Abstract
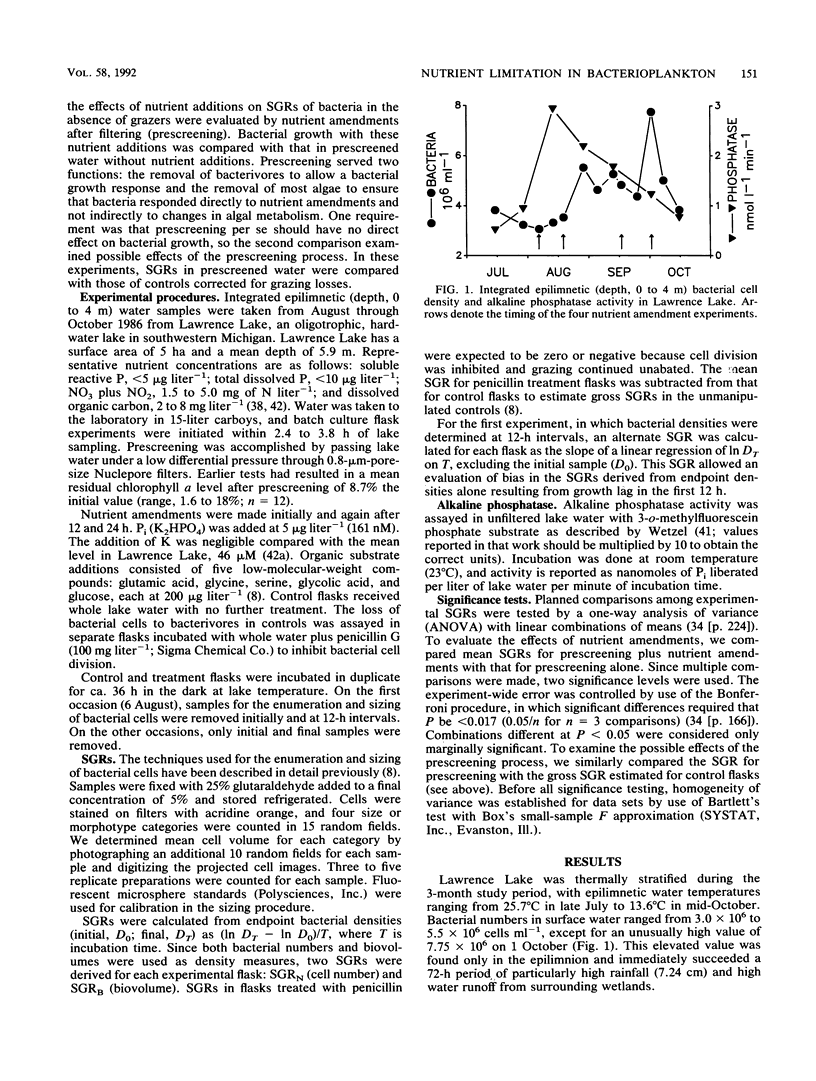
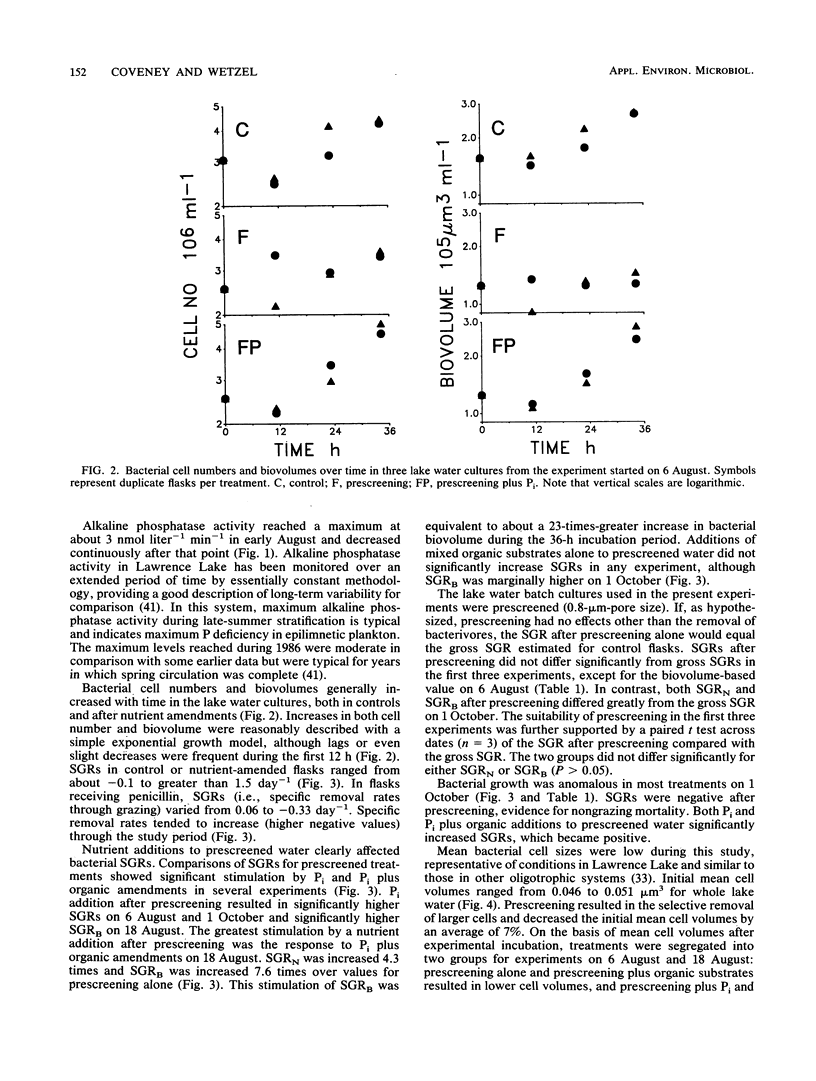
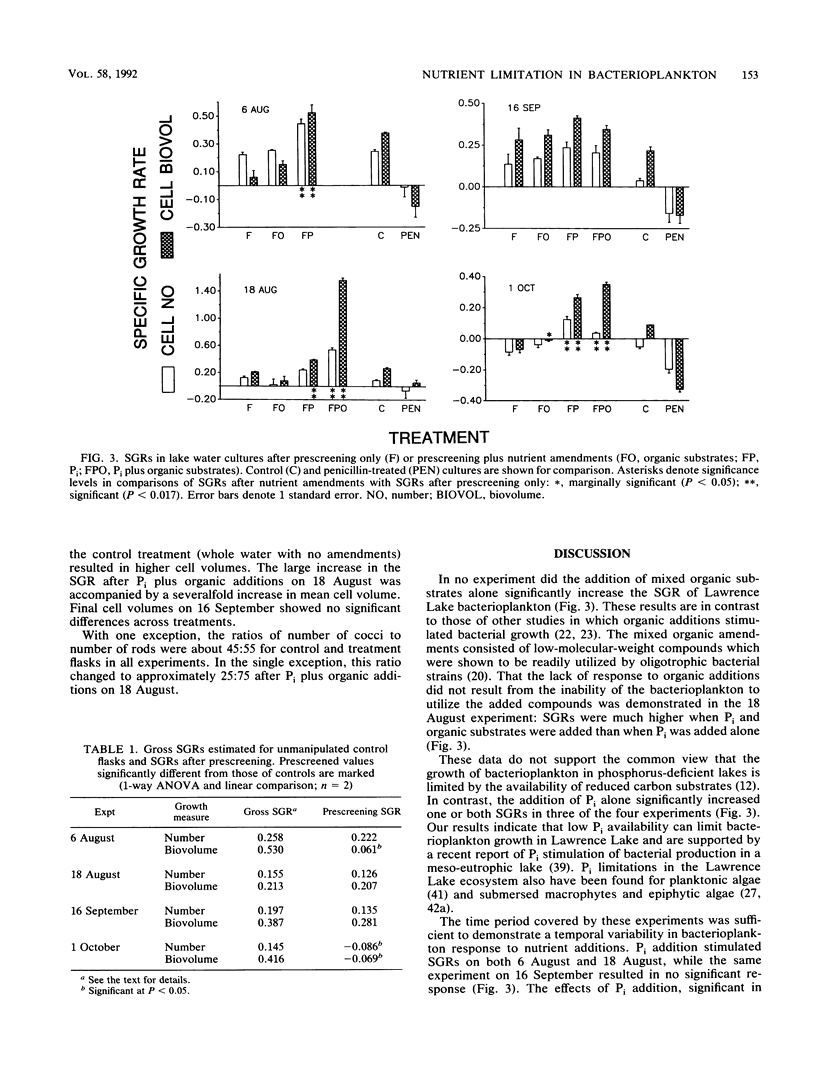
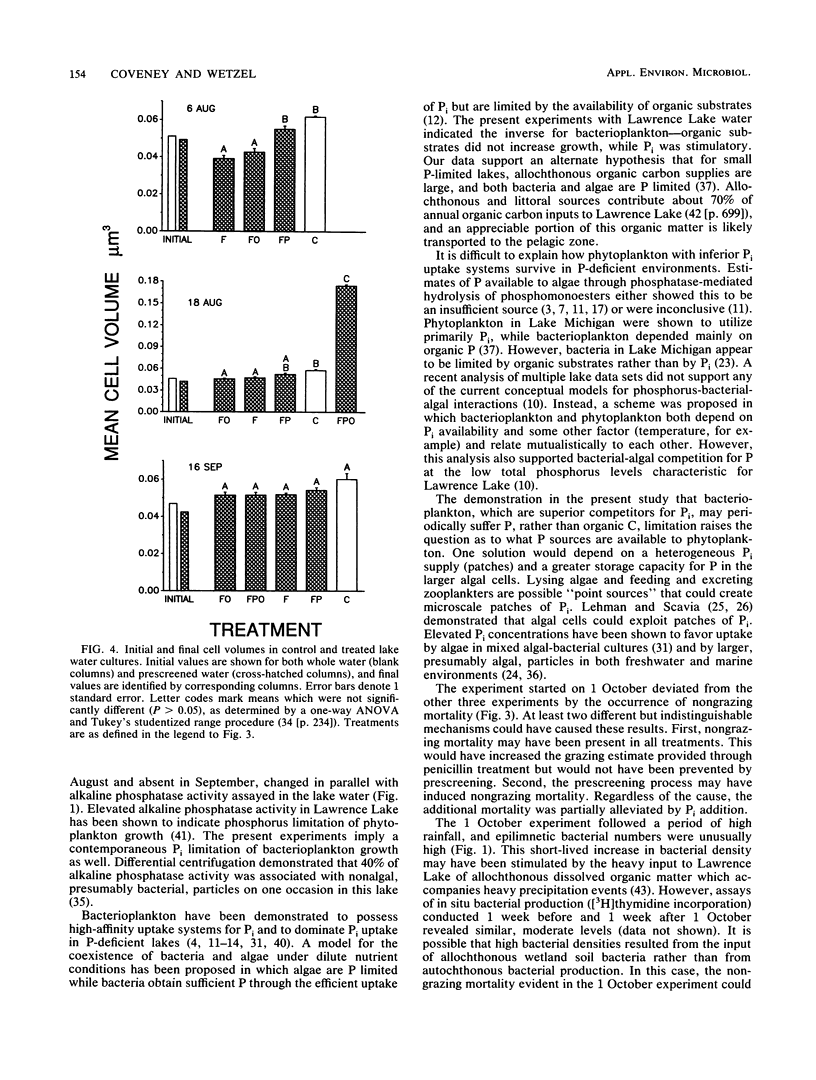
The effects of organic and inorganic nutrient additions on the specific growth rates of bacterioplankton in oligotrophic lake water cultures were investigated. Lake water was first passed through 0.8-μm-pore-size filters (prescreening) to remove bacterivores and to minimize confounding effects of algae. Specific growth rates were calculated from changes in both bacterial cell numbers and biovolumes over 36 h. Gross specific growth rates in unmanipulated control samples were estimated through separate measurements of grazing losses by use of penicillin. The addition of mixed organic substrates alone to prescreened water did not significantly increase bacterioplankton specific growth rates. The addition of inorganic phosphorus alone significantly increased one or both specific growth rates in three of four experiments, and