Abstract
The human immunodeficiency virus type 1 (HIV-1) Tat protein is considered a potential candidate vaccine antigen. In an effort to design a strategy for noninvasive vaccination against HIV-1, we developed transgenic tomatoes expressing the Tat protein. Two independent plants testing positive in transgene detection analysis were selected and grown to maturity. Monoclonal antibodies against Tat recognized a protein of the expected size. Interestingly, expression of Tat seemed to be toxic to the plant, as in all cases the fruit exhibited underdeveloped reproductive structures and no seeds. Nine groups of 10 pathogen-free BALB/c male mice were primed either orally, intraperitoneally, or intramuscularly with 10 mg of tomato fruit extract derived from transgenic or wild-type plants and with 10 μg of Tat86 recombinant protein. Mice were immunized at days 0, 14, and 28, and given boosters after 15 weeks; sera were drawn 7 days after each booster, and the antibody titer was determined by enzyme-linked immunosorbent assay. All three immunization approaches induced the development of a strong anti-Tat immunological response, which increased over time. Isotype subclass determination showed the presence of mucosal (immunoglobulin A) immunity soon after the beginning of the oral immunization protocol, and the data were confirmed by the presence of anti-Tat antibodies in fecal pellets and in vaginal washes. We also demonstrated that sera from immunized mice inhibited with high efficiency recombinant Tat-dependent transactivation of the HIV-1 long terminal repeat promoter. This neutralization activity might be relevant for the suppression of extracellular Tat activities, which play an important role in HIV disease development.
Human immunodeficiency virus (HIV) infection is responsible for a large number of deaths annually and represents a significant threat to global health. The development of an HIV vaccine is an urgent priority and represents the only realistic approach to control the global expansion of the HIV pandemic, particularly in the developing world. Although preventive immunity remains the main goal, secondary endpoints (e.g., block of virus replication and delay of disease onset) are being considered as more achievable aims. Considering that natural transmission of HIV occurs at the mucosae and that mucosa-associated lymphoid tissues may be the earliest target for virus replication (14, 36), successful induction of robust mucosal immunity may require vaccination by a mucosal route. Thus, a successful HIV vaccine may ultimately be a nonreplicating mucosal vaccine consisting of structural or nonstructural immunogens, alone or in combination. Over the last 2 decades, most efforts in HIV vaccine development have been based on the use of the HIV envelope protein (Env), with the goal of inducing sterilizing immunity. However, Env-based vaccines have failed because of the complex structure of Env and the difficulty of generating broadly reactive, high-titer neutralizing antibodies as well as its high variability among viral isolates (20).
In contrast, the Tat protein of HIV-1 shows little variability among HIV subtypes and is highly conserved in both inter- and intrapatient variants (11). Tat is a small regulatory protein composed of 86 to 101 amino acid residues (depending on the viral isolate) encoded by two exons. Several studies have dissected the molecular mechanisms of Tat function and shown that the protein acts as a powerful transcriptional activator of viral gene expression. At the long terminal repeat (LTR) promoter, the protein binds a cis-acting RNA element (transactivation-responsive region), present at the 5′ end of each viral transcript (1). Through this interaction, Tat activates HIV-1 transcription by promoting the assembly of transcriptionally active complexes at the LTR by multiple protein-protein interactions (16, 23). Besides its fundamental role in the control of HIV-1 gene expression, Tat is also released extracellularly from infected cells (8) and endocytosed by neighboring cells, where it translocates to the nucleus in an active form. The uncommon transcellular trafficking of the Tat protein (7, 33, 34) may be responsible for a variety of biological activities exerted by the extracellular Tat in several cell types that might eventually be involved in the pathogenesis of HIV disease and contribute to the onset of AIDS-associated pathologies, including neurological impairment, immunodeficiency, and tumorigenesis (7). Therefore, the development of an effective anti-Tat immune response might not only lead to the specific immune recognition of HIV-1-infected cells but also neutralize the effects of the extracellular protein released by the infected cells.
HIV-1 Tat-based vaccines (both DNA and protein) have proven to be safe and immunogenic in preclinical models and effective in controlling virus replication and blocking the onset of the disease in monkeys (10, 22). Consistent with these findings, the incidence and risk of progression to advanced HIV disease are lower among patients with antibodies against Tat than anti-Tat-negative individuals (29). In addition, sera from individuals infected with different virus clades are able to recognize the same Tat epitopes, supporting the concept of a cross-clade vaccine (3). These data indicate that Tat could be an optimal target for vaccine strategies in populations in which different HIV-1 subtypes are prevalent.
A requisite for the successful control and prevention of HIV in developing countries is the development of a noninvasive-immunization strategy. In this respect, transgenic plants are invaluable as bioreactors for the production of the Tat antigen. Transgenic plants offer several advantages over conventional production systems, such as low-cost inputs, feasibility of scaling up, increased stability, reduction of health risks deriving from contamination with human pathogens, the lack of a need for refrigeration, the lack of a need for syringes, and the fact that plant cells are able to perform complex posttranslational modifications. A wide variety of pharmaceutically valuable proteins have been produced in plants, including antibodies (32), antigens (2, 4), and biopharmaceuticals that fully retain their activity (6, 13, 40). However, with a few exceptions, most biopharmaceuticals have been expressed in tobacco. This represents an obstacle in cases where therapeutic proteins are administered by mucosal routes, because of the presence of alkaloids and other compounds toxic to humans in tobacco plants.
A series of advantageous features that make plants an optimal system for the development of mucosal HIV vaccines were recently reviewed by Horn et al. (15) and Webster et al. (37). Consequently, several HIV antigens have already been expressed in plants, the most common being the envelope protein (Env), alone or in combination (17, 25, 27, 31, 39, 40). Two groups have expressed a full-length Tat, but in one case, using potato, they did not report immunogenicity studies (19), and in the other case, using spinach (18), oral immunization with plant-based Tat did not induce antibody production.
As a first step towards developing a possible plant-derived oral vaccine against HIV, we have generated tomato plants expressing a version of the native Tat gene from which the introns were removed. In this paper, we report fruit-specific expression of the Tat protein, assessment of the activity of Tat by an LTR transactivation assay, immunization of mice by intramuscular, intraperitoneal, and oral routes with the plant-produced Tat, the identification of immunoglobulin G (IgG) subclasses induced by oral immunization, and evaluation of the mucosal response in mice.
MATERIALS AND METHODS
Tomato tissue culture and transformation.
