Abstract
Intracellular protein filaments intermediate in size between actin microfilaments and microtubules are composed of a surprising variety of tissue specific proteins commonly interconnected with other filamentous systems for mechanical stability and decorated by a variety of proteins that provide specialized functions. The sequence conservation of the coiled-coil, alpha-helical structure responsible for polymerization into individual 10 nm filaments defines the classification of intermediate filament proteins into a large gene family. Individual filaments further assemble into bundles and branched cytoskeletons visible in the light microscope. However, it is the diversity of the variable terminal domains that likely contributes most to different functions. The search for the functions of intermediate filament proteins has led to discoveries of roles in diseases of the skin, heart, muscle, liver, brain, adipose tissues and even premature aging. The diversity of uses of intermediate filaments as structural elements and scaffolds for organizing the distribution of decorating molecules contrasts with other cytoskeletal elements. This review is an attempt to provide some recollection of how such a diverse field emerged and changed over about 30 years.
Introduction
Intermediate filaments are approximately 10 nm diameter, intermediate in size between actin microfilaments and microtubules. These filaments are coded for by 65 human genes defining five classes of intermediate filament proteins and two more distal related beaded lens filament proteins [1] (Table 1). The first two classes are the type I and II keratins that represent 54 subunits of these obligate heteropolymers that define epithelial tissues and hair [2]. All 28 type I keratins are clustered on human chromosome 17 except for keratin 18 that is located at one end of the type II keratin cluster on chromosome 12. All 26 type II keratins and K18 are located on human chromosome 12. The third class includes the homopolymeric filament proteins vimentin [3], desmin [4], glial fibrillary acidic protein (GFAP) [5] and peripherin [6]. The three neurofilament subunits NF-L, NF-M and NF-H, [7] established the fourth class of IF proteins to which were added nestin [8], alpha internexin [9], syncoilin [10] and synemin [11]. Up to five nuclear lamins, coded for by three genes, compose the nuclear lamina and define the fifth class of IF proteins.
Table 1.
Some Landmark IF Publications
| Class | Members | Examplesa | Identification/ Isolationc | Sequenced | KO/Mutatione |
|---|---|---|---|---|---|
| 1 | K9–K20 | K10 | 1982 [61] [101] | 1984 [182] | 1992 [176] |
| K14 | 1978 [54] | 1982 [58] | 1991 [59] | ||
| K18 | 1980 [69] | 1986 [75] | 1998 [157] | ||
| K19 | 1981 [66] | 1989 [183] | 2000 [148] | ||
| K23–K28 | [184] | ||||
| K31–K40b | [184] | ||||
| 2 | K1–K8 | K1 | 1982 [61] [101] | 1983 [185] | 1992 [176] |
| K5 | 1978 [54] | 1984 [186] | 1992 [172] | ||
| K8 | 1980 [69] | 1985 [77] | 1991 [124] | ||
| K71–K80 | [184] | ||||
| K81–K86b | K86 | [184] | 1997 [187] | ||
| 3 | vimentin | 1978 [50] | 1983 [188] | 1994 [144] | |
| GFAP | 1972 [5] | 1984 [189] | 1996 [146] | ||
| desmin | 1976 [4] | 1982 [64] | 1996 [146] [145] | ||
| peripherin | 1983 [6] | ||||
| 4 | NF-L | 1978 [52] | 1985 [190] | 1997 [149] | |
| NF-M | 1978 [52] | 1986 [104] | 1991, 1998 [122] [150] | ||
| NF-H | 1978 [52] | 1986 [104] | 1998 [39] [40] | ||
| synemin | 1980 [191] | 1995 [192] | |||
| a-internexin | 1985 [193] | 1990 [194] | 1999 [195] | ||
| nestin | 1990 [8] | ||||
| syncoilin | 2001 [10] | ||||
| 5 | Lamin A/C | 1978 [196] | 1986 [115] | 1997 [163] | |
| lamin B1 | 1978 [196] | 1987 [197] | 2001 [161] | ||
| lamin B2 | 1988 [198] | ||||
| other | filensin | 1991 [199] | 2003 [200] | ||
| phakinin | 1993 [201] | 2002 [199] |
Only a few pertinent examples of the keratin classes are shown for brevity.
Hair and root sheath keratins are not included because of space. Pleaser refer to [183] as a starting place for references on these genes.
In most cases these refer to the date of isolation of the indicated protein or definitive identification of unique species.
The primary amino acid sequence of the protein, determined directly or deduced from the cDNA.
Year of publication of the description of either the mouse knockout or the animal or human mutation first associated with the disease.
The history of intermediate filaments (IF) could be considered to have divergent beginnings, a shared common discovery phase and an interesting and divergent future, something like the structure of intermediate filament proteins. This review is a personal, and undoubtedly (but not intentionally) biased review of some of the highlights of discoveries about IFs that has led to our present understanding and continued curiosity about this very large family of proteins.
Over the last 30 years, many reviews of all aspects of IF have been published. Historically, some of the most highly cited have been provided by Lazarides [12], Fuchs and Weber [13] and Steinert and Roop [14] and more recently Fuchs and Cleveland [15]. In addition, reviews of IF proteins in disease [16], the dynamic properties of IF [17, 18], function in neuronal development and disease [19], keratin diseases [20], IF structure [21–23], lamin diseases [24], IF function in Caenorhabditis elegans [25], IF as signaling platforms [26], and this issue of this journal are all highly recommended. One important early review by Elias Lazarides was titled, “Intermediate filaments as mechanical integrators of cellular space” [27]. This phrase cleverly described the dramatic, filamentous patterns of proteins that had not yet been linked to specific molecules or functions. Indeed the identification of proteins that linked the IF networks to the actin network, membrane anchoring platforms and a variety of decorating proteins has provided the proof of this early speculation.
X-rays and sheep ranches of Australia
IFs in the form of specialized hair keratins have been the target of attention even before the invention of science. The advancements of understanding the composition, structure and function of IF are attributable to a significant degree to the application of new tools or methods that stimulated flurries of scientific activity. It was the application of x-ray diffraction and crystal structure theory that led William T Astbury in 1932 to publish the first data of a periodic structure of keratin [28]. The possibility of determining molecular structure of a highly ordered hard keratin polymer was stimulated by the interpretation of alpha keratin diffraction patterns in light of Linus Pauling’s alpha helix model. This led to the prediction of the alpha helical coiled coil structure by Francis Crick in 1952 [29, 30]. The challenge of interpreting the diffraction pattern of alpha-keratin was pursued through the 1990s. However, it was not until individual proteins were purified and reconstituted that the probability of solving the atomic structure of IF was greatly advanced. The problem of the molecular structure of keratins stimulated the attention of physical biochemists including Australians, George Rogers and his student, Peter Steinert, who started as a wool biochemist and spent his career investigating both the physical and biological aspects of keratins and skin until his passing in 2003. It was his discovery of the polymerization of IFs from denatured, soluble keratin by dialysis into lower ionic solutions [31] that provided the physical assay for assessing the protein subunit requirements necessary for filament formation. This permitted the evaluation by electron microscopy of the heteropolymeric requirements of keratins and neurofilaments and the homopolymeric filaments of other types of IFs. In the 54 years since Crick’s prediction of coiled coil structure of keratins, the problem of an atomic model of the polymerization of IFs into defined diameter filaments has remained a fascinating challenge that may be nearing a solution, ironically, by methods that force parts of the filamentous proteins to adopt crystalline packing arrangements [32–34]. While the structure of IF is the defining characteristic of the gene family and the driving force of the evolutionary conservation of primary structure, the IF structure is used in multiple ways such as structural elements or internal scaffolds for the docking of regulatory proteins.
Electron microscopes and Chilean fishing boats
IFs became real to many when they were visualized by electron microscopy. The electron microscope was invented in 1931 and was available commercially in 1939. However methods for preparation of biological specimens for examination in the electron microscope were not commonly embraced until the 1950s with improved preparation methods and scanning technology. With the widespread examination of cells with electron microscopy, it is likely that a number of investigators in the 1960’s saw IFs in different tissues but failed to recognize them as distinct from smaller actin and larger microtubules. Holtzer and colleagues named IFs during their investigations of muscle [35].
The recognition of filamentous proteins in neuronal axons is likely as old as silver staining for light microscopy. However, the recognition of neurofilaments of about 10 nm in diameter may have started in the sea off the coast of Chile where the ferocious Humboldt or jumbo squid, Dosidicus gigas, was harvested. The large size of the squid giant axon provided a source for axoplasm, axon cell contents, that was investigated by electron microscopy and biochemical methods. While the sensitivity of neurofilaments to calcium dependent proteolysis, and differences between human and other species neurofilament proteins muddled the exact protein composition of neurofilaments for several years, clear microscopic evidence was presented for abundant filaments intermediate in size between actin and microtubules [36].
Gene analysis of NF started when Julien cloned the neurofilament subunits [37]. Julien and Cleveland initiated a careful series of gain and loss of function experiments in mice [19]. The abundance of neurofilaments led to the hypothesis that they might control the caliber of axons, a key characteristic that governs signal conduction velocity. Transgenic over expression, targeted gene mutations and domain switching studies confirmed that axon radial growth was dependent on NF, but surprisingly, it was the C-terminal domains of NF-M that were key for receiving signals from the surrounding glial cells that influence radial diameter [38] much more than the very large repeated arrays of phosphorylation sites of the C-terminal domain of NF-H [39–42]. The influence of NF on axon caliber was due to a more subtle mechanism than filling cytoplasmic space. Julien, Cleveland and others observed effects of over expression of NF subunits and mutant forms on neuron health similar to human disease [43–45]. Mutation of the NF-L subunit is associated with Charcot-Marie-Tooth disease [46]. NF mutations also may be a risk factor but not a primary cause of ALS [47].
