Abstract
Hemidesmosomes (HDs) are multiprotein adhesion complexes that promote attachment of epithelial cells to the basement membrane. The binding of α6β4 to plectin plays a central role in their assembly. We have defined three regions on β4 that together harbor all the serine and threonine phosphorylation sites and show that three serines (S1356, S1360, and S1364), previously implicated in HD regulation, prevent the interaction of β4 with the plectin actin-binding domain when phosphorylated. We have also established that epidermal growth factor receptor activation, which is known to function upstream of HD disassembly, results in the phosphorylation of only one or more of these three residues and the partial disassembly of HDs in keratinocytes. Additionally, we show that S1360 and S1364 of β4 are the only residues phosphorylated by PKC and PKA in cells, respectively. Taken together, our studies indicate that multiple kinases act in concert to breakdown the structural integrity of HDs in keratinocytes, which is primarily achieved through the phosphorylation of S1356, S1360, and S1364 on the β4 subunit.
INTRODUCTION
Hemidesmosomes (HDs) are adhesive superstructures present primarily in the basal cells of epithelial layers. They guarantee stable adhesion of these cells to the underlying basement membrane. HDs are present in complex and (pseudo) stratified epithelia and are composed of at least six proteins: the two subunits of the integrin α6β4 (Stepp et al., 1990; Jones et al., 1991; Sonnenberg et al., 1991), the bullous pemphigoid antigens 180 (Giudice et al., 1992) and 230 (BP230; Stanley et al., 1981), the cytoskeletal linker protein plectin (Hieda et al., 1992; Gache et al., 1996), and the tetraspanin CD151 (Sterk et al., 2000). The integrin α6β4 binds to its ligand laminin-332 (laminin-5) in the basement membrane, whereas the two members of the plakin family, plectin and BP230, link the cell surface receptors α6β4 and BP180 to the intermediate filament cytoskeleton. This ensures resistance of the adhesive contacts of keratinocytes to mechanical separation.
Although HDs present in skin keratinocytes, bladder epithelial cells, and myoepithelial cells contain all six hemidesmosomal proteins (type I), those present in intestinal epithelial cells contain only the integrin α6β4 and plectin (type II; Uematsu et al., 1994; Orian-Rousseau et al., 1996). The binding of plectin to α6β4 is essential for the integrity of the adhesive complex in both HD types, as indicated by patients with mutations in the β4 integrin subunit that prevent this interaction (reviewed in Litjens et al., 2006). Furthermore, it has previously been reported that the recruitment of plectin by α6β4 is one of the first steps in type I HD assembly (Koster et al., 2004). The actin-binding domain (ABD) of plectin binds to the first pair of fibronectin type III (FnIII) domains and a small region of the connecting segment (CS) that separates the first pair of FnIII domains from the second pair on β4 (Geerts et al., 1999). This interaction is strengthened by the binding of the plectin plakin domain to the CS and C-tail of β4 (Koster et al., 2004; Rezniczek et al., 1998). Additionally, in vitro analysis clearly indicates that if the interaction between plectin and α6β4 is disrupted, HD integrity is compromised (Geerts et al., 1999; Koster et al., 2001).
Although HDs are adhesive protein complexes, they nevertheless are dynamic structures because their components are rearranged during cell processes such as migration and division. However, the interaction between plectin and β4 reduces the dynamics when laminin-332 is clustered beneath HDs (Geuijen and Sonnenberg, 2002). Epidermal growth factor (EGF), macrophage-stimulating protein (MSP), and phorbol 12-myristate acetate (PMA) have been implicated in the disassembly of HDs through signaling pathways that result in the phosphorylation of the β4 cytoplasmic domain by protein kinase C (PKC). Activation of PKCα downstream of the EGF receptor has been suggested to lead to phosphorylation of serine residues in the β4 CS that results in a translocation of α6β4 to lamellipodia, where it associates with actin-rich protrusions (Rabinovitz et al., 1999, 2004). Moreover, activation of PKC downstream of the Ron (MSP) receptor also results in a mobilization of α6β4 to actin-rich protrusions in lamellipodia, where it is reported to associate with the Ron receptor through 14-3-3 proteins (Santoro et al., 2003). However, it is clear that other kinases, or mechanisms, are involved in the disassembly of HDs because EGF, MSP, and PMA did not cause complete disassembly of them as appears in these reports.
Previously, Rabinovitz et al. (2004) showed that the serine residues S1356, S1360, and S1364 in the β4 CS are phosphorylated downstream of the EGF receptor, and they suggest that this is mediated by PKCα. Moreover, substitution of these three residues by aspartic acid results in the partial loss of the colocalization of plectin with α6β4 in transfected COS-7 cells. Therefore, we decided to investigate the role of these serines in the disassembly of HDs under more physiological conditions in keratinocytes. By phosphatase inhibition assays three regions of β4 were identified that were heavily phosphorylated on serine and threonine residues in cells, including the region of the CS containing S1356, S1360, and S1364. We further show that activation of endogenous PKC results in the phosphorylation of only S1360.
We also show through phosphomimicry studies that phosphorylation of two or more of these serines prevents the primary interaction of the plectin ABD with β4. Furthermore, we identified S1364 as an endogenous PKA phosphorylation site in keratinocytes and showed that its activation in conjunction with PKC reduces the ability of the plectin ABD to associate with β4. And finally, we established that activation of the EGF receptor results in the phosphorylation of only these three residues in keratinocytes, which leads to a partial disassembly of HDs. Taken together, our results suggest that in keratinocytes, multiple kinases act in concert to induce the disassembly of HDs through the phosphorylation of S1356, S1360, and S1364.
MATERIALS AND METHODS
Cell Lines
PA-JEB (β4-null) keratinocytes have been described previously (Schaapveld et al., 1998) and were maintained in keratinocyte serum-free medium (SFM; Invitrogen, Rockville, MD) supplemented with 50 μg/ml bovine pituitary gland extract, 5 ng/ml EGF, 100 U/ml penicillin, and 100 U/ml streptomycin. Stable expression of β4 mutants in cells was performed as described previously (Sterk et al., 2000; Geuijen and Sonnenberg, 2002). COS-7 cells were grown in DMEM (Invitrogen) containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin. They were transiently transfected using the DEAE-dextran method (Seed and Aruffo, 1987).
β4 and β4 Phospho-specific Antibodies
β4 rabbit polyclonal Abs were raised against the first pair of FNIII repeats and part of the CS (β4A residues 1115–1355) fused to an amino-terminal glutathione S-transferase (GST) moiety. GST preparations were done as previously described (Wilhelmsen et al., 2002), and fusion proteins were eluted from glutathione-Sepharose beads using 15 mM reduced glutathione. The GST moiety was removed by Factor VIII protease treatment followed by column chromatography and subsequently, the β4 fragment was injected into rabbits four times.
