Abstract
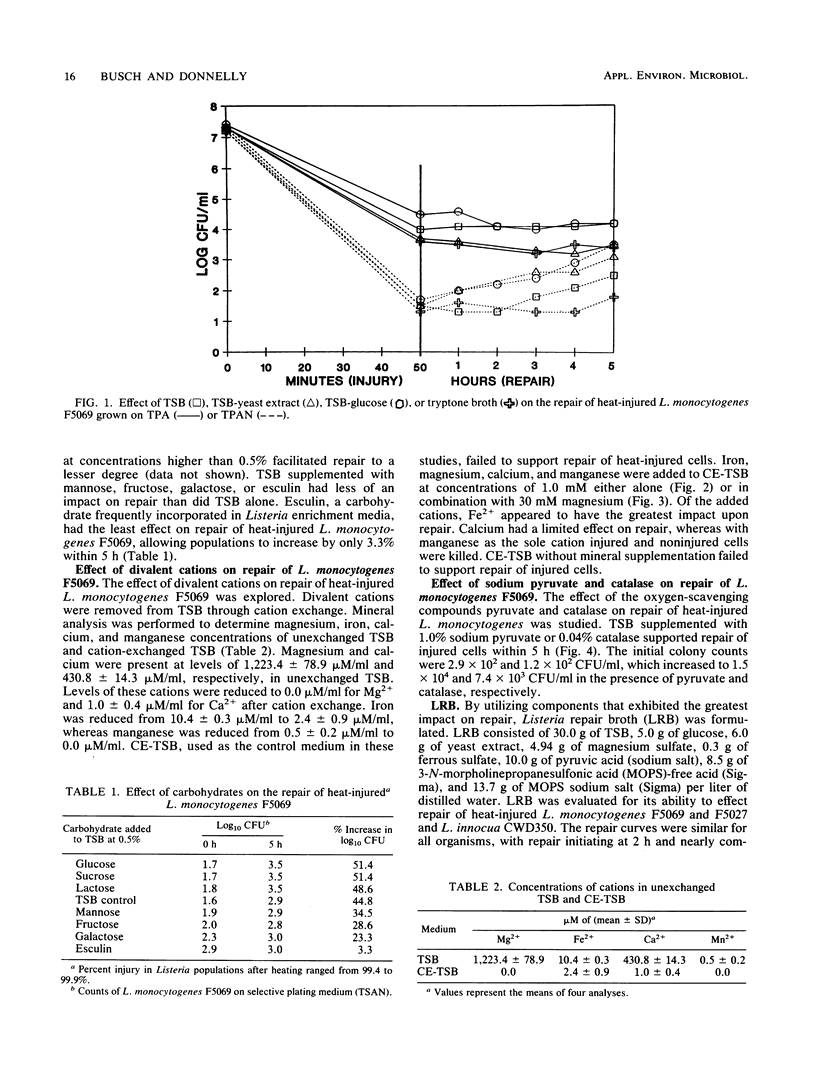
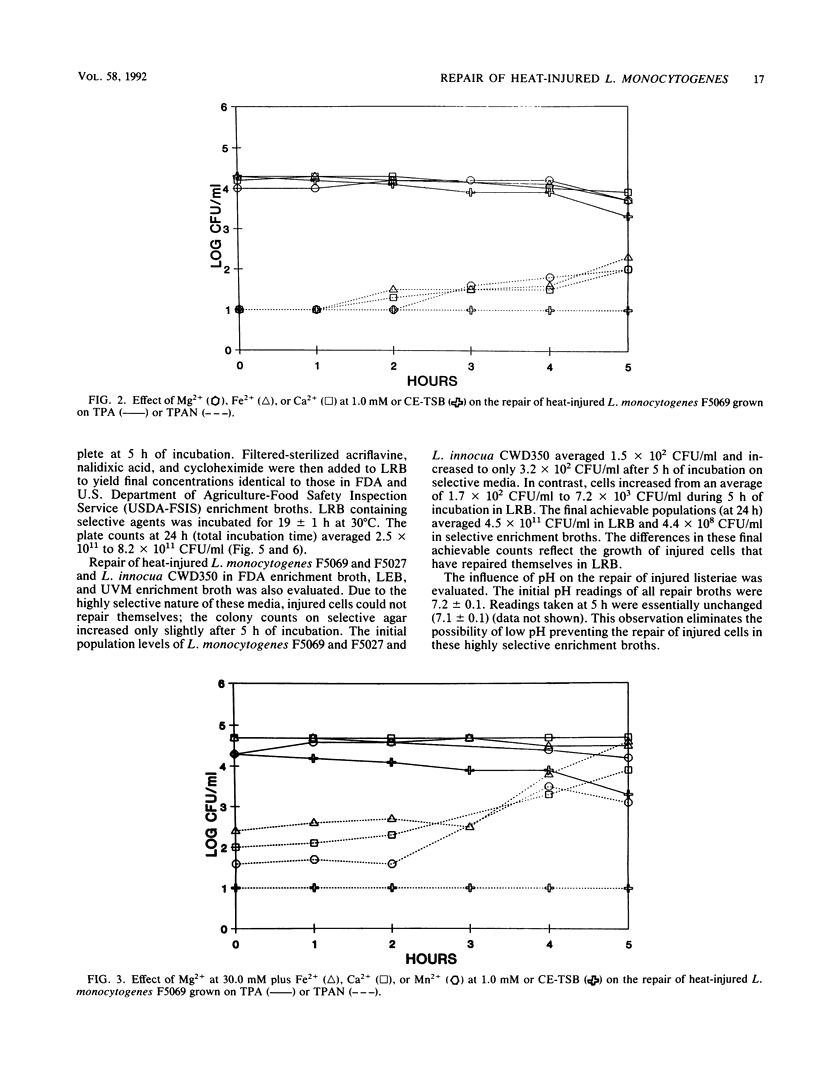
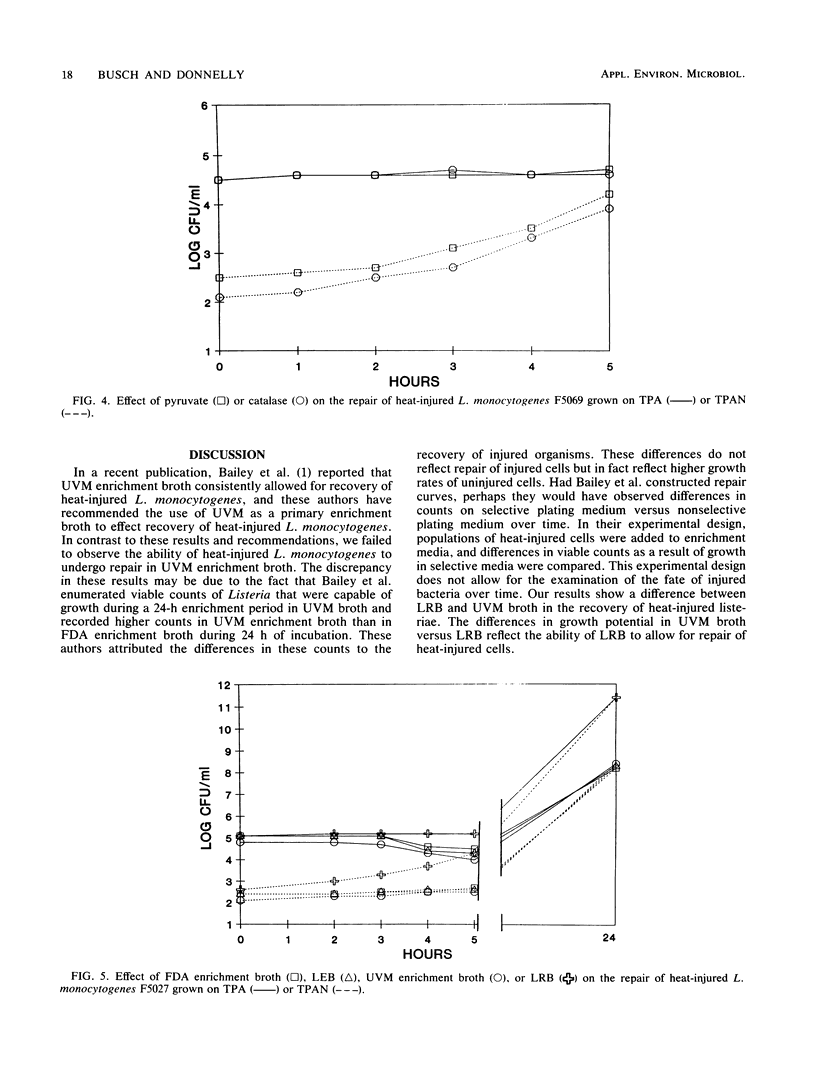
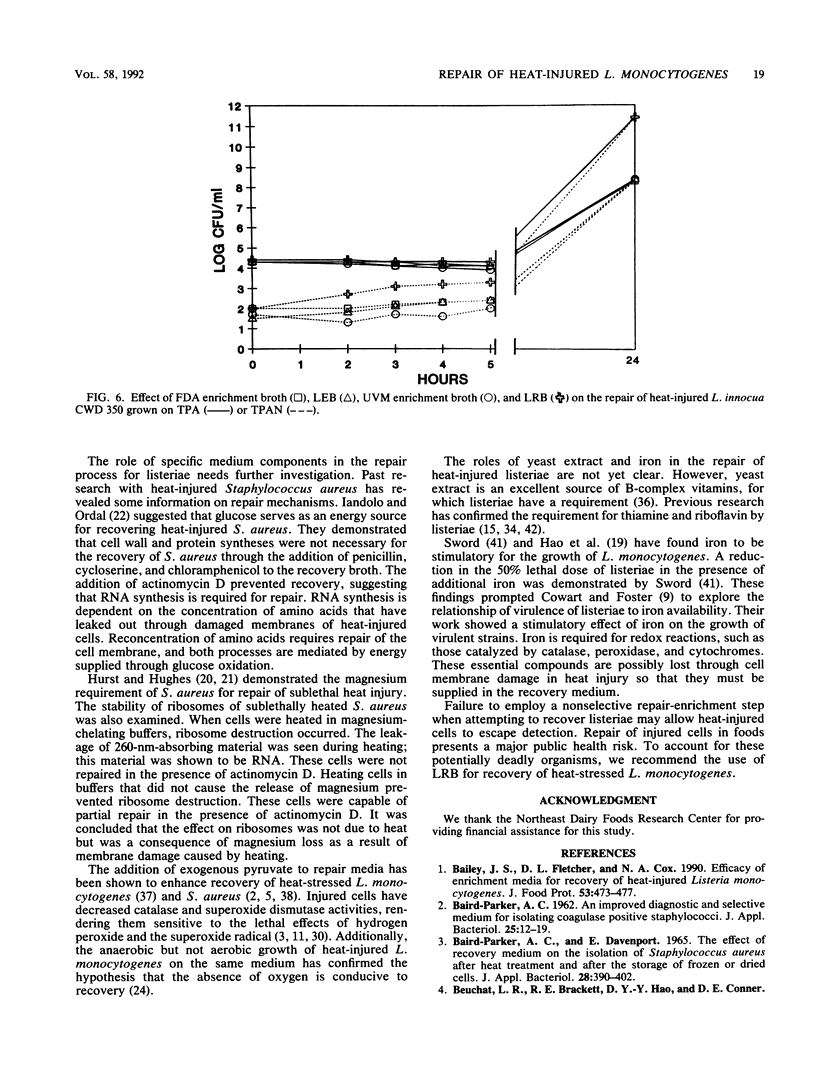
The ability of the divalent cations magnesium, iron, calcium and manganese; yeast extract; pyruvate; catalase; and the carbohydrates glucose, lactose, sucrose, esculin, fructose, galactose, maltose, and mannose to facilitate repair of heat-injured Listeria monocytogenes and Listeria innocua was evaluated. Listeria populations were injured by heating at 56 degrees C for 50 min. To determine the effects on repair, Trypticase soy broth (TSB) was supplemented with each medium component to be evaluated. Repair occurred to various degrees within 5 h in TSB supplemented with glucose, lactose, sucrose, yeast extract, pyruvate, or catalase. Chelex-exchanged TSB was supplemented with divalent cations; magnesium and iron cations were found to have a role in repair. Listeria repair broth (LRB) was formulated by utilizing the components that had the greatest impact upon repair. When incubated in LRB, heat-injured Listeria cells completed repair in 5 h. After the repair, acriflavin, nalidixic acid, and cycloheximide were added to LRB to yield final concentrations identical to those of the selective enrichment broths used in the procedures of the Food and Drug Administration and the U.S. Department of Agriculture. The efficacy of LRB in promoting repair and enrichment of heat-injured Listeria cells was compared with that of existing selective enrichment broths. Repair was not observed in the Food and Drug Administration enrichment broth, Listeria enrichment broth, or University of Vermont enrichment broth. The final Listeria populations after 24 h of incubation in selective enrichment media were 1.7 x 10(8) to 9.1 x 10(8) CFU/ml; populations in LRB consistently averaged 2.5 x 10(11) to 8.2 x 10(11) CFU/ml.