Abstract
A number of neuroimaging and neuropsychology studies have implicated various regions of parietal cortex as playing a critical role in the binding of color and form into conjunctions. The current study investigates the role of two such regions by examining how parietal transcranial magnetic stimulation (TMS) influences binding errors known as ‘illusory conjunctions.’ Participants made fewer binding errors after 1 Hz rTMS of the right intraparietal sulcus (IPS), while basic perception of features (colors and shape) was unaffected. No perceptual effects were found following left IPS stimulation, or stimulation of the right angular gyrus at the junction of the transverse occipital sulcus (IPS/TOS). These results support a role for the parietal cortex in feature binding but in ways that may require rethinking.
Introduction
We experience objects in the world as unified wholes. Yet there is a wealth of evidence to suggest that the brain has specialized processing for different features of the world, such as motion, color, and shape. Veridical perception requires not only the correct registration of these features, but their proper integration into objects.
Some of the most compelling behavioral evidence for a real binding problem in vision comes from the phenomenon of illusory conjunctions. Illusory conjunctions (ICs) occur when viewers incorrectly perceive a feature of one item, such as color, as belonging to another item in a display. Illusory conjunctions were originally reported by Treisman and colleagues, under conditions of distracted attention (Treisman and Schmidt, 1982). On a significant number of trials, participants perceived incorrect combinations of letters and colors. This contributed to the development of feature integration theory (FIT), which proposes that features are registered without spatial attention, but that spatial attention is necessary for correct feature integration to occur. Further studies have extended the situations in which ICs are observed. Even without a distracting attention task or brief exposure duration, ICs can occur frequently when stimuli are presented in peripheral vision, suggesting that poor spatial resolution also hinders the ability of the visual system to bind or co-localize features (Prinzmetal et al., 1995).
Neuropsychological studies support these accounts for ICs. Patients with impaired spatial attention and/or spatial perception have increased IC rates. The most dramatic example of this increase comes from Balint's syndrome, a disorder following bilateral parietal lesions that disrupts spatial processing across the visual environment (see (Robertson, 2003; Robertson, 2004). Patient RM would commonly report illusory conjunctions that were composed of features from different items presented on a computer screen. This even occurred with real objects presented in free view (Friedman-Hill et al., 1995; Robertson et al., 1997). Furthermore, neglect patients with unilateral parietal lesions (Arguin et al., 1994) and a patient with a unilateral pulvinar lesion (Ward et al., 2002), both of which produced spatial processing deficits on the contralateral side to the lesion, were shown to make increased ICs on this side as well.
While patient data have provided the strongest evidence for a critical role of the parietal cortex in feature binding, it does not provide the spatial resolution to determine which areas of the parietal cortex play essential or specific roles in spatial co-localization of features into properly conjoined objects. Other neuroscience methods have found varying parietal regions to be associated with binding. For instance, using fMRI, Donner et al. (2002) found greater activity in the right parietal cortex at the junction of the intraparietal sulcus and transverse occipital sulcus (IPS/TOS) during conjunction search as compared to feature search, even when controlling for overall search difficulty. This area has also been associated with color-form binding in individuals with color-grapheme synesthesia (Esterman et al., 2006; Rich et al., 2003). Unfortunately, imaging only provides correlational evidence. However, transcranial magnetic stimulation (TMS) has been used to ascertain the necessity of the parietal cortex in the search for a conjunction of features. One study found that TMS stimulation over the P4 electrode site in humans caused increased reaction time (RT) in a conjunction visual search task but not a feature visual search task (Ashbridge et al., 1997). This stimulation site was reported as approximately around the right IPS. Other TMS studies have found that stimulation over or around the P4 electrode site induced perceptual neglect-like performance on a variety of visuospatial tasks (Bjoertomt et al., 2002; Hilgetag et al., 2001; Muri et al., 2002). Hilgetag et al. (2001) also found enhanced right (ipsilateral) target detection for P4 stimulation, in addition is increased extinction of contralateral targets for P3 (∼left IPS) and P4 rTMS. In the macaque monkey, temporary deactivation of lateral intraparietal cortex (LIP) led to deficits in conjunction search that were greater than those produced during feature search (Wardak et al., 2004).
In order to further investigate the network involved in color-form integration, the current study used rTMS to disrupt parietal activity while normal perceivers performed a task that produced frequent illusory conjunctions (Esterman et al., 2004). We tested several specific parietal regions that have been associated with feature binding and defined them anatomically for each participant (Figure 2): the right IPS/TOS (Donner et al., 2002; Esterman et al., 2006) and the left and right IPS, anterior to the TOS (Ashbridge et al., 1997). Although this anterior IPS site was selected based on estimates in Ashbridge et al. (1997), the cortex underlying the P4 electrode site varies considerably across participants and studies (see Methods). We also considered it important for this site to be far enough anterior in the IPS to have dissociable effects of TMS from the IPS/TOS site (Esselle and Stuchly, 1992). This site was consequently defined as the midpoint of the IPS adjacent to the supramarginal gyrus/Brodmann area 40 (see Methods for details).
Figure 2.
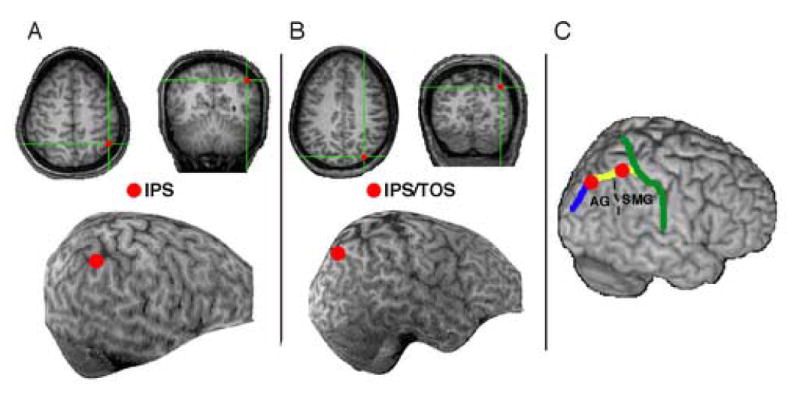
Transcranial magnetic stimulation (TMS) regions of interest. (A) Right intraparietal sulcus (IPS) shown for example participant. (B) Right angular gyrus (AG) at the junction of the transverse occipital sulcus and intraparietal sulcus (IPS/TOS), shown for example participant. (C) Both sites shown on an MNI-template brain. The green line traces the post-central sulcus, the yellow line the IPS, and the blue line the TOS. Supramarginal gyrus (SMG) and AG are labeled. Coordinates in MNI space are estimated as follows: right IPS/TOS: 39, −69, 52; right IPS: 45, −48, 55; left IPS: −37, −52, 58 (not shown).