The phospho-specific β4 rabbit polyclonal antibodies (β4 pS-CS) were raised against a synthetic peptide (sequence: CAQSGEDYDSFLMYSDDVLRpSPSGpSQRPpSVSDD) coupled to an activated mcKLH moiety (Pierce, Rockford, IL) and subsequently, the coupled peptides were injected into rabbits four times. The antibodies were first affinity-purified on a column containing SulfaLink beads (Pierce) cross-linked to the phosphorylated peptides, eluted with 1 M glycine-HCl, pH 2.7, and immediately neutralized with an equal volume of 5% NHCO3. Bovine serum albumin (1%) was then added, and the protein mixture was dialyzed overnight against phosphate-buffered saline (PBS). Finally, the dialyzed antibody sample was run through a column containing SulfaLink beads (Pierce) cross-linked to the equivalent nonphosphorylated peptides and the nonbound fraction collected and stored at 4°C with azide.
cDNA Constructs
For PCR the proofreading Pwo DNA polymerase (Roche Molecular Biochemicals, Indianapolis, IN) was used. All plasmids were verified by sequencing, and protein expression and sizes were confirmed by Western blotting. The full-length β4 cDNA in pUC18 has been described previously (Niessen et al., 1997). Point mutants were generated by the PCR overlap extension method. Subsequently β4 was isolated using the EcoRI restriction site and it was ligated into the retroviral LZRS-IRES-zeo vector as described before (Sterk et al., 2000). BssHII-EcoRV fragments were used for exchange with fragments from IL2R-β4 in pCMV (Nievers et al., 1998) to generate IL2R-β4 mutants. The plasmid pCMV contains a Cytomegalovirus LTR directing the transcription of cDNA sequences. Full-length β4 and deletion mutants of β4 in pCMV have been described before (Niessen et al., 1997). Also, the plectin-1A ABD in pcDNA3-HA (hemagglutinin) has been previously described (Litjens et al., 2003).
Phosphopeptide Mapping
For in vivo phosphopeptide mapping experiments, PA-JEB or COS-7 cells expressing β4 were phosphate-starved for 45 min at 37°C. Subsequently, 2 mCi of [32P]orthophosphate was added and incubated for 3 h at 37°C. Calyculin A (100 nM; Cell Signaling, Beverly, MA), 1 μM PMA (Sigma-Aldrich, St. Louis, MO), 25 μM forskolin (FSK; Calbiochem, San Diego, CA), and 0.1 mM 3-isobutyl-1-methylxanthine (IBMX; Calbiochem) or 50 ng/ml EGF (Sigma-Aldrich) were added for 30 min at 37°C. β4 was immunoprecipitated with the mAb 450-11A (PharMingen International, San Diego, CA). For in vitro phosphopeptide mapping experiments, IL2R-β4 was immunoprecipitated with 450-11A from lysates of transfected COS-7 cells. Subsequently, the isolated protein was phosphorylated in vitro with 20 U of PKA (Sigma-Aldrich) in the presence of 50 μCi [γ-32P]ATP, 40 mM HEPES, pH 7.5, 3 mM MnCl2, 10 mM MgCl2, and 1 mM dithiothreitol (DTT) for 2 h at 30°C. The in vivo and in vitro samples were subjected to SDS-PAGE, and the gel was dried. The film was exposed at room temperature for 10 min for in vitro phosphorylations or 1 h for in vivo phosphorylations. Radioactive β4 was isolated from the gel and digested with trypsin. Phosphopeptide mapping was performed as described previously (van der Geer et al., 1993; Wilhelmsen et al., 2002).
Immunofluorescence
PA-JEB/β4 keratinocytes were seeded on glass coverslips in keratinocyte SFM. After 24 h the medium was replaced by high-calcium medium (Ham's F12/DMEM, 1:3). After overnight incubation, cells were stimulated with 50 ng/ml EGF (Sigma-Aldrich) for 30 min at 37°C. Cells were fixed and permeabilized as described before (Litjens et al., 2003). Primary antibodies used were the rat mAb GoH3 against α6 (Sonnenberg et al., 1987) and the mouse mAb 121 against plectin/HD1 (Hieda et al., 1992), which was a generous gift from Dr. K. Owaribe (Nagoya University, Nagoya, Japan). Secondary antibodies were fluorescein isothiocyanate (FITC)-conjugated goat anti-rat antibodies, purchased from Rockland (Gilbertsville PA), and Cy5-coupled donkey anti-rat and Texas Red–coupled donkey anti-mouse antibodies, obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Secondary antibodies were selected for minimal cross-reactivity against the other species. The coverslips were mounted onto glass slides in Mowiol mounting medium (Calbiochem) containing 2.5% DABCO (Sigma-Aldrich). Immunofluorescence images were taken using a Leica confocal laser scanning microscope (Deerfield, IL).
Scatter Plots and Colocalization Images
Colocalization between α6 and plectin images (eight-bit grayscale values) was investigated by a custom-made Visual Basic (v6.0) program (Microsoft Corp.). Pixel intensity for α6 was plotted against the pixel intensity of plectin in a scatter plot. Multiple occurrences of identical coordinates are color coded as indicated in Figure 5. For a pair of colocalized images, the scatter plot shows a distribution along the diagonal with some divergence because of photon noise. Noncolocalized structures appear as off-diagonal clusters. Superimposed on a grayscale overlay image, the bright colocalized pixels, the noncolocalized α6 pixels, and the noncolocalized plectin pixels are shown in color as indicated in Figure 5.
Figure 5.
The effect of S1356/S1360/S1364 phosphorylation on HD formation and plectin binding. PA-JEB/β4WT (A–J), PA-JEB/β43xA (K–T), or PA-JEB/β43xD (U–D′) cells were either left untreated (A–E, K–O, and U–Y) or treated for 30 min with EGF (F–J, P–T, or Z–D′), and immunofluorescence studies were performed to locate endogenous plectin using the mAb 121 (red) and α6 using GoH3 (green). Colocalization appears as yellow. Scatter plots are produced as described in Materials and Methods and show the amount of colocalization between α6 and plectin. In the right panels, high intensity colocalizing pixels are shown in blue, noncolocalized high intensity α6 pixels are shown in red, whereas noncolocalized high-intensity plectin pixels are shown in yellow.
Pulldown and Phospho-specific Antibody Assays
COS-7 cells were transfected with indicated cDNA constructs. When indicated, COS-7 or PA-JEB/β4 cells were stimulated with either 1 μM PMA (Sigma-Aldrich), 25 μM FSK (Calbiochem), and 0.1 mM IBMX (Calbiochem) or 50 ng/ml EGF (Sigma-Aldrich) for 30 min (or for the indicated times in the time-course stimulation assays) at 37°C. Cells were lysed in M-PER buffer (Mammalian Protein Extraction Reagent; Pierce), containing 1 mM sodium vanadate and a cocktail of protease inhibitors (Sigma-Aldrich). Immunoprecipitations were performed with mAb HA 12CA5 (Santa Cruz Biotechnology, Santa Cruz, CA) and immunoblottings were done with either pAb anti-IL2R (sc-665; Santa Cruz Biotechnology), pAb anti-HA (sc-805; Santa Cruz Biotechnology), mAb anti-pTyr (4G10), pAb anti-EGF receptor (Ab-17; Neomarkers or 1005; Santa Cruz Biotechnology), mAb anti-EGFR pY845 (Cell Signaling, Beverly, MA), a mixture of mAbs antibodies against PKCα, β, and δ (Transduction Laboratories, Lexington, KY) or pAb anti-phospho-PKC (pan; betaII Ser 660; 9371; Cell Signaling) as described previously (Litjens et al., 2003). Gö6983 (Sigma-Aldrich) was used at a concentration of 100 nM for 30 min before PMA addition.
