Abstract
Respiratory syncytial virus (RSV) is a major cause of bronchiolitis and viral pneumonia in young children and a serious health risk in immunocompromised individuals and the elderly. Immunity to RSV is not completely understood. In this work, we established a method for monitoring RSV infection by real-time PCR and applied this method for analysis of RSV replication in vivo in the cotton rat model in naïve animals and in animals rendered immune to RSV by prior RSV infection. We found that even though no virus could be isolated from the lungs of RSV-challenged immune animals, RSV infection in fact took place and an accumulation of viral RNA transcripts was observed. This type of replication, therefore, can be termed “abortive,” as RSV is capable of entering the cells in the lungs of immune animals, yet the production of progeny viruses is impaired. Similar patterns of RSV gene expression gradient were observed between naïve and reinfected animals, indicating that the skewing of mRNA gradient of viral gene expression, a mechanism documented during latent infection by other viruses, is not likely to be responsible for abortive replication of RSV during reinfection. We found that passive administration of antibodies to RSV prevents productive infection normally accompanied by viral release in the lung, but it does not prevent abortive replication of the virus. To the best of our knowledge, this is the first evidence of abortive replication of RSV in vivo.
Respiratory syncytial virus (RSV) is a major cause of bronchiolitis and viral pneumonia in young children, and the primary viral cause of death in infants (38, 43). By the age of 2, most children have been infected with RSV at least once, and half of those children have been reinfected (13). Reinfection with RSV, often occurring with milder symptoms or as asymptomatic, is common. RSV infection in children is associated with recurrent wheezing later in life, pointing to a link between RSV and inflammatory allergic disorders (35, 39, 41). In addition to being a causative agent of serious respiratory disease in pediatric populations worldwide, RSV poses a serious health risk in immunocompromised individuals and the elderly (17, 44).
RSV is an enveloped, nonsegmented, negative-strand RNA virus that belongs to the family Paramyxoviridae. The RSV genome contains 10 genes in the order NS1, NS2, N, P, M, SH, G, F, M2, L from the 3′ end, which give rise to 11 proteins (the M2 open reading frame [ORF] encodes two proteins, M2-1 and M2-2). RSV proteins include three transmembrane surface proteins, G, F, and SH; the virion matrix protein M; nucleocapsid proteins L, N, P, M2-1, and M2-2; and two putative nonstructural proteins, NS1 and NS2. RSV genes are transcribed sequentially from the 3′ end of the genome, with promoter-proximal genes transcribed more frequently than downstream genes (8). This results in a transcription gradient characteristic also of other mononegaviruses. Upon accumulation of virally expressed proteins in infected cells, RSV begins a replicative cycle in which a positive-strand copy of genome, the antigenome, is synthesized as an intermediate. Both genomic and antigenomic RNA get packaged into completed virions in a process dependent on ongoing viral protein synthesis (21).
Immunity to RSV is still not completely defined. The reason for the absence of long-lasting protection against RSV is not known. Clearance of RSV during primary infection is thought to be mediated by CD4+ and CD8+ T lymphocytes; however, the same cells can augment pathology of RSV disease (6, 15). Antibodies appear to be an illness-sparing mechanism and were shown to protect both mice and cotton rats (CRs) from RSV infection administered prophylactically without inducing pathology of the disease (31, 42). Moreover, when administered therapeutically at the height of RSV infection, anti-RSV antibodies were shown to clear virus from the lungs within 24 h (30). Antibodies also appear important for protection against excessive illness during reinfection. Mice depleted of B lymphocytes by anti-μ treatment were still partially protected from RSV replication during reinfection, but the illness score was dramatically increased compared to that of mice with intact humoral responses (14).
Animal studies using the CR model indicate that inflammatory changes in the lung are elicited in animals immune to RSV even in the absence of detectable virus production (32). One possible explanation for that phenomenon could be abortive viral replication triggering an inflammatory response in the lung. We have undertaken experiments to closely monitor the progression of RSV infection in the CR Sigmodon hispidus during primary RSV infection and during reinfection of animals immune to RSV. Our results present evidence of abortive RSV infection in the rechallenge model and may provide an explanation for the phenomenon of recurrent respiratory problems in humans recovering from RSV bronchiolitis.
MATERIALS AND METHODS
Animals.
Inbred S. hispidus CRs were obtained from a colony maintained at Virion Systems, Inc. Animals were housed in large polycarbonate rat cages with a bedding of paper mill by-products and were fed a standard diet of rodent chow and water. The colony was monitored for antibodies to paramyxoviruses, and no such antibodies were found. All studies were conducted under applicable laws and guidelines and after approval from the Virion Systems, Inc. Institutional Animal Care and Use Committee.
Viruses, cells, and viral assays.
The prototype Long strain of RSV was obtained from the American Type Culture Collection (VA). Virus was propagated in HEp-2 cells and serially plaque purified to reduce defective-interfering particles (16). A single pool of virus containing 3.7 × 107 PFU/ml was used. Viral titers in the lungs of infected animals and in the supernatants of infected cells were determined by plaque assay (33). For animal studies, viral titers were expressed as geometric means ± standard errors of means (SEM) for all animals in a group. For cell culture studies, viral titers were expressed as means ± SEM for two different wells per treatment type. Human alveolar epithelial type II cells (A549) were obtained from the American Type Culture Collection. A549 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 IU/ml of penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 10% fetal bovine serum in a humidified 37°C incubator with 5% CO2. Peritoneal macrophages were obtained from S. hispidus as previously described (4).
Drugs.
The humanized monoclonal antibody palivizumab (Synagis; 100 mg/ml) directed against the F protein of RSV and pooled human anti-RSV antibody preparation RSV immune globulin (RSVIG) (RespiGam; 50 mg/ml) were provided by MedImmune, Inc. (Gaithersburg, MD).
RSV infection in vitro.
A549 cells were infected with RSV diluted in plain DMEM medium to yield various multiplicities of infection (MOIs) (0.001 to 0.1). At various times postinfection, cell supernatants were harvested for viral quantification by plaque assay. Cells in the wells from which supernatants were collected were lysed with the RLT buffer with β-mercaptoethanol, and RNA was extracted using the RNeasy purification kit (QIAGEN). For assays of antibody inhibition of RSV infection of A549 cells or macrophages, RSV was incubated for 1 h at room temperature with various concentrations of palivizumab (0 to 600 μg/ml) in plain DMEM or RPMI medium, respectively, and then used to infect the cells. Twenty-four hours after infection, supernatants were harvested for viral quantification and cells were harvested for RNA isolation as described above.