We predicted that cortical inhibition from 1 Hz rTMS over the right IPS and IPS/TOS would both potentially lead to a temporary increase in the number of illusory conjunctions, particularly for stimuli presented in the left (contralateral) visual field.
Materials and Methods
Participants
24 healthy individuals from the local population at the University of California, Berkeley participated in the experiments. 8 participated in the right IPS stimulation group, 8 participated in the right IPS/TOS group and 8 participated in the left IPS group. Two individuals were left handed (1 in the right IPS group and 1 in the left IPS group).
Apparatus
Stimuli were presented using a Macintosh G4 Powerbook connected to a 19-inch CRT monitor (60Hz refresh rate). The software package Psychlab was used to present stimuli with frame accuracy. Responses were made verbally after each trial and recorded by the experimenter. The participant's chair was positioned such that eye level was at midline and approximately 46 cm distance from the computer screen. Eye movements were monitored with a video camera pointed at the participant's eyes. The experimenter monitored the eyes and marked trials when eye movements were detected between the fixation and response period. Trials with eye movements were eliminated from analysis (less than 4% of trials for all participants; mean=0.5%, range= 0% to 3.6%).
Stimuli
Stimuli and procedures were adapted from Esterman et al. (2004), which have been shown to produce relatively high rates of illusory conjunctions. Stimuli consisted of a horizontal string of 4 characters printed in 36 point Geneva font (See Figure 1A). This string was briefly presented in one quadrant on a black screen. The two inner characters were colored, one being the target, the other the distractor. The target was either an L or 7 (a 180 degree rotated ‘L’). The distractor was always an ambiguous letter/digit ‘O’ (a capital letter ‘O’). The target and distractor were always different colors. The colors used were highly distinguishable red (RGB%: 87,4,3), green (13,72,9), and blue (1,1,83). The 2 outer, or flanker characters, were either both ‘S’s or both ‘8’s, and were always white. Characters subtended approximately .79 × 1.19 degrees of visual angle. Eccentricity of the inner edge of the character string began at 5.27 degrees from central fixation (in one of the four quadrants of the screen). Characters were evenly spaced 1 cm apart. Given the experimental procedure subsequently described, eccentricity was increased to 7.01 degrees or 8.74 degrees for certain individuals.
Figure 1.
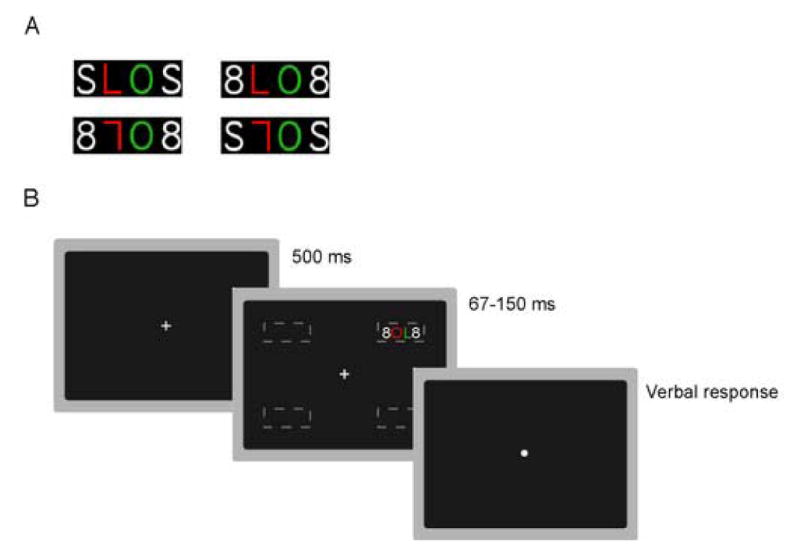
(A)Sample stimuli. Four characters strings consisting of a colored target (‘L’ or ‘7’) presented in one of the two inner positions within the string, a colored distractor (‘O’) and two white flankers (‘S’ or ‘8’). Possible colors were red, green, or blue. (B)Sample trial. A fixation dot was followed by the briefly presented character string (threshold determined for each participant) in one of four peripheral locations (dashed lines, not shown in experiment). Participants responded verbally without a deadline.
Procedure
Participants were instructed to maintain fixation on a central white fixation dot. The dot changed to a cross 500 ms before the character string appeared. Participants were instructed to maintain fixation on the cross. The character string flashed for 150 ms randomly in one of the four quadrants, and the screen then turned black. Participants were asked to identify the target character (L or 7), and its color (red, green, or blue). After their verbal report, another trial began 1000 ms after the experimenter entered the response (Figure 1B). Accuracy was stressed, and this served as the main dependent variable. Unlike full report paradigms examining ICs, the memory requirements of this task were negligible, given that there was minimal memory load and no delay period between display and response.
Training
All participants performed a practice session 1–3 days before the TMS session to familiarize them with the task and reduce response variance during the TMS session. Participants were first given four practice trials, in which character strings were presented for 1000, 800, 600, and 400 ms. Subsequently, the experiment consisted of 192 trials, with each possible character string appearing in each location on the screen. There were four blocks of 48 trials. Each block was counterbalanced to contain the same number of each trial type, colors, and targets. Trials were pseudo-randomized within each block. This practice session served to train participants on the task, maintaining fixation, and establishing optimal presentation time and eccentricity for significant ICs to occur. Eye movements were monitored with feedback. In illusory conjunction experiments, timing and eccentricity must be optimal for significant ICs to occur (Esterman et al., 2004). If conditions are too difficult, numerous letter and color errors result, in which case conjunction errors are not likely to be ‘true’ ICs, but instead due to guessing. To address this problem, criteria were devised to ensure optimal number of conjunction errors and minimal numbers of other errors. For a block of trials to be included in the dataset, it had to reach the criterion of at least 15% conjunction errors (8 of 48) and less than 15% feature errors (misidentifying the target or reporting the absent color).
