Abstract
Puumala virus (PUUV) is the endemic hantavirus in northern Sweden and causes nephropathia epidemica (NE), a milder form of hemorrhagic fever with renal syndrome. There is a need for fast and reliable diagnostics to differentiate the disease from other infections. By aligning virus RNA sequences isolated from 11 different bank voles and one human patient, we designed a real-time reverse transcriptase (RT) PCR method for detection of PUUV RNA. The real-time RT-PCR assay showed linearity from 20 to 2 × 106 virus copies with a correlation coefficient above 0.98 to 0.99 for all experiments. The detection threshold for PUUV cDNA was two copies per reaction. A two-step qualitative RT-PCR to detect PUUV RNA showed 100% concordance with the real-time RT-PCR assay. PUUV RNA viremia was detected in 33 of 34 PUUV immunoglobulin M (IgM)-positive patients with typical clinical NE disease from the region of endemicity. One PUUV IgM-negative sample had PUUV RNA, and 4 days later, the patient was IgM positive. Of samples with indeterminate IgM, 43% were PUUV RNA positive. The kinetics of antibody titers and PUUV viremia were studied, and five of six NE patients displayed a decrease in PUUV viremia a few days after disease outbreak coupled with an increase in PUUV IgM and IgG. In one patient with continuously high PUUV RNA levels but low IgM and no IgG response, the infection was lethal. These findings demonstrated that real-time RT-PCR is a useful method for diagnosis of PUUV viremia and for detecting PUUV RNA at early time points, before the appearance of IgM antibodies.
Members of the family Bunyaviridae cause severe infections in a large and increasing number of people worldwide each year. The family Bunyaviridae contains five genera, and among these, the genus Hantavirus causes two febrile illnesses: hemorrhagic fever with renal syndrome (HFRS) in Europe and Asia and hantavirus pulmonary syndrome (HPS) on the American continent. HFRS accounts for up to 150,000 cases and thousands of deaths annually, while HPS, since its appearance in 1993, has had nearly 2,000 reported cases with a mortality rate above 40% (27). In nature, hantaviruses are maintained in persistently infected rodents, and the virus is transmitted to humans by inhalation of infected rodent material. Puumala virus (PUUV) is endemic in Norway, Sweden, Finland, Russia, and parts of central Europe. Other hantaviruses not found in Sweden that cause HFRS are Seoul virus, Dobrava virus, and Hantaan virus. PUUV infection usually induces a mild form of HFRS called nephropathia epidemica (NE). PUUV infection can occasionally result in a more serious form of the disease characterized by renal failure and circular shock. For NE, there have been reports of fatal outcome (7, 18, 33), although the mortality rate is less than 2% (20).
PUUV is carried by bank voles (Cletrionomys glareolus), and in Sweden, there are 200 to 600 diagnosed cases of NE each year, but the true incidence is considered to be seven to eight times higher (22). Its presence in Sweden is almost exclusively confined to the northern part of the country, and the prevalence of PUUV-specific antibodies was about 9% in a randomized and stratified population study of the adult population in northern Sweden (1). Persons at higher risk, such as farmers and forestry workers in areas of endemicity, have prevalence rates up to 18% (1).
The hantaviruses are spherical, enveloped particles 90 to 120 nm in diameter that contain two glycoproteins, G1 and G2, and enclose three unique negative-stranded RNAs designated L, M, and S, which are associated with the protein (nucleocapsids). The L segment encodes an RNA-dependent RNA polymerase. The M segment encodes G1 and G2, and the S segment codes for a nucleocapsid protein (N). Most PUUV isolates have been characterized from rodent sources. However, a unique PUUV was isolated from an NE patient living outside of Umeå, Västerbotten County, in northern Sweden (16). This isolate has recently been characterized and is the first complete PUUV sequence (PUUV Umeå/hu) from a human source (15). The findings imply that the diversity between different PUUV isolates is high, and consequently, characterization of local PUUV isolates is important for clinical diagnostic work.
Hantavirus diagnosis using cell culture is tedious and takes a long time. To specifically analyze the neutralizing capacity of the patient serum, a plaque reduction neutralization test (3, 28) or a focal reduction neutralization test (10, 29) could be used. Common measures to diagnose hantavirus infections utilize serology, since on admission, most patients have both specific immunoglobulin M (IgM) and IgG. Sensitive and specific detection of hantavirus in patient specimens can be monitored by reverse transcriptase (RT) PCR (12, 23, 24). Recently, real-time RT-PCR techniques have been used for detection of PUUV in tissue culture (2, 9).
In our efforts to follow a PUUV infection in vivo, we used a one-step real-time RT-PCR method for the detection of PUUV RNA in NE patient sera from northern Sweden. The technique was useful to determine the level and duration of viremia in NE patients and to identify patients with PUUV infection before the appearance of antibodies.
MATERIALS AND METHODS
Clinical samples.
Serum samples from patients with a clinical disease suspected to be NE were prospectively analyzed by immunofluorescence (IF) from November 2003 to May 2006. Samples were handled according to routine instructions and analyzed by IF within 1 to 2 days after sampling. A qualitative two-step RT-PCR and a quantitative one-step real-time RT-PCR analysis were performed later on samples stored at −80°C. The project was approved by the Research Ethics Committee of Umeå University, Umeå, Sweden.
Construction of synthetic standard PUUV RNA.
