Abstract
The p12I protein of human T-cell leukemia/lymphoma virus type 1 (HTLV-1) is a small oncoprotein that increases calcium release following protein kinase C activation by phorbol myristate acetate, and importantly, this effect is linker for activation of T cells (LAT) independent. Here, we demonstrate that p12I inhibits the phosphorylation of LAT, Vav, and phospholipase C-γ1 and decreases NFAT (nuclear factor of activated T cells) activation upon engagement of the T-cell receptor (TCR) with anti-CD3 antibody. Furthermore, we demonstrate that p12I localizes to membrane lipid rafts and, upon engagement of the TCR, relocalizes to the interface between T cells and antigen-presenting cells, defined as the immunological synapse. A p12I knockout molecular clone of HTLV-1 expresses more virus upon antigen stimulation than the isogenic wild type, suggesting that, by decreasing T-cell responsiveness, p12I curtails viral expression. Thus, p12I has contrasting effects on TCR signaling: it down-regulates TCR in a LAT-dependent manner on one hand, and on the other, it increases calcium release in a LAT-independent manner. The negative regulation of T-cell activation by p12I may have evolved to minimize immune recognition of infected CD4+ T cells, to impair the function of infected cytotoxic CD8+ T cells, and to favor viral persistence in the infected host.
Human T-cell leukemia/lymphoma virus type 1 (HTLV-1) causes a rare, aggressive hematopoietic malignancy of mature T cells, designated adult T-cell leukemia/lymphoma, as well as a progressive myelopathy defined as HTLV-1-associated myelopathy/tropical spastic paraparesis (18, 20, 25, 42, 55). HTLV-1 infects human CD4+ and CD8+ (22, 33, 35, 39, 43) T cells of memory phenotype and is believed to confer a proliferative and survival advantage on infected memory T-cell clones, with consequent accumulation of somatic mutations and neoplastic transformation (16). The provirus is thought to be mostly latent, mainly in resting T cells (21). However, once T cells are stimulated by antigen, the proviral DNA can be expressed, endangering the survival of the infected cells because of immune recognition.
The HTLV-1 genome, in addition to structural (Gag and Env) and enzymatic (Pol) proteins, encodes positive regulators of viral expression from open reading frames (ORFs) III and IV, such as Tax and Rex, as well as two negative regulators, p30II (ORF II) (40) and HBZ, which is encoded from the minus strand RNA (19). ORF I encodes a 12-kDa protein (p12I) that in cells is localized in the endoplasmic reticulum (ER)/Golgi (13, 28, 32). The p12I protein has been shown to have multiple functions. p12I interacts with the interleukin-2 receptor (IL-2R) β and γc chains, increases STAT-5 activation, and decreases the threshold of the IL-2 requirement for T-cell proliferation (38, 41) in primary human lymphocytes. In the ER/Golgi, p12I binds both the IL-2 receptor chains and the major histocompatibility complex (MHC) class I heavy chain and interferes with their trafficking to the cell surface (28, 38). p12I also binds to calreticulin and calnexin (13) and increases calcium release and nuclear factor of activated T cells (NFAT) activation upon phorbol myristate acetate (PMA) stimulation of T cells. This effect bypasses antigen receptor ligation, as it also occurs in linker for activation of T cells (LAT)-deficient T cells (1, 12, 29).
T-cell receptor (TCR) signaling occurs through a cascade of events (51, 52) that involve activation of the protein tyrosine kinases Lck and Fyn, which phosphorylate the tyrosines in the cytosolic domains of TCRζ and the CD3 subunits. These phosphorylated domains become the docking sites for ZAP70, which is phosphorylated and activated when bound. Activated ZAP70 phosphorylates LAT, which then binds Grb2, phospholipase C-γ1 (PLC-γ1), and the p85 subunit of phosphatidylinositol 3-kinase and, indirectly, Vav, Cb1, and SLP76. The recruitment of these critical molecules to the membrane ultimately results in multiple events, including calcium release, dephosphorylation and nuclear translocation of NFAT, enhancement of transcription, cytokine production, and T-cell proliferation.
These events lead over time to protein and lipid rearrangements that occur during the formation of the immunological synapse between the T cell and the antigen-presenting cells (APCs) (7, 37). These membrane rearrangements include effects on the lipid rafts, a heterogeneous mixture of components on the surfaces of cells that includes the glycosphingolipid- and cholesterol-rich microdomains and proteins that are enriched in these regions (46). The function of immune synapse formation and the various molecular sorting events that accompany it are controversial but may include effects on signaling and down-regulation of various molecules (5, 48).
TCR stimulation of HTLV-1-infected T cells and IL-2 production could be deleterious to viral persistence, as the viral transactivator, Tax, increases viral expression by exploiting CREB/ATF that is activated by IL-2 (50). Thus, viral expression is likely maintained at a very low level in vivo to minimize the elimination of the infected T cells by the adaptive immune response. In support of this notion is the fact that the virus has evolved at least two negative regulators of viral expression: HBZ, which decreases Tax-mediated transcriptional activation (19), and p30II, which decreases the cytoplasmic level of the Tax/Rex mRNA (40).
p12I has been shown to have an effect on T-cell activation. In the presence of strong antigens, such as phytohemagglutinin or PMA, p12I increases STAT-5 activation, NFAT transcriptional activity, and calcium release (12, 29, 41), and the last effect is LAT independent. Surprisingly, we found here that p12I down-regulates NFAT activity in a LAT-dependent manner following engagement of the TCR with either the anti-CD3 antibody or MHC-bound antigen. Furthermore, we demonstrate that p12I accesses and clusters within the lipid rafts (8, 46) and is recruited to the immunological synapse upon engagement of the TCR by MHC-bound antigen. p12I decreases the phosphorylation of PLC-γ1 and Vav molecules involved in proximal TCR signaling and interacts with LAT. Furthermore, genetic knockout (KO) of p12I expression in an HTLV-1 molecular clone resulted in an augmentation of viral production only following engagement of the TCR. Thus, p12I indirectly regulates viral expression. Taken together, these results suggest that HTLV-1 has evolved p12I as a function to favor viral persistence by dampening the responsiveness to TCR stimulation of T cells carrying the provirus.
MATERIALS AND METHODS
Plasmids, immunoprecipitation, and Western blotting.
The pME p12I expression plasmid used here was modified by deleting amino acids S and L within the HA1 tag carboxy terminus of pME p12I as described by Koralnik et al. (31), as these two amino acids revealed an endosomal sorting motif that affects protein expression. The plasmid pCEFL-LAT-Myc was described previously (6, 56, 57). pNFAT-TA-luciferase and pTK-Renilla were obtained from Clontech (Palo Alto, CA) and Promega (Madison, WI), respectively. The anti-HA1 (12CA5) and anti-HA1-horseradish peroxidase (3F10-hrp) antibodies were obtained from Roche (Indianapolis, IN); the anti-Lck (3A5), anti-c-Myc (9E10), anti-PLC-γ1 (E-12), anti-Vav (C-14), and anti-Lyn (H-6) antibodies from Santa Cruz (Santa Cruz, CA); and the anti-CD71 antibody from Zymed (San Francisco, CA). Anti-β-tubulin (TUB 2.1) was from Sigma (St. Louis, MO); anti-LAT (2E9) and anti-PY (4G10) were from Upstate (Lake Placid, NY).