Stimuli in the initial block were presented at the inner most eccentricity, and stimulus presentation time was 150 milliseconds. These conditions represented the easiest setting, and were appropriate for 8 participants throughout the experiment. If performance was worse than criteria (too many feature errors), the subsequent block was performed under the same conditions, since practice typically improved performance. If performance was better than criteria (too few conjunction errors) presentation time was reduced and eccentricity was increased in an alternating fashion as follows: 100 ms, 7.01 deg, 83 ms, 8.74 deg, 67 ms. Following 4 experimental blocks, those blocks that did not reach criteria were repeated in random order, with a maximum of 8 total blocks per participant.
Sixteen participants required reduced presentation time, and 6 others required increased eccentricity to reach criteria. Timing and eccentricity on the final block that reached criteria was used during the TMS session.
TMS session
This session was conducted 1–3 days after the training. Following each block of rTMS (see below), participants performed 96 trials of the illusory conjunction task, which lasted 6–7 minutes. There were four TMS blocks- two with parietal rTMS, and two with sham TMS (see below). These were alternated, and counterbalanced between subjects. A single parietal ROI was tested in each session (two blocks). This yielded 192 trials per session following parietal TMS, and 192 trials following sham TMS. Different parietal ROIs were tested with different participants (8 each) and were determined by MRI for each participant (see below). Eye movements were monitored without feedback during the TMS session (occurring on less than 4% of trials for all participants; mean=0.5%, range= 0% to 3.6%).
Transcranial Magnetic Stimulation
We first obtained high resolution anatomical MRIs for each participant. The images were acquired using a MPFLASH protocol on a Varian INNOVA 4T system at the University of California, Berkeley (2×2×2 isotropic voxels, 128 slices). We identified the target regions for TMS stimulation from each participant's MRI (left and right IPS, right IPS/TOS). The voxel location was marked on a skull-stripped reconstructed image (Figure 2).
The scalp location was determined using a stereotaxic localization system (Brainsight; Rogue-Research Inc., Montreal, Canada). Coil position over the target regions were monitored online during the stimulation epochs. In addition, trajectory estimates of the TMS pulse were estimated intermittently throughout recording. The locations in the present study were identified using the pattern of gross anatomical landmarks of the intraparietal sulcus (IPS), angular gyrus (AG), transverse occipital sulcus (TOS), lateral fissure, and supramarginal gyrus (SMG). IPS/TOS was defined as the junction of the angular gyrus, IPS, and TOS (Figure 2B and 2C; see Esterman et al, 2006).
In order to be consistent with our IPS site, we determined anatomical markers to define the IPS TMS site on each individual's structural MRI, rather than use the P4 electrode site. Estimates of the cortical site below P4 have ranged from anterior IPS/superior parietal cortex (Ashbridge et al., 1997), area 40/supramarginal gyrus (Lewald et al., 2004), post central gyrus (Oliveri et al., 1999), and IPS at the border of angular and supramarginal gyrus (Hilgetag et al., 2001; for review see Chambers and Mattingley 2005). Additionally, we wanted to choose a site that was far enough from the posterior IPS/TOS site (e.g. greater than 1 cm) that we could see dissociable effects of the TMS (Esselle and Stuchly, 1992). We also took notice that other researchers have found dissociable effects of TMS over AG versus SMG (Chambers et al., 2004a; Chambers et al., 2004b). We therefore took into account the estimates of P4, and Ashbridge et al. (1997) in particular, and then specifically defined our region as the midpoint of the IPS along its border with SMG/Brodmann area 40. This is approximately 1/4th of the sulcal distance back from the post-central sulcus along the length of the IPS. We used these guidelines in order to place a maker on the MRI image. This site is anterior to the portion of IPS that is dorsal/adjacent to the AG, and is generally the IPS region dorsal to the dorsolateral projection of the lateral sulcus. An example of this site is shown for 1 participant in Figure 2A. In Figure 2C, both sites are shown on an MNI-template brain with the critical sulci and gyri marked, and coordinates in MNI space are estimated (right IPS/TOS: 39, −69, 52; right IPS: 45, −48, 55; left IPS: −37, −52, 58, not shown).
Repetitive TMS (rTMS) was performed using an iron-cored figure-8 coil (NeoTonus Inc., Marietta, GA; see Epstein and Davey, 2002). Before each TMS session, the participant's active motor-threshold was determined as the point at which 4–6 visible twitches of the thumb were detected following 10 pulses over the motor cortex while the thumb and index finger were held together in a pinch-like posture. Participants' thresholds were between 34–57% maximum stimulator output, and parietal TMS was set to 115% of motor threshold (MT). Each stimulation epoch consisted of 480 consecutive pulses that were delivered at a rate of 1Hz (8 minutes). This low frequency design has been shown to cause a transient inhibition of the underlying cortex, with the duration of the effect roughly equal to the duration of the stimulation at 1 Hz (Pascual-Leone et al., 1998). During rTMS blocks, the coil was oriented tangential to the surface in order to deliver stimulation directly to the target cortical location. The coil was aimed toward the frontal pole. For sham control rTMS blocks, the coil was oriented 90 degrees away from the scalp so that no pulses perturbed underlying neural tissue.
Analysis
Raw response data
In addition to a correct response, there were 5 different kinds of possible errors. If the participant correctly reported the target character, but incorrectly reported the distractor color, this was a conjunction error. These trials were candidates for ICs. If a participant reported the correct target character, but a color that was absent in the display, this was a color feature error. Similarly, the participant may have incorrectly reported the target character, making an alphanumeric feature error. Errors that are not conjunction errors are feature errors. Because they occurred on less than 7% of trials, we collapsed feature errors into one category for the following analyses.