Discovery and coalescence
While seeing IFs convinced investigators of a third cellular filament system, it was the variety of different IF proteins, in contrast to microfilament and microtubules, that stimulated great attention. The many unique proteins responsible for apparently similar sized filaments focused the efforts of many investigators throughout the 1970s and early 1980s. Glial fibrillary acidic protein (GFAP) was the first IF protein to be recognized by a specific antibody [5] and to be used as a reliable marker of glial and astrocyte cell identity. While GFAP was considered a reliable marker of astrocytes, current investigations have implicated GFAP positive cells as candidate source of multipotent neuronal derivatives [48]. Indeed, the use of IF proteins as markers of specific types of cells is arguably the most important, and certainly the most common, application of IF research. Lazarides and Weber, two major figures of IF discovery preceded their IF research with studies of the actin cytoskeleton using specific antibodies to reveal actin filament pattern in cells [49]. It was eight years after Holtzer’s recognition of muscle IF by electron microscopy before the responsible muscle protein, desmin, was purified [4]. Purifying an IF protein, generating specific antibodies and demonstrating its capability to form IF became the essential paradigm.
1978 was a good year for IF. Hynes’ laboratory purified the fibroblast 58kD IF protein, generated distinguishing antibodies and contrasted it with the behavior of actin and microtubule networks [50]. However, it was Franke and colleagues who also purified it and further named the fibroblast IF protein vimentin later the same year [51]. The productive collaborations between Franke, Osborn and Weber demonstrated the power of cell type specific IF antibody tools [51]. In addition, Liem and colleagues [52] resolved ambiguity about the sizes of neurofilament subunits and GFAP. By the end of 1978 the major IF proteins, neurofilaments, GFAP, desmin, vimentin and keratins were all recognized, though sorting out the many keratins and the interaction of the three neurofilament subunits had just begun.
In contrast to the use of purified protein antigens, at least two sets of investigators, separated by the Atlantic Ocean, recognized the implications of cross reaction of keratin antisera with a variety of different cultured epithelial cells and tissues. Howard Green at MIT headed the first group and Werner Franke the second. Green, the inventor of 3T3 cells, was responsible for determining the conditions that permitted the cultivation and differentiation of human epidermal keratinocytes. Sun and Green used the cross reaction of human epidermal keratin antibody with a variety of cultured epithelial cell types to identify intracellular keratins and later contributed widely used, broadly reactive, monoclonal keratin antibodies [53]. The culture system permitted the analysis of the requirements for proliferation and differentiation of keratinocytes. This discovery eventually also resulted in the production of keratinocytes for patient transplantation.
The groups in Boston and Heidelberg took different and complementary investigative strategies on keratins. In Green’s laboratory, Elaine Fuchs with the initial help of Don Cleveland adopted a molecular biology paradigm to sort out the epidermal keratin proteins by cloning the RNAs and genes, and thus providing definitive criteria of identity. Remarkable progress was made in defining the molecular changes of keratin proteins, RNAs and genes [54–58], that set the stage for discovering the molecular basis for the first genetic disease caused by mutations in intermediate filament proteins. The family tree of major contributors on keratin IF continued from the Green lab to the Fuchs laboratory and then to Pierre Coulombe, who continued to explore the physical and functional roles of keratins [16, 18, 59].
Mean while, Werner Franke and Klaus Weber recognized that the cross reaction of multiple keratin antibodies with epithelial cells must reflect significant evolutionary and structural conservation of a family of cytoskeletal proteins related to hard keratins [60]. Franke’s group surveyed the expression of keratin proteins in an amazing diversity of cell types, animals, developmental and pathological states, using two dimensional gel analysis of proteins and a variety of antibodies to eventually produce a catalog of the expression patterns of keratin proteins that may be the most highly cited IF paper [61]. These comparative studies demonstrated a clear distinction between keratins of simple or single layered, epithelial tissues, multilayered or squamous epithelia and hair or hard keratins. Further recognition of the two major protein groups of acidic (Type I) and basic (Type II) keratin subunits and the requirement for at least one of each type to reconstitute 10 nm filaments [31, 62] fit well with the two gene families identified by Fuchs and coworkers [63]. In 1982 Weber discovered the IF tripartite structure of a central alpha-helical domain flanked by non-helical terminal domains by sequencing the desmin protein [64]. From 1978 to 1988, Franke published 112 papers on keratins as well as more than that number on other topics. During this period, the Franke laboratory produced many current, independent IF investigators including Jose Jorcano, Harald Herrmann, Thomas Magin, Rudolf Leube and Roland Moll.
IF and stem cells
In the 1970s and early 1980s, the paths to IFs merged from keratin molecular structure (Steinert, Aebi), epidermal biology (Green, Fuchs, and Roop) and the cytoskeleton (Lazarides, Weber, Franke, Goldman). However, additional investigators arrived at IF via studies of early embryonic development. To generate markers of the earliest differentiated cell types of the mouse embryo, Brulet, Kemler and Jacob made monoclonal antibodies against undifferentiated teratocarcinoma cells and cytoskeletal fractions of differentiated derivatives [65, 66]. Oshima purified mouse K8 (Endo A, cytokeratin A) and K18 (EndoB, cytokeratin D) as markers of the differentiation of mouse embryonal carcinoma cells and in combination with two-dimensional gel electrophoresis found that these proteins were the antigens for two of the monoclonal antibodies prepared by Kemler [67, 68].
The results of the laboratories of Franke, Jacob and Oshima identified the keratin subunits of reactive filaments in the trophectoderm of preimplantation mouse embryos and extraembryonic endoderm [66, 69–73]. Subsequent cloning of the RNAs and genes for both human and mouse K8 and K18 provided tools to investigate the transcriptional regulation of these genes during early teratocarcinoma stem cell differentiation and embryo development [74–82]. However, while the dispersed regulatory elements of the K18 gene has complicated its use as an epithelial specific vector [83, 84], the promoters of other IF genes have been used extensively to drive expression of genes in epidermis [85–90]. A review of the transcriptional regulation of IF genes will need to be a future opportunity.
Nestin, an IF characteristic of neuronal stem cells was discovered by Mckay’s laboratory [8]. This has become a particular important marker used in human ES cell differentiation, neuronal stem cell studies and wound healing [91]. Furthermore, the relatively compact promoter has been used to generate reliable reporter genes in cells and mice [92]. Very recently, a function of nestin as a modulator of survival signaling by titration of Cdk5 has been shown [93]. Both nestin and K8/K18 may utilize the IF structure to sequester regulatory proteins and thus modulate signaling pathways.
IF and pathology
The development and widespread availability of specific IF protein antibodies permitted investigators to query different cells, tissues and states with new specific tools and resulted in a 50-fold increase in the number of annual publications on IFs from 1979 to 1989 (Figure 1). Both Franke and Weber recognized the power of these tools in clinical diagnostics [94–96]. However it was the tool of monoclonal antibodies that provided the opportunity for both biologists and pathologists to reliably identify the same antigens in different tissues or states. Early useful monoclonal antibodies were generated by the laboratories of Kemler [66] Franke and Weber [97], Lane [98, 99], Ramaekers [100], Sun [101] and others. The application of these reagents permitted a degree of standardization that moved into the standard clinical pathology laboratory. The important applications of these and subsequent generations of antibodies aided in the immunocytochemical diagnosis of the tissues and tumors, an application that was recognized as early as 1979 [102]. One key characteristic of keratins and other IF that makes them useful in pathology is the relative stability of expression even after transformation to pathological states. The continued expression of characteristic keratin expression in carcinomas must reflect either an unusually stable molecular mechanism of transcriptional control or a strong selective advantage for continued expression.
Figure 1.
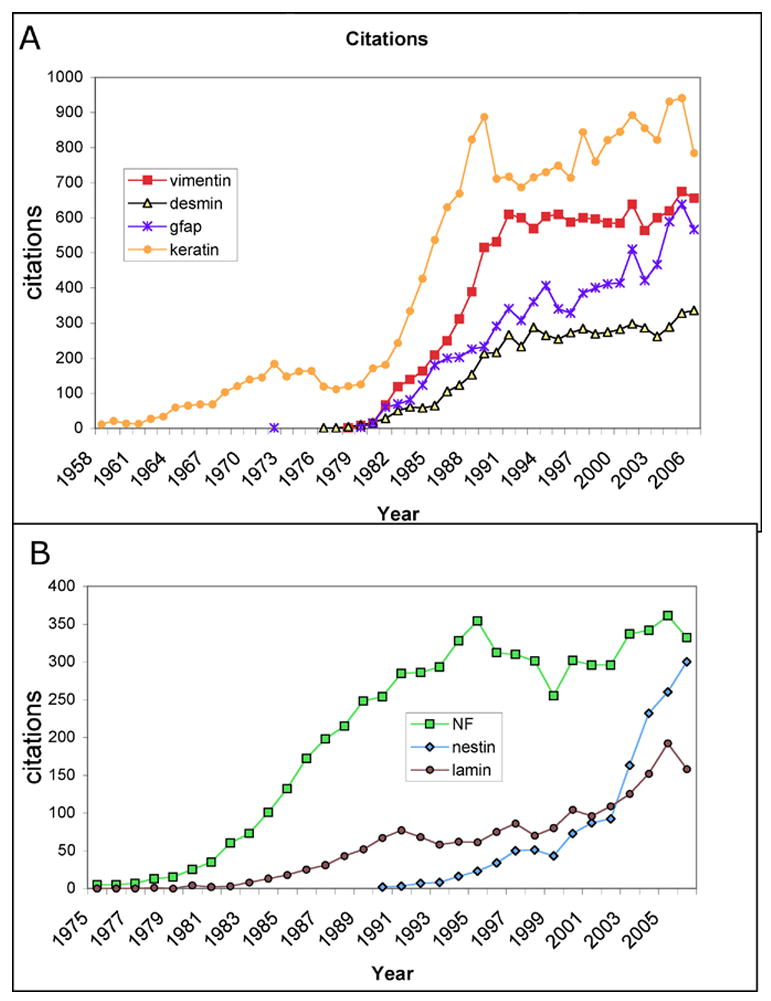
Intermediate filament publications. The PubMed database was searched with the indicated key word and year. A, keratin, vimentin, desmin and glial fibrillary acidic protein (GFAP). B, nestin, neurofilament forms (NF) and lamin citations are shown.
