Abstract
Nonsyndromic cleft lip with or without cleft palate (NSCL/P) is one of the most common congenital facial defects, with an incidence of 1 in 700–1,000 live births among individuals of European descent. Several linkage and association studies of NSCL/P have suggested numerous candidate genes and genomic regions. A genomewide linkage analysis of a large multigenerational family (UR410) with NSCL/P was performed using a single-nucleotide–polymorphism array. Nonparametric linkage (NPL) analysis provided significant evidence of linkage for marker rs728683 on chromosome 18q21.1 (NPL=43.33 and P=.000061; nonparametric LOD=3.97 and P=.00001). Parametric linkage analysis with a dominant mode of inheritance and reduced penetrance resulted in a maximum LOD score of 3.61 at position 47.4 Mb on chromosome 18q21.1. Haplotype analysis with informative crossovers defined a 5.7-Mb genomic region spanned by proximal marker rs1824683 (42,403,918 bp) and distal marker rs768206 (48,132,862 bp). Thus, a novel genomic region on 18q21.1 was identified that most likely harbors a high-risk variant for NSCL/P in this family; we propose to name this locus “OFC11” (orofacial cleft 11).
Cleft lip and palate are birth defects that affect the upper lip and the roof of the mouth. Nonsyndromic cleft lip with or without cleft palate (NSCL/P) is one of the most common congenital craniofacial birth defects, affecting 1 in 700–1,000 newborns in the United States each year.1 Its highest prevalence rates are in Native Americans and Asians.2–4 Nonsyndromic familial NSCL/P represents almost half of facial malformations; the cases can be sporadic or inherited as an autosomal dominant trait. On the other hand, >400 syndromes, including numerous chromosomal anomalies, may include a facial cleft as one of the manifestations. Some of the common syndromes and/or anomalies associated with such clefting include van der Woude (MIM 119300), Apert (MIM 101200), Meckel (MIM 249000), Treacher Collins (MIM 154500), and Zlotogora-Ogur (MIM 225060) syndromes; however, the genes mutated in these disorders are not responsible for NSCL/P.5 Recent reports suggest that several genes on various chromosomal regions have pathogenic mutations associated with NSCL/P, including LHX8 (MIM 604425) on 1p31.1, SKI (MIM *164780) on 1p36.3, MTHFR (MIM *607093) on 1p36.3, IRF6 (MIM *607199) on 1q32-q41, TGFB2 on 1q41, TGFA (MIM *190170) on 2p13.3, GLI2 (MIM *165230) on 2q14.2, SATB2 (MIM *608148) on 2q32-q33, SUMO1 (MIM *601912) on 2q33.1, P63 (MIM *603273) on 3q27-q29, MSX1 (MIM *142983) on 4p16.3-p16.1, SPRY1 (MIM *602465) on 4q28.1, MSX2 (MIM *123101) on 5q34-q35, F13A1 (MIM +134570) on 6p25.3-p24.3, TGFBR1 (MIM *190181) on 9q33-q34, FOXE1 (MIM *602617) on 9q22.33, PVRL1 (MIM *600644) on 11q23-q24, SPRY2 (MIM *602466) on 13q31.1, TGFB3 (MIM *190230) on 14q24.3, JAG2 (MIM *602570) on 14q32.33, GABRB3 (MIM *137192) on 15q11.2-q12, RARA (MIM *180240) on 17q21.1, BCL3 (MIM *109560) on 19q13.31, TGFB1 (MIM *190180) on 19q13.2, TBX1 (MIM *602054) on 22q11.21, and PHF8 (MIM *300560) on Xp11.26–13; however, none of these seem to be a common locus for the majority of sporadic or familial cases of NSCL/P.14–16
Various independent association and linkage studies of different populations have identified 10 loci with evidence of linkage for syndromic and/or nonsyndromic orofacial cleft (OFC): on 6p24.3 (OFC1 [MIM 119530]), 2p13 (OFC2 [MIM 602966]), 19q13 (OFC3 [MIM 600757]), 4q21-q31 (OFC4 [MIM 608371), 4p16.1 (OFC5 [MIM 608874]), 1q32-q41 (OFC6 [MIM 608864]), 11q23-q24 (OFC7 [MIM 600644]), 3q27 (OFC8 [MIM 129400]), 13q33.1-34 (OFC9 [MIM 610361]), and 2q32.2-q33 (OFC10 [MIM 601912]). Pathogenic mutations have been identified in 4 of these 10 loci: MSX1 on 4p16.1, IRF6 on 1q32-q41, PVRL1 on 11q23-q24, and TP73L on 3q27.17–20 Increased incidence of clefts associated with chromosomal aberrations involving chromosomes 13 and 18 have also been reported.21,22
A genomewide linkage analysis was performed on an extended American family (UR410) of self-reported European origin with NSCL/P and likely autosomal dominant mode of inheritance. The family initially was tested for linkage to loci reported elsewhere to be candidates for autosomal dominant forms of NSCL/P (OFC4). Markers analyzed by Beiraghi et al.23 for the OFC4 locus on chromosome 4q provided weak evidence of linkage (LOD score <2.3). The present analysis with use of SNP markers provides significant evidence of a susceptibility locus on a genomic region of ∼5.7 Mb on chromosome 18q21.1.
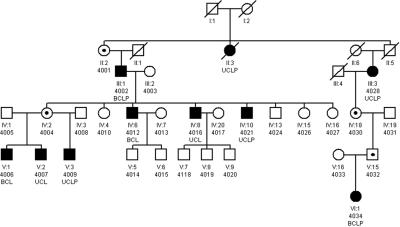
The six-generation NSCL/P-affected family (UR410) with probable autosomal dominant inheritance was first ascertained in Switzerland; however, a branch of 30 individuals residing in the United States has extended the pedigree reported by Ward et al.24 Of the 52 individuals in this family, 11 (7 males and 4 females) showed NSCL/P phenotypes, ranging from unilateral to bilateral NSCL/P. There were no other associated anomalies observed in the individuals of this family. Informed consent was obtained from all subjects who participated in the study, and blood samples were collected from all cooperating family members. Samples from a total of 27 individuals were used for the linkage analysis: 9 affected individuals, 4 obligate carriers, and 14 apparently unaffected individuals (fig. 1).