Conjunction Errors
The raw data strongly suggest that the majority of these conjunction errors were due to errors in feature binding, and not errors in confusing the distractor (‘O’) with the target (Donk, 1999; Prinzmetal et al., 2001). If conjunction errors were due to confusing the distractor with the target, participants would have reported the distractor color, but would have had to guess the target character. Therefore, mistaking the distractor with the target would lead to an equal number of conjunction errors as alphanumeric errors with distractor color, which is far from the case (24% and 2% respectively). It can also be inferred from these data that conjunction errors were not due to misperceiving the target and/or distractor color, and therefore guessing the color. If this were the case, the number of conjunction errors would equal the number of color errors, but they do not (24% and 1% respectively). Nevertheless, formal modeling of data was conducted in order conservatively correct for guessing.
Probability Model
Different procedures have been developed to formally correct for guessing in illusory conjunction experiments. One of the most fruitful of these approaches has been multinomial modeling, which allows independent estimation of the probabilities of correctly perceiving features from the probability of correctly conjoining those same features (Ashby et al., 1996; Prinzmetal et al., 2002). Adapting the model to the data from this experiment yielded 4 parameters. The first was the probability of perceiving the target character (TL). The second and third were the probabilities of perceiving the target color (TC) and/or distractor color (DC). The final parameter was the probability of properly conjoining the target color with the target character (alpha, α). In this model, 1-α is the probability that the participant erroneously conjoined the distractor color with the target character. The model also required enumerating all ways in which each possible response types could be reported. Thus, a probability tree was constructed reflecting each parameter and the subsequent response. For example, a true illusory conjunction would occur if the participant correctly perceived the target character (TL), the target color (TC), and the distractor color (DC), and if the participant erroneously conjoined features (1-α). While this would lead to a conjunction error, other ‘false’ ICs could also lead to a conjunction error, such as failing to perceive the target character (1-TL), the target color (1-TC), and the distractor color (1-DC). On one sixth of these trials, the participant would have happened to guess the target character and conjunction color and produced a conjunction error, without making a true' illusory conjunction. In our version of the model, it is assumed that when the target character is not perceived, participants may not know which element is the target (Esterman et al., 2004). We estimated these parameters with values that best fit the data for each individual participant. The maximum likelihood measurement used to estimate the goodness of fit was G2, as recommended by Riefer and Batchelder (1988) and used by others to model ICs (Ashby et al., 1996; Prinzmetal et al., 2002). Minimizing the G2 led to the best estimation of the 4 parameters.
Results
Raw response data
Response proportions for each of these response types are shown in Figure 3A, 4A, and 5A for the right IPS, right IPS/TOS, and left IPS groups respectively. For each stimulation site and response type (correct responses, conjunction errors, and feature errors), a 2×2 ANOVA was conducted with TMS condition (TMS, sham) and visual field (left, right) as factors.
Figure 3.
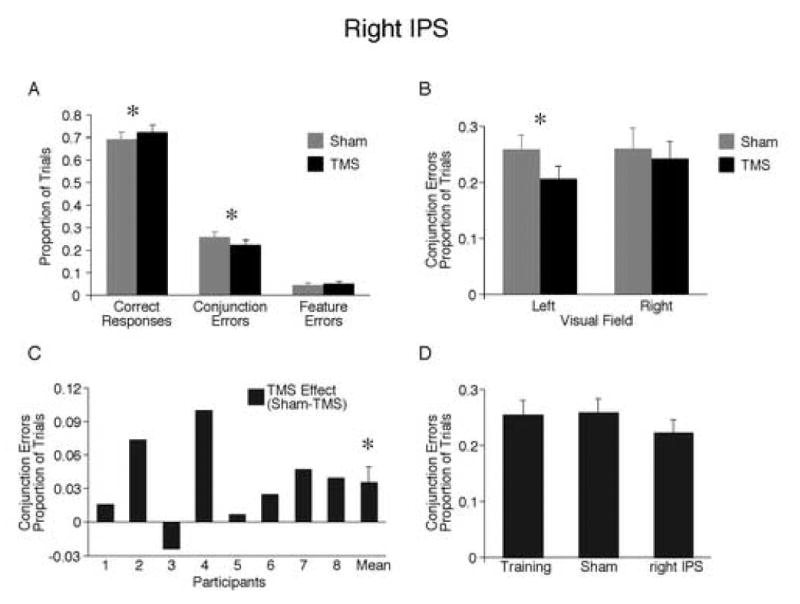
TMS of right IPS. (A) Proportion of all possible response types. Fewer conjunction errors were made after TMS compared to sham. Consistently, a greater proportion of responses were correct after TMS. (B) Effect of TMS on conjunction errors across visual field. (C) Effect of TMS on conjunction errors for each participant. (D) Comparison of conjunction errors on final training, sham, and TMS blocks. Asterisks denote significant differences between conditions, p<0.05.
Figure 4.
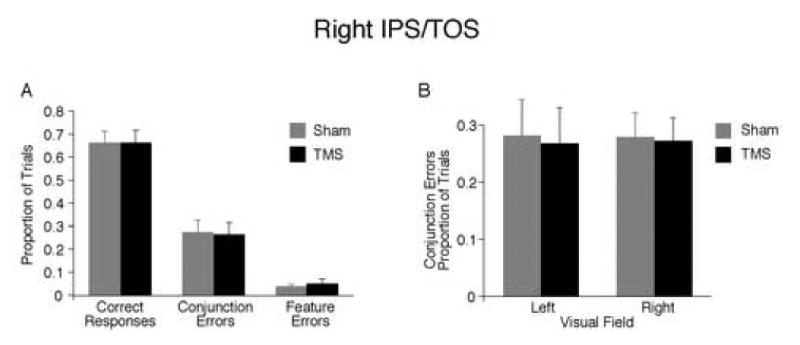
TMS of right IPS/TOS. (A) Proportion of all possible response types. Performance did not differ between sham and TMS. (B) Effect of TMS on conjunction errors across visual field.
Figure 5.
