressive behavior and that ERα in other brain areas, including the MPOA, was not correlated with aggression. These data suggest that the effects of estrogen on aggression may be limited to discrete subnetworks in the brain, although this hypothesis needs to be tested directly. It remains unknown whether individual differences in ERα expression in CD-1 mice are due to genetic or environmental factors. However, recently identified correlations between variability in regulatory regions of the human ERα gene and aspects of personality suggest a possible genetic basis (Comings et al., 1999; Westberg et al., 2003).
Acknowledgments
We thank S.L. Bowers, J.R. Kuhlman, and A.G. Trainor for technical help and Novartis Pharma AG, Basel, Switzerland for generously donating fadrozole. This work was supported by NIH grants MH076313 to B.C.T. and MH57535 to R.J.N.
Abbereviations
- AHA
anterior hypothalamus
- dBNST
anterodorsal bed nucleus of the stria terminalis
- vBNST
anteroventral bed nucleus of the stria terminalis
- LS
lateral septum
- MEA
medial amygdala
- MPOA
medial preoptic area
- LH
lateral hypothalamus
References
- Albert D, Walsh M. Neural systems and the inhibitory modulation of agonistic behavior: a comparison of mammalian species. Neurosci Biobehav Rev. 1984;8:5–24. doi: 10.1016/0149-7634(84)90017-4. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Jonik RH, Walsh ML. Hormone-dependent aggression in male and female rats: experiential, hormonal, and neural foundations. Neurosci Biobehav Rev. 1992;16:177–192. doi: 10.1016/s0149-7634(05)80179-4. [DOI] [PubMed] [Google Scholar]
- Balog J, Kurunczi A, Parducz A. 17beta-Estradiol increases, aging decreases, c-fos expression in the rat accessory olfactory bulb. NeuroReport. 2001;12:3787–3790. doi: 10.1097/00001756-200112040-00037. [DOI] [PubMed] [Google Scholar]
- Caldwell GS, Glickman SE, Smith ER. Seasonal aggression independent of seasonal testosterone in wood rats. Proc Natl Acad Sci U S A. 1984;81:5255–5257. doi: 10.1073/pnas.81.16.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Gustaffson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci U S A. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Johnson P, MacMurray JP. Potential role of estrogen receptor gene (ESR1) in anxiety. Mol Psychiatry. 1999;4:374–377. doi: 10.1038/sj.mp.4000503. [DOI] [PubMed] [Google Scholar]
- Crane JW, Buller KM, Day TA. Evidence that the bed nucleus of the stria terminalis contributes to the modulation of hypophysiotropic corticotropin-releasing factor cell responses to systemic interleukin-1β. J Comp Neurol. 2003;467:232–242. doi: 10.1002/cne.10918. [DOI] [PubMed] [Google Scholar]
- Davis E, Marler C. c-fos changes following an aggressive encounter in female California mice: a synthesis of behavior, hormone changes and neural activity. Neuroscience. 2004;127:611–624. doi: 10.1016/j.neuroscience.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour K, Ferris C. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol Behav. 1996;60:25–29. doi: 10.1016/0031-9384(95)02246-5. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Demas GE, Moffatt CA, Drazen DL, Nelson RJ. Castration does not inhibit aggressive behavior in adult male prairie voles (Microtus ochrogaster) Physiol Behav. 1999;66:59–62. doi: 10.1016/s0031-9384(98)00268-6. [DOI] [PubMed] [Google Scholar]
- Demas GE, Polacek KM, Durazzo A, Jasnow AM. Adrenal hormones mediate melatonin-induced increases in aggression in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2004;46:582–591. doi: 10.1016/j.yhbeh.2004.07.001. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Van Leeuwen FW, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Gold L, De Vries GJ, Potegal M. Evidence for a functional and anatomical relationship between the lateral septum and the hypothalamus in the control of flank marking behavior in golden hamsters. J Comp Neurol. 1990;293:476–485. doi: 10.1002/cne.902930310. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke L. Role of estradiol in alcohol intake and alcohol-related behaviors. J Stud Alcohol. 1999;57:162–170. doi: 10.15288/jsa.1996.57.162. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Scordalakes EM, Rissman EF. Estrogen receptor [alpha] influences socially motivated behaviors. Horm Behav. 2002;42:484–491. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2000;38:102–110. doi: 10.1006/hbeh.2000.1604. [DOI] [PubMed] [Google Scholar]
- Joppa MA, Meisel RL, Garber MA. c-fos expression in female hamster brain following sexual and aggressive behaviors. Neuroscience. 1995;68:783–792. doi: 10.1016/0306-4522(95)00179-m. [DOI] [PubMed] [Google Scholar]
- Kelliher KR, Chang YM, Wersinger SR, Baum MJ. Sex difference and testosterone modulation of pheromone-induced neuronal fos in the Ferret’s main olfactory bulb and hypothalamus. Biol Reprod. 1998;59:1454–1463. doi: 10.1095/biolreprod59.6.1454. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patters of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustaffson JA. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein J, De Vries G. Sex differences in the parental behaviour of adult virgin prairie voles: independence from gonadal hormones and vasopressin. J Neuroendocrinol. 1999;11:441–449. doi: 10.1046/j.1365-2826.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Han YR, Grossman H, Karnik MS, Patel D, Scott N, Specht SM, Gandhi CC. Individual differences in the expression of a “General” learning ability in mice. J Neurosci. 