Standard PUUV RNA was constructed from a pT7Blue-3 vector (Novagen, Darmstadt, Germany) with a cloned 501-bp cDNA fragment of the S gene (strain Umeå/hu; accession no. AY526219). The insert was sequenced to locate the 5′-to-3′ direction and to certify 100% homology to the primers and probe. This was important to ensure the same efficiency in quantitation of the standard curve and the patient samples. The plasmid was linearized with the restriction enzyme SacI and used as a template for RNA transcription using the MEGAscript High Yield Transcription Kit (Ambion, Austin, TX) according to the manufacturer's protocol. The synthesized RNA was incubated with DNase at 37°C for 15 min, purified by phenol-chloroform extraction and isopropanol precipitation, and finally resuspended in 40 μl of nuclease-free water with 40 U recombinant RNasin RNase inhibitor (Promega, Madison, WI). RNA dilutions of 10−4 to 10−9 were analyzed by real-time PCR with and without a prior RT reaction step to ensure a DNA template concentration of less than 0.2%. DNA contamination equal to or above 0.2% required DNase treatment of the RNA. The RNA concentration was measured spectrophotometrically, and RNA at ≥1 × 108 copies/μl was aliquoted and stored at −80°C. A new tube of standard RNA was used for each real-time RT-PCR experiment and diluted to 2 × 106 copies/μl of cDNA prior to the RT reaction. The standard cDNA was finally used in 10-fold dilutions from 2 × 106 to 20 copies.
Primers and probes.
The Primer Express program (Applied Biosystems, Foster City, CA) was used to design primers and probes for both the one-step real-time RT-PCR and the two-step RT-PCR. For the real-time RT-PCR, the selected forward primer, S237 5′ (5′-GGC AGA TGC TGT GTC CAG G-3′), and the reverse primer, S304 3′ (5′-CAT CTG GCT CAA TCC CAG TTG-3′), were synthesized by DNA Technology A/S, Aarhus, Denmark, and positioned between nucleotides 237 and 304 in the S gene of PUUV strain Umeå/hu. The TaqMan MGB probe S263-282 (5′-TGG ATA CAA AGC CTA CTG AT-3′) was labeled with 6-carboxyfluorescein at the 5′ end (Applied Biosystems). For a qualitative method, a two-step RT-PCR was constructed. The same area in the S segment as for the real-time RT-PCR was used as a target. The outer primers amplified a 380-bp fragment using primers S62 5′ (5′-ACC CGC CAT GAA CAA CAA CT-3′) and S442 3′ (5′-TAG GGC TTT CAA AAT AAT AGG TAG-3′) positioned between nucleotides 62 and 442 in the S gene of strain Umeå/hu. The inner primers S160 5′ (5′-GCA AGC AAG GCA ACA GAC AGT-3′) and S347 3′ (5′-TGG CAT TCA CAT CAA GGA CAT T-3′) amplified a 187-bp fragment between nucleotides 160 and 347.
Real-time RT-PCR.
For preparation of total RNA, a QIAamp Viral RNA kit (QIAGEN) was used according to the manufacturer's instructions. The reverse transcription was performed at 42°C for 45 min in a total mixture of 25 μl, containing 12.5 μl eluted RNA, RT buffer (Invitrogen, Carlsbad, CA), 5 mM dithiothreitol, 500 μM deoxynucleoside triphosphates, 12 ng/μl pd(N)6 random hexamer (Amersham, Pharmacia, Biotech Inc.), 20 U rRNasin (Invitrogen), and 200 U Moloney murine leukemia virus (Invitrogen). The quantification was performed in triplets in a 96-well reaction plate using ABI Prism 7900HT Sequence Detection System 2.0 (Applied Biosystems,). The TaqMan assay was performed in a 25-μl final reaction volume containing TaqMan buffer A, 5 mM MgCl2, 200 μM dATP, 200 μM dGTP, 200 μM dCTP, 400 μM dUTP, 0.25 U AmpErase UNG, 1 U AmpliTaq Gold (Applied Biosystems), 1 μl cDNA, 900 nM of each primer, and 225 nM of the 6-carboxyfluorescein-labeled MGB probe. The real-time PCR was performed as follows: initiation for 2 min at 50°C and 10 min at 95°C, followed by 45 cycles at 95°C for 15 s and 60°C for 1 min.
Two-step RT-PCR.
The RNA preparation and reverse transcription were carried out as described above. The PCR was performed in a 50-μl reaction mixture consisting of 10 μl cDNA in the first PCR and 5 μl amplified product in the second PCR, 200 μM deoxynucleoside triphosphates (Amersham Biosciences), Taq buffer, 3.0 mM MgCl2, and 2 U Taq polymerase (Roche). The primer concentration in the first PCR was 0.2 μM, followed by 0.4 μM in the second nested PCR. Cycling conditions, performed on a DNA Engine thermal cycler (MJ Research, Waltham, MA), were 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 50°C for 45 s, and 72°C for 45 s, ending with a 5-min hold at 72°C. The PCR products were detected by electrophoresis in ethidium bromide-stained 2% agarose gels.
IF.
Vero E6 cells, cultured in Dulbecco's modified Eagle's medium (Sigma) supplemented with 5% fetal bovine serum (HyClone, Logan, Utah), were infected with the PUUV Umeå/hu strain, washed, and seeded on spot slides in an appropriate concentration. After the slides were dried overnight at room temperature, cold acetone was used for fixation, and the slides were then stored at −70°C. For IgG analysis, patient serum was added in stepwise dilutions in phosphate-buffered saline (PBS) to the slides and incubated at room temperature for 60 min. The slides were washed with PBS for 10 min and then rinsed carefully with distilled H2O three or four times. They were then incubated for 60 min at 37°C with fluorescein-conjugated rabbit anti-human IgG (F202; DAKO A/S, Glostrup, Denmark) diluted in PBS with Evans blue. After the slides were washed as described above, they were mounted and analyzed in a fluorescence microscope. For IgM analysis, patient serum was pretreated with Rf-absorbent (Virion\Serion GmbH, Würzburg, Germany) to eliminate possible interference of rheumatoid factor and PUUV-specific IgG. The slides with diluted samples were incubated overnight at 37°C. After the slides were washed as described above, fluorescein-conjugated rabbit F(ab′)2 anti-human IgM antibodies (F0317; DAKO A/S, Glostrup, Denmark) diluted in PBS with Evans blue were added and incubated for 60 min at 37°C. Washing, mounting, and analysis were performed as described above for IgG.
Statistics.
