Abstract
Coxiella burnetii, the cause of human Q fever, is an aerosol-borne, obligate intracellular bacterium that targets host alveolar mononuclear phagocytic cells during infection. In all cell types examined, C. burnetii establishes a replicative niche in a lysosome-like parasitophorous vacuole where it carries out a lengthy infectious cycle with minimal cytopathic effects. The persistent and mild nature of C. burnetii infection in vitro suggests that the pathogen modulates apoptosis to sustain the host cell. In the current study, we examined the ability of C. burnetii to inhibit apoptotic cell death during infection of human THP-1 monocyte-derived macrophages and primary monkey alveolar macrophages. C. burnetii-infected cells demonstrated significant protection from death relative to uninfected cells following treatment with staurosporine, a potent inducer of intrinsic apoptosis. This protection correlated with reduced cleavage of caspase-9, caspase-3, and poly(ADP-ribose) polymerase (PARP), all proteolytic events that occur during apoptosis. Reduced PARP cleavage was also observed in cells treated with tumor necrosis factor alpha to induce extrinsic apoptosis. Apoptosis inhibition was a C. burnetii-driven process as infected cells treated with rifampin or chloramphenicol, inhibitors of bacterial RNA and protein synthesis, respectively, showed significantly reduced protection against staurosporine-induced apoptosis. C. burnetii infection affected the expression of multiple apoptosis-related genes and resulted in increased synthesis of the antiapoptotic proteins A1/Bfl-1 and c-IAP2. Collectively, these data suggest that C. burnetii modulates apoptotic pathways to inhibit host cell death, thus providing a stable, intracellular niche for the course of the pathogen's infectious cycle.
Coxiella burnetii is the causative agent of human Q fever, a disease that normally manifests as an acute influenza-like illness but can also progress to severe chronic endocarditis. C. burnetii is transmitted via aerosols and initially targets host alveolar mononuclear phagocytic cells (40). Aerosol transmission of C. burnetii is facilitated by the enhanced environmental stability of the organism (41, 60). This stability is conferred by the small-cell variant (SCV) morphological form of the organism which is part of a biphasic developmental cycle that includes a more metabolically and replicatively active large-cell variant (LCV) form (14, 26). Following phagocytosis, the nascent phagosome interacts with the autophagic pathway, a process that is thought to stall phagosome maturation (30, 48). This interaction may provide a “nutrient pulse” that prepares C. burnetii for survival and replication in its more toxic, mature parasitophorous vacuole (PV) (48, 57). The PV is a lysosome-like compartment that maintains an acidic pH (pH ∼5) and contains a variety of lysosomal hydrolases (2, 12, 27). During a lag phase lasting 1 to 2 days postinfection, SCVs differentiate into LCVs that then replicate exponentially for ∼4 days. LCV replication begins coincident with a dramatic expansion of the PV that eventually encompasses the majority of the host cell cytoplasm and harbors more than 100 organisms (57). Significant LCV-to-SCV morphogenesis begins at ∼6 days postinfection, with the vast majority of C. burnetii organisms in the SCV form by 2 to 4 weeks postinfection (14).
A common theme among bacterial pathogens of monocytes/macrophages is an infectious process that modulates host cell apoptotic signaling (15). Apoptotic cell death involves sequential activation of cysteine proteases, termed caspases, that serve initiator (e.g., caspases-8 and -9) or effector (e.g., caspase-3, -6, and -7) functions. The extrinsic apoptotic pathway acts downstream of cell surface death receptor signaling and involves caspase-8 activation. Intrinsic apoptosis is triggered by intracellular signals and is mediated by release of mitochondrial cytochrome c. This release is regulated by the Bcl-2 family of proteins that control mitochondrial membrane integrity. Members of the BH3-only subfamily of this group (e.g., Bim) sense intracellular stress signals and activate proteins (e.g., Bax) that promote cytochrome c release. Other Bcl-2-like proteins (e.g., Bcl-2) antagonize this proapoptotic activity. Released cytochrome c forms a complex with Apaf-1 and caspase-9, termed the apoptosome. Both the apoptosome and caspase-8 of the extrinsic pathway converge on caspase-3. This “executioner” caspase activates terminal apoptotic effector molecules that mediate morphological events such as chromatin condensation (for a review of apoptotic signaling, see reference 32).
As opposed to necrosis, apoptosis is normally a noninflammatory form of cell death (32). Thus, induction of apoptosis by some intracellular bacterial pathogens is viewed as a strategy that promotes pathogen release and intercellular spread in the presence of an attenuated immune response (42). In contrast, infection by a number of intracellular bacteria including Mycobacterium tuberculosis (38), Chlamydia pneumoniae (59), Legionella pneumophila (1, 39), and Brucella spp. (24, 25) inhibits apoptosis. These pathogens traffic to diverse vacuolar compartments of macrophages and have moderate to slow intracellular growth rates. Consequently, preservation of the host cell via an antiapoptotic mechanism is considered a pathogenic strategy that ensures sufficient bacterial replication.
The infectious process of C. burnetii in macrophages and other host cells is remarkably benign. Minimal lysis is observed following extensive C. burnetii replication and development of the large, spacious PV (3, 47). Moreover, in cell cultures persistently infected with C. burnetii, the host cell cycle and RNA/protein metabolism remain undisturbed (4, 5). Collectively, these observations suggest that C. burnetii employs an active mechanism to sustain viability of the host cell. Here, we show that C. burnetii infection inhibits apoptosis in macrophages. Protection from cell death requires de novo protein synthesis and is likely a mechanism that ensures completion of the pathogen's lengthy infectious cycle.
MATERIALS AND METHODS
C. burnetii and mammalian cell culture.
C. burnetii Nine Mile phase I (RSA493) and phase II (RSA439) strains were propagated in African green monkey kidney (Vero) cells (CCL-81; ATCC, Manassas, VA) and purified as previously described (29). Human monocyte-like (THP-1) cells (TIB-202; ATCC) were maintained in RPMI 1640 medium (Gibco, Carlsbad, CA) supplemented with 10% fetal calf serum (Gibco) at 37°C in 5% CO2. THP-1 cells were used between passages 10 and 20 and were cultured in 96-well, 24-well, or 6-well tissue culture plates or in 25-cm2 tissue culture flasks. Cells were incubated in the presence of 200 nM phorbol 12-myristate 13-acetate (PMA; EMD Biosciences, San Diego, CA) for 24 h to induce differentiation into an adherent, macrophage-like cell. Prior to infection, medium containing PMA was removed, and cells were replenished with fresh RPMI medium lacking PMA. Alveolar macrophages from cynomolgus monkeys (Macaca fascicularis) were isolated by bronchial lavage. Lavage fluid was centrifuged for 10 min at 500 × g, and cells were resuspended in fresh Dulbecco's modified Eagle medium (Gibco) containing 7.5% fetal calf serum. Cells were placed in individual wells of a 24-well CellBIND plate (Corning, Corning, NY) and incubated for 2 h at 37°C in 5% CO2. The medium containing nonadherent cells was then removed, and fresh medium was added to wells. Adherent alveolar macrophages were incubated for an additional 12 h before infection. In all experiments, THP-1 cells and primary alveolar macrophages were infected for 48 h with C. burnetii phase I or phase II strains at a multiplicity of infection (MOI) of 250 or 25, respectively, by the addition of organisms to cell culture medium. (This time point was considered 0 h postinfection.) The phase I MOI has been previously shown to result in initial infection of a high percentage of THP-1 cells with a few bacteria (16, 17). A lower MOI was used for phase II organisms because they are roughly 10-fold more infectious for cultured cells than phase I bacteria (57). Staurosporine (EMD Biosciences) was added to cell cultures at a final concentration of 500 nM to induce intrinsic apoptosis. Tumor necrosis factor alpha (TNF-α; BD Biosciences, San Jose, CA) and cycloheximide (EMD Biosciences) were added to cell cultures at final concentrations of 50 ng/ml and 2 μg/ml, respectively, to induce extrinsic apoptosis. As needed, rifampin or chloramphenicol (final concentrations, 10 μg/ml) was added to cell cultures along with the C. burnetii inoculum. These concentrations of antibiotics have been previously shown to inhibit C. burnetii RNA and protein synthesis, respectively (10, 31). Animal protocols were approved by the Rocky Mountain Laboratories Institutional Animal Care and Use Committee.