RSV infection in vivo.
For primary RSV infection studies, CRs were inoculated once intranasally (i.n.) with 100 μl of RSV suspension, 105.6 PFU per animal. Animals were sacrificed by CO2 inhalation at 6 h, 12 h, and 1, 2, 4, 7, and 14 days after infection (four animals per time point). A group of animals was sacrificed prior to infection (0 h) and served as uninfected controls. For secondary RSV infection studies, CRs were inoculated twice i.n. with 100 μl of RSV suspension, 105.6 PFU per animal, with an interval of 21 days between infections. Animals were sacrificed at 6 h, 12 h, and 1, 2, 4, 7, and 14 days after the second viral challenge. Control (0 h) animals were sacrificed just prior to the second challenge. Animals with primary and secondary infection were age matched at the time of sacrifice. The lungs were removed from the thorax and dissected for viral titrations and RNA extraction.
For experiments for inhibition of RSV replication by palivizumab, animals were administered a single intramuscular injection of palivizumab (15 mg/kg of body weight) and infected with RSV 24 h later (105.6 PFU RSV per animal in 100 μl i.n.). Animals were sacrificed at 0 h (prior to infection), 12 h, and 2, 4, and 14 days postinfection (four animals per time point), and lungs were harvested for viral titrations and RNA extraction. Control animals were infected with RSV without prior inoculation with palivizumab and sacrificed at 0 h, 12 h, and 2, 4, and 14 days postinfection. Treatments with RSVIG and anti-RSV CR serum were performed by inoculating animals i.n. with 100 μl of 50 mg/kg RSVIG or with 100 μl CR serum (RSV neutralizing titer 1:1, 250) 1 h prior to infection. Animals were then infected with 105.6 PFU RSV per animal in 100 μl i.n. Infected animals continued to receive inoculations of RSVIG or CR serum for 3 consecutive days following RSV infection. On day 4, all animals were sacrificed (four animals per group) and lungs were harvested for viral titrations and RNA extraction. Control animals were infected with RSV without any antibody treatment and sacrificed on day 4 postinfection.
Real-time PCR.
Total RNA was extracted from homogenized lung tissue or from infected cells using the RNeasy purification kit (QIAGEN) and treated with DNase (QIAGEN). One microgram of total RNA was used to prepare cDNA using SuperScript II RT (Invitrogen) and one of the following primers: oligo(dT) (for analysis of RSV gene expression and for measurements of housekeeping gene β-actin mRNA), primer 5′ CAATGAACTAGGATATCAAGAC 3′ (for analysis of the amount of RSV genome), or primer 5′ GTCTTGATATCCTAGTTCATTG 3′ (for analysis of the amount of RSV antigenome). The sequences of the last two primers correspond to the positive and negative strands within the intergenic region between the SH and G genes of RSV. This region was chosen for genome/antigenome analysis because of its reported resistance to the transcriptional read-through phenomenon (18).
For all real-time PCRs, the Bio-Rad iQ SYBR green supermix was used in a final volume of 25 μl with a final primer concentration of 0.5 μM. Primer Express software was used to design primers for viral amplicons (Table 1). A 64-nucleotide amplicon in the β-actin transcript was amplified using primers 5′ TACGCCAACACAGTGCTGTCT 3′ (forward) and 5′ TCTGCATCCTGTCGGCAAT 3′ (reverse). Reaction mixtures were set up in duplicates in 96-well trays. Amplifications were performed on a Bio-Rad iCycler for 1 cycle of 95°C for 3 min, followed by 40 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 15 s. Melting curves were completed for each primer pair, and no nonspecific amplification was noted under conditions tested. The baseline cycles and cycle threshold (CT) values were calculated by the iQ5 software in the PCR base line-subtracted curve fit mode.
TABLE 1.
Primers used for the real-time PCR analysis of RSV ampliconsa
| Viral gene | Forward primer | Reverse primer | Start of forward primerb | Amplicon length |
|---|---|---|---|---|
| NS1 | CACAACAATGCCAGTGCTACAA | TTAGACCATTAGGTTGAGAGCAATGT | 231 | 83 |
| N | AAGGGATTTTTGCAGGATTGTTT | CTCCCCACCGTAGCATTACTTG | 719 | 66 |
| M | ATGTGCTAATGTGTCCTTGGATGA | TGATTTCACAGGGTGTGGTTACA | 270 | 68 |
| G | CGGCAAACCACAAAGTCACA | TTCTTGATCTGGCTTGTTGCA | 191 | 64 |
| F | TAAGCAGCTCCGTTATCACATCTC | ATTGGATGCTGTACATTTAGTTTTGC | 1,205 | 74 |
| M2 | CATGAGCAAACTCCTCACTGAACT | TCTTGGGTGAATTTAGCTCTTCATT | 294 | 80 |
| L | CACTCTACAAAACAAAAAGACACAATCA | AGGATGCTGCATTGAACACATT | 529 | 72 |
Primers were designed using Primer Express software.
Shown are the number of nucleotides from the first nucleotide in the start codon (ATG) to the beginning of the amplicon.
Relative quantitation of DNA was applied to all samples. The standard curves were developed using serially diluted cDNA sample most enriched in the transcript of interest (e.g., lungs from day 4 postprimary RSV infection). The CT values were plotted against log10 cDNA dilution factor. These curves were used to convert the CT values obtained for different samples to relative expression units. These relative expression units were then normalized to the level of β-actin mRNA (“housekeeping gene”) expressed in the corresponding sample. For animal studies, mRNA levels were expressed as the geometric means ± SEM for all animals in a group at a given time. For cell culture studies, mRNA levels were expressed as the means ± SEM for two different wells per treatment type.
RESULTS
Development of real-time PCR assay to monitor dynamics of RSV infection.