While IF staining provides pathologists with tools to distinguish tumor and cell types, another application is the recognition of keratin fragments and peptides in the circulation of cancer patients. Weber and colleagues were responsible for recognizing the antigenic relationship of circulating tissue polypeptide antigen and the degradation products of simple epithelial keratins [103]. Nearly all of the antibodies found to be useful for monitoring cancers through patient sera have proven to recognize epitopes of K8, K18 and K19 [104]. The origin of keratin fragments in the circulation is the proteolytic cleavage of tumor cell keratins. The release of keratin fragments into the circulation may reflect an escape from the normally orderly disposal cell contents during apoptosis.
IF and cell death
In dying cells IFs might be considered toxic waste because misfolded proteins and protein aggregates have been implicated in many diseases such as Alzheimer’s and Huntington’s disease, human genetic diseases of epidermal keratins and Mallory bodies in alcoholic liver disease. The path to disposal of IF during programmed cell death was first discovered when lamins A, B and C were identified as substrates of the caspase proteases, activated by apoptosis [105, 106]. Comparison of the protein sequence of the cleavage sites of lamins to other IF revealed that K18 was a potential substrate of caspase cleavage. This prediction was verified [107]. The exact sequence of the second caspase cleavage site of K18 was also identified [108] and cleavage of the second site generates a neo-epitope for a monoclonal antibody now widely used to detect apoptotic epithelial cells[109]. The central cleavage site of K18 is within the linker region between the coiled coil 1B and 2A regions and is conserved in Type I keratins, except for those with extracellular protective functions in the epidermis (K9 and K10) and in Type III IF [110, 111] and in lamins [103,104]. However, the orderly degradation of the keratin cytoskeleton is preceded by the cleavage of plectin, a cytoskeletal cross linking protein, by caspase 8 [112] and is also regulated by the adaptor protein DEDD that associates with both keratins and caspase before they are cleaved [113].
The origins of IF discovered
The similarity of different IF proteins was highlighted in 1981 by the discovery of an antigenic epitope shared by all IF proteins and recognized by a single monoclonal antibody [114]. The protein sequencing of wool keratins and desmin, and the cloning and sequencing of IF mRNAs starting in 1981 [57] revealed the conserved central coiled coil l 1A, 1B and 2A, 2B rod domains [64]. Subsequent cDNA sequences of other IFs and exon gene structure facilitated evolutionary studies. In 1986, McKeon discovered that the nuclear lamins were members of the IF superfamily but contained extended helical segments not found in other IF [115]. The extended helical segments of the nuclear lamins were found in IFs for invertebrates suggesting an evolutionary path from nuclear lamins to cytoplasmic IFs [116]. By examining the existence and structure of IF in a variety of different animals, Weber continues to provide compelling insight into evolutionary appearance, divergence and functional specialization of IFs. Identification of 11 IF genes, 5 of which are uniquely essential, in Caenorhabditis elegans [117] and 65 coding genes in the human genome [1] contrasts with the absence of cytoplasmic IF in Drosophila [25].
The function of Ifs
The exquisite IF patterns observed within cells is a reflection of the interactions of individual filaments, sometimes in dramatically regular patterns such as the tonofilament bundles found for keratins or as the spherical mesh for the nuclear lamina [118]. The great abundance of IF in certain tissues such as neuronal axons and in epidermal differentiating keratinocytes led logically to the expectation that IFs have a structural function. However, proving this idea was harder than expected. Vimentin filaments patterns could be disrupted by the microinjection of vimentin antibody [119]. However, little affect of the disruption on cell appearance or behavior was evident. Furthermore, intracellular antibody-mediated disruption of keratins did not lead to an obvious change of cell behavior [119, 120]. This was a surprise, and contrasted with interfering with either actin or microtubule organization. The first disruption of IF in a multicellular embryo was obtained by injecting keratin antibody into one of two cells of the two-cell stage mouse embryo and examining the development of the blastocyst in culture [121]. Disruption of keratin filaments had no discernible effect on blastocyst formation including the keratin containing trophoblast outer layer of cells.
The first deficiency of an IF in an adult organism was reported in a spontaneous mutant quail that lacked neurofilaments. While these birds were viable, they developed neurological deficiencies including decreased axon caliber [122, 123]. With the development of gene targeting methods it became possible to genetically knockout the expression of IF genes and thus eliminate the lingering concern about residual IF protein present when antibodies disrupted organization of IFs. The first IF gene to be inactivated by gene targeting technology was mouse K8 [124]. K8 deficient ES cells differentiated normally in culture to polarized yolk sac extraembryonic endoderm. In the absence of K8 nearly all K18 also disappeared due to the rapid degradation of excess protein subunit [125]. Most K8 deficient mice died, although some could survive without K8 [126]. Embryonic edema was initially interpreted as a possible structural defect of the liver. However, many genes that affect placental function present with similar, hypoxic phenotypes [127]. The rescue of K8 deficient mice by aggregation with tetraploid embryos capable only of extra-embryonic development, proved that K8 deficient mice died due to placental deficiency, not because of embryonic defects [128]. Two interpretations of this placental defect have been proposed. Based on histological grounds, the placental hematoma formation caused by the loss of both K18 and K19 (that is equivalent to the loss of K8, the major complementary Type II subunit) was interpreted as due to trophoblast fragility [129]. Based on increased sensitivity of some K8 deficient epithelial cell lines and liver to apoptosis induced by TNF or Fas [130, 131], increased sensitivity of K8 deficient placenta to activation of the maternal immune system by concanavalin A [128] and the sensitivity of K8 deficient embryo survival on maternally expressed TNF and TNFR2 [128], K8/K18 were proposed to provide protection of trophoblast cells from maternal immune system dependent, apoptotic challenges. One possible mechanisms is due to K18 titration of TRADD, a TNFR adaptor protein [132]. Recently K17 has also been implicated in resistance to TNF killing, perhaps by sequestering TRADD, an adaptor protein, for the TNF R1 and also down regulating Flip, an inhibitor of caspase 8 [133]. Post-translational regulation of Flip, was first reported in K8 deficient mice [134] but was not confirmed with different antibodies [135]. A protective role of K8 was also revealed in liver disease [136]. Omary later showed that K18 mutations were a risk factor for liver disease in humans [137]. The increased sensitivity of K8 deficient hepatocytes to manipulation [138] and the fragility of hepatocytes over expressing a mutant K18 [139] were interpreted as reflections of structural functions for K8/K18. It is possible that these filament proteins may have both structural and regulatory functions.
The survival of K8 deficient mice to birth was dependent on modifier genes that differ between C57Bl6 and FVB/N mice. Only about half of the expected K8 null mice died before birth in the FVB/N background. Thus K8 is important, but not absolutely essential for placental function. Again this was a surprising result as K8 was the only type II keratin of hepatocytes and was the dominant component in pancreas. However, just like the neurofilament deficient quail, K8 deficient mice proved not to be completely “normal”, as closer inspection revealed bacteria-dependent colonic hyperplasia [140, 141], hypersensitivity of the liver to stress [135, 136, 138] and alterations in intestinal epithelial membrane proteins [142] [143].
Soon joining the ‘escaper” K8 deficient mice, were viable mice deficient in vimentin [144], desmin [145] [146], GFAP [147], K19 [148], NF-L [149], NF-M [150] and NF-H [40, 41, 151]. However, while all of these IF deficient mice were viable, all also revealed phenotypes found either by testing during an appropriate condition or in combination with other deficiencies. For example, desmin deficient mice developed muscle and cardiac defects [145, 146] that were due to decreased myofibril strength [152]. Desmin mutations causing similar conditions in humans were found in 2000 [153]. Vimentin deficient mice were apparently normal [144] but defects in wound healing [154] and endothelial cell stability have recently been discovered [155].
The subtle phenotypes of some single IF knockout mice were due to compensatory functions of other family members. The combination of vimentin deficiency and GFAP deficiency revealed alterations in response to brain injury [156]. In the case of the simple epithelial keratins, both K18 and K19 deficient mice are viable but the combination is lethal [129]. Similarly the combination of K8 and K19 deficiencies increased the penetrance and decreased the time before lethality [148], apparently due to partial protection by low levels of K7 and its preferential polymerization with K18 [157]. Recently, the regulatory role of K17 in cell size [158], K8 phosphorylation in susceptibility to apoptosis [135], and the importance of NF-M in neurons [19] are a few examples of the functions of IF that were not immediately evident in knockout mice. One illustrative test of similar functions for individual members of the keratin family was the attempt to complement K14 deficiency with K18 [159]. The failure of K18 to complement K14 deficiency suggests a different, or at least specialized functions of K18 and K14 just as ectopic expression of desmin filaments in K5 deficient animals failed to complement the skin defects [160]. The specific functions of each of the different types of intermediate filaments are discussed in the accompanying articles. However, clearly, from a historical perspective, expectations that IFs would have similar functions in different tissues, or that IFs would have universal functions, analogous to cellular functions of microfilaments or microtubules required re-evaluation.
