Abstract
Latent membrane protein 1 (LMP1) of Epstein Barr virus (EBV) is important for maintaining proliferation of EBV-infected B cells. LMP1, unlike its cellular counterpart, CD40, signals without a ligand and is largely internal to the plasma membrane. In order to understand how LMP1 initiates its ligand-independent signaling, we focused on a leucine heptad in LMP1's first membrane-spanning domain that was shown to be necessary for LMP1's signaling through NF-κB. LZ1EBV, a recombinant EBV genetically altered to express LZ1, a derivative of LMP1 in which a leucine heptad was replaced with alanines, transformed B cells with 56% of wild-type (wt) EBV's efficiency, demonstrating the importance of this heptad. To elucidate the mechanism by which this domain contributes to the functions of LMP1, the properties of the wt and LZ1 were compared in transfected cells. LZ1 failed to home to lipid rafts as efficiently as did wt LMP1. The distribution of tagged derivatives of LZ1 also differed from that of wt LMP1 in transfected cells. LZ1's defect in homing to lipid rafts and altered trafficking likely underlie the defect in transformation of LZ1EBV. While the third and fourth membrane-spanning domains of LMP1 foster its trafficking to the Golgi, the leucine heptad within the first membrane-spanning domain contributes to its trafficking, particularly to internal rafts. B cells that are successfully transformed by LZ1EBV have the same average number of viral genomes and the same fraction of cells with capped LZ1 at the cell surface but express 50% more of the LZ1 allele than wt infected cells.
Epstein Barr virus (EBV), a human gammaherpesvirus, causes infectious mononucleosis and contributes to many malignancies, including Burkitt's lymphoma, Hodgkin's lymphoma, and nasopharyngeal carcinoma (8, 41). EBV can infect human resting B lymphocytes in vitro and induce their proliferation, which we term here “transformation”; a fraction of these cells can yield lymphoblastic cell lines (LCLs). In these LCLs, the EBV genome is maintained as a plasmid that replicates once per cell cycle, expresses only a subset of its ∼100 genes, and does not produce virus, a state referred to as latency (32). One viral gene, encoding latent membrane protein 1 (LMP1), is expressed during latency in EBV-transformed LCLs and has been shown to be required to maintain proliferation of these infected cells (11, 20). The LMP1 protein has been detected in tumor cells of EBV-associated malignancies, including Hodgkin's lymphoma and a fraction of nasopharyngeal carcinoma (8, 35).
LMP1 is a 386-amino-acid (aa) transmembrane protein with a 25-aa intracellular amino terminus, followed by six membrane-spanning domains (160 aa) and an approximately 200-aa intracellular carboxyl-terminal domain that engages cellular signaling molecules. LMP1 is a viral mimic of CD40, a glycoprotein belonging to the tumor necrosis factor receptor (TNFR) family (for a review, see reference 25). LMP1 activates several signaling pathways, including NF-κB, AP-1, and janus-activated kinase (JAK)-STAT, by binding to TNFR-associated factors (TRAF) and/or tumor necrosis factor receptor-associated death domain-containing protein (TRADD) and JAK3 through its C terminus (1, 10, 14, 15, 17, 19, 23, 33). Genetic studies indicate that LMP1 signaling is essential for maintaining B-cell proliferation in culture (11, 12, 24).
LMP1's targeting to lipid rafts (cholesterol- and spingolipid-rich microdomains also known as detergent-resistant membranes) is important for its signaling. The nature of lipid rafts in live cells, though generally accepted, is still debated (for a review, see reference 30). Lipid rafts can be isolated by their insolubility in Triton X-100 at 0°C and their buoyant density in sucrose or other gradients (2, 13). LMP1 is found in lipid rafts, as defined by their fractionation with detergent-resistant membranes and colocalization with known lipid raft-associated signaling proteins (3, 4, 16, 21). LMP1 also recruits TRAF3 and TRAF2, major downstream effectors of NF-κB signaling, to lipid rafts (16, 21, 26). Both LMP1 and CD40 (when CD40L is present) colocalize with lipid raft markers, such as the B subunit of cholera toxin, which binds to GM1 (21, 26). Posttranslational modifications of proteins with myristoylation or palmitoylation help them to be recruited to lipid rafts (29). LMP1 is palmitoylated at residue cysteine 78 in its third membrane-spanning domain, but mutating that residue to prevent palmitoylation affects neither its association with lipid rafts nor its signaling (16).
Multiple observations support a pivotal role for LMP1's membrane-spanning domains in its signaling. Unlike CD40, LMP1 signals constitutively, i.e., in the absence of a ligand. Interestingly, when the extracellular and transmembrane domains of CD40 were replaced by the LMP1 amino terminus and transmembrane domains, the chimera signaled more efficiently than wild-type (wt) CD40, indicating that a sequence allowing efficient signaling is present in these domains (21). LMP1 signaling requires its oligomerization and targeting to lipid rafts, and both of these properties are mediated through LMP1's transmembrane domains (16, 21, 23). A derivative of EBV with an LMP1 gene lacking the entire transmembrane region transformed B cells with less than 1% of the efficiency of the wt (12). Additionally, the amino terminus and transmembrane domains of LMP1 have been reported to be important for LMP1's ability to induce cytostasis when expressed at high physiological levels (24). The amino terminus is important for LMP1's correct insertion in the membrane, and this orientation is thought to be mediated by the positive charges in LMP1's cytosolic regions (5). Recently, it was found that prenylated Rab acceptor 1 (PRA1) interacts with LMP1 through its third and fourth membrane-spanning domains and affects its trafficking to the Golgi and its signaling through NF-κB (28).
In a search for activities encoded by domains in the transmembrane regions, we found that LMP1's first and sixth membrane-spanning domains have multiple leucine residues potentially similar to a leucine heptad (22). Studies of a heptad of leucine residues in an artificial protein have indicated that this motif can form a structure similar to the leucine zippers found in cytosolic proteins that mediate protein-protein interaction (15a). Replacement of leucines 29, 30, 32, 33, 36, 37, and 40 with alanines in the first (LZ1 [a derivative of LMP1 in which a leucine heptad was replaced with alanines]) but not in the sixth membrane-spanning domain of LMP1 resulted in inefficient LMP1 signaling, indicating their functional importance (22). The importance of these leucines is consistent with the finding that transmembrane domains 1 and 2 are sufficient for LMP1's lipid raft targeting and induction of cytostasis but not sufficient for oligomerization (6). In addition, the aromatic residues in the FWLY motif of the first transmembrane domain of LMP1 are also important for LMP1's localization to rafts and signaling (37, 40), supporting the idea that the first membrane-spanning domain is important for LMP1's trafficking and signaling.
In order to understand the mechanism underlying LZ1's inefficient signaling, we studied LZ1 trafficking based on previous findings. In this study, we demonstrate that LZ1 has a defect in homing to rafts, leading to a defect in signaling through NF-κB. Moreover, when LZ1 was expressed with other viral genes in a recombinant virus, the virus had defects in induction and maintenance of the proliferation of primary B cells or transformation. Thus, the leucine heptad is important for LMP1 trafficking and thus for its signaling.
MATERIALS AND METHODS
Cell culture and plasmids.
721 is an EBV-positive lymphoblastic cell clone (18). 293 cells are derived from human embryonic kidney cells and are likely of neural origin (36). 721 cells and B-cell clones were grown in RPMI 1640 supplemented with 10% fetal bovine serum. 293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. All cell culture media were supplemented with 200 U/ml penicillin and 200 μg/ml streptomycin, and all cells were grown at 37°C in a humidified 7% CO2 atmosphere.
