Abstract
Migration of immature neurons is essential for forming the cortical layers and nuclei. Impairment of migration results in aberrant neuronal cytoarchitecture, which leads to various neurological disorders. Neurons alter the mode, tempo and rate of migration when they translocate through different cortical layers, but little is known about the mechanisms underlying this process. Here we show that endogenous pituitary adenylate cyclase-activating polypeptide (PACAP) has short-term and cortical layer-specific effects on granule cell migration in the early postnatal mouse cerebellum. Application of exogenous PACAP significantly slowed the migration of isolated granule cells and shortened the leading process in the microexplant cultures of the P0-3 cerebella. Interestingly, in the cerebellar slices of P10 mice, application of exogenous PACAP significantly inhibited granule cell migration in the EGL and ML, but failed to alter the movement in the PCL and IGL. In contrast, application of PACAP antagonist accelerated granule cell migration in the PCL, but did not change the movement in the EGL, ML and IGL. Inhibition of the cAMP signaling and the activity of phospholipase C significantly reduced the effects of exogenous PACAP on granule cell migration. The PACAP action on granule cell migration was transient, and lasted for approximately 2 hours. The duration of PACAP action on granule cell migration was determined by the desensitization of its receptors and prolonged by inhibiting the PKC. Endogenous PACAP was present sporadically in the bottom of the ML, intensively in the PCL, and throughout the IGL. Collectively, these results indicated that PACAP acts on granule cell migration as “a brake (stop signal) for cell movement”. Furthermore, these results suggest that endogenous PACAP slows granule cell migration when the cells enter the PACAP-rich PCL, and 2 hours later the desensitization of PACAP receptors allows the cells to accelerate the rate of migration and to actively move within the PACAP-rich IGL. Therefore, endogenous PACAP may provide a cue that regulates granule cell migration in a cerebellar cortical layer-specific manner.
Keywords: cerebellar development, granule cell, neuronal cell migration, cAMP, rate of cell movement, desensitization
In the developing brain, the majority of neurons migrate from their birthplace to their final destinations (Rakic, 1990; Hatten, 1999). This active movement of immature neurons plays a crucial role in establishing the normal neuronal cytoarchitecture (Rakic, 1990; Komuro and Yacubova, 2003). Impairment of this process results in either cell death or improper positioning of neurons, leading to deficiency in a wide variety of brain functions (Rakic, 1988; Flint and Kriegstein, 1997; Gressens, 2006). Recent advances in the real-time observation of neuronal cell migration in the natural cellular milieu reveal that neurons exhibit different modes and speed of migration when they move through different cortical layers (Rakic et al., 1994; Rakic and Komuro, 1995, Komuro and Rakic, 1998b; Komuro et al., 2001; Nadarajah and Parnavelas, 2002; Marin and Rubenstein, 2003; Noctor et al., 2004). For example, in the developing cerebellum, upon completion of their final mitosis, granule cells migrate tangentially in the external granular layer (EGL), then change the direction, and migrate radially along the process of Bergmann glial cells through the molecular layer (ML) (Komuro et al., 2001). After entering the Purkinje cell layer (PCL), the cells detach from the glial cells and slow down their movement (Komuro and Rakic, 1998a). Two hours later the cells speed up their movement and cross the border between the PCL and the internal granular layer (IGL). In the IGL, the cells migrate radially until attaining their final position (Komuro and Rakic, 1998a). Interestingly, the average speed of granule cell migration in the cortical layers decreases in the following order: EGL > ML > IGL > PCL (Komuro and Rakic, 1995, 1998a; Komuro et al., 2001; Komuro and Yacubova, 2003; Yacubova and Komuro, 2003). To date, several molecules, which affect the direction of migration, have been discovered, but little is known about the mechanisms by which the speed of migration is controlled in a cortical layer-specific manner.
In this study we hypothesized that PACAP, which is a member of the secretin/glucagon/vasoactive intestinal polypeptide family, controls the migration of cerebellar granule cells. PACAP has two bioactive products, PACAP38 and PACAP27 (Miyata et al., 1989, 1990; Vaudry et al., 2000). PACAP27 is the N-terminal 27-amino acid sequence of PACAP38 (Miyata et al., 1989, 1990). PACAP exerts pleiotropic physiological functions on multiple targets via a family of three receptors (PAC1, VPAC1 and VPAC2), which belong to the class B G-protein-coupled receptor superfamily (Pisegna and Wank, 1993; Ishihara et al., 1992). The roles of PACAP in the brain development have been suggested from the clinical studies. For example, gain-of function of chromosomal regions including genes in the PACAP signaling pathway leads to developmental disorders of the brain. The PACAP gene (ADCYAP1) resides at 18p11. Fetuses with trisomy 18 develop microcephaly and spina bifida (Salihu et al., 1997). Patients with tetrasomy 18p suffer from microcephaly, mental retardation, and congenital hydrocephalus (Takeda et al., 1989). The 18p11 region is also associated with an increased susceptibility to schizophrenia (Faraone et al., 2005). The PAC1 gene (ADCYAP1R1) is situated at 7p15. A patient with 7p15 duplication exhibits severe mental deficiency with communicating hydrocephalus (Miller et al., 1979). Although these chromosomal regions encode a large number of genes, these lines of evidence indicate that alterations of the PACAP signaling may contribute to some of these neurologic manifestations. Furthermore, recent studies showed that transgenic mice, which overexpress the human PAC1 receptors, develop hydrocephalus-related phenotypes (Lang et al., 2006). Collectively, these studies suggest that PACAP plays a critical role in the formation and function of the brain.
Interestingly, it has been reported that there are specific patterns of PACAP and its receptor expression throughout the developing brain: PACAP is present intensively in differentiating and postmigratory neurons, while neuronal precursors and premigratory neurons express PACAP receptors (Basille et al., 1993, 1994, 2000, 2006; Nielsen et al., 1998a, b; Skoglosa et al., 1999). In the case of developing rodent and human cerebellum, high levels of PACAP are present in differentiating cells and axonal terminals in restricted cortical layers, and granule cells express PACAP receptors, especially PAC1 receptors, prior to an initiation of their migration (Basille et al., 1993, 2000, 2006; Nielsen et al., 1998a; Falluel-Morel et al., 2005). Furthermore, it has been shown that the activation of PAC1 receptors, which are highly present in the immature neurons, including premigrating granule cells, alters the intracellular cAMP- and Ca2+- signaling pathway (Zer and Feltz, 1994; Favit et al., 1995; Gonzalez et al., 1997; Villalba et al., 1997; Mei, 1999; Vaudry et al., 2000). This is very intriguing because the speed of granule cell migration is sensitive to the changes in these second messenger signaling pathways (Komuro and Rakic, 1998b; Kumada and Komuro, 2004; Komuro and Kumada, 2005; Kumada et al., 2006, 2007; Jiang et al., 2007). These lines of evidence suggest that endogenous PACAP may control granule cell migration at the specific cortical layers by activating the PACAP receptors. To examine this possibility, in this study, we examined whether and how exogenous and endogenous PACAP affects the cortical-layer specific changes in granule cell migration in the early postnatal mouse cerebellum.
EXPERIMENTAL PROCEDURES
All animal procedures were approved by the Internal Animal Care and Use Committee of the Cleveland Clinic Foundation and University of Rouen.
Migration of isolated granule cells in microexplant culture
Cerebella of postnatal day 0–3 mice (CD-1) were placed in ice-chilled Hanks’ balanced salt solution, and freed from meninges and choroid plexus (Komuro and Rakic, 1996, 1999; Yacubova and Komuro, 2002a; Kumada and Komuro, 2004; Kumada et al., 2006). Cerebellar slices were then made with a surgical blade, from which white matter and deep cerebellar nuclei were removed. Rectangular pieces (50–100 μm) were dissected out from the remaining tissue, which mainly consisted of cerebellar gray matter, under a dissecting microscope. Small pieces of cerebellum were placed on 35 mm-glass bottom microwell dishes (MatTec Co.) which had been coated with poly-L-lysine (100 μg/ml)/laminin (20 μg/ml), with 50 μl of the culture medium. We used poly-L-lysine and laminin as substrata, since these materials provide scaffold for migrating granule cells and promote their movement (Yacubova and Komuro, 2002a). We were aware that the rate of neuronal cell movement depends on the concentrations of laminin coated on coverslips. Higher (50 to 100 μg/ml) or lower (1 to 5 μg/ml) concentrations of laminin significantly reduced the rate of granule cell movement (Yacubova and Komuro, 2002a). Therefore, we used a concentration of 20 μg/ml of laminin that allows granule cells to migrate at the fastest rate. Each dish was put in a CO2 incubator (37°C, 95% air, 5% CO2). One to two hours after plating, 1 ml of the culture medium was added to each dish. The incubation medium consisted of DMEM/F12 (Invitrogen) with N2 supplement, 90 U/ml penicillin and 90 μg/ml streptomycin. In these cultures more than 95% of the migrating neurons were granule cells, which were easily distinguished from other neurons by the small size of their cell bodies (Komuro and Rakic, 1996; Yacubova and Komuro 2002a). Although granule cells were prepared from the EGL and the IGL of all lobules of the cerebellum, the vast majority of granule cells were derived from the EGL, since at the age of P0–P3 the IGL contains only very small numbers of postmigratory granule cells (Yacubova and Komuro 2002a). Therefore, the majority of granule cells were at the same developmental stage. One day after plating, dishes were transferred into the chamber of a micro-incubator (Medical System Corp.) attached to the stage of a confocal microscope (TCS SP, Leica). Chamber temperature was kept at 37°C, and the cells were provided with a constant gas flow (95% air, 5% CO2). The transmitted images of migrating granule cells at 488 nm were collected every 60 sec for up to 5 hours. To examine the effects of PACAP on granule cell migration, PACAP27 or PACAP38 was added to the culture medium.
Granule cell migration in acute slice preparations
Cerebella of postnatal 10-day-old mice (CD-1) were sectioned transversely or sagittally into 180 μm-thick slices on a vibrating blade microtome (VT1000S, Leica Instruments) (Komuro and Rakic, 1995; 1998a, 1999; Komuro et al., 2001; Kumada and Komuro, 2004; Kumada et al., 2006). To label granule cells, cerebellar slices were incubated for 4 minutes in 2 μM Cell Tracker Green CMFDA (5-chloromethylfluorescein diacetate, Invitrogen), which was added to the culture medium. The culture medium consisted of DMEM/F12 (Invitrogen) with N2 supplement, 90 U/ml penicillin and 90 μg/ml streptomycin. The slices were subsequently washed with the culture medium, and put in a CO2 incubator. Two to ten hours after labeling, slices were transferred into the chamber of a micro-incubator attached to the stage of a confocal microscope (Leica). The rate of cell movement is closely related to the temperature of the medium; lowering the medium temperature slows cell movement (Rakic and Komuro, 1995). Therefore, the chamber temperature was kept at 37.0 ± 0.5°C using a temperature controller (TC-202, Medical System Corp.), and the slices were provided with constant gas flow (95% O2, 5% CO2). To prevent movement of the slice preparations during observation, a nylon net glued to a small silver wire ring was placed over the preparations.