Total cell lysates were extracted with 1% NP-40 RIPA buffer with 50 mM Tris-HCl (7.5) and 150 mM NaCl in the presence of 20 μg/ml leupeptin, 1 mM 4-(2-aminoethyl)benzensulfonyl fluoride hydrochloride (AEBSF), 1 mM Na3VO4, and 20 μg/ml aprotinin, and insoluble materials were removed by centrifugation. Immunoprecipitations were performed, after lysates were precleared with 10 μl of protein G-agarose (Roche), by adding the appropriate antibodies and protein G-agarose and washing them three times in RIPA buffer. In some experiments, 60 mM N-octylglucoside was added in RIPA buffer (Sigma). The sample suspension was boiled in the presence of 10% β-mercaptoethanol and loaded on a gel for electrophoresis.
Transfection and gradient flotation assays.
Jurkat T, J.CaM2.5, and Raji cells were kept in RPMI 1640, and 293T cells were kept in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 5 mM l-glutamine. Jurkat T and J.CaM2.5 cells were transfected with DMRIE-C (Invitrogen, Carlsbad, CA) and 293T cells with Effectene (QIAGEN, Valencia, CA).
For the flotation assay, 2 × 107 cells were lysed at 0°C in 1 ml of 0.2% Triton X-100 in MNE buffer (25 mM MES [morpholineethanesulfonic acid], pH 6.5, 150 mM NaCl, 5 mM EDTA) containing 20 μg/ml of aprotinin, 1 mM AEBSF, and 1 mM sodium orthovanadate; subjected to 10 strokes of a tight-fitting Dounce homogenizer; and mixed with 1 ml of 80% sucrose in MNE buffer. The lysate was transferred to a centrifuge tube and overlaid with 2 ml of 30% sucrose and 1 ml of 5% sucrose. After centrifugation for 20 h at 200,000 × g, 0.4-ml fractions were collected, starting from the top of the gradient buffer. The pellet fraction was suspended in 0.4 ml of 1% Triton in MNE buffer and mixed with sodium dodecyl sulfate sample buffer. Five microliters of individual fractions was subjected to polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane, which was reacted with specific antibody.
T-cell stimulation and detection of the immunological synapse.
Jurkat T cells were spun down and treated with 1 μg/ml or the indicated concentrations of anti-CD3 (UCH-T1; EMD Biosciences, La Jolla, CA), anti-CD28 (BD, Franklin Lakes, NJ), or both in the serum-free medium. In some experiments, cells were treated with PMA at 20 ng/ml. After 1 h of incubation at 37°C, an equal amount of medium with 20% fetal calf serum was overlaid on the cells, and samples were incubated at 37°C to allow luciferase expression. For analysis of phosphorylated proteins following TCR stimulation, cells were lysed in RIPA buffer.
In the cocultivation experiments, Jurkat T and Raji cells were prepared separately at a concentration of 1 × 106 cells/ml in the fresh medium. Raji cells were prepulsed with or without 10 μg/ml of superantigen staphylococcal enterotoxin E (SEE) (Toxin Technology, Sarasota, FL) for 1 h at 37°C. The cells were combined at a 1:1 ratio and fixed by adding 4% paraformaldehyde at each time point. In parallel, cells were incubated for the indicated time and lysates were obtained for the luciferase assay. In some experiments, cells were cocultivated in the presence of TransWell with a pore size of 0.4 μm (Corning, Corning, NY).
Some of the cocultivation experiments were performed in the presence of Zerit (Bristol-Myers Squibb Virology, Princeton, NJ) (24). We treated long terminal repeat (LTR)-driven luciferase-expressing Raji cells with or without 35 μM of Zerit the day before cocultivation with Jurkat T cells. The cocultures were maintained in the presence of Zerit for 72 h.
For the confocal microscopy, 1 × 105 cells were fixed and spun on a glass slide coated with poly-l-lysine. After being washed with phosphate-buffered saline, the cells were permeabilized with 0.1% saponin for 60 min at 37°C. The cells were then stained with fluorescein isothiocyanate (FITC)-conjugated cholera toxin A (Co-Tx) (FITC-Co-Tx) or anti-HA1 and anti-CD3, followed by FITC-labeled or Cy3-labeled anti-mouse antibodies. Cells were visualized with a Zeiss laser scanning confocal microscope (LSM 510; Carl Zeiss MicroImaging, Thornwood, NY) with a 100×/1.3 natural aperture Plan Apochromat oil objective and 3× zoom and visualized as maximum projections of Z stacks of focal-plane sections through entire cell volumes.
Luciferase assay.
The Dual-Luciferase Reporter Assay System was obtained from Promega (Madison, WI), and all the measurements were performed according to the manufacturer's protocol. The same samples were used for the Bio-Rad (Hercules, CA) Protein Assay to determine the amount of protein used.
Quantification of HTLV p19 Gag.
Direct or diluted cell culture supernatants were used in an HTLV p19 Gag enzyme-linked immunosorbent assay using a kit obtained from the ZeptoMetrix Corporation (Buffalo, NY). All the measurements were performed according to the manufacturer's protocol.
RESULTS
The HTLV-1 p12I protein down-regulates TCR signaling.
Others have demonstrated that p12I increases calcium release and NFAT activity in T cells stimulated with PMA, which directly activates a protein kinase C (PKC) isoform downstream of TCR (12, 29). The PMA-mediated activation of NFAT by p12I is LAT independent and occurs in the LAT-deficient Jurkat T cells (J.CaM2.5), in which phosphorylation of PLC-γ1 and Vav is also lost (12).
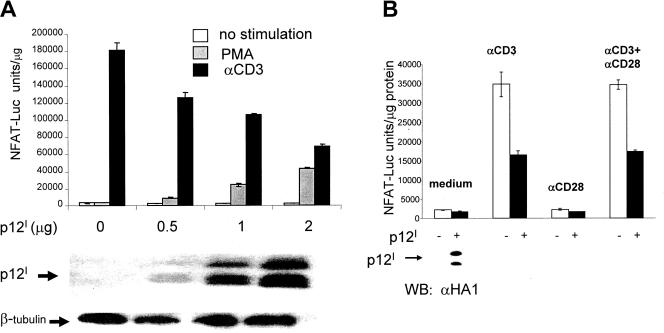
In the present study, we demonstrate that p12I displays opposite effects upon engagement of the TCR through ligation with anti-CD3 antibody and decreases T-cell activation (Fig. 1A). Transfection of Jurkat T cells with p12I resulted in NFAT activation when PMA was used as a stimulus, as observed previously (12, 29); however, CD3 ligation resulted in a decrease in NFAT activation, and both effects were dependent on the dose of p12I (Fig. 1A). Costimulation with anti-CD28 antibody did not restore NFAT activity (Fig. 1B), and the down-regulation of TCR signaling by p12I was LAT dependent, as coexpression of LAT (LAT-Myc) was required in LAT-deficient J.CaM2.5 cells to observe p12I down-regulation of NFAT (data not shown).
FIG. 1.