The expression plasmid for LMP1 was generated by cloning the cDNA of LMP1 from the B95-8 strain of EBV into the pSG5 vector (p1990). The plasmid encoding LZ1 (p2492) encodes a mutation replacing leucines 29, 30, 32, 33, 36, 37, and 40 with alanines in the first membrane-spanning domain of LMP1 (22). LMPΔC-GFP(p2230) is a derivative of LMP-1 that substitutes enhanced green fluorescent protein (GFP) for the C terminus of LMP1 (aa 190 to 386). Full-length LMP1-GFP(p3228), LMP1-RFP(p3440), LZ1-GFP(p3494), and LZ1-RFP(p3493) had either enhanced GFP (Clontech) or monomeric red fluorescent protein (mRFP) (3a) fused at the C terminus of LMP1 or LZ1. The derivatives of LMP1 and LZ1 that express only the first two membrane-spanning domains were generated by deleting the sequence between the BclI and BsaBI sites in the membrane-spanning domains of LMP1 [wt LMP1 1-2TM GFP(p3462); wt LMP1 1-2TM RFP(p3463); LZ1 1-2TM RFP(p3464); LZ1 1-2TM GFP(p3477)]. Δ25-132 is an expression plasmid of LMP1 that has residues 25 to 132 of LMP1 deleted (p540). The FWLY plasmid (kindly provided by E. Keiff) encodes a substitution mutation changing the FWLY motif to AALA in the first membrane-spanning domain of LMP1 (40).
NF-κB reporter assay.
A total of 4 × 106 293 cells were transfected with 0 to 5 μg of plasmids encoding LMPΔC-GFP and/or LZ1 and 100 ng of an NF-κB luciferase reporter that contained four copies of an NF-κB-responsive element upstream of luciferase (p1242), using Lipofectamine (Invitrogen). All transfections were equalized with carrier DNA. The cells were incubated for 48 h and split into two tubes. One half was lysed in passive lysis buffer (Promega) and assayed for light emission on a monolight 3010 luminometer, and the other half was used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and quantitative Western blots. Relative light units were normalized to the measured levels of LZ1.
Immunofluorescent staining.
A total of 4 × 105 293 cells were transfected with 0.5 to 2 μg of expression plasmids encoding derivatives of LMP1 or LZ1 in a six-well plate, using Lipofectamine (Invitrogen). The subcellular localizations of these derivatives were examined 24 to 48 h after transfection. B-cell clones infected with either wt EBV or LZ1EBV (a recombinant EBV genetically altered to express LZ1) were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS), and washed twice with PBS plus 5% calf serum. The cells were stained with affinity-purified rabbit anti-LMP1 at 1:500 for 1 h and then incubated with Texas red-conjugated goat anti-rabbit antibodies (Molecular Probes) at 1:1,000 for 1 h. The cells were washed three times with PBS plus 5% calf serum after being stained with both primary and secondary antibodies.
Images were acquired with an inverted fluorescence microscope (Axiovert 200M; Zeiss) that was equipped with a digital charge-coupled device camera (Axiocam HRm; Zeiss). GFP and mRFP were visualized using specific individual filter sets for fluorescein isothiocyanate and Texas red. Images were collected with a 63×, 1.4 natural aperture oil objective lens (Plan Prochromaro; Zeiss) with 5 to 10 slices of Z stacks. Axiovision software (Zeiss) was used for acquisition and computation of the images.
Flotation assay.
Transiently transfected 293 cells (3 × 107) or B-cell clones (1 × 107 to 3 × 107) were lysed at 0°C in 1 ml of 1% Triton X-100 in MNE buffer (25 mM MES [morpholineethanesulfonic acid], pH 6.5, 150 mM NaCl, 5 mM EDTA) containing a mixture of aprotinin, leupeptin, bestatin, pepstatin A, E-64, and AEBSF (protease inhibitor cocktail; Sigma) for 30 min to 1 h. Cell lysates were subjected to 10 strokes of a tight-fitting Dounce homogenizer and spun at 300 × g, and the supernatant was mixed with 1 ml of 80% sucrose in MNE buffer. The lysate was transferred to a centrifuge tube and overlaid with 0.5 ml of 35%, 30%, 20%, 10%, and 5% (each) sucrose in MNE buffer. After centrifugation for 16 h at 200,000 × g in an SW50 rotor, 11 0.5-ml fractions were collected from the top of the gradient. Cell lysates equivalent to 3 × 104 cells from each fraction were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and probed with the indicated antibodies or subjected to dot blot analysis.
SDS-PAGE, Western blotting, and dot blot analysis.
Cell lysates were separated on 9% or 12% denaturing polyacrylamide gels and transferred electrophoretically to nitrocellulose membranes. The blots were blocked with 5% nonfat milk and probed with appropriate primary antibodies, followed by incubation with secondary antibodies. Primary antibodies were used at the following dilutions: affinity-purified rabbit anti-LMP1 antibodies, 1:500; mouse anti-alpha-tubulin (Sigma), 1:10,000; rat monoclonal anti-EBNA1 IH4-1, 1:50; mouse anti-human transferrin receptor (ZYMED), 1:1,000; mouse anti-EBNA2 (Novocastra; NCL-EBV-PE2), 1:100; rat anti-LMP2A, 1:500 (kindly provided by R. Longnecker); and rabbit anti-IκB, 1:1,000 (Sigma). Alkaline phosphatase-conjugated goat anti-rabbit, alkaline phosphatase-conjugated donkey anti-mouse (Jackson Immunoresearch), or alkaline phosphatase-conjugated anti-rat (Promega) antibody was used at 1:1,000 dilution as a secondary antibody. GM1 in each fraction was probed with horseradish peroxidase-conjugated cholera toxin B subunit (CTxB) (Sigma) at 1:5,000 and detected by enhanced chemi-luminescence. All of the signals were determined by dilution to be in a linear range. The signals were quantified using ImageQuant software.
Recombinant maxi-EBV plasmid.
The complete genome of the B95-8 strain of EBV has been cloned into a mini-F plasmid, allowing facile recombination with EBV (9). This plasmid, which encodes GFP, hygromycin phosphotransferase, and chloramphenicol acetyltransferase for selection in eukaryotic cells and Escherichia coli, is named maxi-EBV (9). To introduce recombinant LZ1 into the EBV genome, a shuttle vector encoding LZ1 with approximately 5 kbp of flanking homologous regions on each side was linearized and electroporated into DH10B cells harboring maxi-EBV. This shuttle vector allows expression of the LZ1 protein under the control of the LMP1 wt promoter and a kanamycin selection marker between the BALF1 gene and the promoter of LMP2A when recombined into maxi-EBV (12). DH10B cells were made competent for recombination by introducing a plasmid that carries the red recombination machinery of lambda under an arabinose promoter. The introduced plasmid also has a temperature-sensitive origin, which supports replication at 30°C but not at 42°C. After the linear fragment was introduced, the bacteria were grown at 30°C to allow recombination for 2 hours, and then the cells were plated on a plate with terrific broth plus chloramphenicol-kanamycin (30 μg/ml each) and incubated at 42°C overnight. Colonies were picked from the selection plate, diluted in 50 μl Tris-EDTA, and vortexed, and 5 μl of this suspension was used for PCR screening for the recombinants. Three clones were chosen based on the PCR results. Isolated DNAs from these three clones were amplified and subjected to another screening with PCR using different primer sets specific for the sequence of either wt LMP1 or LZ1. The transmembrane region of LMP1 was amplified by PCR and sequenced to ensure that the region of interest was correct for each recombinant used.
Production of virus and titering.