Sensitivity, specificity, positive and negative predictive values (PPV and NPV), and 95% confidence intervals were calculated with standard formulas using IgM antibody response analyzed by IF as the reference standard.
RESULTS
Specificity of real-time RT-PCR primers.
PUUV strains circulating in bank voles from Sweden belong to two distinct genetic lineages (11, 21) separated by a contact zone located south of Umeå in central Sweden (13, 30). For a reliable detection of PUUV RNA in NE patients from the region of endemicity in northern Sweden, it was important to design a real-time RT-PCR method able to detect multiple local PUUV isolates that might vary in their sequences. For this purpose, RNA was prepared from lung tissues of 11 different bank voles trapped within the northern Swedish county of Västerbotten. The real-time RT-PCR primers and probe were selected from a viral-RNA region with low sequence variability. The RNA was first reverse transcribed, amplified with primers from the S segment, and finally sequenced (14). An alignment of the sequences obtained, including the human isolate strain Umeå/hu from the same region (15), resulted in the design of the primers and probe described in Materials and Methods with no mismatches allowed.
Linear range, limit of detection, and precision study.
Threshold cycle (CT) values obtained from PUUV cDNA were plotted against the logarithmic concentrations of the serial dilutions. The assay showed linearity over a range from 20 to 2 × 106 read copies, and the correlation coefficient of the analysis was above 0.98 to 0.99 for all the experiments included in this study (Table 1). The reproducibility of the assay was established by measuring inter- and intra-assay variability on PUUV dilutions representing both synthetic plasmid RNA/cDNA and genomic RNA/cDNA. Samples of synthetic RNA representing 2,000,000 copies down to 20 copies and genomic RNA representing 20,000 to 2 copies were tested in triplicate in experiments done for 10 consecutive days. The mean coefficient of variation (CV) of CT values within the experiments (intra-assay) and between different experiments (interassay) were 0.12 to 1.45% and 0.43 to 1.59%, respectively (Table 1).
TABLE 1.
Average CT values for different real-time RT-PCR experiments using PUUV strain UME
| No. of copies | cDNAa | Avg CT value for expt no.:
|
Mean CV (%)c
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Intra-assay | Interassay | ||
| 2 × 106 | P | 18.93 | 19.02 | 19.32 | 19.22 | 19.19 | 19.30 | 19.33 | 18.99 | 19.00 | 19.34 | 0.21 | 0.85 |
| G | NDb | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2 × 105 | P | 22.27 | 22.49 | 23.04 | 22.84 | 22.52 | 22.94 | 22.58 | 22.33 | 22.29 | 22.94 | 0.25 | 1.30 |
| G | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2 × 104 | P | 25.64 | 25.76 | 26.35 | 25.99 | 25.74 | 26.10 | 25.91 | 25.50 | 25.55 | 26.09 | 0.20 | 1.05 |
| G | 24.93 | 25.10 | 25.18 | 25.26 | 25.26 | 25.26 | 25.20 | 25.08 | 24.84 | 25.04 | 0.12 | 0.58 | |
| 2 × 103 | P | 28.65 | 29.06 | 29.71 | 29.22 | 29.12 | 29.32 | 29.31 | 28.77 | 28.91 | 29.50 | 0.28 | 1.12 |
| G | 28.36 | 28.46 | 28.58 | 28.70 | 28.71 | 28.75 | 28.60 | 28.59 | 28.52 | 28.40 | 0.26 | 0.46 | |
| 2 × 102 | P | 32.08 | 32.22 | 33.16 | 32.82 | 32.42 | 32.49 | 32.68 | 32.17 | 32.16 | 32.91 | 0.42 | 1.13 |
| G | 31.88 | 31.84 | 31.80 | 31.95 | 32.27 | 32.04 | 32.10 | 32.03 | 32.00 | 32.06 | 0.37 | 0.43 | |
| 2 × 101 | P | 36.22 | 35.57 | 35.56 | 35.58 | 35.70 | 35.32 | 36.37 | 35.60 | 35.77 | 35.93 | 1.45 | 0.91 |
| G | 34.89 | 35.51 | 34.97 | 35.46 | 35.48 | 34.80 | 34.72 | 35.03 | 35.11 | 35.62 | 1.02 | 0.94 | |
| 2 × 100 | P | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| G | 38.66 | 37.43 | 38.75 | 0.00 | 37.92 | 37.66 | 0.00 | 37.21 | 38.35 | 0.00 | 1.22 | 1.59 | |
P, plasmid cDNA; G, genomic cDNA.
ND, not determined.
Consistency (intra-assay CV) and reproducibility (interassay CV) for the real-time RT-PCR method. The copy numbers were spectrophotometrically calculated for the template RNA used in the plasmid reactions but only approximated for the genomic PUUV RNA/cDNA.
Specificity of the PUUV real-time RT-PCR.
The viral specificity of the real-time RT-PCR was demonstrated by analyzing DNA and RNA viruses that could potentially be found in serum or plasma in humans. The viruses were tested at an approximate concentration equivalent to 103 to 104 viruses/PCR in normal human plasma. All of the following viruses gave negative PCR signals: cytomegalovirus, Epstein-Barr virus, varicella-zoster virus, human herpesvirus 6, adenovirus type 3, parvovirus B19, enterovirus (coxsackievirus B5), influenzavirus A, influenzavirus B, hepatitis B virus, and hepatitis C virus. The probe and the primers involved in both assays were also aligned with all sequences of the GenBank and EMBL databases using the PubMed NCBI BLAST program and showed no significant homology to any organism available except PUUV.
The quantitative one-step real-time RT-PCR compared to a qualitative two-step RT-PCR.
To evaluate our new real-time RT-PCR assay with patient samples, we used a laboratory-designed two-step RT-PCR used previously by us to detect PUUV RNA. We analyzed serum samples from 47 patients with clinical suspicion of NE by both techniques and found 100% concordance between the assays. Both assays detected 34 positive PUUV RNA samples and 13 negative samples (Table 2). One of the 34 samples (no. 11) was deemed weakly PUUV RNA positive by two-step RT-PCR but was clearly positive with the one-step real-time RT-PCR (Table 2).
