Abstract
Oocyte development in the mammalian ovary requires productive interactions with somatic granulosa cells of the ovarian follicle. Proliferating granulosa cells support the progression of follicular growth and maturation, multiplying dramatically as it unfolds. The cell cycle recruitment of granulosa cells is regulated at least in part by hormones such as follicle-stimulating hormone (FSH) and estrogen. Follicles recruited into the growth phase following formation of multiple layers of granulosa cells have two major fates: either to continue proliferation followed by differentiation, or to die by programmed cell death, or atresia. While many of the signaling pathways orchestrating ovarian follicle development are known, the downstream transcriptional regulators that integrate such signals in the mammalian ovary remain to be defined. Recent experiments in diverse organisms have revealed multiple instances of gonad-selective components of the basal transcriptional machinery. One such protein, TAF4b, is a gonadal-enriched coactivator subunit of the TFIID complex required for normal female fertility in the mouse. To determine the etiology of female infertility of the TAF4b-deficient mice, we have determined multiple functions of TAF4b during postnatal ovarian follicle development. Here we demonstrate that the TAF4b protein is expressed in the granulosa cell compartment of the mammalian ovarian follicle. Furthermore, TAF4b-deficient mouse ovaries contain reduced numbers of primordial as well as growing follicles and a concomitant increased proportion of apoptotic follicles in comparison to wild type counterparts. Importantly, TAF4b-null follicles are largely resistant to induction of proliferation in response to multiple hormonal stimuli including estrogen and FSH and demonstrate compromised granulosa cell survival. Together, these data suggest that TAF4b integrates a program of granulosa cell gene expression required for normal ovarian follicle survival and proliferation in response to diverse ovarian signaling events.
Keywords: Folliculogenesis, Granulosa Cells, TAF4b, TFIID, Survival, Proliferation, Atresia
INTRODUCTION
Growth and development of a mammalian oocyte takes place in a specialized compartment of the ovary called the ovarian follicle. These follicles are comprised of multiple cell types: a single germ cell (oocyte) surrounded by multiple layers of somatic granulosa cells, which in turn are enveloped by thecal cells. Follicle growth and oocyte development are closely regulated by an ordered and complex series of signaling events occurring during folliculogenesis. A succession of linked paracrine and endocrine hormonal signals (such as c-Kit, GDF9, FSH, and LH) direct postnatal mammalian follicle development from the initial events of primordial follicle recruitment to ovulation of a mature oocyte decorated with cumulus granulosa cells (reviewed in Matzuk and Lamb, 2002). These irreversible, forward developmental transitions are balanced with the controlled culling of many follicles by regulated follicle death called atresia. Elucidating mechanisms that regulate follicle selection for growth versus atresia is critical for understanding the normal developmental processes occurring during folliculogenesis. While numerous signaling pathways have been shown to be essential for the precise regulation of folliculogenesis and preventing premature ovarian failure (Goswami and Conway, 2005), the mechanisms directing changes in gene expression patterns underlying the execution of these developmental transitions are largely unknown.
To address the molecular mechanisms underlying female fertility and folliculogenesis, we have begun to characterize the ovarian functions of a gonadal-enriched subunit of the TFIID complex, called TAF4b (formerly TAFII105). In eukaryotes, activation of gene transcription results from the combinatorial input of activator proteins and multi-protein co-activator complexes that respond to specific signaling networks (reviewed in Lemon and Tjian, 2000; Taatjes et al., 2004). TFIID is a multi-protein general transcription factor complex that is largely conserved from yeast to humans and is a central player in the activator-dependent recruitment of RNA polymerase II (Pol. II) to specific gene promoters (reviewed in Albright and Tjian, 2000). Rather than directly binding to Pol. II, transcriptional activators utilize a set of diverse coactivators and general transcription factors to guide Pol. II to specific genes. The TFIID complex contains the TATA-box binding protein (TBP) and approximately 14 TBP-associated factors serving as coactivators (TAFs; reviewed in Matangkasombut et al., 2004). Studies in yeast have confirmed the critical role of TAF subunits in recruitment of Pol. II for transcriptional activation (Komarnitsky et al., 1999). In metazoans, the broadly expressed transcriptional activator Specificity Protein 1 (Sp1) has been shown to specifically interact with dTAF4 (formerly Drosophila TAFII110) and hTAF4 (formerly human TAFII130) subunits of TFIID complex in Drosophila and mammals respectively (Hoey et al., 1993; Gill et al., 1994; Tanese et al., 1996; Saluja et al., 1998). Until recently, the regulation of tissue-specific gene expression was mostly attributed to activator proteins that may interact with TAFs and can be expressed in precise cell type-specific patterns. However, recent biochemical and genetic studies suggest that certain members of the basal transcriptional machinery are in fact expressed in a tissue-specific manner and contribute to tissue-selective gene expression patterns (reviewed in Hochheimer and Tjian, 2003; Levine and Tjian, 2003).
Diversification of general transcription factors has been employed in metazoans to regulate gonadal gene expression patterns and reproduction (reviewed in DeJong, 2006). The most notable examples are gonad-selective subunits of TFIID that are critical for reproduction in species as diverse as Drosophila and mice (Hiller et al., 2001; Martianov et al., 2001; Zhang et al., 2001; Hiller et al., 2004). In addition, a testis-specific subunit of TFIIA, called ALF, has been found selectively expressed in mouse germ cells (Upadhyaya et al., 2002). Accordingly, gene expression patterns required for sexual reproduction appear to be executed in part by gonadal forms of core transcription regulatory factors. However, the cellular functions and molecular mechanisms executed by these variant basal transcription factors in the gonads remain poorly understood.
To directly elucidate transcriptional mechanisms operating in the mammalian ovary, we have begun to delineate the in vivo functions of TAF4b in female reproduction. This work was initiated by the discovery that viable and apparently healthy TAF4b-deficient female mice were infertile owing to gross developmental defects of the ovary (Freiman et al., 2001). Recently, we demonstrated the ability of TAF4b to modulate networks of granulosa cell gene expression and siRNA knockdown of TAF4b levels in cultured granulosa cells was shown to disrupt normal granulosa cell morphology (Geles et al., 2006). Together, these studies provide the framework for the potential mechanism of TAF4b as an integrator of ovarian gene expression underlying granulosa cell functions required for robust female fertility.
In the present study, we have elucidated the sub-ovarian expression and function of TAF4b in the postnatal ovary. We show that TAF4b protein is readily detected within the granulosa cell compartment of the mouse ovary. Morphometric analysis of TAF4b-deficient ovaries indicates that TAF4b is required for multiple aspects of folliculogenesis. These include early primordial follicle survival as well as subsequent follicle growth. Most importantly, TAF4b-deficient granulosa cells are significantly impaired in their ability to proliferate in response to multiple hormonal stimuli in vivo. TAF4b-deficient granulosa cells undergo increased apoptosis and TAF4b-deficient follicles display increased rates of atresia. Together, these studies suggest that TAF4b is required for the normal in vivo response to hormonal signaling in the ovary supporting granulosa cell survival and proliferation during folliculogenesis.