Cell death assay.
Cell viability was assessed using a colorimetric Cell Counting Kit-8 according to the manufacturer's protocol (Dojindo Laboratories, Gaithersburg, MD). Briefly, C. burnetii-infected and uninfected THP-1 cells in 96-well plates were treated with staurosporine for 4 or 8 h. WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] reagent was then added to wells, and incubation continued for an additional 4 h at 37°C. Following this incubation, the A450 of cell cultures was measured, and cell viability was calculated using the following formula: [(Atest − Abackground)/(Acontrol − Abackground)] × 100, where A is the absorbance at A450, background refers to medium alone, and control refers to the untreated cell population.
Microscopy and PARP staining.
Light microscopy was performed using a Nikon TE300 microscope equipped with a DXM1200 digital camera (Nikon, Tokyo, Japan). Images were acquired using ACT-1 software (Nikon). For fluorescence microscopy, cells on 12-mm glass coverslips in 24-well tissue culture plates were fixed and permeabilized with 100% ice-cold methanol for 3 min and then blocked for 1 h in phosphate buffered saline ([PBS] 1 mM KH2PO4, 155 mM NaCl, 3 mM Na2HPO4, pH 7.4) containing 5% bovine serum albumin. Cells were then incubated with rabbit anti-cleaved poly(ADP-ribose) polymerase (PARP) (Cell Signaling Technology, Danvers, MA) and guinea pig anti-C. burnetii primary antibodies for 1 h, washed three times in PBS, and then incubated in PBS containing Alexa Fluor-488 anti-rabbit and Alexa Fluor-594 anti-guinea pig immunoglobulin G antibodies (Molecular Probes, Carlsbad, CA) for 1 h. Cellular DNA was stained with DRAQ5 (Alexa Corporation, Lausen, Switzerland). Confocal fluorescence microscopy was conducted with a modified Perkin-Elmer UltraView spinning disk confocal system connected to a Nikon Eclipse TE2000-S microscope. Images were acquired with a 60× oil immersion objective (1.4 numerical aperture; Nikon) and a Photometrics Cascade II:512 digital camera (Princeton Instruments, Trenton, NJ) using Metamorph software (Molecular Devices, Downingtown, PA). One hundred cells were examined to determine the percentage of cleaved PARP-positive (apoptotic) cells within a cell culture.
Immunoblot analysis.
C. burnetii-infected monkey alveolar macrophages or THP-1 cells in six-well plates were directly lysed in buffer containing 50 mM Tris, 5 mM EDTA, and 1% sodium dodecyl sulfate, followed by 10 passages through a 26-gauge syringe needle. The protein concentration of each sample was determined using a detergent-compatible protein assay (Bio-Rad, Hercules, CA). Total protein (10 μg/lane) was separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to an Immobilon-P membrane (Millipore, Bedford, MA). Following transfer, membranes were blocked for 1 h at room temperature in Tris-buffered saline ([TBS] 150 mM NaCl, 100 mM Tris-HCl, pH 7.6) containing 0.1% Tween-20 and 5% nonfat milk. Membranes were then incubated overnight at 4°C in TBS-Tween-20 containing rabbit polyclonal antibodies directed against PARP (cleaved form), caspase-9, caspase-3, A1/Bfl-1, Bad, Bak, Bax, Bik, Bim, or Bmf (Cell Signaling Technology) or monoclonal antibodies directed against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Cell Signaling Technology) or c-IAP2 (clone F30-2285) (BD Biosciences, San Jose, CA). Membranes were washed and then incubated for 1 h at room temperature in TBS-Tween-20 containing anti-rabbit or anti-mouse immunoglobulin G secondary antibody conjugated to horseradish peroxidase (Pierce, Rockford, IL). Reacting proteins were detected via enhanced chemiluminescence using ECL Femto reagent (Pierce).
RT2 Profiler PCR Array.
C. burnetii-infected THP-1 cells in 25-cm2 flasks were solubilized with TRIzol reagent (Invitrogen). Total RNA was isolated by chloroform extraction, and cDNA was synthesized from 1 μg of RNA using a First Strand cDNA synthesis kit (Superarray, Frederick, MD). Reverse transcription-PCR (RT-PCR) was conducted on cDNA using a SYBR Green/ROX kit (Superarray) and the RT2 Profiler PCR Array system according to the manufacturer's instructions (Superarray). RT-PCRs were performed using an ABI7000 machine (Applied Biosystems, Foster City, CA) and the following cycling parameters: 95°C initial denaturation for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min.
RESULTS
C. burnetii-infected THP-1 cells are protected from staurosporine-induced apoptosis.
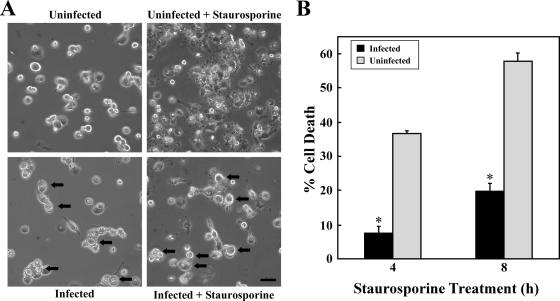
Because C. burnetii-infected macrophages show long-lived viability (3, 47), we hypothesized that the organism can modulate apoptotic signaling. As a model system to examine this possibility, we used infected PMA-differentiated THP-1 cells treated with staurosporine. Differentiated THP-1 cells faithfully mimic the properties of human monocyte-derived macrophages (38), and staurosporine, a protein kinase inhibitor, is a potent inducer of intrinsic apoptosis in this cell type (63). Unless otherwise noted, all experiments were performed using cells infected with the avirulent C. burnetii Nine Mile phase II strain for 48 h. At this time point, the organism is entering the log phase of its growth cycle (14, 53), and PVs are visible by light microscopy. Uninfected cells treated with staurosporine for 4 h displayed obvious characteristics of apoptosis, such as membrane blebbing and cell shrinking, that were not associated with treated infected cells (Fig. 1A). Cell viability following staurosporine treatment was then quantified by staining with WST-8, a dye that is reduced by dehydrogenases in viable cells, leading to the formation of a visible formazan reaction product. As shown in Fig. 1B, 4- and 8-h treatments of uninfected cells with staurosporine resulted in 37% and 58% cell death, respectively. In contrast, C. burnetii-infected cells were significantly protected from cell death, showing only 8% and 20% death following treatments of 4 h and 8 h, respectively.