Both wt maxi-EBV and three independent clones of LZ1EBV plasmids were used to establish virus-producing 293 cell lines as described previously (12). 293 cell clones were transfected with expression plasmids for BZLF1 and BARF4 to induce the lytic cycle. Three days posttransfection, the supernatants were collected and filtered through 0.8-μm-pore-size filters. Raji cells (1 × 105) were infected with dilutions of virus stocks, incubated at 37°C in 24-well plates, and cultured for 3 days to allow expression of GFP. Twenty-four hours after infection, the medium was changed; 48 h postinfection, both tetradecanoyl phorbol acetate and sodium butyrate were added to the cells at final concentrations of 20 ng/ml and 3 mM, respectively; and 72 h postinfection, the percentage of GFP-positive cells was determined by UV microscopy. On the basis of these data, “green Raji units” (GRU) per ml were calculated as a measure of the concentration of infectious maxi-EBV particles in different virus stocks using the following equation: GRU/ml = percentage of GFP-positive Raji cells × number of Raji cells infected (105) × dilution factor.
Proliferation assay.
Primary human B lymphocytes were isolated from freshly drawn human blood by magnetic sorting using the Human B Cell Isolation Kit II (Milteny Biotec). Primary B cells (1 × 104) were exposed to a total of 3 or 10 GRU of virus stock. These 1 × 104 infected B cells were plated in each well of a 96-well plate and monitored for proliferation. After 4 to 6 weeks, the percentage of wells with proliferating cell clones was determined by the Poisson distribution. Clones were picked randomly and propagated for up to 3 months for further analysis.
FISH.
Fluorescent in situ hybridization (FISH) analysis was performed based on the protocol of Lawrence et al. (27) with some modifications. Briefly, 721 cells and B-cell clones were treated with 0.075 M KCl for 20 min at 37°C, fixed in methanol-acetic acid (3:1) for 30 min at room temperature, and spread on frozen slides. The slides were treated with 4× SSC (1 × SSC is 0.15 M NaCl, 0.015 M sodium citrate) containing 0.5% (vol/vol) Nonidet P-40 (Sigma) for 30 min at 37°C, dehydrated in a cold ethanol series (70, 80, and 90%) for 2 min each, air dried, and denatured in 70% formamide-2× SSC for 2 min at 72°C. The slides were dehydrated in a cold ethanol series and air dried. Hybridization probes for detection of EBV plasmids were generated by nick translation using biotin 11-dUTP (Roche). Twenty micrograms of probe was precipitated by ethanol in the presence of 6 μg salmon sperm DNA (Perkin Elmer) and 4 μg human Cot-1 DNA (Invitrogen), resuspended in CEP hybridization buffer (Vysis), and incubated for 10 min at 70°C, for 5 min at 4°C, and for 1 h at 37°C. A hybridization mixture containing 5 ng of probe was placed on each sample and incubated overnight at 37°C in a moist chamber. The slides were washed in 2× SSC containing 50% formamide for 30 min at 50°C and in 2× SSC for 30 min at 50°C. The hybridized probe was detected by incubation with 30 μl detection solution containing streptavidin conjugated to Cy3 (Cytocell) for 20 min at 37°C. The slides were washed twice in 4× SSC containing 0.1% Triton X-100 (Sigma) for 5 min at room temperature. The chromosomes were counterstained with a mounting medium containing DAPI (4′,6′-diamidino-2-phenylindole) (Vector). Images were acquired with an inverted fluorescence microscope (Axiovert 200H; Zeiss) that was equipped with a digital charge-coupled device camera (Axiocam HRm; Zeiss) and a Z motor. Cy3 and DAPI were visualized using specific individual filter sets for Texas red and DAPI. Images were collected with a 63×, 1.4 natural aperture oil objective lens (Plan Prochromaro; Zeiss) with 5 to 10 slices of Z stacks by exposure for 0.01 s or 1 s to detect Cy3 and DAPI individually. Axiovision software (Zeiss) was used for acquisition and computation of the images.
RESULTS
LZ1EBV is inefficient in the induction and maintenance of primary B cells.
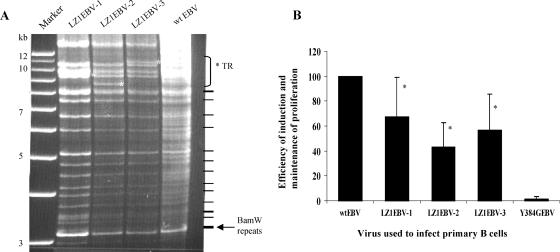
Infection of primary B cells with EBV leads to their proliferation (20, 24), and genetic analyses have shown that LMP1 must signal in order to maintain proliferation of these infected cells (11). Derivatives of LMP1 that have a substitution mutation in the leucine heptad in the first (SubLZ1LMP1, here named LZ1) or both the first and the sixth membrane-spanning domains (SubLZLMP1) could signal at only 3% of the wt LMP1 level through NF-κB on a per-molecule basis in 293 cells and BJAB cells (22). We hypothesized that LZ1 would be defective in its support of the proliferation of B cells in the presence of the other EBV genes normally expressed. We used the maxi-EBV system to generate recombinant EBVs encoding LZ1 instead of LMP1 (9). The recombinants were screened by PCR using primer sets specific to LZ1, and three independent clones were chosen for further study (Fig. 1B). Furthermore, the transmembrane region of LMP1 was amplified by PCR and sequenced to ensure the region of interest was correct (data not shown). We digested the recombinant DNAs with two different restriction enzymes to confirm that the band patterns were similar to those of the parental maxi-EBV (termed wt EBV below) to make sure other repeated elements in the EBV genome were not altered by recombination. For each clone, 10 μg of DNA was digested with either BamHI (Fig. 1A) or BglII (data not shown), and the fragments were separated on a 0.6% agarose gel and stained with ethidium bromide. These digestion patterns showed that throughout the entire EBV genome, no difference from the parental EBV plasmid was detected, with the exception of different numbers of terminal repeats and different-size fragments generated by recombination. The numbers of terminal repeats were confirmed by Southern blot analysis (data not shown). Furthermore, all three clones had numbers of BamHI W repeats similar to that of the parental EBV construct as measured by Southern blot analysis (data not shown), ensuring that the efficiency of inducing and/or maintaining proliferation of B cells was not affected by the numbers of repeats in the picked clones.
FIG. 1.
LZ1EBV is inefficient in the induction and/or maintenance of proliferation of primary B cells. (A) Restriction enzyme analysis of three LZ1EBV(+) clones. For each clone, plasmids were isolated from E. coli after amplification using a modified protocol for low-copy-number plasmids. For each clone, 10 μg of DNA was digested with BamHI, and the fragments were separated on a 0.6% agarose gel and stained with ethidium bromide. Lane 1 (from left), 1-kb-plus ladder; lane 2, LZ1EBV clone no. 1; lane 3, LZ1EBV clone no. 2; lane 4, LZ1EBV clone no. 3; lane 5, wt EBV. A new band appeared above the 12-kb marker in all three clones after recombination. The digestion pattern, other than the 13-kb band, was similar to that of maxi-EBV (wt EBV), indicating that there is no obvious difference in other regions of EBV. (The different digestion pattern in the region from 8.5 kb to 11 kb, marked with an asterisk, resulted from different numbers of terminal repeats [TR] in each plasmid.) Multiple background bands from the E. coli DNA, generated in the process of isolating wt EBV from E. coli, were observed in the wt EBV lane. The predicted band patterns from the digest are shown on the right. Note that the intensities of the BamW repeat bands in LZ1 clones are similar to wt EBV. (B) Primary B cells were infected with either wt EBV or mutant derivatives of EBV. The infected B cells were plated and grown in 96-well plates for at least 4 weeks, and the efficiencies of induction and maintenance of proliferation were determined by Poisson distribution. Three independent isolates of LZ1EBV, named LZ1EBV-1, -2, and -3, induce and/or maintain proliferation of B cells with 67.6% ± 31.2%, 43.1% ± 19.6%, and 56.6% ± 29.1% of the efficiency of wt EBV (n = 5) (LZ1EBV equals, on average, 55.8% ± 27.2%). Y384GEBV was used as a negative control (n = 2). The error bars represent standard deviations of the samples. *, Wilcoxon rank sum test; P value (two-sided) < 0.02.