Real-time PCR analysis has been used in the past to detect RSV infection (10, 11, 20, 29). However, this method has never been used to monitor dynamics of RSV infection in progression. We established conditions for measuring the expression of RSV genes and the production of RSV genome/antigenome in infected cells. This method was verified using cultures of RSV-infected epithelial cells and then applied to monitor the progression of RSV infection in vivo.
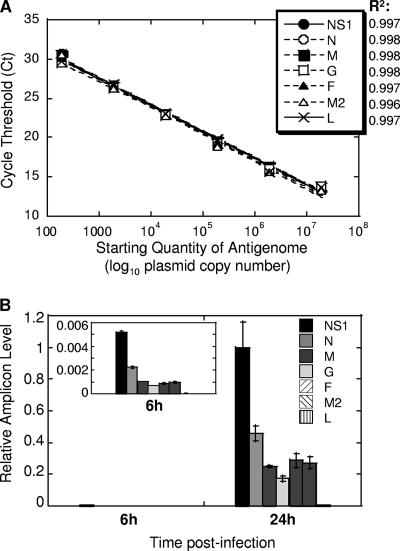
RSV amplicons NS1, N, M, G, F, M2, and L were targeted for quantification. All amplicons were less than 100 nucleotides in size, with the primers defining them shown in Table 1. To compare efficiencies of each primer pair, a standard curve was developed using serial 10-fold dilutions of plasmid containing the entire RSV antigenome (correlation coefficients of 0.996 to 0.998) (Fig. 1A). Each primer pair was efficient in amplifying appropriate DNA fragment, and efficiencies between primer pairs were nearly identical (98 to 99%). To assess validity of real-time PCR assay for monitoring dynamics of RSV infection, human A549 alveolar epithelial cells were inoculated with RSV and RNA was extracted at 6 and 24 h after infection. As shown in Fig. 1B, the infection of A549 cells was slow, with transcripts of RSV genes appearing at 6 h and accumulating considerably by 24 h postinfection, consistent with reports of RSV infection of epithelial cells measured by other methods (3). A pronounced gradient of RSV gene expression was apparent in infected A549 cells, with amounts of transcripts diminishing dramatically in the order from NS1 to L. Reverse transcription with oligo(dT) primer, used in this particular experiment, results in a cDNA pool that contains not only reverse-transcribed copies of individual mRNA produced during RSV transcription but also copies of RSV antigenome. However, because antigenome would be represented similarly in reactions for different RSV genes, the differences between amplicon levels measured by reverse transcription-PCR (RT-PCR) here are attributed to differences in gene expression levels. For simplicity, hereafter we will refer to fluctuations in the different RSV amplicon levels measured in oligo(dT) cDNA as gene expression differences.
FIG. 1.
Establishing real-time PCR assay of RSV replication. (A) Amplification of various RSV genes from RSV antigenome inserted into a vector. Six 10-fold dilutions of antigenome-containing vector were used to generate a standard curve. CT values were plotted against log10 quantity of antigenome used as a template. Correlation coefficients (R2) of the best fit are shown next to the symbols corresponding to the seven RSV genes analyzed. Each point on this graph includes symbols for all seven genes. (B) Expression of RSV genes in infected A549 cells. A549 cells were infected with RSV at an MOI of 0.1. Six and 24 h after infection, total RNA was extracted from infected cells and reverse transcribed using oligo(dT) primer. Expression of mRNA for each RSV gene indicated was measured by real-time PCR. The relative level of each amplicon was normalized by β-actin mRNA level (also measured by real-time PCR) in the corresponding sample and expressed relative to the level of NS1 amplicon at 24 h. The insert shows gradient of RSV gene expression at 6 h on an adjusted y scale to highlight differences between levels of various amplicons. The results represent the means ± SEM for two different wells per time point.
The newly developed RT-PCR assay was next tested for its ability to accurately quantify viral load in infected cells. A549 cells were inoculated with RSV at various MOIs (0.001 to 0.1). Forty-eight hours after infection, cell supernatants were collected for viral titrations, while the cells themselves were lysed for RNA extraction. Viral yield in supernatants of A549 cells accurately represented the level of infection (Table 2). Cells infected with the 10-fold-increasing amount of RSV released virus in the quantities that also differed by ∼10-fold. When viral RNA from these cells was analyzed by RT-PCR using primers for RSV NS1 gene, the difference in the amount of NS1 amplicon detected in A549 cells also differed by ∼10-fold among cultures infected with RSV at MOIs of 0.001, 0.01, and 0.1. We also measured the level of negative-sense (genome) and positive-sense (antigenome) RNA in RSV-infected cells (Table 2). To do so, total RNA of infected cells was reverse transcribed using forward or reverse primer annealing to the genomic or antigenomic strand of RSV, respectively. The amount of reverse-transcribed product was then measured by real-time PCR using primers specific for RSV amplicon G or M proximal to the start of genome or antigenome fragment prepared, respectively. The amount of genome and antigenome detected increased progressively in samples infected with an increasing amount of RSV, similar to the pattern of viral gene expression (Table 2), although reflecting less precisely differences between MOIs of infection and viral yield in infected cells. Real-time PCR analysis of extracts of cells infected with RSV indicated that the RSV genome was about seven- to eightfold more abundant in infected cells than was the antigenome at 24 h and about three- to fourfold more abundant than was the antigenome at 48 h postinfection. These results are consistent with reports for other paramyxoviruses, in which the antigenome constitutes only 10 to 40% of genome in infected cells (23, 24, 34). Overall, these results indicate that the established real-time PCR assay can be used to accurately monitor RSV infection.
TABLE 2.
Comparison of RSV detection by viral culture and real-time PCR in RSV-infected epithelial cells
| Yield or expression level | MOI ata:
|
||
|---|---|---|---|
| 0.001 | 0.01 | 0.1 | |
| Viral yield (log10 PFU/ml) | 2.24 ± 0.06 | 3.26 ± 0.20 | 4.77 ± 0.25 |
| RSV gene expression levelb | 0.004 ± 0.001 | 0.069 ± 0.005 | 1 ± 0.1909 |
| RSV genome levelc | 0.0009 ± 0.0002 | 0.020 ± 0.003 | 1 ± 0.117 |
| RSV antigenome levelc | 0.0003 ± 0.0002 | 0.005 ± 0.002 | 0.269 ± 0.057 |
A549 alveolar epithelial cells were infected with RSV at an MOI of 0.001 to 0.1. Forty-eight hours after infection, cell supernatants were collected for the quantification of viral yield by plaque assay, while cells themselves were lysed and used as a source of RNA for real-time PCR analysis of RSV transcripts.