FIG. 1.
C. burnetii inhibits staurosporine-induced death of THP-1 cells. THP-1 cells were infected with the C. burnetii Nine Mile phase II strain for 48 h. (A) Infected and uninfected cells were treated with staurosporine for 4 h and subsequently visualized by light microscopy. Treated uninfected cells demonstrated morphological changes consistent with apoptosis (e.g., membrane blebbing and cell shrinking) that were not observed with treated infected cells that maintained distinct PVs (arrows). Bar, 20 μm. (B) Infected and uninfected cells were incubated with staurosporine for 4 or 8 h. Cell viability was then assessed via WST-8 staining, and viability was calculated as described in Materials and Methods. Results are expressed as percent cell death compared to untreated cells. Experiments were conducted in triplicate, and error bars represent the standard deviation from the mean. An asterisk indicates a P value of <0.05 in comparison to uninfected cell cultures as determined by a Student's t test. Infected cells demonstrated over 50% less death than uninfected cells when treated with staurosporine.
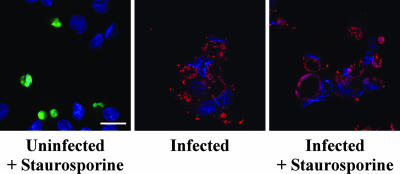
To confirm that C. burnetii infection was protecting cells from apoptotic cell death, infected and uninfected THP-1 cells were treated with staurosporine for 2 h and then analyzed for the presence of cleaved PARP. Proteolytic cleavage of nuclear PARP inactivates the enzyme's DNA repair activity and is considered a classical marker of the terminal stages of apoptosis (32). Treated infected cell cultures displayed dramatically fewer cleaved PARP-positive cells than treated uninfected cell cultures (Fig. 2). Approximately 40% of treated uninfected cells were positive versus ∼3% and ∼1% of treated infected and untreated infected cells, respectively (data not shown). Collectively, these data indicate that C. burnetii inhibits staurosporine-induced death of THP-1 cells primarily through inhibition of apoptosis.
FIG. 2.
C. burnetii inhibits apoptotic cell death of THP-1 cells. THP-1 cells were infected with the C. burnetii Nine Mile phase II strain for 48 h. Infected and uninfected cells were then treated with staurosporine for 2 h to induce apoptosis. Untreated infected cells were used as a control. Cells were immunolabeled for confocal fluorescence microscopy by using antibodies directed against C. burnetii (red) and cleaved PARP (green). DRAQ5 was used to stain DNA (blue). Untreated and treated infected cell cultures contained significantly fewer cleaved PARP-positive (apoptotic) cells than treated uninfected cell cultures. Bar, 10 μm.
C. burnetii-infected THP-1 cells and alveolar macrophages demonstrate reduced caspase processing.
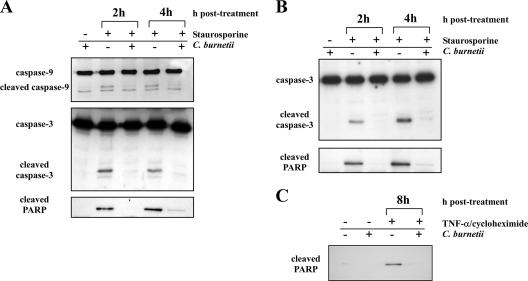
To further examine the antiapoptotic effects of C. burnetii infection, caspase-9 and caspase-3 processing were analyzed by immunoblotting. Caspase-9 is an upstream caspase that is activated in the apoptosome following release of mitochondrial cytochrome c (32). Caspase-3 is downstream of caspase-9 and is an executioner caspase involved in late apoptotic events, such as PARP cleavage (32). Relative to uninfected cells, infected cells treated with staurosporine contained substantially reduced levels of cleaved caspase-9 and caspase-3 (Fig. 3A). In agreement with immunofluorescence results (Fig. 2), the level of cleaved PARP was also dramatically reduced in treated infected cells (Fig. 3A). Substantially reduced PARP cleavage was also observed in infected THP-1 cells treated with TNF-α and cycloheximide for 8 h to induce extrinsic apoptosis (Fig. 3C).
FIG. 3.
C. burnetii infection inhibits caspase and PARP processing in THP-1 cells and monkey primary alveolar macrophages. Uninfected cells or cells infected with the C. burnetii Nine Mile phase II strain for 48 h were treated with staurosporine for 2 or 4 h or with TNF-α for 8 h. Cells were lysed, and equal amounts of protein were subjected to immunoblot analysis. (A) Lysates of staurosporine-treated THP-1 cells probed for caspase-9, caspase-3, and PARP. Cleaved forms of all proteins were dramatically reduced in infected cell lysates. (B) Lysates of staurosporine-treated monkey primary alveolar macrophages probed for caspase-3 and PARP. Similar to THP-1 lysates, cleaved forms of caspase-3 and PARP were substantially reduced in infected cell lysates. (C) Lysates of TNF-α-treated THP-1 cells probed for PARP. Similar to staurosporine-treated cells, cleaved PARP was dramatically reduced in infected cell lysates.
To confirm that the antiapoptotic activity of C. burnetii is not strictly a property of avirulent phase II organisms, PARP and caspase-3 processing was assessed in staurosporine-treated THP-1 cells infected with virulent phase I C. burnetii. Similar to phase II-infected cells, cleaved forms of both proteins were substantially reduced in cells infected with virulent phase I organisms (data not shown). To examine whether the antiapoptotic activity of C. burnetii occurs in primary macrophages, C. burnetii-infected alveolar macrophages from cynomolgus monkeys were treated with staurosporine, and cleavage of caspase-3 and PARP was examined. Consistent with the antiapoptotic activity observed in infected THP-1 cells, infected alveolar macrophages displayed dramatically reduced processing of caspase-3 and PARP relative to uninfected cells following treatment with staurosporine for 2 and 4 h (Fig. 3B). Together, these results indicate that infection of macrophages by both virulent and avirulent C. burnetii inhibits the caspase and PARP processing that occurs during both intrinsic and extrinsic apoptosis.