Contrasting effects of the HTLV-1 p12I protein on TCR and PKC signaling in Jurkat T cells. (A) (Top) Jurkat T cells were cotransfected with p12I at different doses (μg/106 cells) and with NFAT reporter plasmid and stimulated with nothing, with anti-CD3 (αCD3), or with PMA. NFAT activity was measured at 18 h by measuring the levels of luciferase in cell lysates. The level of luciferase activity was normalized to the protein content of the cell lysates. The average and the standard deviation of triplicate samples are shown. This is a representative experiment out of four independent experiments. (Bottom) The expression of the p12IHA1-tagged protein was verified by Western blotting on total cell lysate with antibodies to HA1. Antibody to β-tubulin was also used in Western blotting to verify equivalent protein loading. (B) Jurkat T cells cotransfected with the NFAT reporter plasmid and either p12I (2 μg/106 cells) or control vector were left unstimulated or were stimulated with either anti-CD3 or anti-CD28 alone or both for 18 h. NFAT activity was normalized to the protein content as in panel A, and a duplicate experiment is presented with the standard error. p12I expression was assessed by Western blot (WB) analysis in transfected cells (bottom).
The p12I protein decreases phosphorylation of LAT and proximal TCR signaling molecules.
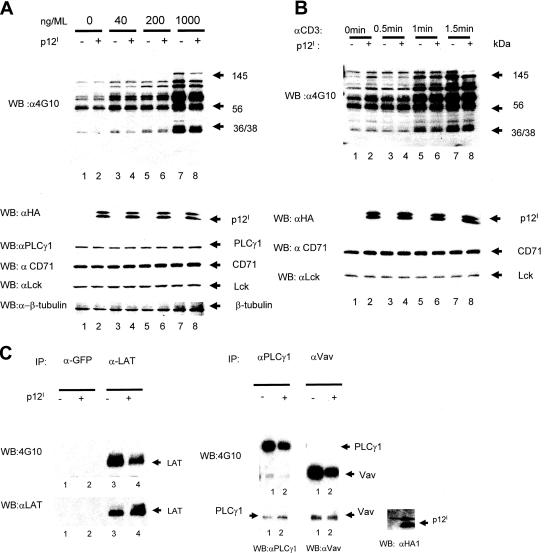
To begin to understand the molecular mechanism of NFAT down-regulation induced by p12I, we investigated whether the phosphorylation of cellular proteins involved in the TCR signaling cascade was affected (51, 52). The lysates of Jurkat T cells expressing p12I or not were stimulated with the indicated doses of anti-CD3 antibody, and cell lysates were reacted with the anti-phosphotyrosine antibody 4G10. We observed a decreased level of phosphorylation of cellular proteins that ranged in mass between 36 and 145 kDa in cells expressing p12I (Fig. 2A), which occurred within 1 min of anti-CD3 stimulation (Fig. 2B). Next, we analyzed by immunoprecipitation and Western blotting the expression and phosphorylation of proximal TCR signaling molecules, such as PLC-γ1, Vav, Lck, and LAT. Western blotting with the specific antibodies was used to control for equivalent levels of immunoprecipitates.
FIG. 2.
The HTLV-1 p12I protein decreases the phosphorylation of PLC-γ1, Vav, and LAT upon TCR engagement. (A) (Top) Jurkat T cells transfected with p12I or sham DNA plasmids were treated with anti-CD3 antibody at increasing concentrations. Total cell lysates were obtained 2 min after pulse and analyzed by Western blotting (WB) using the anti-phosphotyrosine antibody (4G10). (Bottom) The membrane was also treated with anti-hemagglutinin (αHA) to verify p12I expression, as well as anti-PLC-γ1, anti-transferrin receptor, anti-Lck, and anti-β-tubulin to control for equivalent protein loading. (B) Jurkat T cells transfected with p12I or sham DNA plasmids were treated with 1 μg/ml anti-CD3, and cell lysates were harvested at the indicated time points. Equal amounts of lysates were analyzed by Western blotting with 4G10. The same membrane was probed with anti-HA to verify p12I expression and with anti-transferrin receptor (CD71) and anti-Lck to control for equivalent amounts of proteins. (C) The lysates of Jurkat T cells expressing p12I stimulated with anti-CD3 antibody (1 μg/ml) for 2 min were harvested, and aliquots were immunoprecipitated with antibodies against LAT, PLC-γ1, and Vav. The immunoprecipitates were first reacted in Western blotting with the 4G10 antibody to assess the phosphorylation status of each of the proteins. The same membranes were stripped and reacted with their specific antibodies to control for equivalent levels of protein in the immunoprecipitates. p12I expression was confirmed by Western blotting using the anti-HA1 antibody. The anti-green fluorescent protein (GFP) antibody was used as an irrelevant antibody control.
LAT phosphorylation was decreased in the presence of p12I, as demonstrated by the 4G10 anti-phosphotyrosine antibody, and this effect was not due to differential loading of proteins, as the same membrane reacted with anti-LAT antibody demonstrated an equivalent level of LAT in the immunoprecipitates (Fig. 2C, left). Similarly, decreased phosphorylation of PLC-γ1 and Vav, but not Lck, was observed in the presence of p12I (Fig. 2C, right, and data not shown for Lck). Again, Western blotting on the same membranes with the specific antibodies to PLC-γ1 and Vav demonstrated equivalent levels of PLC-γ1 and Vav in the immunoprecipitate (Fig. 2C, right). Expression of the tagged p12I was verified by Western blotting in the Jurkat T-cell lysates using the anti-HA1 antibody (Fig. 2C, right). Thus, p12I expression resulted in decreased phosphorylation of proximal TCR signaling molecules, and these findings provide a mechanistic explanation for the decrease in NFAT activity observed in the presence of p12I (Fig. 1A).
The HTLV-1 p12I protein localizes to lipid rafts.
When p12I is expressed ectopically in transfected cells, it localizes mostly to the ER/Golgi (28, 34). Because TCR signaling involves recruitment of molecules within the lipid rafts (57), we hypothesized that p12I may also translocate to the lipid rafts. To test this hypothesis, we used both immunofluorescence on entire cells and a biochemical approach following cell fractionation.
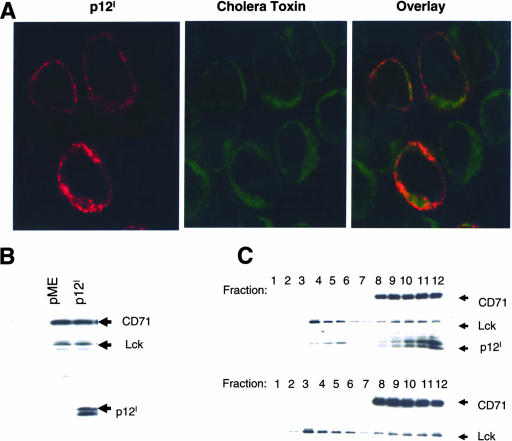
Jurkat T cells transfected with p12IHA were stained with FITC-Co-Tx, which binds to the lipid rafts (2) and the anti-HA1 antibody. A subset of cells that were costained with both FITC-Co-Tx and anti-HA1 and image merging suggested colocalization of p12I and Co-Tx in the lipid rafts (Fig. 3A).
FIG. 3.
p12I localizes to the lipid rafts. (A) p12I colocalization with Co-Tx was visualized by confocal microscopy using immunostaining with anti-HA1, followed by Cy3-labeled secondary antibody and FITC-Co-Tx, which binds to the rafts. (B) Total cell lysates of Jurkat T cells expressing p12I and control were analyzed by Western blotting to demonstrate equivalent amounts of CD71 and Lck. p12I expression was also verified. (C) Equivalent amounts of cell lysates from Jurkat T cells (B) were subjected to flotation assay to separate raft and nonraft fractions by biochemical means. The distribution of p12I, as well as Lck and CD71 as raft and nonraft markers, was visualized by Western blot analysis in the collected fractions.