Figure 1. .
Pedigree of family UR410 with NSCL/P. Affected individuals are shown with blackened symbols, and unaffected individuals are shown with unblackened symbols. Samples included in the analysis are numbered below their pedigree symbols. A dot in the center of a symbol indicates an individual who is an obligate carrier and produced affected children with NSCL/P. BCLP=bilateral cleft lip and cleft palate; UCLP=unilateral cleft lip and cleft palate; BCL=bilateral cleft lip; UCL=unilateral cleft lip.
DNA from peripheral-blood samples was isolated using DNA-extraction kits. A genomewide genotyping was undertaken using GeneChip Mapping 10K XbaI Array containing 10,555 SNPs. These SNP markers are equally distributed in the genome, with mean intermarker distances of 250 kb and an average heterozygosity of 0.38 (Affymetrix). The assay was done using 250 ng of genomic DNA, and >99% of the SNPs were determined unequivocally for each sample. Scanned images were processed with Affymetrix Micro Array Suite Software, and data were analyzed with GDAS v2 software. PedCheck was used for the detection of any Mendelian incompatibility of genotypes.25 SNP genotype data were imported into the linkage-analysis programs GENEHUNTER26 and MERLIN.27 In the initial genome scan, evidence of linkage was assessed with a nonparametric, penetrance-independent, affected-only, and allele-sharing analysis (NPL score). With use of MERLIN, one can convert this into a nonparametric LOD score (LOD*) by maximizing the likelihood with respect to a scalar parameter, δ, that measures the amount of excess sharing of identical-by-descent alleles among affected relatives (with δ=0 corresponding to the null hypothesis of no linkage28). We used the Sall scoring function26,29 and the exponential allele-sharing model to generate the relevant linkage statistic.
When significant evidence of linkage was found by exceeding the predetermined threshold (P<.01), two-point as well as multipoint LOD scores maximized over various plausible genetic model parameters (MOD-score analysis) of the entire pedigree were performed, using a GENEHUNTER_MODSCORE analysis package. The map order and intermarker distances between SNPs were based on data from the National Center for Biotechnology Information (build 35.1). To evaluate the false-positive rate of linkage, simulations were performed to evaluate the results with use of empirical P values. The simulations were designed to match the observed data in marker density and informativeness, pedigree structure, and individual phenotypes. We generated 10,000 replicate data sets under the null hypothesis of no linkage, to estimate the empirical P value. On the basis of our simulations, the empirical threshold for genomewide significance at the 5% level was set at NPL=42.12. Putative haplotypes containing the disease-causing loci were determined by using the critical recombinants across the affected family members, with use of SIMWALK and GENEHUNTER.
Linkage analysis of tightly linked loci can lead to an excess of false-positive results if the markers are in strong linkage disequilibrium (LD) and parents are not available for genotyping.30,31 Therefore, the impact of LD on LOD scores was reassessed at the linked regions, with use of two approaches. First, MERLIN was used to accommodate marker-marker LD in both parametric and nonparametric analyses, by organizing closely located adjacent markers into clusters. Although many studies have shown that the extent and distribution of LD are variable throughout the genome, in most cases, significant LD does not influence markers separated by >0.1 cM in outbred populations.32–34 However, to be conservative, markers within 0.2 cM in a cluster were used. Second, SNPLINK35 was used to calculate LD between markers, and the LD was then removed by considering each set of markers in LD (defined as “sets in which each consecutive marker pair in the set is found to be in LD”) and by retaining one SNP from each set, chosen as the middle SNP from the set. Linkage was then recalculated using the new LD-free set of markers. We used LD values >0.7 and >0.4 for D′ and r2, respectively, to define significant LD, as suggested elsewhere.36,37
Before this genomewide analysis, 19 polymorphic microsatellite markers on chromosome 4q (OFC4) had been genotyped in the region from 117.06 cM to 161.04 cM (GDB Human Genome Database). It was suggested that chromosome 4q was involved in NSCL/P, because a previous limited study23 of a small branch of the UR410 family had detected LOD scores of 2.27 and 1.93 for markers D4S175 and D4S192, respectively. Linkage analysis was performed using LINKAGE, under the assumption of an autosomal dominant inheritance with reduced penetrance.
The linkage analysis of and haplotype data about chromosome 4q determined using samples from the extended pedigree excluded the published candidate genomic region23 (table 1). The genomewide linkage scan with use of an NPL analysis provided significant evidence of linkage for an NSCL/P locus (OFC11) at and around marker rs728683 on chromosome 18q21.1 (NPL=43.33; P=.000061). With use of simulations (see above), this value is equivalent to a genomewide empirical P value of .012. We also converted these NPL values to nonparametric LOD* scores,28 maximizing over δ. The peak linkage corresponds to the Kong and Cox28 LOD* score of 3.97, with P=.00001. These data were also supported by subsequent parametric linkage analysis, with a dominant mode of inheritance and reduced penetrance. The maximum multipoint LOD score of 3.61 at position 47.4 Mb on chromosome 18 was observed. Twenty-three SNP markers, spanning a region of ∼8.2 cM (rs1824683–rs724349), showed parametric LOD scores from 3.61 (NPL=43.30; P=.000061) to 2.92 (NPL=17.33; P=.000244) (table 2). The best-fitted parametric model that was used for obtaining the optimum linkage results was incomplete dominance with 65% penetrance and disease-allele frequency of 0.0001. No additional peak with a statistically significant LOD score was found in the genome (fig. 2). Haplotype analysis for the 18q21.1-linked region was performed using 27 informative SNP markers. Informative crossovers in affected individuals IV-10 (4021) and V-3 (4009), between rs1824683/rs328149 and rs768207/rs768206, respectively, defined the NSCL/P candidate region of 5.7 Mb, bordered by proximal marker rs1824683 (physical map position 42,403,918 bp) and distal marker rs768206 (physical map position 48,132,862 bp) (fig. 3).
Table 1. .
