Abstract
Replication origins in Saccharomyces cerevisiae are spaced at intervals of approximately 40 kb. However, both measurements of replication fork rate and studies of hypomorphic alleles of genes encoding replication initiation proteins suggest the question of whether replication origins are more closely spaced than should be required. We approached this question by systematically deleting replicators from chromosome III. The first significant increase in loss rate detected for the 315-kb full-length chromosome occurred only after all five efficient chromosomal replicators in the left two-thirds of the chromosome (ARS305, ARS306, ARS307, ARS309, and ARS310) had been deleted. The removal of the inefficient replicator ARS308 from this originless region caused little or no additional increase in loss rate. Chromosome fragmentations that removed the normally inactive replicators on the left end of the chromosome or the replicators distal to ARS310 on the right arm showed that both groups of replicators contribute significantly to the maintenance of the originless chromosome. Surprisingly, a 142-kb derivative of chromosome III, lacking all sequences that function as autonomously replicating sequence elements in plasmids, replicated and segregated properly 97% of the time. Both the replication initiation protein ORC and telomeres or a linear topology were required for the maintenance of chromosome fragments lacking replicators.
In eukaryotes, DNA replication initiates at specific sites called replication origins. cis-acting sequences called replicators define the positions and regulate the activity of replication origins by promoting the assembly of prereplicative complexes (pre-RCs) during the G1 phase of the cell cycle. Eukaryotic replicators were first identified in the budding yeast Saccharomyces cerevisiae on the basis of their ability to promote the extrachromosomal maintenance of plasmids (22, 50). The dissection of these autonomously replicating sequence (ARS) elements revealed an essential 11-bp sequence, called the ARS consensus sequence (ACS), that is required for both plasmid and chromosomal replicator activity (reviewed in reference 34). The ACS is the core of the binding site for the highly conserved six-subunit origin recognition complex (ORC), which recruits and assembles the other components of the pre-RC (reviewed in reference 1). Like the ORC, the other components of the pre-RC are highly conserved throughout the eukaryotic kingdom.
Upon entry into S phase, pre-RCs are activated to initiate replication according to a temporal program whose determinants are poorly understood. The activation of replication origins requires the activity of two kinases, a cyclin-dependent kinase composed of the catalytic subunit encoded by CDC28 and regulatory subunits encoded by CLB5 and CLB6, and the Dbf4-dependent kinase, composed of a catalytic subunit encoded by CDC7 and a regulatory subunit encoded by DBF4. During replication initiation, DNA is unwound at origins and replication fork proteins are assembled to form replisomes, which move bidirectionally away from origins (reviewed in reference 2).
ARS elements and replication origins have been mapped in the yeast genome by a variety of approaches. In early studies, ARS elements were identified on chromosomes III and VI by screening overlapping fragments of the chromosomes for ARS activity in the plasmid assay (37, 41, 48). ARS elements were then examined for chromosomal replication origin activity by two-dimensional gel electrophoresis of replication intermediates (9, 15, 18, 20, 23, 24, 41, 55, 56, 67). These studies demonstrated that ARS elements are required for replication origin activity and that not all ARS elements are detectably active as chromosomal replication origins. More recent studies have used genome arrays to identify replication origins. The first approach made use of density transfer experiments to generate a genome-wide replication timing profile (43). Replication origins could be identified as regions that replicated earlier than their neighboring sequences. The second approach was based on the twofold increase in copy number caused by replication (66). Again, regions that doubled in copy number before their neighboring sequences were considered to be replication origins. These timing profiles, which would not detect inefficient replication origins, identified 332 and 247 replication origins, respectively, suggesting an average spacing of 40 kb between replication origins in the 12-Mb yeast genome. This average spacing is consistent with spacing deduced in early electron microscopy and fiber autoradiography studies (35, 44). A third approach used genome arrays to identify the binding sites of two pre-RC components, ORC and the Mcm2-7 complex, by chromatin immunoprecipitation (64). This approach, which has the potential to identify both efficient and inefficient replication origins, identified 429 potential origins in the yeast genome.
Several lines of evidence suggest that replication origins are more closely spaced than should be required. For example, in their genome-wide study of replication dynamics, Raghuraman et al. (43) found that replication forks move at a mean rate of 2.9 kb/min during an S phase of approximately 55 min. Thus, forks from a single bidirectional replication origin that initiates early in S phase should be able to replicate about 320 kb of DNA. Although this calculation does not take into account the presence of replication fork barriers or pause sites, which are known to be present in the yeast genome (5, 10, 19), it suggests that replication origins might be redundant. Consistent with this idea, hypomorphic alleles of genes encoding components of the pre-RC reduce the efficiency of replication origin firing (16, 27) and therefore increase the spacing between replication origins without affecting cell viability. Moreover, a hypomorphic allele of the essential Cdc7p protein kinase, whose activity is required for replication initiation, also causes a reduction in the efficiency of replication origin firing (13), and the deletion of CLB5, one of the two S-phase cyclins, prevents late replication origins from firing (14). Although the length of S phase was extended in each of the replication mutants, these results suggest that a genome-wide increase in origin spacing is tolerated. However, the extent of the increase in origin spacing was not determined in these studies.
Because of the detailed mapping of ARS elements and replication origins on chromosome III, we undertook a study of the effects of deleting replication origins on chromosome stability. In an early study, we found that deleting either of the two efficient replicators, ARS307 and ARS309, from a small circular derivative of chromosome III caused an increase in its loss rate and the circular chromosome could not be maintained in the absence of both ARS307 and ARS309; however, deleting both of these efficient replication origins from the full-length chromosome III had no effect on its loss rate (8). To further examine the effect of deleting replication origins, we continued our analysis of the full-length chromosome. The first significant increase in loss rate detected for the full-length chromosome occurred only after all five efficient chromosomal replicators between the left end of the chromosome and the MAT locus were deleted. The removal of the inefficient replicator ARS308 from this originless region caused little or no additional increase in loss rate. Chromosome fragmentations which removed the normally inactive replicators on the left end of the chromosome and/or the replicators distal to MAT on the right arm showed that both groups of replicators contributed significantly to the maintenance of this chromosome. Surprisingly, a 142-kb derivative of chromosome III, lacking all sequences that function as ARS elements in plasmids, replicated and segregated properly 97% of the time. Both the replication initiator protein ORC and telomeres or a linear topology were required for maintenance of chromosome fragments lacking efficient replicators.
MATERIALS AND METHODS
Strains.
Escherichia coli strains JA226 and DH5α were used for plasmid DNA preparations (56). Yeast strains are listed in Table 1. Strain CF4-16B33 was used for the construction of all ARS deletions and chromosome fragmentations as described below. Strains with a balancer chromosome III derived from Saccharomyces carlsbergensis were made by crossing CN5-19a with the disomic MATa/MATα strains CF4-16B33 and YDN292 and isolating rare prototrophic diploids trisomic for chromosome III. These strains were sporulated and His+ Ade+ Ura− Trp− MATa spores that segregated red colonies that were His− Ade− Ura− Trp− were identified, yielding strains CB08D and YDN288. The S. cerevisiae chromosome III in these strains was subsequently fragmented to the right of ARS310. The orc2-1 and orc5-1 mutations were introduced into strain YKN6 by the two-step gene replacement procedure (3), yielding strains YNS1 and YNS3. Plasmids carrying the orc2-1 and orc5-1 mutations were provided by A. Dillin and J. Rine (University of California, Berkeley). A suppressor mutation that allows some orc2-1 strains to grow at 30°C has been reported (47). The orc2-1 strains used in this study failed to grow at 30°C.
TABLE 1.
Yeast strains
| Strain name | Genotype | Source |
|---|---|---|
| CN5-19a | his4-S8 ura3-52 MATa | This work |
| CF4-16B33a,b,c | trp1-Δ63 ura3-52 ade2-101his4-280 C2G::SUP11-1 MATα | This work |
| his4-290 MATa | ||
| YDN292b | trp1-Δ63 ura3-52 ade2-101his4-280 C2G::SUP11-1 5ORIΔ MATα | This work |
| his4-290 MATa | ||
| YKN6 | trp1-Δ63 ura3-52 lys2-801 ade2-101 his3-Δ200 leu2-Δ1 can1R cyh2R kar1-Δ15 MATα | This work |
| YMS499-1d | trp1-Δ63 ura3-52 lys2-801 ade2-101 his3-Δ200 leu2-Δ1 can1R cyh2R kar1-Δ15 MATa | This work |
| F013b | trp1-Δ63 ura3-52 ade2-101his4-280 C2G::SUP11-1 H9G::TRP1 | This work |
| his4-290 MATa | ||
| F510b | trp1-Δ63 ura3-52 ade2-101his4-280 C2G::SUP11-1 5ORIΔ H9G::TRP1 | This work |
| his4-290 MATa | ||
| CB08Da,b | trp1-Δ63 ura3-52 ade2-101his4-280 C2G::SUP11-1 MATα | This work |
| his4-S8 MATa | ||
| YDN288a,b | trp1-Δ63 ura3-52 ade2-101his4-280 C2G::SUP11-1 5ORIΔ MATα | This work |
| his4-S8 MATa | ||
| CB08D3a,b | trp1-Δ63 ura3-52 ade2-101his4-280 C2G::SUP11-1 H9G::TRP1 | This work |
| his4-S8 MATa | ||
| YDN289a,b | trp1-Δ63 ura3-52 ade2-101his4-280 C2G::SUP11-1 6ORIΔ H9G::TRP1 | This work |
| his4-S8 MATa | ||
| YNS1 | trp1-Δ63 ura3-52 lys2-801 ade2-101 his3-Δ200 leu2-Δ1 can1R cyh2R kar1-Δ15 orc2-1 MATα | This work |
| YNS3 | trp1-Δ63 ura3-52 lys2-801 ade2-101 his3-Δ200 leu2-Δ1 can1R cyh2R kar1-Δ15 orc5-1 MATα | This work |
These strains carry a hybrid balancer chromosome from the brewing strain S. carlsbergensis. his4-S8 complements his4-280.