MATERIALS AND METHODS
Animals
Wild-type and TAF4b-null mice were generated by mating heterozygous TAF4b+/- male and female mice as previously described (Freiman et al., 2001). Offspring were genotyped by PCR analysis of tail-snip genomic DNA amplifying the region targeted by homologous recombination. All animal protocols were reviewed and approved by Brown University institutional animal care and use committee and were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Generation of anti-mouse TAF4b antibodies
A fragment encompassing an amino-terminal variable domain of mouse TAF4b (amino acids 2-98) was expressed in E. coli as a GST-tagged fusion protein and used as immunogen to generate a rabbit polyclonal serum (as per standard procedure; Harlow and Lane, 1999). For affinity purification of the specific anti-TAF4b antibodies, we generated a 6XHis-tagged fusion construct with the same domain of the protein. The expression construct was transformed into SG130009 strain of E. coli, and induced to express the fusion proteins with 1 mM IPTG for 3 hours at 37°C. The cells were harvested, resuspended in the denaturing lysis buffer (8 M urea, 100 mM NaCl, 20 mM Hepes pH 8.0), and lysed by rapid freeze-thaw followed by sonication. Insoluble fractions were removed by centrifugation at 10,000 g for 10 minutes. The supernatants containing fusion proteins were bound to Ni-NTA resin (ProBond, Invitrogen, Carlsbad, CA), and washed with 20 mM imidazol-containing wash buffer. The bound proteins were eluted with increasing concentrations of imidazole (stepwise: 100 mM, 250 mM, 500 mM, and 1 M), the majority of protein eluted with 250 mM imidazole fraction. The purified protein was crosslinked to Affigel 10 matrix as per manufacturer’s protocol (Bio-Rad, Hercules, CA), and the specific antibodies were purified by sequential binding and eluting of the antibody as per the manufacturer’s protocol.
Purified granulosa cell isolation
The ovaries of 8 week old wild type C57/Bl6 mice (Jackson Laboratories, Bar Harbor, ME) were removed and subjected to puncture with a 26 gauge needle to liberate granulosa cells into collection media (DME-F12, 1X penicillin-streptomycin, 0.3% BSA). Resulting granulosa cell suspensions were filtered through 40 μm nylon filter to remove large tissue clumps and oocytes. The granulosa cell-depleted residual ovarian tissue (termed “ovary remnants” here) was washed in PBS and dounced in low salt protein extraction buffer. Purified granulosa cells were spun down, washed in PBS, spun down again, and resuspended in extraction buffer. For total ovary extracts, whole ovaries were dounced in low salt extraction buffer. The resultant extracts were analyzed by western blotting using our affinity-purified anti-TAF4b antibodies.
Morphometric analysis of ovarian follicles
9 μm sections of paraffin-embedded ovaries were fixed in 3.7% formaldehyde and mounted on slides. Routine hematoxylin and eosin staining was performed for histologic examination by light microscopy. The number of follicles for each ovary was counted in 10-15 consecutive largest sections; only follicles with visible oocyte nuclei were counted. Follicles were classified as primordial, primary (surrounded by a single layer of cubiodal granulosa cells), growing preantral (oocytes surrounded by two or more layers of granulosa cells with no antrum), or antral (antrum within the granulosa cells enclosing the oocyte). Follicles were determined to be atretic if the oocyte and/or granulosa cell exhibited pyknotic nuclei and cytoplasmic shrinking, or granulosa cells were pulling away from the basement membrane. Average number of follicles per section was determined for each follicle type in each animal. Data is presented as average ± s.e.m. from at least 3 pairs of mice for each time point (Hu et al., 2004).
Stat3 immunocytochemistry
Stat3 staining was performed as described (Murphy et al., 2005). Sections derived as above were subjected to a microwave antigen retrieval technique by heating for 5 min in 10 mM citrate buffer (pH 6.0). The cooled sections were incubated in 3% H2O2 for 5 min to quench endogenous peroxidase activity, washed in PBS, and blocked in PBS/0.5% Tween-20 with 10% normal goat serum and 10 mg/ml BSA. Sections were then incubated with anti-Stat3 (1:500; C-20 from Santa Cruz Biotechnology, Santa Cruz, CA) in blocking solution overnight at 4°C. Secondary antibody was Goat anti-Rabbit HRP-conjugated from Pierce (Rockford, IL) applied at 1:300 for 2 hours at room temperature. Sections were developed with 3,3’-DAB (Vector kit), lightly counterstained with hematoxylin, dehydrated, and mounted with Permount (Fisher). Follicles were quantified as above.
BrdU incorporation
Assessment of granulosa cell proliferation by BrdU incorporation was performed as previously described by Kadakia et al. 2001 . Hormonally primed mice (5 IU PMSG or 1 μg/g E2 for 24 hours) were injected ip with 1 μg/gm BrdU, and sacrificed after 1 hour. The ovaries were dissected, frozen in OCT, and 7 μm frozen sections were generated. The sections were rinsed in CSK buffer (10 mM Hepes pH 7.4, 300 mM sucrose, 100 mM NaCl, 3 mM MgCl2) once at room temperature, incubated in CSK/Tx-100 (0.5%) buffer for 2 minutes on ice, and fixed in 100% methanol at -20°C for 20 min. The DNA was denatured by incubation in 2N HCl for 90 min at room temperature, and the slides were neutralized by rinsing 2x w/PBS. Endogenous peroxidase activity was quenched by incubating in 3% H2O2 for 5 min. The slides were further washed in PBS, and blocked in PBS/0.5% Tween-20 with 10% normal goat serum and 10 mg/ml BSA for 20 min followed by blocking with PBST supplemented with 200 ug/ml unlabeled goat anti-mouse Fab fragments (to decrease the secondary antibody background). Primary monoclonal anti-BrdU antibody was used at 1:100 dilution; the remaining procedures were carried out as described above. After counterstaining with hematoxylin, the percentages of positive cells per follicle were determined for comparison in 5 follicles per each experimental animal. Preantral follicles were analyzed in the estrogen-treated animals; in the PMSG-treated group, antral follicles induced by hormonal treatment in wild-type and heterozygous animals were included into the analysis as well.
PCNA immunolabeling
Chromatin recruitment of PCNA was assessed as in van Betteraey-Nikoleit et al. (2003), with modifications. Frozen sections were rinsed in cold CSK buffer once, incubated in CSK/Tx-100 (0.5%) buffer for 2 minutes on ice, and fixed in 100% methanol at -20°C for 5 min. Peroxidase quenching, blocking and labeling procedures were as described for BrdU immunolabeling. Primary anti-PCNA monoclonal antibody (PC-10) was used at 1:100 dilution.
Histone H3AcK9 immunolabeling
Frozen sections of the ovaries were fixed in 3.7% formaldehyde. Peroxidase quenching, blocking and labeling procedures were as above. The primary antibody against Histone H3 acetylated on K9 was obtained from Upstate Biotech (Lake Placid, NY), and used at 1:200 dilution.
Caspase-3 immunolabeling
Granulosa cell apoptosis was assessed in frozen ovarian sections (Matikainen et al., 2001). Frozen ovarian sections of estrogen-primed mice fixed in methanol were labeled with rabbit monoclonal antibodies against cleaved caspase-3 (5A1) and against total caspase-3 (8G10), which were obtained from Cell Signaling Technology (Danvers, MA) and used at 1:300 and 1:100 dilution respectively. Secondary goat anti-rabbit Cy-3 conjugated antibody was from Jackson Research Laboratories (Westgrove, PA), and used at 1:300 dilution.
TUNEL assay
DNA fragmentation associated with apoptosis was assessed in frozen sections of the estrogen-primed mouse ovaries fixed with 3.7% formaldehyde using ApoAlert DNA fragmentation kit (BD Biosciences Clontech; San Jose, CA) as per manufacturer’s protocol.
