Abstract
Leptin is an adipocyte-derived pleiotropic hormone that modulates a large number of physiological functions, including control of body weight and regulation of the immune system. In this work, we show that a recombinant strain of the food-grade lactic acid bacterium Lactococcus lactis (LL-lep) can produce and efficiently secrete human leptin. The secreted leptin is a fully biologically active hormone, as demonstrated by its capacity to stimulate a STAT3 reporter gene in HEK293 cells transfected with the Ob-Rb leptin receptor. The immunomodulatory activity of leptin-secreting L. lactis was evaluated in vivo by coexpression with the human papillomavirus type 16 E7 protein. In C57BL/6 mice immunized intranasally with a recombinant L. lactis strain coproducing leptin and E7 antigen, the adaptive immune response was significantly higher than in mice immunized with recombinant L. lactis producing only E7 antigen, demonstrating adjuvanticity of leptin. We then analyzed the effects of intranasally administered LL-lep in obese ob/ob mice. We observed that daily administration of LL-lep to these mice significantly reduced body weight gain and food intake. These results demonstrate that leptin can be produced and secreted in an active form by L. lactis and that leptin-producing L. lactis regulates in vivo antigen-specific immune responses, as well as body weight and food consumption.
Human leptin, the product of the obese (ob) gene, is a 167-amino-acid protein (∼16 kDa) with a 21-amino-acid signal sequence and one disulfide bond (70). Leptin is an adipocyte-secreted hormone that bears structural similarity to the helical cytokine family (43, 69). Treatment with recombinant leptin has been shown to reduce food intake and body weight and to correct metabolic and hormonal perturbations in leptin-deficient ob/ob mice (10, 27, 53). In humans, leptin also plays a crucial role in the regulation of body weight, as demonstrated by morbid obesity in patients with congenital mutations in either leptin or the leptin receptor gene (15, 47, 64, 65). Although leptin treatment induced remarkable weight loss in patients with rare congenital leptin deficiency (19, 20, 25, 42), it showed poor efficiency in most obese patients. Indeed, clinical trials involving the subcutaneous administration of recombinant leptin to obese subjects indicated that a significant reduction of body weight was only observed if serum leptin concentrations were 20- to 30-fold higher than normal physiological levels (29). This poor response was attributed in part to insufficient transport of leptin across the blood-brain barrier in obese patients (11). Since intranasal delivery is an efficient route for the administration of drugs directly to the brain (8, 28, 37), intranasal leptin administration is considered an interesting strategy to bypass the blood-brain barrier in leptin-resistant humans. This has motivated several recent studies demonstrating rapid and effective intranasal leptin administration and transport to the brain (23, 60, 61). These observations suggest that leptin may still be an effective therapeutic agent for the treatment of obesity (33).
In addition to its effects on body weight control and energy metabolism, leptin is now known to be a pleiotropic hormone also involved in the regulation of immunity, sexual maturation and fertility, bone formation, angiogenesis, tumorigenesis, and wound healing (1). Leptin has been shown to be beneficial for the treatment of lipodystrophy (32, 38, 49) and hypothalamic amenorrhea (68). However, it could also have adverse effects on the development of autoimmune diseases (44), atherosclerosis (35, 59), and cancer (13, 14). Therefore, not only leptin, but also leptin antagonists, produced by site-directed mutagenesis of leptin, have the potential for multiple therapeutic applications, depending on the patients and the target tissues considered (50, 52).
Both leptin and leptin antagonists are generally produced in Escherichia coli, after extraction from insoluble inclusion bodies, followed by renaturation and purification of the renatured protein (21). The development of a novel bacterial expression system, allowing the secretion of a soluble, biologically active leptin, may considerably facilitate (i) large-scale production of leptin and (ii) rapid production and evaluation of the activities of leptin mutants. Lactoccocus lactis has been demonstrated to be an efficient vector for the production and secretion of heterologous proteins (39, 40, 46, 62). In addition, L. lactis is a food-grade, gram-positive bacterium that could be used to deliver therapeutic proteins at the mucosal level, including the intranasal mucosa (4, 6, 62, 63).
In this work, we demonstrate that human leptin can be produced and efficiently secreted by L. lactis in a soluble and biologically active form. In addition, we demonstrate that the intranasal administration of a leptin-producing L. lactis strain can modulate the adaptive immune response in C57BL/6 mice and also reduces food intake and body weight gain in leptin-deficient ob/ob mice.
MATERIALS AND METHODS
DNA manipulations.
Plasmid constructions were established in L. lactis by electrotransformation (36). Isolation of plasmid DNA was performed by using a Mini-Scale purification system (QIAGEN S.A.). Lysozyme (10 mg/ml) and mutanolysin (100 ng/ml) were added prior to the lysis step and incubated for 30 min (37°C) to prepare the protoplasts. PCR (Cetus apparatus; Perkin Elmer, Norwalk, CT) was performed using Vent DNA polymerase (Promega), and DNA sequences were confirmed by sequencing (MWG-Biotech AG). Restriction and DNA-modifying enzymes were used according to the supplier's recommendations.
Cloning of the human leptin gene in L. lactis.
A 462-bp DNA fragment encoding mature human leptin (i.e., without the signal peptide) was PCR amplified from the pcDNA3:leptin vector (64) by using primers NsiI-hlep (5′-CCAATGCATCAGTGCCCATCCAAAAAGTCCAAGATGAC-3′) and SpeI-hlep (5′-GGACTAGTCCTCAGCACCCAGGGCTGAGGTCCAGCTGCCACAGCATGTCCTGCAGAGACCCCTG-3′). The resulting fragment was directly digested with NsiI and SpeI enzymes (restriction sites on the primers are indicated in bold and italics) and cloned into purified backbone isolated from the NsiI-SpeI-cut pSEC-E7 vector (7), a derivative of the broad-host-range plasmid pWV01, which replicates in E. coli and several gram-positive bacteria (6), resulting in pSEC:lep (Fig. 1). This plasmid was introduced into L. lactis strain NZ9000 carrying the regulatory genes nisR and nisK (18) to obtain the strain LL-lep. As a negative control, NZ9000 was transformed with the pSEC empty vector to generate strain LL. Recombinant clones were selected by the addition of 10 μg of chloramphenicol per ml. Recombinant L. lactis strains were grown in M17 medium supplemented with 1% glucose (GM17) at 30°C without aeration.