These strains are disomic or partially disomic for chromosome III.
This strain is a trp1 derivative of CF4-16B (8).
This strain is a MATa derivative of YKN6 produced by expressing HO to switch the mating type.
Strains carrying the 61-kb ring chromosome marked with SUP11-1 (8) were made by crossing the kar1-Δ15 strain YMS499-1 with strains PF1-7D (carrying the 0ORIΔ ring) and PF6-5B (carrying the ARS309Δ ring) (8) and identifying Leu+ Canr MATa segregants in which the ring had been transferred to the YMS499-1 nucleus by chromoduction (25). Additional ARS deletions were made in the ring as described below. Ring chromosomes carrying internal telomere repeat sequences (TRS) were made using plasmid pYND125 as described below. The SUP11-1-marked linear 61-kb chromosome fragments were made by first constructing a linear derivative of the 61-kb ring carrying the ARS307Δ in strain 33-4 (8) by using plasmid pNO8. SUP11-1 was then integrated near the ARS307Δ by using C2G::SUP11-1 (8). Additional ARS deletions were introduced as described below.
Plasmids.
For the deletion of ARS elements, we constructed plasmids carrying ARS deletions ranging in size from 0.25 kb to 3.2 kb in the URA3 vectors YIp5 (51) or pRS306 (49) for use in two-step gene replacements (3). The sequences deleted corresponded to the smallest subclones with ARS activity that we had identified (37). Each of the replicator deletion constructs was demonstrated to lack ARS activity, and substitution of the chromosomal copy of the ARS element with the deletion caused the abrogation of chromosomal replicator activity (8, 9). Plasmids for the deletion of ARS307 and ARS309 were described by Dershowitz and Newlon (8), and the plasmid for deletion of ARS306 was described by Deshpande and Newlon (9). The construction of other ARS deletion plasmids is described in the supplemental material. The ARS305 deletion removes a 1.1-kb BamHI-ClaI ARS305 fragment extending from positions 38606 to 39706 of the chromosome III sequence (www.yeastgenome.org). The ARS310 deletion removes either a 3.4-kb KpnI fragment, creating a deletion extending from positions 164426 to 167613 of the chromosome III sequence, or an 846-kb EcoRV fragment extending from positions 166499 to 167344 of the chromosome III sequence (www.yeastgenome.org). ARS308 was removed by replacing CEN3 with CEN4.
To construct plasmids for chromosome fragmentation (62), we inserted a 340-bp C4A2 Tetrahymena telomere fragment in the integrating vectors pRS304 and pRS306 (49) to serve as a seed for S. cerevisiae telomere addition, following linearization of the plasmid by restriction enzyme digestion. Chromosome III sequences were inserted in the polylinker on the other side of the restriction enzyme site to target chromosome fragmentations. Plasmids designed to fragment the right arm at two sites, position 117326, approximately 2.4 kb to the right of CEN3 (pYND97), and positions 174361, 5 kb to the right of ARS310 (pYND56), were made in pRS304. Plasmids designed to fragment the chromosome at three positions on the left arm were made in pRS306. pYND103 fragments the chromosome at position 6582, approximately 5 kb to the left of ARS301. pYND101 fragments the chromosome at position 17033, approximately 2 kb to the right of ARS320. pYND104 fragments the chromosome at position 31871, approximately 1.5 kb to the right of ARS304.
Plasmid pNO8 was used to linearize the 61-kb ring chromosome near ARS309. Plasmid pYND125 was used to insert telomere repeat sequences into the 61-kb ring chromosome and its ARS deletion derivatives using a two-step gene replacement.
Loss rate measurements.
Chromosome loss rates were measured by fluctuation analysis using the colony isolation method previously described (8). To distinguish chromosome loss from mitotic recombination or gene conversion events that caused the loss of SUP11-1, red colonies were tested for histidine prototrophy and their ability to mate. Chromosome loss events produced His− MATa colonies. White and red colonies from the plates used to isolate colonies for loss rate determinations were tested for the presence of the expected ARS deletions by Southern analysis.
Analysis of replication intermediates.
Methods for DNA isolation, two-dimensional gel electrophoresis, and fork direction analysis were described previously (56).
Chromoductions.
Chromoductions (25) were performed by mating a kar1-Δ15 (58) recipient strain with a strain carrying the chromosome III derivative to be transferred. Chromoductants were selected on plates containing 60 μg/ml canavanine and 10 μg/ml cycloheximide but lacking arginine, leucine, and tryptophan.
RESULTS
Deletion of active replicators causes only modest chromosome instability.
To examine the dependence of chromosome stability on the presence of replicators, we systematically deleted replicators from the leftmost 55% of chromosome III and measured the effects on the rates of chromosome loss by fluctuation analysis (8). For these experiments, we made use of a haploid ade2-101 strain that was disomic for chromosome III. The two copies of chromosome III carry complementing his4 alleles and are heterozygous for mating type. The chromosome from which replicators were deleted carried an insertion of the ochre suppressor tRNA, SUP11-1, 7.8 kb to the left of CEN3. Therefore, the disomic strain is a His+ nonmater that forms white colonies as a result of the suppression of ade2-101 by SUP11-1. Loss of the test chromosome is signaled by a red colony or a red sector that is His− Ade− MATa.
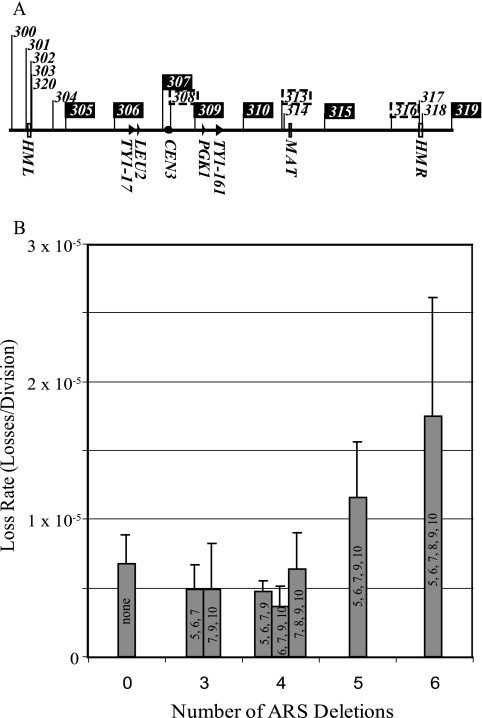
Figure 1A shows the locations and relative efficiencies of replicators along chromosome III. In most cells, this chromosome is replicated by forks that initiate at the seven efficiently used replicators: ARS305, ARS306, ARS307, ARS309, ARS310, ARS315, and ARS319 (36, 41). The removal of up to four of the five efficient replicators between the left telomere and MAT had no effect on the loss rate. Each of the replicator deletions was studied individually and in combination with other deletions (Fig. 1B). Two-tailed t tests, analysis of variance tests, and nonparametric analogs were used to assess the statistical significance of differences in loss rates. The effect of deleting ARS306 and ARS307 (data not shown), which are both on the left arm, was not significantly different from that of deleting ARS307 and ARS309, which flank the centromere (8). These pairs of deletions created regions of approximately 100 kb that lack efficient replicators. Similarly, deleting ARS305, ARS306, and ARS307, which removed all efficient chromosomal replicators from the left arm, or ARS307, ARS309, and ARS310 yielded chromosomes with wild-type loss rates. Chromosomes from which four ARS elements, either ARS305, ARS306, ARS307, and ARS309 or ARS306, ARS307, ARS309, and ARS310 or ARS307, ARS308, ARS309, and ARS310, were deleted also showed wild-type loss rates. It was not until all five efficient replicators in the 194-kb region between the left telomere and MAT (ARS305, ARS306, ARS307, ARS309, and ARS310) were deleted to produce the 5ORIΔ chromosome that there was a modest two- to threefold increase in the loss rate. ARS308 is tightly associated with CEN3 (20). Replacing CEN3 with CEN4, which does not have an associated ARS element, created the 6ORIΔ chromosome and caused no further statistically significant increase in loss rate. The loss rates for chromosomes with zero to four ARS deletions were significantly lower that the loss rates for chromosomes with five or six ARS deletions (P = 0.002). Thus, the 164-kb region of the chromosome between ARS304 and ARS313 can be replicated by forks that initiate outside the region. Because ARS304 and the ARS elements to its left, as well as ARS314, are not detectably active in wild-type strains and ARS313 is only weakly active, these results raise the possibility that the entire 225-kb region to the left of ARS315, which comprises 71% of the chromosome, can be replicated by a fork originating at ARS315.