These three independent isolates of recombinant virus were tested to ensure that any phenotypes encoded by the recombinant EBVs reflected their common engineered genotype. Equal numbers of GRU of wt EBV and LZ1EBV were used to infect primary B cells. B cells (104) exposed to a total of 3 or 10 GRU were plated out per well in a 96-well plate immediately after infection; 4 to 6 weeks later, the wells containing proliferating B cells were counted to determine the efficiency of induction and maintenance of proliferation. In order to meet the statistical requirements for one-hit kinetics, we counted only plates that had less than 63% of wells with proliferating cells. On average, cells infected with the three different isolates of LZ1EBV had only 56% as much ability to yield proliferating progeny as those infected with wt EBV (Fig. 1B). This reduction was statistically significant for each virus isolate (P < 0.02). Y384G-EBV, which has a mutation in the LMP1 CTAR2 domain and is therefore defective in most signaling pathways induced by LMP1, was used as a negative control (12). There was no difference in the sizes of the colonies that grew out when infected with LZ1EBV relative to those that grew out when infected with wt EBV. Clearly, the leucine heptad has a role in EBV's transformation of cells.
LZ1 has a defect in trafficking to rafts.
What function could be mediated by the LMP1 leucine heptad? Because LMP1 signals from lipid rafts in an intracellular compartment (26) and LMP1's first two membrane-spanning domains are sufficient for the trafficking (6), we tested whether LZ1 homes to lipid rafts, using cell fractionation. 293 cells were cotransfected with LMP1, LZ1, or FWLY expression vectors, together with vector that expresses Δ25-132. The cells were then incubated for 48 h and lysed with cold 1% Triton X-100. Flotation assays, using 5% to 35% discontinuous sucrose gradients, were used to separate raft and nonraft components. TfR (transferrin receptor), a protein widely used as a nonraft marker, and glycosphingolipid GM1, a common constituent of lipid rafts, were used to identify fractions enriched in nonrafts and rafts, respectively. A derivative of LMP1, Δ25-132, which has the first four membrane-spanning domains deleted and was previously reported to be found outside of rafts, was used as an internal negative control (6). TfR was almost exclusively found in the bottom fractions, and GM1 was enriched either in the fourth to sixth fractions or the third to fifth fractions of the gradients. We defined fractions that contained at least 70% GM1 and at least 90% TfR as raft and nonraft fractions, respectively. Δ25-132, our internal negative control, was mostly found in the last six nonraft fractions. Among four independent experiments, 14.4% ± 2.5% of wt LMP1 was found in raft fractions, whereas only 3.8% ± 1.1% of LZ1 was found in the same fractions, indicating that the leucine heptad contributes to LMP1's proper trafficking (Fig. 2A). This difference was statistically significant (P = 0.02). Based on our definition of rafts and nonrafts, a greater fraction of FWLY than LZ1 was found in the raft fractions; however, the levels were lower than that of wt LMP1. A similar trend was found in BJAB cells (data not shown).
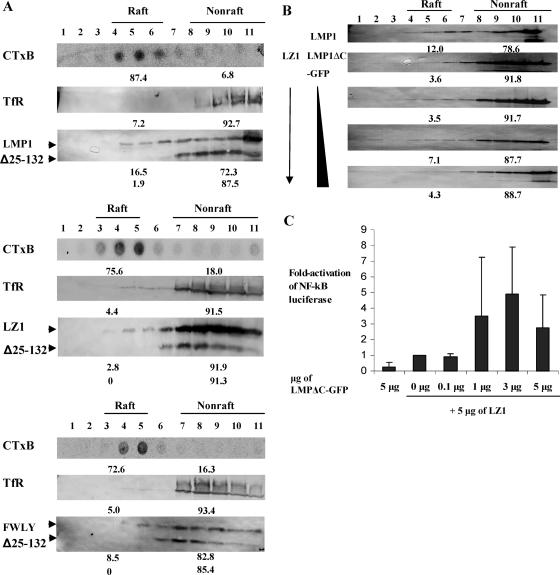
FIG. 2.
LZ1 has a defect in trafficking to lipid rafts. (A) Transiently transfected 293 cells were loaded on a discontinuous sucrose gradient for a flotation assay. The GM1 in cell lysates was probed with horseradish peroxidase-conjugated CTxB and detected by enhanced chemiluminescence. We defined fractions that contained at least 70% GM1 and at least 90% TfR as raft and nonraft fractions, respectively. The numbers below each blot show the percentage of total protein in either the raft or nonraft fraction. On average, 14.4% ± 2.5% of LMP1 and 3.8% ± 1.1% of LZ1 was detected in the raft fractions (n = 4) (Wilcoxon rank sum test; P value [two sided] = 0.02). Shown are representative blots of four independent experiments using Δ25-132 as an internal negative control. The percentage of FWLY found in rafts (8.5%) was greater than LZ1 but lower than wt LMP1. (B) 293 cells were transfected with expression vectors (1 μg of LMP1 or 1 μg of LZ1 with 0, 0.1, 0.3, and 1 μg LMPΔC-GFP), incubated for 48 h, lysed, and loaded on discontinuous sucrose gradients for flotation assays. Equal amounts of cell lysates in each fraction were immunoblotted for LMP1 or LZ1. The percentage of total LMP1 or LZ1 was measured for each fraction, and the numbers show the percentage of each protein in raft (fractions 4 to 6) and nonraft (fractions 8 to 11) fractions in a representative blot. On average, from two independent experiments, the defect of LZ1 in trafficking to rafts was complemented to 67% of the level of wt LMP1 by LMPΔC-GFP. (C) 293 cells were transfected with expression vectors (5 μg of LMPΔC-GFP or 5 μg of LZ1 with 0, 0.1, 1, 3, and 5 μg LMPΔC-GFP), incubated for 48 h, and lysed with passive buffer for an NF-κB reporter assay. Induction (n-fold) of NF-κB activation was measured by normalizing relative light units to the measured levels of LZ1. The value for LZ1 alone was set as 1. On average, from two independent experiments, the defect in LZ1 in signaling was complemented up to fivefold over that of LZ1 alone. Shown are average inductions from two independent experiments, with error bars representing the standard deviation of each data point. Statistical analysis was performed with a distribution-free test for the slope of the regression line (Theil) (31) for six independent experiments, and the P value (two sided) of each set was combined with Fisher's method (P value = 0.015). The induction of NF-κB when 5 μg of both LZ1 and LMPΔC-GFP were transfected was not included in the statistical analysis because LMP1 signaling decreases when high physiological levels of LMP1 are expressed in cells (22).