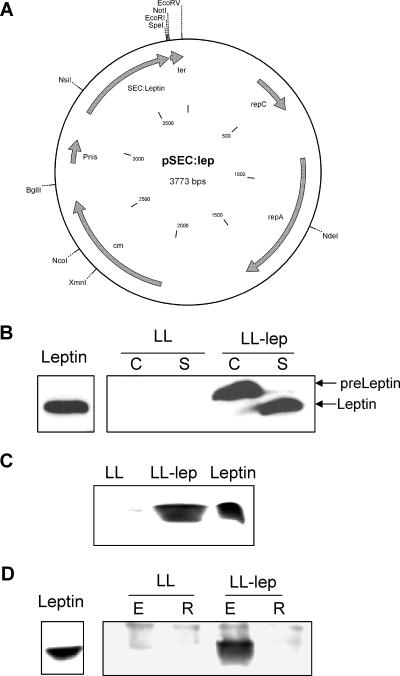
FIG. 1.
Schematic representation of pSEC:lep vector and expression of leptin by L. lactis. (A) A 462-bp DNA fragment encoding mature human leptin was fused in frame with a DNA fragment containing the Usp45 signal peptide (SEC:Leptin), derived from the predominant L. lactis-secreted protein (67). In this plasmid, leptin expression is controlled by the nisin-inducible promoter (PnisA) and harbors the Usp45 ribosome binding site and the rho-independent trpA transcription terminator (ter) (12) for clone stability. The pSEC:Lep carries the pC194 chloramphenicol resistance marker (cm) (34) (B) Strains LL and LL-lep were grown and induced with 10 ng/ml nisin for 1 h. After centrifugation, cell pellet and culture medium were treated as described in Materials and Methods. The antileptin antibody detected a protein in LL-lep culture supernatants with an apparent molecular mass identical to that of the commercial recombinant leptin. C, cell fraction; S, supernatant fraction. (C) Strains LL and LL-lep were grown as described in Materials and Methods. After centrifugation, leptin was immunoprecipitated from 1 ml of culture medium and immunodetected by Western blotting using antileptin antibody. (D) Strain LL or LL-lep culture supernatants were fractionated on 100-kDa centrifugal membranes as described previously (9). Leptin present in retentates (R) and eluates (E) was immunoprecipitated and detected by immunoblotting with antileptin antibody. Commercial leptin (Leptin) was used as a control in the assays.
Inducible expression of leptin.
For the induction of leptin expression from the nisin promoter, strains were grown to an optical density at 600 nm of 0.6, followed by induction with 10 ng of nisin (Sigma) per ml for 1 h. L. lactis culture extraction and immunoblotting assays were performed as follows, using a polyclonal leptin antibody (Bio-Vendor). To quantify leptin production, protein samples were prepared from 2 ml of induced culture. After centrifugation (5 min, 10,000 × g), the cell pellet and supernatant were treated separately. The supernatants were treated with 1 mM phenylmethylsulfonyl fluoride and 10 mM dithiothreitol, followed by the addition of 100 μl of 100% trichloroacetic acid to precipitate proteins. Samples were incubated for 10 min on ice, and proteins were recovered from the pellets after centrifugation at 4°C for 10 min at 13,000 rpm. The cell fraction was obtained by cell lysis in lysis buffer (25% sucrose, 1 mM EDTA, 50 mM Tris-HCl [pH 8.0], and 10 mg/ml lysozyme) complemented with 1 mM phenylmethylsulfonyl fluoride and 10 mM dithiothreitol. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, and immunodetection were performed as previously described (7). Human commercial leptin (PeproTech., Inc.) was used as a control in Western blotting. The concentrations of leptin secreted in the medium and retained in cell fractions were assessed by using an enzyme-linked immunosorbent assay (ELISA) kit (Bio-Vendor).
STAT3 reporter gene activation assay.
Human embryonic kidney (HEK293) cells maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Cergy Pontoise, France) supplemented with 4.5 g/liter glucose and 10% fetal calf serum were seeded at a density of 2.5 × 105 cells per 2-cm2 well. Transient transfections were performed 1 day later using FuGENE 6 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's protocol. HEK293 cells were cotransfected in 2-cm2 wells with 700 ng/well of Ob-Rb receptor, 300 ng/well of a signal transducer and activator of transcription-3 (STAT3)-firefly luciferase reporter gene, and 0.02 ng/well of pcDNA3-Renilla luciferase (used as an internal control between samples) (17). Twenty-four hours after transfection, the culture medium was removed and cells were cultured for an additional 24 h in 0.5 ml of DMEM containing 1% fetal calf serum and either 10 nM human commercial leptin or 50 μl of strain L. lactis culture medium. The cells were then washed with phosphate-buffered saline (PBS) and lysed in passive lysis buffer (Promega) for 15 min at room temperature. Total lysates were centrifuged for 2 min at 15,000 rpm, and the supernatants were used in a dual-luciferase assay system (Promega) using a Berthold Luminometer (Lumat LB 9507).
Administration of live L. lactis strain coproducing leptin and E7 antigen to C57BL/6 mice.
Specific-pathogen-free C57BL/6 mice (females, 6 to 8 weeks of age; Charles River Breeding Laboratories) were housed in groups of four mice per cage in pathogen-free Texler-type isolators (La Calhène, Vélizy, France) under sterile conditions with water and were fed ad libitum in the animal facilities of the Unité d'Ecologie et de Physiologie du Système Digestif at the Institut National de la Recherche Agronomique (INRA, Jouy-en-Josas, France). Food intake and body weight were recorded every day. All experiments were performed according to protocols in accordance with INRA guidelines.
To construct a recombinant L. lactis strain coexpressing leptin and E7 antigen, we introduced a second plasmid encoding a cell wall-anchored form of human papillomavirus type 16 (HPV-16) E7 antigen (16) into the LL-lep strain, resulting in the LL-lep/E7 strain. Live bacterial inocula of the different L. lactis strains were prepared and induced as previously described (4). Briefly, cellular pellets were harvested by centrifugation at 3,000 × g at 4°C and washed three times with sterile PBS. The pellet was suspended in PBS to a final concentration of 1 × 109 CFU. Groups of C57BL/6 mice (five mice per group) were immunized intranasally with 1 × 109 CFU from each induced recombinant lactococcus strain suspended in 10 μl of PBS (5 μl was administered with a micropipette into each nostril) on days 0, 14, and 28. Control mice received identical quantities of L. lactis transformed with an empty vector (strain LL). The inocula were controlled for CFU and leptin and E7 production for all animal administrations. The antibodies used were human antileptin (Bio-Vendor) and anti-E7 (Santa Cruz Biotechnology).