FIG. 1.
Chromosome III replicators and loss rates of ARS deletion chromosomes. (A) Positions of ARS elements are indicated above the line representing chromosome III. ARS elements that function as efficient chromosomal replication origins are indicated as black boxes with white type. ARS elements that function in less than 25% of cell cycles are boxed with dotted lines (41). ARS elements that do not normally function as replication are not boxed.CEN3 is indicated by a filled circle on the map line. (B) ARS elements were deleted from the SUP11-1-marked chromosome of strain CF4-16B33 as described in Materials and Methods, and loss rates were determined by fluctuation analysis. The numbers within the bars indicate the last one or two digits of the names of the ARS elements deleted (e.g., 7 refers to ARS307). Error bars indicate standard deviations.
A chromosome III derivative lacking all ARS elements is surprisingly stable.
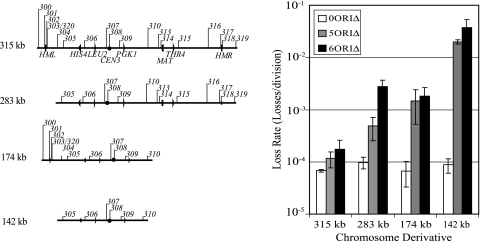
To assess the role of the remaining replicators in maintaining the chromosome, we performed a series of chromosome fragmentations (62) to remove the remaining ARS elements (Fig. 2). The fragmentation of chromosome III just to the right of ARS304, which produced a 283-kb chromosome fragment, did not affect the stability of the chromosome carrying no ARS deletions (Fig. 2). However, the loss rate of the 283-kb 6ORIΔ chromosome, lacking ARS305 through ARS310, increased approximately 37-fold, suggesting that one or more of the six normally inactive replicators on the left end of the chromosome contributes to the replication 6ORIΔ of the chromosome. Consistent with this idea, in the 6ORIΔ strain we found very weak bubble arcs associated with ARS301 and ARS303 in two-dimensional gel patterns of replication intermediates (data not shown), confirming a previous report that these two ARS elements became active replicators in a chromosome from which ARS305 and ARS306 were deleted (63). Replication intermediates of both the balancer chromosome and the 6ORIΔ chromosome were seen in these two-dimensional gel patterns. Therefore, if ARS301 and ARS303 were fully active in the 6ORIΔ chromosome, the intensities of the bubble arcs and Y arcs should have been similar. However, the bubble arcs were much less intense than the Y arcs, suggesting that these replicators function inefficiently in the 6ORIΔ chromosome.
FIG. 2.
Loss rates of chromosomes fragmented near ARS304, ARS310, or both. Diagrams on the left show the 0ORIΔ derivatives of chromosome III used. 0ORIΔ, 5ORIΔ, and 6ORIΔ chromosomes described for Fig. 1 were fragmented as described in Materials and Methods. pYND104 was used for fragmentations near ARS304, producing the 283-kb fragment, and pYND56 was used for fragmentations near ARS310, producing the 174-kb fragment. The 141-kb derivative was fragmented at both ends. Loss rates were determined by fluctuation analysis. Error bars indicate standard deviations.
We also examined the stability of 174-kb chromosomes fragmented 6.9 kb to the right of ARS310 to remove the remaining seven replicators on the right arm (Fig. 2). The results were similar to those found for the fragmentations near ARS304, with no effect on the 0ORIΔ control and a 30-fold increase in the loss rate of the 6ORIΔ chromosome (Fig. 2). These results demonstrate that replicators distal to ARS310 on the right arm also contribute to the replication of the 6ORIΔ chromosome.
Finally, we fragmented the 0ORIΔ and 6ORIΔ chromosomes to the right of ARS304 and to the right of ARS310, producing a 142-kb chromosome III fragment. While the stability of the 0ORIΔ chromosome fragment was unaffected, the loss rate of the 6ORIΔ chromosome fragment increased by more than 200-fold relative to the full-length 6ORIΔ chromosome (Fig. 2). Nevertheless, it is remarkable that the 6ORIΔ doubly fragmented chromosome, which carries no sequence that functions as an ARS element in the plasmid assay, still replicates and segregates correctly in 97% of cell divisions.
Because we had seen no significant difference in the loss rates of the full-length 5ORIΔ and 6ORIΔ chromosomes, we also examined the loss rates of the fragmented 5ORIΔ chromosomes, which retain the CEN3-associated inefficient replicator ARS308 (Fig. 2). While the loss rates of the 5ORIΔ fragments were not significantly different from the loss rates of the 6ORIΔ fragments, each of the 6ORIΔ loss rates was higher than the corresponding 5ORIΔ loss rate, suggesting that ARS308 contributes in a minor way to the maintenance of the 5ORIΔ fragments.
One explanation for the unexpectedly high stabilities of origin deletion chromosomes is that they regained one or more origins by gene conversion or reciprocal recombination with the balancer chromosome. To examine this possibility, we used Southern analysis to test for the presence of ARS deletions and, where appropriate, the presence of the new telomere(s) in both white disomic colonies and red colonies that had lost the SUP11-1-marked chromosome. Colonies from each fluctuation experiment and from the strains created for analysis of chromosome stability were analyzed. Events that restored replication origins were rare. Only 1 of 58 5ORIΔ or 6ORIΔ full-length chromosomes regained an ARS element, and only 4 of 50 singly fragmented 5ORIΔ or 6ORIΔ chromosomes regained one or more ARS elements by gene conversion or reciprocal recombination. We also deleted RAD52, which is required for all homologous recombination. rad52 mutants are known to exhibit increased chromosome loss rates (21, 33). We found that the loss rate of the 0ORIΔ fragment was increased to the same extent as the loss rate of the 5ORIΔ fragment (data not shown). Therefore, the maintenance of these origin deletion chromosomes does not depend on the acquisition of a replication origin.
Further evidence that homologous interaction with the balancer chromosome did not contribute substantially to the maintenance of the 5ORIΔ or 6ORIΔ chromosome was provided by the analysis of strains carrying a homeologous balancer chromosome derived from the alloploid brewing yeast S. carlsbergensis (57, 68) in place of the S. cerevisiae balancer. This brewing yeast chromosome, which can substitute for S. cerevisiae chromosome III function, is a mosaic in which the sequences between the left telomere and MAT were derived from a chromosome diverged in DNA sequence from the S. cerevisiae homeologue, and the sequences distal to the MAT locus on the right arm are S. cerevisiae-like (reviewed in reference 26). Mitotic recombination rates between sequences on this chromosome and S. cerevisiae sequences are reduced 10- to 20-fold in the homeologous regions (42). If homologous interactions were important for the maintenance of the 5ORIΔ or 6ORIΔ chromosome, then their stabilities should be reduced in strains carrying the S. carlsbergensis balancer. Measurements of loss rates of S. cerevisiae chromosome III in this strain background revealed that the loss rates of the 315-kb 0ORIΔ full-length chromosome ([3.7 ± 1.8] × 10−5 loss/division) and 174-kb 0ORIΔ chromosome fragmented at ARS310 ([7.0 ± 2.8] × 10−5 loss/division) were not significantly different from the loss rates measured in strains with the S. cerevisiae balancer (Fig. 2). The 5ORIΔ and 6ORIΔ full-length chromosomes ([3.0 ± 1.0] × 10−4 and [4.4 ± 1.0] × 10−4 loss/division, respectively) and the 174-kb 5ORIΔ chromosome fragmented near the position of ARS310 ([2.4 ± 0.5] × 10−3 loss/division) were lost at rates approximately twofold higher in these strains than in strains with the S. cerevisiae balancer (Fig. 2). The 174-kb 6ORIΔ chromosome fragmented near the position of ARS310 ([1.1 ± 0.3] × 10−2 loss/division) was lost at a rate approximately fivefold higher in these strains. These modest increases in loss rate of the 5ORIΔ and 6ORIΔ chromosomes further indicate that homology with the balancer chromosome is not a major factor in the maintenance of the originless chromosomes, although we cannot exclude the possibility of a role for short regions of homology that are not able to direct efficient homologous recombination.
Replication of the 6ORIΔ fragment.