The function of nuclear lamins is an example of IF with both structural and regulatory functions. While lamin B1 and/or B2 composed the nuclear lamina of all mammalian cells and are essential for viability [161] mutations in the LMNA gene that generates lamins A and C are responsible for a great variety and number of genetic diseases [16, 24]. Nuclei of lamin A deficient or mutant cells have nuclear structure alterations, increased fragility and decreased mechanical stiffness [162–164] but mutations in lamin A also result in alterations in tissue specific gene expression most likely due to the association of the nuclear lamina with chromatin and specific transcription factors [165–167]. Lamin A/C may be important for the perinuculear location of genes that facilitates gene silencing. [168]. The C-terminal, non alpha-helical region of lamin has multiple interaction domains including a terminal, specialized membrane attachment region. Muations that interfere with the processing of the farneslylated CAAX-box cause premature aging, the Hutchison-Gilford progeria syndrome. Thus specialized functions of lamin A reside in the unique domains flanking the helical domains shared with other IF proteins.
If individual IFs have unique functions, filament organization disruption strategies may have different outcomes. For example, the binding of proteins such as Cdk5 [93], Jnk [169] or 14-3-3 [170, 171] to IF might still occur in cells with disorganized filaments but not in cells with none of the protein. However, disorganization of filament organization that primarily provides structural integrity may cause structural failure or cytolysis as occurs with disruption of K14 or K10. Over expression studies of specific mutant protein forms have been revealing in the study of IF regulatory functions, for example, in the titration of regulatory molecules by competitive binding [135].
Human disease and intermediate filaments
The discovery of human diseases that are caused by mutations in IF genes proved the relevance of the basic research efforts and provided evidence for structural functions for epidermal keratins. The first disease of IFs was epidermolysis bullosa simplex (EBS), a rare genetic skin disease characterized by blistering of the skin. The blisters arise due to cytolysis of the basal layer of cells that have disorganized keratin tonofilaments, beneath the more differentiated skin cells. The identification of EBS patients with point mutations in the K14 [59] and K5 [172] genes was preceded by the results of expressing normal and mutant K14 in transgenic mice [173, 174] that had striking resemblance to the human disease. This disease was also observed in a patient with a complete absence of K14 [175], equivalent to a targeted gene knockout. Mutations within keratin 1 and 10 were discovered as the basis of another genetic disease, epidermolytic hyperkeratosis that led to fragility in the upper layers of the skin [176–178]. Subsequently disease-causing or associated mutations have been described in most IF genes of all five classes [16].
More recently laminopathies composed of at least 12 different diseases have been defined by over a hundred individual mutations of the LMNA gene [24, 179]. These diseases affect muscle, adipose, bone, nerve and skin and even the aging process and have provided an entree for understanding the underlying molecular interactions of lamins. Furthermore, the dispensability of lamin A, when lamin C is present provides some hope for treatment by suppression of mutant lamin A expression. [180]. When combined with the near universal use of IF antibodies for diagnosis and the expanding use in stem cell research, it is evident that research investment in intermediate filaments has yielded an excellent return in both health impact, conceptual expansion and independent investigators (Figure 2).
Figure 2. Participants of a research conference on intermediate filaments, July 1992.

1 R. Liem; 2 K. Green; 3 R. Linck; 4 H. Pant; 5 I. Carey; 6 B. McCormick; 7 R. Miller; 8 M. Kiurpakus; 9 K. Albers; 10 P. Opal; 11 J. Erikson; 12 R. Evans; 13 O. Skalli; 14 P. Hornbeck; 15 D. Sun; 16 T. Letai; 17 T. Hashimoto; 18 s. Chin; 19 Y. Chan; 20 S.C. Lee; 21 T. Frappier; 22 C. Sommers; 23 M.K. Lee; 24 R. Presland; 25 M. Monteiro; 26 Z. Xu; 27 J. Rothnagel; 28 E. Glasgow; 29 B. Lu; 30 M. Schecter; 31 M Carden; 32 a. Eckect; 33 B. Druger; 34 H. Herrmann; 35 J. Vickers; 36 A. Trejo; 37 I. Nicholl; 38 E. Rugg; 39 K. Straube; 40 J. Eyer; 41 H. Baribault; 42 U. Aebi; 43 R. Moir; 44 J.M. Navarro; 45 T. Stappenbeck; 46 J.M. Paramio; 47 J. harris; 48 J. Cholberg; 49 R. J. Williams; 50 R. Wu; 51 P. Coulombe; 52 M. Manabe; 53 J.s. Gordon; 54 M. O’Guin; 55 S.Y. Kim; 56 L. Hutton; 57 L. Marekov; 58 Y. Captetanki; 59 J.M. Yang; 60 A. Goldman; 61 D. Paulin; 62 F. Gounari; 63 Y.H. Chou; 64 W. Geraros; 65 J. Nash; 66 W.J. Chen; 67 N. Stuurman; 68 M.M. Portier; 69 F. Landon; 70 K. Yoneda; 71 J Compton; 72 I.G. Kim; 73 D. Mischke; 74 B. Korge; 75 D. Parry; 76 H. Boemendal; 77 M. Sanders; 78 S.S. Lim; 79 G. Ghing; 80 C. CChien; 81 M. Hatzfeld; 82 I. Freedbery; 83 M. Klymkowsky; 84 J. Jones; 85 E. Gardner; 86 K. Laur; 87 A. Steven; 88 P. Steinert; 89 P. Hoffman; 90 N. Jorplawa; 91 T. Belecky-Adams; 92 L. Parysek; 93 G. Wiche; 94 E. Nigg; 95 N. Markova; 96 T. Trevor; 97 B. Lane; 98 D. Roop; 99 B. Dale; 100 I. Makarova; 101 R. Goldman; 102 E. Fuchs; 103 S. Khan; 104 D. Cleveland; 105 W. Ip; 106 R. Oshima; 107 F. McKeon; 108 Z. Zehner; 109 T. Sun; 110 G. Shaw; 111 R. Quinlan; 112 E. White; 113 F. Van de Kluder; 114 S. Georgatos.
Back to the Future
What does the future of IF research hold?
An important question is the pathological consequences of IF protein aggregates. Intracellular inclusions of IF proteins are associated with IF diseases of the liver, muscle and brain. While Mallory body formation may not be necessary for increased toxicity of certain liver disease models [136], protein aggregation and/or precipitation has been implicated in many different human diseases. Even an imbalance of expression of complementary keratin subunits may place a heavy challenge on a cells ability to degrade and dispose of dangerously insoluble proteins. The responses of cells to such material may sensitize them to additional stress. Understanding the contributions of direct functions, such as titrating regulatory proteins or providing strength and secondary responses to imbalanced or aggregated IF proteins will be key to understanding IF associated disease states.
The structure of IFs at atomic resolution still remains to be solved and the determinants of multi-filament organization and branching are of great interest. The roles of IFs in modulating intracellular signaling, both apoptotic and kinase stimulated pathways will likely lead to molecular explanations of additional roles. Finally, the molecular basis of the tissue specific transcriptional regulation of many IF genes remains to be determined. For example, the simple epithelial keratins K8, K18 and K19 were isolated as differentiation markers of pre-implantation embryos and teratocarcinoma stem cells. Human ES cells like human embryonal carcinoma cells [181] and unlike mouse ES cells, express abundant keratin filament RNAs and proteins. (data not shown). The transcriptional regulatory determinants of the differences of mouse and human ES cells are not known.
Great progress in both associating IF gene mutations with human disease and discovering non-structural functions of specific IFs appears to have stimulated renewed interest and potential for an exciting future.
Acknowledgments
I am grateful to my many colleagues over the years and to the editors of this special journal issue for their diligence, expertise and patience. I apologize to my many colleagues whom I have not credited sufficiently because of the breadth of the current field, the objective of personal historical context and limited space and time.