A derivative of LMP1 (LMPΔC-GFP) that lacks its carboxy terminus signaling domain and cannot signal can complement SubLZLMP1 to allow SubLZLMP1 to signal through NF-κB (22). We therefore asked if LMPΔC-GFP could complement LZ1 to traffic to lipid rafts. 293 cells were transfected with a fixed amount of a vector expressing LZ1, along with increasing amounts of a vector expressing LMPΔC-GFP, and cell extracts were isolated and loaded under sucrose gradients for flotation assays. With increasing amounts of the LMPΔC-GFP expression vector cotransfected, the defect of LZ1 in trafficking to rafts was complemented to as high as 67% of the extent of trafficking to rafts observed with wt LMP1 (Fig. 2B). Transfecting high, equal amounts of LMPΔC-GFP and LZ1 sometimes led to a partial decrease in the percentage of LZ1 found in raft fractions. This decrease is consistent with a decrease in LMP1 signaling through NF-κB when high physiological levels of LMP1 are expressed in cells (Fig. 2C) (22). The total level of LZ1 decreases as the level of LMPΔC-GFP cotransfected increases. This decrease is consistent with our previous findings (22), and all are consistent with LMP1's efficient signaling and short half-life being coupled. Other receptors, for example, epidermal growth factor receptor and TNFR-1, also have their signaling and rapid turnover coupled (34, 38). When LZ1 signaling through NF-κB was assayed in a parallel experiment, its defect in signaling was complemented by LMPΔC (Fig. 2C). Thus, complementing LZ1's defect in trafficking to lipid rafts also complements its defect in signaling through NF-κB.
LZ1 differs from wt LMP1 in its distribution in cells.
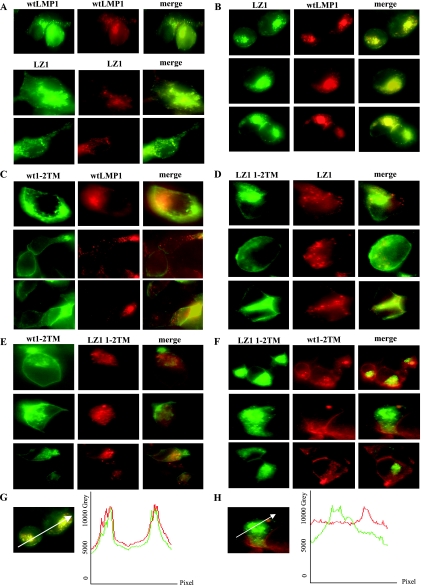
The different partitioning into rafts made it likely that LZ1 would have a different distribution inside the cell than wt LMP1. Testing this possibility with intact LZ1 and wt LMP1 is not possible because coexpressing full-length wt LMP1 and full-length LZ1 in the same cell alters their individual distributions, since they aggregate with each other (Fig. 3B). We therefore first analyzed the distributions of two membrane-spanning domains of LMP1 or LZ1 tagged with either GFP or mRFP (termed wt 1-2TM and LZ1 1-2TM) because they were sufficient for targeting to lipid rafts (6, 40). 293 cells were transiently cotransfected with derivatives of both wt LMP1 and LZ1 and then examined for the colocalization of the tagged molecules. Colocalization of intact wt LMP1 with itself and intact LZ1 with wt LMP1 or itself served as positive controls (Fig. 3A and B). Wt 1-2TM and LZ1 1-2TM did not colocalize, suggesting that the leucine heptad is important for LMP1's proper distribution in the cell (Fig. 3E and F). Full-length wt LMP1 and LZ1 1-2TM or full-length LZ1 and wt 1-2TM did not colocalize either, consistent with our hypothesis and the results from other groups that detected no interaction or a very weak interaction between wt LMP1 and wt 1-2TM (data not shown) (40). However, to our surprise, full-length wt LMP1 and LZ1 also had a distribution different from that of wt 1-2TM and LZ1 1-2TM (Fig. 3C and D). This finding was unexpected, since derivatives with the first two wt membrane-spanning domains have been found to localize to lipid rafts in biochemical assays (6, 28). The full-length molecules localized internally, whereas the derivatives with just the first two membrane-spanning domains localized predominantly at the cell surface. Derivatives of LMP1 missing the first two membrane-spanning domains, in comparison, were found to be primarily internal in 293 cells (data not shown). Our data indicate that either (i) aggregation of LMP1 is important for its localization, since 1-2TM derivatives aggregate poorly (6, 40), or (ii) the third to sixth membrane-spanning domains play roles in LMP1's trafficking, perhaps by interacting with PRA1 at the Golgi (28). The average intensities of GFP and mRFP were similar in the cells that we examined. Also, similar patterns of localization were found with all of the derivatives when the fluorescent tags were switched, indicating that the fluorescent tags did not alter the distribution of the tested proteins. Profiles of the intensity of each fluorescent molecule in a cross-section were measured to determine if the two tagged molecules colocalized (Fig. 3G and H). The profiles confirmed that the distributions of LZ1 and wt LMP1 differ in transfected cells, indicating that the leucine heptad plays a role in LMP1's trafficking.
FIG. 3.
LZ1 differs from wt LMP1 in its distribution in cells. 293 cells were transiently cotransfected with 0.5 to 2 μg of plasmids expressing derivatives of LMP1. Signals from full-length wt LMP1GFP and wt LMP1RFP, LZ1GFP and LZ1RFP (A) and wt LMP1 and LZ1 (B) served as positive controls for colocalization. Wt 1-2TM LMP1 GFP and full-length LMP1 RFP (C) and LZ1 1-2TM GFP and full-length LZ1 RFP (D) did not colocalize. Wt 1-2TM GFP and LZ1 1-2TM RFP (E) and LZ1 1-2TM GFP and wt 1-2TM RFP (F) also failed to colocalize in cells. Similar patterns of localization were found with all of the derivatives when the fluorescent tags were switched. (G and H) Profiles of the intensities of the fluorescent molecules in a cross-section. The white arrows in the merged images indicate the line and direction of the measured intensities. (G) The merged image of cells cotransfected with LZ1 GFP and wt LMP1 RFP (the same image is shown in panel B, first row) is shown as a representative of a colocalized signal. (H) The merged image of a cell cotransfected with LZ1 1-2TM GFP and wt LMP1 1-2TM RFP (the same image is shown in panel F, second row) is shown as a representative of signals that did not colocalize.
LZ1 traffics to lipid rafts and signals through NF-κB as efficiently as wt LMP1 in B cells infected with LZ1EBV that grow.
In order to characterize functions mediated by the leucine heptad of LMP1 in EBV's transformation of cells, clones of cells were randomly picked from B cells that were infected with either wt EBV or LZ1EBV and grown for an additional 6 weeks after infection for further analysis. Not all cells infected and induced to proliferate by EBV survive crisis to become immortal (7, 39). Here, we term cells that are transformed by either wt EBV or LZ1EBV but that have not passed through crisis as wt EBV(+)/LZ1EBV(+) B-cell clones.
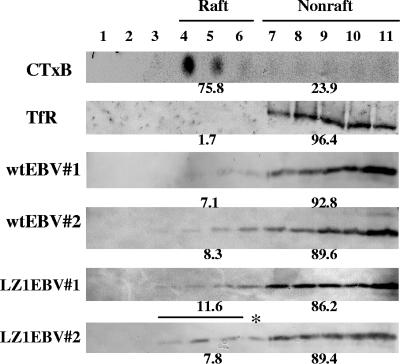
First, to understand how 44% of LZ1EBV(+) cells continued to proliferate to give rise to B-cell clones, we measured the percentage of LZ1 found in rafts in wt EBV(+) and LZ1EBV(+) B-cell clones by flotation assays. Extracts of two wt EBV(+) B-cell clones and three LZ1EBV(+) B-cell clones were subjected to flotation assays. Raft and nonraft fractions were defined as fractions that contained at least 70% total GM1 (detected by CTxB) or at least 90% total TfR, as in earlier assays. On average, 7.7% ± 0.9% of wt LMP1 and 9.2% ± 2.1% of LZ1 was detected in raft fractions (Fig. 4). There was no significant difference in the percentages of LMP1 and LZ1 found in the raft fractions.
FIG. 4.