For transformation experiments, the tomato cultivar TA234, kindly donated by Steve Tanksley, Cornell University, was employed. Eight-day cotyledons were excised and processed as described by Gutierrez-Ortega et al. (13). Cocultivation with Agrobacterium tumefaciens was performed for 24 h at 19°C in the dark with the addition of 100 mg/liter acetosyringone. Shoot regeneration was carried out in the presence of 300 mg/liter ticarcillin-clavulanic acid (Timentin) to inhibit Agrobacterium and 100 mg/liter kanamycin as a selective agent. The Patho Screen NptII enzyme-linked immunosorbent assay (ELISA) kit (AGDIA) was employed to detect nptII expression following the manufacturer's instructions.
Construction of plant expression vectors.
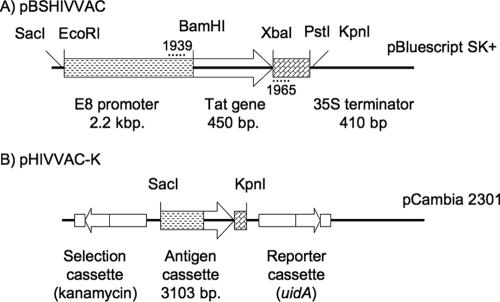
Plasmid pBSHIVVAC was constructed by ligating the following components: (i) a 2.2-kb fragment containing the tomato E8 fruit-specific promoter obtained by EcoRI/BamHI digestion of pUC118 E8 (21); (ii) a 450-bp fragment encoding the Tat cDNA (provided by Mauro Giacca, ICGEB, Trieste, Italy), obtained by BamHI/XbaI digestion of pCMVTat101 (26); (iii) a XbaI/PstI 410-bp fragment containing the cauliflower mosaic virus terminator; and (iv) pBlueScript II SK(+) (Stratagene) digested with PstI/EcoRI. The correct orientation of all components was verified by restriction mapping and sequencing using an ABI PRISM Big Dye terminator system (Applied Biosystems). Subsequently, the whole cassette was excised from pBlueScript and subcloned, as a KpnI/SstI fragment, into the binary vector pCambia 2301 (www.cambia.org), which confers resistance to kanamycin. This vector was called pHIVVAC-K (Fig. 1A). Plasmids were maintained and multiplied in Escherichia coli XL1-Blue. The pHIVVAC-K vector was transferred into A. tumefaciens LBA 4404.
FIG. 1.
Map showing the T-DNA region of plasmids used to generate transgenic tomato plants. (A) pBSHIVVAC was constructed by ligating four fragments: tomato E8 promoter, Tat gene, 35S terminator, and pBluescript SK(+). Dotted lines indicate the positions of the primers employed for amplification: 1939 (forward) and 1965 (reverse) Total size, 5,712 bp. (B) pHIVVAC-K was obtained by cloning the expression cassette from pBSHIVVAC into pCAMBIA 2301. Total size, 14,327 bp.
Genomic DNA isolation and PCR.
Genomic DNA was purified from tomato leaves using a REDExtract-N-Amp plant PCR kit (Sigma). Transgenes were amplified by PCR using 4 μl of leaf extract; 10 μl of REDExtract-N-Amp PCR mix, and 20 pmol each of primers directed to the 3′ end of the E8 promoter (5′ ATCAGACGTATTGGG 3′) and the 5′ end of the 35CaMV terminator (5′ AAGAACCCTAATTCC 3′) in a volume of 50 μl. The primers were designed to include some base pairs from the E8 promoter and from the 35S terminator flanking the Tat gene so that the final size of the amplified product was 520 bp. Amplification conditions were as follows: 1 cycle at 95°C for 5 min; 30 cycles at 95°C for 45 s, 52°C for 1.5 min, and 72°C for 1 min; and 1 cycle at 72°C for 10 min. Amplification products were analyzed on 0.8% agarose gels.
Genomic DNA extraction and Southern hybridization.
Genomic DNA was isolated from young leaves as described by Sambrook and Russell (30) and subsequently digested (15 μg) with either EcoRI or HindIII. Digests were separated on a 0.8% agarose gel, blotted onto a nylon membrane (Amersham), and incubated at 80°C for 2 h. The 32P-labeled probes, specific for the transgene, were prepared using a Redi Prime II random prime labeling system (Amersham) following the manufacturer's instructions. Membranes were hybridized at 65°C overnight, washed twice for 15 min at 65°C with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate (SDS), and exposed to BioMax film (Kodak) at −70°C.
RNA and protein expression analysis.
RNA from fresh fruit was extracted using a Concert kit (Invitrogen) following the manufacturer's instructions. A total of 20 μg of total RNA from each sample was loaded on a denaturing gel and blotted onto a nylon membrane as before. Hybridization was performed as described above.
Total proteins were extracted from fresh fruits by grinding and homogenization in 1 volume (wt/vol) of extraction buffer (10% SDS, 100 mM Tris-Cl [pH 8], 0.1 mg/liter phenylmethylsulfonyl fluoride) at 4°C. Samples were subsequently freeze-dried and stored at 4°C. Expression of the Tat protein was determined by Western blotting. Two hundred fifty micrograms of freeze-dried nontransformed and Tat-expressing tomato extracts was dissolved in radioimmunoprecipitation assay (250 mM NaCl) lysis buffer and incubated at 4°C for 30 min. Protein samples were separated by 10% SDS-polyacrylamide gel electrophoresis, electrotransferred to a nitrocellulose membrane, and probed with monoclonal anti-Tat antibodies. The nitrocellulose membranes were blocked for 1 h in phosphate-buffered saline (PBS) containing 5% dry skim milk and 0.1% Triton X-100. The membranes were probed with anti-Tat primary antibody (mouse monoclonal antibody clone 4672; AIDS Research and Reference Reagent Program, AIDS Program, NIAID, NIH repository), extensively washed with 0.1% Triton X-100 in PBS, and then incubated with horseradish peroxidase-conjugated secondary antibody. Bound antibody was visualized with an enhanced chemiluminescence (ECL) kit (Amersham).
Luciferase transactivation assay.