A laser scanning confocal microscope (TCS SP, Leica) was used to visualize migrating granule cells labeled with Cell Tracker Green CMFDA in the slices (Komuro and Rakic, 1995, 1998a; Rakic and Komuro, 1995; Komuro and Rakic, 1999). The use of this microscope permitted high-resolution imaging of migrating neurons up to 120 μm deep within the tissue slices. The tissue was illuminated with a 488-nm wavelength light from an argon laser through an epifluorescence inverted microscope equipped with a 40x oil-immersion objective (numerical aperture 1.25, Leica), and fluorescence emission was detected at 530 nm ± 15 nm. To clearly resolve the movement of granule cells, image data were typically collected at an additional electronical zoom factor of 1.5–2.0. Time-lapse imaging of live, fluorescently labeled cells can produce phototoxic effects in the imaged cells. Indeed, when well stained cells were imaged with very high incident illumination intensity, or imaged too frequently, we invariably saw changes in the structure or dynamics of the migrating granule cells. However, when the incident illumination was sufficiently attenuated, the labeled specimens could be imaged (5 minute intervals) for many hours without signs of photodynamic damage (Komuro and Rakic, 1995, 1998a; Komuro et al., 2001). To protect the migrating granule cells labeled with Cell Tracker Green CMFDA from any cytotoxic effect of the laser beam, the excitation light level was reduced by 99%. To avoid injured granule cells located near the sectioning surfaces, we examined the shape and behavior of migrating granule cells located 15–50 μm below the surface of the slices. If granule cells showed no evidence of changes in cell shape or motility for more than 300 minutes, the brain slice was discarded. Shock to the tissue during sectioning of slices can disrupt cell movement and prevent cells from migrating. Accordingly, the present study is based on the analysis of approximately 70 % of the healthy slices that had displayed active cell migration. This sampling procedure favored slices in which cells displayed visible and robust movement and alteration of morphology shortly after sectioning. Images of the granule cells in a single focal plane or up to 40 different focal planes along the z-axis were collected with laser scans every 5 to 30 min for up to 20 hours. At the beginning and end of each recording session for each preparation, frame images were recorded with 40x magnification (electronical zoom factor of 1), or 20x magnification (electronical zoom factor of 1) to determine the orientation of the slice preparations, the borders of cortical layers (the EGL, the ML, the PCL, and the IGL) and the position of granule cells by optical sectioning of several different focal planes along the z-axis.
Ca2+ measurements in cerebellar slices
Cerebella of postnatal 10-day-old mice (CD-1) were sectioned sagittally into 180 μm-thick slices on a vibrating blade microtome. To monitor the Ca2+ levels of granule cells, the cerebellar slices were incubated for 30 minutes with a cell-permeant, acetoxymethyl ester form of 1 μM Oregon Green 488 BAPTA-1 (Molecular Probes) diluted in the culture medium, which consisted of DMEM/F12 (Invitrogen) with N2 supplement, 90 U/ml penicillin and 90 μg/ml streptomycin (Kumada and Komuro, 2004; Gudz, et al., 2006; Kumada et al., 2006). It has been shown that the loading of 1 μM Oregon Green 488 BAPTA-1 does not cause any significant noxious effects on granule cell migration (Kumada and Komuro, 2004). The slices were subsequently washed three times with the culture medium, and the dye was allowed to de-esterify for an additional 30–60 minutes in the CO2 incubator. Cerebellar slices were transferred into the chamber of a micro-incubator (Medical System Corp.) attached to the stage of a confocal microscope (Leica). Chamber temperature was kept at 37°C, and the cells were provided with a constant gas flow (95% O2, 5% CO2). Slices loaded with Oregon Green 488 BAPTA-1 were illuminated with a 488-nm light from an argon laser, and the emission was detected at 530 ± 15 nm. Images of granule cells were collected every 1~15 seconds for up to 5 hours. The changes in fluorescence intensity of each granule cell were normalized to its baseline fluorescence intensity.
The movement of granule cell somata to the outside of the focal plane often caused the slow changes in the baseline fluorescence intensity of Oregon Green 488 BAPTA-1. To determine whether the changes in baseline fluorescence intensity reflect the changes in Ca2+ levels or the deviation of granule cell somata from the focal plane, at the beginning and end of each recording session and when slow changes in baseline intensity were detected, the z-axis positions of granule cells were determined by optical sectioning along the z-axis. If the z-axis position of granule cell somata changed by more than 1 μm from the initial position, the experimental data were excluded from this study.
Immunohistochemistry of PACAP
After an anesthetic overdose, postnatal 10-day-old mice (CD-1) were perfused transcardially with phosphate buffer saline (PBS) (pH 7.4), followed by 4 % paraformaldehyde and 0.2 % picric acid in PBS. Brains were quickly dissected and post-fixed overnight at 4°C with the same fixative solution. Tissues were stored for 12 hours in a solution of PBS containing 15 % and 30 % sucrose successively. Thereafter, the tissues were cut at 12 μm in the sagittal plane with a cryostat (CM 3050 S, Leica, Rueil Malmaison, France). Tissue slices were mounted on glass slides coated with 0.5% gelatin and 5% chrome alum. Prior to immunohistochemical staining, tissue sections were digested for 3 minutes at 37°C with 1 mg/ml of pepsin in 0.2 N HCl and rinsed in PBS. The tissue sections then were incubated overnight at 4°C with an antiserum raised in rabbit against PACAP 38 (1:200, Arimura) and an antiserum raised in mouse against calbindin (1:400, Sigma-Aldrich, Saint-Quentin Fallavier, France). The sections were rinsed in three successive baths of PBS and incubated for 90 minutes at room temperature with Alexa 488 conjugated goat anti-rabbit (1:200, Invitrogen, Boulogne Billancourt, France) and rhodamine conjugated goat anti-mouse (1:500, Immunotech, Marseille, France). Finally, the sections were rinsed in PBS (3 times), and mounted in PBS/glycerol (1/1). The preparations were examined on a Leica SP2 upright confocal laser scanning microscope (DM RXA-UV) equipped with Accousto-Optical Beam Splitter (AOBS) system. For confocal images, Alexa 488 and rhodamine were excited respectively at 488 and 594 nm. To study the specificity of the immunoreaction, the following controls were performed: (1) substitution of PACAP38 antiserum with PBS and (2) preincubation of the PACAP38 antiserum with synthetic PACAP38 (10 μM) for 18 hrs.
Statistical analysis
Statistical differences were determined using ANOVA. Statisticalsignificance was defined at P <0.05 or P < 0.01.
RESULTS
Exogenous PACAP27 and PACAP38 slow the migration of isolated granule cells in the microexplant cultures of P0–P3 mouse cerebella
Using microexplant cultures of P0–P3 mouse cerebella (Komuro and Rakic, 1996; Yacubova and Komuro, 2002a), we first examined whether PACAP affects granule cell migration. In these cultures isolated granule cells actively migrate in the absence of cell-cell contact (Yacubova and Komuro, 2002a), and this allows us to determine the direct effects of PACAP on granule cell migration. Application of PACAP38 (1 μM) to the culture medium immediately slowed granule cell movement from 72.7 μm/hr in control to 20.9 μm/hr (Fig. 1A, B). Likewise, application of PACAP27 (1 μM) reduced the rate of granule cell movement from 96.2 μm/hr in control to 53.4 μm/hr (Fig. 1C, D). Isolated granule cells in microexplant cultures are known to exhibit systematic fluctuations in the rate of migration every 2–3 hours (Yacubova and Komuro, 2002a). Therefore, to confirm the effects of PACAP38 and PACAP27 on the rate of granule cell migration, we averaged the data obtained from more than 100 isolated cells in the microexplant cultures of P0–P3 mouse cerebella. As presented in Fig. 1E, application of PACAP38 (1 μM) or PACAP27 (1 μM) reduced the average rate of granule cell migration to 63 % and 61 % of the control values, respectively. Furthermore, we examined whether application of PACAP38 and PACAP27 alters the morphology of the leading process of migrating granule cells because it has been reported that PACAP38 and PACAP27 stimulates the extension (or outgrowth) of the neurite and axon of differentiating and mature neurons (Gonzalez et al., 1997, Yuhara et al., 2001; Guirland et al., 2003; Fukiage et al., 2006; Lang et al., 2006). Interestingly, application of PACAP38 (1 μM) or PACAP27 (1 μM) significantly shortened the leading process of the migrating granule cells in the microexplant cultures (Fig. 1F). In contrast, the number of the leading process branches of migrating granule cells was not affected by application of PACAP38 (1 μM) or PACAP27 (1 μM) (Fig. 1G). These results indicated that application of exogenous PACAP38 or PACAP27 directly slows the migration of cerebellar granule cells and shortens their leading process. Moreover, these results suggest that PACAP38 and PACAP27 act on granule cell migration as “a brake” (stop signal) for cell movement. For the remaining experiments, we used PACAP38 alone. This was because PACAP38 and PACAP27 induced similar effects on granule cell migration, and PACAP38 is by far the predominant form in the brain, while PACAP27 represents less than 10% of the total peptide content (Arimura, 1991; Ghatei et al., 1993; Masuo et al., 1993; Hannibal et al., 1995; Piggins et al., 1996).
Fig. 1.
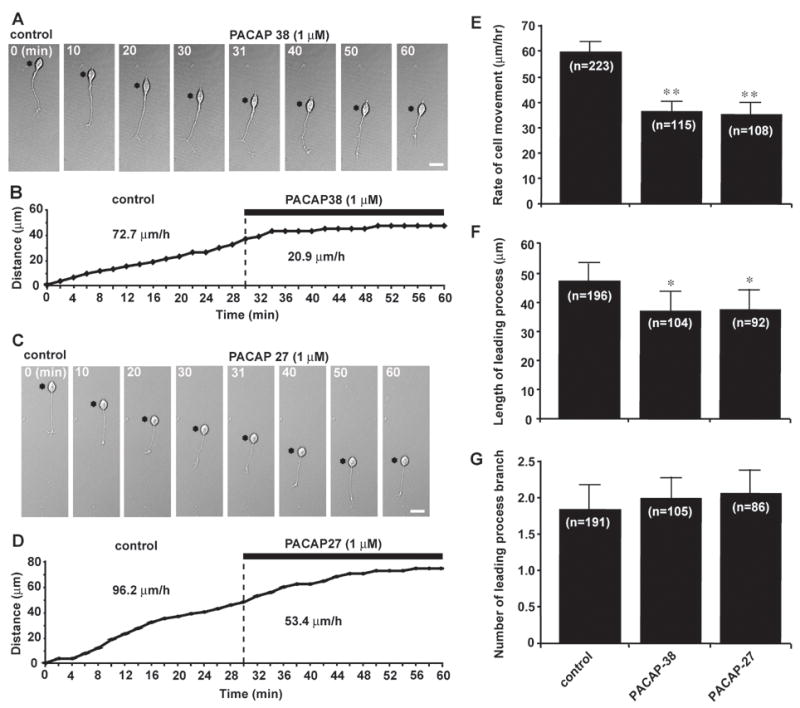
PACAP38 and PACAP27 inhibit granule cell migration in the microexplant cultures of the early postnatal mouse cerebellum. (A and C) Time-lapse images showing that application of 1 μM PACAP38 (A) or 1 μM PACAP27 (C) slows the migration of isolated granule cells in the microexplant cultures of P0–P3 mouse cerebella. Asterisks mark the granule cell somata. Elapsed time (in minutes) is indicated on top of each photograph. Scale bar: 12μm. (B and D) Sequential changes in the distance traveled by the granule cell somata shown in (A) and (C) before and afterapplication of 1 μM PACAP38 (B) or 1 μM PACAP27 (D). (E–G), Histograms presenting the changes in the rates of migration (E), the leading process length (F) and the number of the leading process branch of granule cells by application of 1 μM PACAP38 or 1 μM PACAP27. Bar: S.D. In this and following figures, single (p <0.05) and double (p <0.01) asterisks indicate statistical significance.