Measurement of E7-specific CD4+ and CD8+ T cells.
Splenocytes from immunized C57BL/6 mice were incubated with the E749-57 peptide (major histocompatibility complex [MHC] class I epitope [22]) or E730-67 peptide (MHC class II epitope [66]). The number of E7-specific gamma interferon (IFN-γ)-producing T cells was determined by enzyme-linked immunospot assay (mouse IFN-γ; R&D Systems) as previously described (4).
Administration of live bacterial inocula to ob/ob mice.
Specific-pathogen-free ob/ob C57BL/6 mice (males, 6 to 8 weeks of age; Janvier) were housed as described above. After arriving, the mice were acclimatized for 1 week (including PBS pretreatment during the last 4 days). The room temperature was constant at 25°C, and a 12-h light, 12-h dark cycle was maintained. Mice (five animals per cage) were fed powdered ground rodent chow (food no. R03-40; SAFE, Augy, France) in powdered-food hoppers for mice (UAR, Epinay/Orge, France). Individual body weights and global food intake (food intake per cage) were monitored daily. For the preparation of the live bacterial inocula, LL-lep was grown and induced as described above. Cellular pellets were then harvested by centrifugation at 3,000 × g at 4°C and washed three times with sterile PBS. The pellet was suspended in PBS to a final concentration of 1 × 109 CFU. We previously demonstrated that after a 1-hour nisin pulse induction, nisin-induced protein production and secretion by the recombinant LL will continue for at least 10 h in the absence of nisin (5). Groups of ob/ob C57BL/6 mice were inoculated intranasally with 1 × 109 CFU of the nisin-induced lactococcus strain suspended in 10 μl of PBS (5 μl was administered with a micropipette into each nostril) daily for 19 days. Control mice received either PBS or identical quantities of LL. The quantities of CFU and leptin production were monitored as described above.
Statistical analyses.
Student's t test was used to compare the differences between groups by using JMP statistical software. A P value of <0.05 was considered significant.
RESULTS
Characterization of leptin production by Lactococcus lactis.
Lactococcus strains transformed with an empty vector (LL) or with the expression vector pSEC:lep (LL-lep) (Fig. 1A) were grown, and the leptin expression was induced with nisin as described in Materials and Methods. The ability of LL-lep to produce and secrete human leptin was then tested by Western blot using a polyclonal leptin antibody. As shown in Fig. 1B, bands of ∼18 kDa and 16 kDa were detected in nisin-induced cultures of the LL-lep strain, corresponding, respectively, to SPUsp45-Lep precursor (pre-Leptin), present in the cell fraction, and to mature leptin, present in the supernatant fraction. No signal was detected in the negative-control strain LL (Fig. 1B). In addition, the recombinant leptin secreted by L. lactis could be directly immunoprecipitated from the culture medium of the LL-lep strain, indicating that this protein is properly processed and secreted in an immunoreactive form (Fig. 1C).
Previous studies applying size-exclusion filtration to leptin mutants have shown that improperly folded leptin forms large molecular aggregates that cannot cross a filtration membrane with a cutoff of 100 kDa (9, 56). To ensure that leptin produced by L. lactis does not form such macromolecular aggregates, LL-lep culture medium was submitted to size exclusion membrane filtration. Leptin secreted by LL-lep was recovered in the eluate fraction, whereas no leptin was detected in the retentate fraction of the filtration unit (Fig. 1D). This result indicates that leptin secreted by LL-lep does not form large macromolecular aggregates.
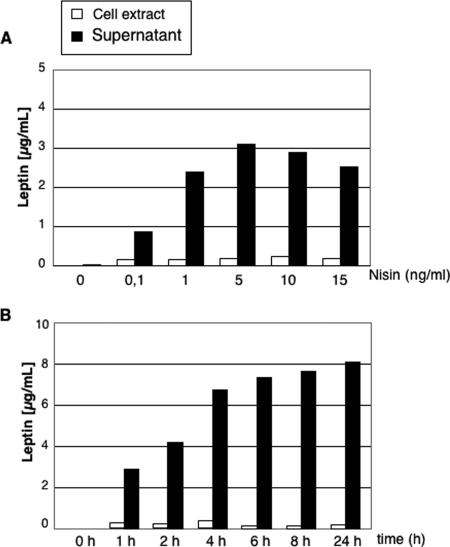
Leptin secretion by the LL-lep strain was further characterized and quantified by ELISA. We first analyzed leptin production and secretion by the LL-lep strain at different nisin inducer concentrations (Fig. 2A). Incubation for 1 h with nisin resulted in a dose-dependent increase in leptin secretion, with a maximal effect obtained at a nisin concentration of 5 ng/ml. The very small amounts of leptin detected in the cell extracts indicated that most of the leptin produced by LL-lep was processed and secreted in the medium. We calculated that, under these experimental conditions, the secretion efficiency (i.e., the percentage of leptin found in the supernatant related to the total amount of leptin found in both the supernatant and cell fraction) was at least 90%. Time-course experiments indicated that, at a concentration of 10 ng/ml of nisin, leptin accumulated in the culture medium for about 6 to 8 h, reaching a maximal concentration of about 7 to 8 μg/ml (Fig. 2B). These experiments demonstrate that LL-lep has a remarkably efficient capacity to secrete leptin in the culture medium.
FIG. 2.
Leptin expression by recombinant lactococci as a function of induction conditions. (A) Leptin quantification by ELISA of cell fractions or supernatant samples from LL-lep cultures induced (at an optical density at 600 nm of 0.6 U) with increasing concentrations of nisin (0 to 15 ng/ml). (B) Leptin quantification by ELISA of cell fractions or supernatant samples from LL-lep cultures induced (at an optical density at 600 nm of 0.6 U) with 10 ng/ml nisin at different times (0 to 24 h). The results of a representative experiment are shown.
Leptin secreted by L. lactis is biologically active.