HeLa cells stably expressing the LTR-luciferase cassette (containing an integrated HIV-1 LTR-Luc gene construct; HL3T1 cells) were seeded into a 24-well plate (40,000 cells/well) and incubated overnight in Dulbecco's modified Eagle's medium (Invitrogen, Cergy-Pontoise, France) supplemented with 10% fetal calf serum. The following day, when cells had reached 70% confluence, recombinant glutathione S-transferase (GST)-Tat protein or lyophilized Tat tomato extracts dissolved in sterile PBS were added; GST recombinant protein and nontransformed tomato extract were used as negative controls. Recombinant GST-Tat, containing the 86-amino-acid Tat protein of HIV-1 clone HXB2 fused to GST, was produced and purified by glutathione-agarose affinity chromatography as described by Marzio et al. (26). The lyophilized Tat tomato extracts were prepared by grinding fruit samples in liquid nitrogen and adding 2 volumes of cold extraction buffer (0.01 M Na2HPO4, 0.003 M KH2PO4, 0.1 M NaCl, 0.025 M sodium ascorbate, 0.5% polyvinyl-pyrrolidone 40,000). Crude extracts were centrifuged in a bench centrifuge at 10,000 rpm for 10 min at 4 C, and cleared supernatants were filtered through sieve cloth and dialyzed against PBS using 12,000- to 14,000-molecular weight dialysis tubing (Spectra-Por). Dialyzed extracts were lyophilized, reconstituted in deionized water, and filter sterilized with 0.2-μm membranes (Millipore). Recombinant Tat was present at approximately 1 μg/mg in tomato fruit extract.
Twelve or twenty-four hours after the addition of proteins and extracts, cells were harvested and lysed with passive lysis buffer (dual-luciferase reporter assay; Promega commercial kit). Luciferase expression was quantified on 10 μl of lysate supernatant by adding 50 μl of a buffer containing 20 mM Tris-HCl, 1.07 mM Mg(CO3)4, 1 mM Mg(OH)2·5H2O, 2.67 mM MgSO4, 0.1 mM EDTA, 33.3 mM dithiothreitol, 270 μM coenzyme A, 470 μM luciferin, and 530 μM ATP.
Light emission was measured by a Lumat LB9501 luminometer (Berthold), and relative light units were calculated versus background activity (untreated cells and cells treated with GST-transformed and nontransformed tomato extracts). Luciferase activity was normalized to the protein concentration, determined with a BCA protein assay kit (Pierce) according to the manufacturer's instructions. Results are averages of at least two different experiments, each done in triplicate.
Immunization protocol.
Four groups of pathogen-free BALB/c female mice (10 animals per group) were primed either intraperitoneally (i.p.) or intramuscularly (i.m.) with 500 μg (equivalent to 500 ng of recombinant Tat) of lyophilized tomato fruit extract derived from transformed or nontransformed plants and emulsified (1:1) in complete Freund's adjuvant (Sigma). Similarly, four other groups of mice were immunized with 1.5 μg GST-Tat recombinant protein (equivalent to 500 ng of pure Tat86) or with a recombinant GST protein as a control. Mice were given boosters on days 14, 28, and 90 using the same antigens in incomplete Freund's adjuvant (Sigma). Sera were drawn 7 days after each booster. Four additional groups of animals (10 per group) were used for oral feeding (Tat tomato, nontransformed tomato, recombinant GST-Tat, and GST proteins). Prior to immunization, mice were starved 1 day and fed with 5 mg (equivalent to 5 μg of recombinant Tat) of lyophilized extract or with 15 μg of recombinant proteins. Throughout the experiment, mice were periodically weighed, and the condition of each mouse was constantly monitored.
Blood sampling.
Blood was withdrawn at weekly intervals from 1 week before immunization with tomato extract until 2 months after the end of the treatment and monthly thereafter for 3 additional months.
Vaginal washes.
Vaginal washes were obtained by flushing the vaginal lumen several times with 30 μl of PBS containing 0.5% bovine serum albumin (BSA) and collecting the effluent. To limit the effect of the estrous cycle on local antibody responses, vaginal washes from 2 consecutive days were collected, pooled, centrifuged to remove particulate matter, and stored at −80°C until use.
Isolation of fecal antibodies.
Feces were removed from cages before and 1 week after each immunization, dissolved in sterile PBS, and centrifuged at top speed for 10 min. Supernatants were collected and stored at −20°C until use.
Evaluation of humoral immune response.
The presence of antibodies in the serum was determined by ELISA. Briefly, 96-well microtiter plates (Nunc) were coated overnight with 5 μg/ml of GST-Tat in 0.05 M carbonate/bicarbonate buffer (pH 9.6) at 37°C. The plates were blocked with 1% BSA in PBS containing 0.05% Tween 20 (PBS-T) at 37°C for 1 h. Following a wash with PBS-T, twofold serial dilutions of each sample in PBS-T containing 0.5% BSA were made (final volume, 100 μl; dilutions ranging from 1:40 to 1:320), and plates were incubated at 37°C for 1 h. At the end of the incubation period, plates were washed with PBS-T, 100 μl Fc-specific horseradish peroxidase-conjugated goat anti-mouse IgG (1:2,000) was added to each well, and wells were incubated at 37°C for 1 h. Unbound conjugate was removed by thoroughly washing the plates with PBS-T, and enzyme activity was determined as previously described. The data for anti-Tat antibody responses are expressed as net optical density (OD) values. The OD values were measured after 5 min of substrate development.
Evaluation of mucosal response.
Mucosal immunity was analyzed by a conventional ELISA (as described above) on Tat-coated plates using the effluent of the vaginal washes and the supernatants of dissolved feces. Samples were processed as previously described and stored at −80°C until assayed for specific antibody reactivity (38).
Determination of Ig isotypes and subclasses.
Tat-specific antibody isotypes and subclasses were determined in mouse sera at the 1/100 dilution by ELISA using specific conjugates and substrates containing specific anti-IgM, anti-IgG1, anti-IgG2a, and anti-IgA antibodies at the 1:300 dilution (Bio-Rad). The OD values were measured after 5 min of substrate development.
Neutralization of extracellular Tat activity.
HL3T1 HeLa cells were seeded in a 24-well plate (40,000 cells/well) and incubated overnight in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum. Increasing amounts (from 0.25 μg/ml to 5 μg/ml) of recombinant GST-Tat protein were diluted in Optimem (final volume of 500 μl). Various dilutions of pooled serum were then added to the HL3T1 cells, and cells were incubated for 24 h at 37°C. A luciferase assay was performed as described above.
Results are expressed as means with standard deviations. The antibody response was analyzed by the nonparametric Mann-Whitney U test using Statview software (Abacus). Results were considered statistically significant if P values were <0.05.
RESULTS
Transformation of tomato plants with Tat.