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bairdparker A. C., Davenport E. The effect of recovery medium on the isolation of Staphylococcus aureus after heat treatment and after the storage of frozen or dried cells. J Appl Bacteriol. 1965 Dec;28(3):390–402. doi: 10.1111/j.1365-2672.1965.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Brewer D. G., Martin S. E., Ordal Z. J. Beneficial effects of catalase or pyruvate in a most-probable-number technique for the detection of Staphylococcus aureus. Appl Environ Microbiol. 1977 Dec;34(6):797–800. doi: 10.1128/aem.34.6.797-800.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan R. L., Smith J. L., Stahl H. G., Archer D. L. Listeria methods development research at the Eastern Regional Research Center, U.S. Department of Agriculture. J Assoc Off Anal Chem. 1988 May-Jun;71(3):651–654. [PubMed] [Google Scholar]
- Bunning V. K., Donnelly C. W., Peeler J. T., Briggs E. H., Bradshaw J. G., Crawford R. G., Beliveau C. M., Tierney J. T. Thermal inactivation of Listeria monocytogenes within bovine milk phagocytes. Appl Environ Microbiol. 1988 Feb;54(2):364–370. doi: 10.1128/aem.54.2.364-370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart R. E., Foster B. G. Differential effects of iron on the growth of Listeria monocytogenes: minimum requirements and mechanism of acquisition. J Infect Dis. 1985 Apr;151(4):721–730. doi: 10.1093/infdis/151.4.721. [DOI] [PubMed] [Google Scholar]