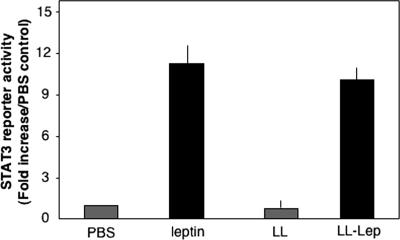
The Ob-Rb leptin receptor is known to activate the JAK/STAT signaling pathway, leading to the stimulation of STAT3 transcriptional activity (24). To determine whether leptin secreted by L. lactis can bind to the leptin receptor and stimulate its downstream signaling pathways, HEK293 cells were cotransfected with the Ob-Rb leptin receptor and a STAT3-firefly luciferase reporter gene (17). After 24 h of transfection, cell cultures were followed for 24 h in the presence of commercial leptin (10 nM) or either strain LL or LL-lep culture medium (50 μl) derived from cells previously induced with 10 ng/ml of nisin for 2 h. Luciferase reporter activity was measured from HEK293 cell protein extracts. Both commercial leptin and culture medium from LL-lep markedly increased firefly luciferase activity, whereas culture medium from LL had no effect (Fig. 3). This result demonstrates that leptin secreted by recombinant lactococci binds to the human leptin receptor expressed in HEK293 cells and activates the JAK/STAT signaling pathway, leading to the stimulation of STAT3 transcriptional activity.
FIG. 3.
In vitro biological activity of leptin produced by L. lactis. HEK293 cells were cotransfected with Ob-Rb receptor, STAT3-firefly luciferase reporter gene, and pcDNA3-Renilla luciferase. Twenty-four hours after transfection, cells were cultured for an additional 24 h in 0.5 ml of DMEM containing 1% serum and either 10 nM commercial leptin or 50 μl of L. lactis culture medium, from leptin-producing or -nonproducing strains. Results are expressed as ratios of activities for firefly luciferase over Renilla luciferase and represent the means of two independent experiments performed in triplicate.
Intranasal administration of L. lactis coexpressing leptin and HPV-16 E7 protein in C57BL/6 mice enhances Th1 immune response.
Previous work showed that the intranasal administration of an L. lactis strain expressing a cell wall-anchored form of HPV-16 E7 antigen induced an antigen-specific T-cell response in mice (3). Moreover, coadministration of an L. lactis strain secreting IL-12 had adjuvant effects, resulting in protection against HPV-16-induced tumors in mice (4). Since leptin is known to regulate immune function and to promote T helper 1 (Th1)-cell differentiation (44), we asked whether the coexpression of leptin with HPV-16 E7 antigen in the same strain could modulate the antigen-specific T-cell response after intranasal administration in mice. Three groups of four C57BL/6 mice were immunized on days 0, 14, and 28 with 1 × 109 CFU of the control L. lactis strain (LL), an L. lactis strain expressing E7 antigen (LL-E7), or an L. lactis strain coexpressing leptin and E7 antigen (LL-lep/E7). One week after the last immunization, splenocytes from immunized animals were used for the detection of IFN-γ, a cytokine characteristic of a Th1 type of immune response. Immunization with LL-lep/E7 resulted in a marked enhancement of IFN-γ secretion by CD4+ and CD8+ lymphocytes in response to E7-derived peptides (Fig. 4). These results further confirm that leptin produced by L. lactis is biologically active and demonstrate the in vivo immunomodulatory effects of intranasal administration of LL-lep in mice.
FIG. 4.
Production levels of IFN-γ from spleen cells in mice immunized with live lactococci expressing human leptin and E7 antigen. Five C57BL/6 mice were intranasally immunized on days 0, 14, and 28 with strains LL, LL-E7, and LL-lep/E7. One week after the last immunization (day 35), splenocytes from immunized mice were pooled and stimulated in vitro with E730-67 peptide (MHC class II epitope) or E749-57 peptide (MHC class I epitope) for identification of IFN-γ-producing CD4+ and CD8+ T cells, respectively, by enzyme-linked immunospot assay. *, differences are statistically significant (P < 0.05).
Intranasal administration of LL-lep reduces food intake and body weight gain in ob/ob mice.
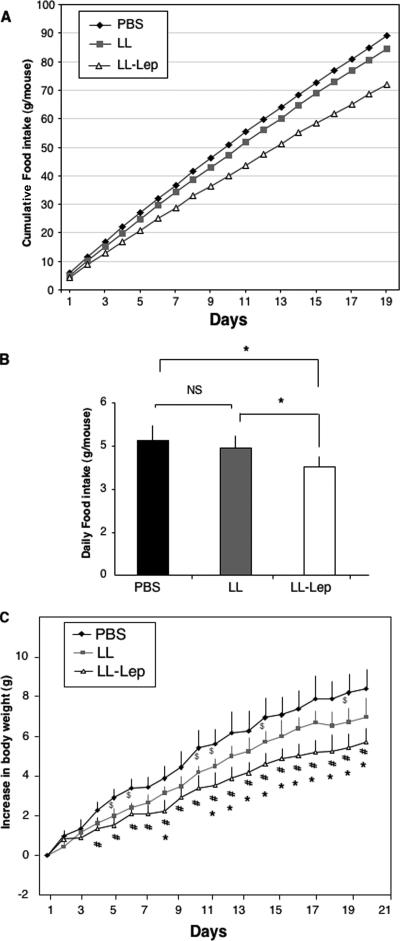
To further evaluate in vivo the biological activity of the leptin produced by L. lactis, we studied the effects of intranasal administration of LL-lep on food intake and body weight in leptin-deficient ob/ob mice. Three groups of five C57BL/6 ob/ob mice were subjected to intranasal administration of PBS or strain LL or LL-lep daily for 19 days. The total amount of food consumed by each group of mice (food intake per cage) was monitored every day during the duration of the treatment. Monitoring of the cumulative food intake at the end of the treatment period showed that, on average, a mouse from the LL-lep-treated group consumed 17 g and 13 g less than a mouse from the PBS-treated or the LL-treated group, respectively (Fig. 5A). Daily food intake was not significantly different between PBS- and LL-treated mice. In contrast, daily food intake was significantly lower in LL-lep-treated mice than in PBS- or LL-treated mice (Fig. 5B). The body weight of each mouse was monitored daily over the test period (Fig. 5C). Body weight gain from baseline values tended to be lower in mice receiving strain LL than in mice receiving PBS, but this effect reached significance at only a few time-points. In contrast, ob/ob mice that received LL-lep gained significantly less weight than PBS- and LL-treated mice. Differences between LL-lep-treated mice and PBS- or LL-treated mice became significant from day 4 and 11, respectively, and remained significant up to the end of the treatment. We also evaluated the effect of daily intranasal administration of 10 μg of commercial leptin (i.e., the maximal amount of recombinant leptin produced in vitro by LL-Lep in 24 h, according to our in vitro experiments), and we did not observe any significant effect on body weight (data not shown). Altogether, our results demonstrate that leptin produced by LL-lep has biological activity in vivo on the regulation of food intake and body weight of ob/ob mice and confirm the interest in using lactococci for leptin delivery.