TABLE 2.
Comparison between detection of PUUV IgM and IgG antibodies and PUUV RNA in serum samples from patients with suspected NE
| Sample no. | Day of sampling after disease outbreak | PUUV IgMa | PUUV IgG titer | PUUV RNAb | PUUV RNAc (copies/ml) |
|---|---|---|---|---|---|
| 1 | 5 | + | <40 | + | 107,125 |
| 2 | Unknown | + | 320 | + | 171 |
| 3 | 4 | + | 160 | + | 12,255 |
| 4 | 2 | + | 80 | + | 56,620 |
| 5 | 4 | + | >640 | + | 40,965 |
| 6 | 4 | + | >640 | + | 37,365 |
| 7 | 1 | + | >640 | + | 11,741 |
| 8 | 8 | + | 80 | + | 5,485 |
| 9 | 5 | + | 320 | + | 4,113 |
| 10 | Unknown | + | >640 | + | 1,543 |
| 11 | 5 | + | 40 | +/− | 3,599 |
| 12 | 7 | + | 160 | + | 74,645 |
| 13 | Unknown | + | 640 | + | 2,999 |
| 14 | 5 | + | <40 | + | 12,684 |
| 15 | 3 | + | 40 | + | 24,167 |
| 16 | 8 | + | 640 | + | 2,828 |
| 17 | 16 | + | 160 | + | 75,587 |
| 18 | 4 | + | 80 | + | 9,429 |
| 19 | Unknown | + | >640 | NDd | 1,371 |
| 20 | 3 | + | 80 | + | 177,485 |
| 21 | 7 | + | <40 | + | 70,274 |
| 22 | Unknown | + | 160 | + | 71,302 |
| 23 | Unknown | + | <40 | + | 2,914 |
| 24 | Unknown | + | >640 | + | 14,912 |
| 25 | 8 | + | 320 | + | 18,854 |
| 26 | 1 | + | <40 | + | 120,494 |
| 27 | 3 | + | 80 | + | 55,705 |
| 28 | 5 | + | 320 | ND | 18,425 |
| 29 | 7 | + | <40 | ND | 29,138 |
| 30 | Unknown | + | 320 | ND | 14,569 |
| 31 | Unknown | + | >640 | ND | 1,542 |
| 32 | 4 | + | 320 | + | 3,000,000 |
| 33 | 7 | + | <40 | ND | 450,000 |
| 34 | 8 | + | 320 | + | 54,000 |
| 35 | 6 | + | >640 | + | 950 |
| 36 | Unknown | + | 320 | + | 100,000 |
| 37 | 4 | + | >640 | + | 240,000 |
| 38 | 7 | + | >640 | - | 0 |
| 39 | Unknown | + | <40 | ND | 0 |
| 40 | Unknown | + | 160 | ND | 0 |
| 41 | Unknown | + | >640 | ND | 0 |
| 42 | 2 | + | >640 | − | 0 |
| 43 | Unknown | + | 320 | − | 0 |
| 44 | Unknown | + | 320 | − | 0 |
| 45 | Unknown | +/− | 40 | + | 244 |
| 46 | 4 | +/− | <40 | ND | 1,800,000 |
| 47 | Unknown | +/− | 160 | + | 74,000 |
| 48 | 17 | +/− | 320 | − | 0 |
| 49 | Unknown | +/− | 320 | − | 0 |
| 50 | 8 | +/− | >640 | − | 0 |
| 51 | 5 | +/− | <40 | − | 0 |
| 52 | 4 | − | <40 | + | 251,958 |
| 53-100 | 1-21 | − | <40 | ND | 0 |
IgM was diluted 1:16 and scored as negative (−), positive (+), or weakly positive (+/−) by IF.
Detected by RT-PCR. After two-step RT-PCR, the amplification product was detected by agarose gel electrophoresis and scored as negative (−) or positive (+).
Detected by real-time RT-PCR.
ND, not determined.
IgM antibody response compared to PUUV viremia detected by real-time RT-PCR.
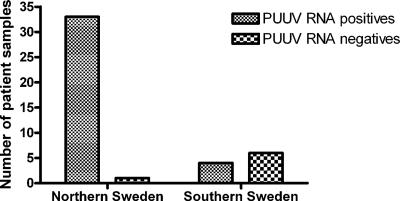
In total, we studied 100 serum samples from 100 patients with clinical suspicion of NE for PUUV antibodies and PUUV RNA. All were analyzed by IF in the routine clinical diagnostics for PUUV IgM, and 44 (44%) patients were clearly IgM positive, 7 were weakly positive, and 49 were negative (Table 2). All 100 samples were eligible for analysis by real-time RT-PCR, and 41 of these (41%) were positive for PUUV RNA (Table 2). When comparing IgM and PUUV RNA results, we found that of the 44 clearly positive IgM patients, 37 were PUUV RNA positive and 7 were negative (Table 2). When we studied the backgrounds of these seven PCR-negative patients, we observed that six of the patients were diagnosed in the southern part of Sweden and their samples had been sent to us for further analysis. The PCR primers and probes we used were specifically designed to detect PUUV sequences isolated from the region of endemicity in northern Sweden. We investigated the specificity of our assay by analyzing 10 IgM-positive NE patients from southern Sweden included in our material. Only 4 of the 10 (40%) patients from southern Sweden were PUUV RNA positive compared to 33 of 34 (97%) from northern Sweden, confirming the specificity of our primers (Fig. 1).
FIG. 1.
Detection of PUUV RNA by real-time RT-PCR in IgM-positive patient samples from different geographical locations.