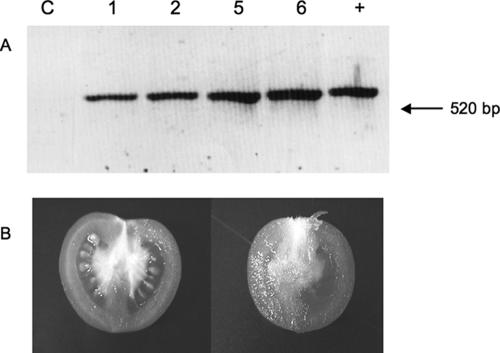
One hundred cotyledons from tomato plantlets were used for the transformation experiment. Fifteen kanamycin-resistant regenerated shoots were selected, rooted, and transferred to soil. Five lines were selected at random and subjected to PCR to determine the presence of the transgene, using primers designed to amplify the complete Tat gene. Specific amplification bands of the expected size (520 bp) were obtained from four plants examined. To confirm that the amplified bands corresponded to Tat, the DNA was transferred to a membrane and hybridized against the Tat gene. The amplified bands did hybridize with the probe (Fig. 2A). All selected lines also tested positive for accumulation of NptII protein by ELISA, whereas regenerated nontransformed plants did not show any NptII accumulation (data not shown).
FIG. 2.
Hybridization of PCR products to Tat and phenotypes of transformed plants. (A) After PCR amplification using genomic DNA from four transformed tomato lines, including a nontransformed line (C), the DNA was transferred to a nylon membrane and hybridized against a 32P-labeled Tat-specific probe. Numbers at the top indicate the transgenic lines. The hybridizing bands which correspond to the amplified bands (520 bp) are clearly visible in all lines. Lane +, amplification from DNA using as template the plasmid employed for transformation. (B) Nontransformed fruit (left) and transformed fruit expressing Tat (right).
Phenotypic analysis of the transformed plants did not reveal any major differences between wild-type and transformed plants in most respects: time to flowering, ripening pattern, and leaf morphology were similar in both groups (data not shown). However, there was a single, albeit important, difference, in that transformed plants did not develop viable seeds. This result was found in all selected lines. At most, a few underdeveloped seed-like structures could be observed (Fig. 2B).
Tat expression in transgenic plants.
One month after the plants were transferred to soil, two plants (lines 2 and 5) were chosen randomly and subjected to Southern blot analysis to verify the presence of Tat transgene in the tomato genome. One hybridizing band was observed for line 2, whereas two hybridizing bands were observed for line 5 (Fig. 3). Southern analysis of the three remaining lines revealed the presence of one to three hybridizing bands (data not shown). Wild-type regenerated plants did not show any hybridization signal. Line 2, which contains only one copy of the transgene, was employed for further studies.
FIG. 3.
Southern blot analysis of transformed tomato lines. DNA samples from two transformed tomato plants (2 and 5) were digested with EcoRI, transferred to a nylon membrane, and hybridized against a 32P-labeled Tat-specific probe. Lane + was loaded with pCMVTat101 DNA. wt, DNA from a nontransformed line. Numbers on the left are sizes, in base pairs. (A) Ethidium bromide-stained gel; (B) autoradiograph.
Total RNA isolated from wild-type and line 2 tomatoes at different ripening stages (green, yellow, orange, and red) was used to determine Tat mRNA expression by Northern blotting. Figure 4 reveals a faint hybridizing signal in green fruit, which increased in intensity as ripening proceeded, whereas no signal was detected in ripe fruits from wild-type plants (Fig. 4). This result indicated that the Tat gene was being transcribed. Analysis from other plant organs (leaf and stem) did not reveal any hybridizing signal, as expected (data not shown).
FIG. 4.
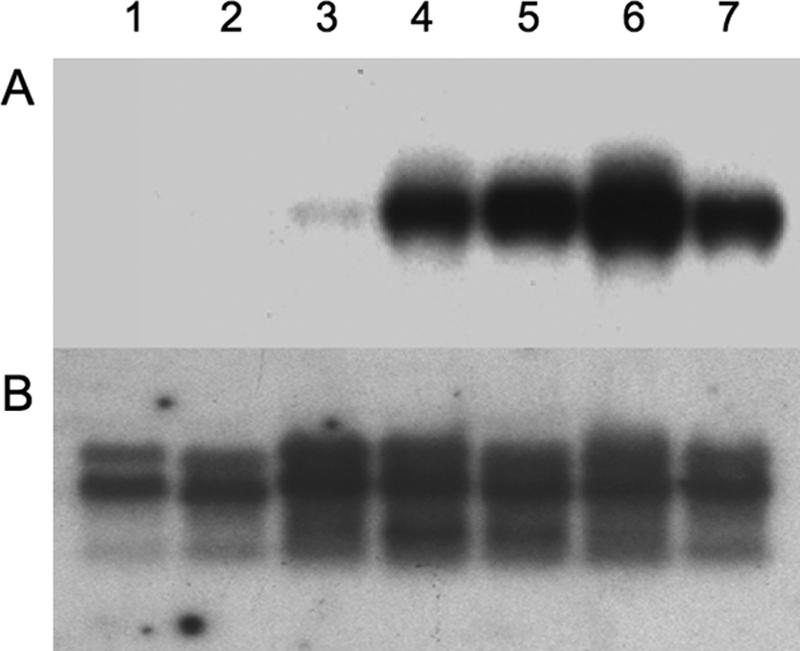
Tat expression at the transcriptional level. (A) Total RNA was extracted from fruits from line 2 at various ripening stages, transferred to a nylon membrane, and hybridized against a 32P-labeled Tat-specific probe. Lane 1, nontransformed green fruit; lane 2, nontransformed ripe fruit; lane 3, transformed green fruit; lane 4, transformed yellow fruit; lane 5, transformed orange fruit; lane 6 transformed red fruit; lane 7 transformed senescent red fruit. (B) Hybridization against a ribosomal 28S gene probe was performed as a loading control.
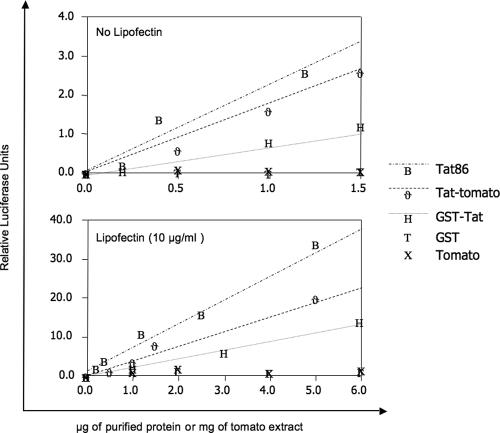
To evaluate whether functional Tat was expressed in tomatoes, we set up a luciferase transactivation assay on HL3T1 cells containing an HIV-1 LTR-Luc gene construct. The experiments were conducted in parallel using increasing amounts of Tat tomato extract (0.5 to 5 mg), recombinant GST-Tat (0.5 to 5 μg), or commercial Tat peptide (0.2 to 5 μg). Nontransformed tomato and purified recombinant GST protein were used as negative controls. All three sources of Tat efficiently transactivated the LTR in a dose-dependent manner (Fig. 5). In a second series of experiments, the same amounts of proteins were delivered to the cells using Lipofectin, which increases protein internalization (35). By using the equations of the regression curves obtained from these experiments, we estimated that functional Tat was present at approximately 1 μg/mg fruit (dry weight) in tomato fruit extract.