FIG. 5.
Effects of daily intranasal administration of LL-Lep on food intake and body weight gain in ob/ob mice. ob/ob mice were inoculated daily with PBS or strain LL or LL-lep (1 × 109 CFU/inoculum). Food intake and body weight gain were measured every day for 19 days. (A) The total amount of food ingested by the mice present in the same cage and receiving the same treatment was measured every day. The figure represents the evolution of cumulative food intake per mouse during the period of treatment. (B) Mean food intake per animal and per day. *, differences are statistically significant (P < 0.05); NS, difference is not significant. (C) Evolution of body weight gain during treatment. Differences are statistically significant (P < 0.05) between LL-lep- and LL-treated mice (*), between LL-lep- and PBS-treated mice (#), and between LL- and PBS-treated mice ($).
DISCUSSION
About 30% of the North American population and 20% of the European population is overweight. Given the world-wide prevalence of this pathology, peptides that regulate appetite and body weight represent major pharmaceutical targets for the next decades. In addition to its implication in the control of food intake and body weight, leptin may also have a high therapeutic potential in other biomedical fields, through its regulatory role on the immune system, for instance. To date, recombinant leptin has been produced essentially in E. coli, necessitating a multistep extraction process that generally includes disruption of the cells, centrifugation, extraction, and refolding of the denatured protein (21, 31, 57). Although in some cases leptin could be extracted in a soluble form from the E. coli periplasm, the recovery procedure involved osmotic shock and several centrifugation steps (26). In addition, all the methods described to date for recombinant leptin production require purification steps before the leptin's biological activity can be evaluated.
In this work, we cloned the leptin gene in our usual expression vector, resulting in a vector allowing rapid and efficient secretion of soluble human leptin by L. lactis. We demonstrated that this recombinant leptin is directly secreted in a biologically active form, as shown by its capacity to efficiently stimulate the STAT3 promoter in HEK293 cells expressing the leptin receptor. This procedure could easily be adapted for the large-scale production of biologically active recombinant leptin. In addition, leptin production in secreted form allows for direct assessment of its biological activity in small volumes of culture medium, without any purification steps. This opens important perspectives for the production and rapid evaluation of the biological activity of leptin mutants, which may act as potent leptin receptor agonists or antagonists, with valuable therapeutic potential (51). Furthermore, in some cases, it may also be important to be capable of rapidly evaluating the biological activity of such mutants in vivo, without having to purify them. We showed that the activity of leptin produced by LL-lep can be studied in vivo in mice, by direct intranasal administration of live lactococci. Indeed, we first demonstrated that leptin produced by LL-lep has immunomodulatory properties in C57BL/6 mice. Intranasal administration of food-grade bacteria expressing antigens has been proposed as an alternative vaccination approach for the stimulation of mucosal and systemic immune responses (45), and concomitant delivery of proinflammatory cytokines reportedly has adjuvant effects (4, 6, 63). Previous works using L. lactis as a live vaccine to immunize mice against E7 antigen have shown that coadministration of two strains, one expressing IL-12 and the other E7, resulted in about two- and sixfold increases, respectively, in CD4+ and CD8+ IFN-γ responses to E7 antigen (4, 6). In the present work, the administration of L. lactis coexpressing leptin and E7 antigen resulted in about 3- and 9.5-fold increases, respectively, in CD4+ and CD8+ IFN-γ responses to E7. This result suggests that leptin may have interesting immunostimulatory properties in the context of mucosal vaccination.
In vivo biological activity of leptin secreted by LL-lep was also demonstrated by the intranasal administration of Lactococci in leptin-deficient ob/ob mice. Several peptidic hormones need to be transported into the cerebrospinal fluid through the blood-brain barrier to exert their effects, and intranasal administration has been described as a means to bypass the blood-brain barrier and deliver active molecules directly into the brain. The nasal route for drug delivery to the brain via the olfactory region has been widely investigated. Various studies indicate that large molecules, such as peptides, can be directly transported from the nasal mucosa into the cerebrospinal fluid, the olfactory bulb, and the brain parenchyma (30). Several recent studies have shown that intranasal delivery of leptin resulted in the reduction of food intake and body weight (60, 61). We observed that intranasal administration of LL-lep significantly reduced food intake and body weight gain compared to administration of LL, suggesting that leptin secreted by LL is biologically active on the central nervous system. At the end of the treatment period, the animals were sacrificed and blood samples were taken for the measurement of peripheral leptin. Using a human leptin ELISA, we observed that virtually no leptin was detected in the plasma of LL-lep-treated ob/ob mice (data not shown). This suggests that the effect of intranasally administered LL-lep may be the result of direct transport of the secreted leptin from the nasal cavity to the brain. In agreement with this, a very recent study, using intranasally administered radioiodinated leptin, clearly supported circumvention of the blood-brain barrier and direct transport of leptin from nose to brain (23).
Interestingly, in mice treated with control strain LL, body weight gain tended to be lower than in PBS-treated mice, although this effect generally did not reach statistical significance, except for a few time-points. Since appetite and food preference are known to depend tightly on olfactory stimuli (2, 41, 55), the presence of LL in the intranasal cavity may somehow affect animal feeding behavior. However, food intake was not significantly decreased in LL-treated mice compared to the intake of PBS controls (Fig. 5B). On the other hand, the presence of a foreign microorganism in the intranasal cavity may induce local production of inflammatory cytokines that could impact on body weight gain through catabolic mechanisms, by directly acting on the central nervous system (48, 54, 58). Further work will be needed to firmly establish whether LL alone has any effect on the regulation of body weight and to elucidate the potential mechanisms involved.
In summary, we have designed an L. lactis strain for highly efficient secretion of biologically active leptin, which can be directly recovered from the culture medium. Biological activity of the leptin produced by L. lactis was also demonstrated in vivo in mice. Indeed, we showed that the intranasal administration of leptin-secreting lactococci can regulate immune responses in mice. Finally, we demonstrated, for the first time, the potential of L. lactis to produce peptides with therapeutic interest for the treatment of obesity.