Quantitative PCR
Total RNA was isolated from whole ovaries of the untreated 17 week old mice using Qiagen RNeasy mini kits, or from the ovaries of estrogen-primed 3 week old mice using Qiagen RNeasy micro kit. 20 μl of cDNA was prepared from approximately 1 μg of total RNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) according to manufacturers directions, using a mix of oligo(dT) and random primers. Real-time PCR reactions were performed in triplicate using 2 μls cDNA template, ABI SYBR green PCR master-mix and primers for Esr2, Fshr and 18S rRNA in ABI 7300 Real Time PCR System. Relative expression levels of Esr2 and Fshr were normalized to 18S ribosomal RNA levels to correct for equivalent total RNA levels.
Primer sequences are as follows:
| 18S F-ccgcggttctattttgttgg | 18S R-ggccgtccctcttaatcatg |
| Esr2 F-aaacagagagaccctgaagagaa | Esr2 R-cctcttggcgcttggactag |
| Fshr F-ccaacctacccaacttgcatg | Fshr R- cagatatcggagactgggaagatt |
Serum hormone levels
FSH, LH, and prolactin in 6-week old female mice were measured by radioimmunoassay using reagents from the National Hormone and Pituitary Program (Harbor-UCLA Medical Center, Torrance, CA). Nine to twelve animals were analyzed per genotype.
RESULTS
Expression of TAF4b in the granulosa cells of the ovarian follicle
To determine the cell types and cellular processes affected by TAF4b gene disruption in the TAF4b-null female mice, we examined the expression pattern of the TAF4b protein in multiple mouse tissue and ovarian extracts. To analyze the expression of TAF4b protein, we generated polyclonal antisera raised against the hypervariable domain of mouse TAF4b compared to TAF4 (first 98 amino acid residues) fused to GST. The crude antisera specifically detects TAF4b, but not TAF4 by western blot analysis (data not shown). Affinity-purified anti-TAF4b antibodies show that similar to its mRNA expression in adult mouse tissues, TAF4b protein is most readily detected in the testis and ovary (Fig. 1A). To test whether TAF4b is enriched in a specific cell type within the ovary, we examined the expression of TAF4b protein in ovarian granulosa cells. We found TAF4b enriched in purified granulosa cells of the adult ovary and, conversely, depleted in ovarian remnants after granulosa cell expulsion (Fig. 1B), in agreement with our previously reported mRNA expression pattern (Freiman et al., 2001). The TAF4b enrichment in granulosa cells is even more pronounced than that of cyclin D2, an established granulosa cell-expressed cell cycle protein (Sicinski et al., 1996). Finally, immunolabeling detected TAF4b-positive granulosa cells in preantral follicles derived from actively cycling mice at 12 weeks of age (Fig. 1C); in contrast, granulosa cells of early preantral follicles appeared negative for TAF4b protein expression. This granulosa cell labeling appeared specific since such late preantral follicle immunostaining was not detected in the TAF4b-null ovaries (Fig. 1D) as opposed to general stromal cell background. Furthermore, the full-length TAF4b-specific band is not detected in the TAF4b-null ovarian extract by western blot analysis (Fig. 1E). We conclude that in adult female mice, TAF4b is readily detected in follicular granulosa cells and that the reproductive defects stemming from disruption of TAF4b may be in part due to disruption of normal pathways regulating gene expression in granulosa cells. A recent report has found TAF4b protein expressed in the oocytes of embryonic and prepubertal mice, suggesting a more dynamic regulation of the TAF4b expression pattern in the ovary (Falender et al., 2005). However, the focus of the present study is to examine the potential functions of TAF4b in the granulosa cell compartment of the ovary.
Figure 1. TAF4b protein is enriched in ovarian granulosa cells.
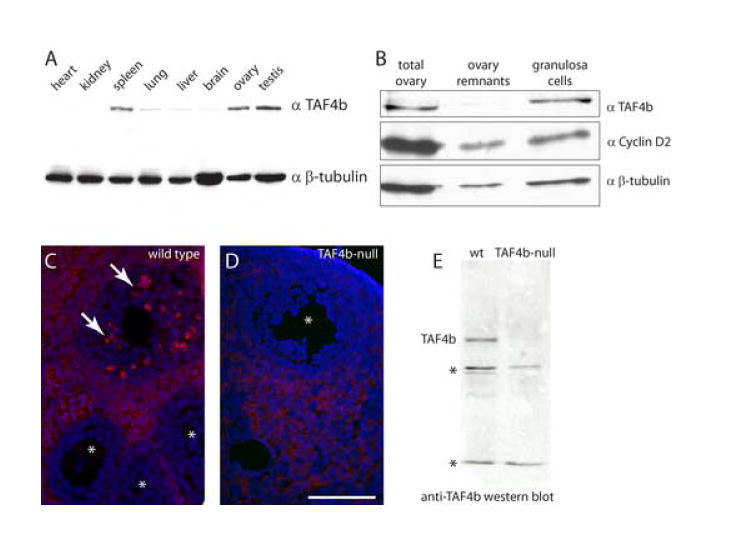
A. Western blot of mouse tissue extracts (~20 μg/lane) probed with anti-TAF4b antibodies; β-tubulin levels serve as a loading control. B. TAF4b is enriched in purified granulosa cells, as opposed to ovary remnants depleted of granulosa cells (ovary remnants). Total ovary protein extract represents starting material without fractionation. Cyclin D2 is a granulosa cell-enriched cell cycle protein and β-tubulin serves as a loading control. C. TAF4b immunolabeling in 12-week old mouse ovary detects protein expression in granulosa cells of selective large preantral follicles (arrows), while granulosa cells of early preantral follicles are not labeled (*). D. Granulosa cell labeling is absent from the large preantral follicles of TAF4b-null females (*); the cytoplasmic stromal-cell labeling has been determined to be non-specific. All follicles in C and D are preantral; size bar in D indicates 100 μm. E. Western blot indicates absence of full-length TAF4b from ovary extracts of TAF4b-null mice, * denotes non-specific cross-reacting bands.
Multiple defects in ovarian folliculogenesis in the TAF4b-null ovaries
TAF4b-null female mice are infertile, at least partially due to defective follicular development. To determine if a particular stage of folliculogenesis is selectively affected in TAF4b-null mice, we characterized ovarian follicles in these animals compared to fertile littermates during the transition from prepubertal to adult ovarian development. Here, we determined follicle composition in the TAF4b-null ovaries as compared to their wild-type and heterozygous littermates at different ages of development. For quantification, we utilized histological sections of the ovaries stained with hematoxylin and eosin (H & E; Fig. 2A-B), or for efficient identification of primordial follicles, we used ovary sections stained with anti-Stat3 antibodies, which highlight oocytes (Murphy et al., 2005), and counter-stained with hematoxylin (Fig. 2C-D). Morphometric analysis of follicular distribution across development was performed at postnatal days 21, 45, and 84, providing a global overview of follicle populations before, during, and after sexual maturation. The numbers of preantral follicles in TAF4b-null mice were significantly lower than in controls (Fig. 2G), suggesting that these either arise from primary follicles at a lower rate due to insufficient proliferation of granulosa cells, or that they are lost due to attrition by apoptosis. Unexpectedly, we also found reduced numbers of primordial follicles in TAF4b-null mice compared to their wild-type and heterozygous littermates (Fig. 2E). In spite of the low numbers of primordial follicles as early as 3 weeks of age, the decrease in primary follicles numbers in TAF4b-null mice was never statistically significant, suggesting similar rates of follicular recruitment into the growing (preantral) phase. The antral follicles of the TAF4b-null mice increase in numbers slowly up to 6 weeks of age, but decline at 12 weeks due to depletion in the primordial and growing oocytes reserves. Collectively, this analysis suggests that TAF4b-deficiency leads to multiple defects throughout folliculogenesis, rather than disruption of a single developmental transition or event.