Seven samples (no. 45 to 51) represented a select group of patients with a weak or diffuse positive response in IgM (Table 2). Three of the seven patients (43%) were confirmed by PCR to have ongoing PUUV infections. All three of these samples had relatively low levels of IgG (samples 45 to 47), indicating detection of PUUV RNA relatively early after infection (Table 2).
In the study, there were 49 patients from northern Sweden with clinical suspicion of NE but no IgM antibodies. In 48 of the 49 IgM-negative samples, there were no IgG antibodies and no PUUV RNA, indicating no PUUV infection. These patients had not received the correct clinical diagnosis and had a disease other than NE, and they could be regarded as negative controls. Interestingly, in one IgM/IgG-negative sample (no. 52), more than 250,000 copies/ml of PUUV RNA were detected in the patient serum (Table 2). This PUUV RNA-positive sample originated from a patient sampled only 4 days after a clinically diagnosed outbreak of NE, and 4 days later, the patient was clearly IgM positive (data not shown). The patient's spouse had NE, and the apprehensive patient visited the hospital as soon as the first weak symptoms appeared. Thus, this sample was collected very early after PUUV infection.
To summarize, all patients we studied were originally clinically diagnosed with suspected NE. At serological analysis, 49 patients from northern Sweden were IgM negative and were considered not to be infected with NE. The real-time RT-PCR confirmed 48 of these samples to be negative, resulting in a clinical specificity of 98% (confidence interval [CI], 93%, 99%), while the clinical sensitivity was 97% (CI, 90%, 99%) (Table 3). The PPV and NPV were 97% (CI, 90%, 99%) and 98% (CI, 93%, 99%), respectively (Table 3). The patient with the IgM-positive sample that was PCR negative had a high IgG response, and possibly the viremic phase had passed. Interestingly, one of the IgM-negative samples was PCR positive, and thus, we could diagnose this patient as suffering from NE by PCR alone, actually increasing the clinical sensitivity of the PCR assay compared to the IgM assay. Also, three of seven patients with indeterminate IgMs were positive for PUUV RNA, which further improved the clinical sensitivity.
TABLE 3.
Correlation between real-time RT-PCR and IgM for detection of PUUV infection in patients from northern Sweden clinically diagnosed with suspected NEa
| PCR result | No. IgM positive | No. IgM negative | Total |
|---|---|---|---|
| Positive | 33 | 1 | 34 |
| Negative | 1 | 48 | 49 |
| Total | 34 | 49 | 83 |
Using IgM as the reference, real-time RT-PCR had a clinical specificity of 98% (CI, 93%, 99%), a clinical sensitivity of 97% (CI, 90%, 99%), a PPV of 97% (CI, 90%, 99%), and an NPV of 98% (CI, 93%, 99%). The statistical analysis was calculated using standard formulas with 95% CI.
Kinetics of PUUV viremia in NE patients detected by real-time RT-PCR.
The pathogenesis of a PUUV infection involves the spread of virus through the blood, and we wanted to study PUUV viremia and antibody levels in patients with clinically diagnosed NE. Since this was a retrospective study and patients were not originally studied with the intent of following the kinetics of PUUV infection, we were fortunate that samples from six patients were accessible for analysis. The patients were followed after their first admittance to the hospital, and five of the patients showed a decrease of PUUV viremia within a few days after disease outbreak (Table 4). The levels of PUUV RNA viremia at admittance varied between patient samples from 1,800,000 copies/ml to 6,000 copies/ml (Table 4). For patient A, the level of virus RNA in serum was very high on the third day after disease outbreak and then dropped to low levels 4 days later. This decrease in PUUV viremia was coupled with an increase in IgM and IgG titers (Table 4). The same pattern of disappearance of PUUV viremia and increase of antibody levels was seen for patients B, C, E, and F independent of the actual virus RNA titer. These five patients all became healthy after their hospital stay. Patient D, who died, had a very high viremia, with 1,800,000 copies/ml of PUUV RNA in serum 4 days after disease outbreak. The virus level dropped to 450,000 copies/ml at day 7, but there was only a moderate increase in IgM and no IgG was detected (Table 4). The patient died 16 days after disease outbreak of a cerebral hemorrhage.
TABLE 4.
Kinetics of PUUV RNA and antibodies in sera of patients clinically diagnosed with suspected NE
| Patient/day after disease outbreak | PUUV RNA detected by real-time RT-PCR (copies/ml) | PUUV IgM titer | PUUV IgG titer |
|---|---|---|---|
| A/3 | 586,445 | 64 | 80 |
| A/5 | 25,281 | 128 | 320 |
| A/7 | 1,285 | 256 | NDb |
| B/3 | ND | +a | 640 |
| B/4 | 8,000 | 128 | 1,280 |
| B/5 | 2,000 | 128 | 2,560 |
| B/6 | 1,300 | 128 | 2,560 |
| B/7 | 2,000 | 128 | 2,560 |
| B/8 | 600 | 256 | 2,560 |
| B/9 | 0 | 265 | 2,560 |
| B/10 | 0 | 256 | 2,560 |
| C/3 | 196,000 | ND | ND |
| C/4 | 83,000 | 16 | 320 |
| C/5 | 11,200 | 64 | 640 |
| C/6 | 0 | 64 | 640 |
| D/4 | 1,800,000 | 8 | <40 |
| D/7 | 450,000 | 32 | <40 |
| E/3 | 6,000 | 8 | <40 |
| E/4 | 430 | 64 | 160 |
| E/5 | 40 | ND | ND |
| E/6 | 30 | 128 | 640 |
| E/7 | 30 | 256 | 1,280 |
| E/8 | 30 | 256 | 640 |
| F/3 | 57,000 | 16 | 40 |
| F/9 | 0 | 256 | 2560 |
IgM was diluted 1:16 and scored as positive (+) by IF.
ND, not determined.