Acknowledgments
We thank Catherine Postic for useful advice and Véronique Fauveau (Plate-forme de microchirurgie, Institut Cochin) for expert technical assistance for some experiments. We also thank Naima Cortes-Perez for useful help in statistical analyses.
L.G.B.-H. was the recipient of a grant from the Association pour la Recherche sur le Cancer.
Footnotes
Published ahead of print on 29 June 2007.
REFERENCES
- 1.Ahima, R. S., and S. Y. Osei. 2004. Leptin signaling. Physiol. Behav. 81:223-241. [DOI] [PubMed] [Google Scholar]
- 2.Amer, A., and T. J. Maher. 2005. Nasal administration of the calcium channel blocker diltiazem decreases food intake and attenuates weight gain in rats. Pharmacol. Biochem. Behav. 82:379-387. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez-Humaran, L. G., N. G. Cortes-Perez, Y. Le Loir, A. Gruss, C. Rodriguez-Padilla, O. Saucedo-Cardenas, P. Langella, and R. Montes de Oca-Luna. 2003. Fusion to a carrier protein and a synthetic propeptide enhances E7 HPV-16 production and secretion in Lactococcus lactis. Biotechnol. Prog. 19:1101-1104. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez-Humaran, L. G., N. G. Cortes-Perez, F. Lefevre, V. Guimaraes, S. Rabot, J. M. Alcocer-Gonzalez, J. J. Gratadoux, C. Rodriguez-Padilla, R. S. Tamez-Guerra, G. Corthier, A. Gruss, and P. Langella. 2005. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J. Immunol. 175:7297-7302. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez-Humaran, L. G., P. Langella, J. Commissaire, S. Gilbert, Y. Le Loir, R. L'Haridon, and G. Corthier. 2003. Controlled intra- or extracellular production of staphylococcal nuclease and ovine omega interferon in Lactococcus lactis. FEMS Microbiol. Lett. 224:307-313. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez-Humaran, L. G., P. Langella, N. G. Cortes-Perez, A. Gruss, R. S. Tamez-Guerra, S. C. Oliveira, O. Saucedo-Cardenas, R. Montes de Oca-Luna, and Y. Le Loir. 2003. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect. Immun. 71:1887-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermudez-Humaran, L. G., P. Langella, A. Miyoshi, A. Gruss, R. T. Guerra, R. Montes de Oca-Luna, and Y. Le Loir. 2002. Production of human papillomavirus type 16 E7 protein in Lactococcus lactis. Appl. Environ. Microbiol. 68:917-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Born, J., T. Lange, W. Kern, G. P. McGregor, U. Bickel, and H. L. Fehm. 2002. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 5:514-516. [DOI] [PubMed] [Google Scholar]
- 9.Boute, N., V. Zilberfarb, L. Camoin, S. Bonnafous, Y. Le Marchand-Brustel, and T. Issad. 2004. The formation of an intrachain disulfide bond in the leptin protein is necessary for efficient leptin secretion. Biochimie 86:351-356. [DOI] [PubMed] [Google Scholar]
- 10.Campfield, L. A., F. J. Smith, Y. Guisez, R. Devos, and P. Burn. 1995. Recombinant mouse ob protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269:546-549. [DOI] [PubMed] [Google Scholar]
- 11.Caro, J. F., J. W. Kolaczynski, M. R. Nyce, J. P. Ohannesian, I. Opentanova, W. H. Goldman, R. B. Lynn, P. L. Zhang, M. K. Sinha, and R. V. Considine. 1996. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348:159-161. [DOI] [PubMed] [Google Scholar]
- 12.Christie, G. E., P. J. Farnham, and T. Platt. 1981. Synthetic sites for transcription termination and a functional comparison with tryptophan operon termination sites in vitro. Proc. Natl. Acad. Sci. USA 78:4180-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleary, M. P., S. C. Juneja, F. C. Phillips, X. Hu, J. P. Grande, and N. J. Maihle. 2004. Leptin receptor-deficient MMTV-TGF-alpha/Lepr (db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp. Biol. Med. (Maywood) 229:182-193. [DOI] [PubMed] [Google Scholar]
- 14.Cleary, M. P., F. C. Phillips, S. C. Getzin, T. L. Jacobson, M. K. Jacobson, T. A. Christensen, S. C. Juneja, J. P. Grande, and N. J. Maihle. 2003. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res. Treat. 77:205-215. [DOI] [PubMed] [Google Scholar]
- 15.Clement, K., C. Vaisse, N. Lahlou, S. Cabrol, V. Pelloux, D. Cassuto, M. Gourmelen, C. Dina, J. Chambaz, J. M. Lacorte, A. Basdevant, P. Bougneres, Y. Lebouc, P. Froguel, and B. Guy-Grand. 1998. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392:398-401. [DOI] [PubMed] [Google Scholar]
- 16.Cortes-Perez, N. G., L. G. Bermudez-Humaran, Y. Le Loir, C. Rodriguez-Padilla, A. Gruss, O. Saucedo-Cardenas, P. Langella, and R. Montes-de-Oca-Luna. 2003. Mice immunization with live lactococci displaying a surface anchored HPV-16 E7 oncoprotein. FEMS Microbiol. Lett. 229:37-42. [DOI] [PubMed] [Google Scholar]
- 17.Couturier, C., and R. Jockers. 2003. Activation of the leptin receptor by a ligand-induced conformational change of constitutive receptor dimers. J. Biol. Chem. 278:26604-26611. [DOI] [PubMed] [Google Scholar]
- 18.de Ruyter, P. G., O. P. Kuipers, M. M. Beerthuyzen, I. van Alen-Boerrigter, and W. M. de Vos. 1996. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J. Bacteriol. 178:3434-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farooqi, I. S., S. A. Jebb, G. Langmack, E. Lawrence, C. H. Cheetham, A. M. Prentice, I. A. Hughes, M. A. McCamish, and S. O'Rahilly. 1999. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341:879-884. [DOI] [PubMed] [Google Scholar]