To confirm biochemically that p12I is in the lipid microdomains, we performed a flotation assay on T-cell lysates. The p12I protein was expressed in Jurkat T cells, and the total cell lysate was reacted in Western blotting with anti-HA1, anti-Lck (a protein located in the lipid rafts), and anti-CD71 (transferrin receptor localized in the cytoplasm and cellular membranes) (Fig. 3B). Cell lysates were prepared in 40% sucrose and overlaid with 30% and 5% sucrose cushions. The flotation assay yields the lipid rafts as a Triton-insoluble and buoyant fraction in the 5% and 30% sucrose interface (fractions 2 to 5), whereas solubilized proteins remain in 40% sucrose at fractions 8 to 12. Following centrifugation, the gradient fractions were analyzed by Western blotting with anti-HA1 and the anti-Lck and anti-CD71 antibodies as controls. Lipid raft fractions (4 to 6) contained Lck, a raft-associated protein in Jurkat T cells, whereas the solubilized fractions (8 to 12) contained CD71 (Fig. 3C, top). Interestingly, p12I was found not only in the soluble fractions 8 to 12, containing the cytoplasmic membrane, as expected, but also in fractions 4 to 6, which are enriched for Lck. No p12I was observed in cells transfected with the pME vector as a control (Fig. 3C, bottom). Lipid raft localization of p12I was also confirmed in 293T cells, where p12I colocalized in fraction 4, together with Lyn, a protein found in the lipid rafts in this cell type (data not shown). These data demonstrate that p12I localizes not only to the ER/Golgi, but also to the lipid rafts of different cell types.
p12I translocates to the immunological synapse upon MHC class II TCR engagement and down-regulates TCR signaling.
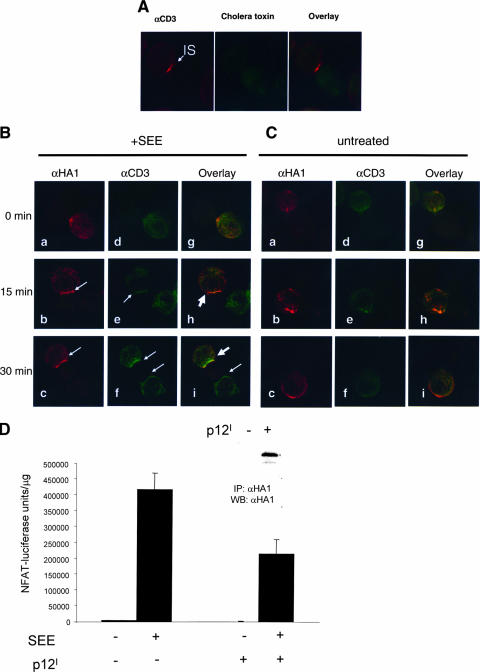
Engagement of the TCR by peptide MHC on APCs leads to formation of immune synapses, complex structures that include the TCR, protein tyrosine kinases, and LAT. Because p12I is found in the lipid rafts and affects TCR signaling, we wished to investigate whether p12I was recruited to the immunological synapse upon TCR engagement. We used Jurkat T cells and Raji cells as an in vitro model of CD4+ T-cell and APC interaction that involves the TCR and MHC class II engagement. Polarization of the TCR could be visualized with the anti-CD3 antibody within 15 min of cocultivation of Jurkat T cells with SEE-prepulsed Raji B cells (Fig. 4A). Transfection of p12I in Jurkat T cells demonstrated that, following cocultivation of the T cells with SEE-prepulsed Raji B cells, the p12I protein was present in the immunological synapse and colocalized with CD3 (Fig. 4B, b, c, e, f, h, and i). The formation of the immunological synapse was not observed in the absence of SEE (Fig. 4C, right).
FIG. 4.
Recruitment of the HTLV-1 p12I protein to the immunological synapse and down-regulation of TCR signaling in T cells. (A) Staining of Jurkat T cells cocultivated with SEE-prepulsed Raji cells for 15 min with the anti-CD3 antibody and FITC-Co-Tx. CD3 molecules aggregated at the immunological synapse (IS) following TCR engagement. (B and C) The distribution of p12I and CD3 was studied by immunofluorescence and microimaging analyses. Jurkat T cells expressing p12I were mixed with prepulsed Raji B cells in the presence (B) or absence (C) of 10 μg/ml SEE and fixed at different time points. The arrows indicate the immunological synapse stained by anti-HA1 (αHA1) antibody (images b and c) or anti-CD3 antibody (images e and f). (D) The effect of p12I expression on NFAT-driven luciferase activity in Jurkat T cells was studied under the same experimental conditions as for panels B and C. Lysates were prepared after 20 h of cocultivation. Luciferase units were normalized for the lysate protein content. The average and the standard deviation of six duplicate samples are shown. Expression of p12I in the lysate was verified by immunoprecipitation (IP) and Western blotting (WB) (inset).
To investigate whether in this in vitro model of TCR stimulation as well, p12I affects TCR signaling and NFAT activity, Jurkat T cells were cotransfected with the NFAT-luciferase reporter gene with or without p12I before cocultivation with Raji cells. p12I expression was verified by Western blotting (Fig. 4D, top). When Raji cells were prepulsed with SEE and cocultivated with Jurkat T cells, there was an increase of luciferase activity that decreased in the presence of p12I (Fig. 4D). Thus, p12I down-regulates TCR signaling, not only following engagement of the TCR by anti-CD3 ligation, but also under more physiological conditions with MHC class II.
p12I binds to exogenous LAT.
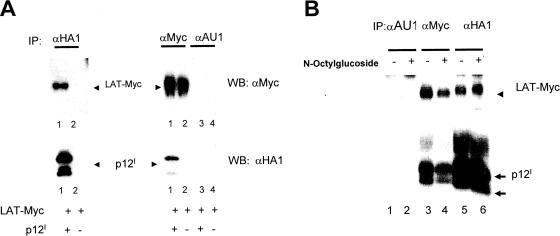
The finding that p12I affects TCR signaling, translocates to the lipid rafts, and participates in immunological-synapse formation led us to formulate the hypothesis that p12I may physically interact with proximal TCR signaling molecules. We focused particularly on LAT, because LAT, like p12I, resides in the lipid rafts. To investigate this possibility, we expressed a LAT-Myc-tagged protein together with p12I in Jurkat T cells. As demonstrated in Fig. 5A, the anti-Myc antibody coprecipitated p12I and the anti-HA1 (mouse monoclonal antibody 12CA5) coprecipitated LAT-Myc, whereas the irrelevant anti-AU1 control antibody precipitated neither p12I nor LAT-Myc (Fig. 5A). Binding of p12I to LAT-Myc was also confirmed in 293T cells, where antibody to p12I coprecipitated LAT-Myc, anti-Myc antibody coprecipitated p12I, and again, the anti-AU1 antibody coprecipitated neither (data not shown). Because p12I is very hydrophobic and is found in the lipid microdomains, there is a possibility that its coprecipitation with LAT is due to the localization of both proteins to the glycosphingolipid-enriched membrane microdomains rather than to a real physical interaction within the cell. To attempt to address this question, Jurkat T cells were cotransfected with both p12I and the tagged LAT-Myc construct and the cell lysate was solubilized with N-octylglucoside, a compound able to solubilize the glycosphingolipid-enriched components of the membrane (6). Treatment with N-octylglucoside did not affect the coprecipitation of p12I and LAT, providing further support to their interaction in cells (Fig. 5B). We next investigated whether p12I was able to coprecipitate endogenous LAT from Jurkat T-cell lysates using the anti-LAT antibody. p12I did not coprecipitate endogenous LAT. While it is possible that the interactive site of p12I with endogenous LAT may interfere with the binding of the anti-LAT antibody, there is also the alternative possibility that this interaction may result from overexpression of highly hydrophobic proteins. Thus, whether the interaction occurs in live cells and whether it is of any biological significance remain uncertain.