Abbreviations
- GFAP
glial gibrillary acidic protein
- NF
neurofilament
- NF-L
neurofilament-low molecular mass protein
- NF-M
neurofilament-medium molecular mass protein
- NF-H
neurofilament-high molecular mass protein
- IF
intermediate filament
- ALS
amyotrophic lateral sclerosis
- ES
embryonic stem
- Cdk5
cyclin-dependent kinase 5
- DEDD
death effector domain containing
- TRADD
tumor necrosis factor receptor type 1-associated death domain protein
- TNFR
tumor necrosis factor receptor
- LMNA
lamin A gene
- EBS
epidermolysis bullosa simplex
- Flip
Flice-like inhibitory protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hesse M, Magin TM, Weber K. Genes for intermediate filament proteins and the draft sequence of the human genome: novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J Cell Sci. 2001;114:2569–75. doi: 10.1242/jcs.114.14.2569. [DOI] [PubMed] [Google Scholar]
- 2.Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–74. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franke WW, Schmid E, Winter S, Osborn M, Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979;123:25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- 4.Lazarides E, Hubbard BD. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A. 1976;73:4344–8. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–35. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 6.Portier MM, de Nechaud B, Gros F. Peripherin, a new member of the intermediate filament protein family. Dev Neurosci. 1983;6:335–44. doi: 10.1159/000112360. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman PN, Lasek RJ. The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol. 1975;66:351–66. doi: 10.1083/jcb.66.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lendahl U, Zimmerman LB, McKay RDG. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan MP, Chin SS, Fliegner KH, Liem RK. Alpha-internexin, a novel neuronal intermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing rat brain. J Neurosci. 1990;10:2735–48. doi: 10.1523/JNEUROSCI.10-08-02735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newey SE, Howman EV, Ponting CP, Benson MA, Nawrotzki R, Loh NY, Davies KE, Blake DJ. Syncoilin, a novel member of the intermediate filament superfamily that interacts with alpha-dystrobrevin in skeletal muscle. J Biol Chem. 2001;276:6645–55. doi: 10.1074/jbc.M008305200. [DOI] [PubMed] [Google Scholar]
- 11.Bellin RM, Sernett SW, Becker B, Ip W, Huiatt TW, Robson RM. Molecular characteristics and interactions of the intermediate filament protein synemin. Interactions with alpha-actinin may anchor synemin-containing heterofilaments. J Biol Chem. 1999;274:29493–9. doi: 10.1074/jbc.274.41.29493. [DOI] [PubMed] [Google Scholar]
- 12.Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annual Review of Biochemistry. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs E, Weber K. Intermediate Filaments: structure, dynamics, function, and disease. Annual Review of Biochemistry. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 14.Steinert PM, Roop DR. Molecular and cellular biology of intermediate filaments. Annual Review of Biochemistry. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- 16.Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- 17.Helfand BT, Chang L, Goldman RD. The dynamic and motile properties of intermediate filaments. Annu Rev Cell Dev Biol. 2003;19:445–67. doi: 10.1146/annurev.cellbio.19.111401.092306. [DOI] [PubMed] [Google Scholar]
- 18.Coulombe PA, Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol. 2004;6:699–706. doi: 10.1038/ncb0804-699. [DOI] [PubMed] [Google Scholar]
- 19.Lariviere RC, Julien JP. Functions of intermediate filaments in neuronal development and disease. J Neurobiol. 2004;58:131–48. doi: 10.1002/neu.10270. [DOI] [PubMed] [Google Scholar]
- 20.Porter RM, Lane EB. Phenotypes, genotypes and their contribution to understanding keratin function. Trends Genet. 2003;19:278–85. doi: 10.1016/s0168-9525(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 21.Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Current Opinion in Cell Biology. 2002;14:110–122. doi: 10.1016/s0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann H, Hesse M, Reichenzeller M, Aebi U, Magin TM. Functional complexity of intermediate filament cytoskeletons: from structure to assembly to gene ablation. Int Rev Cytol. 2003;223:83–175. doi: 10.1016/s0074-7696(05)23003-6. [DOI] [PubMed] [Google Scholar]
- 23.Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu Rev Biochem. 2004;73:749–89. doi: 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- 24.Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat Rev Genet. 2006;7:940–52. doi: 10.1038/nrg1906. [DOI] [PubMed] [Google Scholar]
- 25.Goldman RD. Worms reveal essential functions for intermediate filaments. Proc Natl Acad Sci U S A. 2001;98:7659–61. doi: 10.1073/pnas.151247898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pallari HM, Eriksson JE. Intermediate filaments as signaling platforms. Sci STKE. 2006;2006:pe53. doi: 10.1126/stke.3662006pe53. [DOI] [PubMed] [Google Scholar]
- 27.Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980;283:249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- 28.Astbury WT, Street A. X-Ray studies of the structure of hair, wool, and related fibres. Philosophical Transactions of the Royal Society of London. 1932;230:75–101. [Google Scholar]
- 29.Crick FH. Is alpha-keratin a coiled coil? Nature. 1952;170:882–3. doi: 10.1038/170882b0. [DOI] [PubMed] [Google Scholar]
- 30.Crick FH. The packing of a-helices: simple coiled-coils. Acta Cryst. 1953;6:689–697. [Google Scholar]
- 31.Steinert PM, Idler WW, Zimmerman SB. Self-assembly of bovine keratin filaments in vitro. J Mol Biol. 1976;108:547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- 32.Strelkov SV, Herrmann H, Geisler N, Wedig T, Zimbelmann R, Aebi U, Burkhard P. Conserved segments 1A and 2B of the intermediate filament dimer: their atomic structures and role in filament assembly. EMBO J. 2002;21:1255–66. doi: 10.1093/emboj/21.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann H, Strelkov SV, Feja B, Rogers KR, Brettel M, Lustig A, Haner M, Parry DA, Steinert PM, Burkhard P, Aebi U. The intermediate filament protein consensus motif of helix 2B: its atomic structure and contribution to assembly. J Mol Biol. 2000;298:817–832. doi: 10.1006/jmbi.2000.3719. [DOI] [PubMed] [Google Scholar]
- 34.Strelkov SV, Schumacher J, Burkhard P, Aebi U, Herrmann H. Crystal structure of the human lamin A coil 2B dimer: implications for the head-to-tail association of nuclear lamins. J Mol Biol. 2004;343:1067–80. doi: 10.1016/j.jmb.2004.08.093. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa H, Bischoff R, Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968;38:538–55. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huneeus FC, Davison PF. Fibrillar proteins from squid axons. I. Neurofilament protein. J Mol Biol. 1970;52:415–28. doi: 10.1016/0022-2836(70)90410-9. [DOI] [PubMed] [Google Scholar]
- 37.Julien JP, Meyer D, Flavell D, Hurst J, Grosveld F. Cloning and developmental expression of the murine neurofilament gene family. Brain Res. 1986;387:243–50. doi: 10.1016/0169-328x(86)90030-6. [DOI] [PubMed] [Google Scholar]
- 38.Garcia ML, Lobsiger CS, Shah SB, Deerinck TJ, Crum J, Young D, Ward CM, Crawford TO, Gotow T, Uchiyama Y, Ellisman MH, Calcutt NA, Cleveland DW. NF-M is an essential target for the myelin-directed "outside-in" signaling cascade that mediates radial axonal growth. J Cell Biol. 2003;163:1011–20. doi: 10.1083/jcb.200308159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacomy H, Zhu Q, Couillard-Despres S, Beaulieu JM, Julien JP. Disruption of type IV intermediate filament network in mice lacking the neurofilament medium and heavy subunits. J Neurochem. 1999;73:972–84. doi: 10.1046/j.1471-4159.1999.0730972.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Q, Lindenbaum M, Levavasseur F, Jacomy H, Julien JP. Disruption of the NF-H gene increases axonal microtubule content and velocity of neurofilament transport: relief of axonopathy resulting from the toxin beta, beta′-iminodipropionitrile. J Cell Biol. 1998;143:183–93. doi: 10.1083/jcb.143.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao MV, Houseweart MK, Williamson TL, Crawford TO, Folmer J, Cleveland DW. Neurofilament-dependent radial growth of motor axons and axonal organization of neurofilaments does not require the neurofilament heavy subunit (NF-H) or its phosphorylation. J Cell Biol. 1998;143:171–81. doi: 10.1083/jcb.143.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirokawa N, Takeda S. Gene targeting studies begin to reveal the function of neurofilament proteins. J Cell Biol. 1998;143:1–4. doi: 10.1083/jcb.143.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eyer J, Peterson A. Neurofilament-deficient axons and perikaryal aggregates in viable transgenic mice expressing a neurofilament-beta-galactosidase fusion protein. Neuron. 1994;12:389–405. doi: 10.1016/0896-6273(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 44.Xu Z, Cork LC, Griffin JW, Cleveland DW. Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell. 1993;73:23–33. doi: 10.1016/0092-8674(93)90157-l. [DOI] [PubMed] [Google Scholar]
- 45.Cote F, Collard JF, Julien JP. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of Amyotrophic Lateral Sclerosis. Cell. 1993;73:35–46. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- 46.Mersiyanova IV, Perepelov AV, Polyakov AV, Sitnikov VF, Dadali EL, Oparin RB, Petrin AN, Evgrafov OV. A new variant of Charcot-Marie-Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Am J Hum Genet. 2000;67:37–46. doi: 10.1086/302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia ML, Singleton AB, Hernandez D, Ward CM, Evey C, Sapp PA, Hardy J, Brown RH, Jr, Cleveland DW. Mutations in neurofilament genes are not a significant primary cause of non-SOD1-mediated amyotrophic lateral sclerosis. Neurobiol Dis. 2006;21:102–9. doi: 10.1016/j.nbd.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 49.Lazarides E, Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974;71:2268–72. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hynes RO, Destree AT. 10 nm filaments in normal and transformed cells. Cell. 1978;13:151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- 51.Franke WW, Schmid E, Osborn M, Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Nat Acad Sci USA. 1978;75:5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liem RK, Yen SH, Salomon GD, Shelanski ML. Intermediate filaments in nervous tissues. J Cell Biol. 1978;79:637–45. doi: 10.1083/jcb.79.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun TT, Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978;14:469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- 54.Fuchs E, Green H. The expression of keratin genes in epidermis and cultured epidermal cells. Cell. 1978;15:887–897. doi: 10.1016/0092-8674(78)90273-8. [DOI] [PubMed] [Google Scholar]