- 20.Farooqi, I. S., G. Matarese, G. M. Lord, J. M. Keogh, E. Lawrence, C. Agwu, V. Sanna, S. A. Jebb, F. Perna, S. Fontana, R. I. Lechler, A. M. DePaoli, and S. O'Rahilly. 2002. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Investig. 110:1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fawzi, A. B., H. Zhang, M. van Heek, and M. P. Graziano. 1996. Purification of milligram quantities of human leptin from recombinant E. coli. Horm. Metab. Res. 28:694-697. [DOI] [PubMed] [Google Scholar]
- 22.Feltkamp, M. C., H. L. Smits, M. P. Vierboom, R. P. Minnaar, B. M. de Jongh, J. W. Drijfhout, J. ter Schegget, C. J. Melief, and W. M. Kast. 1993. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur. J. Immunol. 23:2242-2249. [DOI] [PubMed] [Google Scholar]
- 23.Fliedner, S., C. Schulz, and H. Lehnert. 2006. Brain uptake of intranasally applied radioiodinated leptin in Wistar rats. Endocrinology 147:2088-2094. [DOI] [PubMed] [Google Scholar]
- 24.Fruhbeck, G. 2006. Intracellular signalling pathways activated by leptin. Biochem. J. 393:7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson, W. T., I. S. Farooqi, M. Moreau, A. M. DePaoli, E. Lawrence, S. O'Rahilly, and R. A. Trussell. 2004. Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J. Clin. Endocrinol. Metab. 89:4821-4826. [DOI] [PubMed] [Google Scholar]
- 26.Guisez, Y., I. Fache, L. A. Campfield, F. J. Smith, A. Farid, G. Plaetinck, J. Van der Heyden, J. Tavernier, W. Fiers, P. Burn, and R. Devos. 1998. Efficient secretion of biologically active recombinant OB protein (leptin) in Escherichia coli, purification from the periplasm and characterization. Protein Expr. Purif. 12:249-258. [DOI] [PubMed] [Google Scholar]
- 27.Halaas, J. L., K. S. Gajiwala, M. Maffei, S. L. Cohen, B. T. Chait, R. L. Rabinowitz, R. L. Lallone, S. K. Burley, and J. M. Friedman. 1995. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543-545. [DOI] [PubMed] [Google Scholar]
- 28.Hallschmid, M., C. Benedict, J. Born, H. L. Fehm, and W. Kern. 2004. Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol. Behav. 83:55-64. [DOI] [PubMed] [Google Scholar]
- 29.Heymsfield, S. B., A. S. Greenberg, K. Fujioka, R. M. Dixon, R. Kushner, T. Hunt, J. A. Lubina, J. Patane, B. Self, P. Hunt, and M. McCamish. 1999. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282:1568-1575. [DOI] [PubMed] [Google Scholar]
- 30.Illum, L. 2000. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 11:1-18. [DOI] [PubMed] [Google Scholar]
- 31.Imagawa, K., Y. Numata, G. Katsuura, I. Sakaguchi, A. Morita, S. Kikuoka, Y. Matumoto, T. Tsuji, M. Tamaki, K. Sasakura, H. Teraoka, K. Hosoda, Y. Ogawa, and K. Nakao. 1998. Structure-function studies of human leptin. J. Biol. Chem. 273:35245-35249. [DOI] [PubMed] [Google Scholar]
- 32.Javor, E. D., E. K. Cochran, C. Musso, J. R. Young, A. M. Depaoli, and P. Gorden. 2005. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes 54:1994-2002. [DOI] [PubMed] [Google Scholar]
- 33.Kastin, A. J., and W. Pan. 2006. Intranasal leptin: blood-brain barrier bypass (BBBB) for obesity? Endocrinology 147:2086-2087. [DOI] [PubMed] [Google Scholar]
- 34.Kok, J., J. M. van der Vossen, and G. Venema. 1984. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl. Environ. Microbiol. 48:726-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstantinides, S., K. Schafer, J. G. Neels, C. Dellas, and D. J. Loskutoff. 2004. Inhibition of endogenous leptin protects mice from arterial and venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 24:2196-2201. [DOI] [PubMed] [Google Scholar]
- 36.Langella, P., Y. Le Loir, S. D. Ehrlich, and A. Gruss. 1993. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J. Bacteriol. 175:5806-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence, D. 2002. Intranasal delivery could be used to administer drugs directly to the brain. Lancet 359:1674. [DOI] [PubMed] [Google Scholar]
- 38.Lee, J. H., J. L. Chan, E. Sourlas, V. Raptopoulos, and C. S. Mantzoros. 2006. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J. Clin. Endocrinol. Metab. 91:2605-2611. [DOI] [PubMed] [Google Scholar]
- 39.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1998. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J. Bacteriol. 180:1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Loir, Y., S. Nouaille, J. Commissaire, L. Bretigny, A. Gruss, and P. Langella. 2001. Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Appl. Environ. Microbiol. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Magnen, J. 1999. Role of dietary odour in the short-term control of intake in the white rat (first published in French in 1956). Appetite 33:30-32. [DOI] [PubMed] [Google Scholar]
- 42.Licinio, J., S. Caglayan, M. Ozata, B. O. Yildiz, P. B. de Miranda, F. O'Kirwan, R. Whitby, L. Liang, P. Cohen, S. Bhasin, R. M. Krauss, J. D. Veldhuis, A. J. Wagner, A. M. DePaoli, S. M. McCann, and M. L. Wong. 2004. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc. Natl. Acad. Sci. USA 101:4531-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madej, T., M. S. Boguski, and S. H. Bryant. 1995. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. 373:13-18. [DOI] [PubMed] [Google Scholar]
- 44.Matarese, G., S. Moschos, and C. S. Mantzoros. 2005. Leptin in immunology. J. Immunol. 174:3137-3142. [DOI] [PubMed] [Google Scholar]
- 45.Mielcarek, N., S. Alonso, and C. Locht. 2001. Nasal vaccination using live bacterial vectors. Adv. Drug. Deliv. Rev. 51:55-69. [DOI] [PubMed] [Google Scholar]