Two-Point LOD Scores between NSCL/P in family UR410 and Polymorphic Markers from Known Candidate Genomic Region on Chromosome 4q[Note]
| LOD at θ= |
||||||||
| Marker | .00 | .01 | .05 | .10 | .20 | .30 | .40 | Mapping Position (cM) |
| D4S193 | −9.86 | −3.12 | −.59 | .28 | .76 | .71 | .43 | 117.06 |
| D4S1612 | −10.37 | −3.98 | −1.33 | −.31 | .44 | .59 | .42 | 124.45 |
| D4S194 | −4.73 | −1.85 | −.70 | −.28 | −.01 | .02 | .00 | 126.15 |
| D4S2975 | −3.14 | −.46 | .24 | .47 | .51 | .34 | .12 | 126.71 |
| D4S1615 | −1.69 | .77 | 1.27 | 1.31 | 1.08 | .69 | .26 | 128.31 |
| D4S2307 | −3.21 | −.45 | .76 | 1.12 | 1.19 | .96 | .55 | 129.92 |
| D4S429 | −10.72 | −3.30 | −1.35 | −.62 | −.09 | .00 | −.01 | 131.00 |
| D4S3039 | −6.96 | −1.90 | −.62 | −.18 | .10 | .10 | .01 | 132.72 |
| D4S175 | −3.36 | .45 | 1.64 | 1.97 | 1.93 | 1.52 | .87 | 134.74 |
| D4S2939 | −3.58 | −1.94 | −.72 | −.17 | .20 | .20 | .09 | 142.24 |
| D4S192 | −3.11 | −.55 | .16 | .43 | .60 | .54 | .34 | 143.31 |
| D4S424 | −1.22 | .45 | .99 | 1.10 | 1.00 | .76 | .42 | 144.56 |
| D4S2998 | −9.60 | −3.87 | −1.23 | −.23 | .50 | .63 | .44 | 145.98 |
| D4S1586 | −5.77 | −1.98 | −.66 | −.16 | .19 | .25 | .17 | 147.06 |
| D4S3008 | .63 | .62 | .59 | .54 | .42 | .30 | .16 | 152.98 |
| D4S3021 | −7.44 | −5.00 | −2.94 | −1.99 | −1.03 | −.51 | −.19 | 154.63 |
| D4S2631 | −7.21 | −3.25 | −1.31 | −.59 | .07 | .06 | .05 | 155.75 |
| D4S413 | −11.86 | −3.41 | −1.46 | −.73 | −.19 | −.03 | .00 | 157.99 |
| D4S3033 | −9.32 | −2.55 | −.09 | .70 | 1.04 | .84 | .42 | 161.04 |
Note.— Scores were calculated under a dominant model with 75% penetrance and .0001 disease-allele frequency.
Table 2. .
Parametric-Linkage and NPL Scores for the Peak Region at Chromosome 18q21.1[Note]
| Position |
Genome Scan of theNSCL/P-Affected Family |
||||
| Nonparametric LOD |
|||||
| SNP | Genetic (cM) |
Physical (bp) |
Parametric LOD | NPL | P |
| rs726131 | 65.641 | 42,118,566 | −.55 | 10.53 | .000656 |
| rs1824683 | 66.057 | 42,403,918 | 3.48 | 34.40 | .000244 |
| rs328149 | 66.087 | 42,424,469 | 3.51 | 36.10 | .000244 |
| rs645631 | 66.203 | 42,505,993 | 3.61 | 42.82 | .000153 |
| rs1073744 | 66.204 | 42,506,272 | 3.61 | 42.81 | .000153 |
| rs953291 | 67.077 | 43,124,497 | 3.61 | 42.78 | .000153 |
| rs1944574 | 68.548 | 43,979,261 | 3.61 | 42.82 | .000153 |
| rs953570 | 68.567 | 43,988,463 | 3.61 | 42.82 | .000153 |
| rs1944584 | 68.667 | 44,035,980 | 3.61 | 42.82 | .000153 |
| rs1398193 | 68.885 | 44,241,137 | 3.61 | 42.83 | .000153 |
| rs1877412 | 68.885 | 44,241,673 | 3.61 | 42.83 | .000153 |
| rs1943984 | 70.873 | 45,477,909 | 3.61 | 42.76 | .000153 |
| rs1985467 | 71.431 | 45,928,259 | 3.61 | 42.82 | .000153 |
| rs1822466 | 71.519 | 45,999,664 | 3.61 | 42.83 | .000153 |
| rs1373185 | 71.519 | 45,999,849 | 3.61 | 42.83 | .000153 |
| rs768360 | 71.542 | 46,018,071 | 3.61 | 42.83 | .000153 |
| rs2006747 | 71.795 | 46,222,356 | 3.61 | 42.83 | .000153 |
| rs728682 | 71.966 | 46,349,352 | 3.61 | 42.83 | .000153 |
| rs2969972 | 71.989 | 46,362,761 | 3.61 | 42.84 | .000061 |
| rs1822458 | 72.264 | 46,528,403 | 3.61 | 42.94 | .000061 |
| rs1941962 | 72.892 | 46,999,467 | 3.61 | 43.17 | .000061 |
| rs1369766 | 73.228 | 47,272,878 | 3.61 | 43.30 | .000061 |
| rs728683 | 73.339 | 47,362,995 | 3.61 | 43.33 | .000061 |
| rs724349 | 74.247 | 48,019,014 | 2.92 | 17.33 | .000244 |
| rs959655 | 74.477 | 48,132,862 | .23 | 10.74 | .000610 |
| rs768207 | 74.477 | 48,132,968 | −.04 | 10.73 | .000610 |
Note.— The model used for parametric analysis was incomplete dominance with 65% penetrance and a disease-allele frequency of .0001.
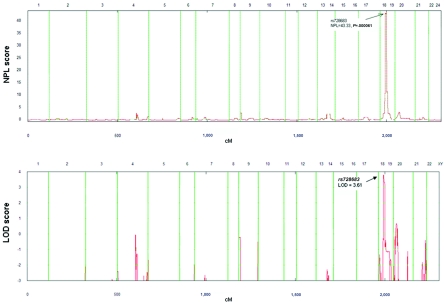
Figure 2. .