C. burnetii protein synthesis is required for antiapoptotic activity.
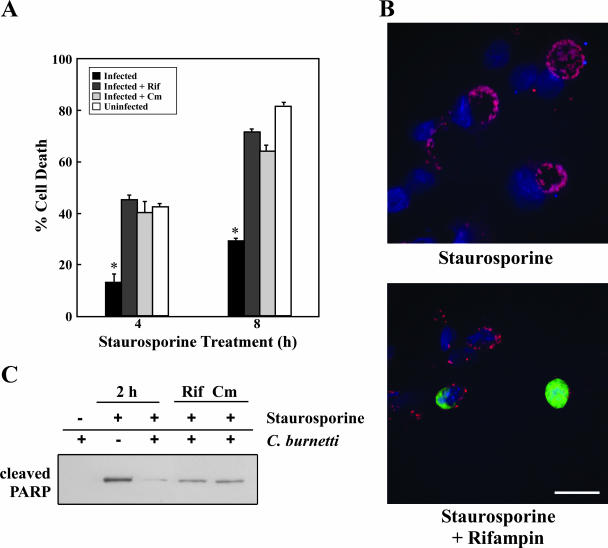
Both preexisting bacterial cell envelope components and bacterial proteins synthesized de novo during the infectious process are known to signal antiapoptotic events (20, 38). Therefore, to examine whether C. burnetii protein synthesis is required for apoptosis inhibition, THP-1 cells were infected with C. burnetii in the presence of rifampin or chloramphenicol, antibiotics that inhibit bacterial RNA and protein synthesis, respectively. Cells infected in the presence of either antibiotic and treated with staurosporine showed ∼40% and ∼70% cell death at 4 h and 8 h posttreatment, respectively (Fig. 4A). These percentages approximated those of treated uninfected cells and were significantly higher than those of treated infected cells cultured in the absence of antibiotics (13% and 29%, respectively). Consistent with apoptotic cell death, a higher percentage of rifampin-treated infected cells contained cleaved PARP than untreated infected cells following a 2-h treatment with staurosporine (Fig. 4B and data not shown). Accordingly, immunoblotting revealed increased levels of cleaved PARP in infected cells incubated in the presence of rifampin or chloramphenicol following a 2-h treatment with staurosporine (Fig. 4C). These results confirm that de novo protein synthesis by C. burnetii is required for antiapoptotic activity.
FIG. 4.
C. burnetii protein synthesis is required for antiapoptotic activity. THP-1 cells were infected with the C. burnetii Nine Mile phase II strain for 48 h in the presence or absence of the antibiotics rifampin (Rif) or chloramphenicol (Cm) to inhibit bacterial RNA and protein synthesis, respectively. (A) Infected and uninfected cell cultures were treated with staurosporine for 4 or 8 h and assessed for viability using WST-8 staining. Experiments were conducted in triplicate, and error bars represent the standard deviation from the mean. An asterisk indicates a P value of <0.05 in comparison to uninfected cells and infected cells treated with antibiotics as determined by a Student's t test. Antibiotic-treated infected cell cultures demonstrated levels of death similar to those of uninfected cell cultures. (B) Cells infected in the presence or absence of rifampin were treated with staurosporine for 2 h. Cells were immunostained for confocal fluorescence microscopy by using antibodies directed against C. burnetii (red) and cleaved PARP (green). DNA (blue) was stained with DRAQ5. The percentage of cells staining positive for nuclear cleaved PARP following staurosporine treatment was significantly higher in infected cell cultures treated with rifampin. Bar, 10 μm. (C) Cells infected in the presence or absence of rifampin or chloramphenicol were treated with staurosporine for 2 h. Cell lysates were harvested, and equal amounts of protein were subjected to immunoblot analysis for cleaved PARP. Antibiotic treatment of infected cells resulted in increased levels of cleaved PARP following staurosporine treatment.
C. burnetii infection does not induce mass degradation of proapoptotic mitochondrial proteins.
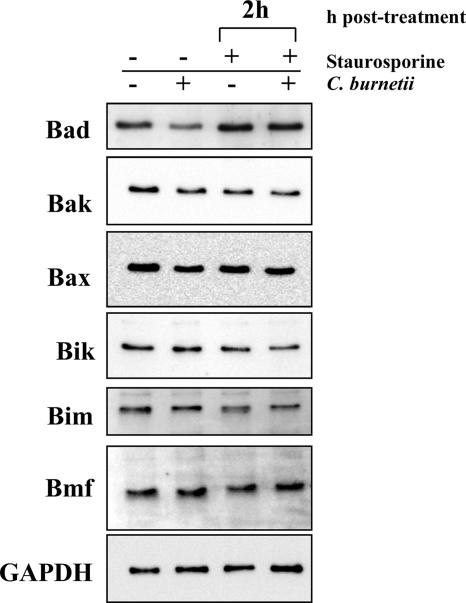
Infection by obligate intracellular Chlamydia trachomatis results in nearly complete degradation of several proapoptotic Bcl-2 BH3-only subfamily proteins that transmit death signals to the mitochondria during intrinsic apoptosis (19, 22, 44, 61). Because C. burnetii infection affects intrinsic apoptotic signaling downstream of mitochondrial release of cytochrome c (e.g., decreased caspase-9 activation), we examined whether a similar process was occurring. Immunoblot analyses did not indicate any broad degradation of the BH3-only proteins Bad, Bik, Bim, and Bmf in C. burnetii-infected THP-1 cells with or without staurosporine treatment (Fig. 5). Thus, unlike C. trachomatis (20), C. burnetii infection confers protection against apoptosis in the presence of proapoptotic BH3-only proteins. The proapoptotic Bcl-2 family proteins Bak and Bax, whose activation leads directly to cytochrome c release, were similarly not degraded (Fig. 5).
FIG. 5.
C. burnetii infection does not induce mass degradation of proapoptotic Bcl-2 family proteins. THP-1 cells were infected with the C. burnetii Nine Mile phase II strain for 48 h. Infected and uninfected cell cultures were then treated with staurosporine for 2 h. Cells were lysed, and equal amounts of protein were subjected to immunoblot analysis using Bad, Bak, Bax, Bik, Bim, or Bmf primary antibodies. Similar levels of these proteins were observed between infected and uninfected cells treated with staurosporine. The GAPDH immunoblot is included as a loading control.