The expression of RSV NS1 amplicon was analyzed by real-time PCR, normalized by the expression of β-actin in the corresponding cell culture, and presented relative to the amount NS1 amplicon in infected culture with an MOI of 0.1 (assigned a relative value of 1).
Expression of RSV genome and antigenome was analyzed by real-time PCR, normalized by the level of β-actin in the corresponding cell culture, and presented relative to the amount genome in infected culture with an MOI of 0.1 (assigned a relative value of 1).
Abortive replication of RSV in the reinfection model.
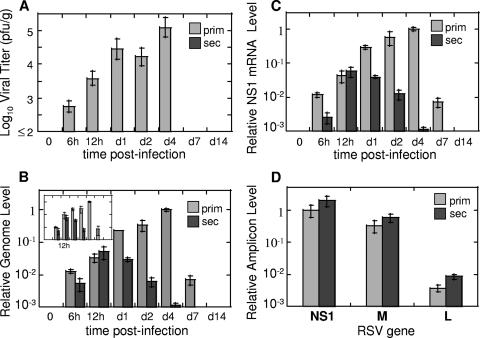
We applied the newly developed real-time PCR method to monitor the progression of RSV infection in vivo to compare the level of infectious virus that could be isolated from the lung to the amount of viral genetic material expressed in the same organ. The infection of naïve CRs with RSV (primary RSV infection) results in efficient virus replication in the lungs that reaches peak on day 4 postinfection and disappears by day 7 (Fig. 2A). If animals inoculated once with RSV are kept for 21 days and then rechallenged with RSV (secondary RSV infection), no virus is detected in the lungs of such animals (Fig. 2A).
FIG. 2.
RSV replication in vivo: evidence of abortive replication in the reinfection model. CRs were infected with RSV once for primary infection studies or twice (with an interval of 21 days) for secondary infection studies. At the indicated time points after the final challenge, lungs were collected for viral titer determination by plaque assay (A) or for analysis of RSV replication by real-time PCR (B to D). (A) Pulmonary viral titers in CRs with primary RSV infection (prim) and secondary RSV infection (sec). Viral titers were determined by plaque assay as previously described (limit of detection 2 log10 PFU/g) (33). (B) RSV genome and antigenome replication in primary and secondary RSV infection. RNA was extracted from the lungs of infected animals and reversed transcribed using primers complementary to the genomic or antigenomic strand of RSV. Relative genome (and antigenome [insert]) amounts were determined by real-time PCR, normalized by the β-actin mRNA level in the corresponding sample, and expressed relative to the level of genome (or antigenome [insert]) detected in the lungs of animals with primary RSV infection on day 4. (C) Expression of RSV genes during primary and secondary RSV infection. The RNA extracted from animals described above was reverse transcribed using oligo(dT) primer. The expression of NS1 mRNA was measured by real-time PCR, normalized by β-actin as described above, and expressed relative to the level of NS1 mRNA detected in the lungs of animals with primary RSV infection on day 4. (D) Gradient of RSV gene expression at 12 h postinfection in primary and secondary RSV disease. NS1, M, and L amplicon levels were measured by real-time PCR in cDNA prepared using oligo(dT) primer. The expression of each amplicon was normalized by β-actin and expressed relative to the level of NS1 mRNA detected in the lungs of animals with primary RSV infection. The results represent the means ± SEMs for four animals per time point for each primary and secondary infection.
Genome and antigenome synthesis in primary RSV infection (Fig. 2B) increased progressively following RSV challenge, reaching its peak on day 4 postinfection and diminishing after that. This pattern of genome/antigenome production paralleled the amount of infectious viral particles detected in the lungs of animals with primary RSV infection (Fig. 2A) on corresponding days. The only difference was seen at day 7, when no virus could be recovered from the lungs, and yet genomic and antigenomic strands of RSV could still be detected. We did not detect the persistence of RSV transcripts beyond day 7 for RSV infection in CRs, although RSV viral persistence has been reported for the BALB/c mouse model of RSV disease (37).
Analysis of viral RNA expression in secondary RSV infection demonstrated surprisingly high levels. As Fig. 2B demonstrates, secondary RSV infection was accompanied by efficient synthesis of both genomic and antigenomic strands of RSV. It reached peak at 12 h postinfection and declined afterwards to an undetectable level by day 7. The production of genomic/antigenomic RNA was indistinguishable between primary and secondary RSV infection at 12 h postinfection. After that, the amount of viral RNA in animals with secondary infection started to decrease, and the differences between primary and secondary infection increased in magnitude with each subsequent time point as infection progressed. Because the scales of changes in viral titer and RSV RNA production in Fig. 2A and B were set to be comparable (spanning 3 orders of magnitude each), the large discrepancy in the extent of viral RNA replication and production of infectious viral particles during secondary RSV infection can be easily appreciated. For example, while the amount of infectious viral particles on day 1 in primary RSV infection was at least 288-fold higher than that in secondary RSV infection (taking the 2 log10 PFU/g detection limit into account and allowing for the possibility of the presence of some undetectable virus in the lungs of the secondarily infected animals), the difference in RSV RNA level between primary and secondary infection was only eightfold. We also analyzed the expression of individual RSV genes during RSV infection in vivo. The expression of NS1, M, and L was followed for several days postinfection during primary and secondary RSV infection of CRs. The dynamics of RSV gene expression changes were very similar to those of genome/antigenome variations noted above and are exemplified by changes in the NS1 amplicon level shown in Fig. 2C. NS1 gene expression in secondary RSV infection increased between 6 and 12 h and declined afterwards, while NS1 expression in primary infection increased progressively past the 12-h time point, reached peak on day 4 postinfection, and declined afterwards. M and L amplicons followed identical patterns of time-dependent changes after RSV infection (data not shown). Similarly to the case for the genome/antigenome changes, the expression levels of RSV genes between primary and secondary infection were very similar at 12 h postinfection for each particular gene analyzed.
Abortive RSV replication is not associated with changes in viral gene expression gradient.