- Crawford R. G., Beliveau C. M., Peeler J. T., Donnelly C. W., Bunning V. K. Comparative recovery of uninjured and heat-injured Listeria monocytogenes cells from bovine milk. Appl Environ Microbiol. 1989 Jun;55(6):1490–1494. doi: 10.1128/aem.55.6.1490-1494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmier A. W., Martin S. E. Catalase and superoxide dismutase activities after heat injury of Listeria monocytogenes. Appl Environ Microbiol. 1988 Feb;54(2):581–582. doi: 10.1128/aem.54.2.581-582.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C. W., Baigent G. J. Method for flow cytometric detection of Listeria monocytogenes in milk. Appl Environ Microbiol. 1986 Oct;52(4):689–695. doi: 10.1128/aem.52.4.689-695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C. W. Historical perspectives on methodology to detect Listeria monocytogenes. J Assoc Off Anal Chem. 1988 May-Jun;71(3):644–646. [PubMed] [Google Scholar]
- FRIEDMAN M. E., ROESSLER W. G. Growth of Listeria monocytogenes in defined media. J Bacteriol. 1961 Oct;82:528–533. doi: 10.1128/jb.82.4.528-533.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Gilchrist J. E., Campbell J. E., Donnelly C. B., Peeler J. T., Delaney J. M. Spiral plate method for bacterial determination. Appl Microbiol. 1973 Feb;25(2):244–252. doi: 10.1128/am.25.2.244-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden D. A., Beuchat L. R., Brackett R. E. Evaluation of selective direct plating media for their suitability to recover uninjured, heat-injured, and freeze-injured Listeria monocytogenes from foods. Appl Environ Microbiol. 1988 Jun;54(6):1451–1456. doi: 10.1128/aem.54.6.1451-1456.