Multipoint linkage analysis with use of NPL in the genomewide scan of multigenerational family UR410 with NSCL/P. In the upper panel, the X-axis represents the chromosome location for the 22 autosomes, and the Y-axis represents the NPL score. The highest peak is on chromosome 18q21.1. In the lower panel, the X-axis shows chromosome location for the 22 chromosomes, and the Y-axis shows the location score, calculated using GENEHUNTER software. The maximal LOD score of 3.61 is observed around marker rs728683.
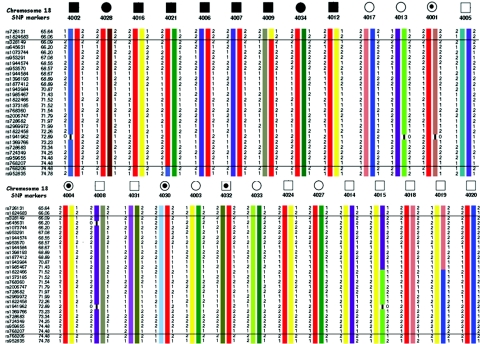
Figure 3. .
Genotypes and haplotypes of chromosome 18q21.1 SNP markers are shown below selected individuals of family UR410. Haplotypes associated with affected status are shown in red. Haplotype analysis indicated that the cosegregating segment of the NSCL/P locus is flanked proximally by marker rs1824682 (position 42403918) and distally by marker rs959655 (position 48132862) on chromosome 18q21.1.
To assess the impact of LD on linkage, we first used markers within 0.1–0.2 cM of each other in a cluster and estimated the linkage scores. Several clusters of 2–6 SNPs demonstrated LD. With the assumption of complete LD within the cluster, MERLIN uses population haplotype frequencies in the calculation of linkage. Interestingly, at chromosome 18, the LOD and NPL scores were virtually the same, especially for the significant markers. We also recalculated the linkage scores by removing the redundant SNPs (D′>0.7 or r2>0.4), using SNPLINK, and arrived at almost similar results.
The etiology of OFCs is complex, related to both genetic and environmental factors.2,38 Although there have been a number of studies undertaken to identify the genetic variations responsible for NSCL/P, the results have not been consistent among populations, and no single susceptibility gene has been identified to date that plays a major role in this disorder.39–44 The majority of these reports used small nuclear families; however, there are a few exceptional large multigenerational families with both an autosomal dominant and autosomal recessive inheritance of NSCL/P that gave significant evidence of linkage to chromosomes 13q,45 17p,46 and multiple loci.47 Previous studies of a subset of pedigree UR410 suggested evidence of linkage on 4q; however, the present study, which includes 19 additional genotyped individuals (2 affected), fails to confirm this region (LOD score <2.3). The previous data were also considered to be a false-positive result by two independent studies.42,48 We report here linkage analysis, with use of an SNP array containing ∼10,000 SNP markers, that provided significant evidence of a novel susceptibility locus on a genomic region of 5.7 Mb on chromosome 18q21.1.
Several genomewide linkage studies conducted using multiplex families of various ethnic origins showed evidence of linkage at chromosome 18; however, these suggested regions either do not include the present locus or do not have statistically significant results.39,40,44,49 The genome-scan meta-analysis conducted by Marazita et al.43 of 388 extended multiplex families with NSCL/P from seven diverse populations yielded positive evidence at multiple loci, including weak evidence at the chromosome 18q21.1 region. Furthermore, the study suggested SMAD2 as a candidate in the mapped genetic interval.
A whole-genome scan, performed using a high-density SNP genotyping assay conducted by Radhakrishna et al.,45 of two large Indian families provided suggestive evidence of linkage on chromosome 18q21.1 and strong evidence on chromosome 13q33.1-34. The SNP marker (rs959655) that gave the highest NPL peak (NPL=3.64; P=.0066) on chromosome 18 in the previous study45 is also supported by parametric LOD score. This SNP marker is in the vicinity of the genetic interval mapped in the present study. Several studies have also found that genetic variation in IRF6 contributes to the development of NSCL/P50–52; however, in the present study, we did not observe any positive linkage.
Numerous reports indicate that chromosome 18–related anomalies are associated with increased incidence of NSCL/P, including deletion of 18q53–57 and full or partial trisomy 18 involving the region 18q21.21,22,58–69 Niedrist et al.22 analyzed 352 cases of trisomy 18 from northeastern Switzerland and found that 14% of the subjects had facial clefts. Schinzel et al.70 reported three subjects with the 18q syndrome associated with various developmental anomalies and cleft palate. An isochromosome 18q in a fetus with congenital megacystis, growth retardation, and cleft lip and palate was reported by Chen et al.59 Partial deletion of 18q in a patient with bilateral complete cleft lip was reported by Fujimoto et al.68 Cody et al.55 analyzed 42 individuals with deletions of 18q and observed several subjects with craniofacial abnormalities, including NSCL/P, associated with breakpoints at 18q21.1, 18q21, and 18q21.2.
