Abstract
The adherence of Plasmodium falciparum-infected red blood cells (IRBCs) in the human placenta is mediated by chondroitin-4-sulfate (C4S). Although IRBC binding to C4S has been unequivocally established, the adherence characteristics of IRBCs at different stages of parasite development and through successive parasite generations after selection for C4S adherence are not known. Here we show that IRBCs acquire a significant capacity to bind to C4S at as early as 14 h and exhibit maximum binding at 22 to 26 h postinvasion. Surprisingly, the IRBC binding ability decreases by ∼50% at the late trophozoite and schizont stages. The binding strength of the IRBCs also gradually decreases during successive generations after selection for C4S binding, and at the 32nd generation, the binding capacity was only ∼31% of that of IRBCs at the 2nd generation, suggesting that IRBCs eventually lose their C4S-adherent capacity. We also tested the susceptibility of the adhesive protein(s) on the IRBC surface to trypsin treatment at different stages of parasite development. The data show that IRBCs with late trophozoites are more resistant to trypsin treatment than those containing early trophozoites, indicating that parasite proteins expressed on the IRBC surface during trophozoite maturation partially mask accessibility of adhesive protein for binding to C4S. These data provide important insights into the expression pattern of the C4S-adhesive protein(s) on the IRBC surface, emphasizing the need for understanding the regulation of genes involved in IRBC binding to C4S. Our data also define the parasite stage at which IRBCs are suitable for studying structural interactions with C4S.
A distinguishing feature of Plasmodium falciparum compared to three other species of human malaria parasites, P. vivax, P. malarie, and P. ovale, is that infection with P. falciparum leads to adherence of infected red blood cells (IRBCs) in the microvascular endothelia of various organs (3, 10, 25, 36, 42, 46). This event is thought to be the key factor responsible for the severity of P. falciparum malaria. Several host cell adhesion molecules, including thrombospondin (TSP), CD36, intercellular adhesion molecule 1 (ICAM1), E-selectin, P-selectin, and platelet endothelial cell adhesion molecule-1/CD31, have been reported to be receptors for IRBC adherence in the microvascular capillaries (3, 11, 25, 40). In the case of pregnant women, chondroitin-4-sulfate (C4S) chains of an unusually low-sulfated chondroitin sulfate proteoglycan (CSPG) mediate IRBC adherence in the placenta, causing maternal malaria which is associated with poor pregnancy outcomes and maternal morbidity and mortality (1, 6, 8, 15, 18, 23, 30).
Members of the var gene family antigenic proteins called P. falciparum erythrocyte membrane protein 1 (PfEMP1) expressed on the IRBC surface have been reported as the adhesins involved in binding of IRBCs to the host endothelial cell receptors or placental C4S (3, 20, 21, 32, 41). The P. falciparum genome contains ∼60 var genes. It has been proposed that the selective expression of one or a subset of PfEMP1 variant members imparts distinct adherent characteristics to IRBCs (32, 35). PfEMP1 antigens on the surface of IRBCs have been shown to undergo clonal variation at a rate of 1 to 2% per generation in vitro and at a much higher rate in vivo (13, 22, 26, 27, 28). This antigenic switching mechanism plays an important role in avoiding the recognition of IRBCs by the host immune system (7, 12, 14, 16, 39). Thus, it appears that depending on the availability of the host receptor and the immune status, as well as the presence or absence of adhesion-specific inhibitory antibodies, parasites with specific adherent phenotypes are selected. Thus, the adherent parasites sequester in the organs where the receptors are expressed and thereafter multiply. Accumulation of a large number of adhesive IRBCs and consequent induction of potent proinflammatory responses lead to clinical manifestations (8).
Several studies have reported that in P. falciparum-infected placentas the adherent IRBCs represent predominantly mature trophozoites and schizonts, and IRBCs with rings and young trophozoites are rarely adhered (4, 13, 24, 28). However, one study has clearly shown that the ring-stage IRBCs can also bind to the placental syncytiotrophoblast surface (34). Despite this observation, it is widely believed that the efficient binding of IRBCs to C4S occurs at the mature trophozoite stage. Accordingly, many investigators have used IRBCs at the mature trophozoite stage to study C4S-IRBC interactions by in vitro cytoadherence assays (2, 4, 5, 13, 24). Studies on the expression pattern of PfEMP1 in CD36- and ICAM1-adherent parasites have shown that maximal PfEMP1 expression occurs at the mid- to late ring stage (21). However, IRBCs maximally adhere to CD36 and to ICAM1 at 18 to 20 h postinvasion, and both types of adherent parasites maintain peak levels of binding until the schizont stage (21). In the case of C4S-adherent IRBCs, the binding characteristics during parasite development are not known. It is also not known how long the C4S binding IRBCs maintain their ability during successive generations after selection for binding. Here, we studied the C4S adherence capacity of IRBCs during various stages of parasite development as well as during successive parasite generations after the selection of IRBCs for C4S binding. Our data demonstrate that the C4S binding ability of the IRBCs is maximal at the late ring and early trophozoite stages and is considerably lower at the late trophozoite and schizont stages. Our data also demonstrate that the binding capacity of IRBCs gradually decreases during successive generations after selecting for C4S binding. To gain insight into the observed low binding capacity of IRBCs at the late trophozoite and schizont stages compared to early stages, we analyzed the binding of IRBCs to C4S after treatment with trypsin. The results indicate that the accessibility of the parasite adhesive protein(s) for C4S binding is partially blocked during the late trophozoite and schizont stages, presumably by other knob-associated proteins expressed during late stages of parasite development.