LZ1 traffics to lipid rafts as efficiently as wt LMP1 in LZ1EBV(+) B-cell clones. Extracts from two wt EBV(+) B-cell clones and three LZ1EBV(+) B-cell clones were loaded on a discontinuous sucrose gradient for flotation assays. Raft/nonraft fractions were defined as fractions that contained at least 70% total GM1 (detected by CTxB) or at least 90% total TfR. Representative blots of CTxB, TfR, and each clone are shown above. On average, 7.7% ± 0.9% of wt LMP1 and 9.2% ± 2.1% of LZ1 was detected in raft fractions. *, raft fractions were from fractions 3 to 5.
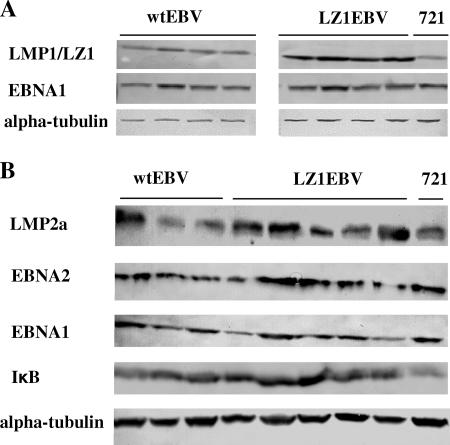
The growth rates of B-cell clones differed from clone to clone; however, LZ1EBV(+) clones did not, on average, have altered growth rates compared to wt EBV(+) clones. We next analyzed NF-κB activities in these cells as a measure of LMP1 signaling. We measured the expression levels of IκB (see Fig. 6B) in B-cell clones as a reflection of NF-κB activities. As shown (see Fig. 6B), there were no detectable difference in the levels of IκB in LZ1EBV(+) clones and those in wt EBV (+) clones. It is likely that we selected clones in which LZ1 traffics to lipid rafts as efficiently as does wt LMP1, thus leading to similar levels of NF-κB activity, because this activity is necessary for B cells to proliferate (11, 43). How LZ1 is altered to traffic similarly to wt LMP1 is still unknown. One hypothesis is that other viral or cellular factors in B-cell clones alter LZ1 trafficking in these clones. We have tested whether LMP2a can affect LZ1 trafficking, but our preliminary data indicate that this hypothesis is unlikely.
FIG. 6.
(A) LZ1 is accumulated in B-cell clones. B-cell strains were established by cloning and propagating primary B cells infected with either wt EBV or LZ1EBV. Cell lysates from equal numbers of four different early passages of wt EBV(+) or LZ1(+) B-cell clones were immunoblotted for LMP1 or LZ1, EBNA1, and alpha-tubulin. LZ1 accumulated approximately 1.5-fold more than wt LMP1 when normalized to the loading control, EBNA1, or alpha-tubulin. Note that the level of LMP1 in wt EBV(+) B-cell clones was comparable to that of 721 (each row shown above is from a single gel). Wilcoxon rank sum test; P value (two-sided) = 0.02. (B) Expression levels of other viral genes in LZ1EBV(+) B-cell clones do not differ from the wt. Cell lysates from equal numbers of at least three different clones of wt EBV(+) and five different LZ1(+) B-cell clones were immunoblotted for LMP2a, EBNA2, IκB, EBNA1, and alpha-tubulin. On average, expression levels relative to alpha-tubulin for LMP2a, EBNA2, IκB, and EBNA1 were 0.22 ± 0.09, 0.62 ± 0.21, 0.97 ± 0.16, and 0.24 ± 0.06 for wt EBV(+) B-cell clones and 0.31 ± 0.13, 0.69 ± 0.41, 1.17 ± 0.25, and 0.20 ± 0.09 for LZ1EBV(+) B-cell clones, respectively. Alpha-tubulin was used a loading control, and 721 cells were used as a positive control.
LZ1EBV(+) and wt EBV(+) B-cell clones have similar percentages of cells with capped LZ1/LMP1 and similar numbers of virus copies per cell.
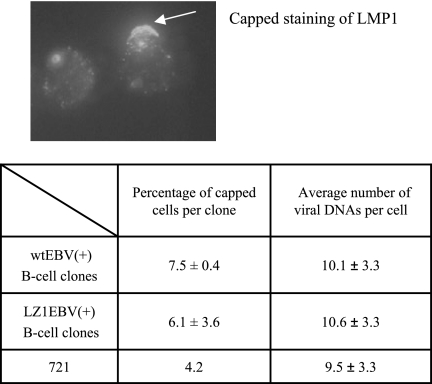
In order to confirm that we had not selected for B-cell clones that had high numbers of copies of viral genomes per cell, we performed FISH analysis. Both wt EBV(+) clones and LZ1EBV(+) clones had, on average, approximately 10 copies of viral DNA per cell, similar to the copy number of 721 cells, which was used as a control (Fig. 5). This measurement indicates that any defects in LZ1 do not select for an altered number of viral genomes per cell.
FIG. 5.
LZ1EBV(+) B-cell clones have percentages of capped cells and virus copy numbers per cell similar to those of wt EBV(+) B-cell clones. Two and three independent clones infected with wt EBV or LZ1EBV, respectively, were fixed, permeabilized, stained with rabbit anti-LMP1, and detected with Texas red-conjugated anti-rabbit antibody. The percentage of cells that had cap-like structures formed by LMP1 was determined. In both B-cell clones, approximately 7% of the cells formed a cap-like structure of LMP1. FISH analysis was performed to determine the average virus copy numbers in B-cell clones. Four wt EBV(+) clones and five LZ1EBV(+) clones were tested, and on average, 10 cells were counted per clone. There was no difference in the average virus copy numbers per cell. 721 cells were used as a positive control.
Our previous findings showed that in 293 and BJAB cells, LMP1 is associated with lipid rafts almost exclusively inside the cell, whereas in 721 cells, LMP1 is found in a fraction of cells at the plasma membrane forming a cap-like structure (26). This structure colocalizes with CTxB, a marker for lipid rafts, and TRAF2, indicating that it may be a site where LMP1 signals (26). We wanted to see if LZ1 has a different distribution than wt LMP1 in B-cell clones. Both wt EBV(+) and LZ1EBV(+) B-cell clones were fixed, permeabilized, and stained with antibody against the C terminus of LMP1 to detect both LMP1 and LZ1. Each strain of cells exhibited cap-like staining of LMP1 in an average of 7% of the cells (Fig. 5). Moreover, there was no other noticeable difference between the distributions of LZ1 and LMP1 in these B-cell clones. The fact that the distributions of LZ1 and LMP1 differ in 293 cells but not in B-cell clones strongly supports the hypothesis that the leucine heptad is important for LMP1 trafficking to internal rafts.
LZ1 is accumulated in B-cell clones.
We next measured the levels of expression of LMP1 or LZ1 protein by immunoblotting in B-cell clones, which were grown for less than 2 months. LZ1 accumulated to 1.5-fold-higher levels than wt LMP1 when normalized to the loading control, EBNA1 or alpha-tubulin (P = 0.02), a smaller difference than the sevenfold increase found in 293 and BJAB cells (Fig. 6A) (22). Both wt LMP1 and LZ1 had broad distributions as measured by fluorescence-activated cell sorter analysis, indicating that the levels of expression of LMP1 or LZ1 differed dramatically among individual cells of each B-cell clone (data not shown), consistent with what had been found earlier (24). The 1.5-fold-increased level of LZ1 may help LZ1EBV(+) B-cell clones to compensate for LZ1's defects.