Migration Assays
PA-JEB, PA-JEB/β4WT, PA-JEB/β4S1356/1360/1364A (β43xA), and PAJEB/β4S1356/1360/1364D (β43xD) keratinocytes were grown to confluence in 24-well tissue culture plates in normal keratinocyte growth medium. Cells were then serum-starved overnight in keratinocyte-SFM and treated with 10 μg/ml mitomycin C (Sigma) 2 h before wounding. Monolayers were scratched with a yellow pipette tip and washed twice in keratinocyte-SFM to remove cell debris, before stimulation with EGF (5 ng/ml). Cells were then incubated at 37°C, and scratched areas were photographed during at least 48 h at 10× magnification. Relative migration was calculated from the scratch area at t = 48 over the scratch area at t = 0 and averaged from three independent experiments done in duplicate.
RESULTS
Phosphorylation of Serine/Threonine on β4 Occurs Primarily in the CS
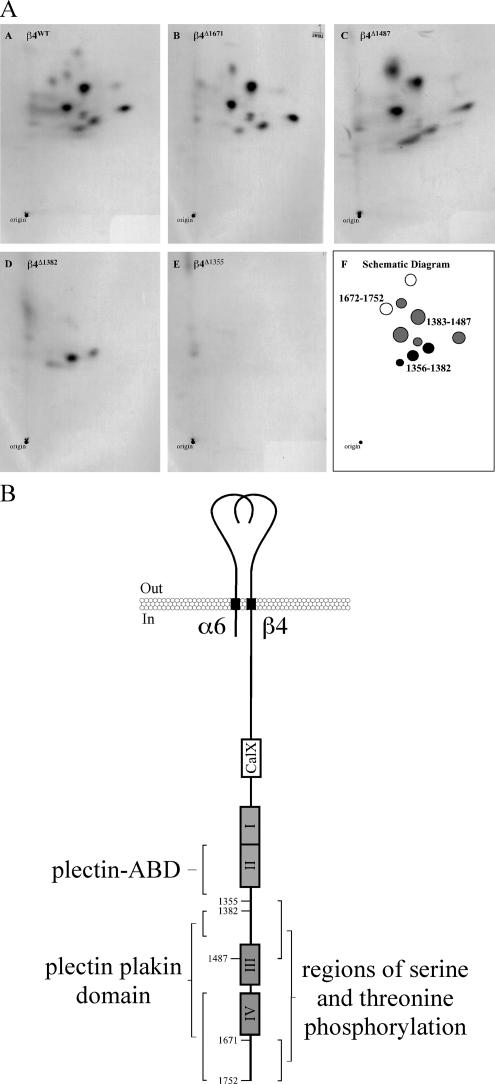
To define the regions of the β4 subunit that are serine- and threonine-phosphorylated, various deletion mutants of the β4 cytoplasmic domain were expressed in COS-7 cells, and subsequently the cells were treated with calyculin A in the presence of [32P]orthophosphate. Calyculin A inhibits protein phosphatase-1 and -2A (Ishihara et al., 1989), which together account for most of the serine/threonine phosphatase activity in a cell. Their inhibition results in an increase in the serine and threonine phosphorylation of proteins. The β4 deletion mutants were then isolated and subjected to phosphopeptide mapping. The results show that by deleting the C-terminal tail (β4Δ1671) two peptides are no longer phosphorylated. Further deletion of the cytoplasmic domain until amino acid 1487 (β4Δ1487) does not result in the obvious loss of phosphorylation of other peptides. However, when the third FnIII domain and part of the CS were deleted (β4Δ1382), five peptides were no longer phosphorylated. The three remaining phosphopeptides were no longer present after further deletion of the β4 cytoplasmic domain to residue 1355 (β4Δ1355; Figure 1A). These results indicate that most of the serine and threonine phosphorylations on β4 occur in the region between residues 1487 and 1355, which comprises a large part of the CS, with some also occurring in the C-terminal tail (Figure 1B).
Figure 1.
Serine/threonine phosphorylation of β4 occurs predominantly in the CS and the C-terminal tail. (A) In vivo phosphopeptide maps of wild type (A) and four sequentially truncated (B–E) β4 subunits derived from COS-7 cells that were treated with calyculin A in the presence of [32P]orthophosphate. (F) A schematic diagram of the results shown in A–E. (B) Schematic drawings of the integrin α6β4 depicting the regions serine and threonine phosphorylated on the β4 subunit after calyculin A treatment and the interaction sites with plectin reported in the literature. Residue numbers indicate where the β4 deletions were made. Roman numerals I–IV show the positions of the four FnIII domains. The CS is between FnIII II and III, whereas the C-terminal tail is after FnIII IV. CalX, Ca-Na exchanger motif. β4 numbering is based on the human β4A sequence.
Phosphorylation of S1356, S1360, and S1364 Disrupts the Bond between β4 with the Plectin-ABD
Previously, Rabinovitz et al. (2004) have shown that substitution of three serine residues at positions 1356, 1360, and 1364 in β4 by phosphomimic aspartic acid residues impairs formation of HDs in transfected COS-7 cells. Our calyculin A studies show that the region between 1356 and 1382 amino acids contains three phosphopeptides, which are most likely related because a laddering pattern of phosphorylation is observed that is consistent with the phosphorylation of different residues on the same peptide (Figure 1A; Boyle et al., 1991). We therefore decided to explore the role of this region in HD regulation in more detail, specifically focusing on the interaction between β4 and plectin.
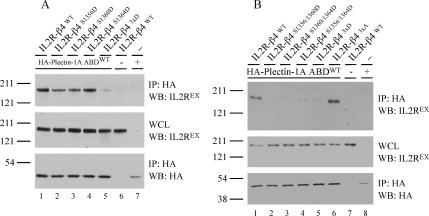
Curiously, the primary site on β4 that interacts with the plectin ABD lies outside the regions that are subject to phosphorylation, whereas those that interact with the plakin domain do not (Figure 1B). As we showed previously, the primary interaction between plectin and β4 occurs between the first pair of FnIII domains on β4 and the plectin ABD (Geerts et al., 1999). In fact, this interaction is sufficient for the formation of normal HD structures in cells (Niessen et al., 1997; Nikolopoulos et al., 2005). The plakin domain of plectin in turn associates with the CS and C-terminal tail of β4, but these regions in β4 are not essential for the localization of plectin in HDs (Niessen et al., 1997; Schaapveld et al., 1998). Therefore, we decided to concentrate on the question whether phosphorylation of S1356, S1360, and/or S1364 influences the binding of the ABD to β4. To this end, we performed coimmunoprecipitation experiments in COS-7 cells using an HA-plectin-1A ABD construct in conjunction with IL2R-β4 fusion proteins containing all possible combinations of aspartic acid residue substitutions of these three serine residues. The results show that the single point mutants IL2R-β4S1356D and IL2R-β4S1360D did not bind as well to the plectin-1A ABD as either the wild-type IL2R-β4 or the IL2R-β4S1364D mutant (Figure 2A), indicating that the phosphorylation of a single residue is not sufficient to prevent the binding of β4 to the ABD. However, when two, or all three, of these residues are replaced by aspartic acid, binding between the two proteins does not occur (Figure 2, A and B). Importantly, binding occurs, and possibly increases, when the three serines are mutated to alanine (Figure 2B), indicating that the loss of binding is not due to mutation of this area of β4 per se. These results indicate that phosphorylation of any two, or all three, of these serine residues on β4 can prevent the interaction with the plectin-1A ABD.