MATERIALS AND METHODS
Materials.
Low-sulfated CSPG was isolated from normal term placentas of women who delivered at the Hershey Medical Center, Hershey, PA, and was purified as described previously (1). Partially sulfated C4S, consisting of 36% 4-sulfated and 64% nonsulfated disaccharides, was prepared by the regioselective 6-O-desulfation of bovine tracheal chondroitin sulfate A (Sigma Chemical Co.) (2). Human blood and plasma for the parasite culturing were obtained from the Blood Bank of Hershey Medical Center, Hershey, PA.
Parasites.
C4S-adherent parasites were selected by panning of FCR3 and 3D7 laboratory strains on plastic petri dishes (Falcon 1058 from Becton-Dickinson Labware) coated with placental CSPG as described previously (2) and were designated FCR3-CSA and 3D7-CSA parasites. The parasites were cultured in RPMI 1640 medium using O-positive erythrocytes and 10% O-positive human plasma. To obtain a tight synchronization, cultures were synchronized within 2 to 3 h of 20 to 30% schizont burst with 5% sorbitol at two consecutive parasite generations (29). Cells were harvested at different time points and used for adherence studies.
Treatment with trypsin.
Cell pellets from parasite cultures were suspended in 10 volumes of phosphate-buffered saline (PBS) (pH 7.2) and treated with trypsin (25 or 50 μg/ml) at 37°C for 1 h. IRBCs were washed three times with PBS (pH 7.2), suspended in 20 volumes of PBS (pH 7.2) containing 2 μg/ml soybean trypsin inhibitor (Sigma Chemical Co.), and allowed to stand at room temperature for 5 min. The pellet was washed five times with PBS (pH 7.2) and used for binding assays.
IRBC binding and inhibition assays.
Circular spots (about 4 mm in diameter) on the surface of plastic petri dishes were coated overnight with purified placental CSPG (0.2 μg/ml) in PBS (pH 7.2) at 4°C. The spots were blocked with 1% bovine serum albumin at room temperature for 2 h and overlaid with 2% suspensions of parasite culture (20 to 30% parasitemia) in PBS (pH 7.2). After 30 min at room temperature, the unbound cells were washed off with PBS (pH 7.2). The bound cells were fixed with 2% glutaraldehyde, stained with Giemsa stain, and counted under a light microscope (2).
For adhesion inhibition assays, 4% suspensions of parasite culture cell pellets in PBS (pH 7.2) were preincubated with an equal volume of 0.16 to 80 μg/ml C4S containing 36% 4-sulfate in the same buffer at room temperature for 30 min. The cell suspensions were layered on CSPG-coated, BSA-blocked spots on petri dishes and allowed to stand at room temperature for 30 min. The unbound cells were washed, and the bound cells fixed, stained, and counted as described above.
Statistical analysis.
Data are presented as mean values ± standard errors. Statistical significance was determined by one-way analysis of variance. P values of <0.05 were considered statistically significant.
RESULTS
Assessment of IRBC binding to placental CSPG at different stages of P. falciparum development.
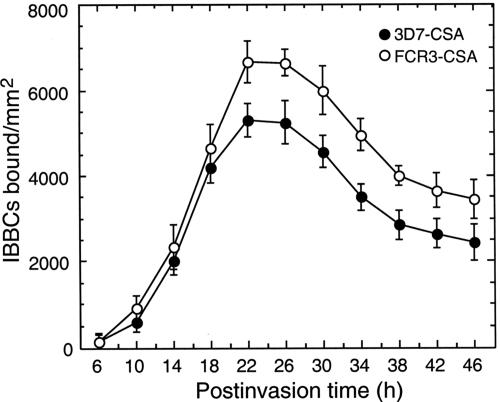
IRBCs from 3D7-CSA and FCR3-CSA parasites were evaluated for their ability to bind to human placental CSPG. At the second generation after selection for C4S binding, the IRBCs were assayed for C4S binding at 4-h intervals during the parasite development, starting from 6 h postinvasion. With both 3D7-CSA and FCR3-CSA, IRBC binding was evident at 14 h postinvasion and thereafter increased, reaching peak levels at 22 to 26 h postinvasion (Fig. 1). The results indicated that the expression of the C4S adhesive protein(s) on the IRBC surface begins at the mid-ring stage and peaks during the early trophozoite stage. These observations are consistent with the previously reported expression pattern of var1/var2 PfEMP1 (4, 13, 21, 24, 28). With both the FCR3-CSA and 3D7-CSA strains, IRBC binding gradually decreased after 26 h postinvasion, by ∼50% of the maximum at the schizont stage (Fig. 1). The results also indicated that compared to the 3D7-CSA strain studied here, FCR3-CSA can bind at a higher capacity.
FIG. 1.
Analysis of P. falciparum IRBC binding to placental CSPG at different stages of parasite development. 3D7-CSA and FCR3-CSA IRBCs with comparable parasitemia (25%) were assayed at the indicated developmental stages for binding to human placental CSPG. The number of IRBCs bound per mm2 of CSPG-coated spots was measured using a light microscope. Values expressed are the means from three independent experiments performed in duplicate; vertical bars indicate standard errors. Both 3D7-CSA and FCR3-CSA IRBCs bound to CSPG in a developmental stage-specific manner. In both cases, IRBCs bound in maximal numbers at 22 to 26 h postinvasion. The binding capacity gradually decreased during trophozoite maturation. Compared to 3D7-CSA parasites, FCR3-CSA bound with higher capacity. The difference in binding capacities of IRBCs from FCR3-CSA and 3D7-CSA is more evident starting from 22 h postinvasion.