C. burnetii infection modulates expression of apoptosis-related genes.
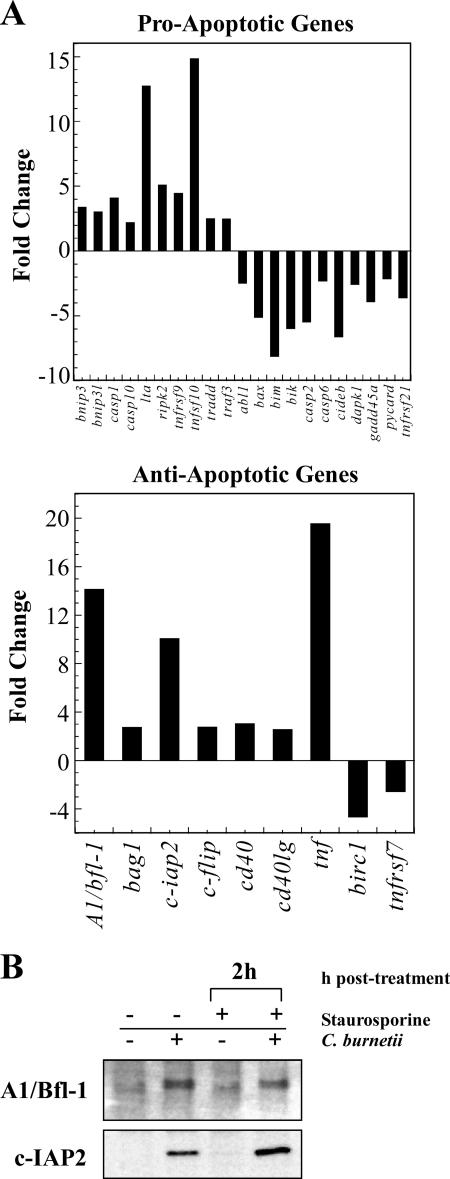
Because C. burnetii-infected cells do not show degradation of proapoptotic proteins, we hypothesized that infection modulates expression of apoptosis-related genes to promote cell survival. To test this hypothesis, a panel of 84 human apoptosis-related genes was examined using RT-PCR to evaluate changes in gene expression during infection of THP-1 cells in the absence of staurosporine. At 48 h postinfection, relative changes in gene expression between uninfected and C. burnetii-infected cells were calculated. Genes showing an increase or decrease in expression greater than or equal to twofold relative to uninfected cells are shown in Fig. 6. Infection resulted in down-regulation of 11 proapoptotic genes, including bax, bim, and bik, which encode proapoptotic Bcl-2 family proteins, as well as two caspase-encoding genes (casp-2 and casp-6). Consistent with antiapoptotic signaling, C. burnetii infection also increased expression of seven antiapoptotic genes including A1/bfl-1, which encodes the antiapoptotic Bcl-2 family protein A1/Bfl-1 that inhibits Bax-mediated release of cytochrome c, and c-iap2, which encodes the inhibitor of apoptosis protein (IAP) c-IAP2 that antagonizes the executioner caspase, caspase-3 (32). In agreement with gene expression data, the levels of both A1/Bfl-1 and c-IAP2 were elevated in infected THP-1 cells with or without staurosporine treatment (Fig. 6B). Up-regulation of c-flip, which encodes a potent inhibitor of caspase-8, and tnf, cd40, cd40lg, and ripk2, which are involved in NF-κB activation, was also observed (32). (TNF-α, encoded by tnf, is also recognized as a proapoptotic cytokine in the absence of NF-κB activation [32].) Interestingly, up-regulated expression of 10 proapoptotic genes was also observed in infected cells. Collectively, these data suggest that C. burnetii counteracts proapoptotic gene expression induced during infection by also stimulating a potent antiapoptotic response, which includes production of proteins such as c-IAP2 that act late in the apoptotic pathway.
FIG. 6.
C. burnetii infection modulates the expression of apoptosis-related genes. (A) Total RNA was extracted from uninfected THP-1 cells and cells infected with the C. burnetii Nine Mile phase II strain for 48 h. RT-PCR was performed using the RT2 Profiler Array. Genes showing an increase or decrease in expression greater than or equal to twofold relative to uninfected cells are shown. Depicted results are representative of two independent experiments. Twenty-nine genes were up- or down-regulated in response to C. burnetii infection, of which 12 indicated a proapoptotic response and 18 indicated an anti-apoptotic response. (B) THP-1 cells were infected with the C. burnetii Nine Mile phase II strain for 48 h. Infected and uninfected cell cultures were treated with staurosporine for 2 h as indicated. Cells were lysed, and equal amounts of protein were subjected to immunoblot analysis using A1/Bfl-1 or c-IAP2 primary antibodies. Consistent with gene expression data, increased levels of A1/Bfl-1 and c-IAP2 were observed in cell lysates of both untreated and staurosporine-treated infected cell cultures relative to lysates of uninfected cells.
DISCUSSION
Here, we demonstrate that C. burnetii infection of THP-1 cells confers pronounced resistance to apoptotic cell death induced by staurosporine, a potent stimulator of intrinsic apoptosis. Protection from apoptosis is associated with substantially reduced levels of cleaved caspase-9, caspase-3, and PARP, indicating diminished stimulation of these proapoptotic responses. The antiapoptotic behavior of C. burnetii-infected cells is consistent with the growth phenotype of the pathogen as minimal cytopathic effects are observed in cells infected for many days (57). Indeed, by occasional subculturing, mouse fibroblast or macrophage-like cell cultures have been maintained for months or even years (5, 47). Persistent in vitro infection is possible because division of an infected host cell frequently results in one daughter cell that contains the PV while the companion daughter cell remains parasite free. This asymmetric cell division thereby supplies new host cells for reinfection by C. burnetii organisms that are occasionally released into the extracellular environment (5, 47).
Inhibition of apoptosis occurs irrespective of the virulence properties of C. burnetii. While this study was primarily conducted with avirulent Nine Mile phase II organisms, infection of THP-1 cells by virulent Nine Mile phase I C. burnetii reduces processing of caspase-3 and PARP to a similar degree. Thus, the shared antiapoptotic activity argues that these strains utilize a common strategy to mediate this process. Indeed, by whole-genome comparisons, the primary difference between Nine Mile phase II and Nine Mile phase I C. burnetii strains is the presence of an ∼26 kb chromosomal deletion that contributes to the synthesis of truncated lipopolysaccharide by phase II organisms (8).
Protection from apoptosis extends to primary macrophages as C. burnetii infection of cultured alveolar macrophages obtained from cynomolgus monkeys by bronchial lavage results in a reduction in caspase-3 and PARP cleavage similar to that observed in THP-1 cells. Pulmonary infection of cynomolgus monkeys is the established non-human primate model of Q fever (58), with alveolar macrophages being a primary target of the pathogen (54). Thus, the in vitro antiapoptotic effect of C. burnetii infection described herein likely has pathophysiologic relevance in vivo.
Differentiated tissue macrophages are long-lived (11) relative to neutrophils and epithelial cells, which are targets of other intracellular pathogens with strong antiapoptotic activities (21, 23). Therefore, C. burnetii antiapoptotic activity is likely necessary to antagonize proapoptotic signals that arise throughout the infectious process. This hypothesis is supported by gene expression data predictive of both pro- and antiapoptotic effects. This behavior is not unprecedented as seemingly antagonistic apoptosis-related gene expression has been reported for host cells infected by other intracellular bacteria that exert strong antiapoptotic effects (1, 39, 55). Among possible proapoptotic signals, expansion of the C. burnetii PV might activate BH3-only proteins (discussed in more detail below). Moreover, a translocated C. burnetii component could bind a cognate nucleotide-binding oligomerization domain-like receptor in the cytosol as part of the host cell's pathogen recognition process, leading to inflammasome formation and activation of the inflammatory caspase, caspase-1 (32).