For some paramyxoviruses (e.g., measles virus), the difference between lytic and abortive infection is thought to be associated with a decrease in the expression of distal viral genes (7). We decided to investigate whether the gradient of RSV gene expression in secondary infection was different from that in primary RSV infection, possibly providing the explanation for the absence of budding virus during reinfection. Twelve hours postinfection was chosen for analysis, as similar levels of NS1 amplicon were detected at this time point between primary and secondary infection (Fig. 2C). The amounts of NS1, M, and L amplicons in animals 12 h after primary RSV infection were quantified by real-time PCR, and the ratio of NS1, M, and L gene transcripts was found to be 1.000:0.326:0.004 (Fig. 2D). An analysis of NS1, M, and L gene transcripts during secondary RSV infection revealed a 1.000:0.298:0.004 ratio. Therefore, similar gradients of RSV gene expression were detected in both primary and secondary RSV infection 12 h postinfection. The same ratio of NS1, M, and L gene transcripts was detected in animals with secondary infection on day 1 (data not shown), in spite of the fact that the overall level of gene expression was considerably lower in secondarily infected animals compared to animals with primary infection (Fig. 2C). This suggests that the pattern of RSV transcription is not impaired during secondary RSV infection and that the lack of productive infection is not associated with an imbalance of various RSV gene expression. Of interest is the fact that the gradient of RSV gene expression in the lung during RSV infection was somewhat different from the gradient of RSV gene expression in infected epithelial A549 cells. On average, there was a difference of about 250- to 900-fold between NS1 and L levels in RSV-infected lungs, while RSV-infected A549 cells had ∼100- to 200-fold more NS1 transcripts than L transcripts. This difference in the gradient of RSV gene expressions might reflect a wider variety of cells that get infected with RSV in the lung.
Antibodies prevent virus production, but not abortive RSV replication in the lung.
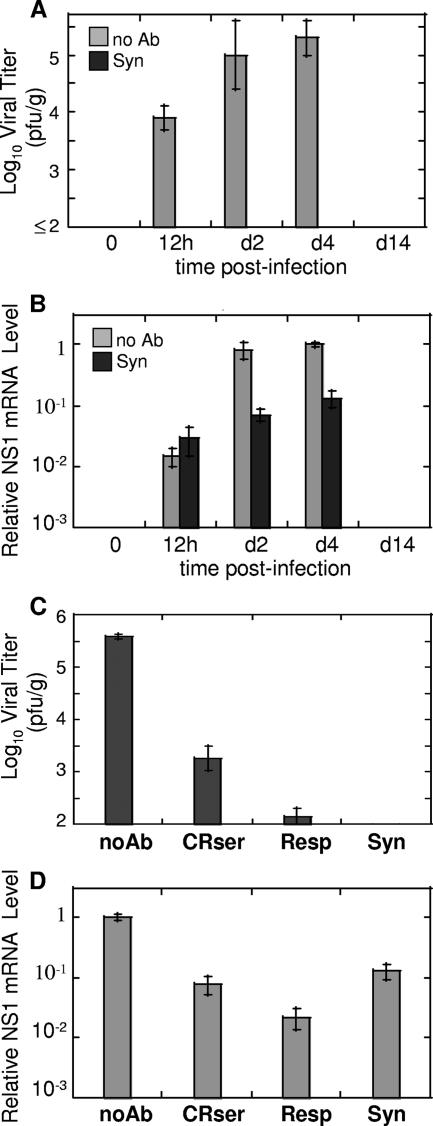
Experiments described above demonstrated that RSV replication occurs in immune animals early after RSV inoculation. This suggested that anti-RSV antibodies do not prevent infection of the lung. To address this issue in a more controlled manner, we used anti-RSV antibodies to block virus production during primary RSV infection. Monoclonal anti-RSV antibody palivizumab, currently used for prophylaxis of severe RSV disease in high-risk infants, was used for these experiments. Animals were inoculated with palivizumab 24 h prior to RSV infection. At various time points after infection (12 h and 2, 4, and 14 days), lungs were collected for analysis of viral load by plaque assay and analysis of RSV replication by real-time PCR. As shown in Fig. 3A, no virus was detected in the lungs of animals inoculated with palivizumab prior to RSV infection. However, when the expression of RSV genes was analyzed, high levels of NS1 amplicon were detected in the lungs of animals infected with RSV following antibody inoculation (Fig. 3B). Viral RNA levels were comparable between antibody-pretreated and untreated animals at 12 h postinfection and remained relatively high in antibody-treated animals on days 2 and 4. In fact, there was difference of only about 10-fold in the level of NS1 gene expression between antibody-treated and untreated animals on day 4 postinfection, a time when over 5 log10 PFU/g RSV was detected in the lungs of animals with primary RSV infection and no virus was recovered from the lungs of RSV-infected animals pretreated with palivizumab. A similar pattern of viral RNA expression was noted for other RSV genes and genome/antigenome in the lungs of palivizumab-treated animals (data not shown). This indicates that anti-RSV antibodies prevent the production of infectious viral particles in infected lungs but do not prevent the replication of viral RNA and the expression of viral genes.
FIG. 3.
Abortive replication of RSV in antibody-treated CRs. (A) Naïve CRs were infected with RSV following treatment with anti-RSV antibody palivizumab (Syn) or in the absence of antibody treatment (no Ab). Animals were sacrificed at the indicated times after infection, and viral titers were determined in the lungs by plaque assay. The results represent the means ± SEM for four animals per time point. No virus was detected in the lungs of animals infected with RSV after palivizumab treatment. (B) Real-time PCR analysis of RSV gene expression in the lungs of animals from the experiment described above. Levels of NS1 amplicon were measured by real-time PCR, normalized by the β-actin level in the corresponding organ, and expressed relative to the level of NS1 detected on day 4 in the lungs of naive animals challenged with RSV. (C and D) Abortive RSV replication in the lungs of CRs treated with anti-RSV immune CR serum (CRser) or with RSVIG (Resp). Control groups included CRs infected with RSV in the absence of antibody treatment (noAb) and CRs infected with RSV after palivizumab treatment (Syn). All animals were sacrificed on day 4 postinfection, and lungs were collected for viral titrations (C) and real-time PCR analysis of RSV NS1 expression (D). NS1 levels were expressed relative to the level of NS1 in the “noAb” group. The results represent the means ± SEM for four animals per treatment type.