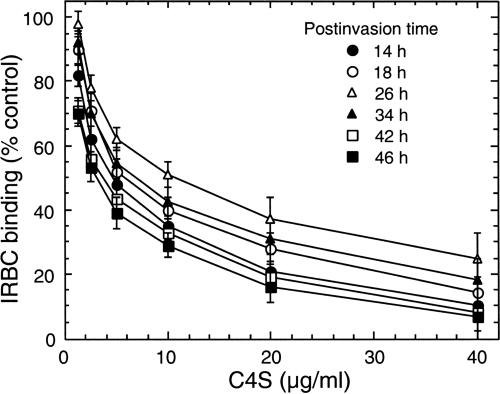
To determine the relative binding strengths of IRBCs at different stages of parasite development, we studied the dose-dependent inhibition of IRBC binding to placental CSPG by a partially sulfated C4S with 36% 4-sulfate content, which has been shown previously to efficiently inhibit IRBC adhesion to placental CSPG (2). With both 3D7-CSA and FCR3-CSA, the strength of IRBC binding to placental CSPG was maximal at 22 to 26 h postinvasion and was significantly lower during 14 to 18 h and 34 to 46 h (P < 0.05) postinvasion (Fig. 2). For the 3D7-CSA clone, the relative binding strengths, as measured by the concentration of C4S required for 50% inhibition of IRBC binding to placental CSPG (IC50), at 14, 18, 22, 26, 30, 34, 38, 42, and 46 h postinvasion were 4.5 ± 1.0, 5.9 ± 0.5, 9.8 ± 0.75, 10.0 ± 1.25, 9.4 ± 0.75, 7.0 ± 0.5, 6.3 ± 0.5, 3.9 ± 1.0, and 3.1 ± 1.0 μg/ml, respectively. Compared to that of 3D7-CSA, the binding strength of FCR3-CSA parasites was 10 to 20% higher at various time points analyzed (not shown), suggesting that the binding capacity of IRBCs is strain dependent as well.
FIG. 2.
Assessment of the CSPG binding strengths of IRBCs at different stages of parasite development. The strength of IRBC binding to human placental CSPG was determined by measuring the dose-dependent inhibition of binding by soluble C4S containing 36% 4-sulfate. 3D7-CSA and FCR3-CSA IRBCs with ∼25% parasitemia were analyzed. Inhibition assay was performed at 14, 18, 22, 26, 30, 34, 38, 42, and 46 h after parasite invasion. The results for IRBCs at 22, 30, and 38 h postinvasion are not presented in the figure. The number of IRBCs bound per mm2 with control untreated samples was in the range of 2,500 to 6,500. At each developmental stage, the plotted values are percent IRBCs bound per mm2 compared to samples not treated with C4S and are means from three independent experiments done in duplicate; vertical bars indicate standard errors. For both 3D7-CSA and FCR3-CSA parasites, the IC50 (binding strength) gradually decreased during trophozoite maturation. Shown are the results for 3D7-CSA parasites; similar stage-specific differences in IRBC binding were also observed for FCR3-CSA parasites (not shown).
Analysis of IRBC binding to placental CSPG at different generations after selection for C4S binding.
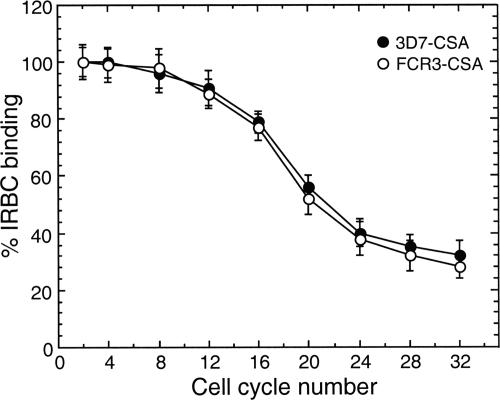
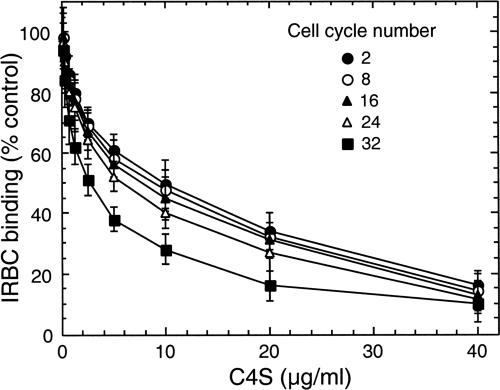
To determine whether the C4S adherence capacity of IRBCs varies during continuous culture of parasites, the binding of 3D7-CSA and FCR3-CSA IRBCs (24 h postinvasion) to the placental CSPG was measured for up to 32 generations. With both parasites, up to eight generations the number of IRBCs bound per unit area of CSPG-coated plates was not noticeably changed, and then it gradually decreased (Fig. 3). We also measured the strength of binding of IRBCs to placental CSPG at 24 h after parasite invasion. At various generations tested, the binding strengths of both 3D7-CSA and FCR3-CSA, as measured by the IC50, were essentially unchanged up to eight generations and then gradually decreased (Fig. 4 and data not shown). For 3D7-CSA parasites, the IC50s at 2, 4, 8, 12, 16, 20, 24, 28, and 32 generations were, respectively, 10.0 ± 0.5, 9.7 ± 1.0, 9.5 ± 0.7, 9.0 ± 0.8, 7.7 ± 0.5, 7.0 ± 0.6, 6.0 ± 0.5, 4.8 ± 1.0, and 3.1 ± 0.5 μg/ml of C4S. From these data, it is evident that the IRBC binding strengths at the 24th and 32nd generations compared to that at the 2nd generation were ∼60% and ∼31%, respectively. At various generations tested, the relative binding strengths of FCR3-CSA were 10 to 20% higher than those of 3D7-CSA, suggesting that the binding strength of IRBCs is strain dependent as well. Taken together, the above results suggested that the level of expression of C4S adhesive protein on the IRBC surface decreases gradually over successive generations.