To test if proliferative signals might be differentially provided by other viral proteins known to be important for transformation in LZ1EBV(+) B cells, we measured the expression levels of LMP2a, EBNA2, and EBNA1 by immunoblotting (Fig. 6B). At least three different clones of wt EBV(+) B-cell clones and five different LZ1EBV(+) B-cell clones were tested. No difference in the levels of any of the viral proteins was found, indicating that these proteins are not detectably altered or upregulated to induce proliferation in the LZ1EBV(+) B-cell clones. This finding, together with localization studies (Fig. 3), shows that the growth of LZ1EBV(+) B-cell clones is likely dependent on LZ1 signaling through NF-κB.
DISCUSSION
Our genetic and biochemical analyses of LMP1 indicate that the leucine heptad in its first membrane-spanning domain is necessary for LMP1's efficient trafficking to lipid rafts, its signaling as measured by its activation of NF-κB, and its contribution to EBV's transformation of primary B lymphocytes. The role of this moiety in trafficking is likely directly related to its support of LMP1's signaling, because the complementation of LZ1, a derivative of LMP1 mutated in this heptad, with a derivative of LMP1 with a wt heptad but no carboxy-terminal signaling domain restored both LZ1 trafficking and its signaling (Fig. 2B and C). LMP1's signaling is known to be necessary for EBV's transformation of B cells. LMP1 appears to have evolved its six membrane-spanning domains to substitute functionally for the role that ligands would have in activating TNFRs, a family to which LMP1 is otherwise related.
LMP1 signals principally from intracellular compartments, which are likely to be Golgi-containing lipid rafts (26, 28), indicating that signaling by LMP1 depends on its subcellular localization. Despite studies showing that LMP1's ability to home to lipid rafts is important for its signaling, little is known about how LMP1 is recruited to lipid rafts. The aromatic residues in the FWLY motif in the first membrane-spanning domain are critical for targeting LMP1 to lipid rafts (40), but the precise mechanism by which they contribute to LMP1 trafficking has yet to be resolved. The leucine in the FWLY motif is mutated in LZ1, indicating that both the adjacent aromatic residues in this motif and the leucine heptad in its first membrane-spanning domain are important for targeting LMP1 to lipid rafts and its dependent signaling. It is not likely that mutations in our study altered LMP1's structure, because (i) substituting a similar motif in the sixth membrane-spanning domain had no phenotype and activated NF-κB as efficiently as the wt (22) and (ii) LMPΔC-GFP can complement LZ1's trafficking and signaling defect through interaction with LZ1.
As an independent approach to confirm LZ1's defect in homing to lipid rafts, we performed a colocalization study with Gap43-GFP and wt LMP1-RFP or LZ1-RFP in 293 cells. The amino terminus of Gap43 is dually palmitoylated, and when fused to GFP (Gap43-GFP), is found both at the plasma membrane and internally, associated with lipid rafts (42). A small percentage of wt LMP1 colocalized with Gap43-GFP both at the periphery and inside the cell, whereas little or no LZ1-RFP colocalized with Gap43-GFP (data not shown). The colocalization of wt LMP1, but not LZ1, with Gap43, together with data from the flotation assay, demonstrates that LZ1 has a defect in homing to lipid rafts.
CD40 is a trimer that can signal on binding a trimer of CD40L. LMP1 achieves its ligand-independent signaling through multifunctional, six-member membrane-spanning domains. The last four membrane-spanning domains mediate self-aggregation, binding to PRA1, and movement to the Golgi (28). A derivative of LMP1 with the last four membrane-spanning domains of LMP1 deleted localizes to ER-like structures (28). Our studies indicate that the first membrane-spanning domain of LMP1, through a leucine heptad and some aromatic residues (40), homes LMP1 to lipid rafts, whether it is at the ER, the plasma membrane, or the Golgi. Self-aggregation, movement to the Golgi, and homing to lipid rafts are all required for LMP1 signaling, and they are all mediated through its six-member membrane-spanning domains. In a fraction of EBV-transformed B cells, LMP1 traffics to the plasma membrane. The mechanism for this trafficking has yet to be fully elucidated, but LMP1's leucine heptad is still required for LMP1's association with lipid rafts.
One hypothesis to explain the mechanism by which the leucine heptad plus the aromatic residues mediate homing is via their binding of another membrane protein that facilitates trafficking to or recruitment of lipid rafts. This mechanism, which would be defective in LZ1, likely contributes to the early events of EBV's inducing and maintaining proliferation on infecting primary B cells. In this context, LZ1 fails to support EBV infection in half of the cells in which wt LMP1 succeeds. LZ1 does cap at the plasma membrane in already proliferating B cells, as does wt LMP1 (Fig. 5), and derivatives of LMP1 that are anchored at the plasma membrane can support proliferation of EBV-infected B cells (11). We speculate that the defect in LZ1 has such a large impact on newly infected cells by affecting signaling internally, where LZ1's alterations in trafficking are most pronounced (Fig. 3).
Many studies show that LMP1 signaling is dependent on its proper trafficking. Understanding in detail how LMP1 traffics inside the cell should illuminate the assembly of its signaling complex and possibly permit the development of tools to inhibit LMP1's function in tumor cells. More generally, this understanding should reveal how ligand-independent receptor-like molecules home to lipid rafts in order to signal.
Acknowledgments
We thank Ulrike Dirmeier and Wolfgang Hammerschmidt for providing maxi-EBV and Y384GEBV and their guidance in making recombinant EBV. We also thank Norman Drinkwater for his help in the statistical analysis and Paul Lambert for his critical review of the manuscript.
This work is supported by NIH grants CA14529 and CA70723. B.S. is an American Cancer Society Research Professor.
Footnotes
Published ahead of print on 20 June 2007.