C. burnetii infection-specific antiapoptotic responses include dramatic increases in expression of A1/bfl-1 (∼14-fold) and c-iap2 (∼10-fold) that correlate with increased synthesis of the encoded proteins. Cellular IAPs are considered the most important negative regulators of caspases. Specifically, c-IAP2 is a potent inhibitor of the executioner caspase, caspase-3, and its up-regulation would confer protection against both extrinsic and intrinsic apoptotic pathways that converge on this caspase. Increased synthesis of the Bcl-2 family protein A1/Bfl-1 would augment the antiapoptotic response by promoting mitochondrial outer membrane integrity and inhibiting release of cytochrome c (32). C. burnetii infection also up-regulated c-flip, which would inhibit caspase-8 activation following death receptor signaling (32). Multiple proapoptotic genes were down-regulated during C. burnetii infection including genes encoding two caspases (casp-2 and casp-6) and Bcl-2 family proteins that promote cytochrome c release from the mitochondria (e.g., bax, bim, and bik). One caveat with this latter group of genes is that their encoded proteins can have relatively long half-lives (43). Collectively, these results suggest that C. burnetii infection modulates host gene expression that favors protection from not only mitochondrion-mediated intrinsic apoptosis but also death receptor-induced extrinsic apoptosis. Indeed, in an initial probe of the extrinsic pathway, we also observed substantially reduced PARP cleavage in C. burnetii-infected THP-1 cells treated with TNF-α. Activation of the antiapoptotic transcription factor NF-κB accompanies protection from cell death during infection by a number of intracellular pathogens including M. tuberculosis (18), Bartonella henselae (33, 50), C. pneumoniae (59), Rickettsia rickettsii (13), and L. pneumophila (1, 39). This transcription factor may play a similar role in C. burnetii-infected cells as c-iap2 and A1/bfl-1, genes that are positively regulated by NF-κB, and tnf, cd40, cd40lg, and ripk2, genes involved in signaling pathways that activate NF-κB (32), are substantially up-regulated during infection.
Certain intracellular pathogens activate an antiapoptotic response in phagocytic cells following interaction of a presynthesized bacterial surface component with its cognate host receptor. For example, binding of the M. tuberculosis cell wall component lipoarabinomannan to Toll-like receptor 2 confers resistance to FasL-induced apoptosis by activating NF-κB, which up-regulates c-flip (38). Thus, de novo protein synthesis following infection is not always required for intracellular bacteria to elicit a protective response. However, here we show that C. burnetii RNA and protein synthesis are both required for a full antiapoptotic response. A C. burnetii inhibitor(s) of apoptosis is likely translocated to the host cytosol via the activity of a type IV secretion system (T4SS) with homology to the Dot/Icm system of L. pneumophila (51, 52). In fact, all intracellular bacterial pathogens with a T4SS (6) examined to date exert potent antiapoptotic effects following infection including L. pneumophila (1, 39), Bartonella spp. (49, 50), Brucella spp. (24, 25), R. rickettsii (13), Anaplasma phagocytophilum (46), and Ehrlichia chaffeensis (46). Among these bacteria, antiapoptotic T4SS substrates have been identified for L. pneumophila (SdhA and SidF) (7, 35) and Bartonella spp. (BepA) (49). Secreted SidF confers antiapoptotic activity by interacting with BNIP3 and Bcl-rambo, which neutralizes the proapoptotic activities of these Bcl-2 family proteins (7). BepA exerts its antiapoptotic effect by triggering a rise in host cell cyclic AMP (49). An intact T4SS is also required by L. pneumophila and Bartonella spp. to activate NF-κB although the precise mechanism of this signaling is unknown (1, 39, 50). Putative C. burnetii T4SS effectors that directly or indirectly inhibit apoptosis would presumably be required throughout the organism's infection cycle. In support of this idea is the observation that expression of dotA, which encodes an integral component of the Dot/Icm secretion apparatus, is detected soon after infection and continues well into the stationary phase of the C. burnetii growth cycle (14).
A direct antiapoptotic mechanism employed by C. trachomatis involves secretion of a chlamydial protease that degrades several proapoptotic Bcl-2 family BH3-only subfamily proteins including Bim, Bik, and Puma (44). These proteins are normally sequestered to cellular sites, such as organelles and the cytoskeleton (e.g., Bim and Bmf), and sense intracellular damage (32). They trigger apoptosis by activating proapoptotic proteins associated with cytochrome c release (45). Thus, broad degradation by chlamydia of BH3-only proteins is thought to provide general protection from a variety of apoptotic stimuli that are likely associated with pathogen replication and inclusion expansion (19, 22, 44, 61). Like chlamydial inclusion formation, generation of the large C. burnetii PV presumably disrupts intracellular structures that may lead to activation of these proapoptotic proteins. However, C. burnetii infection did not induce substantial degradation of the BH3-only proteins Bad, Bik, Bim, or Bmf; thus, another mechanism to negate the death signals of these proteins must be employed.
Like a number of intracellular parasites, growth of C. burnetii is severely inhibited in cells activated with gamma interferon (IFN-γ) (9, 16, 28, 56). The nature of this growth restriction is ill defined, with production of toxic reactive oxygen and nitrogen species implicated (9). Dellacasagrande et al. (16, 17) have also invoked a novel mechanism of IFN-γ-mediated killing of C. burnetii whereby the cytokine induces apoptosis in infected monocytes by up-regulating synthesis of membrane-bound TNF. However, under this scenario, it is unclear how apoptosis per se would kill C. burnetii. This process might actually aid in pathogen dissemination as uptake of apoptotic bodies, which traffic to lysosomes (C. burnetii's intracellular niche), down-regulates macrophage activation (36, 62). Nonetheless, the role of C. burnetii's potent antiapoptotic activity described herein during an immune response involving IFN-γ activation of macrophages remains to be defined.
Dampening of apoptotic cell death may be a strategy to ensure completion of C. burnetii's lengthy biphasic developmental cycle. Coleman et al. demonstrated that significant numbers of the stable SCV begin to appear at ∼6 days postinfection, with increasing numbers thereafter (14). The SCV is considered the environmentally stable form of the organism that initiates natural infections, and an antiapoptotic strategy that promotes its development may be central to C. burnetii pathogenesis. As proposed for Chlamydia spp. (20, 22), antiapoptotic mechanisms may also be involved in C. burnetii's noted ability to establish chronic infections. Indeed, C. burnetii can establish a long-term infection with minimal host cell damage in animal models (34), and human chronic disease often manifests without major pathohistological features (37). Further elucidation of C. burnetii-associated antiapoptotic activity will provide important insight into the virulence potential of this obligate intracellular bacterium.
Acknowledgments
We thank Harlan Caldwell, Olivia Steele-Mortimer, Sonja Best, and Shelly Robertson for critical reading of the manuscript; Kimmo Virtaneva for assistance with RNA isolation and RT-PCR; and Anita Mora for graphic illustrations.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Editor: F. C. Fang
Footnotes
Published ahead of print on 2 July 2007.