FIG. 5.
LTR transactivation activity of Tat from tomato extracts. Freeze-dried extracts from Tat-expressing tomatoes, recombinant GST-Tat, and chemically synthesized Tat peptide were added, either alone or in combination with Lipofectin, to the culture medium of HL3T1 cells, a HeLa cell derivative that contains an integrated HIV-1 LTR-luciferase construct. Luciferase production was assayed at 24 h after protein addition. Values are averages of six experimental results from two independent experiments along with the lines derived from the equations fitting the experimental points.
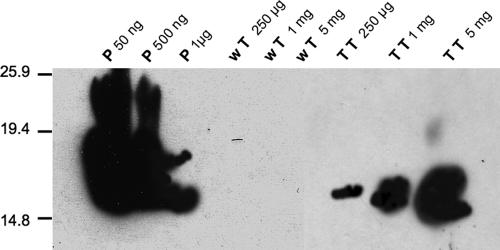
To confirm Tat expression at the protein level, we performed Western blot analysis, which showed a 16- to 17-kDa band in the fruit extract reacting with specific anti-Tat antibodies. The plant-expressed Tat protein retained most of the native immunologic properties, as demonstrated by its immunoreactivity against a Tat-specific monoclonal antibody (Fig. 6).
FIG. 6.
Immunoblot analysis of crude fruit extracts from Tat transgenic lines. Increasing amounts of total soluble protein from transgenic line 2 (TT) and from nontransformed tomato plants (wT) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was incubated with mouse monoclonal antibody clone 4672 (obtained from the AIDS Research and Reference Reagent Program), extensively washed with 0.1% Triton X-100 in PBS, and incubated with horseradish peroxidase-conjugated secondary antibody. Bound antibody was visualized with an ECL kit (Amersham). Molecular masses are indicated on the left, in kilodaltons. Different amounts of authentic, purified Tat protein (P) were included as controls. The numbers at the top indicate the amounts of protein loaded per lane.
Altogether, these experiments clearly indicate that transgenic tomato express a Tat protein of the expected size that is functionally active, supporting its use as a candidate vaccine.
Immunogenicity of plant-expressed Tat in mice.
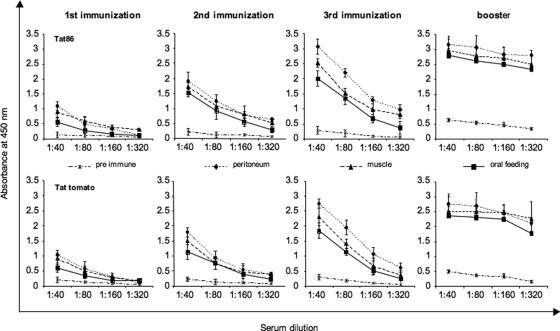
The immunoreactivity of the tomato-expressed Tat was evaluated by injection into immunocompetent BALB/c mice. Animals (n = 120) were divided into 12 groups of 10 animals each, according to immunization route (i.p. injection, i.m. injection, and oral feeding) and immunization agent (extract of nontransformed tomatoes, extract of tomatoes expressing the Tat protein, purified recombinant GST-Tat, and GST, the last two as positive and negative controls, respectively). At days 14 and 28 the animals were further immunized by the same antigen and administration route, and they were given boosters at day 90. Sera were drawn 7 days after each antigen administration, pooled from animals in the same group, and tested for the presence of anti-Tat antibodies by ELISA (Fig. 7). All three immunization approaches induced the development of a strong anti-Tat immunological response, with i.p. administration being the strongest. As expected, the response was enhanced as boosters were applied. Under all conditions, the immunoassay values were comparable to those obtained with purified GST-Tat protein.
FIG. 7.
Detection of anti-Tat antibody in serum by ELISA. Mice were injected i.p. or i.m. or fed with tomato fruit extracts expressing Tat or with GST-Tat86. At days 14 and 28, the animals were given boosters of the same antigen and administration route, and another booster was given at day 90. Sera were drawn 7 days after each antigen administration, pooled from animals within the same group, and tested for the presence of anti-Tat antibodies by ELISA. Data are expressed as net OD values measured after 5 min of substrate development. The results show that all three immunization approaches induce a strong anti-Tat immunological response, which increased over time after each booster. Sera from control animals injected with GST or nontransgenic tomato extracts following the same immunization schedule showed no anti-Tat reactivity.
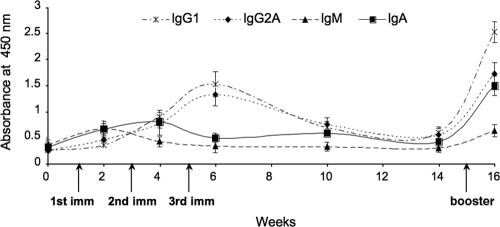
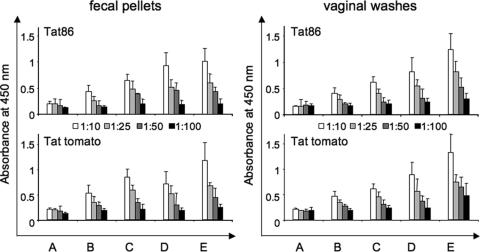
To investigate whether oral feeding was capable of inducing mucosal immunity, we analyzed IgG subclass composition in orally immunized mice. Mucosal IgAs started to increase about a week after the first immunization and declined after the second inoculation, to increase again after the third inoculation (Fig. 8). IgGs also increased dramatically after the second and third immunizations, reaching OD values higher than those of IgAs. To confirm the induction of effective mucosal immunity in these animals, we also determined the presence of anti-Tat IgAs in the feces and effluents of vaginal washes. The results obtained showed a significant reactivity against Tat at low serum dilutions (1:10 and 1:25) in these biological samples, indicating that oral feeding was able to induce Tat-specific IgAs in vaginal and intestinal surfaces (Fig. 9).
FIG. 8.