The 5.7-Mb interval containing the locus for NSCL/P on chromosome 18q21.1 contains 36 annotated transcripts (Ensembl), including several genes implicated in developmental or pathological events in craniofacial birth defects. Candidate genes include protein inhibitor of activated STAT2 (PIAS2 [MIM 603567]), mothers against decapentaplegic and homologs of the Drosophila (MAD) genes (SMAD2 [MIM 601366], SMAD4 [MIM 600993], and SMAD7 [MIM 602932]). Recent reports suggested that the members of the transforming growth factor-β (TGF-β) superfamily that function in the embryonic palate are mediated through the SMAD-signaling system.71 SMAD proteins play a key role in intracellular TGF-β signaling, and mutations in the TGF-β pathway confer resistance to growth inhibition by TGF-β.72 The TGF-β3–knockout mouse resulted in cleft palate due to failure of palatal shelf fusion,73–75 and polymorphisms in the gene for TGF-β3 have been linked to the development of cleft palate in humans.6
Haplotype analysis with critical recombination events allowed us to define a 5.7-Mb disease interval delimited by proximal SNP marker rs824683 (map position 42,403,918 bp) and distal SNP marker rs768206 (map position 48,133,056 bp). The critical interval of the 11th OCF locus (OFC11) could not be further reduced, since additional members of the family were not available. Haplotype analysis identified the risk haplotype shared by all affected individuals that was not found in any of the unrelated unaffected spouses. Since NSCL/P demonstrates reduced penetrance, all unaffected parents (subjects 4001, 4004, 4030, and 4032) who had affected children shared the affected haplotype. Therefore, we speculate that individuals 4018, 4020, 4024, and 4027 with normal phenotype and “at risk” haplotypes may also be at risk of having affected children. The haplotype data also support the autosomal dominant mode of inheritance in this family. Variation in the phenotypic expression—that is, the range from bilateral to unilateral NSCL/P—in members of the same pedigree (UR410) indicates that the genetic variation responsible for these abnormalities at a single locus might have variable phenotypic expression. Few of the positional candidate genes are currently being screened for a possible role in the pathogenesis of NSCL/P, but the results are inconclusive at present. We are also pursuing cytogenetic analysis and array comparative-genomic hybridization that may reveal microdeletions and/or duplications. In summary, this study demonstrates the power of using multigenerational families in the analysis of complex traits. Identification of the pathogenic genetic variation would potentially provide significant insights into the molecular mechanisms underlying the etiology of NSCL/P.
Acknowledgments
We thank the members of family UR410 for their cooperation in the study. The study was supported in part by the Green Cross Blood Bank, Ahmedabad, Gujarat, India. K.M. is supported by a GIS-Institut des Maladies Rare grant. The laboratory of S.E.A. is supported by the Swiss National Science Foundation. S.K.N. was supported by Oklahoma Medical Research Foundation institutional grant 9124, for linkage analysis.
Web Resources
The URLs for data presented herein are as follows:
- Affymetrix, http://www.affymetrix.com/products/arrays/specific/10k.affx
- Ensembl,http://www.ensembl.org/
- GDB Human Genome Database, http://www.gdb.org/
- MERLIN, http://www.sph.umich.edu/csg/abecasis/Merlin/
- National Center for Biotechnology Information, http://www.ncbi.nih.gov/ (for build 35.1)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for van der Woude syndrome, Apert syndrome, Meckel syndrome, Treacher Collins syndrome, Zlotogora-Ogur syndrome, LHX8, SKI, MTHFR, IRF6, TGFA, GLI2, SATB2, SUMO1, P63, MSX1, SPRY1, MSX2, F13A1, TGFBR1, FOXE1, PVRL1, SPRY2, TGFB3, JAG2, GABRB3, RARA, BCL3, TGFB1, TBX1, PHF8, OFC1, OFC2, OFC3, OFC4, OFC5, OFC6, OFC7, OFC8, OFC9, OFC10, PIAS2, SMAD2, SMAD4, and SMAD7)
References
- 1.Tolarova MM, Cervenka J (1998) Classification and birth prevalence of orofacial clefts. Am J Med Genet 75:126–137 [DOI] [PubMed] [Google Scholar]
- 2.Murray JC (2002) Gene/environment causes of cleft lip and/or palate. Clin Genet 61:248–256 10.1034/j.1399-0004.2002.610402.x [DOI] [PubMed] [Google Scholar]
- 3.Murray JC (1995) Face facts: genes, environment, and clefts. Am J Hum Genet 57:227–232 [PMC free article] [PubMed] [Google Scholar]
- 4.Ankola AV, Nagesh L, Hegde P, Karibasappa GN (2005) Primary dentition status and treatment needs of children with cleft lip and/or palate. J Indian Soc Pedod Prev Dent 23:80–82 [DOI] [PubMed] [Google Scholar]
- 5.Houdayer C, Bonaiti-Pellie C, Erguy C, Soupre V, Dondon MG, Burglen L, Cougoureux E, Couderc R, Vazquez MP, Bahuau M (2001) Possible relationship between the van der Woude syndrome (vWS) locus and nonsyndromic cleft lip with or without cleft palate (NSCL/P). Am J Med Genet 104:86–92 [DOI] [PubMed] [Google Scholar]