Figure 2. Morphometric analysis of TAF4b-null ovarian follicles.
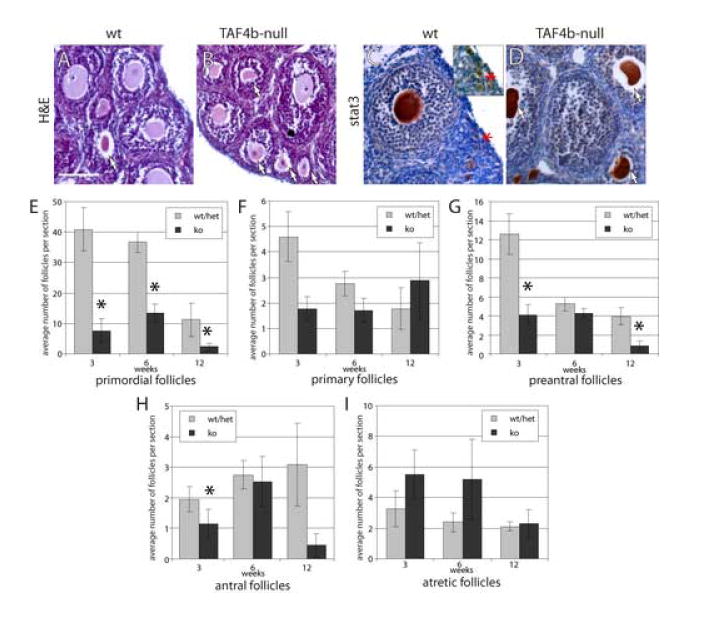
A-D. Representative sections of 21 day old wild type or TAF4b-null ovaries stained with hematoxylin-eosin (A-B) or with anti-stat3 antibodies and hematoxylin (C-D). A: preantral follicles in the wild-type ovary. B: many atretic follicles (arrows) in the TAF4b-null ovary. C: clusters of primordial oocytes are detected by Stat3 label in a wild-type ovary (marked with asterisk, and enlarged in inset). D: atretic and primary follicles are found in a TAF4b-null ovary. Size bar in A indicates 100 μm. E-I. Dynamics of follicle types across different mouse ages. Bars represent average numbers of follicles of each indicated type per section at the ages analyzed. Error bars indicate s.e.m.; 3-5 mice were analyzed per each time point. TAF4b-null values significantly different from wild type numbers (p<0.05) are indicated with asterisks. E: primordial follicles; F: primary follicles; G: preantral follicles; H: antral follicles; I: atretic follicles.
TAF4b promotes granulosa cell proliferation in vivo
To better understand the cellular basis of the observed folliculogenesis defects in the TAF4b-deficient ovaries, we assessed the proliferation of granulosa cells in the presence versus absence of TAF4b. Bromodeoxyuridine (BrdU) incorporation in vivo was utilized to directly label granulosa cells in the S-phase of the cell cycle and measure granulosa cell proliferation. Twenty-four hours prior to BrdU labeling, matched 21 day old TAF4b-null and wild-type or heterozygous female mice were stimulated with a single injection of either estrogen (Kadakia et al., 2001) or PMSG (Robker and Richards, 1998) to stimulate proliferation of follicular granulosa cells. Ovaries were harvested the next day following 1 hour in vivo BrdU treatment, and ovary sections generated from these mice were labeled with anti-BrdU antibodies. BrdU incorporation was readily detectable in granulosa cells derived from wild-type or heterozygous mice (Fig 3A, C) and significantly reduced in the TAF4b-null ovaries in response to both hormonal treatments (Fig. 3B, D, G). PCNA chromatin recruitment was used as an alternative marker of S-phase progression in these hormonally primed mice, and similarly indicated a decreased number of proliferating granulosa cells in TAF4b-null ovaries (Fig 3E-G). Control immunolabeling of acetylated histone H3 (on lysine 9) stained granulosa cell nuclei of each genotype relatively uniformly (Fig 3H, I). Specific reduction of BrdU incorporation and PCNA recruitment in the TAF4b-deficient mice upon hormonal priming are indicative of cell cycle defects in the granulosa cells that lead to aberrant folliculogenesis profiles and infertility.
Figure 3. Granulosa cell proliferation defects in the TAF4b-null ovaries.

A-D. BrdU incorporation into the nuclei of follicular granulosa cells in four week old TAF4b-heterozygous (left column) and null (right column) mice was assessed by immunolabeling. A-B. Mice stimulated with 5 IU PMSG for 24 hours. C-D. Mice stimulated with 1 μg/g estrogen for 24 hours. E-F. PCNA immunolocalization in TAF4b heterozygous (F) or null (G) ovaries detects fewer PCNA-positive nuclei in the null follicles after estrogen stimulation. G. Quantification of percent positive nuclei in follicles in the experiments in A-F. Average values from two independent experiments are presented; error bars indicate s.e.m. Statistically significant differences between TAF4b-null mice and matched controls are indicated with asterisks (p<0.001). H-I. control, uniform immunolabeling with anti-acetylated histone H3 antibody in TAF4b heterozygous (H) or null (I) ovaries. Size bars in the micrographs indicate 100 μm.
The observed lack of proliferative response to either gonadotropin or estrogen stimulation suggested that the defect in granulosa cell cycle progression is downstream of circulating blood levels of these hormones as well as from levels of their corresponding seven-transmembrane follicle stimulating hormone receptor (Fshr), or nuclear hormone estrogen receptor β (Esr2). To directly test this hypothesis, we determined the serum levels of FSH in these mice, and found it to be significantly elevated in the TAF4b-null mice compared to matched heterozygous mice (Fig. 4A). In contrast, serum levels of luteinizing hormone (LH) and prolactin were relatively equivalent between the two genotypes. The pronounced and specific increase in FSH in TAF4b-null mice is consistent with hypogonadal, hypergodanotrophic female infertility seen in many cases of POF and in mouse models of female infertility (reviewed in Goswami and Conway, 2005; Matzuk and Lamb, 2002).
Figure 4. Elevated serum FSH in TAF4b-null mice.

A. Circulating levels of FSH, prolactin, and luteinizing hormone were measured in TAF4b-null mice and in control littermates. Hormonal levels in TAF4b-null mice significantly different from controls are indicated with an asterisk (p<0.001). B-E. Real-time PCR detects normal levels of estrogen receptor beta (Esr2) and FSH receptor (Fshr) mRNAs in TAF4b-null ovarian samples at 3 weeks of age (B, D). At 17 weeks of age, both Esr2 and Fshr mRNA levels are dramatically reduced in TAF4b-null mice (C, E). 18S ribosomal RNA levels were used as a control for normalizing the data. Quantitative real-time PCR was performed in triplicate on the total RNA samples from the ovaries of the wild-type, heterozygous, and TAF4b-null mice at 3 weeks (B, D) or 17 weeks (C, E) of age. The Ct values for Esr2 (B, C) and Fshr (D, E) were normalized to 18S rRNA (ΔCt), and the resultant value was used to calculate the relative expression level. Wild-type expression level values in each experiment were arbitrarily set to 1, and the remaining values were scaled accordingly. Error bars represent 1 SD. 3 week old wild type, TAF4b-null and Het #1 were primed with E2 for 24 hrs. 3 week old Het #2 and all of 17 week old mice were non-treated.