- 55.Fuchs E, Green H. Multiple keratins of cultured human epidermal cells are translated from different mRNA molecules. Cell. 1979;17:573–582. doi: 10.1016/0092-8674(79)90265-4. [DOI] [PubMed] [Google Scholar]
- 56.Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- 57.Fuchs EV, Coppock SM, Green H, Cleveland DW. Two distinct classes of keratin genes and their evolutionary significance. Cell. 1981;27:75–84. doi: 10.1016/0092-8674(81)90362-7. [DOI] [PubMed] [Google Scholar]
- 58.Hanukoglu I, Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982;31:243–252. doi: 10.1016/0092-8674(82)90424-x. [DOI] [PubMed] [Google Scholar]
- 59.Coulombe PA, Hutton ME, Letai A, Hebert A, Paller AS, Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991;66:1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- 60.Franke WW, Weber K, Osborn M, Schmid E, Freudenstein C. Antibody to prekeratin: decoration of tonofilament-like arrays in various cells of epithelial character. Exp Cell Res. 1978;116:429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- 61.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 62.Hatzfeld M, Franke WW. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol. 1985;101:1826–1841. doi: 10.1083/jcb.101.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim KH, Rheinwald JG, Fuchs EV. Tissue specificity of epithelial keratins: differential expression of mRNAs from two multigene families. Mol Cell Biol. 1983;3:495–502. doi: 10.1128/mcb.3.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geisler N, Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO Journal. 1982;1:1649–1656. doi: 10.1002/j.1460-2075.1982.tb01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brulet P, Babinet C, Kemler R, Jacob F. Monoclonal antibodies against trophectoderm-specific markers during mouse blastocyst formation. Proc Natl Acad USA. 1980;77:4113–4117. doi: 10.1073/pnas.77.7.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kemler R, Brulet P, Schnebelen MT, Gaillard J, Jacob F. Reactivity of monoclonal antibodies against intermediate filament proteins during embryonic development. J Embryol Exp Morphol. 1981;64:45–60. [PubMed] [Google Scholar]
- 67.Oshima RG. Identification and immunoprecipitation of cytoskeletal proteins from murine extra-embryonic endodermal cells. J Biol Chem. 1981;256:8124–8133. [PubMed] [Google Scholar]
- 68.Oshima RG. Developmental expression of murine extra-embryonic endodermal cytoskeletal proteins. J Biol Chem. 1982;257:3414–21. [PubMed] [Google Scholar]
- 69.Jackson BW, Grund C, Schmid E, Burke K, Franke W, Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation. 1980;17:161–179. doi: 10.1111/j.1432-0436.1980.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 70.Paulin D, Jakob H, Jacob J, Weber K, Osborn M. In vitro differentiation of mouse teratocarcinoma cells monitored by intermediate filament expression. Differentiation. 1982;22:90–99. doi: 10.1111/j.1432-0436.1982.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 71.Oshima RG, Howe WE, Klier FG, Adamson ED, Shevinsky LH. Intermediate filament protein synthesis in preimplantation murine embryos. Dev Biol. 1983;99:447–455. doi: 10.1016/0012-1606(83)90294-4. [DOI] [PubMed] [Google Scholar]
- 72.Franke WW, Grund C, Kuhn C, Jackson BW, Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis III Primary mesenchymal cells and the first appearance of vimentin filaments. Differentiation. 1982;23:43–59. doi: 10.1111/j.1432-0436.1982.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 73.Jackson BW, Grund C, Winter S, Franke WW, Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis: epithelial differentiation and intermediate-sized filaments in early postimplantation embryos. Differentiation. 1981;20:203–216. doi: 10.1111/j.1432-0436.1981.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 74.Trevor K, Oshima RG. Preimplantation mouse embryos and liver express the same type I keratin gene product. J Biol Chem. 1985;260:15885–15891. [PubMed] [Google Scholar]
- 75.Singer PA, Trevor K, Oshima RG. Molecular cloning and characterization of the Endo B cytokeratin expressed in preimplantation mouse embryos. J Biol Chem. 1986;261:538–547. [PubMed] [Google Scholar]
- 76.Vasseur M, Duprey P, Brulet P, Jacob F. One gene and one pseudogene for the cytokeratin endo A. Proc Natl Acad Sci U S A. 1985;82:1155–9. doi: 10.1073/pnas.82.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duprey P, Morello D, Vasseur M, Babinet C, Condamine H, Brulet P, Jacob F. Expression of the cytokeratin endo A gene during early mouse embryogenesis. Proc Natl Acad Sci U S A. 1985;82:8535–9. doi: 10.1073/pnas.82.24.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ichinose Y, Morita T, Zhang F, Srimahasongcram S, Tondella MLC, Matsumoto M, Nozaki M, Matsushiro A. Nucleotide sequence and structure of the mouse cytokeratin endoB gene. Gene. 1988;70:85–95. doi: 10.1016/0378-1119(88)90107-2. [DOI] [PubMed] [Google Scholar]
- 79.Oshima RG, Abrams L, Kulesh D. Activation of an intron enhancer within the keratin 18 gene by expression of c-fos and c-jun in undifferentiated F9 embryonal carcinoma cells. Genes and Dev. 1990;4:835–848. doi: 10.1101/gad.4.5.835. [DOI] [PubMed] [Google Scholar]
- 80.Fujimura Y, Yamamoto H, Hamazato F, Nozaki M. One of two ets-binding sites in the cytokeratin EndoA enhancer is essential for enhancer activity and binds to ets-2 related proteins. Nuc Acids Res. 1994;22:613–618. doi: 10.1093/nar/22.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takemoto Y, Fujimura Y, Matsumoto M, Tamai Y, Morita T, Matsushiro A, Nozaki M. The promoter of the endo A cytokeratin gene is activated by a 3′ downstream enhancer. Nuc Acids Res. 1991;19:2761–2765. doi: 10.1093/nar/19.10.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seth A, Robinson L, Panayiotakis A, Thompson DM, Hodge DR, Zhang XK, Watson DK, Ozato K, Papas TS. The EndoA enhancer contains multiple ETS binding site repeats and is regulated by ETS proteins. Oncogene. 1994;9:469–77. [PubMed] [Google Scholar]
- 83.Oshima RG, Baribault H, Caulin C. Oncogenic regulation and function of keratin 8 and 18. Cancer Metastasis Rev. 1996;15:445–471. doi: 10.1007/BF00054012. [DOI] [PubMed] [Google Scholar]
- 84.Cecena G, Wen F, Cardiff RD, Oshima RG. Differential sensitivity of mouse epithelial tissues to the polyomavirus middle T oncogene. Am J Pathol. 2006;168:310–20. doi: 10.2353/ajpath.2006.050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lersch R, Stellmach V, Stocks C, Giudice G, Fuchs E. Isolation, sequence, and expression of a human keratin K5 gene: transcriptional regulation of keratins and insights into pairwise control. Mol Cell Biol. 1989;9:3685–3697. doi: 10.1128/mcb.9.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Nat Acad Sci USA. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vassar R, Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- 88.Lu B, Rothnagel JA, Longley MA, Tsai SY, Roop DR. Differentiation-specific expression of human keratin 1 is mediated by a composite AP-1/steroid hormone element. J Biol Chem. 1994;269:7443–7449. [PubMed] [Google Scholar]
- 89.Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14:1030–7. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuisk IR, Li H, Tran D, Capetanaki Y. A single MEF2 site governs desmin transcription in both heart and skeletal muscle during mouse embryogenesis. Dev Biol. 1996;174:1–13. doi: 10.1006/dbio.1996.0046. [DOI] [PubMed] [Google Scholar]
- 91.Frisen J, Johansson CB, Torok C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453–64. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 93.Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J. 2006;25:4808–19. doi: 10.1038/sj.emboj.7601366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Denk H, Franke WW, Eckerstorfer R, Schmid E, Kerjaschki D. Formation and involution of Mallory bodies ("alcoholic hyalin") in murine and human liver revealed by immunofluorescence microscopy with antibodies to prekeratin. Proc Nat Acad Sci USA. 1979;76:4112–4116. doi: 10.1073/pnas.76.8.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bannasch P, Zerban H, Schmid E, Franke WW. Liver tumors distinguished by immunofluorescence microscopy with antibodies to proteins of intermediate-sized filaments. Proc Nat Acad Sci USA. 1980;77:4948–4952. doi: 10.1073/pnas.77.8.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Osborn M, Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983;48:372–94. [PubMed] [Google Scholar]
- 97.Debus E, Weber K, Osborn M. Monoclonal cytokeratin antibodies that distinguish simple from stratified squamous epithelia: characterization on human tissues. EMBO Journal. 1982;1:1641–1647. doi: 10.1002/j.1460-2075.1982.tb01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lane EB, Klymkowsky MW. Epithelial tonofilaments: investigating their form and function using monoclonal antibodies. Cold Spring Harbor Symposia on Quantitative Biology. 1982:387–402. doi: 10.1101/sqb.1982.046.01.038. [DOI] [PubMed] [Google Scholar]
- 99.Lane EB. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982;92:665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramaekers F, Huysmans A, Moesker O, Kant A, Jap P, Herman C, Vooijs P. Monoclonal antibody to keratin filaments, specific for glandular epithelia and their tumors. Lab Invest. 1983;49:353–361. [PubMed] [Google Scholar]
- 101.Tseng CG, Jarvinen MJ, Nelson WG, Huang JW, Woodcock-Mitchell J, Sun TT. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982;30:361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- 102.Franke WW, Schmid E, Weber K, Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979;118:95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- 103.Weber K, Osborn M, Moll R, Wiklund B, Luning B. Tissue polypeptide antigen (TPA) is related to the non-epidermal keratins 8, 18 and 19 typical of simple and non-squamous epithelia: re-evaluation of a human tumor marker. EMBO. 1984;3:2707–2714. doi: 10.1002/j.1460-2075.1984.tb02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stigbrand T, Andres C, Bellanger L, Omary MB, Bodenmiiller H, Bonfrer H, Brundell J, Einarsson R, Erlandsson A, Johansson A, Leca JF, Levi M, Meier T, Nap M, Nustad K, Seguin P, Sjodin A, Sundstrom B, van Dalen A, Wiebelhause E, Wiklund B, Arlestig L, Hilgers J. Epitope specificity of 30 monoclonal antibodies against cytokeratin antigens: the ISOBM TD5-1 workshop. Tumor Biology. 1998;19:132–152. doi: 10.1159/000029984. [DOI] [PubMed] [Google Scholar]