- 6.Lidral AC, Romitti PA, Basart AM, Doetschman T, Leysens NJ, Daack-Hirsch S, Semina EV, Johnson LR, Machida J, Burds A, et al (1998) Association of MSX1 and TGFB3 with nonsyndromic clefting in humans. Am J Hum Genet 63:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein J, Mulliken JB, Stal S, Gasser DL, Malcolm S, Winter R, Blanton SH, Amos C, Seemanova E, Hecht JT (1995) Nonsyndromic cleft lip with or without cleft palate: evidence of linkage to BCL3 in 17 multigenerational families. Am J Hum Genet 57:257–272 [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira AR, Avila JR, Daack-Hirsch S, Dragan E, Felix TM, Rahimov F, Harrington J, Schultz RR, Watanabe Y, Johnson M, et al (2005) Medical sequencing of candidate genes for nonsyndromic cleft lip and palate. PLoS Genet 1:e64 10.1371/journal.pgen.0010064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyszynski DF, Maestri N, McIntosh I, Smith EA, Lewanda AF, Garcia-Delgado C, Vinageras-Guarneros E, Wulfsberg E, Beaty TH (1997) Evidence for an association between markers on chromosome 19q and non-syndromic cleft lip with or without cleft palate in two groups of multiplex families. Hum Genet 99:22–26 10.1007/s004390050303 [DOI] [PubMed] [Google Scholar]
- 10.Martinelli M, Scapoli L, Pezzetti F, Carinci F, Carinci P, Baciliero U, Padula E, Tognon M (1998) Suggestive linkage between markers on chromosome 19q13.2 and nonsyndromic orofacial cleft malformation. Genomics 51:177–181 10.1006/geno.1998.5384 [DOI] [PubMed] [Google Scholar]
- 11.Chenevix-Trench G, Jones K, Green AC, Duffy DL, Martin NG (1992) Cleft lip with or without cleft palate: associations with transforming growth factor alpha and retinoic acid receptor loci. Am J Hum Genet 51:1377–1385 [PMC free article] [PubMed] [Google Scholar]
- 12.Ardinger HH, Buetow KH, Bell GI, Bardach J, VanDemark DR, Murray JC (1989) Association of genetic variation of the transforming growth factor-alpha gene with cleft lip and palate. Am J Hum Genet 45:348–353 [PMC free article] [PubMed] [Google Scholar]
- 13.Alkuraya FS, Saadi I, Lund JJ, Turbe-Doan A, Morton CC, Maas RL (2006) SUMO1 haploinsufficiency leads to cleft lip and palate. Science 313:1751 10.1126/science.1128406 [DOI] [PubMed] [Google Scholar]
- 14.Hecht JT, Wang YP, Blanton SH, Michels VV, Daiger SP (1991) Cleft lip and palate: no evidence of linkage to transforming growth factor alpha. Am J Hum Genet 49:682–686 [PMC free article] [PubMed] [Google Scholar]
- 15.Vintiner GM, Holder SE, Winter RM, Malcolm S (1992) No evidence of linkage between the transforming growth factor-alpha gene in families with apparently autosomal dominant inheritance of cleft lip and palate. J Med Genet 29:393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vintiner GM, Lo KK, Holder SE, Winter RM, Malcolm S (1993) Exclusion of candidate genes from a role in cleft lip with or without cleft palate: linkage and association studies. J Med Genet 30:773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Boogaard MJ, Dorland M, Beemer FA, van Amstel HK (2000) MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat Genet 24:342–343 10.1038/74155 [DOI] [PubMed] [Google Scholar]
- 18.Kondo S, Schutte BC, Richardson RJ, Bjork BC, Knight AS, Watanabe Y, Howard E, de Lima RL, Daack-Hirsch S, Sander A, et al (2002) Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet 32:285–289 10.1038/ng985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sozen MA, Suzuki K, Tolarova MM, Bustos T, Fernandez Iglesias JE, Spritz RA (2001) Mutation of PVRL1 is associated with sporadic, non-syndromic cleft lip/palate in northern Venezuela. Nat Genet 29:141–142 10.1038/ng740 [DOI] [PubMed] [Google Scholar]
- 20.Leoyklang P, Siriwan P, Shotelersuk V (2006) A mutation of the p63 gene in non-syndromic cleft lip. J Med Genet 43:e28 10.1136/jmg.2005.036442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berge SJ, Plath H, Van de Vondel PT, Appel T, Niederhagen B, Von Lindern JJ, Reich RH, Hansmann M (2001) Fetal cleft lip and palate: sonographic diagnosis, chromosomal abnormalities, associated anomalies and postnatal outcome in 70 fetuses. Ultrasound Obstet Gynecol 18:422–431 10.1046/j.0960-7692.2001.00575.x [DOI] [PubMed] [Google Scholar]
- 22.Niedrist D, Riegel M, Achermann J, Schinzel A (2006) Survival with trisomy 18—data from Switzerland. Am J Med Genet A 140:952–959 [DOI] [PubMed] [Google Scholar]
- 23.Beiraghi S, Foroud T, Diouhy S, Bixler D, Conneally PM, Delozier-Blanchet D, Hodes ME (1994) Possible localization of a major gene for cleft lip and palate to 4q. Clin Genet 46:255–256 [DOI] [PubMed] [Google Scholar]
- 24.Ward RE, Bixler D, Jamison PL (1994) Cephalometric evidence for a dominantly inherited predisposition to cleft lip-cleft palate in a single large kindred. Am J Med Genet 50:57–63 10.1002/ajmg.1320500113 [DOI] [PubMed] [Google Scholar]
- 25.O’Connell JR, Weeks DE (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- 27.Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101 10.1038/ng786 [DOI] [PubMed] [Google Scholar]
- 28.Kong A, Cox NJ (1997) Allele-sharing models: LOD scores and accurate linkage tests. Am J Hum Genet 61:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittemore AS, Halpern J (1994) A class of tests for linkage using affected pedigree members. Biometrics 50:118–127 10.2307/2533202 [DOI] [PubMed] [Google Scholar]
- 30.Schaid DJ, McDonnell SK, Wang L, Cunningham JM, Thibodeau SN (2002) Caution on pedigree haplotype inference with software that assumes linkage equilibrium. Am J Hum Genet 71:992–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Q, Shete S, Amos CI (2004) Ignoring linkage disequilibrium among tightly linked markers induces false-positive evidence of linkage for affected sib pair analysis. Am J Hum Genet 75:1106–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawson E, Abecasis GR, Bumpstead S, Chen Y, Hunt S, Beare DM, Pabial J, de Lima RL, Daack-Hirsch S, Sander A, et al (2002) A first-generation linkage disequilibrium map of human chromosome 22. Nature 418:544–548 10.1038/nature00864 [DOI] [PubMed] [Google Scholar]