FIG. 3.
Analysis of P. falciparum IRBC binding to the placental CSPG at different parasite generations. 3D7-CSA and FCR3-CSA IRBCs with 20 to 25% parasitemia were assayed. Binding assay was performed for IRBCs at 2, 4, 8, 12, 16, 20, 24, 28, and 32 cell cycles after selecting for CSPG binding. At each cell cycle, expressed values are percent IRBCs bound per mm2 and are means from three independent experiments done in duplicate; vertical bars indicate standard errors. The binding capacities of both 3D7-CSA and FCR3-CSA IRBCs were more or less similar up to eight generations and then decreased gradually. At the 32nd generation the binding capacity of IRBCs was ∼31% of that at the 2nd generation following selection for CSPG binding.
FIG. 4.
Measurement of CSPG binding strengths of IRBCs at different parasite generations. 3D7-CSA and FCR3-CSA with ∼25% parasitemia at 24 to 26 h postinvasion were studied. The binding strength of parasites at various generations following selection of IRBCs for CSPG binding was measured by assaying dose-dependent inhibition of IRBC binding by C4S. The number of IRBCs bound per mm2 with samples not treated with C4S was in the range of 2,000 to 6,500. At each generation studied, plotted values represent percent IRBCs bound per mm2 compared to control untreated samples and are means from three independent experiments performed in duplicate; vertical bars indicate standard errors. Inhibition assay was performed for IRBCs at 2, 4, 8, 12, 16, 20, 24, 28, and 32 cell cycles after selecting for CSPG binding. The results for IRBCs at 4, 12, 20, and 28 cell cycles are not shown. For both 3D7-CSA and FCR3-CSA parasites, the IC50 (binding strength) was similar up to eighth generation and then gradually decreased. Shown are the results for 3D7-CSA parasites; a similar cell cycle number-specific decrease in binding strength was also observed for FCR3-CSA parasites (not shown).
Effect of trypsin treatment on IRBC binding to the placental CSPG.
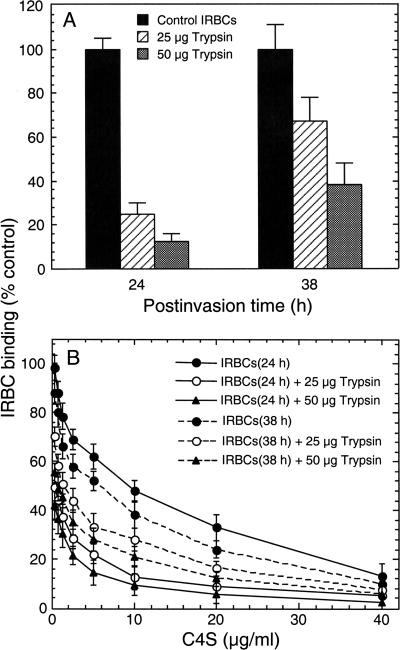
The observed decrease in binding strength of IRBCs during the late trophozoite and schizont stages (Fig. 1) could be due to either diminished levels of the IRBC adhesive protein(s) or steric blocking of the adhesive protein by other proteins expressed on the IRBC surface during the trophozoite stage. To ascertain which of these two factors is contributing, C4S-IRBC binding was analyzed after treatment with trypsin. 3D7-CSA IRBCs at the early trophozoite (24 h postinvasion) and late trophozoite (38 h postinvasion) stages were treated with 25 and 50 μg/ml trypsin for 1 h and assayed for binding to placental CSPG. In the case of IRBCs at 24 h postinvasion, treatment with 25 and 50 μg/ml trypsin resulted in, respectively, ∼75% and ∼88% decreases in IRBC binding to CSPG, compared to untreated control IRBCs (Fig. 5A). In contrast, in the case of IRBCs at 38 h postinvasion, treatment with 25 and 50 μg/ml trypsin caused, respectively, ∼32% and ∼62% decreases in IRBC binding to the CSPG compared to untreated control IRBCs (Fig. 5A). Consistent with these results, the binding strengths of IRBCs at 24 h and 38 h postinvasion were affected differentially by trypsin treatment (Fig. 5B). The decrease in IRBC binding strength was much more pronounced in the case of IRBCs with early trophozoites than in those with late trophozoites, suggesting that the adhesive proteins on the IRBC surface are more readily accessible for cleavage by trypsin during early stages of parasite development than in later stages.
FIG. 5.