To verify that the replication of viral RNA observed in palivizumab-treated animals was not specific to the type of antibody used to inhibit RSV replication, we carried out analogous experiments using serum from RSV-immune CRs or human anti-RSV antibody preparation RSVIG. Animals were treated with CR anti-RSV serum or with RSVIG prior to and following RSV infection and were sacrificed on day 4 for analysis of viral load and viral RNA expression. As shown in Fig. 3C, treatment either with immune CR serum or with RSVIG significantly diminished viral load in the lungs of RSV-infected animals on day 4 postinfection (neither of the antibody preparations, however, was as effective as palivizumab in blocking RSV replication). An analysis of viral RNA expression, exemplified by NS1 amplicon in Fig. 3D, indicated that large amounts of RSV transcripts were present in the lungs of animals treated either with CR immune serum or with RSVIG and infected with RSV. The difference between amounts of RSV transcripts in the lungs was much smaller than the difference in the amount of infectious viral particles recovered from the lungs of these animals. For example, while the amount of NS1 transcripts in the lungs of CR serum-treated animals was ∼10-fold lower than that present in the lungs of untreated, RSV-infected animals (0.079 ± 0.022 versus 1.00 ± 0.101), the amount of virus particles detected in the lungs of animals treated with CR serum was ∼200-fold lower than that present in the lungs of untreated, RSV-infected animals (3.26 ± 0.024 versus 5.58 ± 0.05 log10 PFU/g). Similarly, the difference in the amount of viral transcripts and viral load in RSV-infected animals treated with RSVIG and in untreated animals was ∼50-fold (0.022 ± 0.008 versus 1.00 ± 0.101) and >2,000-fold (2.15 ± 0.015 versus 5.58 ± 0.05 log10 PFU/g), respectively. Furthermore, even though these differences in viral gene expression and pulmonary viral load were less than those seen in palivizumab-treated animals (probably due to the different nature of antibodies used and different routes of antibody administration [30]), they provide another evidence of abortive viral replication of RSV under conditions of preexisting immunity.
Antibodies do not completely prevent infection of epithelial cells and macrophages in vitro.
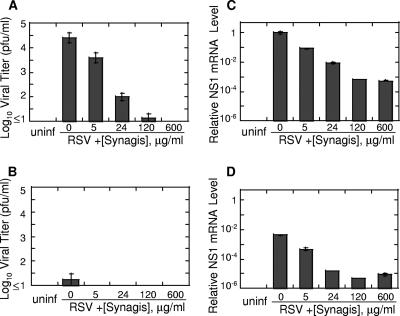
The finding that RSV infection in vivo occurs in immune animals prompted us to explore the effect of neutralizing antibodies on RSV replication in vitro. RSV primarily infects and replicates in the superficial layer of respiratory epithelium (28); however, it is also capable of infecting other cell types, including monocytes, macrophages, and dendritic cells (2, 9, 12). And even though efficient expression of RSV genes in these cells has been noted, minimal to no viral release was documented, suggesting that RSV replication in these cells is abortive (9, 12). We performed experiments to analyze RSV infection of epithelial cells (A549) and macrophages (S. hispidus peritoneal macrophages) in the presence or absence of RSV-neutralizing antibodies (Fig. 4). In agreement with findings from other laboratories (9, 12), we observed little RSV release from infected macrophages, although efficient viral gene expression was seen in these cells. Anti-RSV antibodies inhibited the infection of both epithelial cells and macrophages. The inhibition, however, was incomplete, and a certain level of viral gene expression was visible in cells infected in the presence of antibody concentrations, precluding detectable viral release (e.g., 600 μg/ml palivizumab). These results suggest that a subpopulation of virus might be refractory to the neutralizing effect of antibodies.
FIG. 4.
Antibodies do not completely prevent infection of epithelial cells and macrophages in vitro. Human A549 alveolar epithelial cells (4 × 105 cells/well) (A and C) and CR peritoneal macrophages (2.5 × 106 cells/well) (B and D) were infected with RSV (MOI of 0.1 to 0.5) preincubated with the indicated amounts of palivizumab (Synagis). Twenty-four hours after infection, cell supernatants were harvested for viral quantification by plaque assay (limit of detection, 1 log10 PFU/g) (A and B), while cells were used for analysis of RSV gene expression (C and D). Total RNA was extracted from infected cells and reverse transcribed using oligo(dT) primer. The expression of mRNA for NS1 gene was measured by real-time PCR, normalized by β-actin mRNA level in the corresponding sample, and expressed relative to the level of NS1 amplicon in A549 cells infected with RSV untreated with antibody (0 μg/ml palivizumab). Uninfected cells (uninf) were used as a negative control. The results represent the means ± SEM for two different wells per treatment type.
DISCUSSION
In this work, we established a method for monitoring RSV infection by real-time PCR and applied this method for analysis of RSV replication in vivo in naïve animals and in animals immune to RSV. We found that RSV reinfection of animals infected 21 days earlier results in increasing expression of viral transcripts and genome replication but does not lead to the production of detectable progeny virus. This type of replication, therefore, can be termed “abortive,” as RSV is capable of entering the cells in the lungs of immune animals, yet the production of progeny viruses is impaired. The skewing of gradient of viral gene expression at the level of mRNA, a mechanism documented during latent infection by other viruses (7), is not likely to be responsible for abortive replication of RSV during reinfection, as similar patterns of RSV gene mRNA expression were observed between animals with primary and secondary infection. We found that passive administration of antibodies to RSV prevents productive infection normally accompanied by viral release in the lung, but it does not prevent abortive replication of the virus. To the best of our knowledge, this is the first evidence of abortive replication of RSV in vivo.