REFERENCES
- 1.Brodeur, S. R., G. Cheng, D. Baltimore, and D. A. Thorley-Lawson. 1997. Localization of the major NF-κB-activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs. J. Biol. Chem. 272:19777-19784. [DOI] [PubMed] [Google Scholar]
- 2.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 3.Brown, K. D., B. S. Hostager, and G. A. Bishop. 2001. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1). J. Exp. Med. 193:943-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Campbell, R. E., O. Tour, A. E. Palmer, P. A. Steinbach, G. S. Baird, D. A. Zacharias, and R. Y. Tsien. 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clausse, B., K. Fizazi, V. Walczak, C. Tetaud, J. Wiels, T. Tursz, and P. Busson. 1997. High concentration of the EBV latent membrane protein 1 in glycosphingolipid-rich complexes from both epithelial and lymphoid cells. Virology 228:285-293. [DOI] [PubMed] [Google Scholar]
- 5.Coffin, W. F., III, K. D. Erickson, M. Hoedt-Miller, and J. M. Martin. 2001. The cytoplasmic amino-terminus of the Latent Membrane Protein-1 of Epstein-Barr virus: relationship between transmembrane orientation and effector functions of the carboxy-terminus and transmembrane domain. Oncogene 20:5313-5330. [DOI] [PubMed] [Google Scholar]
- 6.Coffin, W. F., III, T. R. Geiger, and J. M. Martin. 2003. Transmembrane domains 1 and 2 of the latent membrane protein 1 of Epstein-Barr virus contain a lipid raft targeting signal and play a critical role in cytostasis. J. Virol. 77:3749-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Counter, C. M., F. M. Botelho, P. Wang, C. B. Harley, and S. Bacchetti. 1994. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J. Virol. 68:3410-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford, D. H. 2001. Biology and disease associations of Epstein-Barr virus. Phil. Trans. R. Soc. Lond. B 356:461-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delecluse, H. J., T. Hilsendegen, D. Pich, R. Zeidler, and W. Hammerschmidt. 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl. Acad. Sci. USA 95:8245-8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devergne, O., E. D. Cahir McFarland, G. Mosialos, K. M. Izumi, C. F. Ware, and E. Kieff. 1998. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J. Virol. 72:7900-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dirmeier, U., R. Hoffmann, E. Kilger, U. Schultheiss, C. Briseno, O. Gires, A. Kieser, D. Eick, B. Sugden, and W. Hammerschmidt. 2005. Latent membrane protein 1 of Epstein-Barr virus coordinately regulates proliferation with control of apoptosis. Oncogene 24:1711-1717. [DOI] [PubMed] [Google Scholar]
- 12.Dirmeier, U., B. Neuhierl, E. Kilger, G. Reisbach, M. L. Sandberg, and W. Hammerschmidt. 2003. Latent membrane protein 1 is critical for efficient growth transformation of human B cells by Epstein-Barr virus. Cancer Res. 63:2982-2989. [PubMed] [Google Scholar]
- 13.Edidin, M. 2001. Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends Cell Biol. 11:492-496. [DOI] [PubMed] [Google Scholar]
- 14.Eliopoulos, A. G., and A. B. Rickinson. 1998. Epstein-Barr virus: LMP1 masquerades as an active receptor. Curr. Biol. 8:R196-R198. [DOI] [PubMed] [Google Scholar]
- 15.Gires, O., F. Kohlhuber, E. Kilger, M. Baumann, A. Kieser, C. Kaiser, R. Zeidler, B. Scheffer, M. Ueffing, and W. Hammerschmidt. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 18:3064-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Gurezka, R., R. Laage, B. Brosig, and D. Langosch. 1999. A heptad motif of leucine residues found in membrane proteins can drive self-assembly of artificial transmembrane segments. J. Biol. Chem. 274:9265-9270. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi, M., K. M. Izumi, and E. Kieff. 2001. Epstein-Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: protein 1 binds to the cytoskeleton through TNF receptor cytoplasmic factors. Proc. Natl. Acad. Sci. USA 98:4675-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl. Acad. Sci. USA 94:12592-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavathas, P., F. H. Bach, and R. DeMars. 1980. Gamma ray-induced loss of expression of HLA and glyoxalase I alleles in lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 77:4251-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaye, K. M., O. Devergne, J. N. Harada, K. M. Izumi, R. Yalamanchili, E. Kieff, and G. Mosialos. 1996. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-κB activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc. Natl. Acad. Sci. USA 93:11085-11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 90:9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaykas, A., K. Worringer, and B. Sugden. 2001. CD40 and LMP-1 both signal from lipid rafts but LMP-1 assembles a distinct, more efficient signaling complex. EMBO J. 20:2641-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaykas, A., K. Worringer, and B. Sugden. 2002. LMP-1's transmembrane domains encode multiple functions required for LMP-1's efficient signaling. J. Virol. 76:11551-11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieser, A., E. Kilger, O. Gires, M. Ueffing, W. Kolch, and W. Hammerschmidt. 1997. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 16:6478-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam, N., M. L. Sandberg, and B. Sugden. 2004. High physiological levels of LMP1 result in phosphorylation of eIF2 alpha in Epstein-Barr virus-infected cells. J. Virol. 78:1657-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam, N., and B. Sugden. 2003. CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal. 15:9-16. [DOI] [PubMed] [Google Scholar]
- 26.Lam, N., and B. Sugden. 2003. LMP1, a viral relative of the TNF receptor family, signals principally from intracellular compartments. EMBO J. 22:3027-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence, J. B., C. A. Villnave, and R. H. Singer. 1988. Sensitive, high-resolution chromatin and chromosome mapping in situ: presence and orientation of two closely integrated copies of EBV in a lymphoma line. Cell 52:51-61. [DOI] [PubMed] [Google Scholar]
- 28.Liu, H. P., C. C. Wu, and Y. S. Chang. 2006. PRA1 promotes the intracellular trafficking and NF-κB signaling of EBV latent membrane protein 1. EMBO J. 25:4120-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melkonian, K. A., A. G. Ostermeyer, J. Z. Chen, M. G. Roth, and D. A. Brown. 1999. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274:3910-3917. [DOI] [PubMed] [Google Scholar]
- 30.Munro, S. 2003. Lipid rafts: elusive or illusive? Cell 115:377-388. [DOI] [PubMed] [Google Scholar]
- 31.Myles Hollander, D. A. W. 1999. A distribution-free test for the slope of the regression line (Theil), p. 416-420. In N. A. C. C. V. Barnett, N. I. Fisher, I. M. Johnstone, J. B. Kadanae, D. G. Kendall, D. W. Scott, B. W. Silverman, A. F. M. Smith, and J. L. Teugels (ed.), Nonparametric statistical methods, 2nd ed. Wiley-Interscience Publications, New York, NY.
- 32.Rickinson, A. B., and E. Keiff. 1996. Epstein-Barr Virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, vol. 2. Lippencott Raven, New York, NY. [Google Scholar]
- 33.Sandberg, M., W. Hammerschmidt, and B. Sugden. 1997. Characterization of LMP-1's association with TRAF1, TRAF2, and TRAF3. J. Virol. 71:4649-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schutze, S., T. Machleidt, D. Adam, R. Schwandner, K. Wiegmann, M. L. Kruse, M. Heinrich, M. Wickel, and M. Kronke. 1999. Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J. Biol. Chem. 274:10203-10212. [DOI] [PubMed] [Google Scholar]
- 35.Sengupta, S., J. A. den Boon, I. H. Chen, M. A. Newton, D. B. Dahl, M. Chen, Y. J. Cheng, W. H. Westra, C. J. Chen, A. Hildesheim, B. Sugden, and P. Ahlquist. 2006. Genome-wide expression profiling reveals EBV-associated inhibition of MHC class I expression in nasopharyngeal carcinoma. Cancer Res. 66:7999-8006. [DOI] [PubMed] [Google Scholar]
- 36.Shaw, G., S. Morse, M. Ararat, and F. L. Graham. 2002. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 16:869-871. [DOI] [PubMed] [Google Scholar]
- 37.Soni, V., T. Yasui, E. Cahir-McFarland, and E. Kieff. 2006. LMP1 transmembrane domain 1 and 2 (TM1-2) FWLY mediates intermolecular interactions with TM3-6 to activate NF-κB. J. Virol. 80:10787-10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stang, E., L. E. Johannessen, S. L. Knardal, and I. H. Madshus. 2000. Polyubiquitination of the epidermal growth factor receptor occurs at the plasma membrane upon ligand-induced activation. J. Biol. Chem. 275:13940-13947. [DOI] [PubMed] [Google Scholar]
- 39.Sugimoto, M., H. Tahara, T. Ide, and Y. Furuichi. 2004. Steps involved in immortalization and tumorigenesis in human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Res. 64:3361-3364. [DOI] [PubMed] [Google Scholar]
- 40.Yasui, T., M. Luftig, V. Soni, and E. Kieff. 2004. Latent infection membrane protein transmembrane FWLY is critical for intermolecular interaction, raft localization, and signaling. Proc. Natl. Acad. Sci. USA 101:278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young, L. S., and A. B. Rickinson. 2004. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 4:757-768. [DOI] [PubMed] [Google Scholar]
- 42.Zacharias, D. A., J. D. Violin, A. C. Newton, and R. Y. Tsien. 2002. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296:913-916. [DOI] [PubMed] [Google Scholar]
- 43.Zarnegar, B., J. Q. He, G. Oganesyan, A. Hoffmann, D. Baltimore, and G. Cheng. 2004. Unique CD40-mediated biological program in B cell activation requires both type 1 and type 2 NF-κB activation pathways. Proc. Natl. Acad. Sci. USA 101:8108-8113. [DOI] [PMC free article] [PubMed] [Google Scholar]