Isotype subclass determination in mouse sera. Tat-specific antibody isotypes in mouse sera were determined by ELISA using specific anti-IgG1, anti-IgG2a, anti-IgA, and anti-IgM antibodies. The graph shows the results obtained with sera from mice immunized by oral feeding with Tat tomatoes. Mucosal immunity (IgA) was detectable soon after the beginning of the immunization protocol.
FIG. 9.
Evaluation of mucosal immunity. The levels of mucosal anti-Tat antibody were analyzed by conventional ELISA on Tat-coated plates using the effluent of the vaginal washes and the supernatants of dissolved feces after centrifugation. A, preimmunization; B, first immunization; C, second immunization; D, third immunization; E, boost.
Neutralization of Tat transactivation by sera from immunized mice.
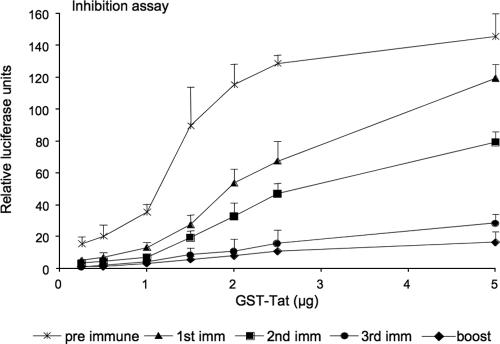
To examine the functional activity of the antibodies detected after i.m., i.p., and oral administration of plant-derived Tat, we developed an in vitro neutralization assay to determine the ability of Tat-specific antibodies to interfere with Tat-driven transactivation of the LTR-dependent HIV-1 luciferase gene. The sera from animals that had been orally immunized with Tat-expressing tomatoes effectively inhibited the activation induced by different doses of recombinant Tat at a 1:100 dilution (Fig. 10). It is interesting that, after the third immunization, Tat activity was almost completely abolished, even with the highest dose of recombinant protein (5 μg/ml). These results indicate that the anti-Tat antibodies induced by anti-Tat immunization via the oral route are able to block the activity of extracellular Tat, an observation that might be of relevance for the suppression of the activities of extracellular Tat. Comparable results were obtained with sera from animals immunized i.p. and i.m., as well as with animals immunized with purified GST-Tat protein as a control (data not shown).
FIG. 10.
Neutralization of Tat transactivation by sera from immunized mice. The graph shows the levels of transactivation of the HIV-1 LTR in HL3T1 cells after the addition of increasing amount of recombinant GST-Tat in the presence of sera from mice orally immunized with tomatoes expressing Tat, obtained at different times along the immunization schedule. Sera were used at a 1:100 dilution. After the third immunization, transactivation by extracellular Tat was almost completely abolished even when the highest concentration of GST-Tat.
DISCUSSION
This work provides several lines of evidence that prove the successful expression of HIV-Tat protein in tomato plants. First, we were able to detect the transgene in the transformed plants by PCR using specific oligonucleotides. Second, Southern analysis of the transformed plants confirmed the stable integration of the transgene into the plant genome, as one or two hybridizing bands were clearly detectable. Southern analyses of additional tomato lines also revealed the presence of the transgene in their genomes (data not shown). Third, Northern blot analysis detected low levels of the Tat mRNA in unripe tomato fruit which increased dramatically upon ripening, as expected considering the promoter used to drive transgene expression. Fourth, an anti-Tat antibody detected a protein of the expected size that was present exclusively in ripe fruit. Finally, the Tat expressed in tomato retains its capacity both to be internalized by the cells and to transactivate an integrated LTR promoter, which indicates preservation of full biological activity.
These experiments yielded lower rates of tomato regeneration and transformation (3% and 1%, respectively) than similar experiments using other antigens in our laboratory (15 to 20% and 5 to 10%, respectively). This could be due to the possible toxic side effects of the protein. Indeed, the Tat transactivator has been known to induce unexpected side effects in several cell types, including alteration of tight-junction protein expression, neuronal degeneration, reactive astrocytosis, and protein oxidation (5). In addition, various reports have indicated that Tat might induce apoptosis in immune cells by activating the classical apoptotic pathway involving mitochondria (9).
Prevention of toxicity was the reason for the use of a tissue-specific promoter in our plants; the seedless phenotype was, however, unexpected. This approach, in theory, should have prevented side effects of Tat in plant cells. Karasev et al. (18) used a chimeric construct consisting of a fusion of Tat with the coat protein of the tobacco mosaic virus and found that the construct induced severe systemic necrosis with stunting in Nicotiana benthamiana, causing the plants to die by 12 to 14 days. This effect, however, was not observed in spinach expressing the same chimeric construct. The reason for this difference is unknown. The expression levels reported were up to 300 μg of Tat per g of spinach leaf tissue. In our case, we obtained expression levels threefold higher than those of Karasev et al., but the fruit-specific promoter may have prevented further alterations in phenotype. It also seems worth reporting that transformed potatoes expressing the Tat protein transduction domain fused to a rotavirus antigen did not exhibit any adverse morphological effect (19). The absence of Tat toxicity in these experiments might be attributed to the fact that only 11 amino acids of the protein were expressed in these experiments, to the possibility that the fusion somehow prevented the toxic effects, or to the low levels at which the protein was expressed, corresponding to 0.0015% of total soluble tuber protein.
Our immunization experiments in mice clearly indicate that Tat expressed in tomatoes is immunogenic. The injection of transgenic tomato extracts either i.m. or i.p. elicited an antibody response against Tat, which increased over time along the immunization schedule. Most notably, oral feeding was also able to induce both systemic and mucosal immunity, a potentially important observation in light of possible applicability for vaccination purposes. Karasev et al. (18) immunized mice orally with 1 g of tissue containing 300 μg of Tat and found that none of the animals developed measurable Tat antibodies. The use of larger amounts of protein in our experiments might have determined the difference in the immune response, considering that mucosal immunization using plant-based compounds requires high doses of antigens (15, 37).
The analysis of Tat-specific antibody isotypes and subclasses revealed that oral immunization induced several types of antibodies, including IgAs, in the gut and in the genital tract, an indication of induction of mucosal immunity. Anti-Tat IgAs were induced shortly after the first immunization, and each booster resulted in an incremental increase in the antibody titer. In contrast, anti-Tat IgGs were induced only after the first booster, although at a higher levels than IgAs. These observations are consistent with the conclusion that oral immunization with extracts from tomatoes transgenic for Tat elicits both mucosal and systemic immunity.