DISCUSSION
In this study, we present the development of a real-time RT-PCR that accurately and specifically detected PUUV RNA from NE patients in northern Sweden. With this technique, we were able to determine and follow the levels of viremia in NE patients. When the antibody analysis resulted in a negative or an indeterminate IgM response, the detection of PUUV RNA was of great use. Specifically, we could show that PUUV RNA viremia appeared earlier than IgM antibodies and then gradually disappeared, coupled with an increase in IgM and IgG levels. Hence, the viremic phase declined, identifying a “narrow time window” for RT-PCR detection.
A hantavirus infection normally has an incubation time of approximately 2 to 4 weeks before the appearance of symptoms, accompanied by an immune response with high levels of hantavirus-specific antibodies (32). By using IF or enzyme-linked immunosorbent assay, antibodies are detected in patient serum, and an IgM response indicates recent exposure. The presence of antibodies does not give any information regarding possible PUUV viremia, and a rapidly disappearing IgM response occasionally occurs. Moreover, it has been suggested that a negative IgM result excludes hantavirus infection only at day 6 after disease outbreak (17). There is serological cross-reactivity between viruses from the genus Hantavirus and both the HPS-causing Sin Nombre virus and the HFRS-causing PUUV and Dobrava, Seoul, and Hantaan viruses (5, 6, 19). By using RT-PCR with specific primers, followed by sequencing, the exact infecting hantavirus type can be determined. Several reports on detection of PUUV RNA by two-step RT-PCR have been published (4, 12, 23, 24, 26, 35), but the quantification and kinetics of hantavirus viremia in patients by real-time RT-PCR have not previously been reported. Two reports have evaluated PUUV by real-time RT-PCR using plasmids and virus samples obtained by cell culture (2, 9). Both studies were performed with primers binding within the S segment of PUUV, a relatively conserved region that we also selected for our primers. By using PUUV RNA, either synthesized in vitro from a plasmid or from the PUUV genome, we showed that the real-time RT-PCR was sensitive, specific, and reliable. We previously developed a qualitative two-step RT-PCR in our laboratory, and we compared this method to our quantitative one-step real-time RT-PCR. For this comparison, we analyzed serum samples from patients with and without clinical suspicion of NE. All patient samples, PUUV positive or negative, yielded the same results with both methods. We concluded that the one-step real-time RT-PCR was favorable as a diagnostic tool for PUUV detection, since we received information regarding the copy number of PUUV RNA and had to perform only a one-step PCR.
The north of Sweden is an area of endemicity for NE, and we based our design of primers and probe for the real-time RT-PCR on PUUV RNA sequences isolated in Västerbotten County in northern Sweden. Since the mutation rate for PUUV is relatively high (21), we wanted to certify a high detection level of NE patients from this area. The genetic characteristics of local PUUVs are of a great importance for the diagnostics of NE. For real-time RT-PCR, the primer sequence is crucial, and great efforts have been made for genetic characterization of different local PUUV isolates of bank vole strains from northern Sweden (14). These sequences were used for the development of our PUUV real-time RT-PCR and were shown to correlate 100% with the two-step RT-PCR, with primers also selected from PUUV isolates from northern Sweden. When we analyzed IgM-positive patients with clinical suspicion of NE from this area, all but one (33/34) were positive for PUUV RNA. The PUUV RNA-negative patient had a high IgG response, and it is possible that there was no longer viremia in this patient. On the other hand, only 4 of 10 IgM-positive patients from southern Sweden were PUUV RNA positive, indicating variations in PUUV RNA sequences. It has been shown that PUUV strains circulating in bank voles from Sweden belong to two distinct genetic lineages (11, 21). A northern and a southern population of bank voles in Sweden can be distinguished by mitochondrial DNA sequences, and they are separated by a contact zone located south of Västerbotten County (13, 30). Distinct PUUV strains in Sweden are separated by the same contact zone; all patient samples from southern Sweden were collected south of this zone, and the patients had not traveled north of the contact zone prior to their illness. Our results indicated that the separate phylogenetic histories of the northern and southern PUUV strains (25) were the reason for the PCR-negative samples from southern Sweden. To certify this, other primers have to be used to amplify southern PUUV strains isolated from voles or patients, and then the resulting PCR products must be sequenced and compared with PUUV strains from voles and patients in northern Sweden.
The appearance of symptoms in NE patients is often accompanied by a significant immune response. However, the IgM response in early stages may be low (17), and real-time RT-PCR could be used to detect PUUV RNA at early time points, before the appearance of IgM antibodies, as well as yielding information regarding PUUV viremia and PUUV copy numbers. We detected a high number of PUUV RNA copies in serum from one IgM-negative patient with clinical suspicion of NE, and the patient was clearly IgM positive 4 days later. Furthermore, three of the patients with a weak or diffuse positive response in IgM were confirmed by PCR to have an ongoing PUUV infection. In Chile, the Andes virus, a species within the genus Hantavirus, was also detected by IF and RT-PCR before the appearance of IgM or IgG antibodies (8). The samples in the study from Chile were from cell cultures infected with serum taken previously from a patient who died from HPS (8).
We were interested in following a subset of NE patients for a longer period and in collecting information regarding both antibody levels and PUUV RNA viremia. In the NE patients, we found that an increase in antibody response was coupled with a decrease in PUUV viremia. Interestingly, for the patient samples that were collected on days 3 and 4 after disease outbreak, the PUUV RNA copy numbers varied between 6,000 copies/ml and 1,800,000 copies/ml. In four of the patients, the PUUV RNA levels dropped to zero or very low at 4 to 9 days after disease outbreak, while the IgM and IgG levels increased from low to high. In infections with Sin Nombre virus, from the genus Hantavirus, it was found by quantitative RT-PCR that HPS patients had high levels of viremia, correlating with the severity of the disease (31), and a recent publication supports these findings and suggests that the initial viral load could be used as a prognostic marker (34). Furthermore, there was a tendency for cases of HPS to be severe rather than moderate when viral RNA was bound to antibodies in immune complexes (32). However, the authors had no information regarding the neutralizing activities of the antibodies. In the six patients followed in our study, immune complex formation was not analyzed, but there was an increase in antibody titers in all patients except for IgG in patient D, where the infection had a fatal outcome. For this patient, the IgM levels increased, but not to high levels, and the PUUV RNA viremia was still 450,000 copies/ml 7 days after disease outbreak.