To confirm the correct transcription of these hormone receptors, we measured the levels of Fshr and Esr-2, mRNAs in the ovaries of TAF4b-null mice and their littermates by quantitative real-time rtPCR at several time points (Fig. 4B-E). We found that at 3 weeks of age, when we administer estrogen and PMSG, TAF4b-null mice displayed no significant reduction of either mRNA (Fig. 4B, D). In contrast, at 17 weeks of age, when TAF4b-null ovaries have only a few recognizable follicle structures, levels of both receptors are decreased compared to wild-type and heterozygous mice (Fig. 4C, E). This reduction could be due to a combination of lower numbers of granulosa cells in the ovary and a downregulation of expression of these mRNAs in the remaining granulosa cells. Together, these data suggest that one of the major limitations of TAF4b-deficient granulosa cells contributing to the lack of preantral follicles in the TAF4b-null mice is that these mutant granulosa cells are incapable of efficient recruitment into proliferation by either endogenously increased FSH levels, or by exogenously supplied hormones, despite the apparent presence of normal levels of corresponding receptors. In agreement with this conclusion, superovulation regimens supplementing animals with PMSG and hCG failed to restore the numbers of pre-ovulatory and ovulation in the TAF4b-knockout females (Freiman et al., 2001). These data indicate that at early time points in the absence of TAF4b, ovaries have lost the capacity to respond to diverse hormonal signaling events in the presence of relatively normal levels of Fshr and Esr2 hormone receptors, and suggest that TAF4b is required to integrate the transcriptional response to hormonal signals.
TAF4b is required for granulosa cell survival
Quantitative analysis of follicular development in the TAF4b-null mice detected similar numbers of primary follicles in TAF4b-null and control mice, suggesting that the follicles enter the preantral growing pool with equivalent efficiency. If the only defect of TAF4b-null granulosa cells was impaired proliferation rate, we would expect to see similar numbers of preantral follicles as in wild type or heterozygous mice; however, such follicles would be of smaller size. Nevertheless, we consistently observed reduced numbers of preantral follicles, despite persistent recruitment of new primary follicles up to 12 weeks of age (Fig. 2F, G), suggesting that the observed decline of preantral follicles numbers stems not only from impaired granulosa cell proliferation, but potentially also from excessive follicular atresia. In fact, we initially observed an increase in atretic follicles in histologically stained sections of the TAF4b-null mice compared to control (Fig 2I).
To directly analyze follicular atresia on a molecular level we assessed caspase-3 activation in granulosa cells, which is a reliable marker of apoptosis in this cell type (Robles et al., 1999; Matikainen et al., 2001). As our data indicated defective response of TAF4b-null granulosa cells to hormonal stimulation, we specifically assessed apoptotic events in preantral follicles following treatment of mice with estrogen. We immunostained ovary sections from 3-4 week old TAF4b-null and control mice with an anti-caspase-3 fragment antibody (Fig. 5A-B), which detects a proteolytic product of the activated caspase-3 indicating progression of the apoptotic pathway in the cell. This assay detected increased granulosa cell apoptosis in the preantral follicles of the TAF4b-null ovaries as compared to a wild type or heterozygous control. Quantification of positively stained cells in the preantral follicles confirmed a significant increase of apoptotic cells in TAF4b-null background (Fig. 5G). As a control, we employed an anti-procaspase-3 antibody, which is expected to be expressed ubiquitously in the ovary (Fig. 5C-D). Procaspase-3 staining was detected uniformly throughout the wild-type ovaries, and appeared diffuse in the cytoplasm, and granular in the nucleus, as previously described (Ramuz et al., 2003). Total caspase-3 staining appeared diminished in the TAF4b-null ovaries; this could be either a general reduction in protein abundance, a consequence of proteolytic processing of procaspase-3 to the active enzyme form, or a reduction of caspase-3 mRNA and protein levels due to increased serum gonadotropin (Flaws et al., 1995; Boone and Tsang, 1998). To further elucidate the significance of the increase in caspase-3 activation in the TAF4b-deficient ovaries, TUNEL assays were performed on the ovarian sections. This assay similarly detected significantly increased numbers of apoptotic granulosa cells in the growing follicles of TAF4b-null mice (Fig. 5E-G). Together, these data indicate an overall reduction in survival of preantral follicles in the TAF4b-deficient ovaries and is consistent with the proposed anti-apoptotic function of TAF4b in B lymphocytes (Yamit-Hezi et al., 2000; Silkov et al., 2002). Taken together, we conclude that TAF4b plays a critical role in maintaining the correct balance between granulosa cell proliferation, survival and apoptosis that is absolutely required for proper mammalian ovarian folliculogenesis
Figure 5. Increased granulosa cell apoptosis in the TAF4b-null ovaries.
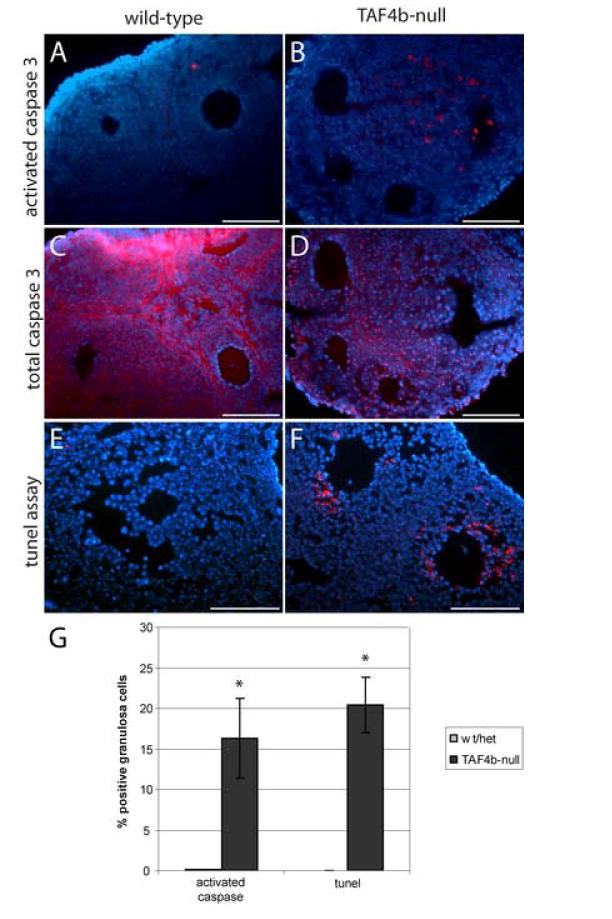
A-B. Activated caspase 3 immunostaining detects increase in the number of atretic follicles in the three week old TAF4b-null ovary. C-D. Total caspase 3 immunolabeling detects similar levels of caspase 3 in TAF4b-null and wild type ovaries (C and D are serial sections corresponding to A and B). Secondary antibody alone generates no detectable signal (data not shown). E-F. TUNEL assay detects increased apoptosis in the TAF4b-null ovaries. Control labeling without terminal transferase yield no detectable signal (data not shown). Size bars in the micrographs indicate 100 μm. G. Quantification of percent positively stained granulosa cells in experiments in A-F. Positive cells counts were performed on growing (preantral) follicles in all genetic backgrounds. Average values from two independent experiments are presented; error bars indicate s.e.m. Statistically significant differences between TAF4b-null mice and matched controls are indicated with asterisks (p<0.01).