One important observation of our study is the indication that the anti-Tat antibodies elicited upon plant-based Tat inoculation were able to neutralize the activity of extracellular Tat. When added to cells, the antisera inhibited LTR transcriptional activation even when very high doses of recombinant Tat were used. These results suggest that the anti-Tat immune response raised by oral immunization with Tat tomatoes might eventually neutralize several of the effects induced by extracellular Tat, especially since this protein is present at very low levels in the extracellular fluids. This conclusion is consistent with previous observations showing that mucosal delivery of Tat protein in mice induced systemic neutralizing antibodies, cytotoxic T lymphocytes, and mucosal IgAs (24, 28). However, one important difference is that those studies were performed with mucosal adjuvants, such as the E. coli heat-labile enterotoxin, whereas we did not use any.
Conclusions.
Our findings demonstrate that Tat antigen can be successfully expressed in tomatoes while retaining the immunological properties of native Tat. We also show that, in immunocompetent mice, orally delivered Tat raises mucosal IgAs and induces serum IgGs with neutralizing activity. Since anti-Tat antibodies may have a role in interfering with Tat activity, and Tat activity is requisite for the massive initial viral output that is thought to enable HIV mutational variants to overwhelm the immune system (12), we conclude that Tat may be an important potential vaccine component and that plants may represent an ideal system for the production of a possible edible HIV vaccine.
Acknowledgments
This research was partially supported by a grant from the Istituto Superiore di Sanità Italy to Mauro Giacca. We are indebted to Conacyt for Ph.D. fellowships 117112 to Y.J.P.R. and 128924 to A.G.O. Y.J.P.R. acknowledges the support of ITSA.
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Berkhout, B., R. H. Silverman, and K. T. Jeang. 1989. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell 59:273-282. [DOI] [PubMed] [Google Scholar]
- 2.Bouche, F. B., E. Marquet-Blouin, Y. Yanagi, A. Steinmetz, and C. P. Muller. 2003. Neutralising immunogenicity of a polyepitope antigen expressed in a transgenic food plant: a novel antigen to protect against measles Vaccine 21:2065-2072. [DOI] [PubMed] [Google Scholar]
- 3.Buttò, S., V. Fiorelli, A. Tripiciano, M. J. Ruiz-Alvarez, A. Scoglio, F. Ensoli, M. Ciccozzi, B. Collacchi, M. Sabbatucci, A. Cafaro, C. A. Guzmán, A. Borsetti, A. Caputo, E. Vardas, M. Colvin, M. Lukwyia, G. Rezza, and B. Ensoli. 2003. Sequence conservation and antibody cross-recognition of the clade B human immunodeficiency virus (HIV) type 1 Tat protein in HIV-1-infected Italians, Ugandans, and South Africans. J. Infect. Dis. 188:1171-1180. [DOI] [PubMed] [Google Scholar]
- 4.Chikwamba, R. K., J. Cunnick, D. Hathaway, J. McMurray, H. Mason, and K. Wang. 2002. A functional antigen in a practical crop: LT-B-producing maize protects mice against Escherichia coli heat labile enterotoxin (LT) and cholera toxin (CT). Transgenic Res. 11:479-493. [DOI] [PubMed] [Google Scholar]
- 5.Crabb, C. 2003. HIV regulatory protein Tat. AIDS 17:N10-N11. [Google Scholar]
- 6.Fischer, R., E. Stoger, S. Schillberg, P. Christou, and R. M. Twyman. 2004. Plant-based production of biopharmaceuticals. Curr. Opin. Plant Biol. 7:152-158. [DOI] [PubMed] [Google Scholar]
- 7.Fittipaldi, A., and M. Giacca. 2005. Transcellular protein transduction using the Tat protein of HIV-1. Adv. Drug Deliv. Rev. 57:597-608. [DOI] [PubMed] [Google Scholar]
- 8.Frankel, A. D., and C. O. Pabo. 1988. Cellular uptake of the Tat protein from human immunodeficiency virus. Cell 55:1189-1193. [DOI] [PubMed] [Google Scholar]
- 9.Giacca, M. 2005. HIV-1 Tat, apoptosis and the mitochondria: a tubulin link? Retrovirology 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein, G., K. Manson, G. Tribbick, and R. Smith. 2000. Minimization of chronic plasma viremia in rhesus macaques immunized with synthetic HIV-1 Tat peptides and infected with a chimeric simian/human immunodeficiency virus (SHIV33). Vaccine 25:2789-2795. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein, G., G. Tribbick, and K. Manson. 2001. Two B cell epitopes of HIV-1 Tat protein have limited antigenic polymorphism in geographically diverse HIV-1 strains. Vaccine 19:1738-1746. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein, G. 1996. HIV-1 Tat protein as a potential AIDS vaccine. Nat. Med. 9:960-964. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez-Ortega, A., C. Sandoval-Montes, M. T. Olivera-Flores, L. Santos-Argumedo, and M. A. Gómez-Lim. 2005. Expression of functional interleukin-12 from mouse in transgenic tomato plants. Transgenic Res. 14:877-885. [DOI] [PubMed] [Google Scholar]
- 14.Heise, C., C. J. Millar, A. Lackner, and S. Dandekar. 1994. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J. Infect. Dis. 169:1116-1120. [DOI] [PubMed] [Google Scholar]
- 15.Horn, M. E., K. M. Pappu, M. R. Bailey, R. C. Clough, M. Barrer, J. M. Jilka, J. A. Howard, and S. J. Streatfield. 2003. Advantageous features of plant-based systems for the development of HIV vaccines. J. Drug Target. 11:539-545. [DOI] [PubMed] [Google Scholar]
- 16.Jeang, K. T., H. Xiao, and E. A. Rich. 1999. Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J. Biol. Chem. 41:28837-28840. [DOI] [PubMed] [Google Scholar]
- 17.Joelson, T., L. Akerblom, P. Oxelfelt, B. Strandberg, K. Tomenius, and T. J. Morris. 1997. Presentation of a foreign peptide on the surface of tomato bushy stunt virus. J. Gen. Virol. 78:1213-1217. [DOI] [PubMed] [Google Scholar]