Analysis of trypsin-treated IRBC binding to placental CSPG. 3D7-CSA IRBCs, harvested at the early (24 h) and late (38 h) trophozoite stages, were treated with 25 and 50 μg/ml trypsin at 37°C for 1 h. Plotted values represent the percent trypsin-treated IRBCs bound per mm2 of CSPG-coated plates compared to untreated IRBCs. (A) IRBCs at 24 h postinvasion are considerably more sensitive to trypsin than IRBCs at 38 h postinvasion. In the case of IRBCs at 24 h postinvasion, treatment with 25 and 50 μg/ml trypsin resulted in, respectively, ∼75% and ∼88% decreases in CSPG binding capacity compared to untreated IRBCs. In contrast, treatment of IRBCs at 38 h postinvasion with 25 and 50 μg/ml trypsin resulted in, respectively, ∼32% and ∼62% decreases in CSPG binding capacity compared to untreated IRBCs. (B) Dose-dependent inhibition of trypsin-treated IRBC binding to human placental CSPG by C4S. The decrease in binding strength of trypsin-treated IRBCs is measured as the IC50 for binding to CSPG-coated plates compared to control untreated IRBCs. The IC50 for IRBCs at 24 postinvasion treated with trypsin is substantially lower than that for IRBCs at 38 h postinvasion treated with trypsin. The IC50 values (μg/ml of C4S) for 24-h IRBCs are as follows: untreated, 9.8 ± 0.5; 25-μg/ml trypsin treated, 0.3 ± 0.1; 50-μg/ml trypsin treated, 0.1 ± 0.05. The IC50 values (μg/ml) for 38-h IRBCs are as follows: untreated, 5.8 ± 0.5; 25-μg/ml trypsin treated, = 1.5 ± 0.25; 50-μg/ml trypsin treated, = 0.6 ± 0.1.
DISCUSSION
Notable findings of this study include the following. (i) The C4S binding strength of IRBCs varies markedly during the parasite developmental stages, with maximum binding capacity at the early trophozoite stage and considerably lower binding capacity at the late trophozoite and schizont stages. The parasite stage-dependent variation in binding strength is strain independent, even though the binding capacities of different strains at a particular developmental stage differ considerably. (ii) The binding capacity of IRBCs gradually decreases during successive generations after acquiring the C4S-adherent phenotype. (iii) Treatment of IRBCs with trypsin differentially affects the adherence capacity of IRBCs with parasites at different developmental stages. Collectively, the data demonstrate that the adhesive protein(s) is maximally expressed on the IRBC surface at the late ring and early trophozoite stages and is partially sterically blocked by other proteins expressed on the IRBC surface during the late trophozoite and schizont stages. These results have important implications for studies aimed at the assessment of structural elements involved in C4S-IRBC interactions.
Our findings that the binding capacity of IRBCs increases gradually from 14 h parasite postinvasion, with maximum binding strength being attained at the late ring and early trophozoite stages, is similar to the reported pattern of the stage-specific binding of IRBCs to TSP, CD36, and ICAM1 during the early phase of the parasite development, which starts at ∼14 h postinvasion and exhibits a peak at ∼18 h (21). It is widely believed that the antigenically variant var gene family PfEMP1s expressed on the IRBC surface mediate IRBC adherence and that the expression of a distinct PfEMP1 confers a specific adherence property to various endothelial cell adhesion molecules, such as TSP, CD36, ICAM1, E-selectin, and P-selectin (3, 20, 21, 41). Transcription of var genes occurs during the ring stage, starting as early as 3 h and with peak levels at 12 h postinvasion, and the appearance of PfEMP1 protein on the IRBCs starts at 14 to 16 h, reaching the maximum level at 24 to 26 h (4, 13, 24, 28). Thus, the parasite developmental stage-specific binding of IRBCs to C4S correlates with the timing of the appearance and expression of PfEMP1 on the IRBC surface, suggesting an important role for PfEMP1 in the IRBC adhesion. Previously, several studies have reported that the DBL-γ domain of a distinct var1 subfamily PfEMP1 mediates the adherence of IRBCs to CSPG (9, 20, 41). However, recently a var2 family PfEMP1 termed VAR2CSA has been implicated as the adhesive protein on the IRBC surface, based on the observation that VAR2CSA is highly transcribed in C4S binding parasites from laboratory strains as well as isolates from infected placentas (37, 38, 44). VAR2CSA has been shown to be expressed selectively at high levels on the surface of C4S-adherent IRBCs, and in populations where malaria is endemic, VAR2CSA is recognized by serum antibodies of women but not by those of men (37, 38, 44, 45). However, it is possible that VAR2CSA might not directly interact with C4S but might play a role in the transport of a novel C4S-adhesive protein(s) onto the IRBCs surface and/or function as a scaffold for the surface display of the adhesive protein(s). Therefore, further detailed studies are required to determine whether var2 PfEMP1 directly binds to C4S or merely plays an indirect role in IRBC binding to C4S.