REFERENCES
- 1.Abu-Zant, A., S. Jones, R. Asare, J. Suttles, C. Price, J. Graham, and Y. A. Kwaik. 2006. Anti-apoptotic signalling by the Dot/Icm secretion system of L. pneumophila. Cell. Microbiol. 9:246-264. [DOI] [PubMed] [Google Scholar]
- 2.Akporiaye, E. T., J. D. Rowatt, A. A. Aragon, and O. G. Baca. 1983. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect. Immun. 40:1155-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baca, O. G., E. T. Akporiaye, A. S. Aragon, I. L. Martinez, M. V. Robles, and N. L. Warner. 1981. Fate of phase I and phase II Coxiella burnetii in several macrophage-like tumor cell lines. Infect. Immun. 33:258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baca, O. G., and H. A. Crissman. 1987. Correlation of DNA, RNA, and protein content by flow cytometry in normal and Coxiella burnetii-infected L929 cells. Infect. Immun. 55:1731-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baca, O. G., T. O. Scott, E. T. Akporiaye, R. DeBlassie, and H. A. Crissman. 1985. Cell cycle distribution patterns and generation times of L929 fibroblast cells persistently infected with Coxiella burnetii. Infect. Immun. 47:366-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backert, S., and T. F. Meyer. 2006. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr. Opin. Microbiol. 9:207-217. [DOI] [PubMed] [Google Scholar]
- 7.Banga, S., P. Gao, X. Shen, V. Fiscus, W. X. Zong, L. Chen, and Z. Q. Luo. 2007. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl-2 protein family. Proc. Natl. Acad. Sci. USA 104:5121-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beare, P. A., J. E. Samuel, D. Howe, K. Virtaneva, S. F. Porcella, and R. A. Heinzen. 2006. Genetic diversity of the Q fever agent, Coxiella burnetii, assessed by microarray-based whole-genome comparisons. J. Bacteriol. 188:2309-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan, R. E., K. Russell, G. Zhang, and J. E. Samuel. 2004. Both inducible nitric oxide synthase and NADPH oxidase contribute to the control of virulent phase I Coxiella burnetii infections. Infect. Immun. 72:6666-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan, R. E., and J. E. Samuel. 2003. Evaluation of Coxiella burnetii antibiotic susceptibilities by real-time PCR assay. J. Clin. Microbiol. 41:1869-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke, B. 2003. Macrophages as novel cellular vehicles for gene therapy. Expert Opin. Biol. Ther. 3:919-924. [DOI] [PubMed] [Google Scholar]
- 12.Burton, P. R., N. Kordova, and D. Paretsky. 1971. Electron microscopic studies of the rickettsia Coxiella burnetii: entry, lysosomal response, and fate of rickettsial DNA in L-cells. Can. J. Microbiol. 17:143-150. [DOI] [PubMed] [Google Scholar]
- 13.Clifton, D. R., R. A. Goss, S. K. Sahni, D. van Antwerp, R. B. Baggs, V. J. Marder, D. J. Silverman, and L. A. Sporn. 1998. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc. Natl. Acad. Sci. USA 95:4646-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman, S. A., E. R. Fischer, D. Howe, D. J. Mead, and R. A. Heinzen. 2004. Temporal analysis of Coxiella burnetii morphological differentiation. J. Bacteriol. 186:7344-7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLeo, F. R. 2004. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 9:399-413. [DOI] [PubMed] [Google Scholar]
- 16.Dellacasagrande, J., C. Capo, D. Raoult, and J. L. Mege. 1999. IFN-gamma-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF. J. Immunol. 162:2259-2265. [PubMed] [Google Scholar]
- 17.Dellacasagrande, J., E. Ghigo, D. Raoult, C. Capo, and J. L. Mege. 2002. IFN-gamma-induced apoptosis and microbicidal activity in monocytes harboring the intracellular bacterium Coxiella burnetii require membrane TNF and homotypic cell adherence. J. Immunol. 169:6309-6315. [DOI] [PubMed] [Google Scholar]
- 18.Dhiman, R., M. Raje, and S. Majumdar. 2007. Differential expression of NF-κB in mycobacteria infected THP-1 affects apoptosis. Biochim. Biophys. Acta 1770:649-658. [DOI] [PubMed] [Google Scholar]
- 19.Dong, F., M. Pirbhai, Y. Xiao, Y. Zhong, Y. Wu, and G. Zhong. 2005. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect. Immun. 73:1861-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer, S. F., C. Schwarz, J. Vier, and G. Hacker. 2001. Characterization of anti-apoptotic activities of Chlamydia pneumoniae in human cells. Infect. Immun. 69:7121-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer, S. F., J. Vier, S. Kirschnek, A. Klos, S. Hess, S. Ying, and G. Hacker. 2004. Chlamydia inhibit host cell apoptosis by degradation of pro-apoptotic BH3-only proteins. J. Exp. Med. 200:905-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge, Y., K. Yoshiie, F. Kuribayashi, M. Lin, and Y. Rikihisa. 2005. Anaplasma phagocytophilum inhibits human neutrophil apoptosis via up-regulation of bfl-1, maintenance of mitochondrial membrane potential and prevention of caspase-3 activation. Cell. Microbiol. 7:29-38. [DOI] [PubMed] [Google Scholar]
- 24.Gross, A., A. Terraza, S. Ouahrani-Bettache, J. P. Liautard, and J. Dornand. 2000. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 68:342-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He, Y., S. Reichow, S. Ramamoorthy, X. Ding, R. Lathigra, J. C. Craig, B. W. Sobral, G. G. Schurig, N. Sriranganathan, and S. M. Boyle. 2006. Brucella melitensis triggers time-dependent modulation of apoptosis and down-regulation of mitochondrion-associated gene expression in mouse macrophages. Infect. Immun. 74:5035-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinzen, R. A., T. Hackstadt, and J. E. Samuel. 1999. Developmental biology of Coxiella burnetii. Trends Microbiol. 7:149-154. [DOI] [PubMed] [Google Scholar]
- 27.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64:796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinrichs, D. J., and T. R. Jerrells. 1976. In vitro evaluation of immunity to Coxiella burnetii. J. Immunol. 117:996-1003. [PubMed] [Google Scholar]
- 29.Howe, D., and R. A. Heinzen. 2006. Coxiella burnetii inhabits a cholesterol-rich vacuole and influences cellular cholesterol metabolism. Cell. Microbiol. 8:496-507. [DOI] [PubMed] [Google Scholar]
- 30.Howe, D., and L. P. Mallavia. 2000. Coxiella burnetii exhibits morphological change and delays phagolysosomal fusion after internalization by J774A.1 cells. Infect. Immun. 68:3815-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howe, D., J. Melnicakova, I. Barak, and R. A. Heinzen. 2003. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell. Microbiol. 5:469-480. [DOI] [PubMed] [Google Scholar]
- 32.Jin, Z., and W. S. El-Deiry. 2005. Overview of cell death signaling pathways. Cancer Biol. Ther. 4:139-163. [DOI] [PubMed] [Google Scholar]
- 33.Kempf, V. A., A. Schairer, D. Neumann, G. A. Grassl, K. Lauber, M. Lebiedziejewski, M. Schaller, P. Kyme, S. Wesselborg, and I. B. Autenrieth. 2005. Bartonella henselae inhibits apoptosis in Mono Mac 6 cells. Cell. Microbiol. 7:91-104. [DOI] [PubMed] [Google Scholar]
- 34.Khavkin, T., and S. S. Tabibzadeh. 1988. Histologic, immunofluorescence, and electron microscopic study of infectious process in mouse lung after intranasal challenge with Coxiella burnetii. Infect. Immun. 56:1792-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laguna, R. K., E. A. Creasey, Z. Li, N. Valtz, and R. R. Isberg. 2006. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc. Natl. Acad. Sci. USA 103:18745-18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laskay, T., G. van Zandbergen, and W. Solbach. 2003. Neutrophil granulocytes—Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 11:210-214. [DOI] [PubMed] [Google Scholar]
- 37.Lepidi, H., P. Houpikian, Z. Liang, and D. Raoult. 2003. Cardiac valves in patients with Q fever endocarditis: microbiological, molecular, and histologic studies. J. Infect. Dis. 187:1097-1106. [DOI] [PubMed] [Google Scholar]
- 38.Loeuillet, C., F. Martinon, C. Perez, M. Munoz, M. Thome, and P. R. Meylan. 2006. Mycobacterium tuberculosis subverts innate immunity to evade specific effectors. J. Immunol. 177:6245-6255. [DOI] [PubMed] [Google Scholar]
- 39.Losick, V. P., and R. R. Isberg. 2006. NF-κB translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J. Exp. Med. 203:2177-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCaul, T. F., T. Hackstadt, and J. C. Williams. 1981. Ultrastructural and biological aspects of Coxiella burnetii under physical disruptions, p. 267-280. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, NY.