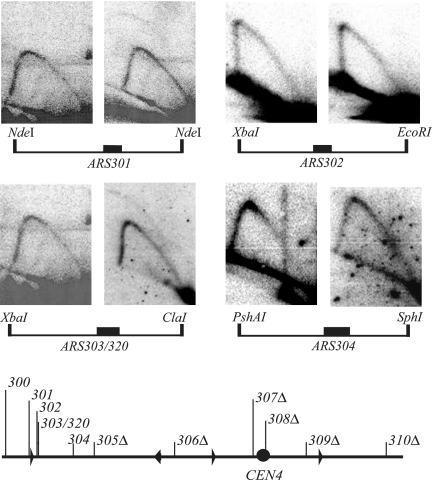
To further explore the role of the six inactive ARS elements in the maintenance of the 6ORIΔ chromosome fragmented to the right of the position of ARS310, we undertook an additional two-dimensional analysis of replication intermediates and constructed additional fragmentations at both ends. For the two-dimensional gel analysis, we used a strain carrying the homeologous S. carlsbergensis chromosome III balancer, which made it possible to find probes that hybridize only to the S. cerevisiae chromosome at high stringency, allowing the visualization of replication intermediates that arise specifically from the 6ORIΔ fragment. Analyses of replication intermediates of ARS301, ARS302, ARS303/320, and ARS304 are shown in Fig. 3. Bubble arcs are present in the ARS301 and ARS303/320 patterns of the 174-kb 6ORIΔ fragment, but not in the 174-kb 0ORIΔ fragment. We have never observed origin activity of ARS302 or ARS304 in its normal chromosomal context.
FIG. 3.
Two-dimensional gel analysis of replication intermediates of ARS301, ARS302, ARS303/ARS320, and ARS304 in 0ORIΔ and 6ORIΔ chromosomes fragmented near ARS310. DNA was prepared from strains CB08D3 (0ORIΔ) and YDN289 (6ORIΔ), which carry the S. carlsbergensis balancer chromosome. A diagram of the restriction fragment examined is shown below each pair of two-dimensional patterns. The filled rectangle represents the position of the ARS element(s). In all cases, the left panel of a pair shows the pattern obtained from the 0ORIΔ chromosome fragment and the right panel shows the pattern obtained from the 6ORIΔ fragment. A diagram of the 174-kb 6ORIΔ chromosome fragment examined is shown at the bottom of the figure. Landmarks on the map line are labeled as described for Fig. 2.
Results of stability measurements on a series of 174-kb 5ORIΔ chromosomes carrying further fragmentations at the left end in strain F510 corroborated the role of the ARS elements flanking HML in the maintenance of the 174-kb 5ORIΔ fragment. Removing 6.5 kb of the left end of the chromosome, including ARS300, caused no increase in the loss rate of this fragment ([1.3 ± 0.3] × 10−3 loss/division) relative to the 174-kb chromosome fragment (Fig. 2). In contrast, the removal of 17 kb of the left end of the chromosome, including HML as well as ARS301, ARS302, and ARS303/ARS320, caused an increase in loss rate equivalent to that seen for the 142-kb 5ORIΔ fragment ([1.8 ± 0.6] × 10−2 loss/division) (Fig. 2). Therefore, of the six inactive ARS elements on the left end of chromosome III, it appears that replication initiations at only ARS301 and ARS303/ARS320 contribute significantly to the maintenance of the 174-kb 5ORIΔ chromosome III fragment.
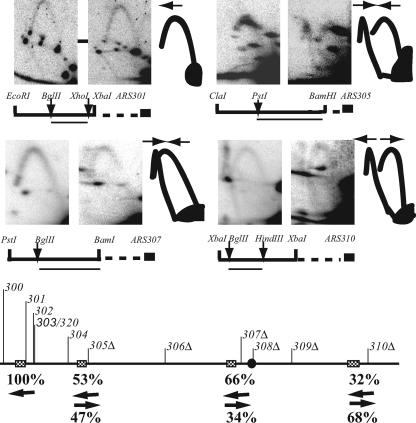
If the 6ORIΔ fragment were replicated by initiations at ARS301 and ARS303/320, then replication forks should diverge from these two ARS elements. To test this hypothesis and to gain insight into how the remainder of the fragment is replicated, we determined the direction of fork movement along the 174-kb 6ORIΔ chromosome by using a modification of the two-dimensional gel procedure (17). Figure 4 shows the analysis of fork movement through fragments immediately to the left of HML, ARS305, ARS307, and ARS310. In the 174-kb 0ORIΔ control fragment, the patterns showed only forks moving to the left from the nearest origin, except near ARS310. The fainter fork moving rightward into ARS310 demonstrates that ARS310 initiates replication in approximately 70% of cell cycles, confirming our previous observations in another strain (56).
FIG. 4.
Analysis of the direction of replication fork movement along the 0ORIΔ and 6ORIΔ chromosomes fragmented near ARS310. DNA was prepared from strains described in the legend for Fig. 3. Diagrams of the restriction fragments analyzed to determine replication fork directions and their relationship to the ARS element or ARS deletion are shown below each set of three panels, with arrows showing the position(s) of the restriction sites used for in-gel digestion prior to the second dimension. The probes used are shown as thin lines below restriction fragment diagrams. In each set of three panels, the left panel shows the pattern obtained from the 0ORIΔ fragment, the middle panel shows the pattern obtained from the 6ORIΔ fragment, and the right panel is a diagram showing the arcs produced by leftward- and rightward-moving forks. How these replication intermediates are resolved depends on the geometry of the site of the in-gel digestion and the probe used to detect them. The directions of fork movement are indicated by arrows above the arcs in the diagrams. The diagram at the bottom of the figure summarizes the quantitation of directions of fork movement through the 6ORIΔ fragment. In this chromosome fragment, ARS308 was deleted by replacing CEN3 with CEN4, which is indicated by a filled circle on the map line. Hatched rectangles indicate the fragments analyzed, and the arrows beneath the hatched rectangles are marked with the percentage of forks going in each direction.
The presence of diverging forks in regions flanking HML in the 174-kb 6ORIΔ fragment provides further support for the idea that replication initiates at latent origins surrounding HML. Since we detected only forks moving to the left from HML in the ARS301 panel but detected forks moving in both directions at approximately equal frequency to the right of HML in the ARS305 panel, we conclude that replication initiates at ARS301 and/or ARS303 only about half of the time.
We also detected forks moving in both directions just to the left of the ARS307 and ARS310 deletions in the 6ORIΔ fragment (Fig. 4). It is particularly striking that at least 30% of the forks that replicate the region adjacent to the ARS310 deletion move leftward through the fragment, indicating that they must have initiated distal to the ARS310 deletion. The new telomere seeded by a C4A2 tract in the fragmentation vector is located only 6.8 kb to the right of the ARS310 deletion, suggesting either that there is a replication origin in this 6.8 kb of chromosome III or that replication is initiating at the new telomere. The fragmentation vector lacks ARS activity, making it unlikely that replication initiates in vector sequences.
A potential mechanism for the introduction of a replicator in this region is the acquisition by the new telomere of a subtelomeric X or Y′ ARS element from another chromosome. The presence of an X element would extend the length of the new telomere by approximately 500 bp, and a Y′ element would add 5 to 6.2 kb of sequence with a characteristic restriction pattern (7). The new telomere was examined by Southern analysis, making use of an EcoRV site in the fragmentation vector and a plasmid probe, to determine the size of the new telomere. The somewhat diffuse band revealed, centered at about 1.6 kb, was predicted by the expected addition of C1-3A repeats to the C4A2 tract of the fragmentation vector by yeast telomerase (54) and is inconsistent with the presence of either a subtelomeric X or a subtelomeric Y′ element (data not shown).
To test whether specific sequences on the right arm of the 174-kb 5ORIΔ fragment contribute to its maintenance, we fragmented this chromosome 2.4 kb to the right of CEN3. The loss rate of the resulting 117-kb fragment ([9.7 ± 0.4 ] × 10−4 loss per division) was not different from the loss rate of the 174-kb 5ORIΔ or 6ORIΔ fragment (Fig. 2), making it unlikely that there is an additional replication origin on the right arm of the 174-kb fragment that contributes to its maintenance.
Taken together, these results are consistent with at least two mechanisms for the maintenance of the chromosome III fragment lacking conventional replication origins. First, in any given chromosome fragment, replication could initiate at one of several replication origins that are so inefficient that they cannot support the maintenance of a plasmid in the ARS assay. Second, replication could initiate by an unknown mechanism at one end or the other of the fragment, but not at both ends in most molecules. Both models would account for the demonstrated bidirectional movement of replication forks at several different places along the 174-kb 6ORIΔ fragment.
Linear topology or telomeres are required for the maintenance of a chromosome fragment lacking ARS elements.