- 33.Phillips MS, Lawrence R, Sachidanandam R, Morris AP, Balding DJ, Donaldson MA, Studebaker JF, Ankener WM, Alfisi SV, Kuo FS, et al (2003) Chromosome-wide distribution of haplotype blocks and the role of recombination hot spots. Nat Genet 33:382–387 10.1038/ng1100 [DOI] [PubMed] [Google Scholar]
- 34.Ke X, Hunt S, Tapper W, Lawrence R, Stavrides G, Ghori J, Whittaker P, Collins A, Morris AP, Bentley D, et al (2004) The impact of SNP density on fine-scale patterns of linkage disequilibrium. Hum Mol Genet 13:577–588 10.1093/hmg/ddh060 [DOI] [PubMed] [Google Scholar]
- 35.Webb EL, Sellick GS, Houlston RS (2005) SNPLINK: multipoint linkage analysis of densely distributed SNP data incorporating automated linkage disequilibrium removal. Bioinformatics 21:3060–3061 10.1093/bioinformatics/bti449 [DOI] [PubMed] [Google Scholar]
- 36.Schaid DJ, Guenther JC, Christensen GB, Hebbring S, Rosenow C, Hilker CA, McDonnell SK, Cunningham JM, Slager SL, Blute ML, et al (2004) Comparison of microsatellites versus single-nucleotide polymorphisms in a genome linkage screen for prostate cancer–susceptibility loci. Am J Hum Genet 75:948–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.John S, Shephard N, Liu G, Zeggini E, Cao M, Chen W, Vasavda N, Mills T, Barton A, Hinks A, et al (2004) Whole-genome scan, in a complex disease, using 11,245 single-nucleotide polymorphisms: comparison with microsatellites. Am J Hum Genet 75:54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Little J, Cardy A, Munger RG (2004) Tobacco smoking and oral clefts: a meta-analysis. Bull World Health Organ 82:213–218 [PMC free article] [PubMed] [Google Scholar]
- 39.Field LL, Ray AK, Cooper ME, Goldstein T, Shaw DF, Marazita ML (2004) Genome scan for loci involved in nonsyndromic cleft lip with or without cleft palate in families from West Bengal, India. Am J Med Genet A 130:265–271 10.1002/ajmg.a.30252 [DOI] [PubMed] [Google Scholar]
- 40.Marazita ML, Field LL, Cooper ME, Tobias R, Maher BS, Peanchitlertkajorn S, Liu YE (2002) Genome scan for loci involved in cleft lip with or without cleft palate, in Chinese multiplex families. Am J Hum Genet 71:349–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz RE, Cooper ME, Daack-Hirsch S, Shi M, Nepomucena B, Graf KA, O’Brien EK, O’Brien SE, Marazita ML, Murray JC (2004) Targeted scan of fifteen regions for nonsyndromic cleft lip and palate in Filipino families. Am J Med Genet A 125:17–22 10.1002/ajmg.a.20424 [DOI] [PubMed] [Google Scholar]
- 42.Wong FK, Hagberg C, Karsten A, Larson O, Gustavsson M, Huggare J, Larsson C, Teh BT, Linder-Aronson S (2000) Linkage analysis of candidate regions in Swedish nonsyndromic cleft lip with or without cleft palate families. Cleft Palate Craniofac J 37:357–362 [DOI] [PubMed] [Google Scholar]
- 43.Marazita ML, Murray JC, Lidral AC, Arcos-Burgos M, Cooper ME, Goldstein T, Maher BS, Daack-Hirsch S, Schultz R, Mansilla MA, et al (2004) Meta-analysis of 13 genome scans reveals multiple cleft lip/palate genes with novel loci on 9q21 and 2q32-35. Am J Hum Genet 75:161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marazita ML, Field LL, Tuncbilek G, Cooper ME, Goldstein T, Gursu KG (2004) Genome-scan for loci involved in cleft lip with or without cleft palate in consanguineous families from Turkey. Am J Med Genet A 126:111–122 10.1002/ajmg.a.20564 [DOI] [PubMed] [Google Scholar]
- 45.Radhakrishna U, Ratnamala U, Gaines M, Beiraghi S, Hutchings D, Golla J, Husain SA, Gambhir PS, Sheth JJ, Sheth FJ, et al (2006) Genomewide scan for nonsyndromic cleft lip and palate in multigenerational Indian families reveals significant evidence of linkage at 13q33.1-34. Am J Hum Genet 79:580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyszynski DF, Albacha-Hejazi H, Aldirani M, Hammod M, Shkair H, Karam A, Alashkar J, Holmes TN, Pugh EW, Doheny KF, et al (2003) A genome-wide scan for loci predisposing to non-syndromic cleft lip with or without cleft palate in two large Syrian families. Am J Med Genet A 123:140–147 10.1002/ajmg.a.20283 [DOI] [PubMed] [Google Scholar]
- 47.Blanton SH, Bertin T, Patel S, Stal S, Mulliken JB, Hecht JT (2004) Nonsyndromic cleft lip and palate: four chromosomal regions of interest. Am J Med Genet A 125:28–37 10.1002/ajmg.a.20423 [DOI] [PubMed] [Google Scholar]
- 48.Blanco R, Suazo J, Santos JL, Carreno H, Palomino H, Jara L (2005) No evidence for linkage and association between 4q microsatellite markers and nonsyndromic cleft lip and palate in Chilean case-parents trios. Cleft Palate Craniofac J 42:267–271 10.1597/03-160.1 [DOI] [PubMed] [Google Scholar]
- 49.Zeiger JS, Hetmanski JB, Beaty TH, VanderKolk CA, Wyszynski DF, Bailey-Wilson JE, de Luna RO, Perandones C, Tolarova MM, Mosby T, et al (2003) Evidence for linkage of nonsyndromic cleft lip with or without cleft palate to a region on chromosome 2. Eur J Hum Genet 11:835–839 10.1038/sj.ejhg.5201052 [DOI] [PubMed] [Google Scholar]
- 50.Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, Ribeiro L, Caprau D, Christensen K, Suzuki Y, Machida J, et al (2004) Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med 351:769–780 10.1056/NEJMoa032909 [DOI] [PubMed] [Google Scholar]