Figure 2.
Phosphomimick aspartic acid substitutions of S1356, S1360, and S1364 in the β4 CS prevents the association with the Plectin-1A ABD. (A) COS-7 cells were transiently transfected with either IL2R-β4WT (lanes 1 and 6), IL2R-β4S1356D (lane 2), IL2R-β4S1360D (lane 3), IL2R-β4S1364D (lane 4), or IL2R-β43xD (lane 5) cDNA constructs or a control plasmid (lane 7) and an expression construct for the HA-plectin-1A ABDWT (lanes 1–5 and 7) or a control plasmid (lane 6). The cells were lysed in M-PER buffer and HA-IPs were probed for the presence of IL2R-β4 (top) and the HA-tagged ABDs (bottom). Whole cell lysates (WCLs) were probed for the expression level of IL2R-β4 proteins (middle). (B) The experiment was performed as in A, except that either IL2R-β4WT (lanes 1 and 7), IL2R-β4S1356/1360D (lane 2), IL2R-β4S1360/1364D (lane3), IL2R-β4S1356/1364D (lane 4), IL2R-β43xD (lane 5), or IL2R-β43xA (lane 6) cDNA constructs, or a control plasmid (lane 8), were instead cotransfected with the HA-tagged plectin-1A ABD cDNA construct (lanes 1–6 and 8) or a control plasmid (lane 7).
PKC Phosphorylates S1360 and PKA Phosphorylates S1364 in Cells
Previous work has suggested that phosphorylation of the β4 CS by PKCα can disrupt HDs (Santoro et al., 2003; Rabinovitz et al., 2004). The phospho-ELM and GPS phosphorylation prediction methods identified several potential PKC phosphorylation sites in the β4 cytoplasmic domain (http://phospho.elm.eu.org/and http://973-proteinweb.ustc.edu.cn/gps/gps_web/predict.php); however, only S1360, of the three residues implicated above, was within a consensus site for PKC phosphorylation (S-X-R; Woodgett et al., 1986). For further investigations, we stably expressed β4WT and β4S1360A in β4-null PA-JEB keratinocytes, reasoning that expression of β4 in these cells would more accurately reflect physiological conditions. Phosphopeptide mapping was then performed on the isolated β4 subunits from control β4WT-expressing cells and β4WT- or β4S1360A-expressing cells after treatment with PMA (to activate endogenous PKC) in the presence of [32P] orthophosphate. The results show that after PMA treatment, phosphorylation of two peptides increases in intensity, but not when S1360 is mutated to alanine (Figure 3A). Interestingly, background phosphorylation of the two tryptic peptides containing S1360A indicates that there is a basal level of phosphorylation of these peptides by other kinases. In fact, these same peptides were no longer phosphorylated after calyculin A treatment of keratinocytes expressing a β4S1356/1360/1364A triple mutant (β43xA), suggesting that the background phosphorylation was due to phosphorylation of S1356 and/or S1364 (Figure 3A). Taken together, the results indicate that S1360 is the only PKC phosphorylation site on β4 in keratinocytes and that kinases other than PKC may phosphorylate S1356 and/or S1364 in these cells.
Figure 3.
S1360 and S1364 are in vivo PKC and PKA phosphorylation sites on β4, respectively, that cooperate to reduce the association with the plectin-1A ABD. (A) In vivo phosphopeptide maps of β4 isolated from either PA-JEB/β4WT cells that were serum-starved (A), treated with PMA (B) or calyculin A (D), PA-JEB/β4S1360A cells that were treated with PMA (C) or PA-JEB/β43xA cells that were treated with calyculin A (E) in the presence of [32P]orthophosphate. (F) A schematic diagram of the results shown in A–E. Gray ovals, peptides containing β4 specific phosphorylation sites. (B) PA-JEB/β4WT cells were either left serum-starved (lane 1), treated with PMA for 30 min (lane 2), or treated with PMA for 30 min in the presence of the compound Gö6983 (lane 3) before lysis in mPER buffer. Similarly, PA-JEB/β43xA (lanes 4 and 5) and PA-JEB/β43xD (lanes 6 and 7) cells were either left serum-starved (lanes 4 and 6) or treated with PMA for 30 min (lanes 5 and 7) before lysis. WCLs were probed for the presence of β4 phosphorylated on residues S1356, S1360, and/or S1364 using our rabbit polyclonal phospho-specific antibody β4 pS-CS (top). The expression level of the β4 proteins was detected using a rabbit polyclonal antibody raised against the first pair of FNIII repeats in β4 (middle). To verify the activation of PKC, WCLs were also probed for the presence of phospho-PKC using pan antibodies raised against phosphorylated Thr 660 of PKCβ II (bottom). (C) Alignment of the human β4A sequence from residue 1352-1368 with the same region in cow, dog, mouse, rat, chicken, and zebrafish. The kinases identified from the phospho-phosphorylation site prediction programs phospho-ELM and GPS are shown below their corresponding residue. The human β4 sequence was used as the input. (D) In vivo phosphopeptide maps of β4 isolated from either serum-starved PA-JEB/β4WT cells (A) or PA-JEB/β4WT (B), PA-JEB/β4S1360/1364A (C) or PA-JEB (an equivalent area of gel were β4 would run was excised; D) cells treated for 30 min with FSK/IBMX in the presence of [32P]orthophosphate. (E) A schematic diagram of the results shown in A–D. Note that the peptides in these maps migrate to a lower part on the thin-layer chromatography plates because the chromatography buffer used was not optimal. Gray ovals, peptides containing β4 specific phosphorylation sites. (E) PA-JEB/β4WT cells were either serum-starved (lane 1) or treated with Forskolin/IBMX for the indicated times (in minutes; lanes 2–7) before lysis in mPER buffer. WCLs were probed for the presence of β4 phosphorylated on residues S1356, S1360, and/or S1364 using our rabbit polyclonal phospho-specific antibody β4 pS-CS (top) and the expression level of the β4 proteins with our rabbit polyclonal antibody raised against the first pair of FNIII repeats in β4 (bottom). (F) COS-7 cells were transiently transfected with either IL2R-β4WT (lanes 1–4 and 7) or IL2R-β4S1360/1364A (lanes 5 and 6) cDNA constructs or a control plasmid (lane 8), and an expression construct for the HA-plectin-1A ABDWT (lanes 1–6 and 8) or a control plasmid (lane 7). The cells were left untreated (lanes 1, 5, 7 and 8) or treated with either FSK/IBMX (lane 2), PMA (lane 3), or all three compounds together (lanes 4 and 6) 30 min before lysis in mPER buffer. HA-IPs were probed for the presence of IL2R-β4 (top) and the HA-tagged ABDs (middle). WCLs were probed for the expression level of IL2R-β4 proteins (bottom). Quantitation was done in ImageJ and is a ratio of the band intensity shown in the top panel to those in the middle panel for each lane, relative to lane 1.