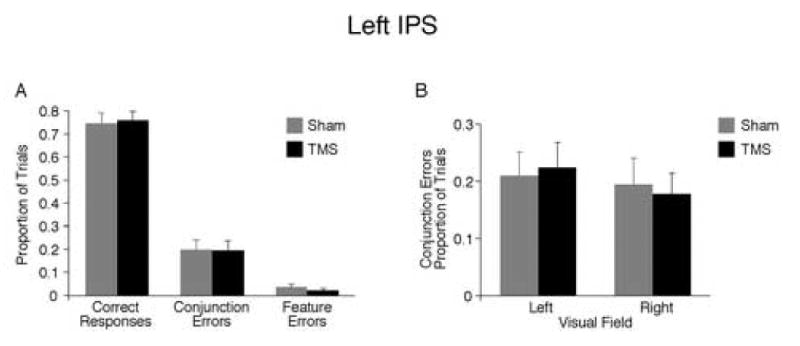
TMS of left IPS. (A) Proportion of all possible response types. Performance did not differ between sham and TMS. (B) Effect of TMS on conjunction errors across visual field.
Right IPS
There was a main effect of TMS condition, such that participants made fewer conjunction errors after TMS of right IPS compared to sham (22% versus 26%; F1,7=6.62, p<0.05; Figure 3A). There was no interaction with visual field (F1,7=3.01, p>0.10; Figure 3B). However, as would be expected, the effect was significant in the contralateral, left visual field (F = 13.59, p<0.01) but not the ipsilateral, right visual field, (p>0.20). Seven of the 8 participants had fewer conjunction errors after right parietal IPS (Figure 3C). 1 As a result of making fewer conjunction errors, participants made significantly more correct responses after TMS than sham (72% versus 69%; F1,7=7.05, p<0.05; Figure 3A). Feature errors were not significantly affected by TMS (Figure 3A), nor did they interact with visual field. This reduction of ICs after TMS was compared to performance on the last training block 1–3 days earlier, which was performed at the same difficulty level as during the TMS session (Figure 3D). Results show that IC rates at the end of training were equivalent to performance in the sham condition (26% in both), while performance after right IPS TMS was better (22% ICs).
Right IPS/TOS
We stimulated the right IPS/TOS because it has been previously associated with color-form binding in fMRI results (Donner et al., 2002). In contrast to the right IPS, there was no effect of stimulation after right IPS/TOS for any response type (Figure 4A), and the rate of conjunction errors did not interact with visual field (Figure 4B), nor did correct responses or feature errors.
Left IPS
There was no effect of TMS for any response type (Figure 5A), and conjunction error rates did not interact with visual field (Figure 5B), nor did correct responses or feature errors. This finding demonstrates that TMS effects on the right IPS is lateralized to the right hemisphere.
Model analysis of right IPS
Results of the probability modeling are shown in Figure 6A and B. The model corroborated the results of analyses of the raw data. After right IPS TMS, the probability of correctly binding (alpha, α) was greater compared to sham stimulation (.77 versus .73, F17=5.88, p<0.05). The probability of perceiving the features (letters and colors) did not differ between TMS and sham (p values > 0.2). Seven of 8 participants showed a higher alpha estimate after right IPS TMS (Figure 6B). This corroborates the raw data in which 7 of 8 participants made fewer conjunction errors after right IPS TMS (Figure 3C versus Figure 6B). The data were also modeled separately for the left and right visual field. As for analysis of the raw data, increased probability of binding was only significant in the left visual field (.79 versus .74, p<0.05).
Figure 6.
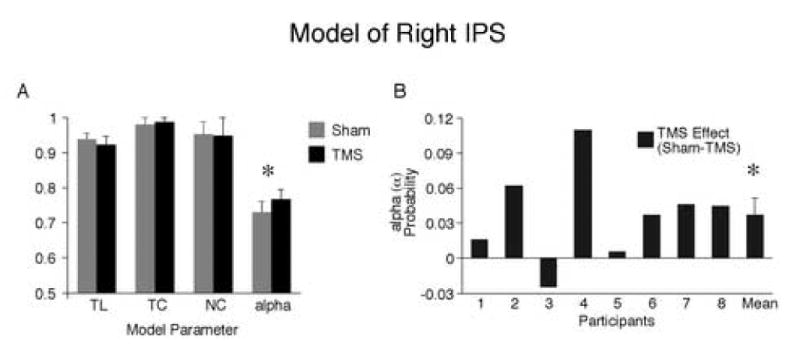
Probability model for right IPS. (A) Model parameters for sham and TMS blocks. TL=probability of perceiving target character; TC=probability of perceiving target color; TC=probability of perceiving distractor color; alpha (α)=probability of correct color-shape binding. Only the binding parameter (α) differed between sham and TMS, with greater probability of binding after TMS. (B) Probability of binding (α) for each participant. Asterisks denote significant differences between conditions, p<0.05.
Discussion
The current results show that offline 1 Hz rTMS of the right IPS reduces feature binding errors immediately following stimulation, and that the significance of this effect was limited to the left, contralateral visual field. Improved binding after right IPS stimulation was indicated by the observation of significantly fewer conjunction errors and a significant increase in the probability of binding (alpha) as estimated by the multinomial probability model. In contrast, stimulation of the left IPS did not influence binding, nor did stimulation of the right IPS/TOS, a region previously associated with binding in an fMRI study (Donner et al., 2002). These results suggest that while these regions may participate in the computations necessary for the perception of integrated objects, their role is not essential. Importantly, TMS did not influence the perception of the features themselves (target characters or colors).
The alteration of feature binding after IPS TMS was restricted to the right hemisphere and contralateral left visual field. This is consistent with previous proposals that the right parietal cortex contains spatial-attentional representations for both visual fields while the left parietal cortex contains only contralateral representations (Heilman and Van Den Abell, 1980; Mesulam, 1981). This pattern of results has also been found in previous TMS studies (Fierro et al., 2000; Muri et al., 2002), such that right TMS influenced contralateral visual spatial abilities, while left TMS did not. This demonstrates that the right parietal cortex can compensate for the altered function of the left parietal, while the left parietal cannot do the same after alterations to the right parietal cortex.