The results of this study show that the binding ability of the C4S-adherent IRBCs decreases during trophozoite maturation by ∼50%. This is in contrast to the pattern of IRBC binding to TSP, CD36, and ICAM1 in that IRBCs maintain maximal binding capacity from the late ring stage up to the schizont stage (21). These results taken together suggest that while the parasite adhesive proteins on the IRBC surface that interacts with CD36 and other endothelial cell adhesion molecules remain accessible with equal efficiency from the late ring through schizont stages, the C4S-adhesive protein(s) is significantly less accessible during the late trophozoite and schizont stages. The most plausible explanation is that the proteins that are expressed on the IRBC surface during the maturation of trophozoites sterically mask the C4S-adhesive protein(s), resulting in decreased IRBC binding capacity. A previous study has shown that a number of proteins are expressed on IRBC surface (17), and presumably many of these are expressed during trophozoite development. This interpretation agrees with the observation that the adhesive protein(s) on the surface of IRBCs is more susceptible to trypsin treatment at the late ring and early trophozoite stages than at the late trophozoite stage. It appears that the parasite has developed an orchestrated mechanism that allows for the efficient binding of IRBCs to the placental CSPG while minimizing the exposure of the adhesive protein(s) for recognition by the host's immune system.
Our data are consistent with a model wherein the parasite expresses the C4S adhesive protein(s) on the IRBC surface prior to many other proteins, which are expressed during its most active metabolic stage. This strategy seems to have at least two selective advantages for parasite survival. (i) Early expression of the adhesive protein(s) on the IRBC surface in a less crowded environment allows the protein to efficiently interact with the C4S dodecasaccharide moiety, a relatively large binding motif of the placental receptor, for maximum IRBC adherence. (ii) Once bound, the expression of other proteins/molecules on the IRBC surface masks the conserved adhesive protein(s) such that it is partially blocked for recognition by the host immune system. This is consistent with the fact that two or more successive pregnancies are required for development of a C4S-IRBC adhesion-inhibitory antibody response (6, 19, 33, 43). Thus, it appears that the host efficiently recognizes dominantly expressed variant PfEMP1 and other antigenic proteins on the IRBC surface, whereas the adhesive proteins (or specific domains) are protected from recognition by steric blockage.
Our observation that IRBCs at different stages of erythrocytic P. falciparum development are differentially susceptible/resistant to trypsin treatment agrees with the fact that IRBCs from different strains differ in their ability to bind CSPG. Previously, several studies have reported that trypsin resistance of IRBCs for C4S binding is strain specific (5, 9, 31). In one study, binding of IRBCs from CS2-CSA and 3D7-CSA to CSA was completely unaffected by treatment with ∼100 μg/ml trypsin, while that of IRBCs from strain HCS3 decreased by 86% upon treatment with a very low concentration (1 μg/ml) of trypsin (5). In another, independent study, 10- and 100-μg/ml trypsin treatment of late-ring-stage IRBCs for 30 min resulted in 50% and 70% loss of binding activity (9). In a very recent study, when matured trophozoites and schizont IRBCs from FCR3-CSA and NF54-CSA were treated with 1 mg/ml trypsin, adhesion of CSA was decreased by >80% and ∼50%, respectively (31). In contrast, IRBCs from strain HB3 completely lost their binding activity upon treatment with 1 mg/ml trypsin under similar conditions (31). The results of the present study agree with these observations. Compared to 3D7-CSA IRBCs, FCR3-CSA IRBCs exhibit a higher binding capacity as well as binding strength at various developmental stages and at different generations after initial selection for CSPG binding. Further, very recently, in a separate study, we found that CSPG-adherent IRBCs from clone 3D7N61 exhibit only ∼30% of binding activity at various developmental stages compared to corresponding stages of 3D7-CSA, used in the present study (R. N. Achur et al., unpublished results). Furthermore, upon treatment with trypsin, 3D7N61-CSA IRBCs almost completely lost their binding activity, clearly indicating a strain-dependent binding capacity. Collectively, these observations demonstrate that CSPG binding IRBCs from different isolates exhibit different binding capacities. In other studies, this was inferred to be due to polymorphisms in VAR2CSA (31). Although this may partly be the case, another reason for differential susceptibility could be differences in the expression of knobs and their associated proteins, leading to variation in the surface exposure of the adhesive protein(s). In any case, it is obvious from the results of this study that IRBCs from different parasite strains bind to CSPG in a developmental stage- and cell cycle number-dependent manner. Our data also clearly indicate that the early-stage trophozoites are ideally suitable for studying C4S-IRBC interactions.
The finding that parasites selected for high-capacity binding to C4S gradually lose their binding strength during successive generations provides an impetus for studying the regulation of the expression of C4S binding protein on the IRBC surface. Our data demonstrate that the level of expression of the adhesive protein(s) decreases over successive generations after initial selection of IRBCs for C4S binding, such that eventually parasites exhibit little or no capacity for binding to C4S. In in vivo situations, parasites that are unable to adhere after several generations are subjected to splenic clearance. Thus, it is likely that in vivo the IRBCs with high binding strengths are constantly selected and only those parasites expressing high levels of adhesive proteins on the IRBC surface are sequestered, while others are cleared.
Previously, several studies have shown that parasites undergo clonal variations to give rise to specific adhesive phenotypes at a rate of 1 to 2% per generation (20, 21, 32, 35, 41). These data, when considered with the results of this study, suggest that only a small population of C4S-adherent parasites in each generation produce high-capacity C4S binding IRBCs. The binding parasites slowly lose their adherent capacity by gradually switching off the adhesive protein gene transcription. Thus, this mechanism may allow for the selection of a population of parasites which by virtue of binding ability can survive efficiently in the host.
Acknowledgments
This work was supported by Public Health Service grant AI45086 from the National Institute of Allergy and Infectious Diseases, NIH.
We thank Larry Taylor for editing the manuscript.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 25 June 2007.