- 105.Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takahashi A, Alnemri ES, Lazebnik YA, Fernandes-Alnemri T, Litwack G, Moir RD. Cleavage of lamin A by Mch2 alpha but not CPP32: multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc Nat Acad Sci USA. 1996;93:8395–8400. doi: 10.1073/pnas.93.16.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial apoptosis. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ku NO, Liao J, Omary MB. Apoptosis generates stable fragments of human type I keratins. J Biol Chem. 1997;272:33197–33203. doi: 10.1074/jbc.272.52.33197. [DOI] [PubMed] [Google Scholar]
- 109.Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorklund P, Ramaekers FC, Bjorklund B, Nap M, Jornvall H, Schutte B. Immunocytochemical detection and mapping of a cytokeratin 18 neo- epitope exposed during early apoptosis. J Path. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 110.Chen F, Chang R, Trivedi M, Capetanaki Y, Cryns VL. Caspase proteolysis of desmin produces a dominant-negative inhibitor of intermediate filaments and promotes apoptosis. J Biol Chem. 2003;278:6848–53. doi: 10.1074/jbc.M212021200. [DOI] [PubMed] [Google Scholar]
- 111.Byun Y, Chen F, Chang R, Trived M, Green KJ, Cryns VL. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001;8:443–450. doi: 10.1038/sj.cdd.4400840. [DOI] [PubMed] [Google Scholar]
- 112.Stegh AH, Herrmann H, Lampel S, Weisenberger D, Andra K, Seper M, Wiche G, Krammer PH, Peter ME. Identification of the cytolinker plectin as a major early in vivo substrate for caspase 8 during CD95- and tumor necrosis factor receptor-mediated apoptosis. Mol Cell Biol. 2000;20:5665–79. doi: 10.1128/mcb.20.15.5665-5679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee JC, Schickling O, Stegh AH, Oshima RG, Dinsdale D, Cohen GM, Peter ME. DEDD regulates degradation of intermediate filaments during apoptosis. J Cell Biol. 2002;158:1051–66. doi: 10.1083/jcb.200112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pruss RM, Mirsky R, Raff MC. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981;27:419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- 115.McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;319:463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- 116.Weber K, Plessmann U, Ulrich W. Cytoplasmic intermediate filament proteins of invertebrates are closer to nuclear lamins than are vertebrate intermediate filament proteins; sequence characterization of two muscle proteins of a nematode. EMBO. 1989;8:3221–3227. doi: 10.1002/j.1460-2075.1989.tb08481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karabinos A, Schmidt H, Harborth J, Schnabel R, Weber K. Essential roles for four cytoplasmic intermediate filament proteins in Caenorhabditis elegans development. Proc Natl Acad Sci U S A. 2001;98:7863–8. doi: 10.1073/pnas.121169998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–4. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 119.Klymkowsky MW. Intermediate filaments in 3T3 cells collapse after intracellular injection of a monoclonal anti-intermediate filament antibody. Nature. 1981;291:249–251. doi: 10.1038/291249a0. [DOI] [PubMed] [Google Scholar]
- 120.Klymkowsky MW, Miller RH, Lane EB. Morphology, behavior, and interaction of cultured epithelial cells after the antibody-induced disruption of keratin filament organization. J Cell Biol. 1983;96:494–509. doi: 10.1083/jcb.96.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Emerson JA. Disruption of the cytokeratin filament network in the preimplantation mouse embryo. Development. 1988;104:219–234. doi: 10.1242/dev.104.2.219. [DOI] [PubMed] [Google Scholar]
- 122.Yamasaki H, Itakura C, Mizutani M. Hereditary hypotrophic axonopathy with neurofilament deficiency in a mutant strain of the Japanese quail. Acta Neuropathol (Berl) 1991;82:427–34. doi: 10.1007/BF00293376. [DOI] [PubMed] [Google Scholar]
- 123.Ohara O, Gahara Y, Miyake T, Teraoka H, Kitamura T. Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J Cell Biol. 1993;121:387–95. doi: 10.1083/jcb.121.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baribault H, Oshima RG. Polarized and functional epithelia can form after the targeted inactivation of both mouse keratin 8 alleles. J Cell Biol. 1991;115:1675–1684. doi: 10.1083/jcb.115.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kulesh DA, Cecena G, Darmon YM, Vasseur M, Oshima RG. Post-translational regulation of keratins: degradation of unpolymerized mouse and human keratins 18 and 8. Mol and Cell Biol. 1989;9:1553–1565. doi: 10.1128/mcb.9.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baribault H, Price J, Miyai K, Oshima RG. Mid-gestational lethality in mice lacking keratin 8. Genes and Development. 1993;7:1191–1202. doi: 10.1101/gad.7.7a.1191. [DOI] [PubMed] [Google Scholar]
- 127.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–48. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 128.Jaquemar D, Kupriyanov S, Wankell M, Avis J, Benirschke K, Baribault H, Oshima RG. Keratin 8 protection of placental barrier function. J Cell Biol. 2003;161:749–756. doi: 10.1083/jcb.200210004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hesse M, Franz T, Tamai Y, Taketo MM, Magin TM. Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO. 2000;19:5060–5070. doi: 10.1093/emboj/19.19.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Caulin C, Ware CF, Magin TM, Oshima RG. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J Cell Biol. 2000;149:17–22. doi: 10.1083/jcb.149.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ku NO, Soetikno RM, Omary MB. Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury. Hepatology. 2003;37:1006–14. doi: 10.1053/jhep.2003.50181. [DOI] [PubMed] [Google Scholar]
- 132.Inada H, Izawa I, Nishizawa M, Fujita E, Kiyono T, Takahashi T, Momoi T, Inagaki M. Keratin attenuates tumor necrosis factor-induced cytotoxicity through association with TRADD. J Cell Biol. 2001;155:415–26. doi: 10.1083/jcb.200103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tong X, Coulombe PA. Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion. Genes Dev. 2006;20:1353–64. doi: 10.1101/gad.1387406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gilbert S, Loranger A, Marceau N. Keratins modulate c-Flip/extracellular signal-regulated kinase 1 and 2 antiapoptotic signaling in simple epithelial cells. Mol Cell Biol. 2004;24:7072–81. doi: 10.1128/MCB.24.16.7072-7081.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ku NO, Omary MB. A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol. 2006;174:115–25. doi: 10.1083/jcb.200602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zatloukal K, Stumptner C, Lehner M, Denk H, Baribault H, Eshkind LG, Franke WW. Cytokeratin 8 protects from hepatotoxicity, and its ratio to cytokeratin 18 determines the ability of hepatocytes to form Mallory bodies. Am J Pathol. 2000;156:1263–1274. doi: 10.1016/S0002-9440(10)64997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ku NO, Darling JM, Krams SM, Esquivel CO, Keeffe EB, Sibley RK, Lee YM, Wright TL, Omary MB. Keratin 8 and 18 mutations are risk factors for developing liver disease of multiple etiologies. Proc Natl Acad Sci U S A. 2003;100:6063–8. doi: 10.1073/pnas.0936165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Loranger A, Duclos S, Grenier A, Price J, Wilson-Heiner M, Baribault H, Marceau N. Simple epithelium keratins are required for maintenance of hepatocyte integrity. Am J Pahol. 1997;151:1673–1683. [PMC free article] [PubMed] [Google Scholar]
- 139.Ku NO, Michie S, Oshima RG, Omary MB. Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J Cell Biol. 1995;131:1303–1314. doi: 10.1083/jcb.131.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baribault H, Penner J, Iozzo RV, Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. doi: 10.1101/gad.8.24.2964. [DOI] [PubMed] [Google Scholar]
- 141.Habtezion A, Toivola DM, Butcher EC, Omary MB. Keratin-8-deficient mice develop chronic spontaneous Th2 colitis amenable to antibiotic treatment. J Cell Sci. 2005;118:1971–1980. doi: 10.1242/jcs.02316. [DOI] [PubMed] [Google Scholar]
- 142.Ameen NA, Figueroa Y, Salas PJ. Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J Cell Sci. 2001;114:563–75. doi: 10.1242/jcs.114.3.563. [DOI] [PubMed] [Google Scholar]
- 143.Toivola DM, Krishnan S, Binder HJ, Singh SK, Omary MB. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J Cell Biol. 2004;164:911–21. doi: 10.1083/jcb.200308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 145.Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–70. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol. 1996;175:362–6. doi: 10.1006/dbio.1996.0122. [DOI] [PubMed] [Google Scholar]
- 147.Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17:607–15. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- 148.Tamai Y, Ishikawa TO, Bosl MR, Mori M, Nozaki M, Baribault H, Oshima RG, Taketo MM. Cytokeratins 8 and 19 in the mouse placental development. J Cell Biol. 2000;151:563–572. doi: 10.1083/jcb.151.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu Q, Couillard-Despres S, Julien JP. Delayed maturation of regenerating myelinated axons in mice lacking neurofilaments. Exp Neurol. 1997;148:299–316. doi: 10.1006/exnr.1997.6654. [DOI] [PubMed] [Google Scholar]
- 150.Elder GA, Friedrich VL, Jr, Bosco P, Kang C, Gourov A, Tu PH, Lee VM, Lazzarini RA. Absence of the mid-sized neurofilament subunit decreases axonal calibers, levels of light neurofilament (NF-L), and neurofilament content. J Cell Biol. 1998;141:727–39. doi: 10.1083/jcb.141.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elder GA, Friedrich VL, Jr, Kang C, Bosco P, Gourov A, Tu PH, Zhang B, Lee VM, Lazzarini RA. Requirement of heavy neurofilament subunit in the development of axons with large calibers. J Cell Biol. 1998;143:195–205. doi: 10.1083/jcb.143.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li Z, Mericskay M, Agbulut O, Butler-Browne G, Carlsson L, Thornell LE, Babinet C, Paulin D. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol. 1997;139:129–44. doi: 10.1083/jcb.139.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dalakas MC, Park KY, Semino-Mora C, Lee HS, Sivakumar K, Goldfarb LG. Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. N Engl J Med. 2000;342:770–80. doi: 10.1056/NEJM200003163421104. [DOI] [PubMed] [Google Scholar]