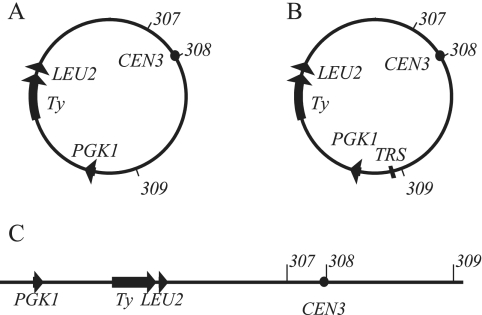
We showed previously that a 61-kb ring chromosome (Fig. 5A) from which the two efficient replication origins, ARS307 and ARS309, were removed could not be stably maintained; at very high frequency, one of the two replication origins was restored by gene conversion (8). To test whether this fragment of chromosome III, which was produced by a recombination between a Ty2 to the left of LEU2 and a Ty1 to the right of PGK1, could be maintained as a linear fragment, we introduced a URA3 gene flanked by C4A2 tracts near ARS309 and selected for linear derivatives of the ring chromosome on plates containing 5-fluoroorotic acid (32). The presence of the expected new telomeres on the 61-kb linear fragment (Fig. 5C) was confirmed by Southern blotting (data not shown). Replicators were then deleted as described above. Consistent with our previous results (8), the deletion of either ARS307 or ARS309 from the 61-kb ring chromosome caused a three- to fourfold increase in loss rate and a derivative lacking both efficient replicators was not recovered (Fig. 5; Table 2). In contrast, the 2ORIΔ linear derivatives from which the efficient replicators ARS307 and ARS309 were deleted or the 3ORIΔ linear derivative lacking all three replicators were maintained and showed only two- or threefold increases in loss rate relative to the linear derivative with only ARS307 deleted. The loss rate of the linear carrying the ARS307 deletion was similar to the loss rate of the ring from which it was derived.
FIG. 5.
Diagrams of 61-kb chromosome III derivatives analyzed. (A) Ring chromosome III (61 kb) formed by recombination between Ty1-17, which is a Ty2 element, and Ty1-161 (Fig. 1A). The hybrid Ty formed by the recombination is designated “Ty” on the maps in the figure. (B) Ring chromosome (61 kb) carrying the 276-bp TRS as described in Materials and Methods. (C) The ring chromosome was linearized as described in Materials and Methods.
TABLE 2.
Comparison of stabilities of 61-kb circular and linear derivatives of chromosome III
| Replicator(s) deleted | Loss rate (losses/division [103])a
|
||
|---|---|---|---|
| Ring | Ring plus TRS | Linear | |
| None | 1.24 ± 0.28 | 3.02 ± 0.68 | Not done |
| ARS307 | 4.52 ± 0.96 | 7.53 ± 0.68 | 3.99 ± 0.71 |
| ARS309 | 4.68 ± 0.88 | 6.10 ± 0.95 | Not done |
| ARS307 and ARS309 | Not recovered | Not recovered | 7.45 ± 1.90 |
| ARS307, ARS308, and AR309 | Not done | Not done | 9.71 ± 1.50 |
See Fig. 5 for diagrams of 61-kb ring and linear chromosomes.
The 61-kb ring chromosome and its linear derivative contain identical DNA sequences except for the telomeres on the linear form. Longtine et al. (30) have reported that TRSs stabilize small ARS plasmids by improving their ability to segregate properly. We undertook two experiments to test the idea that the presence of a TRS would improve the stability of the 61-kb ring chromosome. First, we made use of the SUP11-1 marker on the ring chromosome to determine whether the loss events caused by the deletion of replication origins from the 61-kb ring chromosome and its linear derivatives were due to failure to replicate (1:0 loss events) or failure to segregate (2:0 loss events). The strain carrying a single copy of the 61-kb ring gives rise to colonies that are slightly pink on plates with limiting adenine, due to the incomplete suppression of the ade2-101 allele in the strain, while strains carrying two copies of SUP11-1 give rise to white colonies. Moreover, the sectoring patterns of the colonies are different, with colonies produced by cells with a single copy of the SUP11-1-marked fragment frequently having multiple red sectors, while colonies produced by cells carrying two copies of the marked chromosome fragment infrequently having sectors because the production of a red sector requires independent losses of both marked fragments. Therefore, missegregation events are expected to cause an accumulation of white, nonsectoring colonies along with red colonies that lack the fragment, while failures to replicate are expected to give rise to pinkish sectoring colonies along with red colonies. The number of cells plated for fluctuation analysis was optimized to determine the rate of production of loss events (signaled by red colonies), which include both 1:0 and 2:0 segregations. Nevertheless, it was possible to estimate that the rate of production of white, nonsectoring colonies was (4.1 ± 2.3)× 10−4, a rate 10-fold lower than the rate of loss events. Because reversions of the ade2-101 mutation and new mutations in ADE1 both result in the production of white, nonsectoring colonies, the 10-fold-lower rate of missegregation represents an minimum estimate of the difference. Therefore, simple 1:0 loss events, presumably from failures to replicate, rather than missegregations, constitute the predominant mechanism of loss of the 61-kb ring chromosome.
The second experiment was to insert a 276-bp TRS sequence into 0ORIΔ and 1ORIΔ 61-kb ring chromosomes at the site that was used to linearize the ring (Fig. 5 B) and to measure loss rates of resulting chromosomes (Table 2). The loss rates of all three ring derivatives carrying the TRS were 1.5- to 2-fold higher than the loss rates of the corresponding rings lacking the TRS. Although the differences in loss rate were not significant in two of the cases, these results indicate that the addition of a TRS to the ring chromosomes does not improve their stability. Using the single ARS deletion constructs carrying the TRS, we also attempted to delete the second efficient origin in the presence of the TRS. Both the ARS307 and ARS309 deletion constructs normally yield more ARS deletion popouts than wild-type popouts in the two-step gene replacement procedure, but in this case, we failed to recover the 2ORIΔ chromosome. We analyzed a total of 26 Ura− Leu+ popouts of single ARS deletion rings carrying TRS and found that 19 carried the wild-type copy of the ARS element we were trying to delete. Two of the recovered ring chromosomes carried the wild-type copies of both ARS307 and ARS309, and one carried the deletion we were trying to make but had lost the first deletion. These three-ring chromosomes almost certainly acquired wild-type ARS elements by gene conversion from the balancer chromosome. Finally, we found four very small colonies that grew on plates lacking Leu and containing 5-fluoroorotic acid that were used to select Ura− popouts. These small colonies were Leu− and lacked the ring chromosome when restreaked. It is possible that these four colonies initially carried the 2ORIΔ ring, but it was so unstable that it was lost in subsequent restreaking. These results indicate that internal telomere repeat sequences are insufficient to stabilize the 2ORIΔ 61-kb ring chromosome and that the presence of telomere repeat sequences alone cannot explain the maintenance of the linear chromosome fragment lacking replicators. Instead, it is likely that either the linear topology or the presence of telomeres on the linear fragment supports replication initiation events that cannot occur on the circular chromosome.
ORC is required for the maintenance of five-deletion fragments.
The possibility that replication initiates at or near telomeres on chromosome fragments lacking ARS elements raises the question of whether the normal replication initiation machinery is required for maintenance of these origin-deficient chromosomes. Therefore, we tested whether mutations in the replication initiator protein ORC affected the stability of chromosome fragments from which efficient replicators have been deleted. Temperature-sensitive mutations in two subunits of ORC, orc2-1 and orc5-1, cause defects in replication initiation at the permissive temperature, measured both by plasmid stability assays and by two-dimensional gel analysis of chromosomal replication origins (12, 16, 27), although the orc5-1 defect is more apparent at semipermissive temperatures. We introduced the orc2-1 and orc5-1 mutations into strain YKN6 by two-step gene replacements. Consistent with published results, both mutant strains had a plasmid maintenance defect relative to the wild-type strain, measured as the fraction of plasmid-bearing cells in cultures grown under selection for the plasmid (61). The ARSH4 CEN6 plasmid pRS315 (49) was present in 92.3% ± 7.5% of YKN6 cells, 37.0% ± 2.2% of orc2-1 cells, and 52.6% ± 8.3% of orc5-1 cells.