Our results indicate that real-time RT-PCR is an efficient, specific, and sensitive method for clinical diagnosis of PUUV viremia and for detecting PUUV RNA at early time points, before the appearance of IgM antibodies. The PUUV real-time RT-PCR, combined with immunological techniques, will also be useful for studies of hantavirus pathogenesis and the effects of antiviral treatment.
Acknowledgments
This work was supported by grants from the Swedish Society of Medicine, the County Council of Västerbotten, and the Medical Faculty of Umeå University.
Patrik Johansson and Ann-Christin Verlemyr are greatly acknowledged for their contributions during the initial stages of this study.
Footnotes
Published ahead of print on 30 May 2007.
REFERENCES
- 1.Ahlm, C., P. Juto, B. Stegmayr, B. Settergren, G. Wadell, A. Tarnvik, and F. Elgh. 1997. Prevalence of serum antibodies to hantaviruses in northern Sweden as measured by recombinant nucleocapsid proteins. Scand. J. Infect. Dis. 29:349-354. [DOI] [PubMed] [Google Scholar]
- 2.Aitichou, M., S. S. Saleh, A. K. McElroy, C. Schmaljohn, and M. S. Ibrahim. 2005. Identification of Dobrava, Hantaan, Seoul, and Puumala viruses by one-step real-time RT-PCR. J. Virol. Methods 124:21-26. [DOI] [PubMed] [Google Scholar]
- 3.Chu, Y. K., C. Rossi, J. W. Leduc, H. W. Lee, C. S. Schmaljohn, and J. M. Dalrymple. 1994. Serological relationships among viruses in the Hantavirus genus, family Bunyaviridae. Virology 198:196-204. [DOI] [PubMed] [Google Scholar]
- 4.Dekonenko, A., M. S. Ibrahim, and C. S. Schmaljohn. 1997. A colorimetric PCR-enzyme immunoassay to identify hantaviruses. Clin. Diagn. Virol. 8:113-121. [DOI] [PubMed] [Google Scholar]
- 5.Elgh, F., M. Linderholm, G. Wadell, A. Tärnvik, and P. Juto. 1998. Development of humoral cross-reactivity to the nucleocapsid protein of heterologous hantaviruses in nephropathia epidemica. FEMS Immunol. Med. Microbiol. 22:309-315. [DOI] [PubMed] [Google Scholar]
- 6.Elgh, F., A. Lundkvist, O. A. Alexeyev, H. Stenlund, T. Avsic-Zupanc, B. Hjelle, H. W. Lee, K. J. Smith, R. Vainionpaa, D. Wiger, G. Wadell, and P. Juto. 1997. Serological diagnosis of hantavirus infections by an enzyme-linked immunosorbent assay based on detection of immunoglobulin G and M responses to recombinant nucleocapsid proteins of five viral serotypes. J. Clin. Microbiol. 35:1122-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forslund, T., J. Saltevo, J. Anttinen, S. Auvinen, M. Brummer-Korvenkontio, A. Korhonen, and M. Poutiainen. 1992. Complications of nephropathia epidemica: three cases. J. Intern. Med. 232:87-90. [DOI] [PubMed] [Google Scholar]
- 8.Galeno, H., J. Mora, E. Villagra, J. Fernandez, J. Hernandez, G. J. Mertz, and E. Ramirez. 2002. First human isolate of Hantavirus (Andes virus) in the Americas. Emerg. Infect. Dis. 8:657-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garin, D., C. Peyrefitte, J. M. Crance, A. Le Faou, A. Jouan, and M. Bouloy. 2001. Highly sensitive Taqman PCR detection of Puumala hantavirus. Microbes Infect. 3:739-745. [DOI] [PubMed] [Google Scholar]
- 10.Hörling, J., A. Lundkvist, J. W. Huggins, and B. Niklasson. 1992. Antibodies to Puumala virus in humans determined by neutralization test. J. Virol. Methods 39:139-147. [DOI] [PubMed] [Google Scholar]
- 11.Hörling, J., A. Lundkvist, M. Jaarola, A. Plyusnin, H. Tegelstrom, K. Persson, H. Lehvaslaiho, B. Hornfeldt, A. Vaheri, and B. Niklasson. 1996. Distribution and genetic heterogeneity of Puumala virus in Sweden. J. Gen. Virol. 77:2555-2562. [DOI] [PubMed] [Google Scholar]
- 12.Hörling, J., A. Lundkvist, K. Persson, M. Mullaart, T. Dzagurova, A. Dekonenko, E. Tkachenko, and B. Niklasson. 1995. Detection and subsequent sequencing of Puumala virus from human specimens by PCR. J. Clin. Microbiol. 33:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaarola, M., H. Tegelström, and K. Fredga. 1999. Colonization history in Fennoscandian rodents. Molecular genetics in animal ecology. Biol. J. Linnean Soc. 68:113-127. [Google Scholar]
- 14.Johansson, P. 2005. Implications of local puumala hantavirus genetics and epidemiology for diagnostics and vaccine development. Ph.D. thesis. Umeå University, Umeå, Sweden.