FIG. 5.
p12I interacts with exogenous LAT. (A) Jurkat T cells transfected with p12I and LAT-Myc as indicated were analyzed by immunoprecipitation (IP), followed by Western blotting (WB) to assess the presence of association. (B) Lysates of 293T cells coexpressing p12I and LAT-Myc were used for immunoprecipitation in the presence or absence of N-octylglucoside at 60 mM. Western blotting of the immunoprecipitates is shown.
p12I reduces HTLV-1 expression following TCR engagement by anti-CD3 or MHC class II.
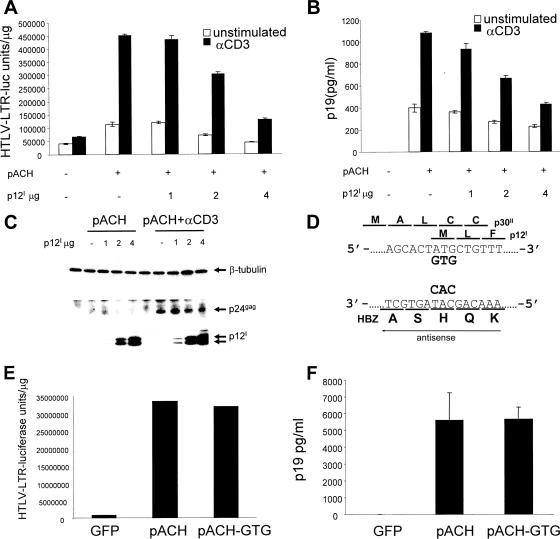
In vivo, HTLV-1 infects CD4+ T cells and, less frequently, CD8+ T cells (17). Because TCR ligation increases Tax-mediated transcription from the HTLV-1 LTR (36), we hypothesized that the ability of p12I to down-regulate TCR signaling may have evolved to limit viral replication. To test this, we transfected Jurkat T cells with the proviral clone pACH (30) without or with increasing amounts of p12I and stimulated them with the anti-CD3 antibody. We observed an increase in the activity of Tax-driven luciferase expression in transfected cells with pACH following TCR ligation, as expected (36) (Fig. 6A), and p12I decreased Tax-driven luciferase levels in a dose-dependent manner (Fig. 6A). Decreased Tax activity was associated with decreased virus production measured as the p19 Gag level in cell supernatants (Fig. 6B). Likewise, while in unstimulated T cells intracellular p24 Gag was barely detectable, in Jurkat T cells stimulated with anti-CD3 antibody, p24 Gag was readily detectable and was decreased by p12I coexpression (Fig. 6C).
FIG. 6.
p12I suppresses viral expression. Jurkat T cells were cotransfected with the pACH clone or control plasmid, together with the HTLV-1 LTR-luciferase reporter gene and increasing amounts of p12I (μg/106 cells). The cells were stimulated or not with 1 μg/ml of anti-CD3 antibody for 50 h. (A) Luciferase activity/μg protein in cell lysates of transfected Jurkat T cells. (B) p19 Gag levels in the supernatants of the transfected Jurkat T cells. (C) Western blot of cell lysates using β-tubulin (to control for equal protein loading), the anti-p24 Gag antibody, or the anti-HA1 antibody. (D) Schematic representation of ORFs I and II and the HBZ ORF in the provirus. The initiation (ATG) codon of ORF I in pACH-GTG was mutagenized to GTG as depicted. (E) Jurkat T cells were transfected with equivalent amounts of pACH or pACH-GTG, together with the HTLV LTR-driven luciferase expression plasmid. The luciferase activity was measured from triplicate samples of cell lysates 44 h after transfection. All values were corrected by the protein content of the cell lysates, and mean values and standard deviations are shown. GFP, green fluorescent protein. (F) p19 Gag levels in the cell supernatants. The error bars indicate standard deviations.
Next, we wished to assess whether the engagement of the TCR by MHC class II in the Jurkat T/Raji B-cell model could affect HTLV-1 replication and whether p12I would affect it. To test this, we used the replication-competent proviral HTLV-1 clone pACH (30), as well as an isogenic clone in which the initiating ATG codon for the p12I protein was mutated (pACH-GTG) without altering the amino acid sequence of either the p30II or HBZ protein, as both of these ORFs overlap in the HTLV-1 genome (Fig. 6D). The pACH and the isogenic p12I KO pACH-GTG clones produce equivalent virus upon transfection in unstimulated Jurkat T cells, as demonstrated by equivalent Tax-driven luciferase levels (Fig. 6E) and by the production of equivalent p19 Gag levels in the supernatants of the transfected Jurkat T cells (Fig. 6F).
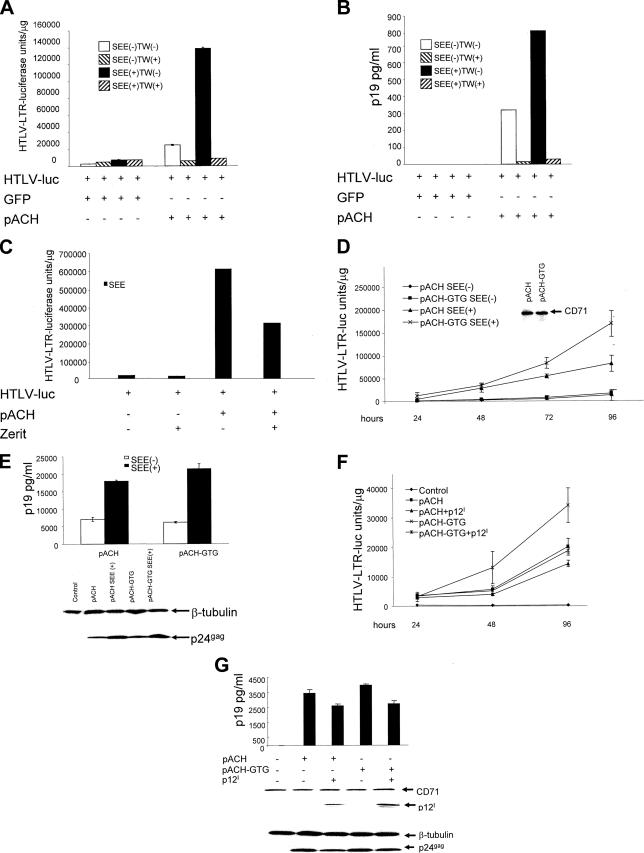
pACH was expressed in Jurkat T cells that were cocultured with Raji B cells transfected with an HTLV-1 LTR-luciferase reporter gene, and the cells were cocultivated or placed in a TransWell system. Luciferase activity was observed only when B cells were cocultured under conditions where cell-to-cell contact could occur (Fig. 7A). Prepulsing of B cells with the bacterial antigen SEE under normal coculture conditions resulted in a 4-fold increase in luciferase activity and a 2.5-fold increase in p19 Gag production (Fig. 7A and B). We excluded the possibility that extracellular Tax may activate LTR-driven luciferase expression in B cells because expression of Tax alone in Jurkat T cells, followed by cocultivation with prepulsed B cells, did not result in activation of the LTR-luciferase reporter gene in B cells (data not shown).