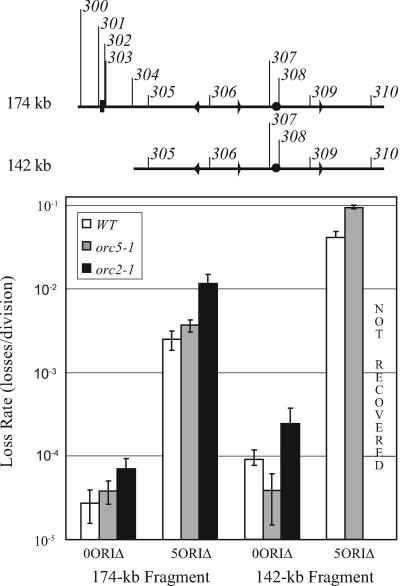
We then introduced both 174-kb and 142-kb 0ORIΔ and 5ORIΔ chromosome fragments into the strains by chromoduction and measured fragment stabilities by fluctuation analysis (Fig. 6). Consistent with the role of ORC in the activation of both efficient and dormant replication origins, the orc2-1 mutation caused a significant increase in the loss rate of the 174-kb 0ORIΔ and 5ORIΔ chromosome fragments and the 142-kb 0ORIΔ chromosome fragment. Strikingly, we were unable to recover chromoductants carrying the 142-kb 5ORIΔ chromosome fragment. In all chromoductants analyzed by Southern blotting, the 142-kb 5ORIΔ fragment had acquired replicators, either by gene conversion of one or more replicators from the balancer chromosome or by events that restored the right or left end of the chromosome, resulting in the loss of one of the telomeres introduced by fragmentation. At the permissive temperature (23°C), the orc5-1 mutation had no effect on the loss rate of either the 174-kb or the 142-kb 0ORIΔ or 5ORIΔ chromosome fragments (data not shown). At the semipermissive temperature of 27°C, the 142-kb 5ORIΔ chromosome fragment, but not the other three fragments tested, was lost at a rate 2.5-fold higher in the orc5-1 strain than in the wild-type strain (Fig. 6). Taken together, these results indicate that ORC is required for maintenance of the chromosome III fragment lacking ARS elements.
FIG. 6.
Stabilities of 0ORIΔ and 5ORIΔ chromosomes fragmented near ARS310 or near ARS304 and ARS310 in orc5-1 and orc2-1 strains. Diagrams of the 174-kb and 142-kb 0ORIΔ fragments are shown at the top of the figure. Strains YKN6 (wild type), YNS1 (orc2-1), and YNS3 (orc5-1) were mated with donor strains F013 and F510, and chromoductants carrying the chromosome III 0ORIΔ and 5ORIΔ fragments were selected as described in Materials and Methods. Loss rates were determined by fluctuation analysis. Loss rates in the wild-type and orc2-1 strains were determined at 23°C, and loss rates in the orc5-1 strain were determined at the semipermissive temperature of 27°C. Error bars indicate standard deviations.
DISCUSSION
Yeast chromosomes are very stable, with loss rates in the range of 10−5 loss per cell division. The loss rate of natural yeast chromosomes is three orders of magnitude lower than the loss rates of centromeric plasmids or yeast artificial chromosomes (YACs) carrying foreign DNA (59, 60), providing an opportunity to measure very small perturbations in their stability. Our systematic analysis of the effects of deleting replicators from yeast chromosome III revealed that replicators are redundant in the sense that single ARS deletions and all combinations of double, triple, and quadruple ARS deletions tested had no detectable effect on chromosome stability. It was not until we deleted the five efficient replicators present in the leftmost 71% of chromosome III that the loss rate of the chromosome increased two- to threefold. This low loss rate raises the possibility that this 225-kb region could be replicated by a fork originating at ARS315. Based on measured fork rates and length of S phase, the replication of this length of DNA by a single fork is feasible (43).
Further analysis of this 315-kb full-length 5ORIΔ chromosome and the 6ORIΔ chromosome that also lacks ARS308 demonstrated that two “dormant” replicators flanking HML, ARS301, and ARS303, which normally do not function as chromosomal replication origins (15), become weakly active and contribute to the maintenance of the chromosome. These results are consistent with those of Vujcic et al. (63), who showed that these two ARS elements become active in a chromosome from which the early-initiating replicators ARS305 and ARS306 had been deleted. The region of chromosome III containing these dormant replicators is normally replicated by a fork that originates at ARS305 and requires about 15 min to traverse the ca. 25-kb region between ARS305 and ARS303. ARS301 initiates replication late in a plasmid context (4), suggesting that it is likely to be replicated by a fork from ARS305 before it initiates replication in the chromosome. Support for this hypothesis is provided by studies on rad53 strains, in which replication forks were induced to collapse by treatment with hydroxyurea (31, 45). Under these conditions, which further slow forks initiated at ARS305, ARS301 initiated 30 to 45 min later than ARS305. These observations suggest that replicators that have evolved to initiate late may play an important role in allowing complete replication of chromosomes in which early-initiating replication forks have collapsed. Because prereplicative complexes competent to initiate replication are assembled only during G1 (reviewed in reference 2), regions near the ends of chromosomes that are normally replicated by forks from interior origins may be particularly vulnerable to replication fork collapse. In this regard it is interesting that Wyrick et al. (64) identified a large number of pro-ARSs, defined as genomic sites that bind both ORC and MCM proteins, in regions within 20 kb of telomeres.
As expected, we found that removing the right arm of the chromosome distal to the ARS310 deletion, which contains efficient replication origins, increased the loss rates of the 5ORIΔ and 6ORIΔ fragments but only to the extent caused by deleting the dormant origins on the left end. An analysis of the direction of fork movement at several places along the 6ORIΔ fragment provided evidence for the activation of the dormant origins flanking HML, which are likely to be the sources of forks moving rightward through the fragment. It also revealed forks moving in both directions in the other three regions analyzed, including a region only 15 kb from the telomere seeded by telomeric repeats in the fragmentation vector, suggesting that replication might initiate at or near the new telomere. Consistent with these results, in their analysis of a YAC in which they created a 174-kb region devoid of replication origins, van Brabant et al. (59) found forks moving in both directions through the originless region, including a region near the right telomere of the YAC. The mechanism by which such initiation events occur remains to be determined, although our findings provide some additional insight into the process.
Our analysis of the maintenance of a 61-kb linear chromosome fragment derived from the 61-kb ring chromosome that was formed by recombination between Ty elements to the left of LEU2 and to the right of PGK1 (52) provides evidence that either a linear topology or the presence of functional telomeres allows replication initiation events that do not occur on the ring chromosome (Fig. 5 and Table 2). One possible explanation for the stability of the 3ORIΔ 61-kb linear chromosome fragment was that it was stabilized by the addition of TRSs, which are added by telomerase during the formation of new telomeres. Longtine et al. (30) found that the addition of a TRS to a small ARS plasmid lacking a centromere increased the stability of the plasmid by improving its segregation efficiency. However, when a TRS was included in a small plasmid carrying a centromere, it caused a reduction in the stability of the plasmid. Consistent with the plasmid studies, we found that the insertion of a TRS in the 0ORIΔ ring or in two 1ORIΔ rings failed to improve the stability of the rings and may have caused a small increase in their loss rates. Therefore, an internal TRS, which binds Rap1p and may recruit factors required for telomeric silencing (29), is not sufficient to stabilize the ring chromosome.
Further experiments will be required to determine the role of telomeres and/or linear topology in the maintenance of the ORIΔ chromosome fragments. One explanation of the role of telomeres is based on the observation that yeast telomeres acquire longer G-strand tails late during S phase, apparently as a result of degradation of the telomeric C-rich strand (reviewed in reference 6). This observation indicates that the lagging strand replication machinery is active at most or all telomeres late in S phase, and it is possible that a replication fork can be formed at telomeres of chromosomes that have not replicated normally during S phase. Another possibility is that ORC, which is known to play a role in the establishment of transcriptionally repressed domains in yeast and Drosophila (reviewed in reference 1), is recruited to telomeres by interaction with one or more telomere binding proteins (see below).
Our finding that ORC is required for the maintenance of the 141-kb 5ORIΔ chromosome suggests that the normal replication initiation machinery is required for the maintenance of the fragment, although we cannot eliminate the possibility that the role ORC plays is distinct from that of replication initiation (see below). It is unlikely that additional ARS elements that contribute to the maintenance of the 141-kb 6ORIΔ are present. In our analysis of overlapping fragments of chromosome III for ARS activity (37, 41), we found no evidence of additional ARS elements, although recent genome-wide analyses of potential replication origins in budding yeast have identified additional potential replicators on chromosome III. In their analyses of ORC and MCM binding sites, Wyrick et al. (64) and Xu et al.(65) found a single ORC-MCM binding site present in the 142-kb chromosome III fragment that was not associated with an ARS element. This site is a few kilobases distal to ARS309, and a fragment amplified from this region did not have ARS activity in the plasmid assay (65). Moreover, initiation events at this site cannot account for the leftward-moving forks seen centromere proximal to the ARS310 deletion (Fig. 4). Therefore, if replication is initiating at internal sites in an ORC-dependent manner, the sites must not bind ORC efficiently enough to be detected in the chromatin immunoprecipitation assay used to identify ORC binding sites.
In another approach to identifying replication origins, Nieduszynski et al. (39) used an analysis of phylogenetic conservation of intergenic regions to identify replication origin sequences in S. cerevisiae. This analysis identified most of the known ARS elements on chromosome III but no additional ones. An additional five phylogenetically conserved intergenic regions with matches to the ACS are present in the chromosome fragment studied here, but they have not been tested for ARS activity (C. A. Nieduszynski, personal communication). These sites were eliminated from the final list of replication origins by one or more of the filters utilized in the study (39). Finally, the results of the whole-genome approaches to mapping replication origins discussed in the introduction have recently been compiled with both the ORC and MCM binding studies and the phylogenetic conservation data in an origin database, OriDB (38). This database lists four potential replication initiation sites on chromosome III that were identified in one or more microarray studies. Only one of these sites was identified as an ORC-MCM binding site, and the fragment containing this region does not have ARS activity.