The abortive RSV replication we observed in vivo might be due to several factors. First, the infection of respiratory epithelial cells during reinfection might proceed to some extent in spite of the presence of high titers of neutralizing antibodies in immune animals, but it would be blocked at some yet-unidentified step required for the production and release of large amounts of progeny virus. While this is an intriguing possibility, it is hard to reconcile with the classical definitions of viral neutralization by antibodies, which state that neutralizing antibodies block viral infectivity by interfering with viral attachment and/or entry (22). For some animal viruses (e.g., poliovirus and Aleutian mink disease parvovirus), however, neutralized virus was shown to penetrate cells at the same ratio and to the same degree as did unneutralized virus (1, 25), suggesting that neutralization of viral infectivity by antibodies might occur on subcellular level. Moreover, our in vitro experiments with cultured cells suggest that a fraction of virus might be refractory to the neutralizing effect of antiviral antibodies and may establish abortive infection in the presence of such antibodies. In immune animals with secondary RSV infection, abortive replication of RSV subsides on day 2 postinfection, while it is seen extending beyond day 2 in naïve animals treated with anti-RSV antibodies prior to infection. Because abortive viral replication in animals with only humoral immunity lasts longer than that in animals with both humoral and cellular immunity, cellular immunity may play an important role in terminating abortive replication of RSV.
Abortive replication of RSV might be important for several basic aspects of RSV biology as we know it. In the simplest case, it may indicate that reinfection with RSV occurs more frequently than currently appreciated. Typically, RSV infection is diagnosed by the isolation of virus from respiratory secretions of infected individuals. These cases would represent children infected with RSV for the first time as well as children and adults reinfected with RSV after a period of time sufficient for the immune protection against virus to wane. However, our results suggest that reinfections might also occur shortly after previous challenge and happen at a time when immune response is sufficiently strong to suppress productive infection, but not abortive replication, of the virus. Clinically, these reinfections would be characterized by the expression of viral antigens in the absence of virus production and may go undetected if viral cultures are used to monitor disease. Notably, results of several recent human studies support the possibility of frequent “abortive” RSV infections. These studies, in which respiratory secretions were analyzed for RSV presence by viral culture and by RT-PCR, revealed that consistently more samples test positive for RSV by RT-PCR than by culture (10, 11, 20, 29). These results might indicate that some individuals with RSV detected by RT-PCR, but not by culture, might have recently recovered from RSV and are currently undergoing reinfection accompanied by abortive replication of the virus.
Abortive replication may have important implications for the establishment of both adaptive and innate immune responses to RSV. RSV is a poor inducer of long-term immunological memory. Repeated expression of RSV antigens during frequent reinfections may impair the balance between the establishment of short-term memory and the establishment of long-term memory. Persistent stimulation by reencountered antigen is known to promote short-term memory (26). Additionally, the capacity of memory lymphocytes to proliferate is not unlimited, and continuous viral antigen stimulation might reduce the memory lymphocyte pool (27). This might result in a suboptimal memory response similar to the one documented for memory responses to intracellular parasites (5). Abortive replication of RSV might also be important for aberrant stimulation of the innate immune system during reinfection. For example, RSV replication and transcription involves the formation of single- and double-stranded RNA replication intermediates, which can serve as viral molecular patterns recognized by Toll-like receptors (e.g., TLR7/8 and TLR3) and can modulate the activation state of a variety of cells, including macrophages, dendritic cells, and natural killer cells (19, 36, 40). Frequent activation of the cells of the innate immune system by repeated infections with RSV in children may result in an altered microenvironment of the lung, predisposing children to inflammatory allergic disorders.
Acknowledgments
We thank Marissa Stock for assistance with viral titrations.
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, grant AI-057575 (J.C.G.B.), and by Virion Systems, Inc., corporate funds.
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Alexandersen, S., S. Larsen, A. Cohn, A. Uttenthal, R. E. Race, B. Aasted, M. Hansen, and M. E. Bloom. 1989. Passive transfer of antiviral antibodies restricts replication of Aleutian mink disease parvovirus in vivo. J. Virol. 63:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold, R., B. Konig, H. Galatti, H. Werchau, and W. Konig. 1995. Cytokine (IL-8, IL-6, TNF-α) and soluble TNF receptor-I release from human peripheral blood mononuclear cells after respiratory syncytial virus infection. Immunology 85:364-372. [PMC free article] [PubMed] [Google Scholar]
- 3.Bermingham, A., and P. L. Collins. 1999. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc. Natl. Acad. Sci. USA 96:11259-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco, J. C., L. Pletneva, M. Boukhvalova, J. Y. Richardson, K. A. Harris, and G. A. Prince. 2004. The cotton rat: an underutilized animal model for human infectious diseases can now be exploited using specific reagents to cytokines, chemokines, and interferons. J. Interferon Cytokine Res. 24: 21-28. [DOI] [PubMed] [Google Scholar]
- 5.Brake, D. A. 2003. Parasites and immune responses: memory illusion? DNA Cell Biol. 22:405-419. [DOI] [PubMed] [Google Scholar]
- 6.Cannon, M. J., P. J. Openshaw, and B. A. Askonas. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 168:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattaneo, R., G. Rebmann, K. Baczko, V. ter Meulen, and M. A. Billeter. 1987. Altered ratios of measles virus transcripts in diseased human brains. Virology 160:523-526. [DOI] [PubMed] [Google Scholar]
- 8.Collins, P. L., and G. W. Wertz. 1983. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc. Natl. Acad. Sci. USA 80:3208-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Graaff, P. M., E. C. de Jong, T. M. van Capel, M. E. van Dijk, P. J. Roholl, J. Boes, W. Luytjes, J. L. Kimpen, and G. M. van Bleek. 2005. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J. Immunol. 175:5904-5911. [DOI] [PubMed] [Google Scholar]