To facilitate our studies and to complement the phosphopeptide mapping data, we produced a rabbit polyclonal antibody raised against a β4-derived peptide containing phosphorylated serines 1356, 1360, and 1364 (β4 pS-CS). To determine the specificity and sensitivity of our new phospho-specific antibody, we blotted lysates derived from β4WT-expressing keratinocytes that were either left serum-starved or treated with PMA in the absence or presence of the PKC inhibitor Gö6983. We used lysates derived from serum-starved or PMA-treated PA-JEB cells expressing β43xA or β43xD as controls. The results show that PMA induces phosphorylation of the β4 subunit, whereas PKC inhibition prevents this (Figure 3B). This suggests that S1360 is specifically phosphorylated by PKC and not by another PMA sensitive kinase. Importantly, the antibody also did not react with either the β43xA or the β43xD subunits before or after PMA addition (Figure 3B). These results highlight both the specificity and sensitivity of our new antibody and independently from the phosphopeptide mapping data indicate that serine 1360 is indeed phosphorylated specifically by PKC.
In search of other kinases that may phosphorylate this region of β4, we again used GPS and phospho-ELM and identified many other kinases that could in principle phosphorylate serines 1356 and 1364, but also 1360 (Figure 3C). We paid particular attention to PKA because this kinase appeared to be the only one capable of phosphorylating S1364 and because there are compounds, FSK and IBMX, that specifically activate this kinase. We first tested the ability of FSK/IBMX to induce phosphorylation of S1364 in cells by using the previously mentioned β4WT-expressing keratinocytes and keratinocytes expressing a β4S1360/1364A double mutant in the presence of [32P]orthophosphate. We used this double mutant to lower the level of background phosphorylation, which would facilitate interpretation of the phosphopeptide maps. Indeed, we see that FSK/IBMX induces phosphorylation of two tryptic peptides, which was abolished by mutation of S1360 and S1364 (Figure 3D). The results were later verified in in vitro phosphopeptide mapping experiments that conclusively show that S1364 is the only PKA phosphorylation site on β4 (Supplementary Figure 1). We also utilized our β4 phospho-specific rabbit polyclonal antibody (β4 pS-CS) to independently determine whether PKA can indeed induce phosphorylation of β4. To this end, a FSK/IBMX time course was performed on β4WT-expressing keratinocytes. The results show that phosphorylation of β4 is maximal at around 30 min after PKA activation, after which it diminishes to background levels by 2 h (Figure 3E). After taking into account the specificity of our new antibody, this data again indicates that the β4 subunit is phosphorylated on S1364 by PKA in keratinocytes.
The results from Figures 2B and 3, A–E, suggest that activation of PKA and PKC should be sufficient to prevent the association of β4 with the plectin-1A ABD. To prove this, we transiently transfected cDNAs expressing the IL2R-β4WT and HA-plectin-1A ABD proteins into COS-7 cells and stimulated the cells with PMA and/or FSK/IBMX. We also utilized an IL2R-β4 construct containing S1360A and S1364A point mutations (IL2R-β4S1360/1364A) as a control because this β4 subunit mutant is not phosphorylated by either PKC or PKA in cells. The results show that PMA and FSK/IBMX by themselves do not influence the binding of the plectin-1A ABD to β4, but together they reduce binding, although only to a degree (40% reduction; Figure 3F). Importantly, the reduction in binding was less with the IL2R-β4S1360/1364A mutant, than for the IL2R-β4WT, after PMA and FSK/IBMX treatment (∼15% reduction; Figure 3F). This indicates that the loss in binding of IL2R-β4WT is in fact mostly due to phosphorylation of S1360 and S1364 by PKC and PKA, respectively.
Effects of EGF Receptor Activation on HD Integrity
The above results suggest the involvement of PKC and PKA in the regulation of the interaction between β4 and plectin in COS-7 cells, but there are other kinases that are predicted to phosphorylate S1356, S1360, and S1364. However, the involvement of these other kinases is difficult to determine because of the lack of activators that specifically activate them, and we therefore decided to focus on the above three serine residues in a more physiological context. Activation of the EGF receptor is known to induce HD disassembly and has been shown to activate some of the kinases capable of phosphorylating S1356, S1360, and/or S1364 (Oda et al., 2005). Therefore, we first tested the ability of EGF to induce phosphorylation of β4 on S1356, S1360, and S1364 in keratinocytes. The β4WT- or β43xA-expressing cells were stimulated with EGF in the presence of [32P]orthophosphate and phosphopeptide mapping was performed on the isolated β4 subunits. The results indicate that after activation of the EGF receptor, one or more of these residues are phosphorylated in cells and importantly, these serine residues are the only ones phosphorylated (Figure 4A).
Figure 4.
EGF induces phosphorylation of β4 only on S1356, S1360, and S1364, which reduces the affinity of β4 for the plectin-1A ABD. (A) In vivo phosphopeptide maps of β4 isolated from either serum-starved PA-JEB/β4WT cells (A) or PA-JEB/β4WT (B), PA-JEB/β43xA (C), or PA-JEB (an equivalent area of gel where β4 would run was excised, D) cells treated for 30 min with EGF in the presence of [32P]orthophosphate. (E) A schematic diagram of the results shown in A–D. Gray ovals, peptides containing β4 specific phosphorylation sites. (B) PA-JEB/β4WT cells were either serum-starved (lane 1) or treated with 50 ng/ml EGF for the indicated times (in minutes; lanes 2–6) before lysis in mPER buffer. WCLs were probed for the presence of β4 phosphorylated on residues S1356, S1360, and/or S1364 using our rabbit polyclonal phospho-specific antibody β4 pS-CS (top), and the expression level of the β4 proteins was detected using a rabbit polyclonal antibody raised against the first pair of FNIII repeats in β4 (second from top panel). To verify the activation of the EGF receptor and PKC isoforms, WCLs were probed using an antibody that recognizes phospho-tyrosine 845 on the activated EGF receptor (third from top panel) and antibodies raised against phosphorylated Thr 660 of PKCβ II (pan-pPKC; second from bottom panel). The expression levels of the EGF receptor and PKC isoforms were detected using an antibody against the EGF receptor (1005; third from bottom panel) and a mixture of antibodies against PKC α, β, and δ (bottom). Interestingly, the phospho-tyrosine 845 EGFR antibody recognizes a cleaved protein fragment of the full-length EGF receptor that contains the phosphorylated cytoplasmic domain. (C) COS-7 cells were transiently transfected with either IL2R-β4WT (lanes 1, 2, and 5) or IL2R-β43xA (lanes 3, 4, and 6) cDNA constructs or a control plasmid (lane 7) or an expression construct for the HA-plectin-1A ABDWT (lanes 1–4 and 7) or a control plasmid (lanes 5 and 6). The cells were left untreated (lanes 1, 3, and 5–7) or treated with EGF (lanes 2 and 4) 30 min before lysis in mPER buffer. HA-IPs were probed for the presence of IL2R-β4 (top) and the HA-tagged ABDs (second from top panel). WCLs were probed for the expression level of IL2R-β4 proteins (third from top panel) and the EGF receptor (second from bottom panel). The activation of the EGF receptor was visualized using phosphotyrosine antibodies (third from bottom panel; the location of the EGFR is denoted by an asterisk) and the classical PKCs using pan antibodies raised against phosphorylated Thr 660 of PKCβ II (bottom panel; the location of the phospho-PKC is denoted by two asterisks). Quantitation was done in ImageJ and is a ratio of the band intensity shown in the top panel to those in the second to top panel for each lane, relative to lane 1.