In addition to its well-defined role in replication initiation and in the establishment of transcriptionally repressed domains in yeast and Drosophila (reviewed in reference 1), ORC is also believed to have a mitotic function. Dillin and Rine (11) first suggested a mitotic role for ORC based on the mitotic delay seen in the orc5-1mutant. The orc2-1 and orc5-1 mutations are each synthetically lethal with deletion mutations causing sister chromatid cohesion defects, including ctf4Δ, ctf8Δ, ctf18Δ, and dcc1Δ (53). In addition, the orc5-1 mutation enhances the cohesion defect caused by scc1-73, a mutation in a cohesin subunit. Finally, ORC has also been shown to play a cohesin-independent role in sister chromatid cohesion in yeast (46). We cannot eliminate the possibility that the role of ORC in maintenance of the 142-kb 5ORIΔ fragment is related to its role in transcriptional silencing or sister chromatid cohesion. To ultimately understand where replication initiates on the 5ORIΔ and 6ORIΔ fragments will require the analysis of single molecules. We are currently using single-molecule analysis of the replicated DNA (40) to analyze the replication of individual originless chromosome III fragments.
In their studies of a YAC carrying human DNA similar in composition to yeast DNA, van Brabant et al. (59) found that an “originless” derivative of this YAC was lost at a rate similar to the loss rate of our 142-kb 6ORIΔ chromosome III fragment lacking both dormant replicators and replicators on the right arm. They found that maintenance of the YAC required RAD9, which encodes a mediator protein required for the DNA damage checkpoint (reviewed in reference 28). In a rad9Δ strain, the YAC was unstable when cells were grown under selection for the YAC, with derivatives that had deleted large portions of the originless region overtaking the population, indicating that the DNA damage checkpoint is important for maintaining the YAC. Consistent with their results, we have found that RAD9 and other genes encoding components of the DNA damage checkpoint pathway are important for the maintenance of the originless chromosome fragment (unpublished data). These findings raise the possibility that DNA damage checkpoint pathway has a role in an alternative route to replication initiation.
Supplementary Material
Acknowledgments
We thank K. Runge, V. Zakian, A. Dillin, and J. Rine for plasmids; Conrad Nieduszynski for additional information about his phylogenetic analysis of conserved sequences on chromosome III; Shakespeare Sajous for technical assistance; Michael Newlon, Carmela Irene, and James Theis for helpful comments on the manuscript; and current and past members of the Newlon lab for helpful discussions.
This work was supported by a NIH grant (GM35679) awarded to C.S.N.
Footnotes
Published ahead of print on 23 April 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bell, S. P. 2002. The origin recognition complex: from simple origins to complex functions. Genes Dev. 16:659-672. [DOI] [PubMed] [Google Scholar]
- 2.Bell, S. P., and A. Dutta. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71:333-374. [DOI] [PubMed] [Google Scholar]
- 3.Boeke, J. D., J. Trueheart, G. Natsoulis, and G. R. Fink. 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154:164-175. [DOI] [PubMed] [Google Scholar]
- 4.Bousset, K., and J. F. Diffley. 1998. The Cdc7 protein kinase is required for origin firing during S phase. Genes Dev. 12:480-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewer, B. J., and W. L. Fangman. 1988. A replication fork barrier at the 3′ end of yeast ribosomal RNA genes. Cell 55:637-643. [DOI] [PubMed] [Google Scholar]
- 6.Chakhparonian, M., and R. J. Wellinger. 2003. Telomere maintenance and DNA replication: how closely are these two connected? Trends Genet. 19:439-446. [DOI] [PubMed] [Google Scholar]
- 7.Chan, C. S., and B. K. Tye. 1983. A family of Saccharomyces cerevisiae repetitive autonomously replicating sequences that have very similar genomic environments. J. Mol. Biol. 168:505-523. [DOI] [PubMed] [Google Scholar]
- 8.Dershowitz, A., and C. S. Newlon. 1993. The effect on chromosome stability of deleting replication origins. Mol. Cell. Biol. 13:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande, A. M., and C. S. Newlon. 1992. The ARS consensus sequence is required for chromosomal origin function in Saccharomyces cerevisiae. Mol. Cell. Biol. 12:4305-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshpande, A. M., and C. S. Newlon. 1996. DNA replication fork pause sites dependent on transcription. Science 272:1030-1033. [DOI] [PubMed] [Google Scholar]
- 11.Dillin, A., and J. Rine. 1998. Roles for ORC in M phase and S phase. Science 279:1733-1737. [DOI] [PubMed] [Google Scholar]
- 12.Dillin, A., and J. Rine. 1997. Separable functions of ORC5 in replication initiation and silencing in Saccharomyces cerevisiae. Genetics 147:1053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson, A. D., W. L. Fangman, and B. J. Brewer. 1998. Cdc7 is required throughout the yeast S phase to activate replication origins. Genes Dev. 12:491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donaldson, A. D., M. K. Raghuraman, K. L. Friedman, F. R. Cross, B. J. Brewer, and W. L. Fangman. 1998. CLB5-dependent activation of late replication origins in S. cerevisiae. Mol. Cell 2:173-182. [DOI] [PubMed] [Google Scholar]
- 15.Dubey, D. D., L. R. Davis, S. A. Greenfeder, L. Y. Ong, J. G. Zhu, J. R. Broach, C. S. Newlon, and J. A. Huberman. 1991. Evidence suggesting that the ARS elements associated with silencers of the yeast mating-type locus HML do not function as chromosomal DNA replication origins. Mol. Cell. Biol. 11:5346-5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox, C. A., S. Loo, A. Dillin, and J. Rine. 1995. The origin recognition complex has essential functions in transcriptional silencing and chromosomal replication. Genes Dev. 9:911-924. [DOI] [PubMed] [Google Scholar]
- 17.Friedman, K. L., and B. J. Brewer. 1995. Analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Methods Enzymol. 262:613-627. [DOI] [PubMed] [Google Scholar]
- 18.Friedman, K. L., B. J. Brewer, and W. L. Fangman. 1997. Replication profile of Saccharomyces cerevisiae chromosome VI. Genes Cells 2:667-678. [DOI] [PubMed] [Google Scholar]
- 19.Greenfeder, S. A., and C. S. Newlon. 1992. Replication forks pause at yeast centromeres. Mol. Cell. Biol. 12:4056-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenfeder, S. A., and C. S. Newlon. 1992. A replication map of a 61-kb circular derivative of Saccharomyces cerevisiae chromosome III. Mol. Biol. Cell 3:999-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwell, L. H., and D. Smith. 1985. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics 110:381-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsiao, C. L., and J. Carbon. 1979. High-frequency transformation of yeast by plasmids containing the cloned yeast ARG4 gene. Proc. Natl. Acad. Sci. USA 76:3829-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, R. Y., and D. Kowalski. 1993. A DNA unwinding element and an ARS consensus comprise a replication origin within a yeast chromosome. EMBO J. 12:4521-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huberman, J. A., J. G. Zhu, L. R. Davis, and C. S. Newlon. 1988. Close association of a DNA replication origin and an ARS element on chromosome III of the yeast, Saccharomyces cerevisiae. Nucleic Acids Res. 16:6373-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji, H., D. P. Moore, M. A. Blomberg, L. T. Braiterman, D. F. Voytas, G. Natsoulis, and J. D. Boeke. 1993. Hotspots for unselected Ty1 transposition events on yeast chromosome III are near tRNA genes and LTR sequences. Cell 73:1007-1018. [DOI] [PubMed] [Google Scholar]
- 26.Kielland-Brandt, M. C., T. Nilsson-Tillgren, C. Gjermansen, S. Holmberg, and M. B. Pederson. 1995. Genetics of brewing yeasts, p. 223-254. In A. E. Wheals, A. H. Rose, and J. H. Harrison (ed.), The yeasts, 2nd ed., vol. 6. Academic Press, London, United Kingdom. [Google Scholar]
- 27.Liang, C., M. Weinreich, and B. Stillman. 1995. ORC and Cdc6p interact and determine the frequency of initiation of DNA replication in the genome. Cell 81:667-676. [DOI] [PubMed] [Google Scholar]
- 28.Longhese, M. P., M. Clerici, and G. Lucchini. 2003. The S-phase checkpoint and its regulation in Saccharomyces cerevisiae. Mutat. Res. 532:41-58. [DOI] [PubMed] [Google Scholar]