Enhanced effects of exogenous PACAP38 on granule cell migration in the ML of P10 mouse cerebella
The next question is whether PACAP38 affects the migration of granule cells in their natural microenvironment. In the developing ML, granule cells migrate along the processes of Bergmann glial cells (Rakic, 1971). The interaction between the granule cells and the Bergmann glial processes is essential for granule cell migration in the ML (Rakic, 1971; Rakic, 1990). Interestingly, Bergmann glial cells also express the PAC1 type of PACAP receptors (Ashur-Fabian et al., 1997). Application of exogenous PACAP38 may affect the functions of Bergmann glial cells via the activation of its receptors (Tatsuno and Arimura, 1994). So, it may be possible that application of exogenous PACAP38 indirectly affects granule cell migration along the Bergmann glial process in the ML by altering the functions of the glial cells via the activation of PAC1 receptors. To address this question, we used acute cerebellar slice preparations of P10 mice, which allow us to examine the effects of exogenous PACAP38 on granule cell migration in their natural cellular environment. The combined use of fluorescent dyes and a confocal microscope makes the observation of granule cell migration in the cerebellar slices possible (Komuro and Rakic, 1995; Komuro and Rakic, 1998a, 1999; Komuro et al., 2001). Application of exogenous PACAP38 (1 μM) to the culture medium immediately slowed the migration of granule cells in the ML (Fig. 2A–C). For example, the granule cells marked (a) and (b) in Fig. 2A reduced the rate of migration in the ML from 17.8 μm/hr and 23.4 μm/hr in control to 2.5 μm/hr and 9.7 μm/hr after application of 1 μM PACAP38, respectively (Fig. 2B1, 2 and C1, 2). The effects of PACAP38 on granule cell migration in the ML were dose-dependent (Fig. 2D). The average rate of granule cell migration in the ML decreased from 13.6±0.6 μm/hr (n=32) in control to 12.1±0.4μm/hr (n=33) in 10 nM PACAP38, 9.3±0.5μm/hr (n=30) in 100 nM PACAP38, and 5.2±0.4μm/hr (n=31) in 1μM PACAP38. Most importantly, the effects of exogenous PACAP (1 μM) on the average rate of granule cell migration in the slices were higher than those in the microexplant cultures. For example, PACAP38 (1 μM) reduced the average rate of migration of isolated granule cells in the microexplant cultures to 63 % of the control value (Fig. 1E), while PACAP38 (1 μM) reduced the average rate of migration in the ML to 38 % of the control value (Fig. 2D). These results suggest that exogenous PACAP38 slows granule cell migration in the ML (1) directly via the activation of its receptors on the plasmalemmal surface of the granule cells and (2) indirectly via altering the interaction between the granule cells-Bergmann glial cells through the activation of PACAP receptors on the glial cells.
Fig. 2.
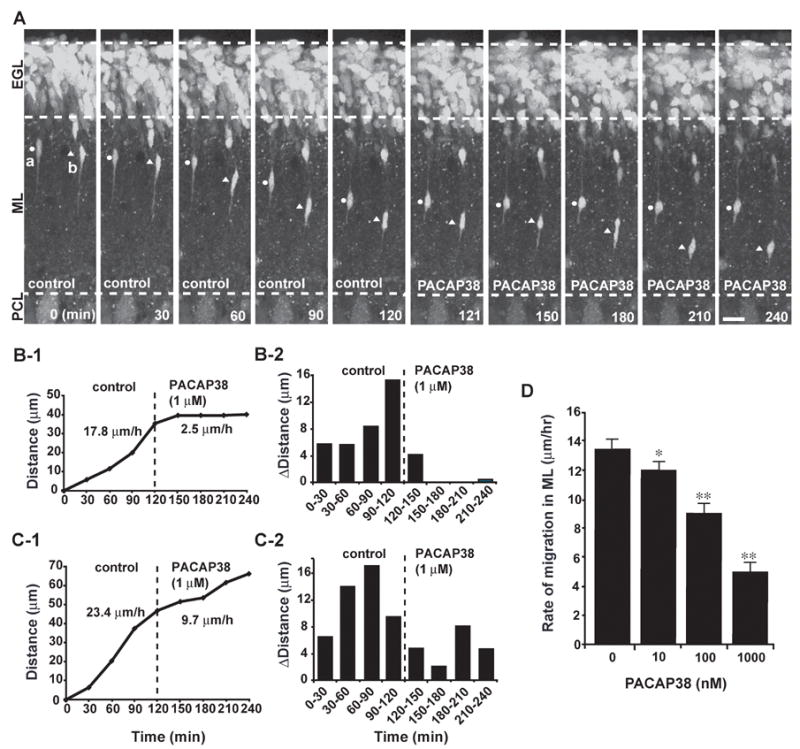
Dose-dependent inhibition of granule cell migration in the ML of P10 mouse cerebellumby PACAP38. (A) Time-lapse images showing that application of 1 μM PACAP38 immediately slows the migration of granule cells in the ML. Elapsed time (in minutes) is indicated on the bottom of each photograph. Circles (a) and triangles (b) mark the cell bodies of migrating granule cells. Scale bar: 16μm. (B1,2 and C1,2) Sequential changes in the total distance (B1 and C1) and the distance (B2 and C2) traveled during each 30 minutes of the testing period by granule cells marked by circles (B) and triangles (C) in A were plotted as a function of elapsed time before and after application of 1 μM PACAP38. (D) Dose-dependent reduction of the rate of granule cell migration in the ML of P10 mouse cerebella by PACAP38. Each column represents the average rate obtained from at least 30 migrating cells. Bar: S.D.
Cellular mechanisms by which PACAP slows granule cell migration
How did PACAP38 slow granule cell migration? Our working hypothesis is that application of exogenous PACAP38 inhibits the migration of granule cells via altering the cAMP and Ca2+ signaling pathways. It has been reported that granule cell migration is highly sensitive to the changes in these second messenger signaling pathways (Komuro and Rakic, 1992, 1993, 1998b; Kumada and Komuro, 2004; Komuro and Kumada, 2005; Kumada et al., 2006, 2007; Jiang et al., 2007). Importantly, it has been shown that PACAP38 affects the cAMP and Ca2+ signaling pathways via the activation of its receptors (Vaudry et al., 2000). For example, the activation of PAC1 receptors stimulates the activity of the adenylate cyclase (AC), which in turn increases the intracellular cAMP levels and enhances the activity of protein kinase A (PKA) (Gonzalez et al., 1994; Basille et al., 1995; Favit et al., 1995; Villalba et al., 1997). Furthermore, PAC1 receptors also stimulate the activity of the phospholipase C (PLC) which leads to enhanced Ca2+ release from intracellular Ca2+ stores (Gonzalez et al., 1996; Mei, 1999). Therefore, to examine the cellular mechanisms by which PACAP38 slows granule cell migration, we inhibited the activity of the cAMP and Ca2+ signaling pathways with the use of its specific inhibitors. As we expected, the inhibition of the cAMP signaling pathways with Rp-cAMPS (100 μM, a cAMP antagonist) or PKI (5 μM, a PKA inhibitor) significantly reduced the effects of 1 μM PACAP38 on the rate of granule cell migration in the ML (Fig. 3A). Likewise, the inhibition of the Ca2+ signaling pathways with U73122 (1 μM, a PLC antagonist) significantly reduced the PACAP38 action on granule cell migration in the ML (Fig. 3A). These results suggest that PACAP38 slows the migration of granule cells in the ML via altering the cAMP and Ca2+ signaling pathways.
Fig. 3.
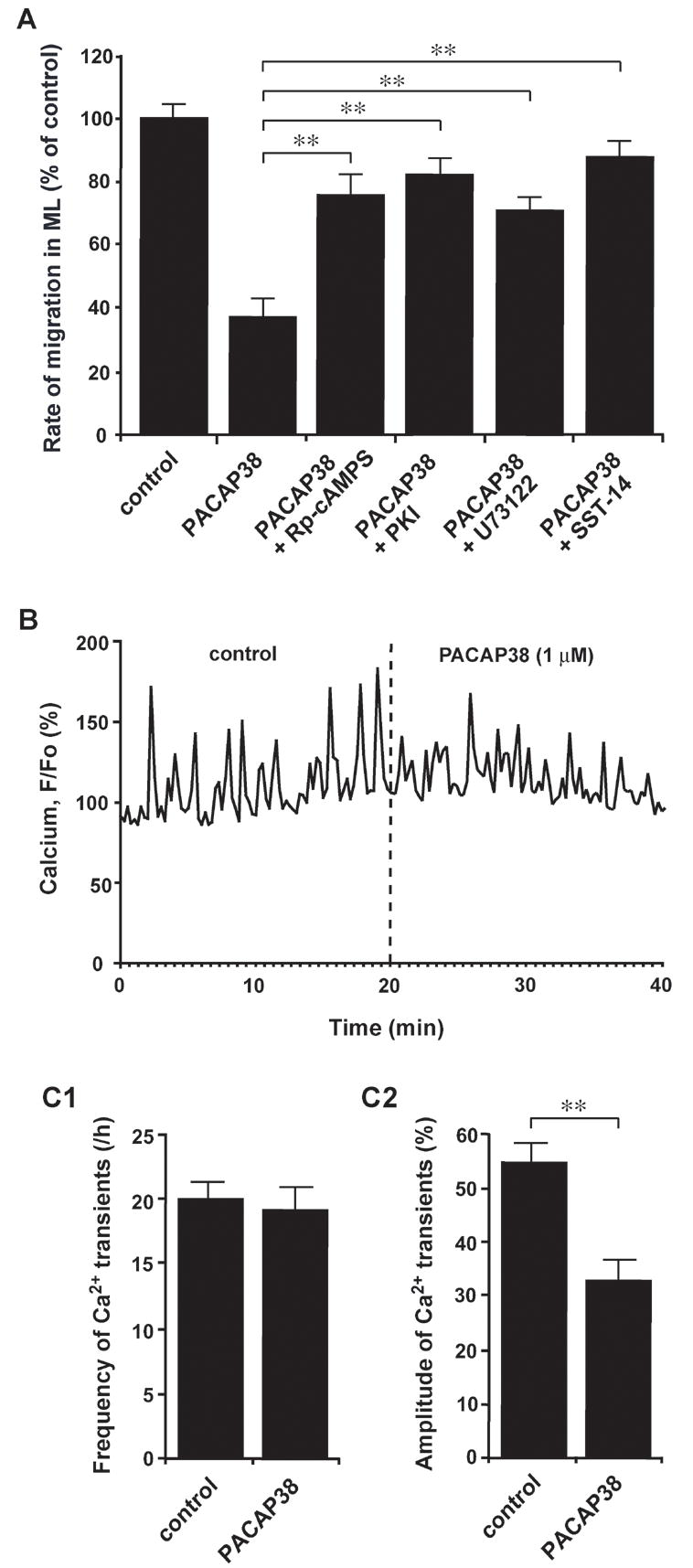
The action of PACAP38 on granule cell migration in the ML of P10 mouse cerebella depends on the activity of the cAMP- and Ca2+- signaling pathways. (A) Histograms showing the alterations of the effects of 1μM PACAP38 on the rate of granule cell migration in the ML by application of 100 μM Rp-cAMPS, 5 μM PKI, 1 μM U73122, and 1 μM somatostatin 14 (SST-14). Each column represents the average values obtained from at least 30 migrating cells. Bar: S.D. (B) Changes in intracellular Ca2+ transients of the somata of migrating granule cells in the ML of the P10 mouse cerebella by application of 1 μM PACAP38. (C1 and C2) Histograms showing the changes in the frequencies (C1) and amplitudes (C2) of Ca2+ transients in the granule cell somata in the ML of the P10 mouse cerebella by application of 1 μM PACAP38. Each column represents the average values obtained from at least 30 migrating cells. Bar: S.D.
It has been shown that somatostatin, which is another neuropeptide, also affects the migration of granule cells by inhibiting the cAMP signaling pathway (Yacubova and Komuro, 2002b). So, it is interesting to examine whether the effects of PACAP38 on granule cell migration are modulated by somatostatin. Importantly, application of somatostatin-14 (1 μM) to the culture medium significantly reduced the effects of 1 μM PACAP38 on granule cell migration in the ML (Fig. 3A). These results further support the hypothesis that PACAP38 slows granule cell migration by stimulating the cAMP signaling pathway.
There is a paradox in the mechanisms by which PACAP38 slows granule cell migration in the ML. This is because the stimulation of the Ca2+ mobilization accelerates the migration of isolated granule cells in the microexplant cultures (Komuro and Rakic, 1996; Kumada and Komuro, 2004). Meanwhile, the stimulation of the cAMP signaling pathway slows the migration of isolated granule cells (Kumada et al., 2006, 2007). Interestingly, it has been shown that in the migrating granule cells the Ca2+ and cAMP signalings interact with each other (Kumada et al., 2006, 2007; Jiang et al., 2007). For example, the stimulation of the cAMP signaling pathway resulted in suppression of the Ca2+ signaling pathway (Kumada et al., 2006). Indeed, the stimulation of the cAMP signaling pathway significantly reduced the frequency of spontaneous Ca2+ transients in the migrating granule cells and inhibited the migration (Kumada et al., 2006). Therefore, we examined whether PACAP38 alters the intracellular Ca2+ levels of migrating granule cells in the ML of P10 mouse cerebella. We found that application of PACAP38 (1 μM) to the culture medium fails to change the frequency of Ca2+ transients in the granule cell somata, but decrease the amplitude (Figs. 3B and C1, C2). These results suggest that PACAP38 slows the migration of granule cells in the ML by reducing the size of Ca2+ transients because it has been shown that lowering the amplitude of Ca2+ transients impedes the movement of isolated granule cells in the microexplant cultures (Komuro and Rakic, 1996).