- 10.Falsey, A. R., M. A. Formica, J. J. Treanor, and E. E. Walsh. 2003. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. J. Clin. Microbiol. 41:4160-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falsey, A. R., M. A. Formica, and E. E. Walsh. 2002. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J. Clin. Microbiol. 40:817-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franke-Ullmann, G., C. Pfortner, P. Walter, C. Steinmuller, M. L. Lohmann-Matthes, L. Kobzik, and J. Freihorst. 1995. Alteration of pulmonary macrophage function by respiratory syncytial virus infection in vitro. J. Immunol. 154:268-280. [PubMed] [Google Scholar]
- 13.Glezen, W. P., L. H. Taber, A. L. Frank, and J. A. Kasel. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140:543-546. [DOI] [PubMed] [Google Scholar]
- 14.Graham, B. S., L. A. Bunton, J. Rowland, P. F. Wright, and D. T. Karzon. 1991. Respiratory syncytial virus infection in anti-μ-treated mice. J. Virol. 65:4936-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Investig. 88:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta, C. K., J. Leszczynski, R. K. Gupta, and G. R. Siber. 1996. Stabilization of respiratory syncytial virus (RSV) against thermal inactivation and freeze-thaw cycles for development and control of RSV vaccines and immune globulin. Vaccine 14:1417-1420. [DOI] [PubMed] [Google Scholar]
- 17.Han, L. L., J. P. Alexander, and L. J. Anderson. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. Infect. Dis. 179:25-30. [DOI] [PubMed] [Google Scholar]
- 18.Hardy, R. W., S. B. Harmon, and G. W. Wertz. 1999. Diverse gene junctions of respiratory syncytial virus modulate the efficiency of transcription termination and respond differently to M2-mediated antitermination. J. Virol. 73:170-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornung, V., M. Guenthner-Biller, C. Bourquin, A. Ablasser, M. Schlee, S. Uematsu, A. Noronha, M. Manoharan, S. Akira, A. de Fougerolles, S. Endres, and G. Hartmann. 2005. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 11:263-270. [DOI] [PubMed] [Google Scholar]
- 20.Hu, A., M. Colella, J. S. Tam, R. Rappaport, and S. M. Cheng. 2003. Simultaneous detection, subgrouping, and quantitation of respiratory syncytial virus A and B by real-time PCR. J. Clin. Microbiol. 41:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, Y. T., and G. W. Wertz. 1982. The genome of respiratory syncytial virus is a negative-stranded RNA that codes for at least seven mRNA species. J. Virol. 43:150-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klasse, P. J., and Q. J. Sattentau. 2002. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J. Gen. Virol. 83:2091-2108. [DOI] [PubMed] [Google Scholar]
- 23.Kolakofsky, D., and A. Bruschi. 1975. Antigenomes in Sendai virions and Sendai virus-infected cells. Virology 66:185-191. [DOI] [PubMed] [Google Scholar]
- 24.Kolakofsky, D., P. F. Spahr, and H. Koprowski. 1974. Comparison of 6-94 virus and Sendai virus RNA by RNA-RNA hybridization. J. Virol. 13:935-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandel, B. 1967. The interaction of neutralized poliovirus with HeLa cells. I. Adsorption. Virology 31:238-247. [DOI] [PubMed] [Google Scholar]
- 26.Masopust, D., J. Jiang, H. Shen, and L. Lefrancois. 2001. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166:2348-2356. [DOI] [PubMed] [Google Scholar]
- 27.Moskophidis, D., E. Laine, and R. M. Zinkernagel. 1993. Peripheral clonal deletion of antiviral memory CD8+ T cells. Eur. J. Immunol. 23:3306-3311. [DOI] [PubMed] [Google Scholar]
- 28.Neilson, K. A., and E. J. Yunis. 1990. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr. Pathol. 10:491-502. [DOI] [PubMed] [Google Scholar]
- 29.Perkins, S. M., D. L. Webb, S. A. Torrance, C. El Saleeby, L. M. Harrison, J. A. Aitken, A. Patel, and J. P. DeVincenzo. 2005. Comparison of a real-time reverse transcriptase PCR assay and a culture technique for quantitative assessment of viral load in children naturally infected with respiratory syncytial virus. J. Clin. Microbiol. 43:2356-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prince, G. A., V. G. Hemming, R. L. Horswood, P. A. Baron, and R. M. Chanock. 1987. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J. Virol. 61:1851-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince, G. A., V. G. Hemming, R. L. Horswood, and R. M. Chanock. 1985. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 3:193-206. [DOI] [PubMed] [Google Scholar]
- 32.Prince, G. A., A. B. Jenson, V. G. Hemming, B. R. Murphy, E. E. Walsh, R. L. Horswood, and R. M. Chanock. 1986. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J. Virol. 57:721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prince, G. A., A. B. Jenson, R. L. Horswood, E. Camargo, and R. M. Chanock. 1978. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am. J. Pathol. 93:771-791. [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson, W. S. 1970. Self-annealing of subgroup 2 myxovirus RNAs. Nature 225:944-945. [DOI] [PubMed] [Google Scholar]
- 35.Schauer, U., S. Hoffjan, J. Bittscheidt, A. Kochling, S. Hemmis, S. Bongartz, and V. Stephan. 2002. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur. Respir. J. 20:1277-1283. [DOI] [PubMed] [Google Scholar]
- 36.Schröder, M., and A. G. Bowie. 2005. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 26:462-468. [DOI] [PubMed] [Google Scholar]
- 37.Schwarze, J., D. R. O'Donnell, A. Rohwedder, and P. J. Openshaw. 2004. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am. J. Respir. Crit. Care Med. 169:801-805. [DOI] [PubMed] [Google Scholar]
- 38.Shay, D. K., R. C. Holman, G. E. Roosevelt, M. J. Clarke, and L. J. Anderson. 2001. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among U.S. children, 1979-1997. J. Infect. Dis. 183:16-22. [DOI] [PubMed] [Google Scholar]
- 39.Sigurs, N., R. Bjarnason, F. Sigurbergsson, and B. Kjellman. 2000. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 161:1501-1507. [DOI] [PubMed] [Google Scholar]
- 40.Sioud, M. 2005. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence dependent and requires endosomal localization. J. Mol. Biol. 348:1079-1090. [DOI] [PubMed] [Google Scholar]
- 41.Stein, R. T., D. Sherrill, W. J. Morgan, C. J. Holberg, M. Halonen, L. M. Taussig, A. L. Wright, and F. D. Martinez. 1999. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354:541-545. [DOI] [PubMed] [Google Scholar]
- 42.Taylor, G., E. J. Stott, M. Bew, B. F. Fernie, P. J. Cote, A. P. Collins, M. Hughes, and J. Jebbett. 1984. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology 52:137-142. [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, N. Cox, L. J. Anderson, and K. Fukuda. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179-186. [DOI] [PubMed] [Google Scholar]
- 44.Wendt, C. H., and M. I. Hertz. 1995. Respiratory syncytial virus and parainfluenza virus infections in the immunocompromised host. Semin. Respir. Infect. 10:224-231. [PubMed] [Google Scholar]