To independently verify the results of the phosphopeptide mapping experiments, we used our phospho-specific β4 antibody to determine the extent of β4 phosphorylation after treating PA-JEB cells stably expressing the wild-type subunit with EGF for varying lengths of time. The activation of the endogenous EGF receptor and the classical PKCs after EGF treatment was confirmed by Western blotting with antibodies that recognize either phosphotyrosine 845 of the EGF receptor or the phosphorylated forms of the conventional PKCs (Figure 4B). The results show that there is robust phosphorylation of the β4 subunit within 10 min of EGF treatment, which diminishes between 1 to 2 h after the growth factor was added (Figure 4B). This suggests that kinases activated downstream of the EGF receptor can indeed phosphorylate the β4 subunit at residues S1356, S1360, and/or S1364.
Our next aim was to determine whether EGF-induced phosphorylation of serines 1356, 1360, and/or 1364 prevents the association of β4 with the plectin-1A ABD. Again, transient transfection experiments in COS-7 cells were used. IL2R-β4WT or IL2R-β43xA mutant chimeras were coexpressed with the HA-plectin-1A ABD and were left untreated or were treated with EGF for 30 min. The activation of the endogenous EGF receptor was confirmed by Western blotting with phosphotyrosine antibodies. The results show that after EGF treatment, there is approximately a 55% reduction of the binding of the IL2R-β4WT with the HA-plectin-1A ABD, whereas there is no loss of binding when S1356, S1360, and S1364 are replaced by alanine (Figure 4C). In addition, we used antibodies that recognize the phosphorylated, and thus activated, forms of the classical PKCs to show that at least one isoform is indeed activated after EGF treatment (Figure 4C). These results confirm that EGF-induced phosphorylation of these residues reduces the affinity of the plectin-1A ABD for β4.
To see whether EGF can induce the disassembly of HDs in keratinocytes through the phosphorylation of S1356, S1360, and S1364, we used scatter plot analysis to quantify the results of immunofluorescence assays. Briefly, if the colocalization of two proteins is perfect, all pixels in the scatter plot will align along the diagonal and the further the pixels deviate from this line, the weaker the colocalization of the two proteins. Steady-state β4WT-expressing PA-JEB cells show typical hemidesmosomal patterning with strong colocalization of plectin and α6 (blue pixels; Figure 5, A–E). However, after EGF treatment, HDs are still present, but significant proportions of plectin (yellow pixels) and α6 (red pixels) are no longer colocalized, which is highlighted in the scatter plot (Figure 5, F–J). Analysis of their subcellular distribution revealed that the noncolocalized α6 occurred in clusters, whereas the noncolocalized plectin (yellow) was diffusely spread over the cytoplasm (Figure 5J). These results indicate that activation of the EGF receptor causes partial disruption of HDs.
To establish the contribution of β4 phosphorylation to the dissociation of α6 and plectin from HDs after EGF treatment, we used β43xA and β43xD-expressing PA-JEB keratinocytes. The results show that in the PA-JEB/β43xA cells, colocalization of α6 and plectin remains strong after EGF treatment (Figure 5, K–T), which would be expected, if phosphorylation of S1356, S1360, and/or S1364 is responsible for the decrease in α6 and plectin colocalization. In fact, the PA-JEB/β43xD cells already show a maximally reduced colocalization of α6 and plectin in the steady-state situation, which is not further decreased by EGF treatment (Figure 5, U–D′). Together, these results demonstrate that EGF induces partial HD disassembly in keratinocytes by activating downstream kinases that can phosphorylate β4 on S1356, S1360, and/or S1364, which subsequently disrupt the association between plectin and α6β4. Because HD disassembly is a prerequisite for keratinocyte migration, we next tested the ability of the PA-JEB/β4WT, PA-JEB/β43xA, and PA-JEB/β43xD keratinocytes to migrate in a scratch assay. However, the results indicated that there is no significant difference between the migration rates of the different cell lines (data not shown), and therefore we have to conclude that the CS of β4 containing S1356, S1360, and S1364 does not regulate keratinocyte migration.
DISCUSSION
In this study we have assessed the importance of serine phosphorylation of the integrin β4 subunit for the integrity of HDs. We have identified three regions of β4 that are phosphorylated in cells after treatment with calyculin A and show that activation of PKC and PKA in cells induces phosphorylation of S1360 and S1364 on β4, respectively. We also show that activation of the EGF receptor results in the phosphorylation of only S1356, S1360, and/or S1364, and this causes a partial disassembly of HDs in keratinocytes by disrupting the association between β4 with plectin. The results of the in vitro assays indicate that this is due to the inability of the plectin ABD to bind to the first pair of FnIII domains.
Independently from Rabinovitz et al. (2004), we identified S1360 as an important PKC phosphorylation site in β4. In contrast to their interpretation, however, we believe that this is the only PKC phosphorylation site on β4. Our phosphopeptide maps clearly demonstrate that replacement of S1360 by alanine prevents increased phosphorylation of the tryptic peptides derived from this region of β4 in cells upon PMA addition. We believe that background phosphorylation and a different stoichiometry of phosphorylation through the basal activity of other kinases are the cause of the phosphorylation of two tryptic phosphopeptides that contain S1360. This was supported in our calyculin A–derived maps that showed that mutation of S1356, S1360, and S1364 completely prevents phosphorylation of these two tryptic phosphopeptides, consistent with previous results after EGF treatment (Rabinovitz et al., 2004). However, it cannot be ruled out that the in vitro PKCα phosphorylation studies performed by Rabinovitz and coworkers resulted in the phosphorylation of residues, other than S1360, that are not normally phosphorylated on β4 in keratinocytes in cells or that there were nonradiolabeled phosphorylated residues in this region of β4 before isolation. Additionally, the presence of two phosphotryptic peptides indicates that phosphorylation of S1360 possibly augments the phosphorylation of either S1356 or S1364 by another kinase that is activated downstream of PMA or that the above result is due to incomplete digestion of a tryptic peptide containing only phosphoserine 1360.
We also demonstrated that S1364 is the sole PKA phosphorylation site on β4 in keratinocytes. Our studies using phosphomimic aspartic acid residues indicate that at least two of the serines at positions 1356, 1360, and 1364 on β4 must be phosphorylated to prevent it from binding to the plectin-1A ABD, which is consistent with the results obtained previously that suggest that more than one phosphorylation event in this region of β4 is necessary to cause disassembly of HDs (Rabinovitz et al., 2004). Therefore, we reasoned that activation of PKA and PKC might be sufficient to prevent the interaction. However, in our COS-7 studies we only saw a limited decrease of the binding between β4 and the ABD. This may be explained in several ways: First, the phosphorylation of the S1360 and S1364 sites is weak in our experiments and, therefore, binding is not prevented to the same extent as in the phosphomimicry assays. Second, activation of PKA and/or PKC may not be sufficient to prevent this association in cells and that still another kinase is necessary for the phosphorylation of S1356. And lastly, in COS-7 cells activation of PKA and PKC at the β4 cytoplasmic domain is not spatially coordinated (e.g., a scaffolding protein is absent). The role of PKC in β4 phosphorylation and HD disassembly has been well reported, but the possibility that PKA is not involved cannot be ruled out. Interestingly, if PKA would not be involved, another kinase(s) must be and it (they) most likely phosphorylate(s) S1356 because no other kinases are known that can phosphorylate S1364.