FIG. 7.
TCR MHC class II antigen engagement increases HTLV-1 replication. (A and B) The HTLV-LTR-driven luciferase expression in Raji cells was used as a reporter of viral expression. Jurkat T cells expressing pACH or green fluorescent protein (GFP) were cocultured with Raji cells in the presence or absence of SEE under normal coculture conditions [TW(−)] or in the TransWell (TW) system. After 43 h, reporter cells and culture media were analyzed by triplicate dual-luciferase assays (A) and p19 Gag antigen capture assay (B), respectively. (C) Jurkat T cells were transfected with the pACH clone and cocultivated with prepulsed B cells transfected with the HTLV-1 LTR-luciferase control. The cells were cultured in the presence or absence of 35 μM of Zerit for 72 h. Cell lysates were analyzed for the level of luciferase. (D and E) Equivalent numbers of Jurkat T cells transfected with the pACH or pACH-GTG clone were cocultured with Raji cells with or without SEE. Control for an equal input of cells was performed using the anti-CD71 antibody by Western blotting (D, inset). Cells, as well as culture media, were harvested at each time point up to 96 h, and the luciferase levels (D) and p19 Gag values at 48 h (E) from triplicate samples are shown with standard deviations. Expression of p24 Gag in cell lysates at 96 h is shown in the lower part of panel E. (F and G) pACH and pACH-GTG were expressed with or without p12I in Jurkat T cells, which in turn were cocultured with SEE-prepulsed B cells transfected with the LTR-luciferase reporter gene. p12I protein expression and intracellular p24 Gag were assessed by Western blotting at 96 h. Luciferase expression (F) and p19 Gag (G) in the supernatants were measured at 48 h from triplicate samples. Standard deviations are shown.
To discriminate between cell fusion and/or transmission of HTLV-1 particles to the B cells, we treated the Jurkat T-cell and SEE-prepulsed B-cell cocultures with the reverse transcriptase inhibitor Zerit. As demonstrated in Fig. 7C, Zerit decreased Tax-mediated luciferase activity in B cells, providing support to the notion that Tax activity is dependent on the reverse transcription of the viral genome.
Next, we transfected equivalent amounts of wild-type pACH and the pACH-GTG molecular clone in Jurkat T cells. Jurkat T cells were cocultivated with B cells transfected with the HTLV-1 LTR-luciferase reporter gene prepulsed or not with SEE. Measurement of Tax activity in the pACH or GTG clone over time demonstrated that the luciferase levels were higher in cocultures that expressed the pACH-GTG virus over time, suggesting that ablation of p12I results in more efficient viral expression and therefore a higher level of transmission to B cells upon antigen stimulation (Fig. 7D). Accordingly, the levels of p19 Gag in the supernatants, as well as those of p24 Gag in cell lysates, were highest in cocultures producing the mutant pACH-GTG virus (Fig. 7E). Furthermore, coexpression of p12I with either pACH or the pACH-GTG clone resulted in a decrease in luciferase, p19 Gag, and p24 Gag production from both the pACH and the pACH-GTG transfectants (Fig. 7F and G), as expected. Thus, it appears that, in the absence of p12I, more virus was expressed in T cells, and in turn, more virus was transmitted to B cells. Also, in this model of TCR engagement by MHC class II, the overexpression of p12I decreased viral production.
DISCUSSION
The engagement of peptide MHC with the TCR triggers a cascade of events that leads to phosphorylation of the TCR, the TCR proximal signaling molecule, and LAT, which bridges proximal-to-distal effector molecules for T-cell activation. These events also lead to the formation of a polarized cell-cell junction designated the immunological synapse (26). Although the biochemical events peak before the formation of a mature synapse, the stability and length of this contact are thought to be important in the determination of whether T cells enter the cell cycle following antigen stimulation. We observed in our study that the p12I protein encoded by HTLV-1, a virus associated with immune suppression and autoimmune and proliferative syndromes, perturbs TCR signaling in CD4+ T cells. We obtained evidence that p12I may interact directly or indirectly with a critical signaling molecule, LAT. However, the biological significance of this finding remains uncertain, as we were unable to demonstrate the interaction of p12I with endogenous LAT.
Here, we demonstrated that the HTLV-1 p12I protein upon antigen stimulation decreases TCR signaling by inhibiting LAT phosphorylation and, consequently, the phosphorylation of PLC-γ1 and Vav. The result is a decrease in NFAT activation. The specificity of LAT targeting by p12I is suggested by the fact that ZAP70 phosphorylation, which is LAT independent, is not affected by p12I (data not shown). There is no contrast between our data and the positive effect of p12I on NFAT activation that has been previously reported to follow PMA stimulation. In those experiments, PMA stimulation bypassed TCR ligation, and we have reproduced these findings here (Fig. 1A). TCR stimulation drives T cells from a resting state to early G1, whereas PKC activation may occur later in cell cycle progression, suggesting that p12I may have differential effects in different phases of the cell cycle. Indeed, p12I has also been demonstrated to increase STAT-5 activation and responsiveness to IL-2, and both events are associated with the S phase of the cell cycle.
The importance of the physical recruitment of p12I to the immunological synapse in TCR signaling remains unclear, but collectively, the data presented here indicate that HTLV-1 has evolved a function to decrease TCR signaling. There are, however, limitations in this model, as we overexpressed p12I in some of our experiments. However, the hypothesis that p12I may dampen TCR signaling and viral replication is also supported by the finding that selective ablation of p12I expression by mutation of the initiation methionine codon of ORF I results in an increase in viral production upon TCR stimulation, as demonstrated in Fig. 7E.
Other viruses that infect lymphocytes perturb TCR signaling and encode proteins that also associate with lipid rafts. These proteins include the Epstein-Barr virus LMP1 protein (23), human immunodeficiency virus type 1 Nef (58), measles virus proteins (3), the human herpesvirus 8 K5 protein (11), and herpes simplex virus and herpesvirus saimiri (9, 47).
Interestingly, the HIV-1 Nef and p12I proteins have opposite effects on T-cell activation and viral production. Nef associates with the lipid rafts and is thought to transport cholesterol to them and to increase human immunodeficiency virus infectivity. p12I is also in the lipid rafts, but it decreases viral expression. Both Nef and p12I are recruited to the immunological synapse, but Nef potentiates TCR signaling (15) while p12I dampens it. Nef expression is associated with an increase in Cbl phosphorylation (54), whereas p12I decreases it (our unpublished data). The Nef effect is upstream of PKC activation, as it is abolished by PMA, a direct activator of PKC. The negative effect of p12I is not observed when T cells are treated with PMA (12). Nef physically interacts with CD3ζ of the TCR complex (49, 53) and, through its proline-rich motif, mediates interaction with phosphatidylinositol 3-kinase, Vav, and Lck (4, 10, 14, 44). p12I likely interacts directly or indirectly with LAT and, by decreasing its phosphorylation, decreases Vav and PLC-γ1 phosphorylation.