- 18.Karasev, A. V., S. Foulke, C. Wellens, A. Rich, K. J. Shon, I. Zwierzynski, D. Hone, H. Koprowski, and M. Reitz. 2005. Plant based HIV-1 vaccine candidate: Tat protein produced in spinach. Vaccine 23:1875-1880. [DOI] [PubMed] [Google Scholar]
- 19.Kim, T. G., and W. H. Langridge. 2004. Synthesis of an HIV-1 Tat transduction domain-rotavirus enterotoxin fusion protein in transgenic potato. Plant Cell Rep. 22:382-387. [DOI] [PubMed] [Google Scholar]
- 20.Letvin, N. L. 2006. Progress and obstacles in the development of an AIDS vaccine. Nat. Rev. Immunol. 6:930-939. [DOI] [PubMed] [Google Scholar]
- 21.Lincoln, J. E., S. Cordes, E. Read, and L. F. Robert. 1987. Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc. Natl. Acad. Sci. USA 84:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggiorella, M. T., S. Baroncelli, Z. Michelini, E. Fanales-Belasio, S. Moretti, L. Sernicola, A. Cara, D. R. Negri, S. Butto, V. Fiorelli, A. Tripiciano, A. Scoglio, A. Caputo, A. Borsetti, B. Ridolfi, R. Bona, P. ten Haaft, I. Macchia, P. Leone, M. R. Pavone-Cossut, F. Nappi, M. Ciccozzi, J. Heeney, F. Titti, A. Cafaro, and B. Ensoli. 2004. Long-term protection against SHIV89.6P replication in HIV-1 Tat vaccinated cynomolgus monkeys. Vaccine 22:3258-3269. [DOI] [PubMed] [Google Scholar]
- 23.Marcello, A., M. Zoppe, and M. Giacca. 2001. Multiple modes of transcriptional regulation by the HIV-1 Tat transactivator. IUBMB Life 51:175-181. [DOI] [PubMed] [Google Scholar]
- 24.Marinaro, M., A. Riccomi, R. Rappuoli, M. Pizza, V. Fiorelli, A. Tripiciano, A. Cafaro, B. Ensoli, and M. T. DeMagistris. 2003. Mucosal delivery of the human immunodeficiency virus-1 Tat protein in mice elicits systemic neutralizing antibodies, cytotoxic T lymphocytes and mucosal IgA. Vaccine 21:3972-3981. [DOI] [PubMed] [Google Scholar]
- 25.Marusic, C., P. Rizza, L. Lattanzi, C. Manzini, M. Spada, F. Belardelli, E. Benvenuto, and I. Capone. 2001. Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J. Virol. 75:8434-8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marzio, G., M. Tyagi, M. I. Gutierrez, and M. Giacca. 1998. HIV-1 Tat transactivator recruits p300 and CREB-binding protein histone acetyltransferases to the viral promoter. Proc. Natl. Acad. Sci. USA 95:13519-13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLain, L., N. Porta, G. Lomonossoff, Z. Durrani, and N. J. Dimmock. 1995. Human immunodeficiency virus type 1 neutralising antibodies raised to a gp41 peptide expressed on the surface of a plant virus. AIDS Res. Hum. Retrovir. 11:327-334. [DOI] [PubMed] [Google Scholar]
- 28.Partidos, C. D., E. Moreau, O. Chaloin, M. Tunis, J. P. Briand, C. Desgranges, and S. Muller. 2004. A synthetic HIV-1 Tat protein breaches the skin barrier and elicits Tat-neutralizing antibodies and cellular immunity. Eur. J. Immunol. 34:3723-3731. [DOI] [PubMed] [Google Scholar]
- 29.Rezza, G., V. Fiorelli, M. Dorrucci, M. Ciccozzi, A. Tripiciano, A. Scoglio, B. Collacchi, M. Ruiz-Alvarez, C. Giannetto, A. Caputo, L. Tomasoni, E. Castelli, M. Sciandra, A. Sinicco, F. Ensoli, S. Buttò, and B. Ensoli. 2005. The presence of anti-Tat Ab is predictive of long-term non-progression to AIDS or severe immunodeficiency: findings from a cohort of HIV seroconverters. J. Infect. Dis. 191:1321-1324. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Shchelkunov, S. N., R. K. Salyaev, S. G. Pozdnyakov, N. I. Rekoslavskaya, A. E. Nesterov, T. S. Ryzhova, V. M. Sumtsova, N. V. Pakova, U. O. Mishutina, T. V. Kopytina, and R. W. Hammond. 2006. Immunogenicity of a novel, bivalent, plant-based oral vaccine against hepatitis B and human immunodeficiency viruses. Biotechnol. Lett. 28:959-967. [DOI] [PubMed] [Google Scholar]
- 32.Stoger, E., C. Vaquero, E. Torres, M. Sack, L. Nicholson, J. Drossard, S. Williams, D. Keen, Y. Perrin, P. Christou, and R. Fischer. 2000. Cereal crops as viable production and storage systems for pharmaceutical scFv antibodies. Plant Mol. Biol. 42:583-590. [DOI] [PubMed] [Google Scholar]
- 33.Tasciotti, E., and M. Giacca. 2005. Fusion of the human immunodeficiency virus type 1 Tat protein transduction domain to thymidine kinase increases bystander effect and induces enhanced tumor killing in vivo. Hum. Gene Ther. 16:1389-1403. [DOI] [PubMed] [Google Scholar]
- 34.Tasciotti, E., M. Zoppe, and M. Giacca. 2003. Transcellular transfer of active HSV-1 thymidine kinase mediated by an 11-amino-acid peptide from HIV-1 Tat. Cancer Gene Ther. 10:64-74. [DOI] [PubMed] [Google Scholar]
- 35.Tyagi, M., M. Rusnati, M. Presta, and M. Giacca. 2001. Internalization of HIV-1 Tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 276:3254-3261. [DOI] [PubMed] [Google Scholar]
- 36.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Jonson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 37.Webster, D. E., M. C. Thomas, R. Pickering, A. Whyte, I. A. Dry, P. R. Gorry, and S. L. Wesselingh. 2005. Is there a role for plant-made vaccines in the prevention of HIV/AIDS? Immunol. Cell Biol. 83:239-247. [DOI] [PubMed] [Google Scholar]
- 38.Xu-Amano, J., H. Kimono, R. J. Jackson, H. F. Staats, K. Fujihashi, P. D. Burrows, C. O. Elson, S. Pillai, and J. R. McGhee. 1993. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 178:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yusibov, V., A. Modelska, K. Steplewski, M. Agadjanyan, D. Weiner, D. C. Hooper, and H. Koprowski. 1997. Antigens produced in plants by infection with chimeric plant viruses immunize against rabies virus and HIV-1. Proc. Natl. Acad. Sci. USA 94:5784-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, G. G., L. Rodrigues, B. Rovinski, and K. A. White. 2002. Production of HIV-1 p24 protein in transgenic tobacco plants. Mol. Biotechnol. 20:131-136. [DOI] [PMC free article] [PubMed] [Google Scholar]