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao D. Y., Beuchat L. R., Brackett R. E. Comparison of media and methods for detecting and enumerating Listeria monocytogenes in refrigerated cabbage. Appl Environ Microbiol. 1987 May;53(5):955–957. doi: 10.1128/aem.53.5.955-957.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A., Hurst A. Magnesium requirement of Staphylococcus aureus for repair from sublethal heat injury. Can J Microbiol. 1976 Aug;22(8):1202–1205. doi: 10.1139/m76-177. [DOI] [PubMed] [Google Scholar]
- Hurst A., Hughes A. Stability of ribosomes of Staphylococcus aureus S6 sublethally heated in different buffers. J Bacteriol. 1978 Feb;133(2):564–568. doi: 10.1128/jb.133.2.564-568.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger J. D., Johnson A., Croan D., Flynn P., Whippie K., Kimball M., Lawrie J., Curiale M. Comparative studies of nucleic acid hybridization assay for Listeria in foods. J Assoc Off Anal Chem. 1988 May-Jun;71(3):669–673. [PubMed] [Google Scholar]
- Knabel S. J., Walker H. W., Hartman P. A., Mendonca A. F. Effects of growth temperature and strictly anaerobic recovery on the survival of Listeria monocytogenes during pasteurization. Appl Environ Microbiol. 1990 Feb;56(2):370–376. doi: 10.1128/aem.56.2.370-376.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., McClain D. Improved Listeria monocytogenes selective agar. Appl Environ Microbiol. 1986 Nov;52(5):1215–1217. doi: 10.1128/aem.52.5.1215-1217.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnan M. J., Mascola L., Lou X. D., Goulet V., May S., Salminen C., Hird D. W., Yonekura M. L., Hayes P., Weaver R. Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med. 1988 Sep 29;319(13):823–828. doi: 10.1056/NEJM198809293191303. [DOI] [PubMed] [Google Scholar]