Our finding of improved binding after right IPS TMS was contrary to our prediction-that attenuating neural activity in the region would increase erroneous binding. Instead, it actually decreased erroneous binding. If the right IPS region we stimulated normally performs a function that is not optimal for the task or inhibits another process in some way, then decreasing its excitability could lead to better performance. For example, if the right IPS plays a role in spreading or distributing attention over a wide area of the visual field, inhibiting this function may create a bias to focus attention more locally, which would in turn decrease illusory conjunction rates (Prinzmetal, 1995; Prinzmetal et al., 1986; Treisman, 1996). Chong and Treisman (2005) demonstrated that extracting the gist, or statistical properties of a scene, is easier when attention is spread across the display, rather than focused on individual items. This type of ‘distributed’ attention appears to be beneficial for extracting certain information (gist) but is detrimental to bind features to individual items (more ICs). If the right IPS region is engaged in distributed attention, TMS should impair statistical processing or global form processing.
Neighboring regions of the right inferior parietal cortex have been associated with globally distributing attention (Robertson et al., 1988; Weissman and Woldorff, 2005). This explanation may also fit with the findings of a study of right IPS TMS and conjunction visual search (Ashbridge et al., 1997). In that study, right IPS single-pulse TMS delayed conjunction search but not feature ‘popout’ search. It is likely that distributed attention is advantageous when performing a serial visual search for targets that are spread across a visual field full of distractors. Distributing attention globally would therefore be beneficial in a difficult visual search and harmful when performing the current illusory conjunction task.
It has also been demonstrated that the role of the right parietal cortex in conjunction visual search is dynamic, and can be modulated by practice. In a series of studies by Walsh and colleagues, single-pulse right IPS TMS disrupted conjunction search prior to training, however following training, the effects of TMS were absent (Walsh et al., 1998; Walsh et al., 1999). Critically, this effect occurred even when participants were still performing a serial search, meaning that practice had not made the task an efficient ‘popout’ search (Walsh et al., 1999). Furthermore, when the stimulus features were altered, such that a different conjunction was now the target, IPS TMS once again impaired performance. This pattern was also observed in a functional imaging study (Sigman et al., 2005). Activity in the parietal lobe decreased with practice on a hard visual search task, even when that task was still effortful. Nevertheless when the search stimuli were altered, the parietal activation increased. These findings suggest that right parietal cortex may help in learning effortful search for certain conjunctions of features, and may engage strategic processing that helps to improve performance. After practice, once the task is more automated, parietal involvement decreases. In the current study, participants received about 200–400 practice trials in the training session with a very limited set of stimuli. It may be that strategic processing of the parietal lobe is critical for learning the task, but is not optimal once the task is learned. Under such conditions it would be possible that IPS TMS disengaged strategic processing and made binding performance better. However, this account would predict that performance on the sham blocks would be worse than on the last block during the preliminary training, as TMS should bring performance back to ‘baseline’. This was not the case (Figure 3D). Illusory conjunctions in the sham and training were equivalent, while illusory conjunctions after TMS were reduced.
The effects observed in the current study could also be indirect, such that that the right IPS region may have inhibitory connections to other regions critical for binding. If this is the case, then TMS would result in the release of inhibition from these regions, leading to increased cortical excitability in other regions critical for correct binding. Parietal cortex has connections to and from visual, auditory, and somatosensory cortex, as well as to various regions of frontal cortex. A recent study using diffusion-weighted imaging found that IPS regions have connections to the superior colliculus as well as ventral premotor cortex (Rushworth et al., 2005). This hypothesis could be tested by combining methods such as rTMS and EEG, or rTMS and fMRI. Such techniques have shown that TMS induces changes not only in the underlying cortex, but also in interconnected areas (Bestmann et al., 2005; Taylor et al., 2006).
A final explanation of our data is that the 1 Hz TMS we used induced cortical excitation rather than inhibition. There are multiple lines of evidence from studies of rTMS and motor evoked potentials (MEPs) that demonstrate that 1Hz TMS can reduce cortical excitability (Pascual-Leone et al., 1998; Siebner et al., 2004; Todd et al., 2006). This has also been supported by fMRI results immediately following 1 Hz rTMS (Pascual-Leone et al., 1998). However, the consistency of these findings has generated some debate, complicating the interpretation of all 1 Hz rTMS experimental designs. One study found that the intensity level of stimulation was critical, such that 1 Hz at 80% motor threshold (MT) led to decreased MEP amplitude, while 90% did not (Todd et al., 2006). Following 90% MT 1 Hz rTMS, approximately half of the subjects had increases in MEP amplitudes, while the others had decreases. This evidence suggests that rTMS above certain intensity levels can reverse the effects on cortical excitability. In the present study, we stimulated at 115% of motor threshold, opening the possibility that our stimulation led to facilitation rather than inhibition of IPS. Another study found that when direct inhibitory electrical stimulation of motor cortex preceded 1 Hz rTMS, TMS increased cortical excitability as measured by MEPs. Conversely, when preceded by excitatory electrical stimulation, rTMS led to decreases in motor cortex excitability (Siebner et al., 2004). This demonstrates that when motor activity is below a certain baseline, 1 Hz rTMS can lead to excitation. As discussed above, parietal activity is decreased after visual search training with a limited stimulus set (Sigman et al., 2005). As a result, it is possible that after training, decreased cortical activity in the parietal lobe leads to excitatory effects of rTMS, which may differ from its effects in other paradigms and at different levels of expertise. Hyperexcitability of the right parietal cortex could facilitate visuospatial functioning (Kim et al., 2005).