one experiment showed a secondary stimulation by organic substrates. The stimulatory effects of phosphorus addition were greatest concurrently with the highest alkaline phosphatase activity in the lake water. Because bacteria have been shown to dominate inorganic phosphorus uptake in other P-deficient systems, the demonstration that phosphorus, rather than organic carbon, can limit bacterioplankton growth suggests direct competition between phytoplankton and bacterioplankton for inorganic phosphorus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coveney M. F., Wetzel R. G. Experimental evaluation of conversion factors for the [h]thymidine incorporation assay of bacterial secondary productivity. Appl Environ Microbiol. 1988 Aug;54(8):2018–2026. doi: 10.1128/aem.54.8.2018-2026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. G., Chrzanowski T. H. Impact of storms on heterotrophic activity of epilimnetic bacteria in a southwestern reservoir. Appl Environ Microbiol. 1986 Jun;51(6):1259–1263. doi: 10.1128/aem.51.6.1259-1263.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. T., Scavia D. Microscale nutrient patches produced by zooplankton. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5001–5005. doi: 10.1073/pnas.79.16.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. T., Scavia D. Microscale patchiness of nutrients in plankton communities. Science. 1982 May 14;216(4547):729–730. doi: 10.1126/science.216.4547.729. [DOI] [PubMed] [Google Scholar]
- Pomeroy L. R., Deibel D. Temperature regulation of bacterial activity during the spring bloom in newfoundland coastal waters. Science. 1986 Jul 18;233(4761):359–361. doi: 10.1126/science.233.4761.359. [DOI] [PubMed] [Google Scholar]
- Toolan T., Wehr J. D., Findlay S. Inorganic phosphorus stimulation of bacterioplankton production in a meso-eutrophic lake. Appl Environ Microbiol. 1991 Jul;57(7):2074–2078. doi: 10.1128/aem.57.7.2074-2078.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