REFERENCES
- 1.Achur, R. N., M. Valiyaveettil, A. Alkhalil, C. F. Ockenhouse, and D. C. Gowda. 2000. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta, J. Biol. Chem. 275:40344-40356. [DOI] [PubMed] [Google Scholar]
- 2.Alkhalil, A., R. N. Achur, M. Valiyaveettil, C. F. Ockenhouse, and D. C. Gowda. 2000. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J. Biol. Chem. 275:40357-40364. [DOI] [PubMed] [Google Scholar]
- 3.Baruch, D. I., J. A. Gormely, C. Ma, R. J. Howard, and B. L. Pasloske. 1996. Plasmodium falciparum erythrocyte membrane protein-1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule-1. Proc. Natl. Acad. Sci. USA 93:3497-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beeson, J. G., N. Amin, M. Kanjala, and S. J. Rogerson. 2002. Selective accumulation of mature asexual stages of Plasmodium falciparum-infected erythrocytes in the placenta. Infect. Immun. 70:5412-5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeson, J. G., and G. V. Brown. 2004. Plasmodium falciparum-infected erythrocytes demonstrate dual specificity for adhesion to hyaluronic acid and chondroitin sulfate A and have distinct adhesive properties. J. Infect. Dis. 189:169-179. [DOI] [PubMed] [Google Scholar]
- 6.Beeson, J. G., and P. E. Duffy. 2005. The immunology and pathogenesis of malaria during pregnancy. Curr. Top. Microbiol. Immunol. 297:187-227. [DOI] [PubMed] [Google Scholar]
- 7.Beeson, J. G., E. J. Mann, T. J. Byrne, A. Caragounis, S. R. Elliott, G. V., Brown, and S. J. Rogerson. 2006. Antigenic differences and conservation among placental Plasmodium falciparum-infected erythrocytes and acquisition of variant-specific and cross-reactive antibodies. J. Infect. Dis. 193:721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brabin, B. J., C. Romagosa, S. Abdelgalil, C. Menendez, F. H. Verhoeff, R. McGready, K. A. Fletcher, S. Owens, U. D'Alessandro, F. Nosten, P. R. Fischer, and J. Ordi. 2004. The sick placenta—the role of malaria. Placenta 25:359-378. [DOI] [PubMed] [Google Scholar]
- 9.Buffet, P. A., B. Gamain, C. Scheidig, D. Baruch, J. D. Smith, R. Hernandez-Rivas, B. Pouvelle, S. Oishi, N. Fujii, T. Fusai, D. Parzy, L. H. Miller, J. Gysin, and A. Scherf. 1999. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc. Natl. Acad. Sci. USA 96:12743-12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Q., M. Schlichtherle, and M. Wahlgren. 2000. Molecular aspects of severe malaria. Clin. Microbiol. Rev. 13:439-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke, B., R. Coppel, and M. Wahlgren. 2000. Falciparum malaria: sticking up, standing out and out-standing. Parasitol. Today 16:416-420. [DOI] [PubMed] [Google Scholar]
- 12.David, P. H., M. Hommel, L. H. Miller, I. U. Udeinya, and L. D. Oligino. 1983. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc. Natl. Acad. Sci. USA 80:5075-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy, M. F., G. V. Brown, W. Basuki, E. O. Krejany, R. Noviyanti, A. F. Cowman, and J. C. Reeder. 2002. Transcription of multiple var genes by individual, trophozoite-stage Plasmodium falciparum cells expressing a chondroitin sulfate A binding phenotype. Mol. Microbiol. 43:1285-1293. [DOI] [PubMed] [Google Scholar]
- 14.Duffy, M. F., J. C. Reeder, and G. V. Brown. 2003. Regulation of antigenic variation in Plasmodium falciparum: censoring freedom of expression? Trends Parasitol. 19:121-124. [DOI] [PubMed] [Google Scholar]
- 15.Duffy, P. E., and M. Fried. 2003. Plasmodium falciparum adhesion in the placenta. Curr. Opin. Microbiol. 6:371-376. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez, V., M. Hommel, Q. Chen, P. Hagblom, and M. Wahlgren. 1999. Small, clonally variant antigens expressed on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune response. J. Exp. Med. 190:1393-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florens, L., X. Liu, Y. Wang, S. Yang, O. Schwartz, M. Peglar, D. J. Carucci, J. R. Yates, and Y. Wu. 2004. Proteomics approach reveals novel proteins on the surface of malaria-infected erythrocytes. Mol. Biochem. Parasitol. 135:1-11. [DOI] [PubMed] [Google Scholar]
- 18.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 19.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 20.Gamain, B., S. Gratepanche, L. H. Miller, and D. I. Baruch. 2002. Molecular basis for the dichotomy in Plasmodium falciparum adhesion to CD36 and chondroitin sulfate Proc. Natl. Acad. Sci. USA 99:10020-10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner, J. P., R. A. Pinches, D. J. Roberts, and C. I. Newbold. 1996. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 93:3503-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatton, M. L., J. M. Peters, E. V. Fowler, and Q. Cheng. 2003. Switching rates of Plasmodium falciparum var genes: faster than we thought? Trends Parasitol. 19:202-208. [DOI] [PubMed] [Google Scholar]