A possible criticism of our results is that our TMS protocol simply caused arousal or a general enhancement of attention that would influence performance in any task, rather than influencing feature binding specifically. There are reasons to doubt this alternative. First, although feature errors are relatively infrequent, they are actually non-significantly more frequent after right IPS TMS than Sham (5.1% versus 4.6%). Also, of the 3 model estimates of the perception of features (TL, TC, NC), 2 of the 3 decrease after right IPS TMS, indicating not even a trend towards better feature perception after TMS. Our data does not support the fact that our TMS had a general influence on all errors or task performance. Furthermore, other studies find that right posterior parietal rTMS leads to impaired contralateral target detection (Hilgetag et al., 2001; Thut et al., 2005), contralateral sound localization (Lewald et al., 2004), and angle discrimination and mental imagery tasks (Sack et al., 2002). Studies that do find enhancements after TMS find them ipsilateral to the side of TMS and not contralateral (Chambers et al., 2006; Hilgetag et al., 2001). The fact that in other spatial attention demanding tasks, right PPC TMS leads to contralateral deficits, suggests that our effects are not due to a more general effect of all TMS of the right PPC. In our task, the most challenging aspect is the binding of features, and this appears to get better after right IPS TMS. Finding which tasks show improvement and which show impairment after right PPC TMS will help elucidate the multiple functions of this region.
The current study links feature binding to the IPS region of the right parietal cortex. When right IPS was stimulated, the rate of illusory conjunctions was affected, albeit in the opposite direction that was predicted. It is not clear how right IPS stimulation created decreases in conjunction errors, but the fact that it did cannot be denied. The reason could be indirect as in narrowing the scope of attention, which would inhibit flanking distractors and lead to fewer ICs. It could also be indirect if cortical inhibition of right IPS leads to excitation of other connected regions critical for binding. Finally, it is possible that our TMS protocol caused excitation. If this occurred our results would strongly suggest that the right IPS region plays a direct role in representing the space necessary for the co-localization of features into correctly bound objects. In any of these cases, the role of the right IPS in a network responsible for spatially integrating color and form has been supported, but the specific function it plays in binding such features into objects clearly calls for further study.
Acknowledgments
We would like to thank Alice Albrecht, Alexandra List, Rich Ivry, and Alva Noe for their useful comments and help.
Footnotes
To assure the reliability of this effect we tested an additional 3 participants under this condition. All three participants made fewer conjunction errors after TMS (not shown). The proportion of conjunctions errors for each of these participants after TMS was 17%, 13% and 44% as compared to 25%, 14% and 45% after sham.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arguin M, Cavanagh P, Joanette Y. Visual feature integration with an attentional deficit. Brain and Cognition. 1994;24:44–56. doi: 10.1006/brcg.1994.1003. [DOI] [PubMed] [Google Scholar]
- Ashbridge E, Walsh V, Cowey A. Temporal aspects of visual search studied by transcranial magnetic stimulation. Neuropsychologia. 1997;35:1121–31. doi: 10.1016/s0028-3932(97)00003-1. [DOI] [PubMed] [Google Scholar]
- Ashby F, Prinzmetal W, Ivry R, Maddox T. A formal theory of illusory conjunctions. Psychological Review. 1996;103:165–192. doi: 10.1037/0033-295x.103.1.165. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner H, Rothwell J, Frahm J. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage. 2005;28:22–9. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Bjoertomt O, Cowey A, Walsh V. Spatial neglect in near and far space investigated by repetitive transcranial magnetic stimulation. Brain. 2002;125:2012–22. doi: 10.1093/brain/awf211. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Payne JM, Stokes MG, Mattingley JB. Fast and slow parietal pathways mediate spatial attention. Nature Neuroscience. 2004a;7:217–8. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Stokes MG, Janko NE, Mattingley JB. Enhancement of visual selection during transient disruption of parietal cortex. Brain Research. 2006:1097. doi: 10.1016/j.brainres.2006.04.084. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Stokes MG, Mattingley JB. Modality-specific control of strategic spatial attention in parietal cortex. Neuron. 2004b;44:925–30. doi: 10.1016/j.neuron.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Chong SC, Treisman A. Attentional spread in the statistical processing of visual displays. Perception and Psychophysics. 2005;67:1–13. doi: 10.3758/bf03195009. [DOI] [PubMed] [Google Scholar]
- Donk M. Illusory conjunctions are an illusion: the effects of target-nontarget similarity on conjunction and feature errors. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1207–53. [Google Scholar]
- Donner TH, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt SA. Visual feature and conjunction searches of equal difficulty engage only partially overlapping frontoparietal networks. Neuroimage. 2002;15:16–25. doi: 10.1006/nimg.2001.0951. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Davey KR. Iron-core coils for transcranial magnetic stimulation. Journal of Clinical Neurophysiology. 2002;19:376–81. doi: 10.1097/00004691-200208000-00010. [DOI] [PubMed] [Google Scholar]
- Esselle KP, Stuchly MA. Neural stimulation with magnetic fields: analysis of induced electric fields. IEEE Transactions on Bio-Medical Engineering. 1992;39:693–700. doi: 10.1109/10.142644. [DOI] [PubMed] [Google Scholar]
- Esterman M, Prinzmetal W, Robertson L. Categorization influences illusory conjunctions. Psychonomic Bulletin & Review. 2004;11:681–6. doi: 10.3758/bf03196620. [DOI] [PubMed] [Google Scholar]
- Esterman M, Verstynen T, Ivry R, Robertson L. Coming unbound: disrupting automatic integration of synesthetic color and graphemes by TMS of the right parietal lobe. Journal of Cognitive Neuroscience. 2006;18:1570–6. doi: 10.1162/jocn.2006.18.9.1570. [DOI] [PubMed] [Google Scholar]
- Fierro B, Brighina F, Oliveri M, Piazza A, Bua VL, Buffa D, et al. Contralateral neglect induced by right posterior parietal rTMS in healthy subjects. Neuroreport. 2000;11 [PubMed] [Google Scholar]
- Friedman-Hill S, Robertson L, Treisman A. Parietal contributions to visual feature binding: evidence from a patient with bilateral lesions. Science. 1995;269:853–5. doi: 10.1126/science.7638604. [DOI] [PubMed] [Google Scholar]