To simplify our studies, we decided to use EGF receptor activation to definitively determine the importance of these three phosphoserine residues in HD disassembly. We conclusively show that in keratinocytes activation of the EGF receptor induces the phosphorylation of only the peptide containing S1356, S1360, and S1364 in cells, and these events can disrupt the association of plectin with α6β4, leading to the disassembly of type II HDs as has been previously documented in COS-7 cells, and likely also of type I HDs because their formation is critically dependent on the association of β4 with plectin (Geerts et al., 1999; Koster et al., 2001; Rabinovitz et al., 2004). Because EGF is not the only factor necessary to induce cell migration, other growth factors or cytokines might activate downstream kinases that result in a more robust phosphorylation of β4 at S1356, S1360, and/or S1364 or activate kinases necessary to phosphorylate β4 in other regions to induce HD disassembly. Although our calyculin A phosphopeptide maps clearly show that other serine or threonine residues can be phosphorylated on β4, these studies are complicated by the fact that there are 36 of these residues in the region between amino acids 1382–1487 in β4, and therefore we initially decided to focus on S1356, S1360, and S1364 because they had been previously implicated in HD disassembly. Furthermore, it cannot be ruled out that in addition to the phosphorylation of the above serines, phosphorylation of other components of HDs or other mechanisms altogether might lead to their disassembly. In fact, plectin is phosphorylated at its C-terminus by both p34cdc2 and PKA (Malecz et al., 1996), whereas PKC phosphorylates BP180 and thus dissociates it from HDs (Kitajima et al., 1999).
Interestingly, activation of the EGF receptor has been shown to induce the phosphorylation of β4 on multiple residues, including tyrosine, in carcinoma cells (Mainiero et al., 1996; Mariotti et al., 2001). Because β4 is often not localized at the basal side of carcinoma cells, as it is in keratinocytes, but is instead distributed over the entire plasma membrane, β4 has access to kinases that it does normally not have access to at the basal side of keratinocytes. The resulting aberrant phosphorylation of the β4 subunit most likely explains its assumed function as an adapter protein for tyrosine kinases and their associated signaling proteins in cancer cells (reviewed in Wilhelmsen et al., 2006). In any case, these studies highlight the necessary tight control and spatiotemporal regulation of the phosphorylation of β4 in basal keratinocytes.
We found it interesting that the regions of β4 that are phosphorylated after calyculin A, PMA, FSK/IBMX, or EGF treatment are not directly involved in binding to the plectin ABD, although they regulate this binding. In fact, it has been previously shown that the regions that are phosphorylated can be completely deleted, while the ability of β4 to bind plectin and to form type II HDs is retained (Niessen et al., 1997). There are several possible explanations for this regulation of binding: First, electrostatic repulsion weakens the affinity of β4 for plectin. Second, upon phosphorylation, the β4 cytoplasmic domain may adopt a conformation that is suboptimal for plectin binding. In fact, we have shown previously that the conformation of the β4 cytoplasmic domain may be important for plectin recruitment (Koster et al., 2004). Thirdly, and the most intriguing explanation to us, the phosphorylated CS may compete with plectin for binding to the first pair of FnIII domains. The second FnIII domain contains two arginine residues (R1225 and R1281) that are essential for plectin binding (Koster et al., 2001). In fact, mutation of these residues in human patients causes a nonlethal form of junctional epidermolysis bullosa (Pulkkinen et al., 1998; Nakano et al., 2001). It is spatially possible that two of the three phosphorylated residues in the CS bind to these arginine residues, thereby sterically interfering with plectin binding, and thus regulating the interaction between plectin and β4.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. K. Owaribe for the mAb 121 against HD1/plectin. This work was supported by a grant from the Dutch Cancer Society.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-04-0306) on July 5, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Bertotti A., Comoglio P. M., Trusolino L. β4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth. J. Cell Biol. 2006;175:993–1003. doi: 10.1083/jcb.200605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–148. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Gache Y., Chavanas S., Lacour J. P., Wiche G., Owaribe K., Meneguzzi G., Ortonne J. P. Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J. Clin. Invest. 1996;97:2289–2298. doi: 10.1172/JCI118671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts D., Fontao L., Nievers M. G., Schaapveld R. Q., Purkis P. E., Wheeler G. N., Lane E. B., Leigh I. M., Sonnenberg A. Binding of integrin α6β4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J. Cell Biol. 1999;147:417–434. doi: 10.1083/jcb.147.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuijen C. A., Sonnenberg A. Dynamics of the α6β4 integrin in keratinocytes. Mol. Biol. Cell. 2002;13:3845–3858. doi: 10.1091/mbc.02-01-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice G. J., Emery D. J., Diaz L. A. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J. Invest. Dermatol. 1992;99:243–250. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- Hieda Y., Nishizawa Y., Uematsu J., Owaribe K. Identification of a new hemidesmosomal protein, HD1, a major, high molecular mass component of isolated hemidesmosomes. J. Cell Biol. 1992;116:1497–1506. doi: 10.1083/jcb.116.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., et al. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Commun. 1989;159:871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Kurpakus M. A., Cooper H. M., Quaranta V. A function for the integrin α6β4 in the hemidesmosome. Cell Regul. 1991;2:427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima Y., Aoyama Y., Seishima M. Transmembrane signaling for adhesive regulation of desmosomes and hemidesmosomes, and for cell-cell detachment induced by pemphigus IgG in cultured keratinocytes: involvement of protein kinase C. J. Investig. Dermatol. Symp. Proc. 1999;4:137–144. doi: 10.1038/sj.jidsp.5640197. [DOI] [PubMed] [Google Scholar]
- Koster J., Kuikman I., Kreft M., Sonnenberg A. Two different mutations in the cytoplasmic domain of the integrin β4 subunit in nonlethal forms of epidermolysis bullosa prevent interaction of β4 with plectin. J. Invest. Dermatol. 2001;117:1405–1411. doi: 10.1046/j.0022-202x.2001.01567.x. [DOI] [PubMed] [Google Scholar]
- Koster J., van Wilpe S., Kuikman I., Litjens S. H., Sonnenberg A. Role of binding of plectin to the integrin β4 subunit in the assembly of hemidesmosomes. Mol. Biol. Cell. 2004;15:1211–1223. doi: 10.1091/mbc.E03-09-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litjens S. H., de Pereda J. M., Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–383. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Litjens S. H., Koster J., Kuikman I., van Wilpe S., de Pereda J. M., Sonnenberg A. Specificity of binding of the plectin actin-binding domain to β4 integrin. Mol. Biol. Cell. 2003;14:4039–4050. doi: 10.1091/mbc.E03-05-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F., Pepe A., Yeon M., Ren Y., Giancotti F. G. The intracellular functions of α6β4 integrin are regulated by EGF. J. Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecz N., Foisner R., Stadler C., Wiche G. Identification of plectin as a substrate of p34cdc2 kinase and mapping of a single phosphorylation site. J. Biol. Chem. 1996;271:8203–8208. doi: 10.1074/jbc.271.14.8203. [DOI] [PubMed] [Google Scholar]