- 23.Gowda, D. C. 2006. Role of chondroitin 4-sulfate in pregnancy associated malaria. Pharmacology 153:375-400. [DOI] [PubMed] [Google Scholar]
- 24.Gysin, J., B. Pouvelle, N. Fievet, A. Scherf, and C. Lepolard. 1999. Ex vivo desequestration of Plasmodium falciparum-infected erythrocytes from human placenta by chondroitin sulfate A. Infect. Immun. 67:6596-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heddini, A. 2002. Malaria pathogenesis: a jigsaw with an increasing number of pieces. Int. J. Parasitol. 32:1587-1598. [DOI] [PubMed] [Google Scholar]
- 26.Horrocks, P., R. Pinches, Z. Christodoulou, S. A. Kyes, and C. I. Newbold. 2004. Variable var transition rates underlie antigenic variation in malaria. Proc. Natl. Acad. Sci. USA 101:11129-11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyes, S., P. Horrocks, and C. Newbold. 2001. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55:673-707. [DOI] [PubMed] [Google Scholar]
- 28.Kyes, S. A., Z. Christodoulou, A. Raza, P. Horrocks, R. Pinches, J. A. Rowe, and C. I. Newbold. 2003. A well-conserved Plasmodium falciparum var gene shows an unusual stage-specific transcript pattern. Mol. Microbiol. 48:1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 30.Menendez, C., J. Ordi, M. R. Ismail, P. J. Ventura, J. J. Aponte, E. Kahigwa, F. Font, and P. L. Alonso. 2000. The impact of placental malaria on gestational age and birth weight. J. Infect. Dis. 181:1740-1745. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen, M. A., M. Resende, M. Alifrangis, L. Turner, L. Hviid, T. G. Theander, and A. Salanti. 2007. Plasmodium falciparum: VAR2CSA expressed during pregnancy-associated malaria is partially resistant to proteolytic cleavage by trypsin. Exp. Parasitol. [Epub ahead of print.] doi: 10.1016/j.exppara.2007.03.002. [DOI] [PubMed]
- 32.Noviyanti, R., G. V. Brown, M. E. Wickham, M. F. Duffy, A. F. Cowman, and J. C. Reeder. 2001. Multiple var gene transcripts are expressed in Plasmodium falciparum infected erythrocytes selected for adhesion. Mol. Biochem. Parasitol. 114:227-237. [DOI] [PubMed] [Google Scholar]
- 33.O'Neil-Dunne, I., R. N. Achur, S. T. Agbor-Enoh, M. Valiyaveettil, R. S. Naik, C. F. Ockenhouse, A. Zhou, R. Megnekou, R. Leke, D. W. Taylor, and D. C. Gowda. 2001. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect. Immun. 69:7487-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pouvelle, B., P. A. Buffet, C. Lepolard, A. Scherf, and J. Gysin. 2000. Cytoadhesion of Plasmodium falciparum ring-stage-infected erythrocytes. Nat Med. 6:1264-1268. [DOI] [PubMed] [Google Scholar]
- 35.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachs, J., and P. Malany. 2002. The economic and social burden of malaria. Nature 415:680-685. [DOI] [PubMed] [Google Scholar]
- 37.Salanti, A., M. Dahlback, L. Turner, M. A. Nielsen, L. Barfod, P. Magistrado, A. T. Jensen, T. Lavstsen, M. F. Ofori, K. Marsh, L. Hviid, and T. G. Theander. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200:1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salanti, A., T. Staalsoe, T. Lavstsen, A. T. Jensen, M. P. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49:179-191. [DOI] [PubMed] [Google Scholar]
- 39.Sharling, L., A. Enevold, K. M. Sowa, T. Staalsoe, and D. E. Arnot. 2004. Antibodies from malaria-exposed pregnant women recognize trypsin resistant epitopes on the surface of Plasmodium falciparum-infected erythrocytes selected for adhesion to chondroitin sulfate A. Malaria J. 3:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman, I. W., S. Eda, and E. Winograd. 2003. Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect. 5:897-909. [DOI] [PubMed] [Google Scholar]
- 41.Smith, J. D., G. Subramanian, B. Gamain, D. I. Baruch, and L. H. Miller. 2000. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 110:293-310. [DOI] [PubMed] [Google Scholar]
- 42.Snow, R. W., M. Craig, U. Diechmann, and K. Marsh. 1999. Estimating mortality, morbidity and disability due to malaria among Africa's non-pregnant population. Bull. W. H. O. 77:624-640. [PMC free article] [PubMed] [Google Scholar]
- 43.Staalsoe, T., R. Megnekou, N. Fievet, C. H. Ricke, H. D. Zornig, R. Leke, D. W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]
- 44.Tuikue Ndam, N. G., A. Salanti, G. Bertin, M. Dahlback, N. Fievet, L. Turner, A. Gaye, T. Theander, and P. Deloron. 2005. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J. Infect. Dis. 192:331-335. [DOI] [PubMed] [Google Scholar]
- 45.Tuikue Ndam, N. G., A. Salanti, J. Y. Le-Hesran, G. Cottrell, N. Fievet, L. Turner, S. Sow, J. M. Dangou, T. Theander, and P. Deloron. 2006. Dynamics of anti-VAR2CSA immunoglobulin G response in a cohort of Senegalese pregnant women. J. Infect. Dis. 193:713-720. [DOI] [PubMed] [Google Scholar]
- 46.Weatherall, D. J., L. H. Miller, D. I. Baruch, K. Marsh, O. K. Doumbo, C. Casals-Pascual, and D. J. Roberts. 2002. Malaria and the red cell. Hematology Am. Soc. Hematol. Edu. Program 2002:35-57. [DOI] [PubMed] [Google Scholar]