2003;23:6423–6433. doi: 10.1523/JNEUROSCI.23-16-06423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, de Bold JF, de Almeida RMM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and γ-aminobutyric acid systems. Psychopharmacology. 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Miller MA, Devries GJ, Alshamma HA, Dorsa DM. Decline of vasopressin immunoreactivity and messenger-RNA levels in the bed nucleus of the stria terminalis following castration. J Neurosci. 1992;12:2881–2887. doi: 10.1523/JNEUROSCI.12-08-02881.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ERβ: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Chiavegatto S. Molecular basis of aggression. Trends Neurosci. 2001;24:713–719. doi: 10.1016/s0166-2236(00)01996-2. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- Newman S. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nomura M, Durbak I, Chan J, Gustafsson JA, Smithies O, Korach KS, Pfaff DW, Ogawa S. Genotype/age interactions on aggressive behavior in gonadally intact estrogen receptor beta knockout (ERKO) male mice. Horm Behav. 2002;41:288–296. doi: 10.1006/hbeh.2002.1773. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci U SA. 1998;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson J, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (βERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Nomura M, Choleris E, Pfaff D. The role of estrogen receptors in the regulation of aggressive behaviors. In: Nelson RJ, editor. Biology of Aggression. Oxford Univ Press; New York: 2005. pp. 231–249. [Google Scholar]
- Parducz A, Perez J, Garcia-Segura L. Estradiol induces plasticity of GABAergic synapses in the hypothalamus. Neuroscience. 1993;53:395–401. doi: 10.1016/0306-4522(93)90203-r. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotoxic Coordinates. 2. Academic Press; New York: 2002. [Google Scholar]
- Pinxten R, De Ridder E, De Cock M, Eens M. Castration does not decrease nonreproductive aggression in yearling male European starlings (Sturnus vulgaris) Horm Behav. 2003;43:394–401. doi: 10.1016/s0018-506x(03)00012-6. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci. 2003;23:4479–4490. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou F, Amara D, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot M, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. Aromatase activity in quail brain: correlation with aggressiveness. Endocrinology. 1989;124:437–443. doi: 10.1210/endo-124-1-437. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. Aromatization mediates aggressive behavior in quail. Gen Comp Endocrinol. 1990;79:39–53. doi: 10.1016/0016-6480(90)90086-2. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression in male mice lacking functional estrogen receptor α. Behav Neurosci. 2003;117:38–45. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly R, Chang C, Muramatsu M, Swanson L. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simon NG. Hormonal processes in the development and expression of aggressive behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain, and Behavior. Academic Press; New York: 2002. pp. 339–392. [Google Scholar]
- Soma KK, Tramontin AD, Wingfield JC. Oestrogen regulates male aggression in the non-breeding season. Proc R Soc Lond, B Biol Sci. 2000;267:1089–1096. doi: 10.1098/rspb.2000.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J Neurobiol. 2003;56:209–221. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepian-drosterone metabolism by 3{beta}-hydroxysteroid dehydrogenase/{Delta} 5-{Delta}4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology. 2004;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male–male aggression in mice deficient for TRP2. Science. 2002;295:1493–1522. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Bird IM, Marler CA. Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm Behav. 2004;45:115–121. doi: 10.1016/j.yhbeh.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: how interactions between aromatase and the environment modulate aggression. Front Neuroendocrinol. doi: 10.1016/j.yfrne.2005.11.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Coolen LM, de Jong TR, Joosten HW, de Doer SF, Koolhaas JM, Olivier B. Do similar neural systems subserve aggressive and sexual behavior in male rats? Insights from c-fos and pharmacological studies. Eur J Pharmacol. 2005;526:226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Wang Z, De Vries G. Testosterone effects on paternal behavior and vasopressin immunoreactive projections in prairie voles (Microtus ochrogaster) Brain Res. 1993;631:156–160. doi: 10.1016/0006-8993(93)91203-5. [DOI] [PubMed] [Google Scholar]
- Wang J, Cheng C, Zhou J, Smith A, Weickert C, Perlman W, Becker K, Powell D, Bondy C. Estradiol alters transcription factor gene expression in primate prefrontal cortex. J Neurosci Res. 2004;76:306–314. doi: 10.1002/jnr.20076. [DOI] [PubMed] [Google Scholar]
- Wersinger S, Ginns E, O’Carroll A, Lolait SYW., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Westberg L, Melke J, Landen M, Nilsson S, Baghaei F, Rosmond R, Jansson M, Holm G, Bjorntorp P, Eriksson E. Association between a dinucleotide repeat polymorphism of the estrogen receptor alpha gene and personality traits in women. Mol Psychiatry. 2003;8:118–122. doi: 10.1038/sj.mp.4001192. [DOI] [PubMed] [Google Scholar]
- White R, Lees JA, Needham M, Ham J, Parker M. Structural organization of the mouse estrogen receptor. Mol Endocrinol. 1987;1:735–744. doi: 10.1210/mend-1-10-735. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamsters. Neuroendocrinology. 1995;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]