DISCUSSION
Regulation of ovarian-specific gene expression networks in the mammalian oocyte and surrounding granulosa cells are necessary for appropriate progression through folliculogenesis. A number of critical paracrine and endocrine signaling pathways direct oocyte growth and maturation from primordial follicle assembly to ovulation in the postnatal ovary (Matzuk and Lamb, 2002). These highly coordinated developmental transitions require the integrated function of various somatic cell types providing the microenvironment, or niche, where oocytes can grow and mature to become functional gametes. Granulosa cells which are intimately associated by gap junction connections with the oocyte in the mammalian ovary provide the oocyte with essential signals and metabolites required for oocyte growth and maturation. It is only through reciprocal signaling events resulting from intimate granulosa cell-oocyte communication that proper folliculogenesis and ovulation can be achieved (Matzuk et al., 2002). While many of the signaling molecules that orchestrate ovarian follicle progression have been identified, the mechanisms of downstream transcriptional integrators that execute the correct transcriptional responses to multiple signaling pathways remain to be characterized.
We have identified a transcriptional regulatory protein called TAF4b that is required for proper folliculogenesis in the postnatal mouse ovary. TAF4b-deficient female mice are completely infertile owing to a complex series of ovarian follicle defects that results in the inability of TAF4b-deficient mice to produce functional eggs. To begin to understand the sub-ovarian developmental processes regulated by TAF4b in the postnatal mouse ovary, we have characterized the normal expression and function of TAF4b in the granulosa cells of the mouse ovary. Our results are consistent with the notion that TAF4b integrates a subset of granulosa cell gene expression networks in response to diverse signaling pathways in the ovary. In this report, we demonstrate TAF4b protein expression in granulosa cells by immunostaining wild-type ovarian sections with affinity-purified anti-TAF4b antibodies, as well as by Western blot analysis of purified granulosa cell extracts. In addition to granulosa cell expression, a recent report has revealed the potential expression of TAF4b in oocytes (Falender et al., 2005). The difference between the patterns observed previously and in this study may result from utilization of different antibodies, fixation conditions, and/or immunostaining procedures. Alternatively, TAF4b may be expressed in multiple cell types within the mouse ovary including granulosa cells and oocytes. Strikingly, TAF4b has been recently found to be expressed and posttranscriptionally modified in a human ovarian granulosa cell tumor line (Wu et al., 2005). Following our detection of TAF4b expression in the granulosa cells, we then set out to examine the functional significance of TAF4b during folliculogenesis in the postnatal ovary.
The in-depth analysis of folliculogenesis of TAF4b-null females before, during, and after sexual maturation described here reveals a number of related defects that are collectively associated with the etiology of female infertility observed in the TAF4b-deficient mice. At three weeks of age, immature TAF4b-null mice display a striking reduction of primordial follicle pools in comparison to matched heterozygous or wild-type mice. At present, we cannot distinguish whether this defect is due to abnormal development of primordial follicles or to premature culling of these follicles from the ovary by excessive follicular atresia. In addition to detecting reduction in primordial follicle numbers, we observed elevated numbers of atretic follicles and fewer preantral follicles at all time points examined. This observation suggests that TAF4b-null follicles may undergo proliferative defects as well as survival defects. As some TAF4b-null follicles are selected for further maturation to antral follicles, we conclude that TAF4b disruption does not result in a complete block of folliculogenesis at any single time point. Rather, our data support the hypothesis that TAF4b-defcient ovaries are defective at multiple steps required throughout folliculogenesis. This phenotype may reflect a pleiotropic effect of the loss of a key gonadal transcription factor, TAF4b, and inability of the cells to fully and reliably compensate for its absence, even though a related TAF4 protein is still present. Similarly, TAF4b itself has been found unable to compensate for TAF4 depletion in mouse embryonic fibroblasts, suggesting that TFIID complexes containing only TAF4 may regulate a distinct though overlapping set of genes than those containing TAF4b (Mengus et al., 2005). The exact biochemical properties and mechanistic functions of TAF4b-containing TFIID complexes still remain to be explored.
Although the number of primordial follicles in the TAF4b-null mice is reduced, this deficiency is not accompanied by a statistically significant reduction in the number of primary follicles. This observation is consistent with previous reports that the number of primordial follicles in the resting pool does not alter the rate of follicle growth initiation (Hirshfield, 1994; Ratts et al., 1995; Perez et al., 1999). Prenatal exposure of rats to a germ cell toxicant busulfan results in a significant reduction of the oocyte reserve; the treated animals then exhibit a shortened reproductive lifespan. However, at 3 weeks of age, the ovaries of such rats contain similar numbers of preantral follicles compared with controls, even though the primordial follicle stock is nearly depleted (Hirshfield, 1994). Likewise, genetic deficiencies reducing the numbers of primordial follicles at birth, as in the Bcl2-null mice, do not lead to a significant decrease in the numbers of primordial and preantral follicles (Ratts et al., 1995). Conversely, genetic manipulations increasing primordial oocyte pool, as in the Bax-null mice, prolong ovarian lifespan while maintaining similar rates of follicle recruitment into the growing phase as compared to wild-type (Perez et al., 1999). Based on these observations, we hypothesize that the low numbers of preantral follicles in the TAF4b-null mouse represent an independent deficiency from a reduction in the primordial follicle pool.
Consistent with this hypothesis, we observe relatively normal recruitment of the primordial follicles of the TAF4b-null mice into the growing phase, manifested by normal numbers of primary follicles. These primary follicles go on to form preantral follicles, but instead of increasing in size and forming an antrum, they predominantly undergo atresia. TAF4b requirement for granulosa cell survival is consistent with the observed expression of TAF4b-protein in the preantral follicles of the wild-type mice (Fig. 1C). It is furthermore highlighted by greater incidence of apoptotic processes in the preantral follicles of TAF4b-null mice subsequent to estrogen treatment (Fig. 5G) as compared to wild type controls, where estrogen provides a pro-survival signal. Collectively, these data suggest that TAF4b might be a marker for follicular selection, if not simply a requisite component of the selection pathway. Protection of granulosa cells from apoptosis is a well-documented consequence of hormonal signaling both in vivo and in vitro (Robles et al., 1999; Matikainen et al., 2001; Quirk et al., 2006). Furthermore, increased resistance of the dominant (selected) follicles to apoptosis is dependent on proliferative response of their constituent granulosa cells (Quirk et al., 2004). Therefore, the increased atresia as well as deficient granulosa cell proliferation observed in TAF4b-null follicles may both be explained by an aberrant response to hormonal signaling.