- 51.Srichomthong C, Siriwan P, Shotelersuk V (2005) Significant association between IRF6 820G→A and non-syndromic cleft lip with or without cleft palate in the Thai population. J Med Genet 42:e46 10.1136/jmg.2005.032235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanton SH, Cortez A, Stal S, Mulliken JB, Finnell RH, Hecht JT (2005) Variation in IRF6 contributes to nonsyndromic cleft lip and palate. Am J Med Genet A 137:259–262 [DOI] [PubMed] [Google Scholar]
- 53.Wertelecki W, Gerald PS (1971) Clinical and chromosomal studies of the 18q- syndrome. J Pediatr 78:44–52 10.1016/S0022-3476(71)80262-7 [DOI] [PubMed] [Google Scholar]
- 54.Subrt I, Pokorny J (1970) Familial occurrence of 18q. Humangenetik 10:181–187 10.1007/BF00295518 [DOI] [PubMed] [Google Scholar]
- 55.Cody JD, Ghidoni PD, DuPont BR, Hale DE, Hilsenbeck SG, Stratton RF, Hoffman DS, Muller S, Schaub RL, Leach RJ, Kaye CI (1999) Congenital anomalies and anthropometry of 42 individuals with deletions of chromosome 18q. Am J Med Genet 85:455–462 [DOI] [PubMed] [Google Scholar]
- 56.Day EJ, Marshall R, MacDonald PA, Davidson WM (1967) Deleted chromosome 18 with paternal mosaicism. Lancet 2:1307 10.1016/S0140-6736(67)90418-7 [DOI] [PubMed] [Google Scholar]
- 57.Kline AD, White ME, Wapner R, Rojas K, Biesecker LG, Kamholz J, Zackai EH, Muenke M, Scott CI Jr, Overhauser J (1993) Molecular analysis of the 18q– syndrome—and correlation with phenotype. Am J Hum Genet 52:895–906 [PMC free article] [PubMed] [Google Scholar]
- 58.Bertollini R, Mastroiacovo P, Segni G (1985) Maternal epilepsy and birth defects: a case-control study in the Italian Multicentric Registry of Birth Defects (IPIMC). Eur J Epidemiol 1:67–72 10.1007/BF00162315 [DOI] [PubMed] [Google Scholar]
- 59.Chen CP, Chern SR, Lee CC, Town DD (1998) Isochromosome 18q in a fetus with congenital megacystis, intra-uterine growth retardation and cloacal dysgenesis sequence. Prenat Diagn 18:1068–1074 [DOI] [PubMed] [Google Scholar]
- 60.Chen CP, Tzen CY, Chang TY, Lin CJ, Wang W, Lee CC, Chen LF, Chen WL (2003) Prenatal diagnosis of de novo mosaic distal 18q deletion associated with congenital anomalies. Ultrasound Obstet Gynecol 21:202–204 10.1002/uog.45 [DOI] [PubMed] [Google Scholar]
- 61.Chen CP, Chern SR, Hung FY, Hsu CY, Chang TY, Lee CC, Town DD, Chen WL, Chen LF, Tzen CY, et al (2006) Prenatal diagnosis of pure distal 18q deletion. Prenat Diagn 26:184–185 10.1002/pd.1367 [DOI] [PubMed] [Google Scholar]
- 62.Habecker-Green JG, Naeem R, Gold H, O’Grady JP, Kanaan C, Bayer-Zwirello L, Murray MS, Cohn GM (1998) Prenatal diagnosis and clinical features of an individual with tetrasomy 18p and trisomy 18q mosaicism. J Perinatol 18:395–398 [PubMed] [Google Scholar]
- 63.Moore CA, Harmon JP, Padilla LM, Castro VB, Weaver DD (1988) Neural tube defects and omphalocele in trisomy 18. Clin Genet 34:98–103 [DOI] [PubMed] [Google Scholar]
- 64.Pluchon E, Giovangrandi Y, Labbe F, Le Bris MJ, Collet M, Brettes JP, Riviere D, Riviere MR (1993) Prenatal diagnosis of a fetus with partial monosomy 7(q34→qter) and partial trisomy 18(q21→qter). Prenat Diagn 13:983–988 10.1002/pd.1970131013 [DOI] [PubMed] [Google Scholar]
- 65.Hafner E, Sterniste W, Scholler J, Schuchter K, Philipp K (1997) Prenatal diagnosis of facial malformations. Prenat Diagn 17:51–58 [DOI] [PubMed] [Google Scholar]
- 66.Perrotin F, de Poncheville LM, Marret H, Paillet C, Lansac J, Body G (2001) Chromosomal defects and associated malformations in fetal cleft lip with or without cleft palate. Eur J Obstet Gynecol Reprod Biol 99:19–24 10.1016/S0301-2115(01)00347-5 [DOI] [PubMed] [Google Scholar]
- 67.Meins M, Bohm D, Grossmann A, Herting E, Fleckenstein B, Fauth C, Speicher MR, Schindler R, Zoll B, Bartels I, et al (2004) First non-mosaic case of isopseudodicentric chromosome 18 (psu idic(18)(pter→q22.1::q22.1→pter) is associated with multiple congenital anomalies reminiscent of trisomy 18 and 18q- syndrome. Am J Med Genet A 127:58–64 10.1002/ajmg.a.20644 [DOI] [PubMed] [Google Scholar]
- 68.Fujimoto S, Hida K, Tateishi A, Nakashima T, Kameyama Y, Yamada N (1991) 18q-syndrome with cleft lip and palate: a clinically diagnosed case. J Craniomaxillofac Surg 19:61–63 [DOI] [PubMed] [Google Scholar]
- 69.Tongsong T, Sirichotiyakul S, Wanapirak C, Chanprapaph P (2002) Sonographic features of trisomy 18 at midpregnancy. J Obstet Gynaecol Res 28:245–250 10.1046/j.1341-8076.2002.00053.x [DOI] [PubMed] [Google Scholar]
- 70.Schinzel A, Hayashi K, Schmid W (1975) Structural aberrations of chromosome 18. II. The 18q- syndrome. Humangenetik 26:123–132 [PubMed] [Google Scholar]
- 71.Greene RM, Nugent P, Mukhopadhyay P, Warner DR, Pisano MM (2003) Intracellular dynamics of Smad-mediated TGFβ signaling. J Cell Physiol 197:261–271 10.1002/jcp.10355 [DOI] [PubMed] [Google Scholar]
- 72.Blobe GC, Schiemann WP, Lodish HF (2000) Role of transforming growth factor beta in human disease. N Engl J Med 342:1350–1358 10.1056/NEJM200005043421807 [DOI] [PubMed] [Google Scholar]
- 73.Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T (1995) Transforming growth factor-β3 is required for secondary palate fusion. Nat Genet 11:409–414 10.1038/ng1295-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J (1995) Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 11:415–421 10.1038/ng1295-415 [DOI] [PubMed] [Google Scholar]
- 75.Taya Y, O’Kane S, Ferguson MW (1999) Pathogenesis of cleft palate in TGF-β3 knockout mice. Development 126:3869–3879 [DOI] [PubMed] [Google Scholar]