- Mariotti A., Kedeshian P.A., Dans M., Curatola A. M., Gagnoux-Palacios L., Giancotti F. G. EGF-R signaling through Fyn kinase disrupts the function of integrin α6β4 at hemidesmosomes: role in epithelial cell migration and carcinoma. invasion. J. Cell Biol. 2001;155:447–458. doi: 10.1083/jcb.200105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A., Pulkkinen L., Murrell D., Rico J., Lucky A. W., Garzon M., Stevens C. A., Robertson S., Pfendner E., Uitto J. Epidermolysis bullosa with congenital pyloric atresia: novel mutations in the β4 integrin gene (ITGB4) and genotype/phenotype correlations. Pediatr. Res. 2001;49:618–626. doi: 10.1203/00006450-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos S. N., Blaikie P., Yoshioka T., Guo W., Puri C., Tacchetti C., Giancotti F. G. Targeted deletion of the integrin β4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-κB, causing defects in epidermal growth and migration. Mol. Cell. Biol. 2005;25:6090–6102. doi: 10.1128/MCB.25.14.6090-6102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen C. M., Hulsman E. H., Oomen L. C., Kuikman I., Sonnenberg A. A minimal region on the integrin β4 subunit that is critical to its localization in hemidesmosomes regulates the distribution of HD1/plectin in COS-7 cells. J. Cell Sci. 1997;110:1705–1716. doi: 10.1242/jcs.110.15.1705. [DOI] [PubMed] [Google Scholar]
- Niessen C. M., Hulsman E. H., Rots E. S., Sanchez-Aparicio P., Sonnenberg A. Integrin α6β4 forms a complex with the cytoskeletal protein HD1 and induces its redistribution in transfected COS-7 cells. Mol. Biol. Cell. 1997;8:555–566. doi: 10.1091/mbc.8.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievers M. G., Kuikman I., Geerts D., Leigh I. M., Sonnenberg A. Formation of hemidesmosome-like structures in the absence of ligand binding by the α6β4 integrin requires binding of HD1/plectin to the cytoplasmic domain of the β4 integrin subunit. J. Cell Sci. 2000;113:963–973. doi: 10.1242/jcs.113.6.963. [DOI] [PubMed] [Google Scholar]
- Nievers M. G., Schaapveld R. Q., Oomen L. C., Fontao L., Geerts D., Sonnenberg A. Ligand-independent role of the β4 integrin subunit in the formation of hemidesmosomes. J. Cell Sci. 1998;111:1659–1672. doi: 10.1242/jcs.111.12.1659. [DOI] [PubMed] [Google Scholar]
- Oda K., Matsuoka Y., Funahashi A., Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 2005;1:2005–0010. doi: 10.1038/msb4100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian-Rousseau V., Aberdam D., Fontao L., Chevalier L., Meneguzzi G., Kedinger M., Simon-Assmann P. Developmental expression of laminin-5 and HD1 in the intestine: epithelial to mesenchymal shift for the laminin γ2 chain subunit deposition. Dev. Dyn. 1996;206:12–23. doi: 10.1002/(SICI)1097-0177(199605)206:1<12::AID-AJA2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L., Rouan F., Bruckner-Tuderman L., Wallerstein R., Garzon M., Brown T., Smith L., Carter W., Uitto J. Novel ITGB4 mutations in lethal and nonlethal variants of epidermolysis bullosa with pyloric atresia: missense versus nonsense. Am. J. Hum. Genet. 1998;63:1376–1387. doi: 10.1086/302116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz I., Toker A., Mercurio A. M. Protein kinase C-dependent mobilization of the α6β4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J. Cell Biol. 1999;146:1147–1160. doi: 10.1083/jcb.146.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz I., Tsomo L., Mercurio A. M. Protein kinase C-alpha phosphorylation of specific serines in the connecting segment of the β4 integrin regulates the dynamics of type II hemidesmosomes. Mol. Cell. Biol. 2004;24:4351–4360. doi: 10.1128/MCB.24.10.4351-4360.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezniczek G. A., de Pereda J. M., Reipert S., Wiche G. Linking integrin α6β4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the β4 subunit and plectin at multiple molecular sites. J. Cell Biol. 1998;141:209–225. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. M., Gaudino G., Marchisio P. C. The MSP receptor regulates α6β4 and α3β1 integrins via 14-3-3 proteins in keratinocyte migration. Dev. Cell. 2003;5:257–271. doi: 10.1016/s1534-5807(03)00201-6. [DOI] [PubMed] [Google Scholar]
- Schaapveld R. Q., Borradori L., Geerts D., van Leusden M. R., Kuikman I., Nievers M. G., Niessen C. M., Steenbergen R. D., Snijders P. J., Sonnenberg A. Hemidesmosome formation is initiated by the β4 integrin subunit, requires complex formation of β4 and HD1/plectin, and involves a direct interaction between β4 and the bullous pemphigoid antigen 180. J. Cell Biol. 1998;142:271–284. doi: 10.1083/jcb.142.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc. Natl. Acad. Sci. USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., et al. Integrin α6β4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J. Cell Biol. 1991;113:907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Janssen H., Hogervorst F., Calafat J., Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J. Biol. Chem. 1987;262:10376–11083. [PubMed] [Google Scholar]
- Stanley J. R., Hawley-Nelson P., Yuspa S. H., Shevach E. M., Katz S, I. Characterization of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981;24:897–903. doi: 10.1016/0092-8674(81)90115-x. [DOI] [PubMed] [Google Scholar]
- Stepp M. A., Spurr-Michaud S., Tisdale A., Elwell J., Gipson I. K. α6β4 integrin heterodimer is a component of hemidesmosomes. Proc. Natl. Acad. Sci. USA. 1990;87:8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterk L. M., Geuijen C. A., Oomen L. C., Calafat J., Janssen H., Sonnenberg A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin α6β4 and may regulate the spatial organization of hemidesmosomes. J. Cell Biol. 2000;149:969–982. doi: 10.1083/jcb.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu J., Nishizawa Y., Sonnenberg A., Owaribe K. Demonstration of type II hemidesmosomes in a mammary gland epithelial cell line, BMGE-H. J. Biochem. (Tokyo) 1994;115:469–476. doi: 10.1093/oxfordjournals.jbchem.a124361. [DOI] [PubMed] [Google Scholar]
- van der Geer P., Luo K., Sefton B. M., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis on cellulose thin-layer plates. In: Hardie D. G., editor. Protein Phosphorylation; A Practical Approach. Oxford: IRL Press; 1993. pp. 31–59. [Google Scholar]
- Wilhelmsen K., Burkhalter S., van der Geer P. C-Cbl binds the CSF-1 receptor at tyrosine 973, a novel phosphorylation site in the receptor's carboxy-terminus. Oncogene. 2002;21:1079–1089. doi: 10.1038/sj.onc.1205166. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K., Litjens S. H., Sonnenberg A. Multiple functions of the integrin α6β4 in epidermal homeostasis and tumorigenesis. Mol. Cell. Biol. 2006;26:2877–2886. doi: 10.1128/MCB.26.8.2877-2886.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur. J. Biochem. 1986;161:177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.