Exogenous PACAP38 differentially alters granule cell migration in different cortical layers of the early postnatal mouse cerebellum
In the early postnatal cerebellum, granule cells exhibit dynamic changes in the speed of migration when they move through different cortical layers (Komuro and Rakic, 1995, 1998a; Komuro et al., 2001; Komuro and Yacubova, 2003; Kumada and Komuro, 2004). For example, in the P10 mouse cerebella, the average rate of granule cell migration was 14.8±0.8 μm/hr (n=31) in the EGL, 13.5±0.9 μm/hr (n=35) in the top of the ML, 9.8±0.8 μm/hr (n=32) in the bottom of the ML, 5.2±0.4μm/hr (n=36) in the PCL, 11.4±0.8 μm/hr (n=39) in the top of the IGL, and 6.5±0.5 μm/hr (n=35) in the bottom of the IGL (Fig. 4A, B). These results indicated that granule cells significantly slow down their migration, when they enter the PCL. The next question is whether PACAP plays a role in cortical layer-specific changes in the speed of granule cell migration. To address this question, we determined whether application of exogenous PACAP38 (1 μM) differentially affects granule cell migration in the different cortical layers. Interestingly, the effects of PACAP38 (1 μM) on the speed of granule cell migration depended on the position where the cells migrate. During a period of 60 minutes after application of PACAP38 (1 μM), the speed of granule cell migration was reduced by 62 % in the EGL, 62 % in the top of the ML, 52 % in the bottom of the ML, 8 % in the PCL, 5 % in the top of the IGL, and 5 % in the bottom of the IGL (Fig. 4A, B). The decreases in the speed of granule cell migration in the EGL and the top and bottom of the ML by PACAP38 were statistically significant, while the changes in the PCL and the top and bottom of the IGL were not statistically significant. These results indicated that application of PACAP38 significantly slows granule cell migration in the EGL and ML, but fails to alter their movement in the PCL and IGL of the early postnatal mouse cerebellum.
Fig. 4.
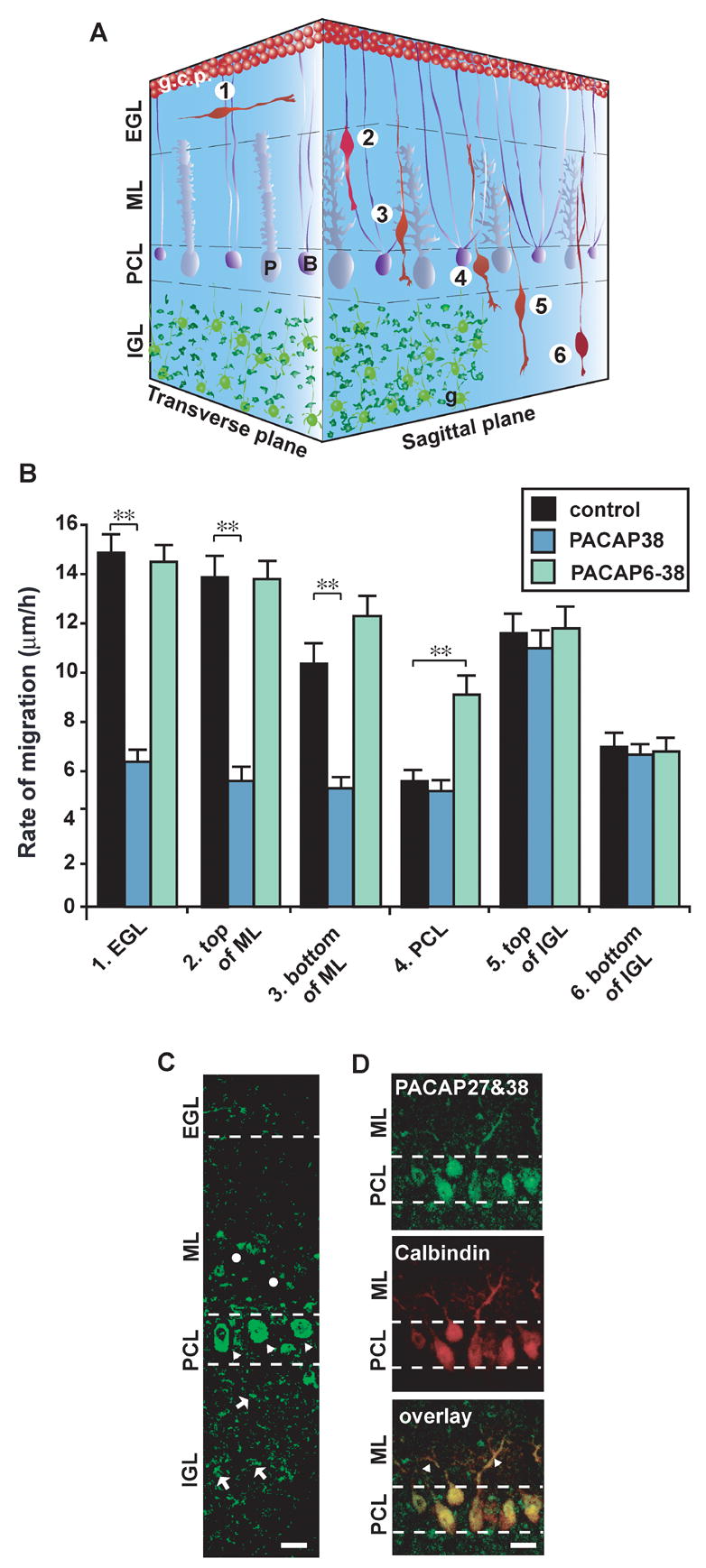
Cortical layer-specific effects of PACAP38 and its antagonist, PACAP6–38, on granule cell migration in the P10 mouse cerebella. (A) Three-dimensional representation of granule cell migration from the EGL to the IGL in the early postnatal mouse cerebellum. 1, Tangential migration in the EGL; 2, Bergmann glia-associated radial migration at the top of the ML; 3, Bergmann glia-associated radial migration at the bottom of the ML; 4, stationary state or slowdown of migration in the PCL; 5, glia-independent radial migration at the top of the IGL; 6, glia-independent radial migration at the bottom of the IGL. Abbreviations: B, Bergmann glial cells; g.c.p., granule cell precursors; P, Purkinje cells; g, postmigratory granule cells. (B)Differential effects of 1 μM of PACAP38 or PACAP6-38 on granule cell migration in the different cortical layers (the EGL, ML, PCL and IGL) of P10 mouse cerebella. Each column represents the average values obtained from at least 30 migrating cells. Bar: S.D. (C) Expression of endogenous PACAP (27 and 38) in the P10 mouse cerebellum. Circles represent a sporadic staining in the bottom of the ML. Triangles represent an intensive staining in the Purkinje cell somata in the PCL. Arrows represent numerous small stainings, possibly mossy fiber terminals, in the IGL. Scale bar: 11 μm. (D) Co-localization of the endogenous PACAP (27 and 38) and calbindin in the dendrite and somata of the Purkinje cells. Triangles represent the double-staining dendrites of the Purkinje cells in the bottom of the ML. Scale bar: 11 μm.
Endogenous PACAP plays a role in the slowdown of granule cell migration in the PCL of the early postnatal mouse cerebellum
Why did exogenous PACAP38 differentially affect the rate of granule cell migration in different cortical layers? Why did exogenous PACAP38 fail to alter the speed of granule cell migration in the PCL and IGL? We hypothesized that endogenous PACAP may be involved in the cortical-layer specific effects of exogenous PACAP on granule cell migration. To address this question, we first examined the distribution of endogenous PACAP in the P10 mouse cerebellum with the use of polyclonal antibody that recognized both PACAP27 and PACAP38. We found that endogenous PACAP is present sporadically in the bottom of the ML, intensively in the PCL, and throughout the IGL (Fig. 4C). Double-staining of PACAP 27 and calbindin revealed that the dendrite of the Purkinje cells in the ML and the somata of the Purkinje cells in the PCL express the endogenous PACAP 38 and/or PACAP27 (Fig. 4D). The PACAP staining observed throughout the IGL most likely represented the mossy fiber terminals (Nielsen et al., 1998a). Collectively, these results indicated that endogenous PACAP 38 and/or PACAP27 are differentially present in different cortical layers of the early postnatal mouse cerebellum.
Second, with the use of PACAP6–38, which is the most potent antagonist for PACAP38 (Robberecht, 1992), we examined the role of endogenous PACAP38 in controlling granule cell migration in the early postnatal cerebellum. Most importantly, application of PACAP6–38 (1 μM) to the culture medium altered the rate of granule cell migration in a cortical-layer specific manner. For example, during a period of 60 minutes after application of PACAP6–38 (1 μM), the speed of granule cell migration was reduced by 10 % in the EGL, and 1 % in the top of the ML, but increased by 19 % in the bottom of the ML, 68 % in the PCL, 1 % in the top of the IGL (Fig. 4A, B). Moreover, application of PACAP6–38 (1 μM) reduced granule cell migration in the bottom of the IGL by 3 % (Fig. 4A, B). Increases in the speed of granule cell migration in the PCL by PACAP6–38 were statistically significant, while the changes in the EGL, ML and IGL were not statistically significant. These results indicated that inhibiting the activity of PACAP receptors by application of its antagonist significantly increases the speed of granule cell migration in the PCL. This is very intriguing because in the microexplant cultures of P0–P3 mouse cerebella, application of PACAP6–38 (1 μM) to the culture medium did not significantly change the rate of granule cell migration (data not shown). Therefore, the results suggest that the slowdown of the granule cell migration observed in the PCL of the early postnatal mouse cerebellum is caused by endogenous PACAP38 and/or PACAP27 through the activation of its receptors. The reason why exogenous PACAP 38 failed to slow granule cell migration in the PCL could be that PACAP receptors on the granule cells in the PCL have already been activated by endogenous PACAP38 and/or PACAP27.
Loss of the receptiveness of granule cells to endogenous and exogenous PACAP by the desensitization of its receptors
At the top of the IGL of the early postnatal mouse cerebellum, where the high levels of PACAP38 and/or PACAP27 are present, granule cells actively migrate at a speed comparable to that observed in the ML (Fig. 4B) (Komuro and Rakic, 1995; 1998a). Application of PACAP38 and its antagonist, PACAP6–38, failed to alter the speed of granule cell migration in the top and bottom of the IGL, suggesting that granule cells lost the receptiveness to the PACAP38 before entering the IGL. We hypothesized that granule cells in the IGL lose the response to PACAP38 via the desensitization of PACAP receptors. This is because PACAP receptors are known to undergo a rapid desensitization after an initial activation, as seen in other G protein-coupled receptors (Dautzenberg and Hauger, 2001; Shintani, 2000). To examine the role of the desensitization of PACAP receptors in granule cell migration, in the following experiments, we chose the granule cells, which were migrating near the EGL-ML border of the ML, at the time PACAP38 (1 μM) was applied to the culture medium. This is because in the top of the ML the expression levels of endogenous PACAP38 and PACAP27 were low, and granule cells exhibited large responses for the application of the exogenous PACAP38 (Fig. 4B). Furthermore, it took approximately 10–11 hours for the granule cells to reach the endogenous PACAP-rich PCL, which allow us to observe the changes in granule cell migration for several hours after application of PACAP38. To test our hypothesis, we examined the sequential changes in the speed of granule cell migration during the prolonged application of PACAP38 (Fig. 5A1, 2). We found that after an initial slowdown in the speed of migration by application of PACAP38 (1 μM), the granule cells gradually increase the speed to the levels comparable to control values (Fig. 5A1, 2). For example, an average rate of granule cell migration was 13.3±1.6 μm/hour (n=10) during 2 hours of control period (a period of C in Fig. 5A1), and reduced to 5.1±0.8 μm/hour (n=10) during the first 2 hours after application of 1 μM PACAP38 (a period of P1a in Fig. 5A1). Thereafter, the rate increased to 12.7±1.5 μm (n=10) during the second 2 hours after application of PACAP38 (a period of P1b in Fig. 5A1). Although the rate of migration was significantly reduced during the first 2 hours after application of PACAP38, there is no statistical significance in the rate of migration between the 2 hours of control period and the second 2 hours after application of PACAP38. The average time that required the granule cells to return to the control speed after application of PACAP38 (1 μM) was 2.1±0.2 hours (n=10) with a range of 1.5–3.0 hours. These results suggest that the PACAP receptors on the plasmalemmal surface of the migrating granule cells undergo desensitization within 1.5–3.0 hours after application of PACAP38.