Recruitment of cells into the cell cycle is most often regulated by the accumulation of G1 regulating D-type cyclins. For example, ovarian granulosa cells depend on expression of cyclin D2 for normal proliferation in response to FSH (Sicinski et al., 1996). Strikingly, TAF4b is a regulator of cyclin D2 transcription, functioning by directly binding to its core promoter (Geles et al., 2006). In agreement with this observation, expression of cyclin D2 mRNA is reduced in the TAF4b-null ovaries (Freiman et al., 2001). Here, we also detect decreased levels of cyclin D2 protein in ovarian granulosa cells of TAF4b-null ovaries (data not shown). Together, these data suggest that TAF4b may be part of an important regulatory cascade involved in the modulation of cyclin D2 expression. In addition, we have recently shown c-Jun to be a direct target of TAF4b regulation in granulosa cells by promoter recruitment (Geles et al., 2006). C-Jun is an important regulator of cell proliferation and phospho-c-Jun expression is reported in the mitotic granulosa cells of preantral mouse ovarian follicles (Oktay and Oktay, 2004). Given the potential roles of c-Jun and cyclin D2 in carcinogenesis (Chu et al., 2002; Eferl and Wagner, 2003; Nateri et al., 2005) and the role of TAF4b in promoting granulosa cell proliferation documented here, we speculate that deregulated expression of TAF4b in the ovary may be associated with subtypes of ovarian cancer in women, especially during granulosa cell tumorigenesis. In fact, a recent report has demonstrated the elevated levels and posttranslational modifications of TAF4b in a human granulosa cell tumor line (Wu et al., 2005). Taken together, we hypothesize that the over-expression of TAF4b in the granulosa cells of the human ovary may be relevant to the etiology of granulosa cell tumorigenesis in women.
In addition to defects in proliferation, we observed increased granulosa cell apoptosis in the TAF4b-deficient ovaries in comparison to wild-type counterparts. This is consistent with previously described anti-apoptotic functions of TAF4b in B and T lymphocytes (Yamit-Hezi et al., 2000; Silkov et al., 2002). While the precise molecular mechanisms of apoptotic suppression in granulosa cells remain to be elucidated, it is likely that TAF4b-containing TFIID complexes specifically regulate expression of a subset of gene targets involved in executing the apoptotic pathway. This could be directly through activator binding and/or promoter recruitment or indirectly through modulating the expression of a downstream regulator of apoptosis. Alternatively, TAF4b may play a novel and more direct role in the regulation of apoptosis. Experiments discriminating between these diverse molecular mechanisms are required in the future.
In conclusion, we posit that TAF4b is a prominent integrator of granulosa cell gene expression programs that are required for granulosa cell proliferation and survival throughout folliculogensis. These processes are most likely disrupted in the absence of TAF4b because of pleiotropic defects associated with interruption of normal development of ovarian follicles. As the granulosa cell-oocyte interactions falter, follicle growth and maturation dwindle until severe follicle depletion is observed in the adult TAF4b-null mice. Why the addition of TAF4b variant to the canonical TFIID complex changes its transcriptional output still remains a mystery. TAF4b may bind to cell type-specific activators and/or ubiquitous activators to generate granulosa cell-specific gene expression patterns. It also may bind to other gonadal-enriched members of core transcriptional regulatory complexes including TFIIA (Xiao et al., 2006). Alternatively, in combination with its function within TFIID, TAF4b may function in a TFIID-independent fashion in the ovary. Perhaps TAF4b is monitoring the signaling or cell cycle status of granulosa cells and responds to certain cellular events by incorporation into TFIID complexes to modulate gene expression patterns accordingly. Further molecular and cellular dissection of the granulosa cell- and oocyte-specific functions of TAF4b, and the combination thereof in transcription and oogenesis is necessary to advance our understanding of fundamental principles regulating female reproductive development in mammals. Such knowledge may have profound implications for the treatment of ovarian pathologies including premature ovarian failure and certain subtypes of ovarian cancers in women.
Acknowledgments
The authors would like to thank Gary Wessel, John Coleman, Kim Boekelheide, and Mary Hixon for sharing reagents, equipment and for helpful suggestions. We thank Melissa Pepling for sharing unpublished data and we are grateful to Eli Adashi and Peter Fuller for insightful comments on the manuscript. We thank Ken Geles, Mike Marr and Shuang Zheng for helpful input throughout these studies. This research was supported in part by NIH/NCRR COBRE and Ellison Medical Foundation awards to R.N.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript.The manuscript will undergo copyediting, typesetting, and review of the resulting proofbefore it is published in its final citable form. Please note that during the productionprocess errorsmaybe discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
References
- Albright SR, Tjian R. TAFs revisited: more data reveal new twists and confirm old ideas. Gene. 2000;242:1–13. doi: 10.1016/s0378-1119(99)00495-3. [DOI] [PubMed] [Google Scholar]
- Boone DL, Tsang BK. Caspase-3 in the rat ovary: localization and possible role in follicular atresia and luteal regression. Biol Reprod. 1998;58:1533–9. doi: 10.1095/biolreprod58.6.1533. [DOI] [PubMed] [Google Scholar]
- Chu S, Rushdi S, Zumpe ET, Mamers P, Healy DL, Jobling T, Burger HG, Fuller PJ. FSH-regulated gene expression profiles in ovarian tumours and normal ovaries. Mol Hum Reprod. 2002;8:426–33. doi: 10.1093/molehr/8.5.426. [DOI] [PubMed] [Google Scholar]
- Dejong J. Basic mechanisms for the control of germ cell gene expression. Gene. 2006;366:39–50. doi: 10.1016/j.gene.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–68. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- Falender AE, Shimada M, Lo YK, Richards JS. TAF4b, a TBP associated factor, is required for oocyte development and function. Dev Biol. 2005;288:405–19. doi: 10.1016/j.ydbio.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Kugu K, Trbovich AM, DeSanti A, Tilly KI, Hirshfield AN, Tilly JL. Interleukin-1 beta-converting enzyme-related proteases (IRPs) and mammalian cell death: dissociation of IRP-induced oligonucleosomal endonuclease activity from morphological apoptosis in granulosa cells of the ovarian follicle. Endocrinology. 1995;136:5042–53. doi: 10.1210/endo.136.11.7588240. [DOI] [PubMed] [Google Scholar]
- Freiman RN, Albright SR, Zheng S, Sha WC, Hammer RE, Tjian R. Requirement of tissue-selective TBP-associated factor TAFII105 in ovarian development. Science. 2001;293:2084–7. doi: 10.1126/science.1061935. [DOI] [PubMed] [Google Scholar]
- Geles KG, Freiman RN, Liu WL, Zheng S, Voronina E, Tjian R. Cell-type-selective induction of c-jun by TAF4b directs ovarian-specific transcription networks. Proc Natl Acad Sci U S A. 2006;103:2594–2599. doi: 10.1073/pnas.0510764103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G, Pascal E, Tseng ZH, Tjian R. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci U S A. 1994;91:192–6. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Using antibodies : a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor,NY: 1999. [Google Scholar]