- 46.Miyoshi, A., I. Poquet, V. Azevedo, J. Commissaire, L. Bermudez-Humaran, E. Domakova, Y. Le Loir, S. C. Oliveira, A. Gruss, and P. Langella. 2002. Controlled production of stable heterologous proteins in Lactococcus lactis. Appl. Environ. Microbiol. 68:3141-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montague, C. T., I. S. Farooqi, J. P. Whitehead, M. A. Soos, H. Rau, N. J. Wareham, C. P. Sewter, J. E. Digby, S. N. Mohammed, J. A. Hurst, C. H. Cheetham, A. R. Earley, A. H. Barnett, J. B. Prins, and S. O'Rahilly. 1997. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387:903-908. [DOI] [PubMed] [Google Scholar]
- 48.Nandi, J., M. M. Meguid, A. Inui, Y. Xu, I. G. Makarenko, T. Tada, and C. Chen. 2002. Central mechanisms involved with catabolism. Curr. Opin. Clin. Nutr. Metab. Care 5:407-418. [DOI] [PubMed] [Google Scholar]
- 49.Oral, E. A., V. Simha, E. Ruiz, A. Andewelt, A. Premkumar, P. Snell, A. J. Wagner, A. M. DePaoli, M. L. Reitman, S. I. Taylor, P. Gorden, and A. Garg. 2002. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 346:570-578. [DOI] [PubMed] [Google Scholar]
- 50.Peelman, F., H. Iserentant, S. Eyckerman, L. Zabeau, and J. Tavernier. 2005. Leptin, immune responses and autoimmune disease. Perspectives on the use of leptin antagonists. Curr. Pharm. Des. 11:539-548. [DOI] [PubMed] [Google Scholar]
- 51.Peelman, F., K. Van Beneden, L. Zabeau, H. Iserentant, P. Ulrichts, D. Defeau, A. Verhee, D. Catteeuw, D. Elewaut, and J. Tavernier. 2004. Mapping of the leptin binding sites and design of a leptin antagonist. J. Biol. Chem. 279:41038-41046. [DOI] [PubMed] [Google Scholar]
- 52.Peelman, F., W. Waelput, H. Iserentant, D. Lavens, S. Eyckerman, L. Zabeau, and J. Tavernier. 2004. Leptin: linking adipocyte metabolism with cardiovascular and autoimmune diseases. Prog. Lipid Res. 43:283-301. [DOI] [PubMed] [Google Scholar]
- 53.Pelleymounter, M. A., M. J. Cullen, M. B. Baker, R. Hecht, D. Winters, T. Boone, and F. Collins. 1995. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540-543. [DOI] [PubMed] [Google Scholar]
- 54.Plata-Salaman, C. R. 2000. Central nervous system mechanisms contributing to the cachexia-anorexia syndrome. Nutrition 16:1009-1012. [DOI] [PubMed] [Google Scholar]
- 55.Ramirez, I. 1993. Role of olfaction in starch and oil preference. Am. J. Physiol. 265:R1404-R1409. [DOI] [PubMed] [Google Scholar]
- 56.Rau, H., B. J. Reaves, S. O'Rahilly, and J. P. Whitehead. 1999. Truncated human leptin (delta133) associated with extreme obesity undergoes proteasomal degradation after defective intracellular transport. Endocrinology 140:1718-1723. [DOI] [PubMed] [Google Scholar]
- 57.Rentsch, J., N. Levens, and M. Chiesi. 1995. Recombinant ob-gene product reduces food intake in fasted mice. Biochem. Biophys. Res. Commun. 214:131-136. [DOI] [PubMed] [Google Scholar]
- 58.Repa, A., C. Grangette, C. Daniel, R. Hochreiter, K. Hoffmann-Sommergruber, J. Thalhamer, D. Kraft, H. Breiteneder, A. Mercenier, and U. Wiedermann. 2003. Mucosal co-application of lactic acid bacteria and allergen induces counter-regulatory immune responses in a murine model of birch pollen allergy. Vaccine 22:87-95. [DOI] [PubMed] [Google Scholar]
- 59.Schafer, K., M. Halle, C. Goeschen, C. Dellas, M. Pynn, D. J. Loskutoff, and S. Konstantinides. 2004. Leptin promotes vascular remodeling and neointimal growth in mice. Arterioscler. Thromb. Vasc. Biol. 24:112-117. [DOI] [PubMed] [Google Scholar]
- 60.Schulz, C., K. Paulus, and H. Lehnert. 2004. Central nervous and metabolic effects of intranasally applied leptin. Endocrinology 145:2696-2701. [DOI] [PubMed] [Google Scholar]
- 61.Shimizu, H., I. S. Oh, S. Okada, and M. Mori. 2005. Inhibition of appetite by nasal leptin administration in rats. Int. J. Obes. (London) 29:858-863. [DOI] [PubMed] [Google Scholar]
- 62.Steidler, L., W. Hans, L. Schotte, S. Neirynck, F. Obermeier, W. Falk, W. Fiers, and E. Remaut. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 63.Steidler, L., K. Robinson, L. Chamberlain, K. M. Schofield, E. Remaut, R. W. Le Page, and J. M. Wells. 1998. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 66:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strobel, A., T. Issad, L. Camoin, M. Ozata, and A. D. Strosberg. 1998. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat. Genet. 18:213-215. [DOI] [PubMed] [Google Scholar]
- 65.Strosberg, A. D., and T. Issad. 1999. The involvement of leptin in humans revealed by mutations in leptin and leptin receptor genes. Trends Pharmacol. Sci. 20:227-230. [DOI] [PubMed] [Google Scholar]
- 66.Tindle, R. W., G. J. Fernando, J. C. Sterling, and I. H. Frazer. 1991. A “public” T-helper epitope of the E7 transforming protein of human papillomavirus 16 provides cognate help for several E7 B-cell epitopes from cervical cancer-associated human papillomavirus genotypes. Proc. Natl. Acad. Sci. USA 88:5887-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Asseldonk, M., G. Rutten, M. Oteman, R. J. Siezen, W. M. de Vos, and G. Simons. 1990. Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis subsp. lactis MG1363. Gene 95:155-160. [DOI] [PubMed] [Google Scholar]
- 68.Welt, C. K., J. L. Chan, J. Bullen, R. Murphy, P. Smith, A. M. DePaoli, A. Karalis, and C. S. Mantzoros. 2004. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 351:987-997. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, F., M. B. Basinski, J. M. Beals, S. L. Briggs, L. M. Churgay, D. K. Clawson, R. D. DiMarchi, T. C. Furman, J. E. Hale, H. M. Hsiung, B. E. Schoner, D. P. Smith, X. Y. Zhang, J. P. Wery, and R. W. Schevitz. 1997. Crystal structure of the obese protein leptin-E100. Nature 387:206-209. [DOI] [PubMed] [Google Scholar]
- 70.Zhang, Y., R. Proenca, M. Maffei, M. Barone, L. Leopold, and J. M. Friedman. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425-432. [DOI] [PubMed] [Google Scholar]