- 15.Johansson, P., M. Olsson, L. Lindgren, C. Ahlm, F. Elgh, A. Holmstrom, and G. Bucht. 2004. Complete gene sequence of a human Puumala hantavirus isolate, Puumala Umea/hu: sequence comparison and characterisation of encoded gene products. Virus Res. 105:147-155. [DOI] [PubMed] [Google Scholar]
- 16.Juto, P., F. Elgh, C. Ahlm, O. A. Alexeyev, K. Edlund, A. Lundkvist, and G. Wadell. 1997. The first human isolate of Puumala virus in Scandinavia as cultured from phytohemagglutinin stimulated leucocytes. J. Med. Virol. 53:150-156. [DOI] [PubMed] [Google Scholar]
- 17.Kallio-Kokko, H., O. Vapalahti, A. Lundkvist, and A. Vaheri. 1998. Evaluation of Puumala virus IgG and IgM enzyme immunoassays based on recombinant baculovirus-expressed nucleocapsid protein for early nephropathia epidemica diagnosis. Clin. Diagn. Virol. 10:83-90. [DOI] [PubMed] [Google Scholar]
- 18.Linderholm, M., B. Settergren, C. Ahlm, L. A. Burman, S. Traff, U. Backlund, and P. Juto. 1991. A Swedish fatal case of nephropathia epidemica. Scand. J. Infect. Dis. 23:501-502. [DOI] [PubMed] [Google Scholar]
- 19.Lundkvist, Å., M. Hukic, J. Horling, M. Gilljam, S. Nichol, and B. Niklasson. 1997. Puumala and Dobrava viruses cause hemorrhagic fever with renal syndrome in Bosnia-Herzegovina: evidence of highly cross-neutralizing antibody responses in early patient sera. J. Med. Virol. 53:51-59. [PubMed] [Google Scholar]
- 20.Lundkvist, Å., and A. Plyusnin. 2002. Molecular epidemiology of hantavirus infections. Kluwer Academic Publishers, Norwell, MA.
- 21.Lundkvist, Å., D. Wiger, J. Horling, K. B. Sjolander, A. Plyusnina, R. Mehl, A. Vaheri, and A. Plyusnin. 1998. Isolation and characterization of Puumala hantavirus from Norway: evidence for a distinct phylogenetic sublineage. J. Gen. Virol. 79:2603-2614. [DOI] [PubMed] [Google Scholar]
- 22.Olsson, G. E., F. Dalerum, B. Hornfeldt, F. Elgh, T. R. Palo, P. Juto, and C. Ahlm. 2003. Human hantavirus infections, Sweden. Emerg. Infect. Dis. 9:1395-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plyusnin, A., J. Horling, M. Kanerva, J. Mustonen, Y. Cheng, J. Partanen, O. Vapalahti, S. K. Kukkonen, J. Niemimaa, H. Henttonen, B. Niklasson, A. Lundkvist, and A. Vaheri. 1997. Puumala hantavirus genome in patients with nephropathia epidemica: correlation of PCR positivity with HLA haplotype and link to viral sequences in local rodents. J. Clin. Microbiol. 35:1090-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plyusnin, A., J. Mustonen, K. Asikainen, A. Plyusnina, J. Niemimaa, H. Henttonen, and A. Vaheri. 1999. Analysis of puumala hantavirus genome in patients with nephropathia epidemica and rodent carriers from the sites of infection. J. Med. Virol. 59:397-405. [DOI] [PubMed] [Google Scholar]
- 25.Plyusnin, A., and S. P. Morzunov. 2001. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. Curr. Top. Microbiol. Immunology. 256:47-75. [DOI] [PubMed] [Google Scholar]
- 26.Puthavathana, P., H. W. Lee, and C. Y. Kang. 1992. Typing of Hantaviruses from five continents by polymerase chain reaction. Virus Res. 26:1-14. [DOI] [PubMed] [Google Scholar]
- 27.Schmaljohn, C., and B. Hjelle. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmaljohn, C. S., S. E. Hasty, S. A. Harrison, and J. M. Dalrymple. 1983. Characterization of Hantaan virions, the prototype virus of hemorrhagic fever with renal syndrome. J. Infect. Dis. 148:1005-1012. [DOI] [PubMed] [Google Scholar]
- 29.Tanishita, O., Y. Takahashi, Y. Okuno, K. Yamanishi, and M. Takahashi. 1984. Evaluation of focus reduction neutralization test with peroxidase-antiperoxidase staining technique for hemorrhagic fever with renal syndrome virus. J. Clin. Microbiol. 20:1213-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tegelstrom, H., P. I. Wyoni, H. Gelter, and M. Jaarola. 1988. Concordant divergence in proteins and mitochondrial DNA between two vole species in the genus Clethrionomys. Biochem. Genet. 26:223-237. [DOI] [PubMed] [Google Scholar]
- 31.Terajima, M., J. D. Hendershot III, H. Kariwa, F. T. Koster, B. Hjelle, D. Goade, M. C. DeFronzo, and F. A. Ennis. 1999. High levels of viremia in patients with the Hantavirus pulmonary syndrome. J. Infect. Dis. 180:2030-2034. [DOI] [PubMed] [Google Scholar]
- 32.Terajima, M., O. Vapalahti, H. L. Van Epps, A. Vaheri, and F. A. Ennis. 2004. Immune responses to Puumala virus infection and the pathogenesis of nephropathia epidemica. Microbes Infect. 6:238-245. [DOI] [PubMed] [Google Scholar]
- 33.Valtonen, M., M. Kauppila, P. Kotilainen, J. Lahdevirta, C. M. Svartback, O. Kosunen, J. Nurminen, H. Sarkkinen, and M. Brummer-Korvenkontio. 1995. Four fatal cases of nephropathia epidemica. Scand. J. Infect. Dis. 27:515-517. [DOI] [PubMed] [Google Scholar]
- 34.Xiao, R., S. Yang, F. Koster, C. Ye, C. Stidley, and B. Hjelle. 2006. Sin Nombre viral RNA load in patients with hantavirus cardiopulmonary syndrome. J. Infect. Dis. 194:1403-1409. [DOI] [PubMed] [Google Scholar]
- 35.Xiao, S. Y., R. Yanagihara, M. S. Godec, Z. A. Eldadah, B. K. Johnson, D. C. Gajdusek, and D. M. Asher. 1991. Detection of hantavirus RNA in tissues of experimentally infected mice using reverse transcriptase-directed polymerase chain reaction. J. Med. Virol. 33:277-282. [DOI] [PubMed] [Google Scholar]