- Heilman K, Van Den Abell T. Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30 doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- Hilgetag C, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nature Neuroscience. 2001;4:953–7. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Kim Y, Min S, Ko M, Park J, Jang S, Lee P. Facilitating visuospatial attention for the contralateral hemifield by repetitive TMS on the posterior parietal cortex. Neuroscience Letters. 2005;382:280–5. doi: 10.1016/j.neulet.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Lewald J, Wienemann M, Boroojerdi B. Shift in sound localization induced by rTMS of the posterior parietal lobe. Neuropsychologia. 2004;42:1598–1607. doi: 10.1016/j.neuropsychologia.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Muri RM, Buhler R, Heinemann D, Mosimann UP, Felblinger J, Schlaepfer TE, et al. Hemispheric asymmetry in visuospatial attention assessed with transcranial magnetic stimulation. Experimental Brain Research. 2002;143 doi: 10.1007/s00221-002-1009-9. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Rossini PM, Traversa R, Cicinelli P, Filippi MM, Pasqualetti P, et al. Left frontal transcranial magnetic stimulation reduces contralesional extinction in patients with unilateral right brain damage. Brain. 1999;122:1731–39. doi: 10.1093/brain/122.9.1731. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. Journal of Clinical Neurophysiology. 1998;15:333–43. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Prinzmetal W. Visual feature integration in a world of objects. Current Directions in Psychological Science. 1995;4:90–94. [Google Scholar]
- Prinzmetal W, Diedrichsen J, Ivry R. Illusory conjunctions are alive and well: a reply to Donk (1999) Journal of Experimental Psychology: Human Perception and Performance. 2001;27:538–41. doi: 10.1037//0096-1523.27.3.538. [DOI] [PubMed] [Google Scholar]
- Prinzmetal W, Henderson D, Ivry R. Loosening the constraints on illusory conjunctions: assessing the roles of exposure duration and attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:1362–1375. doi: 10.1037//0096-1523.21.6.1362. [DOI] [PubMed] [Google Scholar]
- Prinzmetal W, Ivry R, Beck D, Shimizu N. A measurement theory of illusory conjuntions. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:251–69. [PubMed] [Google Scholar]
- Prinzmetal W, Presti DE, Posner MI. Does attention affect visual feature integration? . Journal of Experimental Psychology: Human Perception and Performance. 1986;12:361–9. doi: 10.1037//0096-1523.12.3.361. [DOI] [PubMed] [Google Scholar]
- Rich AN, Puce A, Syngeniotis A, Williams MA, Howard MA, McGlone F, et al. Colour my brain: a functional neuroimaging study of color-graphemic synaesthesia. Annual meeting of the Cognitive Neuroscience Society; New York, NY. 2003. [Google Scholar]
- Riefer DM, Batchelder WH. Multinomial modeling and the measurement of cognitive processes. Psychological Review. 1988;95:318–39. [Google Scholar]
- Robertson L, Lamb M, Knight R. Effects of lesions of temporal-parietal junction on perceptual and attentional processing in humans. Journal of Neuroscience. 1988;8:3757–69. doi: 10.1523/JNEUROSCI.08-10-03757.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L, Treisman A, Friedman-Hill S, Grabowecky M. The interaction of spatial and object pathways: evidence from Balint's syndrome. Journal of Cognitive Neuroscience. 1997;9:295–317. doi: 10.1162/jocn.1997.9.3.295. [DOI] [PubMed] [Google Scholar]
- Robertson LC. Binding, spatial attention and perceptual awareness. Nature Reviews Neuroscience. 2003;4:93–102. doi: 10.1038/nrn1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LC. Space, Objects, Minds and Brains. New York: Psychology Press; 2004. [Google Scholar]
- Rushworth M, Behrens T, Johansen-Berg H. Connection patterns reveal three regions of the human parietal cortex. Cerebral Cortex. 2005 doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Sack AT, Sperling JM, Prvulovic D, Formisano E, Goebel R, Di Salle F, et al. Tracking the mind's image in the brain II: transcranial magnetic stimulation reveals parietal asymmetry in visuospatial imagery. Neuron. 2002;35:195–204. doi: 10.1016/s0896-6273(02)00745-6. [DOI] [PubMed] [Google Scholar]
- Siebner H, Lang N, Rizzo V, Nitsche M, Paulus W, Lemon R, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. Journal of Neuroscience. 2004;24:3379–85. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, Pan H, Yang Y, Stern E, Silbersweig D, Gilbert C. Top-down reorganization of activity in the visual pathway after learning a shape identification task. Neuron. 2005;46:823–35. doi: 10.1016/j.neuron.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Nobre A, Rushworth M. FEF TMS affects visual cortical activity. Cerebral Cortex. 2006 doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Pascual-Leone A. Dorsal posterior parietal rTMS affects voluntary orienting of visuospatial attention. Cerebral Cortex. 2005;15:628–38. doi: 10.1093/cercor/bhh164. [DOI] [PubMed] [Google Scholar]
- Todd G, Flavel S, Ridding M. Low intensity repetitive transcranial magnetic stimulation decreases motor cortex excitability in humans. Journal of Applied Physiology. 2006 doi: 10.1152/japplphysiol.01399.2005. [DOI] [PubMed] [Google Scholar]
- Treisman A. The binding problem. Current Opinion in Neurobiology. 1996;6:171–9. doi: 10.1016/s0959-4388(96)80070-5. [DOI] [PubMed] [Google Scholar]
- Treisman A, Schmidt H. Illusory conjunctions in the perception of objects. Cognitive Psychology. 1982;14:107–41. doi: 10.1016/0010-0285(82)90006-8. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ashbridge E, Cowey A. Cortical plasticity in perceptual learning demonstrated by transcranial magnetic stimulation. Neuropsychologia. 1998;36:363–7. doi: 10.1016/s0028-3932(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Ashbridge E, Cowey A. The role of the parietal cortex in visual attention — hemispheric asymmetries and the effects of learning: a magnetic stimulation study. Neuropsychologia. 1999;37:245–51. doi: 10.1016/s0028-3932(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Ward R, Danziger S, Owen V, Rafal R. Deficits in spatial coding and feature binding following damage to spatiotopic maps in the human pulvinar. Nature Neuroscience. 2002;5:99–100. doi: 10.1038/nn794. [DOI] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel J. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–8. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- Weissman D, Woldorff M. Hemispheric assymetries for different components of global/local attention occur in distinct temporo-parietal loci. Cerebral Cortex. 2005;15:870–6. doi: 10.1093/cercor/bhh187. [DOI] [PubMed] [Google Scholar]