Measles virus also interferes with T-cell activation by directly interacting with the T-cell lipid rafts and causing alteration in the recruitment of proteins central to TCR activation, such as Vav and the AKT kinase (3). The K5 protein of Kaposi sarcoma virus affects T-cell activation by down-regulating ICAM-1 and B7-2 in B cells (11). Herpes simplex virus, like HTLV-1 p12I, decreases LAT phosphorylation, whereas herpesvirus saimiri targets ZAP70. Thus, usurping the TCR signaling pathway, albeit by different mechanisms, is a shared feature of viruses that infect lymphocytes and probably reflects a successful adaptation to the host immune response.
The recent observation that HTLV-1 spreads to T cells through polarization of the cytoskeleton (27) at an interface between cells defined as the viral synapse raises questions about the possible role of p12I in cell-to-cell transmission of HTLV-1. The data presented here suggest that p12I reduces viral replication indirectly by affecting T-cell activation, as demonstrated by the fact that, in unstimulated Jurkat T cells, the wild type and p12I KO molecular clones produce equivalent amounts of virus. The experimental model of viral transmission used here (Jurkat T/B cells) is not suited to direct quantitation of viral transmission. The increased viral transmission to B cells when cocultured with antigen-stimulated Jurkat T cells expressing the p12I KO molecular clone could be ascribed simply to an increased level of virus produced by T cells following antigen stimulation, rather than increased transmission per se.
HTLV-1 has been reported to also infect CD8+ T cells and to impair their function. As TCR signaling is simultaneously required for degranulation by cytotoxic T lymphocytes (45), p12I could affect the cytolytic activity of infected CD8+ T cells by down-regulating TCR signaling. Thus, p12I may play an essential role in viral persistence by down-regulating TCR signaling, viral production, and MHC class I presentation in infected CD4+ T cells (28) on one hand and, on the other, by impairing the function of infected virus-specific CD8+ cytotoxic T cells. Hopefully, further elucidation of the domains of p12I and LAT involved in the perturbation of TCR signaling may provide rational approaches to limit or eliminate the vicious cycle that causes viral persistence in the host and to further minimize the chance for disease development.
Acknowledgments
We thank Robyn Washington Parks and Annika Lindgren for assistance with some of the experiments; Vibeke Andresen, Jean-Michel Heraud, and Susan Sharrow for critical reading of the manuscript; and Steven J. Snodgrass for patient editorial assistance (all from the National Cancer Institute, Bethesda, MD).
We have no conflicting financial interests.
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH. R. Fukumoto was partially supported by the Japan Society for the Promotion of Science. C. Nicot is supported by NIH grant CA 115398.
Footnotes
Published ahead of print on 20 June 2007.
REFERENCES
- 1.Albrecht, B., C. D. D'Souza, W. Ding, S. Tridandapani, K. M. Coggeshall, and M. D. Lairmore. 2002. Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12I. J. Virol. 76:3493-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, H. A., E. M. Hiltbold, and P. A. Roche. 2000. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat. Immunol. 1:156-162. [DOI] [PubMed] [Google Scholar]
- 3.Avota, E., N. Muller, M. Klett, and S. Schneider-Schaulies. 2004. Measles virus interacts with and alters signal transduction in T-cell lipid rafts. J. Virol. 78:9552-9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer, M., M. Lucchiari-Hartz, R. Maier, G. Haas, B. Autran, K. Eichmann, R. Frank, B. Maier, and A. Meyerhans. 1997. Structural constraints of HIV-1 Nef may curtail escape from HLA-B7-restricted CTL recognition. Immunol. Lett. 55:119-122. [DOI] [PubMed] [Google Scholar]
- 5.Bi, K., Y. Tanaka, N. Coudronniere, K. Sugie, S. Hong, M. J. van Stipdonk, and A. Altman. 2001. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat. Immunol. 2:556-563. [DOI] [PubMed] [Google Scholar]
- 6.Bosselut, R., W. Zhang, J. M. Ashe, J. L. Kopacz, L. E. Samelson, and A. Singer. 1999. Association of the adaptor molecule LAT with CD4 and CD8 coreceptors identifies a new coreceptor function in T cell receptor signal transduction. J. Exp. Med. 190:1517-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bromley, S. K., W. R. Burack, K. G. Johnson, K. Somersalo, T. N. Sims, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375-396. [DOI] [PubMed] [Google Scholar]
- 8.Brown, D. A., and E. London. 1998. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 164:103-114. [DOI] [PubMed] [Google Scholar]
- 9.Cho, N. H., P. Feng, S. H. Lee, B. S. Lee, X. Liang, H. Chang, and J. U. Jung. 2004. Inhibition of T cell receptor signal transduction by tyrosine kinase-interacting protein of Herpesvirus saimiri. J. Exp. Med. 200:681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collette, Y., H. Dutartre, A. Benziane, M. Ramos, R. Benarous, M. Harris, and D. Olive. 1996. Physical and functional interaction of Nef with Lck. HIV-1 Nef-induced T-cell signaling defects. J. Biol. Chem. 271:6333-6341. [DOI] [PubMed] [Google Scholar]
- 11.Coscoy, L., and D. Ganem. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Investig. 107:1599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding, W., B. Albrecht, R. E. Kelley, N. Muthusamy, S. J. Kim, R. A. Altschuld, and M. D. Lairmore. 2002. Human T-cell lymphotropic virus type 1 p12I expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J. Virol. 76:10374-10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding, W., B. Albrecht, R. Luo, W. Zhang, J. R. Stanley, G. C. Newbound, and M. D. Lairmore. 2001. Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12I: association with calreticulin and calnexin. J. Virol. 75:7672-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fackler, O. T., W. Luo, M. Geyer, A. S. Alberts, and B. M. Peterlin. 1999. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell 3:729-739. [DOI] [PubMed] [Google Scholar]
- 15.Fenard, D., W. Yonemoto, C. de Noronha, M. Cavrois, S. A. Williams, and W. C. Greene. 2005. Nef is physically recruited into the immunological synapse and potentiates T cell activation early after TCR engagement. J. Immunol. 175:6050-6057. [DOI] [PubMed] [Google Scholar]
- 16.Franchini, G. 1995. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood 86:3619-3639. [PubMed] [Google Scholar]
- 17.Franchini, G., C. Nicot, and J. M. Johnson. 2003. Seizing of T-cells by human T-cell leukemia/lymphoma type 1. Adv. Cancer Res. 89:69-132. [DOI] [PubMed] [Google Scholar]
- 18.Gallo, R. C. 1986. The first human retrovirus. Sci. Am. 255:88-98. [DOI] [PubMed] [Google Scholar]