- Hiller M, Chen X, Pringle MJ, Suchorolski M, Sancak Y, Viswanathan S, Bolival B, Lin TY, Marino S, Fuller MT. Testis-specific TAF homologs collaborate to control a tissue-specific transcription program. Development. 2004;131:5297–308. doi: 10.1242/dev.01314. [DOI] [PubMed] [Google Scholar]
- Hiller MA, Lin TY, Wood C, Fuller MT. Developmental regulation of transcription by a tissue-specific TAF homolog. Genes Dev. 2001;15:1021–30. doi: 10.1101/gad.869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN. Relationship between the supply of primordial follicles and the onset of follicular growth in rats. Biol Reprod. 1994;50:421–8. doi: 10.1095/biolreprod50.2.421. [DOI] [PubMed] [Google Scholar]
- Hochheimer A, Tjian R. Diversified transcription initiation complexes expand promoter selectivity and tissue-specific gene expression. Genes Dev. 2003;17:1309–20. doi: 10.1101/gad.1099903. [DOI] [PubMed] [Google Scholar]
- Hoey T, Weinzierl RO, Gill G, Chen JL, Dynlacht BD, Tjian R. Molecular cloning and functional analysis of Drosophila TAF110 reveal properties expected of coactivators. Cell. 1993;72:247–60. doi: 10.1016/0092-8674(93)90664-c. [DOI] [PubMed] [Google Scholar]
- Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci U S A. 2004;101:11209–14. doi: 10.1073/pnas.0404372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadakia R, Arraztoa JA, Bondy C, Zhou J. Granulosa cell proliferation is impaired in the Igf1 null ovary. Growth Horm IGF Res. 2001;11:220–4. doi: 10.1054/ghir.2001.0201. [DOI] [PubMed] [Google Scholar]
- Komarnitsky PB, Michel B, Buratowski S. TFIID-specific yeast TAF40 is essential for the majority of RNA polymerase II-mediated transcription in vivo. Genes Dev. 1999;13:2484–9. doi: 10.1101/gad.13.19.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon B, Tjian R. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 2000;14:2551–69. doi: 10.1101/gad.831000. [DOI] [PubMed] [Google Scholar]
- Levine M, Tjian R. Transcription regulation and animal diversity. Nature. 2003;424:147–51. doi: 10.1038/nature01763. [DOI] [PubMed] [Google Scholar]
- Martianov I, Fimia GM, Dierich A, Parvinen M, Sassone-Corsi P, Davidson I. Late arrest of spermiogenesis and germ cell apoptosis in mice lacking the TBP-like TLF/TRF2 gene. Mol Cell. 2001;7:509–15. doi: 10.1016/s1097-2765(01)00198-8. [DOI] [PubMed] [Google Scholar]
- Matangkasombut O, Auty R, Buratowski S. Structure and function of the TFIID complex. Adv Protein Chem. 2004;67:67–92. doi: 10.1016/S0065-3233(04)67003-3. [DOI] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Zheng TS, Kluzak TR, Rueda BR, Flavell RA, Tilly JL. Caspase-3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology. 2001;142:2468–80. doi: 10.1210/endo.142.6.8078. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–80. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4(Suppl):s41–9. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- Mengus G, Fadloun A, Kobi D, Thibault C, Perletti L, Michel I, Davidson I. TAF4 inactivation in embryonic fibroblasts activates TGFβ signalling and autocrine growth. Embo J. 2005;24:2753–67. doi: 10.1038/sj.emboj.7600748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Carvajal L, Medico L, Pepling M. Expression of Stat3 in germ cells of developing and adult mouse ovaries and testes. Gene Expr Patterns. 2005;5:475–82. doi: 10.1016/j.modgep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–5. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- Oktay KH, Oktay MH. Immunohistochemical analysis of tyrosine phosphorylation and AP-1 transcription factors c-Jun, Jun D, and Fos family during early ovarian follicle development in the mouse. Appl Immunohistochem Mol Morphol. 2004;12:364–9. doi: 10.1097/00129039-200412000-00014. [DOI] [PubMed] [Google Scholar]
- Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–3. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- Quirk SM, Cowan RG, Harman RM. The susceptibility of granulosa cells to apoptosis is influenced by oestradiol and the cell cycle. J Endocrinol. 2006;189:441–53. doi: 10.1677/joe.1.06549. [DOI] [PubMed] [Google Scholar]
- Quirk SM, Cowan RG, Harman RM, Hu CL, Porter DA. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J Anim Sci. 2004;82(ESuppl):E40–52. doi: 10.2527/2004.8213_supplE40x. [DOI] [PubMed] [Google Scholar]
- Ramuz O, Isnardon D, Devilard E, Charafe-Jauffret E, Hassoun J, Birg F, Xerri L. Constitutive nuclear localization and initial cytoplasmic apoptotic activation of endogenous caspase-3 evidenced by confocal microscopy. Int J Exp Pathol. 2003;84:75–81. doi: 10.1046/j.1365-2613.2003.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995;136:3665–8. doi: 10.1210/endo.136.8.7628407. [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol. 1998;12:924–40. doi: 10.1210/mend.12.7.0138. [DOI] [PubMed] [Google Scholar]
- Robles R, Tao XJ, Trbovich AM, Maravel DV, Nahum R, Perez GI, Tilly KI, Tilly JL. Localization, regulation and possible consequences of apoptotic protease-activating factor-1 (Apaf-1) expression in granulosa cells of the mouse ovary. Endocrinology. 1999;140:2641–4. doi: 10.1210/endo.140.6.6931. [DOI] [PubMed] [Google Scholar]
- Saluja D, Vassallo MF, Tanese N. Distinct subdomains of human TAFII130 are required for interactions with glutamine-rich transcriptional activators. Mol Cell Biol. 1998;18:5734–43. doi: 10.1128/mcb.18.10.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–4. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- Silkov A, Wolstein O, Shachar I, Dikstein R. Enhanced apoptosis of B and T lymphocytes in TAFII105 dominant-negative transgenic mice is linked to nuclear factor-kappa B. J Biol Chem. 2002;277:17821–9. doi: 10.1074/jbc.M200696200. [DOI] [PubMed] [Google Scholar]
- Taatjes DJ, Marr MT, Tjian R. Regulatory diversity among metazoan co-activator complexes. Nat Rev Mol Cell Biol. 2004;5:403–10. doi: 10.1038/nrm1369. [DOI] [PubMed] [Google Scholar]
- Tanese N, Saluja D, Vassallo MF, Chen JL, Admon A. Molecular cloning and analysis of two subunits of the human TFIID complex: hTAFII130 and hTAFII100. Proc Natl Acad Sci U S A. 1996;93:13611–6. doi: 10.1073/pnas.93.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya AB, Khan M, Mou TC, Junker M, Gray DM, DeJong J. The germ cell-specific transcription factor ALF. Structural properties and stabilization of the TATA-binding protein (TBP)-DNA complex. J Biol Chem. 2002;277:34208–16. doi: 10.1074/jbc.M204808200. [DOI] [PubMed] [Google Scholar]
- van Betteraey-Nikoleit M, Eisele KH, Stabenow D, Probst H. Analyzing changes of chromatin-bound replication proteins occurring in response to and after release from a hypoxic block of replicon initiation in T24 cells. Eur J Biochem. 2003;270:3880–90. doi: 10.1046/j.1432-1033.2003.03769.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lu Y, Hu Y, Li R. Cyclic AMP-dependent modification of gonad-selective TAF(II)105 in a human ovarian granulosa cell line. J Cell Biochem. 2005;96:751–9. doi: 10.1002/jcb.20577. [DOI] [PubMed] [Google Scholar]
- Xiao L, Kim M, Dejong J. Developmental and cell type-specific regulation of core promoter transcription factors in germ cells of frogs and mice. Gene Expr Patterns. 2006;6:409–19. doi: 10.1016/j.modgep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Yamit-Hezi A, Nir S, Wolstein O, Dikstein R. Interaction of TAFII105 with selected p65/RelA dimers is associated with activation of subset of NF-kappa B genes. J Biol Chem. 2000;275:18180–7. doi: 10.1074/jbc.275.24.18180. [DOI] [PubMed] [Google Scholar]
- Zhang D, Penttila TL, Morris PL, Teichmann M, Roeder RG. Spermiogenesis deficiency in mice lacking the Trf2 gene. Science. 2001;292:1153–5. doi: 10.1126/science.1059188. [DOI] [PubMed] [Google Scholar]