Fig. 5.
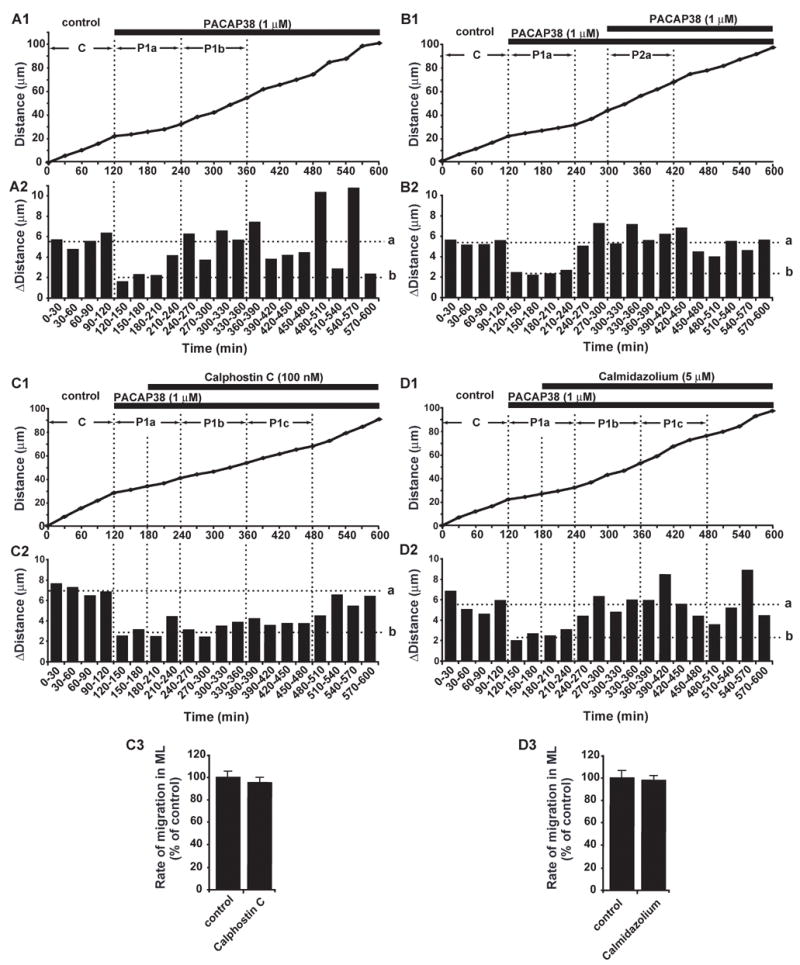
Recovery of granule cells from PACAP38-induced inhibition of migration by desensitization of PACAP receptors. (A1,2) Sequential changes in granule cell migration in the ML of the P10 mouse cerebella after application of 1 μM PACAP38. In A1, C, P1a and P1b indicate the 2 hours control period, the first 2 hours period and the second 2 hours period after application of PACAP38, respectively. (B1,2) Loss of granule cell response in the ML to the 2nd application of 1 μM PACAP38 after prolonged application of 1 μM PACAP38. In B1, C, P1a and P2a indicate the 2 hours control period, the first 2 hours period after first application of PACAP38, and the first 2 hours period after second application of PACAP38, respectively. (C)Delay in the recovery of granule cells from the PACAP38 (1 μM)-induced inhibition of migration in the ML by application of 100 nM calphostin C. In C1, C, P1a, P1b, and P1c indicate the 2 hours control period, the first 2 hours period, the second 2 hours period and third 2 hours period after application of PACAP38, respectively. (D) Application of 5 μM calmidazolium did not alter the recovery of granule cells from the PACAP38-induced slowdown of migration in the ML. In D1, C, P1a, P1b and P1c indicate the 2 hours control period, the first 2 hours period, the second 2 hours period and third 2 hours period after application of PACAP38, respectively. In (A)–(D), the total distance (A1, B1, C1 and D1) and the distance (A2, B2, C2 and D2) traveled by the granule cell somata during each 30 minutes of the testing period were plotted as a function of elapsed time. The horizontal dotted lines (a) in A2, B2, C2 and D2 represent the average distances traversed by granule cells during each 30 minutes in the control periods. The horizontal dotted lines (b) in A2, B2, C2 and D2 represent the average distances traversed by granule cells during each 30 minutes in the first hour after application of 1 μM PACAP38. C3 and D3, Effect of 100 nM calphostin C (C3) and 5 μM calmidazolium (D3) on the rate of granule cell migration in the ML in the absence of exogenous PACAP38. In C3 and D3, each column represents the average rate obtained from at least 30 migrating cells. Bar is S.D.
The recovery of the rate of granule cell migration may be due to lowering the concentrations of extracellular PACAP38 by the cleavage of the peptides and/or clearance from the extracellular space (Vaudry et al., 2000). To test this possibility, we added additional PACAP38 to the medium when the rate of granule cell migration returned to control values after an initial application of PACAP38. We found that second application of PACAP38 (1 μM) 3 hours after the first application of PACAP38 (1 μM) fails to change the rate of migration (Fig. 5B1, 2). For example, an average rate of granule cell migration was 12.9±1.2μm/hour (n=11) during 2 hours of control period (a period of C in Fig. 5B1), and reduced to 4.7±0.6μm/hour (n=11) during the first 2 hours after application of PACAP38 (a period of P1a in Fig. 5B1). Interestingly, the rate was 13.3±1.6 μm (n=11) during the first 2 hours after the second application of PACAP38 (a period of P2a in Fig. 5B1). Theses results demonstrate that the rate of granule cell migration during the first 2 hours after the second application of PACAP38 is significantly faster than that during the first 2 hours after the first application of PACAP38 (p < 0.01). There is no statistical significance in the rate of granule cell migration between the first 2 hours of control period and the first 2 hours after the second application of PACAP38. Collectively, these results support our hypothesis that the loss of granule cell response to the application of PACAP38 is due to the desensitization of its receptors.
It has been reported that the G-protein-coupled receptor kinases, which mediates the desensitization of PACAP receptors, are sensitive to changes in the activity of protein kinases C (PKC) and calmodulin (Chuang et al., 1996; Shintani et al., 2000; Dautzenberg and Hauger, 2001). Therefore, to further examine whether the loss of granule cell response to PACAP38 is due to the desensitization of its receptors, we inhibited the activity of PKC or calmodulin 1 hour after PACAP38 application. Although application of 100 nM calphostin (the PKC inhibitor) did not alter the rate of granule cell migration in the ML in the absence of exogenous PACAP38 (Fig. 5C3), calphostin significantly delayed the recovery of granule cells from the PACAP38-induced slowdown of the migration (Fig. 5C1, 2). For example, an average rate of granule cells was 14.2±1.7μm/hour (n=11) during 2 hours of control period (a period of C in Fig. 5C1), and reduced to 5.3±0.9μm/hour (n=11) during the first 2 hours after application of 1 μM PACAP38 (a period of P1a in Fig. 5A1). The reduction of the rate of migration continued for an additional 4–6 hours in the presence of 100 nM calphostin: 5.9±1.0μm/hour (n=11) during the second 2 hours after application of PACAP38 (a period of P1b in Fig. 5C1), and 6.5±1.1 μm/hour (n=11) during the third 2 hours after application of PACAP38 (a period of P1c in Fig. 5C1). These results demonstrated that the duration of the effective period of PACAP38 for controlling granule cell migration depends on the activity of PKC. This suggests the involvement of the desensitization of PACAP receptors in the recovery of granule cells from the PACAP38-induced slowdown of migration. In the case of inhibiting the calmodulin, application of calmidazolium (5 μM, the calmodulin inhibitor) did not change the recovery of granule cells from the PACAP38-induced slowdown of the migration (Fig. 5D1–3). For example, an average rate of granule cell migration was 12.4±1.4μm/hour (n=10) during 2 hours of control period (a period of C in Fig. 5D1), and reduced to 4.9±0.9μm/hour (n=10) during the first 2 hours after application of 1 μM PACAP38 (a period of P1a in Fig. 5D1). Subsequently, the rate increased to 12.2±1.6μm (n=10) during the second 2 hours after PACAP38 application (a period of P1b in Fig. 5A1), and to 12.4±1.7 μm (n=10) during the third 2 hours after PACAP38 application (a period of P1c in Fig. 5A1). These results suggest that the activity of calmodulin does not involve the desensitization of PACAP receptors of migrating granule cells.
Collectively, these results indicated that application of exogenous PACAP38 immediately inhibits granule cell migration and the effects last for 1.5–3.0 hours. The desensitization of PACAP receptors via PKC-dependent mechanisms was involved in the loss of granule cell response to PACAP 38. These results suggest that after an initial response to endogenous PACAP38 and/or PACAP27 in the PCL, the PACAP receptors of granule cells undergo desensitization, which allows the cells to gradually increase the speed of migration, and to actively move within the endogenous PACAP-rich IGL.
DISCUSSION
It has been thought for a long time that external cues play a role in cortical-layer specific changes in the mode, tempo, and direction of granule cell migration in the developing cerebellum (Hatten and Mason, 1990; Rakic, 1990; Hatten and Heintz, 1995; Rakic and Komuro, 1995; Komuro and Rakic, 1998b; Komuro and Yacubova, 2003; Yacubova and Komuro 2003). However, little is known about the mechanisms underlying the cortical-layer specific changes in granule cell migration. In this series of experiments we examined a role of PACAP in granule cell migration in the early postnatal mouse cerebellum. In the cerebellum the content of PACAP is very high at birth and during the first postnatal weeks and then decreases gradually from P20 to adulthood (Basille et al., 1993; Nielsen et al., 1998a; Skoglosa et al., 1999). Cerebellar granule cells express PACAP receptors prior to their migration (Falluel-Morel et al., 2005). In this study, application of exogenous PACAP38 and PACAP27 inhibited the migration of isolated granule cells in the microexplant cultures, indicating that PACAP directly acts as “a brake” in granule cell migration. Furthermore, application of exogenous PACAP38 at the levels of as low as 10 nM significantly slowed granule cell migration in the ML of the postnatal 10-day-old mice.
Exogenous PACAP38 affected granule cell migration via altering the Ca2+ and cAMP signaling pathways. Interestingly, the effects of exogenous PACAP38 and its antagonist on granule cell migration depended on the cortical layer where the cells translocated. For example, application of exogenous PACAP38 significantly inhibited granule cell migration in the EGL and ML, but failed to alter the movement in the PCL and IGL. In contrast, application of PACAP antagonist significantly accelerated granule cell migration in the PCL, but did not change the movement in the EGL, ML and IGL. Immunohistochemical studies revealed that endogenous PACAP is present sporadically in the bottom of the ML, intensively in the PCL, and throughout the IGL. Sequential observation of granule cell migration after application of exogenous PACAP38 demonstrated that the effects of PACAP38 on granule cell migration last for 1.5–3.0 hours. After the effective period, granule cells accelerated the migration and retuned to the rate before application of PACAP38. Thereafter, granule cells migrate at the same speed with or without exogenous PACAP38, indicating that the PACAP receptors undergo desensitization.
Collectively, present studies suggest that endogenous PACAP38 and/or PACAP27 play a role in the slowdown of granule cell migration in the PCL, where the cells detach from the surface of the Bergmann glial cells, and alter the mode of migration from glia-associated to glia-independent prior to entering the IGL. The role of endogenous PACAP, possibly released from the collateral axons of the Purkinje cells and/or mossy fiber terminals, in the slowdown of granule cell migration in the PCL, was supported by the evidence that the stationary period (1.9 hours) observed in the granule cells in the PCL (Komuro and Rakic, 1998a) is similar to the time (2.1 hours) required for the recovery from the PACAP-induced slowdown. Desensitization of the PACAP receptors allows the granule cells to migrate within the PACAP-rich IGL. Therefore, endogenous PACAP plays a crucial role in cortical layer-specific changes in granule cell migration through the activation and desensitization of PACAP receptors.