- Lovett J. Isolation and identification of Listeria monocytogenes in dairy products. J Assoc Off Anal Chem. 1988 May-Jun;71(3):658–660. [PubMed] [Google Scholar]
- Martin S. E., Flowers R. S., Ordal Z. J. Catalase: its effect on microbial enumeration. Appl Environ Microbiol. 1976 Nov;32(5):731–734. doi: 10.1128/aem.32.5.731-734.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly J. A., Butman B. T., Plank M. C., Durham R. J., Robison B. J. Rapid monoclonal antibody-based enzyme-linked immunosorbent assay for detection of Listeria in food products. J Assoc Off Anal Chem. 1988 May-Jun;71(3):679–681. [PubMed] [Google Scholar]
- McClain D., Lee W. H. Development of USDA-FSIS method for isolation of Listeria monocytogenes from raw meat and poultry. J Assoc Off Anal Chem. 1988 May-Jun;71(3):660–664. [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Sorrells K. M., Speck M. L., Warren J. A. Pathogenicity of Salmonella gallinarum after metabolic injury by freezing. Appl Microbiol. 1970 Jan;19(1):39–43. doi: 10.1128/am.19.1.39-43.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sword C. P. Mechanisms of pathogenesis in Listeria monocytogenes infection. I. Influence of iron. J Bacteriol. 1966 Sep;92(3):536–542. doi: 10.1128/jb.92.3.536-542.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELSHIMER H. J. VITAMIN REQUIREMENTS OF LISTERIA MONOCYTOGENES. J Bacteriol. 1963 May;85:1156–1159. doi: 10.1128/jb.85.5.1156-1159.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. T., Klaenhammer T. R. Influence of Calcium and Manganese on Dechaining of Lactobacillus bulgaricus. Appl Environ Microbiol. 1983 Oct;46(4):785–792. doi: 10.1128/aem.46.4.785-792.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