- 154.Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci. 2000;113:2455–62. doi: 10.1242/jcs.113.13.2455. [DOI] [PubMed] [Google Scholar]
- 155.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–62. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 156.Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisen J. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol. 1999;145:503–14. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Magin TM, Schroder R, Leitgeb S, Wanninger F, Zatloukal K, Grund C, Melton DW. Lessons from keratin 18 knockout mice: formation of novel keratin filaments, secondary loss of keratin 7 and accumulation of liver-specific keratin 8-positive aggregates. J Cell Biol. 1998;140:1441–1451. doi: 10.1083/jcb.140.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–5. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 159.Hutton E, Paladini RD, Yu QC, Yen M, Coulombe PA, Fuchs E. Functional differences between keratins of stratified and simple epithelia. J Cell Biol. 1998;143:487–499. doi: 10.1083/jcb.143.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kirfel J, Peters B, Grund C, Reifenberg K, Magin TM. Ectopic expression of desmin in the epidermis of transgenic mice permits development of a normal epidermis. Differentiation. 2002;70:56–68. doi: 10.1046/j.1432-0436.2002.700106.x. [DOI] [PubMed] [Google Scholar]
- 161.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–65. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 162.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, Gruenbaum Y, Khuon S, Mendez M, Varga R, Collins FS. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2004;101:8963–8. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–20. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–8. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mounkes L, Kozlov S, Burke B, Stewart CL. The laminopathies: nuclear structure meets disease. Curr Opin Genet Dev. 2003;13:223–30. doi: 10.1016/s0959-437x(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 166.Spann TP, Goldman AE, Wang C, Huang S, Goldman RD. Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J Cell Biol. 2002;156:603–8. doi: 10.1083/jcb.200112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shumaker DK, Lopez-Soler RI, Adam SA, Herrmann H, Moir RD, Spann TP, Goldman RD. Functions and dysfunctions of the nuclear lamin Ig-fold domain in nuclear assembly, growth, and Emery-Dreifuss muscular dystrophy. Proc Natl Acad Sci U S A. 2005;102:15494–9. doi: 10.1073/pnas.0507612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Andrulis ED, Neiman AM, Zappulla DC, Sternglanz R. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature. 1998;394:592–595. doi: 10.1038/29100. [DOI] [PubMed] [Google Scholar]
- 169.He T, Stepulak A, Holmstrom TH, Omary MB, Eriksson JE. The intermediate filament protein keratin 8 is a novel cytoplasmic substrate for c-Jun N-terminal kinase. J Biol Chem. 2002;277:10767–74. doi: 10.1074/jbc.M111436200. [DOI] [PubMed] [Google Scholar]
- 170.Liao J, Omary MB. 14-3-3 proteins associate with phosphorylated simple epithelial keratins during cell cycle progression and act as a solubility cofactor. J Cell Biol. 1996;133:345–357. doi: 10.1083/jcb.133.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ku NO, Liao J, Omary MB. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. EMBO. 1998;17:1892–1906. doi: 10.1093/emboj/17.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lane EB, Rugg EL, Navsaria H, Leigh IM, Heagerty AHM, Ishida-Yamamoto A, Eady RAJ. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992;356:244–236. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- 173.Vassar R, Coulombe PA, Degenstein L, Albers K, Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991;64:365–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]
- 174.Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Nat Acad Sci USA. 1989;86:1563–1567. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chan Y, Anton-Lamprecht I, Yu Q, Jackel A, Zabel B, Ernst J, Fuchs E. A human keratin 14 "knockout": the absence of K14 leads to severe epidermolysis bullosa simplex and a function for an intermediate filament protein. Genes and Dev. 1994;8:2574–2587. doi: 10.1101/gad.8.21.2574. [DOI] [PubMed] [Google Scholar]
- 176.Rothnagel JA, Dominey AM, Dempsey LD, Longley MA, Greenhalgh DA, Gagne TA, Huber M, Frenk E, Hohl D, Roop DR. Mutations in the rod domains of keratins 1 and 10 in epidermolytic hyperkeratosis. Science. 1992;257:1128–30. doi: 10.1126/science.257.5073.1128. [DOI] [PubMed] [Google Scholar]
- 177.Cheng J, Syder AJ, Yu QC, Letai A, Paller AS, Fuchs E. The genetic basis of epidermolytic hyperkeratosis: a disorder of differentiation-specific epidermal keratin genes. Cell. 1992;70:811–819. doi: 10.1016/0092-8674(92)90314-3. [DOI] [PubMed] [Google Scholar]
- 178.Chipev CC, Korge BP, Markova N, Bale SJ, DiGiovanna JJ, Compton JG, Steinert PM. A leucine proline mutation in the H1 subdomain of keratin 1 causes epidermolytic hyperkeratosis. Cell. 1992;70:821–828. doi: 10.1016/0092-8674(92)90315-4. [DOI] [PubMed] [Google Scholar]
- 179.Mattout A, Dechat T, Adam SA, Goldman RD, Gruenbaum Y. Nuclear lamins, diseases and aging. Curr Opin Cell Biol. 2006;18:335–41. doi: 10.1016/j.ceb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 180.Fong LG, Ng JK, Lammerding J, Vickers TA, Meta M, Cote N, Gavino B, Qiao X, Chang SY, Young SR, Yang SH, Stewart CL, Lee RT, Bennett CF, Bergo MO, Young SG. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J Clin Invest. 2006;116:743–52. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Damjanov I, Clark RK, Andrews PW. Expression of keratin polypeptides in human embryonal carcinoma cells. Intermediate Filaments: Annals of the New York Academy of Sciences. 1985;455:732–733. [Google Scholar]
- 182.Steinert PM, Parry DAD, Racoosin EL, Idler WW, Steven AC, Trus BL, Roop DR. The complete cDNA and deduced amino acid sequence of a type II mouse epidermal keratin of 60,000 Da: analysis of sequence differences between type I and type II keratins. Proc Nat Acad Sci USA. 1984;81:5709–5713. doi: 10.1073/pnas.81.18.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ichinose Y, Hashido K, Miyamoto H, Nagata T, Nozaki M, Morita T, Matsushiro A. Molecular cloning and characterization of cDNA encoding mouse cytokeratin No. 19. Gene. 1989;80:315–323. doi: 10.1016/0378-1119(89)90295-3. [DOI] [PubMed] [Google Scholar]
- 184.Langbein L, Schweizer J. Keratins of the human hair follicle. Int Rev Cytol. 2005;243:1–78. doi: 10.1016/S0074-7696(05)43001-6. [DOI] [PubMed] [Google Scholar]
- 185.Steinert PM, Rice RH, Roop DR, Trus BL, Steven AC. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature. 1983;302:794–800. doi: 10.1038/302794a0. [DOI] [PubMed] [Google Scholar]
- 186.Jorcano JL, Franz JK, Franke WW. Amino acid sequence diversity between bovine epidermal cytokeratin polypeptides of the basic (type II) subfamily as determined from cDNA clones. Differentiation. 1984;28:155–163. doi: 10.1111/j.1432-0436.1984.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 187.Winter H, Rogers MA, Langbein L, Stevens HP, Leigh IM, Labreze C, Roul S, Taieb A, Krieg T, Schweizer J. Mutations in the hair cortex keratin hHb6 cause the inherited hair disease monilethrix. Nat Genet. 1997;16:372–4. doi: 10.1038/ng0897-372. [DOI] [PubMed] [Google Scholar]
- 188.Quax-Jeuken YE, Quax WJ, Bloemendal H. Primary and secondary structure of hamster vimentin predicted from the nucleotide sequence. Proc Natl Acad Sci U S A. 1983;80:3548–52. doi: 10.1073/pnas.80.12.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lewis SA, Balcarek JM, Krek V, Shelanski M, Cowan NJ. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984;81:2743–6. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Julien JP, Ramachandran K, Grosveld F. Cloning of a cDNA encoding the smallest neurofilament protein from the rat. Biochim Biophys Acta. 1985;825:398–404. doi: 10.1016/0167-4781(85)90067-3. [DOI] [PubMed] [Google Scholar]
- 191.Granger BL, Lazarides E. Synemin: a new high molecular weight protein associated with desmin and vimentin filaments in muscle. Cell. 1980;22:727–38. doi: 10.1016/0092-8674(80)90549-8. [DOI] [PubMed] [Google Scholar]
- 192.Becker B, Bellin RM, Sernett SW, Huiatt TW, Robson RM. Synemin contains the rod domain of intermediate filaments. Biochem Biophys Res Commun. 1995;213:796–802. doi: 10.1006/bbrc.1995.2200. [DOI] [PubMed] [Google Scholar]
- 193.Pachter JS, Liem RK. alpha-Internexin, a 66-kD intermediate filament-binding protein from mammalian central nervous tissues. J Cell Biol. 1985;101:1316–22. doi: 10.1083/jcb.101.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fliegner KH, Ching GY, Liem RK. The predicted amino acid sequence of alpha-internexin is that of a novel neuronal intermediate filament protein. Embo J. 1990;9:749–55. doi: 10.1002/j.1460-2075.1990.tb08169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Levavasseur F, Zhu Q, Julien JP. No requirement of alpha-internexin for nervous system development and for radial growth of axons. Brain Res Mol Brain Res. 1999;69:104–12. doi: 10.1016/s0169-328x(99)00104-7. [DOI] [PubMed] [Google Scholar]
- 196.Gerace L, Blum A, Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978;79:546–66. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wolin SL, Krohne G, Kirschner MW. A new lamin in Xenopus somatic tissues displays strong homology to human lamin A. EMBO J. 1987;6:3809–18. doi: 10.1002/j.1460-2075.1987.tb02717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hoger TH, Krohne G, Franke WW. Amino acid sequence and molecular characterization of murine lamin B as deduced from cDNA clones. Eur J Cell Biol. 1988;47:283–90. [PubMed] [Google Scholar]
- 199.Merdes A, Brunkener M, Horstmann H, Georgatos SD. Filensin: a new vimentin-binding, polymerization-competent, and membrane-associated protein of the lens fiber cell. J Cell Biol. 1991;115:397–410. doi: 10.1083/jcb.115.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG. Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin. Invest Ophthalmol Vis Sci. 2003;44:5252–8. doi: 10.1167/iovs.03-0224. [DOI] [PubMed] [Google Scholar]
- 201.Merdes A, Gounari F, Georgatos SD. The 47-kD lens-specific protein phakinin is a tailless intermediate filament protein and an assembly partner of filensin. J Cell Biol. 1993;123:1507–16. doi: 10.1083/jcb.123.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]