Three PACAP receptors have been cloned so far and termed PAC1, VPAC1, and VPAC2 receptors (Vaudry et al., 2000). In the early postnatal cerebellum, granule cells express the PAC1 receptors and the VPAC1 receptors prior to their migration, but are devoid of the VPAC2 receptors (Basille et al., 1993, 1994, 2000; Nielsen et al., 1998a; Falluel-Morel et al., 2005). The expression levels of PAC1 receptors are two to three times higher than those of the VPAC1 receptors (Basille et al., 1994, 2000). The ontogeny of PACAP receptors has been investigated in detail in the rat cerebellum during postnatal development (Basille et al., 1994, 2000). In the EGL, the density of PAC1 receptors is high from birth to P12, and markedly decreases from P12 to P25. In the ML and IGL, PAC1 receptors are first detected at P8. In the IGL the density of PAC1 receptors slightly decreases during the second and third postnatal weeks, but remains elevated in adults. In the ML the density of PAC1 receptors rapidly decreases during the second and third postnatal weeks, and virtually disappears after P25. The low levels of the VPAC1 receptors are only present in the EGL during first and second postnatal weeks of the rat cerebellum (Basille et al., 2000). In contrast, the VPAC2 receptors are not present in the early postnatal rat cerebellum (Basille et al., 2000). In the case of the developing human cerebellum, the high levels of PAC1 receptors are observed in the EGL of the 16-week-old fetus (Basille et al., 2006). At this stage of development, the moderate levels of PAC1 receptors are present in the IGL (Basille et al., 2006). In the 24-week-old human fetus, the expression levels of PAC1 receptors are high in both the EGL and IGL, but low in the ML (Basille et al., 2006). In the 16- and 24-week-old human fetuses, the expression patterns of the VPAC1 receptors are very similar to those of the PAC1 receptors, although the levels of the VPAC1 receptor expression are much lower than those of the PAC1 receptors (Basille et al., 2006). In contrast, in the 16- and 24-week-old human fetuses, the VPAC2 receptors are absent (Basille et al., 2006). Collectively, these results suggest that in both the humans and rats, immature granule cells express the PAC1 receptors as well as the VPAC1 receptors before they start their migration. During the period of migration from the EGL to their final destination within the IGL, granule cells continuously express the PAC1 receptors and the VPAC1 receptors. These lines of evidence suggest that PACAP receptors play critical roles in granule cell migration in the early postnatal rodent cerebellum as well as the human fetus cerebellum.
How does PACAP control granule cell migration? Present results suggest that PACAP exerts its effects on granule cell migration via altering the cAMP and Ca2+ signaling pathway through the activation of its receptors (Basille et al., 1993, 1995). The cAMP signaling pathway plays critical roles in the PACAP action on granule cell migration. It has been reported that PACAP increases the intracellular cAMP levels via stimulating the activity of adenylate cyclase (Vaudry et al., 2000). The elevations of intracellular cAMP levels are known to inhibit granule cell migration (Kumada et al., 2006). In fact, present results demonstrated that the inhibition of the cAMP signaling pathway significantly reduces the PACAP action on granule cell migration (Fig. 3A). In the case of the Ca2+ signaling pathway, it has been reported that PACAP stimulates the activity of PLC, leading to the stimulation of PKC activity and the increase in the intracellular Ca2+ levels (Vaudry et al., 2000). Present studies revealed that the inhibition of PLC activity reduces the PACAP action on granule cell migration. However, the mechanisms by which the inhibition of the PLC activity eliminates the PACAP action on granule cell migration may not be simple, because the Ca2+ and cAMP signaling pathway interact with each other and the activation of the cAMP signaling pathway suppresses the Ca2+ signaling pathway in granule cells (Kumada et al., 2006). For example, although it has been shown that PACAP38 induces the mobilization of internal Ca2+ (Gonzalez et al., 1997; Mei, 1999; Vaudry et al., 2000), present results indicated that application of exogenous PACAP38 does not stimulate the transient elevations of intracellular Ca2+ levels in the granule cell somata, but significantly decreases the amplitude of Ca2+ transients (Figs. 3B and C). Collectively, present results suggest that PACAP slows granule cell migration by stimulating the cAMP signaling pathway and inhibiting the Ca2+ transients through the activation of its receptors. Moreover, PACAP also slows granule cell migration by altering the activity of Erk/MAPK. This is because it has been shown that PACAP inhibits the programmed cell death in the developing brain through the activation of the cAMP signaling pathway, leading to phosphorylation of the extracellular signal-regulated (Erk)-type of mitogen-activated protein kinase (MAPK) (Gonzalez et al., 1997; Villalba et al., 1997; Vaudry et al., 1998, 2000). Interestingly, granule cell migration is sensitive to changes in the activity of MAPK (Kumada et al., 2006). So, it may be possible that the activity of Erk/MAPK may be one of the downstream targets for the PACAP action on granule cell migration.
PACAP may also affect granule cell migration by altering the activity of the N-methyl-D-aspartate (NMDA) type glutamate receptors. It has been shown that migrating granule cells exhibit spontaneous activation of NMDA receptors, and the inhibition and stimulation of the NMDA receptors alter the rate of granule cell migration (Komuro and Rakic, 1992, 1996; Rossi and Slater, 1993; Kumada et al., 2006). Interestingly, recent studies revealed that PACAP38 enhances the activity of the NMDA receptors by stimulating (1) the Gs subunit of Gα-coupled receptors and PKA, and (2) the Gq subunit of Gα-coupled receptors and PKC (Macdonald et al., 2005; Michel et al., 2006). The possible involvement of the NMDA receptors in PACAP-induced alterations of granule cell migration is intriguing. This is because glutamate, which is released from the parallel fibers and the mossy fibers, is well colocalized with the distribution of the endogenous PACAP in the early postnatal mouse cerebellum. Furthermore, it suggests that PACAP may control granule cell migration in a reciprocal manner. For example, endogenous PACAP may inhibit granule cell migration via stimulating the cAMP signaling pathway, but also may stimulate granule cell migration via enhancing the activity of NMDA receptors presented in the plasma membrane of the cells.
It may be possible that the action of endogenous PACAP on granule cell migration is modulated by other neuropeptides, such as somatostatin and Galanin. It has been reported that endogenous somatostatin controls granule cell migration in the early postnatal mouse cerebellum through the activation of its receptors (Yacubova and Komuro, 2002b, 2003). Granule cells express all five types of somatostatin receptors prior to an initiation of migration, and endogenous somatostatin is present in Purkinje cells, Golgi cells, climbing fibers and mossy fiber terminals (Yacubova and Komuro, 2002b). Somatostatin inhibits the cAMP signaling pathway via stimulating the Gi subunit of Gα-coupled receptors (Tentler et al., 1997). Importantly, present results demonstrated that application of somatostatin-14 significantly reduces the effects of PACAP38 on granule cell migration in the ML. Collectively, these results suggest that somatostatin acts as an “opposite signal” against PACAP in controlling granule cell migration via inhibiting the cAMP signaling pathway.
Galanin, which is a 29–30 amino acid peptide, is abundantly expressed in the CNS (Branchek et al., 2000; Simen et al., 2001). Migrating granule cells express high levels of galanin receptor1 (GalR1), and galanin is present in the PCL and IGL, where endogenous PACAP is present (Jungnickel et al., 2005). GalR1 is negatively coupled to the adenylate cyclase via the Gi subunit of Gα-coupled receptors, and can influence Ca2+ currents, K+ current and MAPK (Wang et al., 1998; Branchek et al., 2000; Simen et al., 2001). These lines of evidence are very intriguing because the action of PACAP on granule cell migration depends on the activity of the Ca2+ and cAMP signaling pathway. So, we expected that galanin may alter the effects of PACAP on granule cell migration. Indeed, although application of 1 μM galanin alone did not alter granule cell migration, co-application of 1 μM galanin with 1 μM PACAP38 significantly reduced the effects of PACAP38 on granule cell migration (data not shown). These results suggest that the PACAP action on granule cell migration is modified by endogenous galanin through the activation of galanin receptors.
PACAP may also indirectly affect granule cell migration by altering surrounding microenvironments, especially Bergmann glial cells. Bergmann glial cells are members of astrocytes, and provide the scaffold to granule cells for their radial migration in the ML of the developing cerebellum (Rakic, 1971). It seems likely that PACAP alters the physiological function of Bergmann glial cells by mobilizing Ca2+ from intracellular Ca2+ stores and activating a quinine-sensitive K+ outward current (Tatsuno and Arimura, 1994; Ichinose et al., 1998). Furthermore, PACAP stimulates the proliferation of glial precursor cells, possibly by activation of the MAP kinase Erk2 (Just et al., 1998; Moroo et al., 1998). Importantly, PACAP increases the production of neurotrophic factors, which could affect proliferation, migration and differentiation of immature neurons (Ashur-Fabian et al., 1997). Therefore, PACAP may indirectly affect granule cell migration by controlling the function of Bergmann glial cells.
The continuing presence of high levels of PACAP often induces the desensitization of PACAP receptors, which is termed homologous desensitization (McArdle and Forrest-Owen, 1997; Shintani et al., 2000; Dautzenberg and Hauger, 2001; Niewiadomski et al., 2002). In general, homologous desensitization begins with the selective recruitment of a G-protein-coupled receptor kinase (GRK) that phosphorylates serine and/or threonine residues in the agonist-activated G-protein-coupled receptor’s cytoplasmic tail and/or third intracellular loop. Next, β-arrestin binds to the phosphorylated G-protein-coupled receptors, decreasing the receptor’s affinity for its cognate heterotrimeric G-protein, and thereby uncoupling it from the Gα subunit by steric hindrance. The activity of GRK is often modulated by the cAMP and Ca2+ signaling pathways (Chuang et al., 1996; Dautzenberg and Hauger, 2001; Luo and Benovic, 2003). To date, little is known about how the homologous desensitization of the PACAP receptors mediated by GRK affects the neuronal development and migration. Importantly, present results suggest that PACAP receptors of migrating granule cells undergo homologous desensitization after continuous applications of exogenous PACAP38 (Fig. 5). Furthermore, present results also suggest that the homologous desensitization of PACAP receptors requires the activity of PKC. This is interesting because migrating granule cells in the IGL exhibit the high frequency of the spontaneous activation of NMDA receptors (Rossi and Slater, 1993), which potentially lead to the activation of PKC through the increase in Ca2+ influxes. Moreover, in the IGL, the granule cells exhibit dynamic oscillations of the intracellular Ca2+ levels (Kumada and Komuro, 2004), leading to the stimulation of the PKC activity. Therefore, it may be possible that the homologous desensitization of PACAP receptors in the granule cells is maintained during the migration within the endogenous PACAP-rich IGL by the continuous stimulation of the PKC activity.
It has been suggested that the polarity of neurons undergoes dynamic changes as they migrate through different regions of the developing cerebellum (Powell et al., 1997; Kawaji et al., 2004; Kerjan et al., 2005; Solecki et al., 2006). To date, little is known about whether PACAP induces the changes in neuronal polarity. Present studies did not reveal that application of exogenous PACAP27 and PACAP38 induces the changes in granule cell polarity in the microexplant cultures. However, it has been reported that vasoactive intestinal polypeptide, which belongs to the same family of PACAP, alters the polarity of cultured human pancreatic cancer cells (Hollande et al., 1995). So, whether PACAP controls granule cell polarity during their migration in the early postnatal mouse cerebellum is remained to be examined.