- 42.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moulding, D. A., C. Akgul, M. Derouet, M. R. White, and S. W. Edwards. 2001. Bcl-2 family expression in human neutrophils during delayed and accelerated apoptosis. J. Leukoc. Biol. 70:783-792. [PubMed] [Google Scholar]
- 44.Pirbhai, M., F. Dong, Y. Zhong, K. Z. Pan, and G. Zhong. 2006. The secreted protease factor CPAF is responsible for degrading pro-apoptotic BH3-only proteins in Chlamydia trachomatis-infected cells. J. Biol. Chem. 281:31495-31501. [DOI] [PubMed] [Google Scholar]
- 45.Puthalakath, H., and A. Strasser. 2002. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 9:505-512. [DOI] [PubMed] [Google Scholar]
- 46.Rikihisa, Y. 2006. Ehrlichia subversion of host innate responses. Curr. Opin. Microbiol. 9:95-101. [DOI] [PubMed] [Google Scholar]
- 47.Roman, M. J., P. D. Coriz, and O. G. Baca. 1986. A proposed model to explain persistent infection of host cells with Coxiella burnetii. J. Gen. Microbiol. 132:1415-1422. [DOI] [PubMed] [Google Scholar]
- 48.Romano, P. S., M. G. Gutierrez, W. Beron, M. Rabinovitch, and M. I. Colombo. 2006. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell. Microbiol. 9:891-909. [DOI] [PubMed] [Google Scholar]
- 49.Schmid, M. C., F. Scheidegger, M. Dehio, N. Balmelle-Devaux, R. Schulein, P. Guye, C. S. Chennakesava, B. Biedermann, and C. Dehio. 2006. A translocated bacterial protein protects vascular endothelial cells from apoptosis. PLOS Pathogens 2:1083-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmid, M. C., R. Schulein, M. Dehio, G. Denecker, I. Carena, and C. Dehio. 2004. The VirB type IV secretion system of Bartonella henselae mediates invasion, pro-inflammatory activation, and anti-apoptotic protection of endothelial cells. Mol. Microbiol. 52:81-92. [DOI] [PubMed] [Google Scholar]
- 51.Segal, G., M. Feldman, and T. Zusman. 2005. The Icm/Dot type-IV secretion systems of Legionella pneumophila and Coxiella burnetii. FEMS Microbiol. Rev. 29:65-81. [DOI] [PubMed] [Google Scholar]
- 52.Sexton, J. A., and J. P. Vogel. 2002. Type IVB secretion by intracellular pathogens. Traffic 3:178-185. [DOI] [PubMed] [Google Scholar]
- 53.Shannon, J. G., D. Howe, and R. A. Heinzen. 2005. Virulent Coxiella burnetii does not activate human dendritic cells: role of lipopolysaccharide as a shielding molecule. Proc. Natl. Acad. Sci. USA 102:8722-8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein, A., C. Louveau, H. Lepidi, F. Ricci, P. Baylac, B. Davoust, and D. Raoult. 2005. Q. fever pneumonia: virulence of Coxiella burnetii pathovars in a murine model of aerosol infection. Infect. Immun. 73:2469-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sukumaran, B., J. A. Carlyon, J. L. Cai, N. Berliner, and E. Fikrig. 2005. Early transcriptional response of human neutrophils to Anaplasma phagocytophilum infection. Infect. Immun. 73:8089-8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turco, J., H. A. Thompson, and H. H. Winkler. 1984. Interferon-gamma inhibits growth of Coxiella burnetii in mouse fibroblasts. Infect. Immun. 45:781-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voth, D. E., and R. A. Heinzen. 2007. Lounging in a lysosome: the intracellular lifestyle of Coxiella burnetii. Cell. Microbiol. 9:829-840. [DOI] [PubMed] [Google Scholar]
- 58.Waag, D. M., W. R. Byrne, J. Estep, P. Gibbs, M. L. Pitt, and C. M. Banfield. 1999. Evaluation of cynomolgus (Macaca fascicularis) and rhesus (Macaca mulatta) monkeys as experimental models of acute Q. fever after aerosol exposure to phase-I Coxiella burnetii. Lab. Anim. Sci. 49:634-638. [PubMed] [Google Scholar]
- 59.Wahl, C., F. Oswald, U. Simnacher, S. Weiss, R. Marre, and A. Essig. 2001. Survival of Chlamydia pneumoniae-infected Mono Mac 6 cells is dependent on NF-κB binding activity. Infect. Immun. 69:7039-7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams, J. C. 1991. Infectivity, virulence, and pathogenicity of Coxiella burnetii for various hosts, p. 21-71. In J. C. Williams and H. A. Thompson (ed.), Q. fever: the biology of Coxiella burnetii. CRC Press, Boca Raton, FL.
- 61.Ying, S., B. M. Seiffert, G. Hacker, and S. F. Fischer. 2005. Broad degradation of proapoptotic proteins with the conserved Bcl-2 homology domain 3 during infection with Chlamydia trachomatis. Infect. Immun. 73:1399-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zamboni, D. S., and M. Rabinovitch. 2004. Phagocytosis of apoptotic cells increases the susceptibility of macrophages to infection with Coxiella burnetii phase II through down-modulation of nitric oxide production. Infect. Immun. 72:2075-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu, H., H. O. Fearnhead, and G. M. Cohen. 1995. An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP. 1 cells. FEBS Lett. 374:303-308. [DOI] [PubMed] [Google Scholar]