- 29.Longtine, M. S., S. Enomoto, S. L. Finstad, and J. Berman. 1993. Telomere-mediated plasmid segregation in Saccharomyces cerevisiae involves gene products required for transcriptional repression at silencers and telomeres. Genetics 133:171-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longtine, M. S., S. Enomoto, S. L. Finstad, and J. Berman. 1992. Yeast telomere repeat sequence (TRS) improves circular plasmid segregation, and TRS plasmid segregation involves the RAP1 gene product. Mol. Cell. Biol. 12:1997-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes, M., C. Cotta-Ramusino, A. Pellicioli, G. Liberi, P. Plevani, M. Muzi-Falconi, C. S. Newlon, and M. Foiani. 2001. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412:557-561. [DOI] [PubMed] [Google Scholar]
- 32.Lundblad, V., and J. W. Szostak. 1989. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 57:633-643. [DOI] [PubMed] [Google Scholar]
- 33.Mortimer, R. K., R. Contopoulou, and D. Schild. 1981. Mitotic chromosome loss in a radiation-sensitive strain of the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 78:5778-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newlon, C. S., and J. F. Theis. 1993. The structure and function of yeast ARS elements. Curr. Opin. Genet. Dev. 3:752-758. [DOI] [PubMed] [Google Scholar]
- 35.Newlon, C. S., and W. G. Burke. 1980. Replication of small chromosomal DNAs in yeast, p. 339-409. In B. Alberts and C. F. Fox (ed.), Mechanistic studies of DNA replication and recombination, vol. 19. Academic Press, New York, NY. [Google Scholar]
- 36.Newlon, C. S., I. Collins, A. Dershowitz, A. M. Deshpande, S. A. Greenfeder, L. Y. Ong, and J. F. Theis. 1993. Analysis of replication origin function on chromosome III of Saccharomyces cerevisiae. Cold Spring Harbor Symp. Quant. Biol. 58:415-423. [DOI] [PubMed] [Google Scholar]
- 37.Newlon, C. S., L. R. Lipchitz, I. Collins, A. Deshpande, R. J. Devenish, R. P. Green, H. L. Klein, T. G. Palzkill, R. B. Ren, S. Synn, and S. T. Woody. 1991. Analysis of a circular derivative of Saccharomyces cerevisiae chromosome III: a physical map and identification and location of ARS elements. Genetics 129:343-357. (Erratum, 130:235, 1992.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieduszynski, C. A., S. Hiraga, P. Ak, C. J. Benham, and A. D. Donaldson. 2007. OriDB: a DNA replication origin database. Nucleic Acids Res. 35:D40-D46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieduszynski, C. A., Y. Knox, and A. D. Donaldson. 2006. Genome-wide identification of replication origins in yeast by comparative genomics. Genes Dev. 20:1874-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norio, P., and C. L. Schildkraut. 2001. Visualization of DNA replication on individual Epstein-Barr virus episomes. Science 294:2361-2364. [DOI] [PubMed] [Google Scholar]
- 41.Poloumienko, A., A. Dershowitz, J. De, and C. Newlon. 2001. Completion of replication map of Saccharomyces cerevisiae chromosome III. Mol. Biol. Cell 12:3317-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priebe, S. D., J. Westmoreland, T. Nilsson-Tillgren, and M. A. Resnick. 1994. Induction of recombination between homologous and diverged DNAs by double-strand gaps and breaks and role of mismatch repair. Mol. Cell. Biol. 14:4802-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raghuraman, M. K., E. A. Winzeler, D. Collingwood, S. Hunt, L. Wodicka, A. Conway, D. J. Lockhart, R. W. Davis, B. J. Brewer, and W. L. Fangman. 2001. Replication dynamics of the yeast genome. Science 294:115-121. [DOI] [PubMed] [Google Scholar]
- 44.Rivin, C. J., and W. L. Fangman. 1980. Replication fork rate and origin activation during the S phase of Saccharomyces cerevisiae. J. Cell Biol. 85:108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santocanale, C., K. Sharma, and J. F. Diffley. 1999. Activation of dormant origins of DNA replication in budding yeast. Genes Dev. 13:2360-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimada, K., and S. M. Gasser. 2007. The origin recognition complex functions in sister-chromatid cohesion in Saccharomyces cerevisiae. Cell 128:85-99. [DOI] [PubMed] [Google Scholar]
- 47.Shimada, K., P. Pasero, and S. M. Gasser. 2002. ORC and the intra-S-phase checkpoint: a threshold regulates Rad53p activation in S phase. Genes Dev. 16:3236-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shirahige, K., T. Iwasaki, M. B. Rashid, N. Ogasawara, and H. Yoshikawa. 1993. Location and characterization of autonomously replicating sequences from chromosome VI of Saccharomyces cerevisiae. Mol. Cell. Biol. 13:5043-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stinchcomb, D. T., K. Struhl, and R. W. Davis. 1979. Isolation and characterisation of a yeast chromosomal replicator. Nature 282:39-43. [DOI] [PubMed] [Google Scholar]
- 51.Struhl, K., D. T. Stinchcomb, S. Scherer, and R. W. Davis. 1979. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc. Natl. Acad. Sci. USA 76:1035-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Surosky, R. T., and B. K. Tye. 1985. Resolution of dicentric chromosomes by Ty-mediated recombination in yeast. Genetics 110:397-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suter, B., A. Tong, M. Chang, L. Yu, G. W. Brown, C. Boone, and J. Rine. 2004. The origin recognition complex links replication, sister chromatid cohesion and transcriptional silencing in Saccharomyces cerevisiae. Genetics 167:579-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szostak, J. W., and E. H. Blackburn. 1982. Cloning yeast telomeres on linear plasmid vectors. Cell 29:245-255. [DOI] [PubMed] [Google Scholar]
- 55.Theis, J. F., and C. S. Newlon. 1997. The ARS309 chromosomal replicator of Saccharomyces cerevisiae depends on an exceptional ARS consensus sequence. Proc. Natl. Acad. Sci. USA 94:10786-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theis, J. F., and C. S. Newlon. 2001. Two compound replication origins in Saccharomyces cerevisiae contain redundant origin recognition complex binding sites. Mol. Cell. Biol. 21:2790-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theis, J. F., C. Yang, C. B. Schaefer, and C. S. Newlon. 1999. DNA sequence and functional analysis of homologous ARS elements of Saccharomyces cerevisiae and S. carlsbergensis. Genetics 152:943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vallen, E. A., M. A. Hiller, T. Y. Scherson, and M. D. Rose. 1992. Separate domains of KAR1 mediate distinct functions in mitosis and nuclear fusion. J. Cell Biol. 117:1277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Brabant, A. J., C. D. Buchanan, E. Charboneau, W. L. Fangman, and B. J. Brewer. 2001. An origin-deficient yeast artificial chromosome triggers a cell cycle checkpoint. Mol. Cell 7:705-713. [DOI] [PubMed] [Google Scholar]
- 60.van Brabant, A. J., W. L. Fangman, and B. J. Brewer. 1999. Active role of a human genomic insert in replication of a yeast artificial chromosome. Mol. Cell. Biol. 19:4231-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Houten, J. V., and C. S. Newlon. 1990. Mutational analysis of the consensus sequence of a replication origin from yeast chromosome III. Mol. Cell. Biol. 10:3917-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vollrath, D., R. W. Davis, C. Connelly, and P. Hieter. 1988. Physical mapping of large DNA by chromosome fragmentation. Proc. Natl. Acad. Sci. USA 85:6027-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vujcic, M., C. A. Miller, and D. Kowalski. 1999. Activation of silent replication origins at autonomously replicating sequence elements near the HML locus in budding yeast. Mol. Cell. Biol. 19:6098-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wyrick, J. J., J. G. Aparicio, T. Chen, J. D. Barnett, E. G. Jennings, R. A. Young, S. P. Bell, and O. M. Aparicio. 2001. Genome-wide distribution of ORC and MCM proteins in S. cerevisiae: high-resolution mapping of replication origins. Science 294:2357-2360. [DOI] [PubMed] [Google Scholar]
- 65.Xu, W., J. G. Aparicio, O. M. Aparicio, and S. Tavare. 2006. Genome-wide mapping of ORC and Mcm2p binding sites on tiling arrays and identification of essential ARS consensus sequences in S. cerevisiae. BMC Genomics 7:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yabuki, N., H. Terashima, and K. Kitada. 2002. Mapping of early firing origins on a replication profile of budding yeast. Genes Cells 7:781-789. [DOI] [PubMed] [Google Scholar]
- 67.Yamashita, M., Y. Hori, T. Shinomiya, C. Obuse, T. Tsurimoto, H. Yoshikawa, and K. Shirahige. 1997. The efficiency and timing of initiation of replication of multiple replicons of Saccharomyces cerevisiae chromosome VI. Genes Cells 2:655-665. [DOI] [PubMed] [Google Scholar]
- 68.Yang, C., J. F. Theis, and C. S. Newlon. 1999. Conservation of ARS elements and chromosomal DNA replication origins on chromosomes III of Saccharomyces cerevisiae and S. carlsbergensis. Genetics 152:933-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.