Although in this study we focused on the role of PACAP in controlling granule cell migration in the early postnatal mouse cerebellum, we are confident that the information obtained from granule cell migration can apply to neuronal migration in other brain areas, especially the developing cerebrum and hippocampus. This is because in the many regions of the developing brain where neurons exhibit cortical layer-specific changes in their migration (Nadarajah and Parnavelas, 2002; Marin and Rubenstein, 2003; Noctor et al., 2004; Morozov et al., 2006), PACAP and its receptors are transiently and highly expressed in the neurons and neuronal precursors during a period of the early brain development (Shuto et al., 1996; Waschek et al., 1998; Skoglosa et al., 1999). For example, PACAP mRNA is present in differentiating neurons of the mouse brain as early as embryonic day 9.5 (E9.5) (Shuto et al., 1996; Waschek et al., 1998). Thereafter, the PACAP mRNA level increases during the prenatal period to reach a maximum at birth, when the majority of immature neurons actively migrate towards the cortical plate in the developing cerebrum. Likewise, the PAC1 receptor (PAC1-R) mRNA is first detected in the neural tube in E9.5 mouse embryos (Waschek et al., 1998; Jaworski and Proctor, 2000). From E9.5 to E11 the PAC1-R mRNA levels increase in the neuroepithelia of the mesencephalon and rhombencephalon (Shuto et al., 1996). At E13 PAC1-R mRNA is expressed in the basal telencephalon and in the neuroepithelia of the hippocampal formation, cerebral cortex, and cerebellum (Zhou et al., 1999). Other PACAP receptors, VPAC1 receptors (VPAC1-R) and VPAC2 receptors (VPAC2-R), are also found in the brain at the early embryonic stage, and the density of these receptors increases during postnatal development (Roth and Beinfeld, 1985; Waschek et al., 1996). Interestingly, the distribution pattern of VPAC1-R mRNA exhibits striking similarities with that of PAC1-R mRNA, although the expression level of VPAC1-R mRNA is much lower than that of PAC1-R mRNA (Pei, 1997). Moreover, the distribution of PACAP and PACAP receptors in the developing brain reveals the existence of a specific correlation between the localization of the peptide and its receptors in all germinative neuroepithelia. For example, neuronal precursors and premigratory neurons express high levels of PACAP receptors, and PACAP is highly present in the migratory pathways (Skoglosa et al., 1999; Jaworski and Proctor, 2000; Vaudry et al., 2000). Therefore, we expect that endogenous PACAP may provide traffic signals to neurons at the specific points of the migratory routes where they change the speed of their movement.
Acknowledgments
We thank A. Arimura for providing PACAP38 antibody. H.K. was supported by National Institutes of Health Grant AA 13613 and Whitehall Foundation Grant 2001-12-35.
Abbreviations
- AC
adenylate cyclase
- EGL
external granular layer
- ML
molecular layer
- IGL
internal granular layer
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PBS
phosphate buffer saline
- PCL
Purkinje cell layer
- PKA
protein kinase A
- PKC
protein kinase C
- PKI
PKA inhibitor fragment 14–22 myristoylated trifluoracetate salt
- PLC
phospholipase C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–1789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Ashur-Fabian O, Giladi E, Brennemam DE, Gozes I. Identification of VIP/PACAP receptors on rat astrocytes using oligodeoxynucleotides. J Mol Neurosci. 1997;9:11–22. doi: 10.1007/BF02800503. [DOI] [PubMed] [Google Scholar]
- Basille M, Cartier D, Vaudry D, Lihrmann I, Fournier A, Freger P, Gallo-Payet N, Vaudry H, Gonzalez BJ. Localization and characterization of pituitary adenylate cyclase-activating polypeptide receptors in the human cerebellum during development. J Com Neurol. 2006;496:468–478. doi: 10.1002/cne.20934. [DOI] [PubMed] [Google Scholar]
- Basille M, Gonzalez BJ, Leroux P, Jeandel L, Fournier A, Vaudry H. Localization and characterization of PACAP receptors in the rat cerebellum during development: evidence for a stimulatory effect of PACAP on immature cerebellar granule cells. Neuroscience. 1993;57:329–338. doi: 10.1016/0306-4522(93)90066-o. [DOI] [PubMed] [Google Scholar]
- Basille M, Gonzalez BJ, Fournier A, Vaudry H. Ontogeny of pituitary adenylate cyclase-activating polypeptide (PACAP) receptors in the rat cerebellum: a quantitative autoradiographic study. Dev Brain Res. 1994;82:81–89. doi: 10.1016/0165-3806(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Basille M, Gonzalez BJ, Desrues L, Demas M, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates adenylyl cyclase and phospholipase C activity in rate cerebellar neuroblasts. J Neurochem. 1995;65:1318–1324. doi: 10.1046/j.1471-4159.1995.65031318.x. [DOI] [PubMed] [Google Scholar]
- Basille M, Vaudry D, Coulouarn Y, Jegou S, Lihrmann I, Fournier A, Vaudry H, Gonzalez BJ. Comparative distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) binding sites and PACAP receptor mRNAs in the rat brain during development. J Com Neurol. 2000;425:495–509. [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–116. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- Chuang TT, Paolucci L, Blasi A. Inhibition of G protein-coupled receptor kinase subtypes by Ca2+/calmodulin. J Biol Chem. 1996;271:28691–28696. doi: 10.1074/jbc.271.45.28691. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. G-protein-coupled receptor kinase 3- and protein kinase C-mediated desensitization of the PACAP receptor type 1 in human Y-79 retinoblastoma cells. Neuropharmacology. 2001;40:394–407. doi: 10.1016/s0028-3908(00)00167-2. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A, Vaudry D, Aubert N, Galas L, Benard M, Basille M, Fontaine M, Fournier A, Vaudry H, Gonzalez BJ. Pituitary adenylate cyclase-activating polypeptide prevents the effects of ceramides on migration, neurite outgrowth, and cytoskeleton remodeling. Proc Natl Acad Sci USA. 2005;102:2637–2642. doi: 10.1073/pnas.0409681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Skol AD, Tsuang DW, Young KA, Haverstock SL, Prabhudesai S, Mena F, Menon AS, Leong L, Sautter F, Baldwin C, Bingham S, Weiss D, Collins J, Keith T, Vanden Eng JL, Boehnke M, Tsuang MT, Schellenberg GD. Genome scan of schizophrenia families in a large Veterans Affairs Cooperative Study sample: evidence for linkage to 18p11.32 and for racial heterogeneity on chromosomes 6 and 14. Am J Med Genet B Neuropsychiatr Genet. 2005;139:91–100. doi: 10.1002/ajmg.b.30213. [DOI] [PubMed] [Google Scholar]
- Favit A, Scapagnini U, Canonico PL. Pituitary adenylate cyclase-activating polypeptide activates different signal transducing mechanisms in cultured cerebellar granule cells. Neuroendocrinology. 1995;61:377–382. doi: 10.1159/000126859. [DOI] [PubMed] [Google Scholar]
- Flint AC, Kriegstein AR. Mechanisms underlying neuronal migration disorders and epilepsy. Curr Opin Neurol. 1997;10:92–97. doi: 10.1097/00019052-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Fukiage C, Nakajima TA, Takayama YO, Minagawa YO, Shearer TH, Azuma MI. PACAP induces neurite outgrowth in cultured trigeminal ganglion cells and recovery of corneal sensitivity after flap surgery in rabbits. Am J Ophthalmol. 2006 Nov 27; doi: 10.1016/j.ajo.2006.10.034. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, Bloom SR. Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptides and its precursor encoding messenger RNA in human and rat tissue. J Endocrinol. 1993;136:156–166. doi: 10.1677/joe.0.1360159. [DOI] [PubMed] [Google Scholar]
- Gonzalez BJ, Leroux P, Basille M, Bodenant C, Vaudry H. Somatostatin and pituitary adenylate cyclase-activating polypeptide (PACAP): Two neuropeptides potentially involved in the development of the rat cerebellum. Ann Endocrinol. 1994;55:243–247. [PubMed] [Google Scholar]
- Gonzalez BJ, Basille M, Vaudry D, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience. 1997;78:419–430. doi: 10.1016/s0306-4522(96)00617-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez BJ, Basille M, Mei YA, Vaudry D, Fournier A, Cazin L, Vaudry H. Ontogeny of PACAP and PACAP receptors in the rat brain: role of PACAP in the cerebellum during development. Ann NY Acad Sci. 1996;805:302–314. doi: 10.1111/j.1749-6632.1996.tb17492.x. [DOI] [PubMed] [Google Scholar]
- Gressens P. Pathogenesis of migration disorders. Curr Opin Neurol. 2006;19:135–140. doi: 10.1097/01.wco.0000218228.73678.e1. [DOI] [PubMed] [Google Scholar]
- Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration via an αv-integrin/myelin proteolipid protein complex. J Neurosci. 2006;26:2458–2466. doi: 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirland C, Buck KB, Gibney JA, DiCicco-Bloom E, Zheng JQ. Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J Neurosci. 2003;23:2274–2283. doi: 10.1523/JNEUROSCI.23-06-02274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Mikkelsen JD, Clausen H, Holst JJ, Wulff BS, Fahrenkrug J. Gene expression of pituitary adenylate cyclase-activating polypeptide (PACAP) in the rat hypothalamus. Regul Pept. 1995;55:133–148. doi: 10.1016/0167-0115(94)00099-j. [DOI] [PubMed] [Google Scholar]
- Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Mason CA. Mechanisms of glial-guided neuronal migration in vitro and in vivo. Experientia. 1990;46:907–916. doi: 10.1007/BF01939383. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- Hollande E, Fanjul M, Claret S, Forgue-Lafitte ME, Bara J. Effects of VIP on the regulation of mucin secretion in cultured human pancreatic cells (Capan-1) In Vitro Cell Dev Biol Anim. 1995;31:227–233. doi: 10.1007/BF02639438. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Asai M, Sawada M. Activation of outward current by pituitary adenylate cyclase activating polypeptide in mouse microglial cells. J Neurosci Res. 1998;51:382–390. doi: 10.1002/(SICI)1097-4547(19980201)51:3<382::AID-JNR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Jaworski DM, Proctor MD. Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Dev Brain Res. 2000;120:27–39. doi: 10.1016/s0165-3806(99)00192-3. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Kumada T, Cameron DB, Komuro H. Cerebellar granule cell migration and the effects of alcohol. Dev Neurosci. 2007 doi: 10.1159/000109847. in press. [DOI] [PubMed] [Google Scholar]
- Jungnickel SRF, Yao M, Shen PJ, Gundlach AL. Induction of galanin receptor-1 (GalR1) expression in external granule cell layer of post-natal mouse cerebellum. J Neurochem. 2005;92:1452–1462. doi: 10.1111/j.1471-4159.2004.02992.x. [DOI] [PubMed] [Google Scholar]
- Just L, Olenik C, Meyer DK. Glial expression of the proenkephalin gene in slice cultures of the subventricular zone. J Mol Neurosci. 1998;11:57–66. doi: 10.1385/JMN:11:1:57. [DOI] [PubMed] [Google Scholar]
- Kawaji K, Umeshima H, Eiraku M, Hirano T, Kengaku M. Dual phases of migration of cerebellar granule cells guided by axonal and dendritic leading processes. Mol Cell Neurosci. 2004;25:228–240. doi: 10.1016/j.mcn.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Kerjan G, Dolan J, Haumaitre C, Schneider-Maunoury S, Fujisawa H, Mitchell KJ, Chedotal A. The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat Neurosci. 2005;8:1516–1524. doi: 10.1038/nn1555. [DOI] [PubMed] [Google Scholar]
- Komuro H, Kumada T. Ca2+ transients control CNS neuronal migration. Cell Calcium. 2005;37:387–393. doi: 10.1016/j.ceca.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Selective role of N-type calcium channels in neuronal migration. Science. 1992;257:806–809. doi: 10.1126/science.1323145. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Dynamics of granule cell migration: a confocal microscopic study in acute cerebellar slice preparations. J Neurosci. 1995;15:1110–1120. doi: 10.1523/JNEUROSCI.15-02-01110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17:275–285. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Distinct modes of neuronal migration in different domains of developing cerebellar cortex. J Neurosci. 1998a;18:1478–1490. doi: 10.1523/JNEUROSCI.18-04-01478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol. 1998b;37:110–130. [PubMed] [Google Scholar]