- 19.Gaudray, G., F. Gachon, J. Basbous, M. Biard-Piechaczyk, C. Devaux, and J. M. Mesnard. 2002. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J. Virol. 76:12813-12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gessain, A., F. Barin, J.-C. Vernant, O. Gout, L. Maurs, A. Calendar, and G. de The. 1985. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 21.Gessain, A., A. Louie, O. Gout, R. C. Gallo, and G. Franchini. 1991. Human T-cell leukemia-lymphoma virus type I (HTLV-I) expression in fresh peripheral blood mononuclear cells from patients with tropical spastic paraparesis/HTLV-I-associated myelopathy. J. Virol. 65:1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanon, E., J. C. Stinchcombe, M. Saito, B. E. Asquith, G. P. Taylor, Y. Tanaka, J. N. Weber, G. M. Griffiths, and C. R. Bangham. 2000. Fratricide among CD8+ T lymphocytes naturally infected with human T cell lymphotropic virus type I. Immunity 13:657-664. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi, M., K. M. Izumi, and E. Kieff. 2001. Epstein-Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: protein 1 binds to the cytoskeleton through TNF receptor cytoplasmic factors. Proc. Natl. Acad. Sci. USA 98:4675-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill, S. A., P. A. Lloyd, S. McDonald, J. Wykoff, and D. Derse. 2003. Susceptibility of human T cell leukemia virus type I to nucleoside reverse transcriptase inhibitors. J. Infect. Dis. 188:424-427. [DOI] [PubMed] [Google Scholar]
- 25.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huppa, J. B., and M. M. Davis. 2003. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3:973-983. [DOI] [PubMed] [Google Scholar]
- 27.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 28.Johnson, J. M., C. Nicot, J. Fullen, V. Ciminale, L. Casareto, J. C. Mulloy, S. Jacobson, and G. Franchini. 2001. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12I protein. J. Virol. 75:6086-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, S. J., W. Ding, B. Albrecht, P. L. Green, and M. D. Lairmore. 2003. A conserved calcineurin-binding motif in human T lymphotropic virus type 1 p12I functions to modulate nuclear factor of activated T cell activation. J. Biol. Chem. 278:15550-15557. [DOI] [PubMed] [Google Scholar]
- 30.Kimata, J. T., F. H. Wong, J. J. Wang, and L. Ratner. 1994. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology 204:656-664. [DOI] [PubMed] [Google Scholar]
- 31.Koralnik, I., A. Gessain, M. E. Klotman, A. Lo Monico, Z. N. Berneman, and G. Franchini. 1992. Protein isoforms encoded by the pX region of the human T-cell leukemia/lymphotropic virus type I. Proc. Natl. Acad. Sci. USA 89:8813-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koralnik, I. J., J. Fullen, and G. Franchini. 1993. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J. Virol. 67:2360-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubota, R., Y. Furukawa, S. Izumo, K. Usuku, and M. Osame. 2003. Degenerate specificity of HTLV-1-specific CD8+ T cells during viral replication in patients with HTLV-1-associated myelopathy (HAM/TSP). Blood 101:3074-3081. [DOI] [PubMed] [Google Scholar]
- 34.Lairmore, M. D., B. Albrecht, C. D'Souza, J. W. Nisbet, W. Ding, J. T. Bartoe, P. L. Green, and W. Zhang. 2000. In vitro and in vivo functional analysis of human T cell lymphotropic virus type 1 pX open reading frames I and II. AIDS Res. Hum. Retrovir. 16:1757-1764. [DOI] [PubMed] [Google Scholar]
- 35.Lim, D. G., B. K. Bieganowska, G. J. Freeman, and D. A. Hafler. 2000. Examination of CD8+ T cell function in humans using MHC class I tetramers: similar cytotoxicity but variable proliferation and cytokine production among different clonal CD8+ T cells specific to a single viral epitope. J. Immunol. 165:6214-6220. [DOI] [PubMed] [Google Scholar]
- 36.Lin, H. C., M. Hickey, L. Hsu, D. Medina, and A. B. Rabson. 2005. Activation of human T cell leukemia virus type 1 LTR promoter and cellular promoter elements by T cell receptor signaling and HTLV-1 Tax expression. Virology 339:1-11. [DOI] [PubMed] [Google Scholar]
- 37.Monks, C. R., H. Kupfer, I. Tamir, A. Barlow, and A. Kupfer. 1997. Selective modulation of protein kinase C-theta during T-cell activation. Nature 385:83-86. [DOI] [PubMed] [Google Scholar]
- 38.Mulloy, J. C., R. W. Crowley, J. Fullen, W. J. Leonard, and G. Franchini. 1996. The human T-cell leukemia/lymphotropic virus type I p12I protein binds the interleukin-2 receptor β and γc chains and affects their expression on the cell surface. J. Virol. 70:3599-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai, M., Y. Yamano, M. B. Brennan, C. A. Mora, and S. Jacobson. 2001. Increased HTLV-I proviral load and preferential expansion of HTLV-I Tax-specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann. Neurol. 50:807-812. [DOI] [PubMed] [Google Scholar]
- 40.Nicot, C., M. Dundr, J. M. Johnson, J. R. Fullen, N. Alonzo, R. Fukumoto, G. L. Princler, D. Derse, T. Misteli, and G. Franchini. 2004. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat. Med. 10:197-201. [DOI] [PubMed] [Google Scholar]
- 41.Nicot, C., J. C. Mulloy, M. G. Ferrari, J. M. Johnson, K. Fu, R. Fukumoto, R. Trovato, J. Fullen, W. J. Leonard, and G. Franchini. 2001. HTLV-1 p12I protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mononuclear cells. Blood 98:823-829. [DOI] [PubMed] [Google Scholar]
- 42.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G. Dalgleish. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 14:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen, A., L. G. Puente, and H. L. Ostergaard. 2005. Tyrosine kinase activity and remodelling of the actin cytoskeleton are co-temporally required for degranulation by cytotoxic T lymphocytes. Immunology 116:276-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 47.Sloan, D. D., J. Y. Han, T. K. Sandifer, M. Stewart, A. J. Hinz, M. Yoon, D. C. Johnson, P. G. Spear, and K. R. Jerome. 2006. Inhibition of TCR signaling by herpes simplex virus. J. Immunol. 176:1825-1833. [DOI] [PubMed] [Google Scholar]
- 48.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283:680-682. [DOI] [PubMed] [Google Scholar]
- 49.Wang, J. K., E. Kiyokawa, E. Verdin, and D. Trono. 2000. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl. Acad. Sci. USA 97:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, T., J. R. Sheppard, and J. E. Foker. 1978. Rise and fall of cyclic AMP required for onset of lymphocyte DNA synthesis. Science 201:155-157. [DOI] [PubMed] [Google Scholar]
- 51.Wange, R. L., and L. E. Samelson. 1996. Complex complexes: signaling at the TCR. Immunity 5:197-205. [DOI] [PubMed] [Google Scholar]
- 52.Weiss, A., and D. R. Littman. 1994. Signal transduction by lymphocyte antigen receptors. Cell 76:263-274. [DOI] [PubMed] [Google Scholar]
- 53.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J. Exp. Med. 189:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, P., and A. J. Henderson. 2005. Nef enhances c-Cbl phosphorylation in HIV-infected CD4+ T lymphocytes. Virology 336:219-228. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83-92. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, W., R. P. Trible, and L. E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 9:239-246. [DOI] [PubMed] [Google Scholar]
- 58.Zheng, Y. H., A. Plemenitas, T. Linnemann, O. T. Fackler, and B. M. Peterlin. 2001. Nef increases infectivity of HIV via lipid rafts. Curr. Biol. 11:875-879. [DOI] [PubMed] [Google Scholar]