- Komuro H, Rakic P. In vitro analysis of signal mechanisms involved in neuronal migration. In: Haynes LW, editor. The neuron in tissue culture. New York: John Wiley & Sons; 1999. pp. 57–69. [Google Scholar]
- Komuro H, Yacubova E, Yacubova E, Rakic P. Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci. 2001;21:527–40. doi: 10.1523/JNEUROSCI.21-02-00527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Yacubova E. Recent advances in cerebellar granule cell migration. Cell Mol Life Sci. 2003;60:1084–1098. doi: 10.1007/s00018-003-2248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T, Komuro H. Completion of neuronal migration regulated by loss of Ca2+ transients. Proc Natl Acad Sci USA. 2004;101:8479–8484. doi: 10.1073/pnas.0401000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T, Lakshmana MK, Komuro H. Reversal of neuronal migration in a mouse model of fetal alcohol syndrome by controlling second-messenger signalings. J Neurosci. 2006;26:742–756. doi: 10.1523/JNEUROSCI.4478-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T, Jiang Y, Cameron DB, Komuro H. How does alcohol impair neuronal migration? J Neurosci Res. 2007;85:465–470. doi: 10.1002/jnr.21149. [DOI] [PubMed] [Google Scholar]
- Lang B, Song B, Davidson W, MacKenzie A, Smith N, McCaig CD, Harmar AJ, Shen S. Expression of the human PAC1 receptor leads to dose-dependent hydrocephalus-related abnormalities in mice. J Clin Invest. 2006;116:1924–1934. doi: 10.1172/JCI27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Benovic JL. G protein-coupled receptor kinase interaction with Hsp90 mediated kinase maturation. J Biol Chem. 2003;278:50908–50914. doi: 10.1074/jbc.M307637200. [DOI] [PubMed] [Google Scholar]
- Macdonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, Orser BA, MacDonald JF. Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires Gαq, protein kinase C, and activation of Src. J Neuroscience. 2005;25:11374–11384. doi: 10.1523/JNEUROSCI.3871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Suzuki N, Matsumoto H, Tokito F, Matsumoto Y, Tsuda M, Fujino M. Regional distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res. 1993;602:57–63. doi: 10.1016/0006-8993(93)90241-e. [DOI] [PubMed] [Google Scholar]
- McArdle CA, Forrest-Owen W. Pituitary adenylate cyclase-activating polypeptide (PACAP) actions on αT3-1 gonadotrophs show desensitization. J Neuroendocrinol. 1997;9:893–901. doi: 10.1046/j.1365-2826.1997.00657.x. [DOI] [PubMed] [Google Scholar]
- Mei YA. High-voltage-activated calcium current and its modulation by dopamine D4 and pituitary adenylate cyclase-activating polypeptide receptors in cerebellar granule cells. Chung Kuo Yao Li Hsueh Pao. 1999;20:3–9. [PubMed] [Google Scholar]
- Michel S, Han JH, Gniotczynski K, Colwell CS. Regulation of glutamatergic signaling by PACAP in the mammalian surprachismatic nucleus. BMC Neurosci. 2006;16:7–15. doi: 10.1186/1471-2202-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Kaufman G, Reed G, Bilenker R, Schinzel A. Familial, balanced insertional translocation of chromosome 7 leading to offspring with deletion and duplication of the inserted segment, 7p15 leads to 7p21. Am J Med Genet. 1979;4:323–332. doi: 10.1002/ajmg.1320040403. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Moroo I, Tatsuno I, Uchida D, Tanaka T, Saito Y, Hirai A. Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates mitogen-activated protein kinase (MAPK) in cultured rat astrocytes. Brain Res. 1998;795:191–196. doi: 10.1016/s0006-8993(98)00291-1. [DOI] [PubMed] [Google Scholar]
- Morozov M, Ayoub AE, Rakic P. Translocation of synaptically connected interneurons across the dentate gyrus of the early postnatal rat hippocampus. J Neurosci. 2006;26:5017–5027. doi: 10.1523/JNEUROSCI.0272-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci. 2002;3:423–432. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Hannibal J, Fahrenkrug J. Expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the postnatal and adult rat cerebellar cortex. NeuroReport. 1998a;9:2639–2642. doi: 10.1097/00001756-199808030-00039. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Hannibal J, Fahrenkrug J. Embryonic expression of pituitary adenylate cyclase-activating polypeptide in sensory and autonomic ganglia and in spinal cord of the rat. J Comp Neurol. 1998b;394:403–415. doi: 10.1002/(sici)1096-9861(19980518)394:4<403::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Niewiadomski P, Nowark JZ, Sedkowska P, Zawilska JB. Rapid desensitization of receptors for pituitary adenylate cyclase-activating polypeptide (PACAP) in chick cerebral cortex. Pol J Pharmacol. 2002;54:717–721. [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Pei L. Genomic structure and embryonic expression of the rat type1 vasoactive intestinal polypeptide receptor gene. Regul Pept. 1997;71:153–161. doi: 10.1016/s0167-0115(97)01012-4. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Stamp JA, Burns J, Rusak B, Semba K. Distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdale of the rat. J comp Neurol. 1996;376:278–294. doi: 10.1002/(SICI)1096-9861(19961209)376:2<278::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Pisegna JR, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci USA. 1993;90:6345–6349. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SK, Rivas RJ, Rodriguez-Boulan E, Hatten ME. Development of polarity in cerebellar granule neurons. J Neurobiol. 1997;32:223–236. doi: 10.1002/(sici)1097-4695(199702)32:2<223::aid-neu7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Rakic P. Defects of neuronal migration and the pathogenesis of cortical malformations. Prog Brain Res. 1988;73:15–37. doi: 10.1016/s0079-6123(08)60494-x. [DOI] [PubMed] [Google Scholar]
- Rakic P. Principles of neuronal cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- Rakic P, Komuro H. The role of receptor/channel activity in neuronal cell migration. J Neurobiol. 1995;26:299–315. doi: 10.1002/neu.480260303. [DOI] [PubMed] [Google Scholar]
- Rakic P, Cameron SR, Komuro H. Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol. 1994;4:63–69. doi: 10.1016/0959-4388(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Gourlet P, De Neef P, Woussen-Colle M-C, Vandermeers-Piret M-C, Vandermeers A, Christophe J. Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Discovery of PACAP(6–38) as a potent antagonist. Eur J Biochem. 1992;207:239–246. doi: 10.1111/j.1432-1033.1992.tb17043.x. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Slater NT. The developmental onset of NMDA receptor channel activity during neuronal migration. Neuropharmacology. 1993;32:1239–1248. doi: 10.1016/0028-3908(93)90018-x. [DOI] [PubMed] [Google Scholar]
- Roth BL, Beinfeld MC. The postnatal development of VIP binding sites in rat forebrain and hindbrain. Peptides. 1985;6:27–30. doi: 10.1016/0196-9781(85)90071-3. [DOI] [PubMed] [Google Scholar]
- Salihu HM, Boos R, Schmidt W. Antenatally detectable markers for the diagnosis of autosomally trisomic fetuses in at-risk pregnancies. Am J Perinatol. 1997;14:257–261. doi: 10.1055/s-2007-994139. [DOI] [PubMed] [Google Scholar]
- Shintani N, Hashimoto H, Kunugi A, Koyama Y, Yamamoto K, Tomimoto S, Mori W, Matsuda T, Baba A. Desensitization, surface expression, and glycosylation of a functional, epitope-tagged type I PACAP (PAC(1)) receptor. Biochem Biophys Acta. 2000;1509:195–202. doi: 10.1016/s0005-2736(00)00295-9. [DOI] [PubMed] [Google Scholar]
- Shuto Y, Uchida D, Onda H, Arimura A. Ontogeny of pituitary adenylate cyclase activating polypeptide and its receptor mRNA in the mouse brain. Regul Pept. 1996;67:79–83. doi: 10.1016/s0167-0115(96)00116-4. [DOI] [PubMed] [Google Scholar]
- Simen AA, Lee CC, Simen BB, Bindokas VP, Miller RJ. The C-terminus of the Ca channel α1B subunit mediates selective inhibition by G-protein-coupled receptors. J Neurosci. 2001;21:7587–7597. doi: 10.1523/JNEUROSCI.21-19-07587.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki DJ, Govek E-E, Tomoda T, Hatten ME. Neuronal polarity in CNS development. Genes Dev. 2006;20:2639–2647. doi: 10.1101/gad.1462506. [DOI] [PubMed] [Google Scholar]
- Skoglosa Y, Takei N, Lindholm D. Distribution of pituitary adenylate cyclase activating polypeptide mRNA in the developing rat brain. Mol Brain Res. 1999;65:1–13. doi: 10.1016/s0169-328x(98)00294-0. [DOI] [PubMed] [Google Scholar]
- Takeda K, Okamura T, Hasegawa T. Sibs with tetrasomy 18p born to a mother with trisomy 18p. J Med Genet. 1989;26:195–197. doi: 10.1136/jmg.26.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuno I, Arimura A. Pituitary adenylate cyclase-activating polypeptide (PACAP) mobilizes intracellular free calcium in cultured rat type-2, but not type-1, astrocytes. Brain Res. 1994;662:1–10. doi: 10.1016/0006-8993(94)90790-0. [DOI] [PubMed] [Google Scholar]
- Tentler JJ, Hadcock JR, Gutierrez-Hartmann A. Somatostatin acts by inhibiting the cyclic 3′,5′-adenosine monophosphate (cAMP)/protein kinase A pathway, cAMP response element-binding protein (CREB) phosphorylation, and CREB transcription potency. Mol Endocrinol. 1997;11:859–866. doi: 10.1210/mend.11.7.9943. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Basille M, Anouar Y, Fournier A, Vaudry H, Gonzalez BJ. The neurotrophic activity of PACAP on rat cerebellar granule cells is associated with activation of the protein kinase A pathway and c-fos gene expression. Ann NY Acad Sci. 1998;865:92–99. doi: 10.1111/j.1749-6632.1998.tb11167.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Hashemi T, Fried S, Clemmons AL, Hawes BE. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry. 1998;37:6711–6717. doi: 10.1021/bi9728405. [DOI] [PubMed] [Google Scholar]
- Waschek JA, Ellison J, Bravo DT, Handley V. Embryonic expression of vasoactive intestinal polypeptide (VIP) and VIP receptor genes. J Neurochem. 1996;66:1762–1765. doi: 10.1046/j.1471-4159.1996.66041762.x. [DOI] [PubMed] [Google Scholar]
- Waschek JA, Csillas RA, Nguyen TB, DiCicco-Bloom EM, Carpenter EM, Rodriguez WI. Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: potential role in patterning and neurogenesis. Proc Natl Acad Sci USA. 1998;95:9602–9607. doi: 10.1073/pnas.95.16.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubova E, Komuro H. Intrinsic program for migration of cerebellar granule cells in vitro. J Neurosci. 2002a;22:5966–5981. doi: 10.1523/JNEUROSCI.22-14-05966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubova E, Komuro H. Stage-specific control of neuronal migration by somatostatin. Nature. 2002b;415:77–81. doi: 10.1038/415077a. [DOI] [PubMed] [Google Scholar]
- Yacubova E, Komuro H. Cellular and molecular mechanisms of cerebellar granule cell migration. Cell Biochem Biophys. 2003;37:213–234. doi: 10.1385/cbb:37:3:213. [DOI] [PubMed] [Google Scholar]
- Yuhara A, Nishio C, Abiru Y, Hatanaka H, Takei N. PACAP has a neurotrophic effect on cultured basal forebrain cholinergic neurons from adult rats. Dev Brain Res. 2001;131:41–45. doi: 10.1016/s0165-3806(01)00249-8. [DOI] [PubMed] [Google Scholar]
- Zerr P, Feltz A. Forskolin blocks the transient K current of rat cerebellar granule neurons. Neurosci Lett. 1994;181:153–157. doi: 10.1016/0304-3940(94)90582-7. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Shinoda S, Shibanuma M, Nakajo S, Funahashi H, Nakai Y, Arimura A, Kikuyama S. Pituitary adenylate cyclase-activating peptide receptors during development: Expression in the rat embryo at primitive streak stage. Neuroscience. 1999;93:375–391. doi: 10.1016/s0306-4522(99)00108-6. [DOI] [PubMed] [Google Scholar]


